Highlights
-
•
Early recognition of FIRES is key to provide possibly disease-modifying therapies.
-
•
Serum IL-1RA dosage may help to support treatment intensification.
-
•
Anakinra and ketogenic diet represent encouraging immunomodulatory strategies.
-
•
Their effect may be synergistic but further evidence in support is needed.
-
•
Structured neuropsychological testing is a relevant outcome measure of treatments.
Keywords: FIRES, NORSE, Interleukin, Anakinra, Ketogenic diet
Abstract
Febrile infection-related epilepsy syndrome (FIRES) is a challenging condition with unfavorable outcome in most cases. Preliminary evidence suggests that some interleukins, in particular IL-1 Receptor Antagonist (IL-1RA), could be elevated due to a functional deficiency of anti-inflammatory pathways. Therefore, treatment strategies acting on innate immunity could represent a targeted treatment.
We describe the case of an 11-year-old child with super-refractory status epilepticus (SE), lasting more than two months. After being treated aggressively with antiseizure medications, anesthetics and empiric treatment for autoimmune encephalitis without success, she responded to anakinra and ketogenic diet. Escalation of the therapy was supported by the finding of a very high serum level of IL-1RA. This immunomodulatory approach allowed to discharge the child from intensive care 48 days after the SE onset. After more than one year follow-up the patient has moderate intellectual disability but with good language skills; she is seizure free and without motor deficits.
This case suggests that serum IL-1RA serum levels may help to support treatment escalation. Moreover, anakinra and ketogenic diet represent encouraging immunomodulatory strategies which deserve further studies and could potentially have a synergistic effect. Finally, structured neuropsychological testing is an important outcome measure that will help to define the effectiveness of different treatment strategies.
1. Introduction
Febrile infection-related epilepsy syndrome (FIRES) is a subtype of new-onset refractory status epilepticus (SE) defined by the occurrence of refractory SE without an evident structural, toxic or metabolic cause, following a febrile infection occurring between 2 weeks and 24 h before seizure onset [1]. The exact mechanism of FIRES is still unknown and, by definition, no etiologic cause is found in the diagnostic evaluation [1]. However, preliminary evidence suggests that some interleukins (ILs), in particular a IL-1 receptor antagonist (IL-1RA), the main antagonist of the proinflammatory ictogenic IL-1ß, could be elevated in FIRES due to a functional deficiency of anti-inflammatory pathways [2]. Therefore, anakinra, a recombinant version of the IL-1RA, could represent a targeted treatment in FIRES, but only few cases have been reported so far [3]. Similarly, the ketogenic diet is thought to exert its anti-inflammatory properties through the inhibition of IL-1ß [4] and it was reported to be effective in FIRES, although no controlled studies are available [5].
To date, due to the lack of an effective therapeutic approach, most of the cases have a poor outcome with major neurological sequela in case of survival and chronic epilepsy in virtually all patients reported [6].
Herein, we describe a patient with FIRES, detailing clinical and laboratory findings, including an ILs panel. We also report the response to immunomodulating therapy such as anakinra and ketogenic diet, discussing the implications for the development of a treatment strategy.
2. Case report
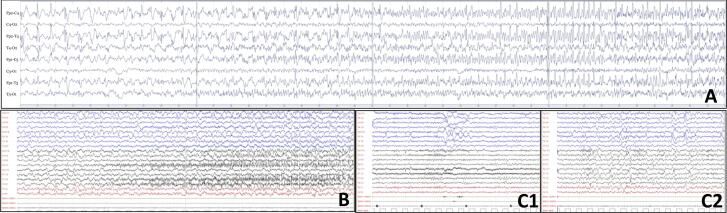
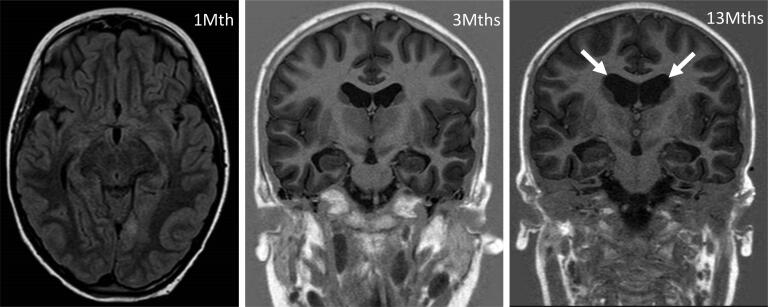
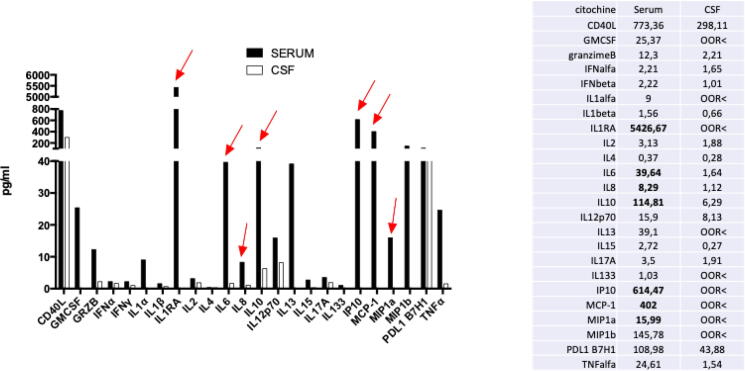
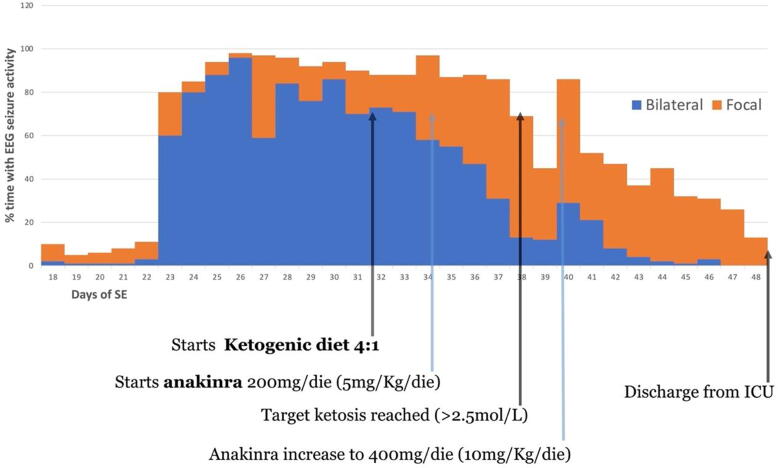
An 11-year-old female presented with repetitive focal to bilateral tonic-clonic seizures, two days after a febrile illness associated with abdominal pain and vomiting. She had normal neurodevelopment, regularly attended 6th grade in school, and had an unremarkable personal and family medical history, Serial seizures began with right version that rapidly evolved into SE that was refractory to first- and second-line antiseizure treatment. Therefore, she was transferred to the intensive care unit (ICU), sedated, intubated and mechanically ventilated. The EEG on day 2 showed a slow background with extreme delta brushes. An extensive diagnostic work-up was performed, to exclude infectious, autoimmune and metabolic causes (Table 1). All investigations were unrevealing except for the identification of hyperproteinorrachy and the isolation of rhinovirus DNA in the PCR panel on nasal exudate. On day 23, her clinical picture was critical; despite an escalation of anesthetics, she experienced continuous convulsive seizures with focal clonic jerks of the right hemibody at onset followed by bilateral clonic jerking. From day 18 to day 48 she was underwent continuously EEG monitoring. The seizure burden peaked on day 26 with abundant epileptiform EEG activity and focal to bilateral tonic-clonic seizures for >90% of the time of recording (Fig. 1A). Most of the seizures arose from the left occipital region but there were also other less active seizure onset regions (temporal left, central left, occipital right). The brain MRI was negative on the first day of seizure recurrence though on day 7 showed T2-FLAIR bilateral hyperintensity of the claustra, more prominent on the left side. This finding was still present but less marked at the control on day 13. On day 34 a T2-FLAIR hyperintensity was evident in the left cuneus (Fig. 2). After the failure of several anesthetics (midazolam, thiopental, propofol, ketamine), antiseizure medications (topiramate, cannabidiol, valproate, ospolot, phenobarbital, phenytoin, lacosamide, brivaracetam) and immunotherapy (metylprednisone 1 g/day for 5 days, IgIV for 5 days, Plasmapheresis for 5 days, every other day), other treatment strategies were considered. The escalation of the anti-inflammatory therapy was also supported by the results of a multiplex cytokine assay. Luminex® Platform (R&D Systems, Minneapolis, MN US) that detected high plasma levels of IL-6, IL-8, IL-10, IP10, MCP-1, MIP1a in the serum and very elevated levels of IL1RA (5327 pg/ml, Fig. 3). The ketogenic diet was started on day 32, reaching the target ketonemia (>2.5 mmol/L) after one week. Anakinra was considered early in the course, but its administration was delayed by the appearance of fever and raised inflammatory biomarkers and was finally started on day 34 at the dose of 200 mg/day and increased to 400 mg/day on day 40. After 3 days of anakinra treatment, seizures were reduced by 50% and stopped after 10 days. On day 48, only focal seizures persisted and she was discharged from the ICU. On day 56 anakinra was stopped due to candida albicans sepsis. This was without an increase in focal seizure frequency from an hourly rate of recurrence. After day 48, she was kept under continuous intravenous (IV) midazolam because of subtle focal seizures manifest as eye deviation to the right (Fig. 1B). This occurred until day 90, when it was stopped and she regained awareness. The focal seizures progressively became less frequent, and she was able to be transferred to rehabilitation on day 137. On day 217, she had a relapse of seizures during a febrile illness due to gastroenteritis, which were rapidly controlled with IV midazolam. On day 270 she was discharged home with the following therapy: lacosamide 400 mg/day, phenobarbital 100 mg/day and clobazam 25 mg/day (see Fig. 4).
Table 1.
Diagnostic investigations.
| Infectious | Serum IgM for common viruses (CMV, EBV, measles): negative | |
| Bacterial and fungal cultures, HIV immunoassay: negative | ||
| PCR multiplex for neurotropic virus on CSF (EBV, CMV, HSV1 e 2, VZV, parvovirus enterovirus, parechovirus HHV6, HHV7, HHV8, adenovirus, JC virus, BK): negative | ||
| PCR multiplex for bacteria on CSF (E. coli, H. influentiae. N. meningitidis, L. monocytogenes, S. agalactiae, S. pneumoniae): negative | ||
| West Nile virus RNA RT-PCR: negative | ||
| PCR panel for respiratory viruses on nasal exudate: positive for rhinovirus | ||
| CSF profile: normal except hyperproteinorrachy (80 mg/dL) | ||
| Metabolic | Serum lactate, ammonia, pyruvate: normal | |
| CSF lactate: normal | ||
| Serum acylcarnitine: profile normal | ||
| Plasma amino acids: normal | ||
| Urine organic acids: normal | ||
| Autoimmune | CSF and serum NMDA-R Ab: negative | |
| Immunoistochemistry on CSF and serum for Neuronal surface antigen antibodies: negative | ||
| ANA, ASMA, anti-endomyseal, anti-onconeural Ab: negative | ||
| Oligoclonal bands of CSF: absent | ||
| MRI | Day 1 | Normal |
| Day 7 | T2-FLAIR hyperintensity of the claustra bilaterally left > right | |
| Day 34 | T2-FLAIR hyperintensity of the left cuneus (Fig. 2) | |
| 3 months | Normal | |
| 1 year | Mild cortical atrophy (>parieto-occipital) | |
Fig. 1.
EEG findings. A) day 30, the beginning of one of the repetitive focal to bilateral motor seizures with EEG onset in the left posterior head region showing typical evolution of ictal activity. B) day 71, a focal left occipital seizure associated with right eye deviation. C) 8 months, normal EEG awake (C1) and during sleep (C2).
Fig. 2.
MRI findings. Left panel: T2-FLAIR hyperintensity of the left cuneus at 1 month from seizure onset. Mid and right panel: mild cortical atrophy from 3 to 13 months (T1-IR) as highlighted by lateral ventricles (arrows) enlargement.
Fig. 3.
Cytokines panel results. On the left, histogram reporting the levels of the various cytokines. The red arrows point on the results that are markedly beyond those observed in healthy controls. On the right, table with the numerical results in serum and csf. CSF: cerebrospinal fluid.
Fig. 4.
Clinical course during ICU stay in relation with anakinra and ketogenic diet.
At thirteen months from the onset, no clinical seizures appeared, no motor disability was present, just a moderate intellectual disability with a milder impact on verbal functioning (WISC-IV verbal comprehension 56, perceptual reasoning 48 working memory 42 processing speed 47, total IQ 40) was noted. She was able to go back to her original school but with a support teacher. She progressively weaned the ketogenic diet due to compliance issues and continues now on a low carbohydrate diet.
3. Discussion
We present the case of an 11-year-old child with FIRES that, despite a very high seizure burden necessitating ICU admission for 48 days, achieved a relatively good outcome at one year follow-up. Although FIRES is a clinical syndrome and not a defined disease [1], in recent years increasing efforts were dedicated finding biomarkers helping to identify early FIRES and guide treatment escalation. In particular, IL-1RA, the receptor antagonist of the cornerstone pro-inflammatory cytokine IL-1, was found to be markedly elevated in patients with FIRES compared to controls and patients with drug-resistant epilepsy [2]. This elevation was thought to be compensatory to a functional deficiency of the IL-1 receptor signaling and proposed as a specific biomarker of FIRES. Moreover, ictal EEG was described as characterized by prolonged focal fast activity ad hemispheric shifting which was proposed as typical, although not confronted with other etiologies of super-refractory SE [7]. In our patient, both EEG features and the elevated levels of the IL-1RA in the serum helped to suspect the diagnosis and guide the treatment but the cytokine panel on the cerebrospinal fluid was negative.
In 2016, anakinra, the recombinant version of the human IL-1RA, was first proposed to be effective in a case report of FIRES for which, a few days after reaching the dose of 10 mg/kg/d, a marked seizure reduction was observed [3]. Subsequently, a retrospective cohort of 25 children proved that earlier initiation of anakinra was associated with shorter duration of hospital stay [8]. However, concomitant use of the ketogenic diet was the rule with the daily dose lower (median 5 mg/kg/day) and highly variable, as was the treatment duration (median 86 days, range 13–257). Moreover, the neuropsychological outcome was qualitatively defined and generally bad with only 3/20 patients with no or mild disability. All patients had chronic epilepsy at a median follow-up of about a year. Other case reports showed similar results [9], [10], [11], [12]. Anakinra was suggested to be effective in 5/6 patients with chronic drug resistant epilepsies of unknown etiology [13]. The inhbition of another proinflammatory cytokine receptor (anti-IL6, tocilizumab) was reported to be effective in terminating SE in 6/7 patients with new-onset refractory status epilepticus but only 3/7 had a moderate disability or less as defined by the modified Rankin scale and all except one had chronic epilepsy. None of the patients had the ketogenic diet.
In 2010, the ketogenic diet was first proposed as an effective treatment in 9 patients with FIRES [5] and this finding was replicated in other case series [14].
In our case too, it is difficult to disentangle the efficacy of anakinra and ketogenic diet from other therapies over time. In our patient, the withdrawal of anakinra 22 days after its introduction due to sepsis did not lead to seizure aggravation. This could be explained either by the fact that it exerts its effects in the acute phase and that its continuation may be unnecessary, or that the concomitant effect of ketogenic diet could help keep neuroinflammation under control. To the best of our knowledge, this is the only case where anakinra was used at 20 mg/Kg/day, with the exception of one other reported case who had a good outcome where tocilizumab was administered concomitantly [15]. In our patient, anakinra and the ketogenic diet were administered relatively late in the course of the SE and therefore we cannot exclude that the seizures would have stopped anyway. At the same time, we are convinced that the relatively good outcome despite the long duration of SE and ICU stay argues against this hypothesis.
With respect to the clinical outcome, this is the first case in which no seizures were reported at one year follow-up. A structured neuropsychological follow-up was performed, showing that visuo-perceptive skills and executive function were more severely affected than language, which was relatively spared. This illustrates that a formal neuropsychological assessment helps explore the degree of cognitive dysfunction that could be masked by intact language skills.
In conclusion, our observation suggests that the determination of serum ILs may support earlier administration of immunomodulating therapies targeting autoinflammatory pathways. Nevertheless, longitudinal measurement in large cohorts of patients with superrefractory SE and correlation with clinical and EEG features are needed to establish their sensitivity and specificity and their role to guide treatment duration. Moreover, anakinra and the ketogenic diet may have a synergistic beneficial effect. These encouraging data from our patient and with previous reports provide support for inflammatory biomarker profiling and may suggest early immunomodulatory therapeutic interventions.
Ethical statement
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Conflict of interest
None of the authors has any conflict of interest to disclose.
References
- 1.Hirsch L.J., Gaspard N., van Baalen A., Nabbout R., Demeret S., Loddenkemper T., et al. Proposed consensus definitions for new-onset refractory status epilepticus (NORSE), febrile infection-related epilepsy syndrome (FIRES), and related conditions. Epilepsia Published online. 2018;59(4):739–744. doi: 10.1111/epi.14016. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson B.D.S., LaFrance‐Corey R.G., Kahoud R.J., Farias‐Moeller R., Payne E.T., Howe C.L. Functional deficiency in endogenous interleukin-1 receptor antagonist in patients with febrile infection-related epilepsy syndrome. Ann Neurol. 2019;85(4):526–537. doi: 10.1002/ana.25439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kenney-Jung D.L., Vezzani A., Kahoud R.J., LaFrance-Corey R.G., Ho M.-L., Muskardin T.W., et al. Febrile infection-related epilepsy syndrome treated with anakinra. Ann Neurol. 2016;80(6):939–945. doi: 10.1002/ana.24806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Youm Y.-H., Nguyen K.Y., Grant R.W., Goldberg E.L., Bodogai M., Kim D., et al. The ketone metabolite β-hydroxybutyrate blocks NLRP3 inflammasome-mediated inflammatory disease. Nat Med. 2015;21(3):263–269. doi: 10.1038/nm.3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nabbout R., Mazzuca M., Hubert P., Peudennier S., Allaire C., Flurin V., et al. Efficacy of ketogenic diet in severe refractory status epilepticus initiating fever induced refractory epileptic encephalopathy in school age children (FIRES) Epilepsia. 2010;51(10):2033–2037. doi: 10.1111/j.1528-1167.2010.02703.x. [DOI] [PubMed] [Google Scholar]
- 6.Gaspard N., Foreman B.P., Alvarez V., Cabrera Kang C., Probasco J.C., Jongeling A.C., et al. New-onset refractory status epilepticus: Etiology, clinical features, and outcome. Neurology. 2015;85(18):1604–1613. doi: 10.1212/WNL.0000000000001940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Farias-Moeller R., Bartolini L., Staso K., Schreiber J.M., Carpenter J.L. Early ictal and interictal patterns in FIRES: The sparks before the blaze. Epilepsia. 2017;58(8):1340–1348. doi: 10.1111/epi.13801. [DOI] [PubMed] [Google Scholar]
- 8.Lai Y.-C., Muscal E., Wells E., Shukla N., Eschbach K., Hyeong Lee K.i., et al. Anakinra usage in febrile infection related epilepsy syndrome: an international cohort. Ann Clin Transl Neurol. 2020;7(12):2467–2474. doi: 10.1002/acn3.51229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sa M., Singh R., Pujar S., D'Arco F., Desai N., Eltze C., et al. Centromedian thalamic nuclei deep brain stimulation and Anakinra treatment for FIRES – Two different outcomes. Eur J Paediatr Neurol. 2019;23(5):749–754. doi: 10.1016/j.ejpn.2019.08.001. [DOI] [PubMed] [Google Scholar]
- 10.Yang J.H., Nataraj S., Sattar S. Successful treatment of pediatric FIRES with anakinra. Pediatr Neurol. 2021;114:60–61. doi: 10.1016/j.pediatrneurol.2020.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Palacios-Mendoza M., Gómez A., Prieto J., Barrios J.C., Orera M., Massot-Tarrús A. Response to anakinra in new-onset refractory status epilepticus: A clinical case. Seizure. 2021;2022(94):92–94. doi: 10.1016/j.seizure.2021.11.014. [DOI] [PubMed] [Google Scholar]
- 12.L’Erario M., Roperto R.M., Rosati A. Sevoflurane as bridge therapy for plasma exchange and Anakinra in febrile infection–related epilepsy syndrome. Epilepsia Open. 2021;6(4):788–792. doi: 10.1002/epi4.12545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yamanaka G., Ishida Y., Kanou K., Suzuki S., Watanabe Y., Takamatsu T., et al. Towards a treatment for neuroinflammation in epilepsy: Interleukin-1 receptor antagonist, anakinra, as a potential treatment in intractable epilepsy. Int J Mol Sci. 2021;22(12):6282. doi: 10.3390/ijms22126282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Peng P., Peng J., Yin F., Deng X., Chen C., He F., et al. Ketogenic diet as a treatment for super-refractory status epilepticus in febrile infection-related epilepsy syndrome. Front Neurol. 2019;10 doi: 10.3389/fneur.2019.00423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stredny CM, Case S, Sansevere AJ, Son M, Henderson L, Gorman MP. Interleukin-6 blockade with tocilizumab in anakinra-refractory febrile infection-related epilepsy syndrome (FIRES). Child Neurol Open 2020;7:2329048X2097925. doi:10.1177/2329048x20979253. [DOI] [PMC free article] [PubMed]