Abstract
A novel coronavirus, severe acute respiratory syndrome coronavirus-2, was isolated from patients’ lower respiratory tracts in December 2019. As of May 19, 2021, there were over 33 million reported infections and almost 600,000 deaths in the United States. The infection, coronavirus disease-19 (COVID-19), can lead to cytokine storm, with elevations in interleukin-6 (IL-6), IL-10, tumor necrosis factor-α, nuclear factor-kappaB (NF-kappaB), and glutathione reductase. NF-kappaB activation is necessary for further transcription of other pro-inflammatory markers. Glutathione may play a role in modulation of NF-kappaB activation and elevated glutathione reductase may indicate glutathione depletion. Administration of N-acetylcysteine (NAC) may replenish spent glutathione and attenuate over-activation of NF-kappaB. This retrospective case series included 10 patients who were COVID-19 positive and received intravenous NAC in an attempt to attenuate the cytokine storm. Patients’ outcomes were graded based on the World Health Organization symptom severity scale from 0, no evidence of infection, to 8, death. Overall, the median WHO Scale prior to NAC was 6.5, and increased by day seven, which indicated clinical worsening. This retrospective case series showed no benefit of NAC; however, further studies are needed to elucidate if differences in drug regimens would lead to positive results.
Keywords: coronavirus, COVID-19, cytokine storm, glutathione, N-acetylcysteine
Introduction
A novel coronavirus, severe acute respiratory syndrome coronavirus-2, was isolated from patients’ lower respiratory tracts as early as December 2019 and was found to be transmissible through aerosolized droplets from coughing and sneezing in both asymptomatic and symptomatic patients.1,2 As of May 19, 2021, there were over 33 million reported infections and almost 600,000 deaths in the United States. 3
Patients with more severe disease can progress to severe pneumonia, acute respiratory distress syndrome (ARDS), multi-organ dysfunction, cytokine storm, and death. 4 Disease progression and promulgation of cytokine storm is marked by a rise in inflammatory cytokines, interleukin-6 (IL-6), IL-10, tumor necrosis factor-α, nuclear factor-kappaB (NF-kappaB), the acute phase reactants C-reactive protein (CRP), lactate dehydrogenase (LDH), ferritin, and D-dimer.5-8 Liver transaminases, lactate, creatinine, and glutathione reductase may also be elevated. 9 It has been shown that NF-kappaB activation is necessary for transcription of downstream pro-inflammatory mediators leading to ARDS. 5 Meanwhile, glutathione has been linked to regulation of NF-kappaB signaling (Figure 1). 6 Greater levels of glutathione reductase in patients may indicate increased oxidative stress, glutathione depletion, and reduced NF-kappaB modulation.5,6,9
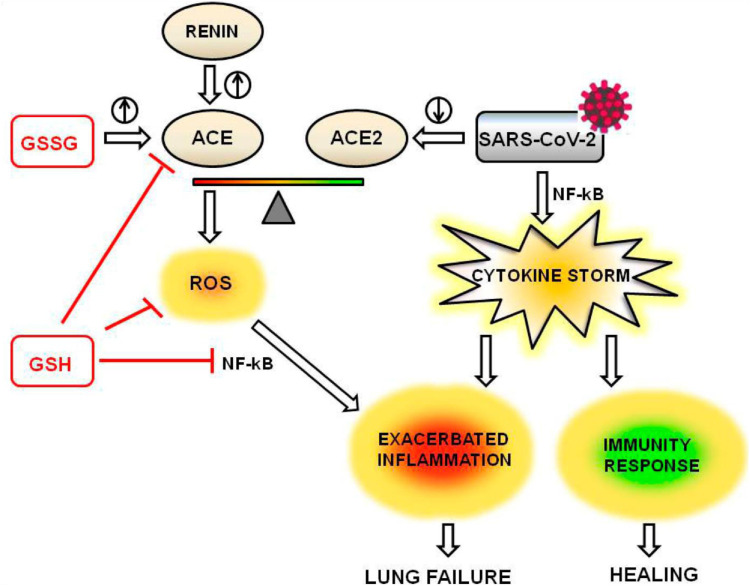
Figure 1.
Proposed pathway of reduced glutathione ameliorating SARS-CoV-2 cytokine storm. ACE, angiotensin converting enzyme; ACE2, angiotensin 2 converting enzyme; GSH, reduced glutathione; GSSG, oxidized glutathione; NF-kB, nuclear factor kappaB; ROS, reactive oxygen species; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2. N-acetylcysteine supplementation may increase supply of GSH. The anti-inflammatory effects of GSH are mediated through decreased production of ROS and reduced activation of NF-kB, signified by the red lines. Figure reprinted with permission by Silvagno et al.
N-acetylcysteine (NAC) is a mucolytic often used in respiratory diseases that act by disrupting mucoprotein disulfide bonds. NAC is also used in management of acetaminophen toxicity through glutathione regeneration. Similarly, NAC may replenish spent glutathione, reduce further oxidative and inflammatory damage, and prevent initiation or progression of cytokine storm in COVID-19 infection. We performed a retrospective evaluation of patients who received intravenous NAC supplementation in management of COVID-19-associated cytokine storm. The objective of this study is to assess clinical outcomes at day 7 after NAC treatment.
Methods
The Institutional Review Board approved this retrospective case series and a waiver of informed consent was received. Patients admitted in April 2020 were included if they were 18 years and older, had a positive COVID-19 PCR test, and had received at least one dose of intravenous (IV) NAC. IV NAC was prescribed at the discretion of the treating physician. Dosage was based on initial treatment for acetaminophen toxicity, 150 mg/kg, with a maximum recommended dose of 10 g based on institution COVID-19 taskforce recommendations. The total number of doses and duration of treatment were determined through provider discretion. Outcomes were assessed up to 7 days after the last dose of IV NAC or until death or discharge, whichever was earlier. Outcomes were censored at 7 days on the assumption that attributable beneficial effects would present acutely. Information collected from the electronic medical record included patient demographics, treatment regimen, inflammatory marker levels, arterial blood gases, and ventilation modality.
Laboratory values were recorded as close to the administration time of IV NAC as possible for pre-administration values and at least 8 hours after infusion and as close to the seventh day after completing IV NAC therapy for post-administration values. The primary outcome was any improvement from baseline on an 8-point ordinal illness severity scale as recommended by the World Health Organization (WHO): 0, no clinical or virological evidence of infection; 1, no limitation of activities; 2, limitation of activities; 3, hospitalized, no oxygen therapy; 4, oxygen by mask or nasal prongs; 5, non-invasive ventilation or high-flow oxygen; 6, intubation and mechanical ventilation; 7, and ventilation plus additional organ support (vasopressors, renal replacement therapy, and extracorporeal membrane oxygenation); 8, death. We also assessed lengths of intensive care and hospital stays, transfer to intensive care, ventilation status on day 7, vasopressor and hemodialysis requirements, and mortality. 10 Missing lab values and measurements were not included and we reported the number of patients whose information was available. Descriptive statistics were used to evaluate all outcomes.
Results
Baseline Characteristics
Ten patients received IV NAC during the study period. All patients were included in the evaluation. Patients had a median age of 62 years, weight of 70.8 kg, and 80% had a history of hypertension (Table 1). Median time from symptom onset to hospital admission was 6 days. Acute phase reactants ferritin, LDH, and CRP were all elevated (Table 2). Half of the patients were on vasopressors and 60% required invasive mechanical ventilation at baseline. Of the patients on mechanical ventilation, the median PaO2/FiO2 (PF) ratio was 177.5. Half of the patients were in an intensive care setting prior to being given NAC. At baseline, patients had a median [IQR] WHO scale score of 6.5 [5–7] indicating a range from non-invasive ventilation modalities to mechanical ventilation plus vasopressor support.
Table 1.
Baseline Characteristics Before Last N-Acetylcysteine Dose.
| Characteristic | Median (IQR) or Proportion | N |
|---|---|---|
| Age | 62 (57–73) | 10 |
| Sex, male | 40% | 10 |
| Weight, kg | 70.75 (60.95–88.75) | 10 |
| Diabetes | 30% | 10 |
| Hypertension | 80% | 10 |
| Time from symptoms to ED presentation, days | 6 (1.25–7) | 10 |
| Vasopressors | 50% | 10 |
| Hemodialysis | 10% | 10 |
| Other drugs administered | ||
| Ascorbic acid | 100% | 10 |
| Melatonin | 10% | 10 |
| Thiamine | 70% | 10 |
| Zinc | 90% | 10 |
Data presented as median (IQR) or proportion.
Abbreviations: N, number of patients with available results; IQR, interquartile range; ED, emergency department.
Table 2.
Treatment Characteristics Before N-Acetylcysteine Dose and at 7 Days After Final N-Acetylcysteine Dose.
| Characteristic | Value Prior to NAC | N | Value Post NAC | N |
|---|---|---|---|---|
| Ferritin, ng/mL | 1779 (1554–2430.3) | 8 | 1566 (1349–2254) | 5 |
| LDH, unit/L | 484 (477–721) | 9 | 423 (422–554) | 5 |
| CRP, mg/dL | 14 (12–17.4) | 9 | 11.7 (8.92–17.3) | 7 |
| D-dimer, ng/mL | >1050 | 1 | >1050 (>1050–>1050) | 5 |
| PCT, ng/mL | .29 (.18–.36) | 6 | No results | 0 |
| Mode of ventilation | ||||
| Room air | 0% | 10 | 10% | 10 |
| Nasal cannula | 10% | 10 | 0% | 10 |
| Non-rebreather | 10% | 10 | 0% | 10 |
| High-flow nasal cannula | 20% | 10 | 0% | 10 |
| Mechanical ventilation | 60% | 10 | 90% | 10 |
| Arterial blood gas | ||||
| pH | 7.4 (7.36–7.45) | 9 | 7.35 (7.23–7.43) | 8 |
| pCO2, mmHg | 46 (38–50) | 9 | 50.5 (46.75–51.25) | 8 |
| pO2, mmHg | 108 (80–133) | 9 | 110 (79–170) | 8 |
| CO3, mmol/L | 26 (24 – 28) | 9 | 23.5 (20–29.25) | 8 |
| Respiratory rate, min−1 | 19 (15–23.5) | 10 | 20 (20–22) | 10 |
| Tidal volume, mL | 450 (450–500) | 7 | 450 (395–500) | 8 |
| FiO2 (mechanical ventilation), % | 70 (60–100) | 7 | 76.5 (50–85) | 8 |
| PF ratio | 177.5 (127–185.17) | 6 | 202.11 (146.5–252) | 8 |
| WHO scale | 6.5 (5–7) | 10 | 7 (6.25–7.75) | 10 |
Data presented as median (IQR) or proportion.
Abbreviations: N, number of patients with available results; IQR, interquartile range; NAC, N-acetylcysteine; LDH, lactate dehydrogenase; CRP, C-reactive protein; PCT, procalcitonin.
Treatment Course
NAC was administered at a median dose of 10,000 mg (median 141.67 mg/kg) every 12 hours for 1 to 6 doses. NAC was administered a median of 3.88 days after hospital admission and half of all patients received NAC outside the intensive care unit. The other five patients received NAC a median of 2.69 days after transfer to intensive care (Table 3).
Table 3.
N-Acetylcysteine Treatment Information.
| Characteristic | Median (IQR) or Proportion | N |
|---|---|---|
| NAC dose, mg | 10000 (9317.5–10000) | 10 |
| NAC dose/body weight, mg/kg | 141.67 (102.28–149.11) | 10 |
| Number of NAC doses | 1.5 (1–3.5) | 10 |
| Time from symptoms to NAC, days | 8.44 (4.9–15.52) | 10 |
| Time from admit to NAC, days | 3.88 (1.18–5.64) | 10 |
| Critical care prior to NAC | 50% | 10 |
| Time to ICU from admit, days | 3.12 (.82–6.91) | 9 |
| Time from NAC to ICU, days | 3.05 (2.17–3.55) | 4 |
Data presented as median (IQR) or proportion.
Abbreviations: N, number of patients with available results; IQR, interquartile range; NAC, N-acetylcysteine; ICU, intensive care unit.
Outcomes
There was a median increase of 0.5 point in the WHO scale on day 7 post NAC administration, indicating clinical worsening. Five patients (50%) worsened, 4 (40%) had no change, and 1 (10%) showed improvement (Table 4). Of the 5 patients outside of intensive care when given NAC, 4 were subsequently escalated to intensive care. The median intensive care and hospital lengths of stay were 6.44 (2.98–10.14) and 10.72 (8.18–12.41) days, respectively.
Table 4.
Primary and Secondary Outcomes at 7 Days After Final N-Acetylcysteine Dose.
| Primary outcome | Median (IQR) or Proportion | N |
|---|---|---|
| Change in WHO scale | .5 (0–1) | 10 |
| Percent worsening | 50% | 10 |
| Percent no change | 40% | 10 |
| Percent with improving scale | 10% | 10 |
| Secondary outcomes | ||
| Upgraded to ICU | 80% | 5 |
| Progression to mechanical ventilation | 75% | 4 |
| Vasopressors | 70% | 10 |
| New vasopressors started | 3 (60%) | 5 |
| Continued on vasopressors | 4 (80%) | 5 |
| Vasopressors stopped | 1 (20%) | 5 |
| New hemodialysis | 0% | 9 |
| Mortality | 30% | 10 |
| ICU Length of stay, days | 6.44 (2.98–10.14) | 9 |
| Hospital length of stay, days | 10.72 (8.18–12.41) | 10 |
Data presented as median (IQR) or proportion.
Abbreviations: N, number of patients with available results; IQR, interquartile range; WHO, World Health Organization; ICU, intensive care unit.
Four patients were not on invasive mechanical ventilation at baseline; however, three progressed to invasive mechanical ventilation. On day 7 post NAC, 90% were on mechanical ventilation. Five patients were on vasopressors at baseline. Four remained on vasopressors at the end of the assessment period. Of those that were not on vasopressors at baseline, three progressed to requiring at least one vasopressor.
Inflammatory markers declined slightly and were not clinically significant. Ferritin decreased from a median of 1,779 ng/mL (IQR 1,554–2,430.25 ng/mL) to 1566 ng/mL (1,349–2,254 ng/mL), LDH from 484 units/L (477–721 units/L) to 423 units/L 422–554 units/L), and CRP from 14 mg/dL (12–17.4 mg/dL) to 11.7 mg/dL (8.9–17.25 mg/dL). D-dimer remained elevated at >1,050 ng/mL. Patient-specific laboratory values pre- and post-NAC were reported where available (Table 5).
Table 5.
Patient-Specific Laboratory Values and Disposition.
| Prior to NAC Therapy | Post NAC Therapy Completion Day 7 | Final Disposition, Hospital Day | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Patient | Ferritin, ng/mL | LDH, unit/L | CRP, mg/dL | D-Dimer, ng/mL | Mode of Ventilation | PF Ratio | Ferritin, ng/mL | LDH, unit/L | CRP, mg/dL | D-Dimer, ng/mL | Mode of Ventilation | PF Ratio | |
| Patient 1 | 270 | 394 | 17.4 | — | NRB | — | — | — | — | — | Ventilator | 240 | Expired day 11 |
| Patient 2 | 1152 | 884 | 12.9 | — | HFNC | — | — | — | 33.5 | — | Ventilator | 70 | Expired day 12 |
| Patient 3 | 1805 | 484 | 18.4 | — | HFNC | 133 | — | — | 5.6 | >1050 | Ventilator | 288 | Expired day 38 |
| Patient 4 | — | 477 | 11.2 | — | Ventilator | 192 | — | — | — | — | Ventilator | — | To VA day 3 |
| Patient 5 | — | — | — | — | Ventilator | 121 | — | — | — | — | Ventilator | 177 | Expired day 8 |
| Patient 6 | 4174 | 510 | 17.2 | — | Ventilator | 178 | 2956 | 421 | 19.8 | >1050 | Ventilator | 228 | To LTC day 91 |
| Patient 7 | 16500 | 2665 | 9.2 | — | NC | — | 1317 | 422 | 14.7 | >1050 | Room air | — | To SAR day 20 |
| Patient 8 | 1688 | 721 | 12 | — | Ventilator | 240 | 1566 | 554 | 11.7 | >1050 | Ventilator | 324 | To SAR day 29 |
| Patient 9 | 1753 | 480 | 43.6 | >1050 | Ventilator | 104 | 2254 | 613 | 9.8 | >1050 | Ventilator | 56 | Expired day 45 |
| Patient 10 | 1849 | 449 | 14 | — | Ventilator | 178 | 1349 | 423 | 8 | — | Ventilator | 172 | Expired day 37 |
Data are single lab values taken prior to or after completing NAC therapy. Missing data represented by hyphens. LDH, lactate dehydrogenase; CRP, C-reactive protein; NRB, non-rebreather mask; HFNC, high-flow nasal cannula; NC, nasal cannula; VA, transferred to Veteran’s Affairs hospital; LTC, transferred to long-term care facility; SAR, transferred to subacute rehabilitation facility.
At the end of the study period, three patients expired and one patient was transferred to another facility to continue care. No patients were discharged by day 7 of follow-up. Ultimately, a total of six patients expired while receiving ICU care and the remaining four were transferred to outside facilities (Table 5).
Discussion
Based on this evaluation, IV NAC had no significant impact on clinical outcomes at day 7. Although acute phase reactants generally decreased and PF ratios increased overall (Table 2 and Table 5), 90% of patients were in critical care at the end of follow up with attributed 30% mortality (Table 4). The changes in inflammatory markers were likely associated with disease progression rather than IV NAC. These patients were heterogeneous in characteristics and not chosen with specific inclusion criteria, which limit both external and internal validity. Patients were prescribed IV NAC on the basis of prescriber discretion and impression of disease severity. There is little literature regarding use of NAC for respiratory viral illnesses and thus guidance for our usage of IV NAC was based on risk-benefit and safety of IV NAC in acetaminophen overdose. Some in vitro and animal studies support the use of NAC in reducing influenza viral replication, but evidence of translation to humans is scarce and uncertain.11,12 Quantitative viral load testing was not performed during the study period, which precluded this observation in our patients.
A case series by Ibrahim et al. of one glucose 6-phosphate dehydrogenase (G6PD) deficient COVID-19 patient and 9 other non-G6PD deficient COVID-19 patients showed clinical improvement after administration of IV NAC. Nine of the 10 patients required extracorporeal membrane oxygenation (ECMO). NAC dosing was 30,000 mg and 20,000 mg IV over two days for the first two patients and 600 mg every 12 hours for 4–9 days for the remaining 8 patients. Inflammatory markers such as CRP and ferritin markedly decreased after NAC treatment. CRP decreased from a mean of 160 mg/dL to 31 mg/dL and ferritin decreased from a mean of 3,630 ng/mL to 1,543 ng/mL with sustained reductions during the treatment course. A total of 8 patients were discharged home and 2 remained hospitalized by the end of the study period. 13 With respect to the present study, it is uncertain if outcomes would have changed had ECMO been an available treatment option. Another notable difference was that inflammatory markers in our patients did not respond nearly as greatly, possibly owing to differences in disease severity or dosing regimen.
A retrospective cohort study by Assimakopoulos et al. evaluated patients with moderate or severe COVID-19 randomized to either standard of care plus oral NAC 600 mg twice a day for 14 days (n = 42) or standard of care (n = 40). Treatment with NAC led to a significantly lower rate of progression to severe respiratory failure requiring mechanical ventilation (P < .01). NAC treatment also significantly decreased 14-day and 28-day mortality, 0% compared to 25% (P < .001), and 4.8% compared to 30% (P < .01), respectively. 14 Interestingly, patients in this study were given NAC orally at a substantially lower dose but over a longer duration than in our current study with improved mortality. However, its retrospective cohort design limits its generalizability.
The most robust study of IV NAC in COVID-19 patients to date was conducted by de Alencar et al. The double-blind, placebo-controlled trial randomized 135 patients to either IV NAC (n = 68), 14 g over 4 hours after enrollment followed by another 7 g over the next 16 hours, or placebo (n = 67). At baseline, 30% of the IV NAC group and 32% of the placebo group did not require supplemental oxygen, 68% of both groups required supplemental oxygen, and 1% and 0% required non-invasive ventilation or high-flow oxygen. The primary endpoint of progression to mechanical ventilation occurred in 24% of IV NAC patients and 21% of placebo patients (P > .05). The difference in the secondary endpoint of ICU admission was also not statistically significant, occurring in 43% and 47% of patients, respectively. 15 Patients in this study were less severe by WHO criteria than those in the present study but demonstrated that IV NAC administration prior to requiring high-flow oxygen or mechanical ventilation prevented neither those outcomes nor ICU admission.
Last, a study of seroconversion to symptomatic influenza virus randomized 262 patients to either 600 mg NAC by mouth twice daily or placebo for 6 months. Both groups demonstrated similar seroconversion, but only 25% of the treatment group developed symptoms compared to 79% of the placebo group. 16 Although NAC may improve cell-mediated immunity, therapy may require prolonged treatment to produce an effect as opposed to large boluses over a short period as in our study and in the study by de Alencar et al.
Limitations of this study include a low number of evaluated patients and selection bias due to the retrospective, observational design. These results are hypothesis generating and thus preclude definitive conclusions. All patients also concomitantly received other medications with purported anti-inflammatory and free radical scavenging properties: ascorbic acid, melatonin, thiamine, and zinc (Table 1).17-19 Notably, patients in this case series did not receive dexamethasone since the results of the RECOVERY trial were not available at the time. 20 Larger, randomized control studies are needed to assess intervention efficacy. This study, however, found no acute signal that NAC improves the clinical status of COVID-19 patients.
Conclusion
Routine NAC should not be recommended for COVID-19-associated cytokine storm at this time. Further studies are needed to evaluate whether different dosing regimens and timing of drug administration can affect clinical outcomes.
Acknowledgments
We would like to acknowledge all hospital staff of Clara Maass Medical Center for their support and continued efforts during this COVID-19 pandemic response.
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Brandon Chen https://orcid.org/0000-0002-2766-6123
References
- 1.Park SE. Epidemiology, virology, and clinical features of severe acute respiratory syndrome-coronavirus-2 (SARS-CoV-2; Coronavirus Disease-19). Clin Exp Pediatr. 2020;63(4):119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rothe C, Schunk M, Sothmann P, et al. Transmission of 2019-ncov infection from an asymptomatic contact in Germany. N Engl J Med. 2020;382(10):970-971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dong E, Du H, Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533-534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoenfeld Y. Corona (COVID-19) time musings: our involvement in COVID-19 pathogenesis, diagnosis, treatment and vaccine planning. Autoimmun Rev. 2020;19(6):102538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen G, Wu D, Guo W, et al. Clinical and immunological features of severe and moderate coronavirus disease 2019. J Clin Invest. 2020;130(5):2620-2629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Silvagno F, Vernone A, Pescarmona GP. The role of glutathione in protecting against the severe inflammatory response triggered by covid-19. Antioxidants. 2020;9(7):624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Singhal T. A review of Coronavirus Disease-2019 (COVID-19). Indian J Pediatr. 2020;87(4):281-286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Von bismarck P, Klemm K, García Wistädt C-F, et al. Selective NF-kappaB inhibition, but not dexamethasone, decreases acute lung injury in a newborn piglet airway inflammation model. Pulm Pharmacol Therapeut. 2009;22(4):297-304. [DOI] [PubMed] [Google Scholar]
- 9.Cao M, Zhang D, Wang Y, et al. Clinical features of patients infected with the 2019 novel coronavirus (COVID-19) in Shanghai, China. medRxiv. doi: 10.1101/2020.03.04.20030395. Published online March 6, 2020 [DOI]
- 10.Marshall JC, Murthy S, Diaz J, et al. A minimal common outcome measure set for COVID-19 clinical research. Lancet Infect Dis. 2020;20(8):e192-e197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Geiler J, Michaelis M, Naczk P, et al. N-acetyl-L-cysteine (NAC) inhibits virus replication and expression of pro-inflammatory molecules in A549 cells infected with highly pathogenic H5N1 influenza a virus. Biochem Pharmacol. 2010;79(3):413-420. [DOI] [PubMed] [Google Scholar]
- 12.Ghezzi P, Ungheri D. Synergistic combination of N-acetylcysteine and ribavirin to protect from lethal influenza viral infection in a mouse model. Int J Immunopathol Pharmacol. 2004;17(1):99-102. [DOI] [PubMed] [Google Scholar]
- 13.Ibrahim H, Perl A, Smith D, et al. Therapeutic blockade of inflammation in severe COVID-19 infection with intravenous N-acetylcysteine. Clin Immunol. 2020;219:108544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Assimakopoulos SF, Aretha D, Komninos D, et al. N-acetyl-cysteine reduces the risk for mechanical ventilation and mortality in patients with COVID-19 pneumonia: a two-center retrospective cohort study. Inf Disp. 2021;53(11):847-854. [DOI] [PubMed] [Google Scholar]
- 15.de Alencar JCG, Moreira CL, Müller AD, et al. Double-blind, randomized, placebo-controlled trial with N-acetylcysteine for treatment of severe acute respiratory syndrome caused by COVID-19. Clin Infect Dis. 2020;72(11):e736-e741. doi: 10.1093/cid/ciaa1443. Published online September. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De flora S, Grassi C, Carati L. Attenuation of influenza-like symptomatology and improvement of cell-mediated immunity with long-term N-acetylcysteine treatment. Eur Respir J. 1997;10(7):1535-1541. [DOI] [PubMed] [Google Scholar]
- 17.Zhang R, Wang X, Ni L, et al. COVID-19: melatonin as a potential adjuvant treatment. Life Sci. 2020;250:117583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marik PE, Khangoora V, Rivera R, Hooper MH, Catravas J. Hydrocortisone, vitamin C, and thiamine for the treatment of severe sepsis and septic shock: A retrospective before-after study. Chest. 2017;151(6):1229-1238. [DOI] [PubMed] [Google Scholar]
- 19.Prasad AS, Beck FW, Bao B, et al. Zinc supplementation decreases incidence of infections in the elderly: effect of zinc on generation of cytokines and oxidative stress. Am J Clin Nutr. 2007;85(3):837-844. [DOI] [PubMed] [Google Scholar]
- 20.Dexamethasone in hospitalized patients with covid-19. N Engl J Med. 2021;384(8):693-704. [DOI] [PMC free article] [PubMed] [Google Scholar]