Abstract
Coffea arabica (Rubiaceae) is a basic drink for all Gulf societies, especially Saudi Arabia, it is the main part of the Saudi tradition. This investigation was carried out to track the chemical composition, caffeine content by UV–visible spectrophotometer, acrylamide content by using a gas chromatograph, free radical scavenging capacity by DPPH methods as well as determined the browning index and separated the volatiles compounds using GC–MS for the most common three degree of roasted Arabic coffee; light (180 ± 10 °C; 6.0 ± 1.0 min), medium (180 ± 10 °C; 8.0 ± 1.0 min), and dark (180 ± 10 °C; 10.0 ± 1.0 min). Data revealed that light roasted coffee has the highest significant (p < 0.05) value of moisture content (4.80%), crude protein (13.05%), and lowest value of ether extract (10.39%) and crude fiber (24.24%). The caffeine content was found to be 1.13% in light coffee, which increased to 1.17% in medium coffee, then decreased to 1.08% in dark coffee. The quantity of acrylamide detected in light roasted coffee (0.41 mg/100 g) was the greatest, whereas medium roasted coffee comparatively produced low amounts (0.31 mg/100 g). The light roasted coffee gave the highest antioxidant activity (88.72 mg TE/g), while the dark roasted coffee gave the least activity (78.76 mg TE/g). Browning index increases with roasting time. Hydrocarbons, alcohols, and esters were the most represented in roasted coffee headspace. Silanes and sec-butyl nitrite compounds were absent in the medium roasted headspace. Except for amines, all 11 classes of volatile compounds were present in the headspace of dark roasted coffee.
Keywords: Roasted Arabic coffee, Caffeine, Acrylamide, Antioxidant activity, Aroma
1. Introduction
Arabic coffee Coffea Arabica (Rubiaceae) is considered the most consumed hot beverage in Saudi Arabia and forms part of Saudi traditions (Al-Mssallem & Brown, 2013). It is mainly made from Arabica coffee beans (Butt & Sultan, 2011). The most popular Arabic coffee beans, and the ones commonly used by people in Saudi Arabia, are the beans that come from the Jizan region, Yemen, and the Harar area of Ethiopia (Butt and Sultan, 2011, Al-Abdulkader et al., 2018, Al Doghaither and Al-Malki, 2017). According to data obtained from the International Coffee Organization, the global intake of coffee estimated to be 1.4 billion cups per day (Statistics, 2015). In Saudi Arabia, coffee consumption has increased dramatically with 18,000 tons per year imported, with a total cost $54 million (Statistics, 2015). The average consumption of Arabic coffee in Saudi by a typical adult was estimated to be between 60 and 300 mL in one sitting, and an individual annually consumes the equivalent of 1.6 kg of coffee (Butt & Sultan, 2011). Coffee consumption has some positive effect on health, such as improving some functions including memory, mood, and cognitive performance (Borota et al., 2014, Olson et al., 2010, Nehlig et al., 2010, Farah, 2018). Several studies on humans have demonstrated that coffee intake is effective in decreasing the hazard of liver cancer incidence by 40% compared with others who do not consume coffee (Bravi et al., 2013). On the other hand, Arabic coffee can increase the risk of cardiovascular disease by increasing body total cholesterol and LDL-C (Badkook & Shrourou 2013). Drinking Arabic coffee among Saudi females aged > 40 years was associated with high osteoporosis (AlQuaiz et al., 2014). There are different kinds of Arabic coffee in the Saudi market roasting to various degrees (Alqarni et al., 2018). The roasting degree of coffee influenced by the roasting temperature and roasting time, and these two elements are responsible for the color of the coffee, whether it be a light, medium, or dark roast coffee (Somporn et al., 2011). Although Arabic coffee contains multiple antioxidant compounds in high concentrations, such as phenolic and flavonoid compounds (Ahmed et al., 2013), the roasting process can make a significant change to the biological activity of the coffee and the chemical composition. For example, some elements can be lost, such as natural phenolic compounds, and other elements can be formed, such as antioxidant compounds, including Maillard reaction products and, therefore, antioxidant activity can be maintained or increased (Wang et al., 2011). Increasing the roasting degree of coffee reduces the antioxidant activity and caffeine content as well (Alqarni et al., 2018, Cho et al., 2014). Antioxidant capacity is higher in coffee obtained from light roasting than coffee obtained from hard roasting, due to the high polyphenol content (Vignoli et al., 2014).
The literature showed that there is no comprehensive study that compared the effect of roasting degree on Arabic coffee in chemical composition, antioxidant activity, and aroma volatile compounds. Therefore, the current study aims to compare the difference in chemical composition, antioxidant activity, color attributes, and aroma volatile compounds in three roasting degrees of Arabic coffee to facilitate in conducting a biological study in the nest stage.
2. Materials and methods
2.1. Materials
About (9 kg) fermented and dried beans of local Arabic coffee cultivar namely Kholani were purchased from a series of supermarket at Tabuk city, Tabuk region, Saudi Arabia. Coffee beans were roasted using drum roasters (The most popular roasters for coffee), where the seeds contact a hot surface of which the temperature reaches 180 ± 10 °C. According to Ku Madihah et al., 2013, Chung et al., 2013, Darsef, 2014, the optimum roasting temperature is 180 °C to achieve high quality. Therefore, 180 °C was chosen as the roasting degree in our research. The coffee was roasted for varying time periods to obtain the three degrees of roasting most popular in Saudi Arabia, which are light, medium, and dark, according to the following roasting conditions (180 ± 10 °C; 6.0 ± 1.0 min), (180 ± 10 °C; 8.0 ± 1.0 min) and (180 ± 10 °C; 10.0 ± 1.0 min), respectively. Then, the roasted coffee was well-ground in the coffee grinder (model GVX212, Krupps, Essen, Germany) with a screen size of 0.30 mm and maintained in the refrigerator in an airtight jar until analysis. The chemicals and indicators utilized in this investigation were of analytical quality level, and obtained from Sigma – Aldrich, USA.
2.2. Chemical analysis
A proximate chemical analysis of roasted coffee powder was analyzed for moisture content, ether extract, crude protein, crude fiber and ash content according to AOAC methods: 925.10, 945.16 968.06, 962.09 and 923.03
(AOAC, 2007), respectively. The nitrogen-free extract was computed by deducting the summation percentages of crude protein, ether extract, crude fiber, and ash content from one hundred.
Moisture, fat, and protein contents and ash of UTCS and WTCS were determined by AOAC
2.3. Determination of caffeine by UV–visible spectrophotometer
According to a procedure reported by Belay et al., (2008), about 50 mg of sieved coffee was dissolved in a temperature range of 80–90 °C in 100 mL of distilled water. To extract caffeine using a magnetic stirrer for 1/2hr and gently heated the solution, get rid of solid particle by using filter paper 25 mL of coffee extract was mixed with 25 mL dichloromethane, stirred the mixture for 10 min. The organic phase, containing the most caffeine (140 mg/mL of caffeine solubility in dichloromethane), was isolated from the water phase with a separate funnel from the mixture. According to Haenen, et al., (1999), the most active solvent for extract 98 – 99% of caffeine from coffee is dichloromethane. Watched the water phase with 25 mL dichloromethane and isolated the organic phase, repeat watching and isolating for 4 times. Then collected the fractions from the organic phase and mixed together. The absorption was measured on a spectrophotometer with 274 nm UV/Vis by using a 10 mm quartz cuvette. 100 mg of pure caffeine was dissolved in 100 mL of distilled water to prepare the caffeine stock standard solution. Running solutions prepared by series dilution of the stock in 25 mL volumetric flasks for 0, 10, 20, 40, 60, and 80 mg/L of caffeine and add 1.0 mL HCl, topping it with distilled water up to the mark.
2.4. Determination of acrylamide
Acrylamide was determined according to (Wendie et al., 2005) by using a Gas Chromatograph with a flame ionization detector at a temperature of 260 °C. An RTX-5 column (30 m lenght × 0.25 mm I.D. × 0.25 m film thickness) was employed with a 260 °C injector temperature, helium gas at constant pressure as a carrier gas, and an oven temperature of 100 °C (held 0.5 min) to 200 °C, at 15 °C/min.
2.5. Free radical scavenging capacity
The free radical scavenging capacity of coffee samples was established by using the stable 1,1-Diphenyl-2-picryl-hydrazyl (DPPH) according to Yusufoglu et al., (2018). The absorbance at 517 nm was measured by UV–Vis spectrophotometer (model UV-180, Shimadzu, Japan) against a blank of pure methanol. The antioxidant activity was expressed as mg of trolox equivalent (TE) per g of powder coffee.
2.6. Browning index
The absorption of the five-fold diluted coffee brew solution was measured by a spectrophotometer UV–Vis (Labomed Inc., USA) at 420 nm, as reported by Chung et al., (2013). This index measures brown compounds, including melanoidins, formed during caramelization, and Maillard reactions.
2.7. Color measurements
Chromameter (CR 400) was used to evaluate the color calculation for roasted coffee. International Commission of Illumination (CIE) reports that the data were represented in L*, a* and b* those indicate to reflected light and coordinated chromatic, red–green axis, and yellow–blue axis, respectively.
2.8. Estimation of volatiles compounds
Volatiles compounds were separated by Gas chromatography-mass spectrometry (Hewlett-Packard 6890 GC/HP 5973 MS Agilent Technologies) fitted with silica capillary column INNOWax (60 m length, 0.2 mm I.D., 0.25 μm film thickness), Helium was the carrier gas. GC–MS conditions stated by Buffo & Cardelli-Freire, 2004, the column gas flow rate was 1 mL min−1, as defined in the GC–MS conditions. The oven program was increased from 40 to 200 °C, with an initial and holding time of 5 °C min−1, at 5 and 45 min, respectively. The conditions were as follows: interface temperature, 280 °C; ionizing force, 70 eV; mass range, 30–330 amu; scanning rate, 2.2 scan sec-1. The amount of injection in splitless mode was 1 μL. Comparing mass spectra with the WILEY and NIST mass spectral (MS) libraries to established volatile compounds.
2.9. Statistical analysis
Results were expressed as mean ± SD. To analyze differences in statistical significance, ANOVA was used by SPSS version 9.0. The results were considered significant at p < 0.05.
3. Results
Proximate chemical composition content in different roasting grades of coffee is presented in Table 1. Data revealed that light roasted coffee have the highest value of moisture content (4.80%) and crude protein (13.05%), and lowest value of ethyl ether extract (10.39%) and crude fiber (24.24%). These values are significantly different (p < 0.05) among dark roasted coffee samples, which have the lowest value of moisture content (3.89%), a small decrease crude protein (11.10%), and the highest values of ether extract (10.65%), crude fiber (28.40%), and ash content (4.10%).
Table 1.
Proximate chemical composition (% in dry matter) content in light, medium, and dark coffee.
| Degree | Light coffee | Medium coffee | Dark coffee |
|---|---|---|---|
| Moisture content % | 4.80 ± 0.24a | 4.30 ± 0.17b | 3.89 ± 0.28c |
| Ether extract % | 10.39 ± 0.30b | 10.47 ± 0.19b | 10.65 ± 0.22a |
| Crude protein % | 13.05 ± 0.14a | 12.36 ± 0.24ab | 11.10 ± 0.06b |
| Crude fiber % | 24.24 ± 0.47b | 28.31 ± 0.31a | 28.40 ± 0.42a |
| Ash content % | 3.95 ± 0.26b | 3.89 ± 0.08b | 4.10 ± 0.17a |
| Nitrogen free extract (NFE) % | 48.37 | 44.97 | 45.76 |
Results are presented as mean ± SD (n = 3). Values followed by the different letters in rows are significantly different at (p < 0.05).
Table 2 shows the quantity of caffeine, acrylamide and the DPPH radical scavenger activities at various roasting degrees. The caffeine level of the three degrees of roasted coffee is not significantly different, according to the findings. The caffeine content was found to be 1.13% in light coffee, increased to 1.17% in medium coffee, and then decreased to 1.08% in dark coffee. The highest amounts of acrylamide were obtained in light roasted coffee (0.41 mg/100 g), whereas medium roasted coffee produced comparatively produced low amounts (0.31 mg/100 g). Regards to the DPPH radical scavenger activities, the light roasted coffee had the highest antioxidant activity (88.72 mg TE/g) while the dark roasted coffee had the lowest activity (78.76 mg TE/g).
Table 2.
Caffeine content, acrylamide, and free radical scavenging capacity (DPPH) in light, medium, and dark coffee.
| Degree | Light coffee | Medium coffee | Dark coffee |
|---|---|---|---|
| Caffeine content % | 1.13 ± 0.02a | 1.17 ± 0.07a | 1.08 ± 0.06a |
| Acrylamide(mg/100 g) | 0.41 ± 0.086a | 0.31 ± 0.063b | 0.36 ± 0.048ab |
| DPPH (mg TE/g) | 88.72 ± 2.91a | 84.61 ± 1.76a | 78.76 ± 2.49b |
Results are presented as mean ± SD (n = 3). Values followed by the different letters in rows are significantly different at (p < 0.05).
The changes in the browning index, L*, a*, and b* values with increasing of roasting time at the same roasting temperature (180 ± 10 °C) are shown in Table 3. Dark roasted coffee (10 min) showed the highest browning index (1.84), and light roasted coffee (6 min) had the lowest browning index (0.45). The whiteness roasted coffee (L* value) tends to decrease significantly (p < 0.05) with increased roasting time. The redness of roasted coffee is expressed by the value a*, which tends to increase throughout the roasting process. The b* value (degree of yellowness) for roasted coffee increased at lower roasting temperatures (180 °C; 6–8 min) but decreased significantly at higher roasting temperatures (180 °C; 10 min).
Table 3.
Browning index and color characteristics in light, medium, and dark coffee.
| Degree | Light coffee | Medium coffee | Dark coffee | |
|---|---|---|---|---|
|
Browning index (420 nm) |
0.4540 ± 0.13b | 0.8600 ± 0.13b | 1.8400 ± 0.24a | |
| Color | L * | 58.62 ± 2.73a | 48.83 ± 1.73b | 41.04 ± 3.06b |
| a * | 9.75 ± 0.44b | 13.04 ± 0.07a | 13.93 ± 0.62a | |
| b * | 31.74 ± 1.21a | 32.21 ± 0.75a | 29.80 ± 1.52b | |
Results are presented as mean ± SD (n = 3). Values followed by the different letters in rows are significantly different at (p < 0.05).
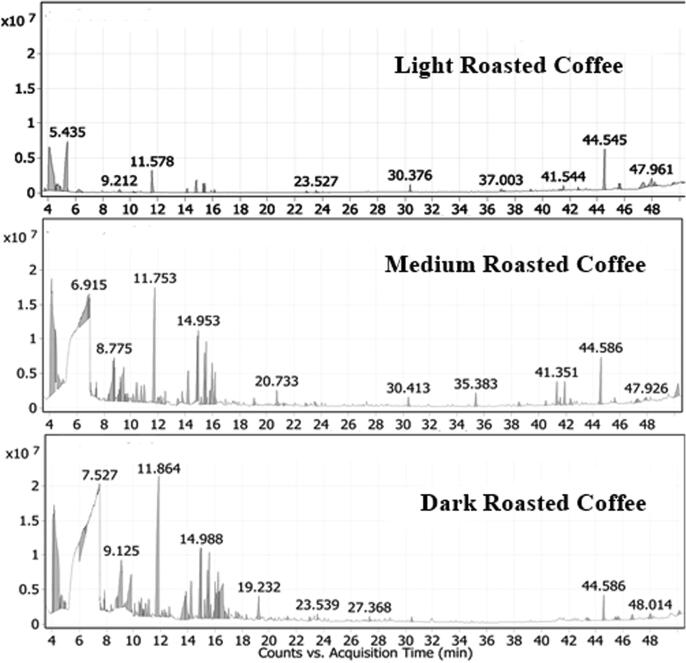
The gas chromatograms of the volatile compounds of the different levels of roasting conducted in the current study are shown in Fig. 1. Also, the volatile compounds in coffee extracts are shown in Table 4. Generally, seventy-nine compounds were identified in the headspace of the three roasting grades of coffee Fig. 2..
Fig. 1.
Gas chromatograms of the volatiles in headspace of light, medium, and dark roasted coffee headspace.
Table 4.
The percentage of volatile compounds in headspace of light, medium, and dark roasted coffee headspace.
| No | Name | Formula | Light coffee | Medium coffee | Dark coffee |
|---|---|---|---|---|---|
| 1 | 4,4-dimethyl-3-hexanol | C8H18O | 0.61 | ND | ND |
| 2 | 3-methylheptane | C8H18 | 32.87 | 24.61 | 21.98 |
| 3 | Carbonic acid, nonyl prop-1-en-2-yl ester | C13H24O3 | ND | 1.42 | ND |
| 4 | 3-Hexanol | C6H14O | ND | 0.23 | ND |
| 5 | (2R,3R)-2-Methyl-3-propyloxirane | C6H12O | 1.41 | ND | ND |
| 6 | Octane | C8H18 | ND | ND | 0.19 |
| 7 | 6,6-dideutero-5-methyl-Undecane | C12H24D2 | ND | ND | 1.56 |
| 8 | 6,8-dioxabicyclo (3.2.1) octan-4. β.-ol-2-D1 | C6H9DO3 | 2.71 | ND | ND |
| 9 | sec-Butyl nitrite | C4H9NO2 | 1.48 | ND | 0.37 |
| 10 | Trimethylsilyl methanol | C4H12OSi | 21.08 | 7.25 | 4.47 |
| 11 | 4-methyl-Octane | C9H20 | 1.66 | ND | ND |
| 12 | 1-Butaneboronic acid | C4H11BO2 | ND | 0.22 | 0.20 |
| 13 | 2-(hydroxymethyl)-1-Hexanol | C7H16O2 | 1.01 | ND | ND |
| 14 | Acetic acid, hexyl ester | C8H16O2 | ND | 0.34 | 0.29 |
| 15 | 2-Ethoxy-3-chlorobutane | C6H13ClO | ND | 0.42 | 0.32 |
| 16 | 3-(1-ethoxyethoxy)-2-methyl-butanal | C9H18O3 | ND | 4.37 | 0.69 |
| 17 | 1-(1-ethoxyethoxy)-Propane | C7H16O2 | ND | 6.29 | 6.22 |
| 18 | 2,2′-Bi-1,3-dioxolane | C6H10O4 | ND | 0.47 | ND |
| 19 | 3,4-dimethyl- 2-Hexanol | C8H18O | ND | 0.26 | 0.23 |
| 20 | 3,5-dimethyl- 3-Hexanol | C8H18O | ND | 0.94 | ND |
| 21 | tetramethyl-silane | C4H12Si | ND | ND | 4.57 |
| 22 | Octanal | C8H16O | ND | 1.26 | 0.4 |
| 23 | 5-ethyl-2-Heptanol | C9H20O | ND | 1.02 | 1.36 |
| 24 | 2,4-dimethyl-3-Pentanol | C7H16O | 0.43 | 4.16 | 3.24 |
| 25 | D-Limonene | C10H16 | 4.68 | 8.52 | 13.07 |
| 26 | Emylcamate | C7H15NO2 | ND | 0.33 | ND |
| 27 | 5-(1-ethoxy-ethoxy)-4-methyl-hex-2-enal | C11H20O3 | ND | ND | 0.32 |
| 28 | 3-pentanol, 3-methyl-, carbamate | C7H15NO2 | ND | ND | 0.35 |
| 29 | 1-ethoxy- octane | C10H22O | ND | 0.90 | 0.58 |
| 30 | [S- (R*, R*)]- 1,2,3,4-butanetetrol | C4H10O4 | ND | 0.54 | 3.65 |
| 31 | 1-[1-ethoxyethoxy]-, (E)- 3-hexene | C10H20O2 | ND | 0.70 | ND |
| 32 | 1-chloro-3-(1-methylethoxy)- 2-propanol | C6H13ClO2 | ND | ND | 1.98 |
| 33 | 4,5-diethyl- Octane | C12H26 | 4.18 | 8.92 | 8.96 |
| 34 | 1-[1-ethoxyethoxy]-, (E)- 3-hexene | C10H20O2 | ND | ND | 0.93 |
| 35 | 5,6-dimethyl-decane | C12H26 | 2.63 | 5.69 | 5.32 |
| 36 | Acetic acid, ethyl ester | C4H8O2 | ND | 0.63 | ND |
| 37 | 1-Hexyl-4,4-D2 acetate | C8H14D2O2 | ND | 3.95 | ND |
| 38 | 5-methyl- undecane | C12H26 | 0.42 | 1.53 | ND |
| 39 | Z-limonene-1,2-epoxide | C10H16O | ND | ND | 0.5 |
| 40 | Camphor | C10H16O | ND | ND | 0.25 |
| 41 | 3,6-dimethyl-octan-2-one | C10H20O | ND | 0.52 | 1.40 |
| 42 | 2,3-dihydroxy-, (S)-Propanal | C3H6O3 | ND | ND | 1.36 |
| 43 | Methoxy acetic acid, octyl ester | C11H22O3 | ND | 0.73 | 2.20 |
| 44 | Glycerin | C3H8O3 | ND | ND | 5.35 |
| 45 | limonene dioxide 1 | C10H16O2 | ND | ND | 0.65 |
| 46 | 11-Dodecyn-1-ol acetate | C14H24O2 | ND | ND | 0.58 |
| 47 | alpha., alpha.,4-trimethyl-, (S)-3-Cyclohexene-1-methanol | C10H18O | ND | ND | 0.4 |
| 48 | 1-Cyclohexyl-3-ethoxy-butan-2-one | C12H22O2 | ND | 0.47 | ND |
| 49 | 3-Tetradecanynoic acid | C14H24O2 | ND | ND | 0.19 |
| 50 | 2-Ethoxy-3-chlorobutane | C6H13ClO | ND | ND | 1.50 |
| 51 | 1-methoxy-4-(2-propenyl)-Benzene | C10H12O | ND | 1.03 | ND |
| 52 | 3-ethoxy-3,7-dimethyl- 1,6-Octadiene | C12H22O | ND | 0.49 | 0.21 |
| 53 | Propanoic acid, 2-methyl-, 2,2-dimethyl-1-(2-hydroxy-1-methylethyl) propyl ester | C12H24O3 | 0.38 | ND | ND |
| 54 | Propanoic acid, 2-methyl-, 3-hydroxy-2,2,4-trimethylpentyl ester | C12H24O3 | 0.51 | ND | ND |
| 55 | 1,2–15,16-Diepoxyhexadecane | C16H30O2 | ND | ND | 0.29 |
| 56 | [1-(5-hexenyl)-2-methylenecyclopropyl] trimethyl-silane | C13H24Si | ND | ND | 0.18 |
| 57 | 3,7-dimethyl-, isobutyrate, (Z)-2-octen-1-ol | C14H26O2 | ND | ND | 0.2 |
| 58 | [1,1′-bicyclopropyl]-2-octanoic acid, 2′-hexyl-, methyl ester | C21H38O2 | ND | 0.23 | ND |
| 59 | 2,2,4-Trimethyl-1,3-pentanediol diisobutyrate | C16H30O4 | 1.47 | 0.50 | 0.23 |
| 60 | Benzoic acid, 2-ethylhexyl ester | C15H22O2 | ND | 0.90 | ND |
| 61 | O-(phenylmethyl)- L-serine | C10H13NO3 | ND | 0.33 | ND |
| 62 | Benzene propionic acid, 3-tridecyl ester | C22H36O2 | ND | 0.26 | ND |
| 63 | 2H-Pyran-3-ol, tetrahydro-2,2,6-trimethyl-6-(4-methyl-3-cyclohexen-1-yl)-, [3S-[3α.,6. α.(R*)]]- | C15H26O2 | 0.92 | ND | ND |
| 64 | Tetraneurin - α – diol | C15H20O5 | ND | ND | 0.3 |
| 65 | 1-(3-Methyl-2-butenoxy)-4-(1-propenyl) benzene | C14H18O | ND | 1.4 | ND |
| 66 | Phthalic acid, butyl undecyl ester | C23H36O4 | 0.79 | 0.38 | ND |
| 67 | 2,3-dihydro-1H-Inden-5-ol | C9H10O | ND | 1.33 | ND |
| 68 | 2-methyl-benzo[e]-1,3,4-triazo-cyclohepta-7-en-3-one | C9H9N3O | ND | 0.51 | ND |
| 69 | Dibutyl phthalate | C16H22O4 | 7.93 | 2.87 | 1.13 |
| 70 | 9,12-Octadecadienoic acid (Z, Z)-, 2-hydroxy-1-(hydroxymethyl)ethyl ester | C21H38O4 | 3.39 | ND | 0.26 |
| 71 | 7-Methyl-Z-tetradecen-1-ol acetate | C17H32O2 | 1.66 | 2.05 | 0.64 |
| 72 | Hexadecadienoic acid, methyl ester | C17H30O2 | ND | 0.25 | ND |
| 73 | 9-Octadecenoic acid (Z)- | C18H34O2 | 1.61 | ND | ND |
| 74 | 2,6,10-trimethyl- tetradecane | C17H36 | 0.95 | ND | ND |
| 75 | 4,7-Octadecadiynoic acid, methyl ester | C19H30O2 | ND | ND | 0.45 |
| 76 | 1-Heptatriacotanol | C37H76O | ND | ND | 0.47 |
| 77 | Glycidyl oleate | C21H38O3 | 3.73 | ND | ND |
| 78 | 9,12,15-Octadecatrienoic acid, 2,3-dihydroxypropyl ester, (Z, Z, Z)- | C21H36O4 | ND | 0.46 | ND |
| 79 | Oleic Acid | C18H34O2 | 1.46 | 0.29 | ND |
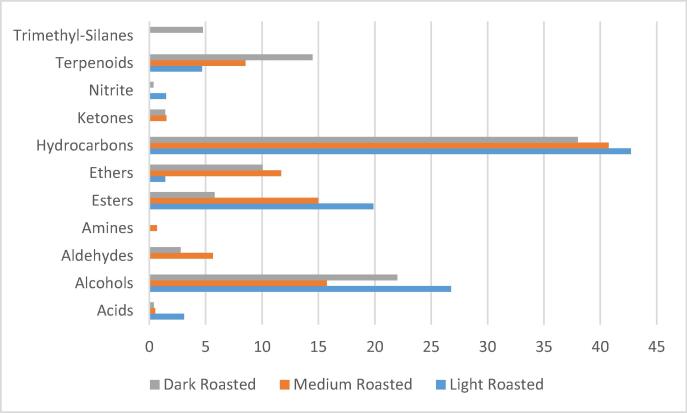
Fig. 2.
The total area percentages of the main chemical classes of volatile components in the headspace of roasted coffee.
4. Discussion
The increase in roasting time is accompanied by a decrease in moisture content caused by a high percent of dry matter. Due to being relatively heat stable, a slight increase was obtained in ether extract. During the roasting process, a part of the coffee protein is degraded into free amino acids and peptides, which are consumed by Strecker reactions and result in a decrease in protein concentration. These data are in agreement with those reported by Oliveira, 2006, Hadipernata and Nugraha, 2018 for roasted coffee; except for moisture levels (1.5%), which do not agree with our data may be due to different roasting condition. Nogaim and Gowri, 2013 found an average content of moisture, crude proteins, total lipids, carbohydrates, and ash in Arabic coffee were 6.99, 10.95, 6.13, 22.12 and 4.16%, respectively, in agreement with our data. In addition, Vasconcelos et al., (2007) reported that protein concentrations stayed the same during the different level of roasting, a decrease in ash content and a minor increase in oil content after roasting probably due to the difference in variety and cultivation conditions. Caffeine content in light, medium, and dark coffee is not significantly different among the three degrees of roasted coffee, but due to sublimation, small losses can occur. Nonetheless, the loss of other compounds may be the cause of increased caffeine. The caffeine content results among the three grads of roasted coffee are logical, as caffeine may be partially lost during roasting, which is supported by the findings of Fuller and Rao, 2017, Alqarni et al., 2018. These data are in agreement with the findings of Farah and Donangelo, 2006, Trandafir et al., 2013 Mar. However, Daglia et al., (1994) reported higher values of caffeine content, measuring 1.8%–3.0% of caffeine in green beans, 1.7%–2.1% in medium roasted beans, and 1.6%–1.9% in strongly roasted beans. Caffeine was listed as a safe substance by the Food and Drug Administration, as harmful doses exceed 10 g for an average adult. Caffeine consumption is usually related to encouraged alertness, learning skills, workout performance, and possibly better mood, as it is used as a stimulant for the nervous system. However, high doses may adversely affect glucose tolerance and increase urinary excretion of minerals such as calcium, as stated by Alqarni et al., 2018. Anis et al (2010) stated roasting results in unhealthy compounds such as acrylamide. Acrylamide is mostly generated when food is cooked at high temperatures, resulting in a Maillard reaction between the amino group in asparagine and the carbonyl source, as in baking, roasting and frying. It is worth noting that at a light roast, the acrylamide content exceeds maximum values then decreases as the time of roasting increases. That result is congruent with the theory of forming during early Maillard reaction. According to Bortolomeazzi et al., (2012) acrylamide was found to range from 150 to 327 μg/kg in coffee (powder, instant, and ground). International Agency for Research on Cancer as well as many research has shown that the acrylamide has a mutagenic effect on laboratory animals, rendering it a possible carcinogenic substance to humans. The acceptable level of acrylamide in coffee products is still being determined and needs further research. In this context, the Food and Drug Administration announced that the acceptable level of consumption in fries is 0.077 mg/kg. Granby & Fagt (2004) recorded 1 g/100 g acrylamide in medium roasted coffee and the content was reduced to 0.5 g/100 g in dark roasted coffee. Alves et al., (2010) also mentioned that the concentration of acrylamide was reduced by about 25% in espresso prepared from medium compared with the acrylamide content in dark roast coffee. In agreement with the conclusion of Soares et al., (2009) that the content of acrylamide in coffee after slow roasting (ie roasting at low temperatures for a long time) is lower than roasting at high temperatures for a short time. As described by Ku Madihah et al., 2013, the optimum roasting condition of Arabic coffee is 180 °C for 26 min, producing low amounts of acrylamide (0.23 mg/100 g). It is clear that the DPPH radical scavenger activities decreased significantly when the roasting level increased, in agreement with Trandafir et al., 2013 Mar, Herawati et al., 2019. In the meantime, Del Castillo et al., 2002 recorded maximum antioxidant activity to be in medium roasted coffee (233 °C/3 min), in Colombian Arabica coffee beans, while dark coffee (240 °C/3 min) had lower antioxidant activity. In accordance with the degradation of the bioactive compounds during roasting, antioxidant compounds are thermally unstable (Vignoli et al., 2014), so reducing such compounds is consistent with a more intense roasting process where the antioxidants are destroyed during heating as concluded by Giuffrè et al., (2018). However, as the grade of roasting increases, large quantities of Maillard and Strecker reaction products are produced and established to enhance the overall antioxidant properties and help to compensate for phenolic losses in antioxidant activity (Ludwig et al., 2014, Vignoli et al., 2014).
Color development is among the factors often used to determine the level of roasting and one of the measures of final product quality (Shan et al., 2016). Several reactions occur during the roasting process, and the main reactions responsible for the formation of color and brown compounds are Maillard reaction and oxidative polymerization or degradation of phenolic compounds. The browning index measures the purity of the browning, as both enzymatic and non-enzymatic browning occurs during roasting. Yen et al., 2005 similarly found a decreased browning index in aqueous extracts of soluble spent coffee grounds compared to roasted coffee. The L* value indicates to the whiteness of roasted coffee. The L* value tends to decrease significantly (p < 0.05) with increased roasting time, this might be due to the brown compounds produced by non-enzymatic browning (Wang et al., 2011, Shan et al., 2016). The redness of roasted coffee (a* value) tended to increase over the entire roasting period. As investigated by Gökmen & Şenyuva (2006), the CIE a* color value is correlated with the acrylamide content in coffee. As previously mentioned, dark and medium roasted coffee contains less acrylamide than light coffee, in agreement with Alves et al., 2010.
The b*value (degree of yellowness) of roasted coffee increased at the lower roasting grade (180 ± 10 °C; 6 to 8 min) but decreased significantly with a higher roasting grade (180 ± 10 °C; 10 min). According to Afoakwa et al., 2014, increasing roasting time causes an increase in the b-value due to the thermal oxidation of polyphenols and Maillard products formation.
Seventy-nine compounds were identified by the gas chromatograms in the headspace of the three roasting grade of coffee. They are distributed into 11 chemical classes as follows: 20 esters, 19 alcohols, 12 ethers, eight hydrocarbons, four acids, four aldehydes, four terpenoids, three ketones, two amines, two trimethyl- silanes and sec-butyl nitrites (Fig. 2). The light roasted coffee had twenty-four compounds, which were distributed into seven classes, while vehicles belonged to aldehydes, ketones, amines, and trimethyl silanes. Hydrocarbons were the most representative, with 42.71% in light roasted coffee headspace, followed by alcohols (26.76%) and esters (19.86%). In the medium roasted headspace, silanes and sec-butyl nitrite compounds were absent, while hydrocarbons, alcohols, and esters were the major proportion (40.75%, 15.73%, and 14.97%, respectively). Except for amines, all 11 classes of volatile compounds were present in the headspace of dark roasted coffee. While hydrocarbons and alcohols amount to 38.01% and 22%, respectively, terpenoids reached 14.47%. They increased significantly compared to the light and medium samples, which had 4.68% and 8.52% of terpenoids, respectively. In a similar way, volatile aldehydes and ketone percentage increased in medium and dark coffee headspace compared to light coffee. Contrariwise, the percentages of volatile acids and esters were recorded to be significantly decreased in dark roasted coffee compared to light and medium roasted samples. Semmelroch and Grosch (1995) reported that the major odorants in both Arabica and Robusta coffee powders were 2,3-butanedione, 2,3-pentanedione, 3-methyl-2-butenthiol (I), methional, 2-furfurylthiol (II), and 3-mercapto-3-methylbutylformate (III).
The identification of volatile compounds in roasted coffee may become useless due to of the difficulty in identifying the chemicals effectively responsible for coffee aroma (Buffo & Cardelli-Freire, 2004).
5. Conclusion
Coffee is classified as a functional food based on the results, owing to its high quantity of compounds that have antioxidant and other biologically beneficial properties. The medium Arabic coffee showed good quality in most chemical composition parameters, including low acrylamide content. These results provide a basis for all interested researchers who may want to carry out further analyses.
Fund
It is self-funded research.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Footnotes
Peer review under responsibility of King Saud University.
References
- Afoakwa E.O., Budu A.S., Mensah-Brown H., Takrama J.F., Ofosu-Ansah E. Effect of roasting conditions on the browning index and appearance properties of pulp pre-conditioned and fermented cocoa (Theobroma Cacao) beans. J. Nutritional Health Food Sci. 2014;1:1–5. [Google Scholar]
- Ahmed G.M., El-Ghamery H.E., Samy M.F. Effect of green and degree of roasted Arabic coffee on hyperlipidemia and antioxidant status in diabetic rats. Adv. J. Food Sci. Technol. 2013;5:619–626. [Google Scholar]
- Al Doghaither H., Al-Malki E. The Addition of Herbal Additives Influences the Antioxidant Activity of Traditional Arabic Coffee. World Appl. Sci. J. 2017;35(3):393–398. [Google Scholar]
- Al-Abdulkader A.M., Al-Namazi A.A., AlTurki T.A., Al-Khuraish M.M., Al-Dakhil A.I. Optimizing coffee cultivation and its impact on economic growth and export earnings of the producing countries: The case of Saudi Arabia. Saudi J. Biol. Sci. 2018;25(4):776–782. doi: 10.1016/j.sjbs.2017.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Al-Mssallem M., Brown J. Arabic coffee increases the glycemic index but not insulinemic index of dates. Saudi Med J. 2013;34(9) [PubMed] [Google Scholar]
- Alqarni MH, Alam P, Salkini MA and Abdel-Kader MS. Roasting Effect on the Caffeine Contents and Antioxidant Potential of Different Coffee Grades Available In the Saudi Market., Indo Am. J. P. Sci, 2018; 05(12).
- AlQuaiz A.M., Kazi A., Tayel S., Shaikh S.A., Al-Sharif A., Othman S., Habib F., Fouda M., Sulaimani R. Prevalence and factors associated with low bone mineral density in Saudi women: a community based survey. BMC Musculoskelet Disord. 2014;15(1) doi: 10.1186/1471-2474-15-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alves R.C., Soares C., Casal S., Fernandes J.O., Oliveira M.B.P.P. Acrylamide in espresso coffee: influence of species, roast degree and brew length. Food Chem. 2010;119:929–934. [Google Scholar]
- Badkook M., Shrourou R. Arabic coffee with two doses of cardamom: effects on health biomarkers in healthy women. Int. J. Nutrition Food Sci. 2013;2(6):280–286. [Google Scholar]
- Belay A., Ture K., Redi M., Asfaw A. Measurement of caffeine in coffee beans by UV- Vis spectrometer. Food chem. 2008;108:310–315. [Google Scholar]
- Borota D., Murray E., Keceli G., Chang A., Watabe J.M., Ly M., Toscano J.P., Yassa M.A. Post-study caffeine administration enhances memory consolidation in humans. Nat. Neurosci. 2014;17(2):201–203. doi: 10.1038/nn.3623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bortolomeazzi R., Munari M., Anese M., Verardo G. Rapid mixed mode solid phase extraction method for the determination of acrylamide in roasted coffee by HPLC-MS/MS. Food Chem. 2012;135(4):2687–2693. doi: 10.1016/j.foodchem.2012.07.057. [DOI] [PubMed] [Google Scholar]
- Bravi F., Bosetti C., Tavani A., Gallus S., La Vecchia C. Coffee reduces risk for hepatocellular carcinoma: An updated meta-analysis. Clin. Gastroenterol. Hepatol. 2013;11(11):1413–1421.e1. doi: 10.1016/j.cgh.2013.04.039. [DOI] [PubMed] [Google Scholar]
- Buffo R.A., Cardelli-Freire C. Coffee flavour: An overview. Flavour and Fragrance Journal. 2004;19:99–104. [Google Scholar]
- Butt M.S., Sultan M.T. Coffee and its consumption: benefits and risks. Crit Rev Food Sci Nutr. 2011;51(4):363–373. doi: 10.1080/10408390903586412. [DOI] [PubMed] [Google Scholar]
- Cho A., Park K., Kim K., Kim S. Influence of Roasting Conditions on the Antioxidant Characteristics of Colombian Coffee (Coffea Arabica L.) Beans. J. Food Biochem. 2014;38(3):271–280. [Google Scholar]
- Chung H.S., Kim D.H., Youn K.S., Lee J.B., Moon K.D. Optimization of Roasting Conditions according to Antioxidant Activity and Sensory Quality of Coffee Brews. Food Sci. Biotechnol. 2013;22(1):23–29. doi: 10.1007/s10068-013-0004-1. [DOI] [Google Scholar]
- Daglia M., Cuzzoni M.T., Dacarro C. Antibacterial activity of coffee: Relationship between biological activity and chemical markers. J. Agric. Food Chem. 1994;42(10):2273–2277. [Google Scholar]
- Darsef R.S. Optimization of roasting robusta sukamakmur coffee with of response surface methodology. BEST: Int. J. Manage. Inf. Technol. Eng. 2014;22(6):35–42. [Google Scholar]
- Del Castillo M.D., Ames J.M., Gordon M. Effect of roasting on the antioxidant activity of coffee brews. J. Agric. Food Chem. 2002;50:3698–3703. doi: 10.1021/jf011702q. [DOI] [PubMed] [Google Scholar]
- Farah A. Nutritional and health effects of coffee. 2018. Federal University of Rio de Janeiro, Brazil.
- Farah A., Donangelo C.M. Phenolic compounds in coffee. Braz. J. Plant Physiol. 2006;18:23–36. [Google Scholar]
- Fuller M., Rao N.Z. The Effect of Time, Roasting Temperature, and Grind Size on Caffeine and Chlorogenic Acid Concentrations in Cold Brew Coffee. Sci Rep. 2017;7:17979. doi: 10.1038/s41598-017-18247-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giuffrè A.M., Caracciolo M., Zappia C., Capocasale M., Poiana M. Effect of heating on chemical parameters of extra virgin olive oil, pomace olive oil, soybean oil and palm oil. Ital. J. Food Sci. 2018;30(4) [Google Scholar]
- Gökmen V., Şenyuva H.Z. Study of color and acrylamide formation in coffee, wheat flour and potato chips during heating. Food Chem. 2006;99(2):238–243. [Google Scholar]
- Granby K., Fagt S. Analysis of acrylamide in coffee and dietary exposure. Analytica Chimica Acta. 2004;52:177–182. [Google Scholar]
- Hadipernata M., Nugraha S. Process technology of luwak coffee through bioreactor utilization. IOP Conf. Ser.: Earth Environ. Sci. 2018;102:012092. doi: 10.1088/1755-1315/102/1/012092. [DOI] [Google Scholar]
- Haenen M., den Berg H.V., Bast A. Applicability of an improved Trolox equivalent antioxidant capacity (TEAC) assay for evaluation of capacity measurements of mixtures. Food Chem. 1999;66:511–517. [Google Scholar]
- Herawati D., Giriwono P.E., Dewi F.N.A., Kashiwagi T., Andarwulan N. Critical roasting level determines bioactive content and antioxidant activity of Robusta coffee beans. Food Sci. Biotechnol. 2019;28(1):7–14. doi: 10.1007/s10068-018-0442-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku Madihah K.Y., Zaibunnisa A.H., Norashikin S., Rozita O., Misnawi J. Optimization of roasting conditions for high-quality Arabica coffee. Int. Food Res. J. 2013;20(4):1623–1627. [Google Scholar]
- Ludwig I.A., Mena P., Calani L., Cid C., Del Rio D., Lean M.E.J., Crozier A. Variations in caffeine and chlorogenic acid contents of coffees: what are we drinking? Food Funct. 2014;5(8):1718–1726. doi: 10.1039/c4fo00290c. [DOI] [PubMed] [Google Scholar]
- Nehlig A., Cunha R.A., de Mendonça A. Is caffeine a cognitive enhancer? J. Alzheimer’s Dis. 2010;20(s1):S85–S94. doi: 10.3233/JAD-2010-091315. [DOI] [PubMed] [Google Scholar]
- Nogaim Q and Gowri P. (2013). Determination of ochratoxin A in Yemeni green coffee. Scholars Academic Journal of Biosciences. 1: 253–262 Google Scholar.
- Oliveira, G. S. Comparação química dos grãos de café (Coffea arábica) sadio e seus grãos PVA (pretos, verdes, ardidos) oriundos do Sul de Minas e do Cerrado Mineiro, submetidos a diferentes graus de torração. Uberlândia, 2006. 113f. Dissertação - (Mestrado em Química), Instituto de Química, Universidade Federal de Uberlândia.
- Olson C.A., Thornton J.A., Adam G.E., Lieberman H.R. Effects of 2 adenosine antagonists, quercetin and cafeïne, on vigilance and mood. J. Clin. Psychopharmacol. 2010;30(5):573–578. doi: 10.1097/JCP.0b013e3181ee0f79. [DOI] [PubMed] [Google Scholar]
- Semmelroch P., Grosch W. Analysis of roasted coffee powders and brews by gas chromatography-olfactometry of headspace samples. LWT-Food Sci. Technol. 1995;28(3):310–313. [Google Scholar]
- Shan O., Zzaman W., Yang T. Impact of Different Temperature-Time Profiles during Superheated Steam Roasting on Some Physical Changes of Robusta Coffee. Pertanika J. Trop. Agric. Sci. 2016;39(3):311–320. [Google Scholar]
- - Soares, C., Farah, A., Fernandes, F., Fernandes, J. O. Influence of the roasting conditions on the formation of acrylamide in Brazilian coffee: preliminary results. Proc. 22nd Int. Conf. Coffee Sci. ASIC, 239–241. 2009. Campinas, SP, Brazil.
- Somporn c, A. Kamtuo, P. Theerakulpisut, S. SiriamornpunEffects of roasting degree on radical scavenging activity, phenolics and volatile compounds of Arabica coffee beans (Coffea Arabica L. cv. Catimor). Int. J. Food Sci. Technol. 46 (2011), pp. 2287–2296.
- Statistics. International Coffee Organization (ICO), 2015. Retrieved from http://www.ico.org.
- Trandafir I., Nour V., Ionica M.E. Antioxidant capacity, phenolic acids and caffeine contents of some commercial coffees available on the Romanian market. Arch Latinoam Nutr. 2013 Mar;63(1):87–94. [PubMed] [Google Scholar]
- Vasconcelos A.L.S., Franca A.S., Glória M.B.A., Mendonça J.C.F. A comparative study of chemical attributes and levels of amines in defective green and roasted coffee beans. Food Chem. 2007;101(1):26–32. [Google Scholar]
- Vignoli J., Viegas M., Bassoli D., Benassi M. Roasting process affects differently the bioactive compounds and the antioxidant activity of Arabica and robusta coffees. Food Res. Int. 2014;61:279–285. [Google Scholar]
- Wang H, H. Qian, W.-R. YaoMelanoidins produced by the Maillard reaction: Structure and biological activity. Food Chem. 128 (2011), pp. 573–584.
- Wendie L.C., Kristel D.V., Marc E.H. Quantifying the formation of carcinogens during food processing: acrylamide. Trends Food Sci. Technol. 2005;16:181–193. [Google Scholar]
- Yen W.J., Wang B., Chang L., Duh P. Antioxidant Properties of Roasted Coffee Residues. J. Agric. Food Chem. 2005;53:2658–2663. doi: 10.1021/jf0402429. [DOI] [PubMed] [Google Scholar]
- Yusufoglu H.S., Soliman G.A., Foudah A.I., AbdelKader M.S., Alqarni M.H., Alam A., Salkini M.A. Standardization and Antioxidant Studies of Arnebia hispidissima. Int. J. Pharmac. 2018;14:428–436. [Google Scholar]