Abstract
Introduction
and importance: Neurological ailments are reported during and after SARS-COV-2 infection.
Case presentation
We report a 67-year-old Iranian man with COVID-19 infection and Acute Disseminated Encephalomyelitis (ADEM) whose neurological symptoms appeared before clinical and radiological pulmonary manifestations.
Clinical discussion
COVID-19 can cause neurological complication without entering the CNS via para infectious inflammatory mechanisms.
Conclusions
This report shows that ADEM might be among primary presentations of COVID-19.
Keywords: COVID-19, ADEM, Acute disseminated encephalomyelitis, Central nervous system, Brain, Neurologic
Highlights
-
•
Para-infectious central nervous system (CNS) syndromes have been defined in cases of SARS-COV-2.
-
•
ADEM might be the first presentation of Covid-19.
Abbreviations
- Central nervous system
(CNS)
- Peripheral nervous system
(PNS)
- emergency department
(ED)
- magnetic resonance imaging
(MRI)
- intensive care unit
(ICU)
- C-reactive protein
(CRP)
- Prothrombin Time
(PT)
- International normalized ratio
(INR)
- Partial Thromboplastin Time
(PTT)
- Cerebrospinal fluid
(CSF)
- Acute disseminated encephalomyelitis
(ADEM)
- Intravenous Immunoglobulin
(IVIG)
- Acute Hemorrhagic Leukoencephalitis
(AHLE)
- angiotensin-converting enzyme type 2
(ACE-2)
1. Introduction
Coronavirus disease 2019 (COVID-19) caused by SARS-CoV-2 emerged as pandemic in 2020 which is commonly presented by fever and respiratory symptoms [1]. With advancements in evidences, multi-organ dysfunction is also reported with the infection [2,3]. Neurological complications associated with central nervous system (CNS) and peripheral nervous system (PNS) such as encephalitis and Guillen Barre disease have been reported with the infection [[3], [4], [5]]. Despite diverse CNS presentations, detection of virus in cerebrospinal fluid has been scarcely reported, indicating that various mechanisms are responsible for neurological manifestation of the disease [17]. In this report, we describe a rare presentation of COVID-19 as a result of multifocal injuries in a 67-year-old male patient.
2. Case description
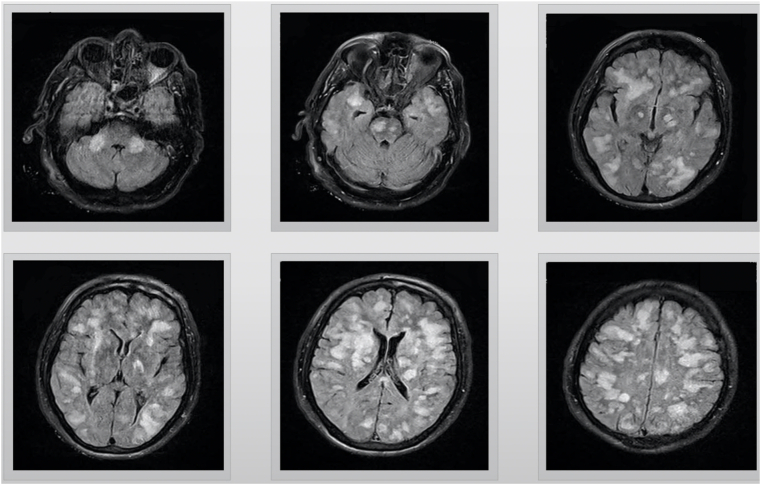
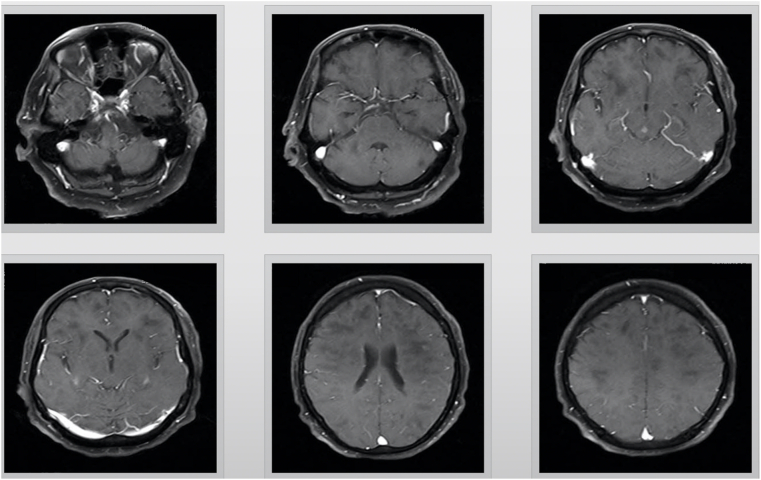
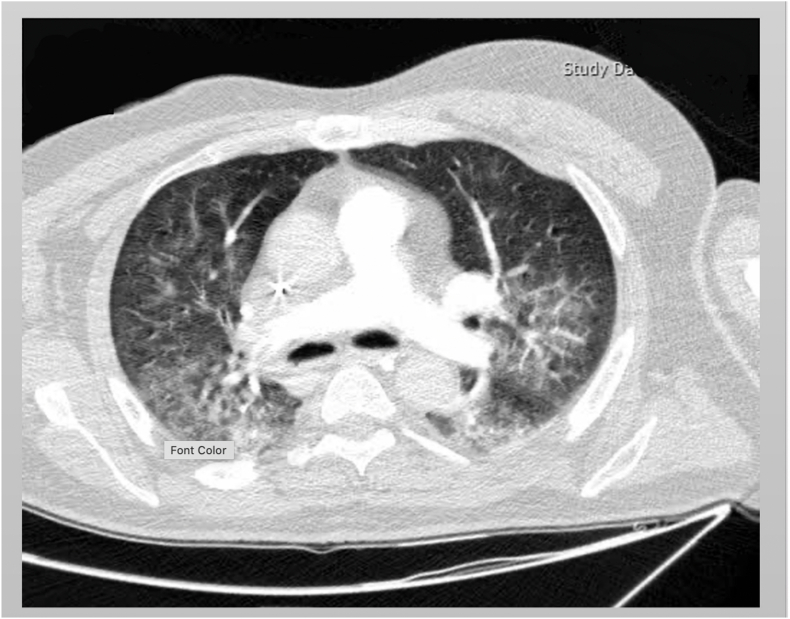
A 67-year-old male was presented to emergency department (ED) with decreased level of consciousness. Symptoms initiated with drowsiness, two days before admission. Past medical history was insignificant. At the time of referral, his body temperature was 37.1 °C. He was drowsy and could not speak consistently, and could not obey simple tasks. Deep tendon reflexes were brisk and plantar reflexes were upward. Magnetic resonance imaging (MRI) of brain revealed extensive high signal lesions in T2W and FLAIR sequences on bilateral cerebral hemispheres, para ventricular and subcortical white matter, middle cerebellar peduncles, centrum semi vale, corpus callosum, the basal ganglia, thalami, midbrain, and pons (Fig. 1). Post-contrast MRI showed sparse enhancements on midpart of the midbrain and left parietal lobe (Fig. 2) COVID-19 infection was suggested by positive nasopharyngeal RT-PCT test result. Contrast enhanced CT thorax scan was normal at the time of admission. One day after admission, loss of consciousness along with respiratory distress, ultimately leading to the requirement of endotracheal intubation and admission to intensive care unit (ICU). Spiral CT scan for chest revealed diffused bilateral patchy ground glass opacities in both lungs along with few subpleural consolidations, indicating COVID-19 (Fig. 3). Patient did not have any previous viral infection based on his asymptomatic history.
Fig. 1.
T2 flare views, axial magnetic resonance imaging (MRI), showing multifocal T2 hyperintense lesions with sparse enhancement on bilateral cerebral hemispheres, para ventricular and subcortical white matter, middle cerebellar peduncles, centrum semiovale, corpus callosum, thevbasal ganglia, thalami, midbrain and brain stem.
Fig. 2.
T1 postcontrast (gadolinium) views, axial magnetic resonance imaging (MRI), showing multifocal T2 hyperintense lesions with sparse enhancemen on bilateral cerebral hemispheres para ventricular and subcortical white matter, middle cerebellar peduncles, centrum semiovale, corpus callosum, thevbasal ganglia, thalami, midbrain and brain stem.
Fig. 3.
Chest CT scan shown ground glass opacities on the right middle lobe. There is also visible the reticular pattern on both lungs' fields.
Laboratory results from admission showed elevated C-reactive protein (CRP) (106 mg/L) and ESR (44 mm/h). Prothrombin Time (PT), International normalized ratio (INR) and Partial Thromboplastin Time (PTT) were in normal. Lymphopenia was present. Cerebrospinal fluid (CSF) analysis was normal for cell count, glucose and protein. CSF was negative for SARS-COV-2 and other viruses including Epstein-Barr virus, herpes simplex, cytomegalovirus, varicella-zoster. No oligoclonal bands were present.
Clinical and Image findings coupled with laboratory data, led to the diagnosis of Acute disseminated encephalomyelitis (ADEM) associated with COVID-19. Along with COVID-19 treatment, methylprednisolone 1 g IV daily for 5 days was instituted. Neurologic status was grossly unchanged. Thus, Intravenous Immunoglobulin (IVIG) 0.4 g/kg daily was administered for 5 more consecutive days after Methylprednisolone pulse therapy.
The patient eventually died after 4 weeks of hospital admission due to worsening of his respiratory and neurologic conditions.
The methods are stated in accordance with SCARE 2020 guidelines [6].
3. Discussion
In this patient, the clinical presentation coupled with imaging characteristics is consistent with a diagnosis of an acute demyelinating event. Our patient was diagnosed with COVID-19 based on positive RT-PCR for SARS-CoV-2 virus on admission. Signs of lung involvement evolved only after CNS involvements. We hypothesize that SARS-CoV-2 infection might have been responsible for the post-infectious demyelinating CNS lesions, an entity known to occur in conjunction to many viral infections, and is in the spectrum of ADEM. This report is important, because it suggests that ADEM might be among primary presentations of SARS-COV-2. Nevertheless, establishing a coincidence or a cause-and-effect relationship may not be easily possible. This similar to some reports wherein the neurological syndrome appeared before clinical and/or radiological pulmonary manifestations. In some instances, the obvious clinical pulmonary symptoms never developed [7, 16].
ADEM,also known as postinfectious encephalomyelitis, is an infrequent para- or postinfectious autoimmune complications [7]. It is mainly a disorder of children. However, few cases in adults have also been reported. Clinically, it is heterogenous by nature and generally causes multifocal neurologic deficits in an acute manner, which often progresses rapidly. Typical MRI lesions consists of FLAIR hyperintensities in deep white matter and at the grey/white matter interface. Post-contrast enhancement is not always detected and usually in the form of punctate or rim enhancing pattern. Restriction can be detected in DWI sequence, especially in early course of disease [8]. ADEM, as an immune-mediated demyelinating monophasic disorder, typically presents between 2 and 4 weeks after viral infections or immunizations [8, 9] Thus, the temporal relationship between COVID-19 symptoms and the onset of ADEM seems to be shorter than the classical picture described for other viral infections.
Acute Hemorrhagic Leukoencephalitis (AHLE), as a severe form of ADEM has been also reported in a COVID-19 patient [9]. ADEM and AHLE share similar histologic features, however, in our patient, size of the lesions, lack of hemorrhage in the images, and normal CSF analysis are all in favor of ADEM diagnosis rather than AHLE, although the natural history was as grave as AHLE.
To our knowledge, ADEM has been reported in association with COVID-19 in a few children and adult patients [[10], [11], [12], [13], [14], [15]]. The prognosis of these patients has been mostly favorable. However, our case had a poor outcome because of severe pulmonary involvement and respiratory distress as a result of COVID-19. We believe the presentation of COVID-19 as ADEM, which was not reported previously, may imply the severity of viral and/or inflammatory multi-system COVID-19 infection and can be considered an index for poor outcome. However, large scale multi-center studies are required in this regard.
It is likely that more than one immune mechanism is involved in triggering ADEM in susceptible patients and the exact mechanism of ADEM in case of COVID-19 infection is largely hypothetical and needs further research.
One of the potential underlying mechanisms of ADEM in association with SARS-CoV-2 is the cytokine pathway activation, which is known to be involved in the pathology of ADEM. It has been stated to result from T cells affected by SARS-CoV-2 inducing a storm of cytokines, especially IL-6 [[16], [17], [18]]. This results in sequences of immune responses that may play an important role in the pathogenesis of ADEM [19].
Other mechanisms being discussed elsewhere includes post-infectious inflammatory process leading to the production autoantibody production to neuronal antigens [20].This mechanism is stated as “molecular mimicry” whereby an infectious organism contains numerous epitopes mimicking the structure of endogenous myelin epitopes [21]. Some of the T-cells points toward the infectious epitopes during an immune response against the entering organism. As an undesirable consequence, some T-cells become skilled at cross-reacting with self-myelin peptides [21]. These cross-reactive T-cells grow in numbers in response to self-antigenic stimulation and release chemokines that further recruit added lymphocytes and macrophages to the site of activation all of which enhances the demyelination process and neuronal injury [7, 21]. Based on few histopathological studies [22], the corresponding mechanism has been noticed in six post-mortem patients in forms of lymphocytic panencephalitis and meningitis along with brainstem perivascular and interstitial inflammatory alteration with neuronal loss as major features [7, 21].
It has long been accepted that the term ADEM should not be utilized in cases of acute encephalitis, which are caused by direct invasion of the viruses into the CNS [7]. Still, few evidence of direct viral invasion with supportive histopathological features have been claimed, albeit very rare [23,24]. It has been hypnotized that SARS-CoV-2 dictates tissue tropism using the angiotensin-converting enzyme type 2 (ACE-2) receptor to bind to cells. The ACE-2 receptor can be found in nervous system tissue as well as endothelial cells among tissues of many other organs [25,26]. In the current report, the COVID-19 RNA was not detected using CSF, using RT-PCR method, thereby this mechanism may not comply to this case.
In addition, some studies have showed vascular-thrombotic complications associated with COVID-19 infection [6]. Histopathological findings of the postmortem brain study of a patient with diagnosis of SARS-COV-2 without specific signs of ADEDM suggested a vascular origin with secondary myelin loss, and the neocortical microscopic infarcts identified raise the possibility of micro thromboembolic events, probably related to COVID-19 related complications. However, in that study, the clinical presentation of ADEM was not apparent and the biopsy solely resembled an acute disseminated encephalomyelitis (ADEM)-like histological appearance.
Since we did not perform a postmortem study, we cannot verify the exact reason, although the pattern of DWI and ADC-map sequences of brain MRI may suggest micro thromboembolic events.
For treatment, high dose corticosteroid followed by IVIG seemed to be effective [12]. However, controversy remains regarding the optimal treatment options including the use of high dose corticosteroids in viremic, and often lymphopenic patients. The potential risks of using IVIG for ADEM, in patients with pro-thrombotic risk factors such as elevated D-dimer levels should be taken into consideration [27]. Since this condition is potentially associated with high mortality, ADEM should be taken into account as a serious possible complication of COVID-19 infection during pandemic and prompt treatment should be initiated.
Our understanding of the Para infectious neuropathology of SARS CoV-2 continues to evolve, but as a cause of devastating outcome in many patients that do survive, there must be special attention to the phenomenology, pathophysiology and treatment of CNS involvement in COVID-19 patients [28].
4. Conclusion
ADEM might be among the primary extra-pulmonary manifestation of COVID-19 infection. Special awareness is warranted in patients presenting with any possible neurological manifestation in the current pandemic situation to prevent mortality and severe morbidity.
Sources of funding
No funding was secured for this study.
Ethical approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Consent to participate
Patient consent was obtained prior to the surgery.
Contributors’ statement
Dr. Sara Esmaeili and Dr. Mahin Jamshidi Makiani and DrHossein Nazarian.: conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript. Dr. Mohammad Hossein Abbasi and Dr. Maziar Emamikhah: Designed the data collection instruments, collected data, carried out the initial analyses, and reviewed and revised the manuscript. Dr. Mohammad Taghi Joghataei and Dr. Zahra Mirzaasgari: Coordinated and supervised data collection, and critically reviewed the manuscript for important intellectual content.
Availability of data and material
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.
Consent for publication
Written informed consent was obtained from the patient for publication of this case report and accompanying images. A copy of the written consent is available for review by the Editor-in-Chief of this journal on request.
Research registration
N/A.
Guarantor
Sara Esmaeili.
Provenance and peer review
Not commissioned, externally peer-reviewed.
Declaration of competing interest
The authors deny any conflict of interest in any terms or by any means during the study.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.amsu.2022.103511.
Contributor Information
Zahra Mirzaasgari, Email: Zahra.Mirzaasgari@gmail.com.
Hossein Nazarian, Email: md.h.nazarian@gmail.com.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.Guan W-j, Ni Z-y, Hu Y., Liang W-h, Ou C-q, He J-x, et al. Clinical characteristics of coronavirus disease 2019. China. 2020;382(18):1708–1720. doi: 10.1056/NEJMoa2002032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Baig A.M., Khaleeq A., Ali U., Syeda H. Evidence of the COVID-19 virus targeting the CNS: tissue distribution, host–virus interaction, and proposed neurotropic mechanisms. ACS Chem. Neurosci. 2020;11(7):995–998. doi: 10.1021/acschemneuro.0c00122. [DOI] [PubMed] [Google Scholar]
- 3.Ellul M.A., Benjamin L., Singh B., Lant S., Michael B.D., Easton A., et al. Neurological associations of COVID-19. Lancet Neurol. 2020;19(9):767–783. doi: 10.1016/S1474-4422(20)30221-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aghagoli G., Gallo Marin B., Katchur N.J., Chaves-Sell F., Asaad W.F., Murphy S.A. Neurological involvement in COVID-19 and potential mechanisms: a review. Neurocritical Care. 2020:1–10. doi: 10.1007/s12028-020-01049-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mao L., Jin H., Wang M., Hu Y., Chen S., He Q., et al. Neurologic manifestations of hospitalized patients with coronavirus disease 2019 in wuhan, China. JAMA Neurol. 2020;77(6):683–690. doi: 10.1001/jamaneurol.2020.1127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agha R.A., Franchi T., Sohrabi C., Mathew G., Kerwan A. The SCARE 2020 guideline: updating consensus surgical CAse REport (SCARE) guidelines. Int. J. Surg. 2020;84:226–230. doi: 10.1016/j.ijsu.2020.10.034. [DOI] [PubMed] [Google Scholar]
- 7.Javed A., Khan O. Acute disseminated encephalomyelitis. Handb. Clin. Neurol. 2014;123:705–717. doi: 10.1016/B978-0-444-53488-0.00035-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Marin S.E., Callen D.J. The magnetic resonance imaging appearance of monophasic acute disseminated encephalomyelitis: an update post application of the 2007 consensus criteria. Neuroimaging Clin. 2013;23(2):245–266. doi: 10.1016/j.nic.2012.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yong M.H., Chan Y.F.Z., Liu J., Sanamandra S.K., Kheok S.W., Lim K.C., et al. A rare case of acute hemorrhagic leukoencephalitis in a COVID-19 patient. J. Neurol. Sci. 2020;416:117035. doi: 10.1016/j.jns.2020.117035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Parsons T., Banks S., Bae C., Gelber J., Alahmadi H., Tichauer M. COVID-19-associated acute disseminated encephalomyelitis (ADEM) J. Neurol. 2020;267(10):2799–2802. doi: 10.1007/s00415-020-09951-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Abdi S., Ghorbani A., Fatehi F. The association of SARS-CoV-2 infection and acute disseminated encephalomyelitis without prominent clinical pulmonary symptoms. J. Neurol. Sci. 2020;416:117001. doi: 10.1016/j.jns.2020.117001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Afshar H., Yassin Z., Kalantari S., Aloosh O., Lotfi T., Moghaddasi M., et al. Multiple sclerosis and related disorders. Vol. 43. 2020. Evolution and resolution of brain involvement associated with SARS- CoV2 infection: a close Clinical - paraclinical follow up study of a case; p. 102216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Utukuri P.S., Bautista A., Lignelli A., Moonis G. 2020. Possible Acute Disseminated Encephalomyelitis Related to Severe Acute Respiratory Syndrome Coronavirus 2 Infection. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pezzini A., Padovani A. Lifting the mask on neurological manifestations of COVID-19. Nat. Rev. Neurol. 2020;16(11):636–644. doi: 10.1038/s41582-020-0398-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Demirci Otluoglu G., Yener U., Demir M.K., Yilmaz B. Encephalomyelitis associated with Covid-19 infection: case report. Br. J. Neurosurg. 2020:1–3. doi: 10.1080/02688697.2020.1787342. [DOI] [PubMed] [Google Scholar]
- 16.Mehta P., McAuley D.F., Brown M., Sanchez E., Tattersall R.S., Manson J.J. COVID-19: consider cytokine storm syndromes and immunosuppression. Lancet. 2020;395(10229):1033–1034. doi: 10.1016/S0140-6736(20)30628-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Alizadeh R., Aghsaeifard Z. Does COVID19 activates previous chronic pain? A case series. Ann. Med. Surg. (Lond). 2021;61:169–171. doi: 10.1016/j.amsu.2020.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vaziri-Harami R., Delkash P. Can l-carnitine reduce post-COVID-19 fatigue? Ann. Med. Surg. (Lond) 2022;73:103145. doi: 10.1016/j.amsu.2021.103145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li Y.C., Bai W.Z., Hashikawa T. The neuroinvasive potential of SARS-CoV2 may play a role in the respiratory failure of COVID-19 patients. J. Med. Virol. 2020;92(6):552–555. doi: 10.1002/jmv.25728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Angileri F., Legare S., Marino Gammazza A., Conway de Macario E., Jl Macario A., Cappello F. Molecular mimicry may explain multi-organ damage in COVID-19. Autoimmun. Rev. 2020;19(8):102591. doi: 10.1016/j.autrev.2020.102591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fujinami R.S., Oldstone M.B. Amino acid homology between the encephalitogenic site of myelin basic protein and virus: mechanism for autoimmunity. Science. 1985;230(4729):1043–1045. doi: 10.1126/science.2414848. [DOI] [PubMed] [Google Scholar]
- 22.von Weyhern C.H., Kaufmann I., Neff F., Kremer M. Early evidence of pronounced brain involvement in fatal COVID-19 outcomes. Lancet. 2020;395(10241):e109. doi: 10.1016/S0140-6736(20)31282-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ann Yeh E., Collins A., Cohen M.E., Duffner P.K., Faden H. Detection of coronavirus in the central nervous system of a child with acute disseminated. Encephalomyelitis. 2004;113(1):e73–e76. doi: 10.1542/peds.113.1.e73. [DOI] [PubMed] [Google Scholar]
- 24.Espíndola OM, Brandão CO, Gomes YCP, Siqueira M, Soares CN, Lima MASD, et al. Cerebrospinal fluid findings in neurological diseases associated with COVID-19 and insights into mechanisms of disease development. Int. J. Infect. Dis. [DOI] [PMC free article] [PubMed]
- 25.Berger J.R. COVID-19 and the nervous system. J. Neurovirol. 2020;26(2):143–148. doi: 10.1007/s13365-020-00840-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Aghsaeifard Z., Alizadeh R. vol. 22. 2022. pp. 6–14. (The Role of Angiotensin-Converting Enzyme in Immunity: Shedding Light on Experimental Findings. Endocrine, Metabolic & Immune Disorders - Drug Targets(Formerly Current Drug Targets - Immune, Endocrine & Metabolic Disorders)). 1. [DOI] [PubMed] [Google Scholar]
- 27.Paterson R.W., Brown R.L., Benjamin L., Nortley R., Wiethoff S., Bharucha T., et al. The emerging spectrum of COVID-19 neurology: clinical, radiological and laboratory findings. Brain. 2020;143(10):3104–3120. doi: 10.1093/brain/awaa240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reichard R.R., Kashani K.B., Boire N.A., Constantopoulos E., Guo Y., Lucchinetti C.F. Neuropathology of COVID-19: a spectrum of vascular and acute disseminated encephalomyelitis (ADEM)-like pathology. Acta Neuropathol. 2020;140(1):1–6. doi: 10.1007/s00401-020-02166-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analyzed during the current study.