Abstract
COVID‐19‐associated pulmonary aspergillosis (CAPA) is a recently recognized entity associated with the COVID‐19 pandemic and known post‐viral pneumonia complications. More data are awaited and there has been a recent consensus criteria published hoping to generate more research and registries to inform clinical decision‐making. Nevertheless, it is clear that CAPA imposes a worsening disease course of COVID‐19 pneumonia with added morbidity and mortality. We present two cases with differing outcomes managed within the limitations of our institute and make reference to the recent consensus criteria. We hope to highlight the importance of considering empirical treatment in the correct clinical context while awaiting the results of microbiological workup as ascertaining the diagnosis of proven CAPA is challenging in the real‐world setting.
Keywords: aspergillosis, COVID‐19, COVID‐associated pulmonary aspergillosis, infection, inflammation
COVID‐19‐associated pulmonary aspergillosis (CAPA) is a recently recognized entity associated with the COVID‐19 pandemic and known post‐viral pneumonia complications. We present two cases with differing outcomes managed within the limitations of our institute and make reference to the recent consensus criteria. We hope to highlight the importance of considering empirical treatment in the correct clinical context while awaiting the results of microbiological workup as ascertaining the diagnosis of proven CAPA is challenging in the real‐world setting.
INTRODUCTION
Viral pneumonia predisposes patients to secondary bacterial or fungal infections. Consequently, there have been emerging reports of COVID‐19‐associated pulmonary aspergillosis (CAPA) which contributes to higher mortality in COVID infection. 1
We report two cases diagnosed to have CAPA based on clinical, microbiological and radiological evidence, and hope to illustrate the importance of recognizing this entity.
CASE REPORT
Patient 1
A 62‐year‐old lady with comorbidity of dyslipidaemia was admitted with severe COVID‐19 pneumonia needing mechanical ventilation. She had her second dose of vaccination (Sinovac) 12 days prior to admission. She was initiated on intravenous methylprednisolone 250 mg daily for 5 days followed by dexamethasone 16 mg daily for 5 days and then 8 mg daily for another 5 days. She was successfully extubated but remained oxygen‐dependent via Venturi mask. Thereafter, she was on tapering oral prednisolone for over 6 weeks.
A repeated high‐resolution computed tomography (CT) thorax (day 40) showed a cavitary lesion in the right upper lobe with an intra‐cavitary hyperdense lesion not seen in the previous CT thorax (day 8) (Figure 1). CAPA was suspected and voriconazole was initiated empirically while awaiting diagnostic workup. Bronchoscopy was not performed due to the considerable risk of worsening respiratory failure.
FIGURE 1.
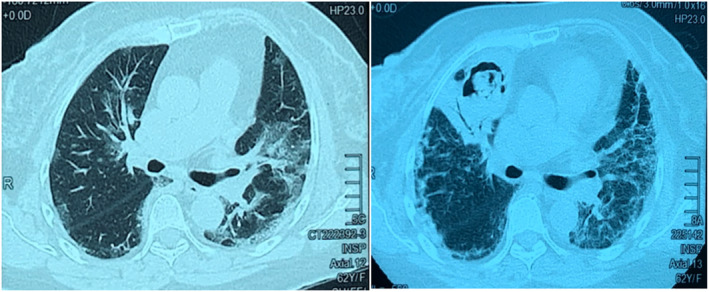
Patient 1. Computed tomography (CT) thorax on day 8 of COVID‐19 infection (left panel) showed bilateral peri‐bronchovascular ground‐glass opacity. On day 40 of COVID‐19 infection (right panel), CT showed new cavitating lesion at the anterior segment of the right upper lobe with soft tissue within it. Consolidative changes were noted adjacent to the cavity
She showed marked improvement after the initiation of intravenous voriconazole 6 mg/kg for two doses followed by 4 mg/kg twice daily before converting to oral voriconazole 4 mg/kg. She was able to be discharged with an oxygen concentrator (1 L/min). Her sputum and blood microbiological workup (including sputum GenXpert MTB DNA) were negative. Her serum galactomannan (galactomannan enzyme immuno‐assay [GM‐EIA], with a turnaround time of 5 days) was positive later on, with index of 2.351. Her contrast CT thorax 1 month after aspergillosis treatment showed reduction in the size of the intra‐cavitary lesion. She was on voriconazole treatment planned for a total duration of 6 months with weekly therapeutic drug monitoring.
Patient 2
A 56‐year‐old lady with comorbidity of diabetes mellitus was admitted for severe COVID‐19 pneumonia. She had not been vaccinated against COVID‐19. She was treated with intravenous methylprednisolone 250 mg daily on admission and then with intravenous dexamethasone 16 mg daily for 7 days and 8 mg daily for 3 days before converting to tapering oral prednisolone course.
Post‐acute phase, she became dependent on high‐flow nasal cannula oxygen therapy due to multiple bacterial nosocomial pneumonias. She developed haemoptysis on day 30 of illness. CT thorax showed cavities in bilateral upper lobes containing soft tissue (Figure 2). She was suspected to have CAPA and voriconazole was initiated empirically. She was similarly deemed to be at high risk for a bronchoscopy.
FIGURE 2.
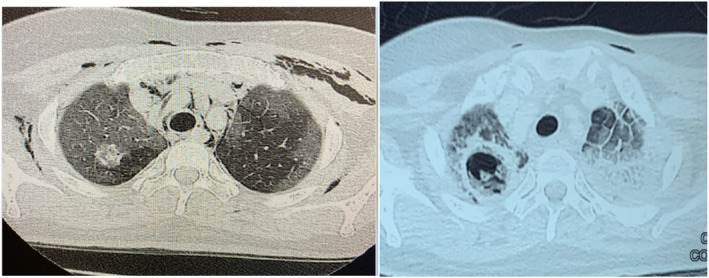
Patient 2. Computed tomography thorax on day 13 of COVID‐19 infection showed peripheral ground‐glass opacity with extensive pneumomediastinum and subcutaneous emphysema. Round heterogenous hyperdensity of the right upper lobe was noted raising the suspicion of infection (tuberculosis, fungal or bacterial) or malignancy, although less likely. On day 32 of COVID‐19 infection, a new cavitating lesion was noted at the right upper lobe where the nodule was seen. Previously seen ground‐glass opacity at the left lung appeared more dense
A full microbiological workup was performed (including sputum GenXpert MTB DNA). Unfortunately, she continued to deteriorate and succumbed after 1 week. Her final bacterial microbiology workup as well as the tuberculosis samples (acid fast bacilli direct smears, GenXpert) were negative. Her serum galactomannan (GM‐EIA) was positive with index of 4.155, and her sputum culture (with a turnaround time of 42 days) recovered Aspergillus niger. Her clinical picture towards the end was dominated by the poor lung reserve (post‐COVID‐19 lung sequelae) and other superimposed infections, possibly explaining the lack of response to voriconazole (Table 1).
TABLE 1.
Demographics and presentation of patients
| Age/ gender | Comorbidity | Mechanical ventilation | Respiratory sample (number of positive sample) | Radiological evidence of aspergillosis | Serum galactomannan | Other organisms | Treatment | Outcome | |
|---|---|---|---|---|---|---|---|---|---|
| Patient 1 | 62/F | None | Yes | Not sent | Day 40 of illness | Positive | Pseudomonas aeruginosa |
Voriconazole Steroid |
Alive |
| Patient 2 | 56/F | Diabetes | No | Aspergillus niger (1) | Day 32 of illness | Positive | Stenotrophomonas maltophilia |
Voriconazole Steroid |
Dead |
DISCUSSION
Prior to the era of COVID pandemic, co‐infection of aspergillosis in pulmonary viral infection has been well recognized in influenza infection. 2 Influenza‐associated pulmonary aspergillosis (IAPA) was thought to be due to the damaged epithelial of airway from viral infection resulting in invasion of aspergillosis. We believed CAPA shares the similar pathogenesis with IAPA.
Clinical manifestations of CAPA are not well described yet. One of our patients had no specific symptoms, while the other patient reported haemoptysis. The time duration between admission and positive culture specimen sent for patients with CAPA in the literature was a median of 8 days. There was delayed diagnosis in both patients as they were diagnosed with CAPA at day 40 and day 30, respectively, after the onset of COVID‐19. This could be due to a low index of suspicion as this entity of CAPA was not well recognized at that point of time.
It is recommended that in COVID‐19 patients with respiratory failure who remained critically ill for 5–14 days despite receiving all support, the following clinical presentation can trigger diagnostic investigations for CAPA: (1) refractory fever for more than 3 days duration or a new fever after a period of defervescence of longer than 48 h during appropriate antibiotic therapy, in the absence of any other obvious cause; (2) worsening respiratory status; (3) haemoptysis; and (4) pleural friction rub or chest pain. 3 Risk factors of CAPA include prolonged steroid use, diabetes mellitus, chronic kidney disease and chronic lung disease. 4
Diagnosis of CAPA was proposed to be made at three different grades as per a recent consensus definition: proven, probable and possible; based on microbiological, radiological and clinical evidence. 3 However, for microbiological criterion, the proposed diagnostic algorithm required lung biopsy for histology examination and tissue culture to reach proven diagnosis of CAPA. This certainly reduces the rate of diagnosis as a significant number of patients are not medically fit for invasive procedure when they are suspected to have CAPA.
Although not being the most common Aspergillus seen in CAPA, in one case series of nine patients, three were attributed to A. niger. 5 Given the positive serum galactomannan result and the radiological findings and exclusion of other causes for deterioration, the most likely explanation is CAPA. A positive serum GM result (≥0.5) would be highly suspicious for CAPA but a negative result should not be used to exclude the diagnosis. 6
Whilst biomarkers are usually validated for bronchoalveolar lavage and serum, these are not validated in tracheal aspirate, sputum and non‐bronchoscopic lavage specimens. Therefore, cut‐off values are not established, results should be interpreted with caution and their value in patient classification is uncertain presently. For patients with COVID‐19 who are at high risk of IPA, consensus criteria can be used as they were specifically developed to challenge the difficulties of non‐validated specimens for key tests in CAPA diagnosis. Aspergillus infection or colonization can both give a positive culture from lower respiratory tract sampling. To differentiate these two conditions, evaluation of predisposing factors, in conjunction with immunological tests and examination of bronchoscopic specimens, is important. In addition to that, Aspergillus antibody maybe helpful as 55% of the patients with Aspergillus infection will have a positive anti‐Aspergillus antibody, whereas this will be negative in almost all the cases of colonization. 7
Radiological findings in CAPA, including nodules with cavities and dendritic signs, air crescent, reverse halo sign, nodular consolidation, ground‐glass opacities, crazy‐paving pattern, pleural effusion and pulmonary cysts, were reported among patients with CAPA by other reports. 8 Our patients developed new cavities with soft tissues within it, forming a typical crescent sign. Sputum fungal culture of patient 1 was not available. However, she demonstrated significant clinical improvement after the initiation of voriconazole added on to the confidence level of diagnosis of CAPA in this case. Serum galactomannan for both patients was strongly positive. As discussed earlier, serum galactomannan is highly specific in non‐neutropaenic patients. Other microbiological evidence that supports the diagnosis of CAPA are histopathological examination of lung biopsy, bronchioalveolar lavage or lower respiratory tract specimen for fungal culture and Aspergillus polymerase chain reaction (PCR), serum lateral flow assay and Aspergillus PCR. 3
CAPA is associated with higher mortality rate in COVID infection especially in critically ill patients. 2 Complications of CAPA include acute respiratory distress syndrome, liver damage and acute kidney injury. Treatment of CAPA is voriconazole loading dose at 6 mg/kg twice a day for two doses, followed by 4 mg/kg twice a day. Daily isavuconazole treatment has similar clinical activity to voriconazole but with less adverse effect. Other options of treatment include amphotericin B and posaconazole. Echinocandin is not recommended to be used alone. However, combination of echinocandin with azole group anti‐fungal may have therapeutic advantage in azole‐resistant cases. 3
The two patients described above were not known to have the typical risk factor of aspergillosis infection prior to COVID infection. However, there is possible associated risk of corticosteroid treatment for CAPA. Alveolar macrophages are the primary innate cellular defence against inhaled Aspergillus conidia. Corticosteroids suppresses nuclear factor kappa B transcription and inhibits oxidative and non‐oxidative mechanisms that are important for damaging Aspergillus conidia and hyphae. In addition, pro‐inflammatory (tumour necrosis factor‐alpha and interferon‐gamma) and anti‐inflammatory (IL‐10) cytokines were reduced in polymorphonuclear neutrophils (PMN), abolishing PMN‐mediated damage of Aspergillus hyphae. 8
In both cases, we initiated empirical voriconazole on the grounds of strong clinical suspicion and in the best interest of the patients due to the concern that delayed treatment could have deleterious impact. Nevertheless, we strongly endorse the practice of pursuing microbiological evidence to achieve a definitive diagnosis. Of note, both our cases are classified as ‘probable CAPA’ as per 2020 European Confederation of Medical Mycology (ECMM) and the International Society for Human and Animal Mycology (ISHAM) ‐ consensus definition. 3 It would have been desirable to pursue a histology or a tissue culture but our patients' condition did not permit that.
We agree on the role of screening for CAPA in the intensive care unit (ICU). As early treatment can be instituted, mortality rates can be reduced. The ECMM/ISHAM 2020 consensus recommended screening with serum GM‐EIA, lateral flow assay or lateral flow device (if GM‐EIA is unavailable) three times per week, if locally available, until discharge from the ICU or defervescence for longer than 7 days with improved lung function. Ideally, this type of screening can be accompanied by regular screening of respiratory samples (such as non‐bronchoscopic lavage, tracheal aspirate or sputum) with culture, PCR and galactomannan. However, the cost effectiveness of the diagnostic test and infectious risk to health care workers during the diagnostic screening need to be evaluated. We hope more data will surface to better inform us the best clinical practice when faced with this new entity.
Increasing reports of CAPA indicate that this entity is not negligible and should be part of our differentials in the post‐acute COVID‐19 setting. With the presence of a consensus definition, it is hoped more research is undertaken, and registries are initiated. These two cases illustrate the importance of early suspicion and consideration for empirical treatment while awaiting microbiological evidence.
CONFLICT OF INTEREST
None declared.
AUTHOR CONTRIBUTION
Jin Lee Lim, Inn Shih Khor and Kumaresh Raj Lachmanan conceptualized the case writing. Jin Lee Lim, Cheng Keat Moh and Yi Min Chan drafted the manuscript and furnished the supplementary documents. Inn Shih Khor, Yoke Fong Lam, and Kumaresh Raj Lachmanan revised the manuscript. All authors read the final manuscript.
ETHICS STATEMENT
The authors declare that appropriate written informed consent was obtained for the publication of this manuscript and accompanying images.
Lim JL, Khor IS, Moh CK, Chan YM, Lam YF, Lachmanan KR. Two cases of COVID‐19‐associated pulmonary aspergillosis (CAPA). Respirology Case Reports. 2022;10:e0940. 10.1002/rcr2.940
Associate Editor: Jonathan Williamson
DATA AVAILABILITY STATEMENT
No data are available.
REFERENCES
- 1. Bartoletti M, Pascale R, Cricca M, Rinaldi M, Maccaro A, Bussini L, et al. Epidemiology of invasive pulmonary aspergillosis among intubated patients with COVID‐19: a prospective study. Clin Infect Dis. 2021;73(11):e3606–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Liu WL, Yu WL, Chan KS, Yang CC, Wauters J, Verweij PE. Aspergillosis related to severe influenza: a worldwide phenomenon? Clin Respir J. 2019;13(8):540–2. [DOI] [PubMed] [Google Scholar]
- 3. Koehler P, Bassetti M, Chakrabarti A, Chen SC, Colombo AL, Hoenigl M, et al. Defining and managing COVID‐19‐associated pulmonary aspergillosis: the 2020 ECMM/ISHAM consensus criteria for research and clinical guidance. Lancet Infect Dis. 2021;21(6):e149–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Wang J, Yang Q, Zhang P, Sheng J, Zhou J, Qu T. Clinical characteristics of invasive pulmonary aspergillosis in patients with COVID‐19 in Zhejiang, China: a retrospective case series. Crit Care. 2020;24:1–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Nasir N, Farooqi J, Mahmood SF, Jabeen K. COVID‐19‐associated pulmonary aspergillosis (CAPA) in patients admitted with severe COVID‐19 pneumonia: an observational study from Pakistan. Mycoses. 2020;63(8):766–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Armstrong‐James D, Youngs J, Bicanic T, Abdolrasouli A, Denning DW, Johnson E, et al. Confronting and mitigating the risk of COVID‐19 associated pulmonary aspergillosis. Eur Respir J. 2020;56(4):2002554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Uffredi ML, Mangiapan G, Cadranel J, Kac G. Significance of Aspergillus fumigatus isolation from respiratory specimens of nongranulocytopenic patients. Eur J Clin Microbiol Infect Dis. 2003;22(8):457–62. [DOI] [PubMed] [Google Scholar]
- 8. Lewis RE, Kontoyiannis DP. Invasive aspergillosis in glucocorticoid‐treated patients. Med Mycol. 2009;47(Suppl 1):S271–81. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
No data are available.


