Short abstract
Content available: Author Audio Recording
Abbreviations
- BMI
body mass index
- CT
computed tomography
- DEXA
dual‐energy x‐ray absorptiometry
- FFA
free fatty acid
- MRI
magnetic resonance imaging
- NAFLD
nonalcoholic fatty liver disease
- NASH
nonalcoholic steatohepatitis
- PPAR
peroxisome proliferator‐activated receptor
- SAT
subcutaneous adipose tissue
- TLR
Toll‐like receptor
- TZD
thiazolidinediones
- VAT
visceral adipose tissue
- WC
waist circumference
Listen to an audio presentation of this article.
Abdominal obesity describes multiple fat compartments including visceral adipose tissue (VAT) and subcutaneous adipose tissue (SAT), each of which carry significantly different metabolic risk. SAT is the largest adipose tissue depot and serves largely as protective lipid storage. When this storage capacity is exceeded, fat deposits outside of subcutaneous tissue such as into visceral tissue, the epicardium, and the liver. These ectopic fats promote insulin resistance, local inflammation, and atherogenic changes. 1 , 2 , 3 , 4 VAT in particular is associated with cardiovascular risk, insulin resistance, metabolic unhealthy obesity, and hepatic steatosis. 1 , 2 The distribution of lipids among SAT, VAT, and liver tissue has been implicated in metabolic hemostasis and progression of nonalcoholic fatty liver disease (NAFLD). 1 , 4 , 5
Current clinical tools for estimating fat do not differentiate between these fat depots, and adipose reduction therapy is still aimed at overall weight loss rather than tissue‐targeted therapy. The goal of this review is to describe the role of VAT in the development of NAFLD, clinical tools aimed at estimating VAT, and future therapies being evaluated for NAFLD treatment via VAT reduction.
Pathogenesis of VAT in NAFLD Progression
VAT is a well‐demonstrated independent risk factor for type 2 diabetes as well as for cardiovascular and metabolic disease via its important role in atherogenic dyslipidemia, insulin resistance, pro‐inflammatory states, and elevated blood pressure 1 , 2 , 3 , 4 (Fig. 1). 3 Visceral fat releases increased free fatty acids (FFAs) and triglycerides into the circulation and can cause inhibition of glucose uptake, glucose oxidation, and glycogen synthesis via several mechanisms as seen in Fig. 2. Increased FFAs to the liver via the portal vein also act as ligands for Toll‐like receptor (TLR) 4 and induce cytokine production, thereby contributing to inflammation pathways associated with NAFLD. 5 , 6
FIG 1.
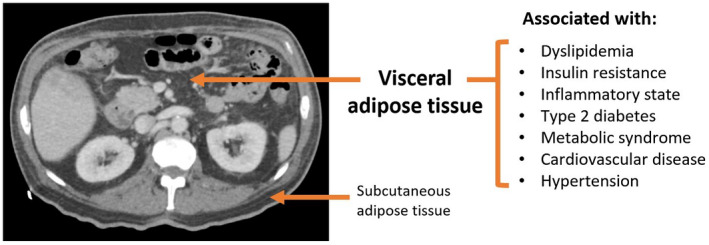
Cross‐section of abdomen with visceral versus subcutaneous fat.
FIG 2.
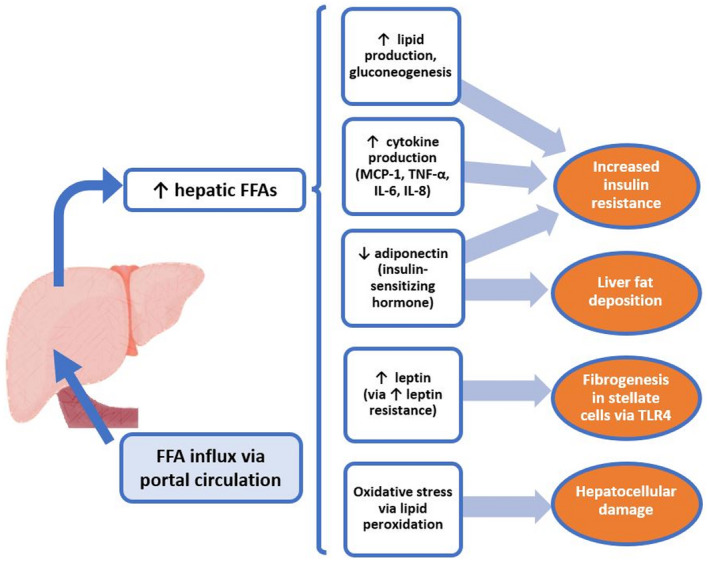
NAFLD as a function of impaired lipid metabolism.
VAT‐associated increases in immune cell activity and markers of inflammation within liver tissue can lead to altered production of adiponectin, a key protein in insulin sensitivity regulation. 4 This impairment leads to lipotoxic accumulation of fatty acids directly into the liver through portal circulation, known as ectopic fat storage, reducing hepatic insulin clearance and increasing gluconeogenesis, dyslipidemia, and hepatic fat deposition (Fig. 3). 4 , 5 , 7
FIG 3.
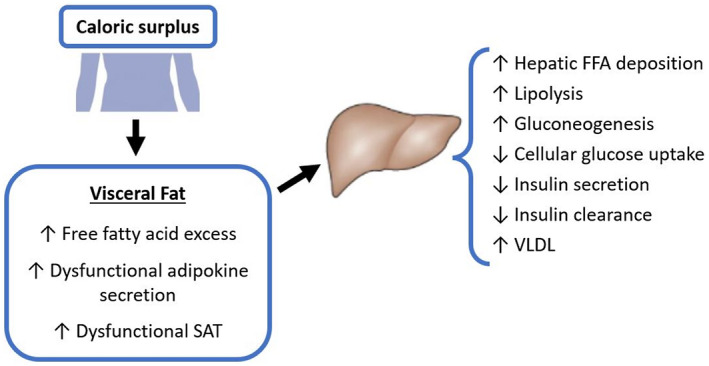
Role of VAT in dysfunctional lipid regulation.
Clinical Measurements of Visceral Fat
Despite its known limitations, body mass index (BMI) is still the most widely used metric for obesity and metabolic‐related risk. However, BMI alone is insufficient to properly assess health risk associated with increased adiposity, does not distinguish between fat mass and lean mass, and can have extreme variation among individuals of the same weight with different levels of visceral fat. 6 , 8 , 9 More focused measures of adiposity and body composition are needed to capture individual health risk. Radiologic assessment of body composition, such as through computed tomography (CT), magnetic resonance imaging (MRI), or dual‐energy x‐ray absorptiometry (DEXA) has allowed for improved calculation of VAT and has been validated against direct metrics such as histology. 10 , 11 However, these modalities are costly and typically reserved for research purposes; as such, surrogate anthropometric measures have been developed (Table 1).
TABLE 1.
Anthropometric Measures of Visceral Adipose Tissue for Use in NAFLD
| Anthropometric Marker | Abbreviation | Advantages | Disadvantages | Correlation With VAT |
|---|---|---|---|---|
| Body mass index | BMI | Large amount of evidence, simple to calculate | Low correlation to VAT within certain subgroups and overall | 0.67‐0.84 |
| Waist circumference | WC | Well documented in literature, use in clinic | Variability of measurement location without consensus, WC alone cannot distinguish SAT from VAT | 0.50‐0.87 |
| Waist‐to‐height ratio | WHtR | Index of central obesity, well studied, simple to calculate | Not superior to WC alone | 0.52‐0.81 |
| Waist‐to‐hip ratio | WHR | Well studied, easy to use in clinic setting | Not superior to WC alone | 0.67‐0.71 |
| Neck circumference | NC | Easy to obtain measurement | Not well documented in literature | 0.63‐0.82 |
| Homeostatic model of insulin resistance | HOMA‐IR | Established association with metabolic dysfunction | Requires less common serologies (fasting insulin, fasting glucose) | 0.42‐0.69 |
| Visceral adiposity index | VAI | Effective marker for stratifying obesity phenotypes, gender‐specific | Not yet widely validated in NAFLD | – |
| Lipid accumulation product | LAp | Stratification of obesity phenotypes, established screening tool for metabolic syndrome, gender‐specific | Not yet widely validated in NAFLD | – |
| Product of triglycerides and glucose | TyG | Stratification of diabetes versus prediabetes and metabolic risk | Not yet widely validated in NAFLD | – |
Waist circumference (WC) is predictive of increased VAT among people with the same BMI, and has been shown to be more strongly associated with amount of VAT than waist‐to‐hip ratio. 7 , 12 When WC is paired with triglyceride levels, it has been shown to be predictive of increased VAT levels and metabolic syndrome with approximately 80% probability. 2 Additionally, measured hyperinsulinemia and elevated apolipoprotein B concentrations have been closely related to VAT accumulation, suggesting that a combination of anthropometric and serologic measures more accurately captures VAT and associated risk of NAFLD. 13 Visceral adiposity index (VAI), a model computed by both anthropometric (BMI and WC) and laboratory parameters such as triglycerides and high‐density lipoprotein cholesterol, serves as an indicator of visceral fat function associated with insulin resistance and cardiometabolic risk. 14 VAI has been implicated as a valuable predictor of NAFLD; other potential indices of insulin resistance such as lipid accumulation product (LAp) and product of triglycerides and glucose (tyG) are reliable indices to discriminate between prediabetes and diabetes among the general population. 14 These markers also reflect visceral fat dysfunction and metabolic unhealthy obesity. As research in this area continues, measures of abdominal obesity and markers of metabolic syndrome associated with increased VAT could refine NAFLD risk assessment beyond current methods used in clinical practice.
Targeted Treatment to Improve Visceral Fat Function
There is a critical need for effective medical therapies for patients with NAFLD. Weight loss and regular exercise combined with a healthy diet is strongly associated with VAT reduction and improvement of hepatic steatosis independent of age, sex, and ethnicity. 3 , 4 , 8 In addition to behavioral changes, therapeutic options to reduce VAT beyond nutritional and activity habits are of great interest to NAFLD treatment researchers (Table 2). Promising targets include adiponectin and leptin, hormones released by visceral adipose tissue that are strongly inversely associated with risk of metabolic syndrome, body composition, and hepatic dysfunction. Increasing levels of these adipose mediators are associated with improvement of visceral fat function and hindrance of NAFLD development and progression. 15 Peroxisome proliferator‐activated receptors (PPAR)‐γ agonists, known as thiazolidinediones (TZDs), reduce fat accumulation in visceral depot and liver and increase fat storage of SAT. This is mediated by increased release of adiponectin, thereby alleviating inflammatory cascades within visceral fat via PPAR‐γ activation. 16 A systemic meta‐analysis shows that pioglitazone 30 mg daily or 45 mg daily improves NAFLD activity scores and fibrosis in both diabetic and non‐diabetic patients. 17 However, side effects of TZDs, including weight gain, fluid retention, and risk of bone fracture, have greatly limited their tolerance.
TABLE 2.
Novel Therapeutic Strategies to Reduce Visceral Adipose Tissue for Use in NAFLD
| Novel Therapeutic Target | Mechanism of action |
|---|---|
| Adiponectin replacement | Increase fatty acid oxidation, reduce oxidative stress and liver fibrosis |
| Leptin replacement | Promote lipid mobilization, reduce liver fat deposition |
| TZDs (PPAR‐γ agonist) | Increase circulating adiponectin levels, regulate inflammation, improve fibrosis |
| SGLT‐2 inhibitor | Reduce ectopic fat, reverse hepatic fibrosis |
Ipragliflozin, a sodium‐glucose co‐transporter‐2 inhibitor, has also been shown to reduce epicardial fat in both obese and non‐obese patients with type 2 diabetes; further studies should clarify whether a similar effect is seen in visceral liver fat. 18 Newer therapeutic targets aimed at improving visceral fat function and reducing VAT volume in conjugation of lifestyle modification may improve metabolic outcome and halt progression of NAFLD and NASH.
Conclusion
Anatomically adjacent to the liver, VAT plays an important role in NAFLD pathogenesis via its diabetogenic, atherogenic, and pro‐inflammatory functions. Many anthropometric metrics and insulin‐resistance markers are being studied to gauge VAT function, with the goal of predicting metabolic risk and NAFLD surveillance. There is significant unmet need to improve accuracy of current clinical models and develop effective therapies that enhance VAT function as a target for NAFLD treatment.
Author Contributions
Concept and Design: C.H., L.Y. Drafting and Revision of Manuscript: C.H., L.Y.
Potential conflict of interest: Liyun Yuan recieved grants from Genfit, Intercept and Enanta.
References
- 1. Neeland IJ, Ross R, Després J‐P, et al. Visceral and ectopic fat, atherosclerosis, and cardiometabolic disease: a position statement. Lancet Diabetes Endocrinol 2019;7:715‐725. [DOI] [PubMed] [Google Scholar]
- 2. Lemieux I, Poirier P, Bergeron J, et al. Hypertriglyceridemic waist: A useful screening phenotype in preventive cardiology? Can J Cardiol 2007;23:23B‐31B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Expert Panel on Detection E . Executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA 2001;285:2486‐2497. [DOI] [PubMed] [Google Scholar]
- 4. McGarry JD. Banting lecture 2001: dysregulation of fatty acid metabolism in the etiology of type 2 diabetes. Diabetes 2002;51:7‐18. [DOI] [PubMed] [Google Scholar]
- 5. Seppälä‐Lindroos A, Vehkavaara S, Häkkinen A‐M, et al. Fat accumulation in the liver is associated with defects in insulin suppression of glucose production and serum free fatty acids independent of obesity in normal men. J Clin Endocrinol Metab 2002;87:3023‐3028. [DOI] [PubMed] [Google Scholar]
- 6. Tchernof A, Després J‐P. Pathophysiology of human visceral obesity: an update. Physiol Rev 2013;93:359‐404. [DOI] [PubMed] [Google Scholar]
- 7. Snijder M, Van Dam R, Visser M, Seidell J. What aspects of body fat are particularly hazardous and how do we measure them? Int J Epidemiol 2006;35:83‐92. [DOI] [PubMed] [Google Scholar]
- 8. Neeland IJ, Poirier P, Després J‐P. Cardiovascular and metabolic heterogeneity of obesity: clinical challenges and implications for management. Circulation 2018;137:1391‐1406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ross R, Neeland IJ, Yamashita S, et al. Waist circumference as a vital sign in clinical practice: a Consensus Statement from the IAS and ICCR Working Group on Visceral Obesity. Nat Rev Endocrinol 2020;16:177‐189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Linge J, Borga M, West J, et al. Body composition profiling in the UK Biobank Imaging Study. Obesity 2018;26:1785‐1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kaul S, Rothney MP, Peters DM, et al. Dual‐energy X‐ray absorptiometry for quantification of visceral fat. Obesity 2012;20:1313‐1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Nazare J‐A, Smith J, Borel A‐L, et al. Usefulness of measuring both body mass index and waist circumference for the estimation of visceral adiposity and related cardiometabolic risk profile (from the INSPIRE ME IAA study). Am J Cardiol 2015;115:307‐315. [DOI] [PubMed] [Google Scholar]
- 13. Lemieux S, Prud'Homme D, Tremblay A, Bouchard C, Despres J. Anthropometric correlates to changes in visceral adipose tissue over 7 years in women. Int J Obes Relat Metab Disord 1996;20:618‐624. [PubMed] [Google Scholar]
- 14. Ahn N, Baumeister SE, Amann U, et al. Visceral adiposity index (VAI), lipid accumulation product (LAP), and product of triglycerides and glucose (TyG) to discriminate prediabetes and diabetes. Sci Rep 2019;9:1‐11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Boutari C, Mantzoros CS. Adiponectin and leptin in the diagnosis and therapy of NAFLD. Metab‐Clin Exp 2020;103:154028. [DOI] [PubMed] [Google Scholar]
- 16. Polyzos SA, Mantzoros CS. Adiponectin as a target for the treatment of nonalcoholic steatohepatitis with thiazolidinediones: a systematic review. Metabolism 2016;65:1297‐1306. [DOI] [PubMed] [Google Scholar]
- 17. Musso G, Cassader M, Paschetta E, Gambino R. Thiazolidinediones and advanced liver fibrosis in nonalcoholic steatohepatitis: a meta‐analysis. JAMA Intern Med 2017;177:633‐640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Fukuda T, Bouchi R, Terashima M, et al. Ipragliflozin reduces epicardial fat accumulation in non‐obese type 2 diabetic patients with visceral obesity: a pilot study. Diabetes Therapy 2017;8:851‐861. [DOI] [PMC free article] [PubMed] [Google Scholar]


