Abstract
Hyperglycemia is a central trait of diabetes mellitus (DM) and is linked to an increase in free radical generation and oxidative stress in the testes, resulting in testicular tissue damage and male infertility. Synthetic medicines are commonly used to manage diabetes; however, they are costly and associated with adverse effects. As a result, the search for a safer and affordable alternative from medicinal plants that contain antioxidants has become imperative to scavenge free radicals caused by hyperglycaemia, thereby alleviating male reproductive dysfunction. Therefore, the present aimed to investigate the ameliorative effects of Anchomanes difformis aqueous extract against oxidative stress in the testes and epididymis of streptozotocin-induced diabetic male Wistar rats. A total of 64 male Wistar rats (eight weeks old) weighing 180 ± 10 mg/kg were divided into seven groups at random. Type 2 diabetic mellitus (T2DM) was induced by streptozotocin (STZ) and a 10% fructose injection intraperitoneally using 40 mg/kg body weight rats. The levels of malondialdehyde (MDA), catalase (CAT), and superoxide dismutase (SOD) activity, reduced glutathione (GSH) concentration, and ferric reducing antioxidant (FRAP) as well as 2, 2-diphenyl-1-picrylhydrazyl (DPPH) values were used to establish the testicular oxidative status. It was found that A. difformis extract significantly (p < 0.05) lowered MDA levels in diabetic rats. Both CAT and SOD activity were significantly (p < 0.05) lower following induction of DM and increased (p < 0.05) after treating with A. difformis. The findings of this study show that A. difformis extract could be a promising source of lead compounds for the development of a therapeutic agent to treat male infertility caused by DM complications.
Keywords: Anchomanes difformis, Oxidative stress, Free radicals, Diabetes mellitus, Hyperglycaemia, Male reproductive dysfunction, Antioxidants, Endogenous antioxidant enzymes
1. Introduction
Diabetes mellitus (DM) is a metabolic disease caused by complications in the secretion or action of insulin that leads to hyperglycemia (Johnson et al., 2019, Temidayo and Stefan, 2017). Due to the increased prevalence of DM worldwide (Cho et al., 2018), chemical drugs such as metformin and glibenclamide were produced for the treatment of DM for lowering blood glucose levels (Liu et al., 2018). However, side effects associated with the usage of these synthetic drugs have raised great concern and hence, the quest for a safer alternative and cost-effective drugs to treat DM has been increasing over the years (Khaki et al., 2014). Hyperglycemia causes excessive production of free radicals (Silva et al., 2020, Tian et al., 2020). A new approach in the treatment of DM emerged after the discovery of oxidative stress as the main instigator of the complications accompanied by DM (Nna et al., 2019). One of the major complications that can be caused by DM is male infertility (Alsenosy et al., 2019). According to Nna and colleagues (2017), there is a relationship between the percentage of diabetic men and the prevalence of male infertility.
The administration of antioxidant-rich medicinal plants to reduce oxidative stress in diabetes mellitus is the focus of the current research, especially since oxidative stress is linked with complications that arise in DM, including male infertility. Oxidative stress leads to the destruction of the male gonads (testes and epididymis) which affects the production and storage of the spermatozoa, further leading to male infertility (Tian et al., 2020). Most of the non-enzymatic antioxidant compounds constitute some plants (Alabi et al., 2020). Due to this knowledge, several studies have investigated the effect of certain medicinal plants on DM complications targeting the antioxidant pathway (Nna et al., 2019, Ostovan et al., 2017). Medicinal plants play an important role in developing countries as therapeutic remedies for complications caused by diabetes (Ataman and Idu, 2015). Sustainable development and medical research continue with further research on the benefits that some plants have on diabetic complications, and the possibility of these plants to treat diabetes with fewer side effects (Tchicaillat-Landou et al., 2018).
Anchomanes difformis (A. difformis), also known as Blume, is an Araceae plant that grows in tropical areas and is mostly found in African countries such as Nigeria, Togo, and Ivory Coast (Ahmed, 2018, Ataman and Idu, 2015). It is a large green-stem plant with white color at the bottom of the stem (Ataman and Idu, 2015). Its prickly stem contains watery or milky latex that can grow long from up to 0.8 m to 2 m in height (Ataman and Idu, 2015). This plant has been well known in traditional medicine for its use in treating different diseases (Alabi et al., 2020). A. difformis roots were used in Benin republic to treat anal and oral wounds and for the treatment of DM and its complications (Aliyu et al., 2013). The roots of the plant were also reported for treating dysentery in Nigeria (Aliyu et al., 2013). Other diseases and ailments such as asthma (Alabi et al., 2020, Oghale and Idu, 2016), malaria (Olanlokun et al., 2017), cough and throat related issues (Ataman and Idu, 2015), were treated by extracts from the plant were recorded. A. difformis contains nutritional components together with some compounds such as alkaloids and phenolic compounds, which are components of antioxidants (Alabi et al., 2021).
Previous studies have yielded positive results regarding the effect of some plants for male infertility treatment as a complication caused by oxidative stress (Asadi et al., 2017, Oyenihi et al., 2020). A. difformis is one of the medicinal plants investigated and found to have positive effects on diabetic parameters, due to its antioxidant content (Alabi et al., 2020). Furthermore, studies on the antioxidant, anti-inflammatory, antidiabetic activities of A. difformis have been documented (Alabi et al., 2020, Ataman and Idu, 2015). Despite the numerous reported biological potentials of A. difformis, there is not enough data on its protective role in restoring low endogenous antioxidant enzymes in male infertility. Therefore, this study specifically focuses on the effects of A. difformis against oxidative stress on the male reproductive organs.
2. Materials and methods
2.1. Chemicals
Streptozotocin (STZ) was supplied by Biocom Africa, Cape Town, South Africa. The following chemicals were procured from Sigma-Aldrich, USA: Na2HPO4·7H2O and NaH2PO4·H2O, sodium acetate and acetic acid, iron chloride (FeCl3), 2,4,6- tripyridyl-S-triazine (TPTZ), 2,2-diphenyl-1-picrylhidrazyl (DPPH), Trolox, ascorbic acid, sodium chloride, diethylenetriaminepentaacetic acid (DETAPAC), while the following chemicals were purchased from Merck, MA, USA: hydrogen chloride (HCl), hydrogen peroxide (H2O2), butylated hydroxytoluene (BHT), phosphoric acid, thiobarbituric acid, butanol, and ethanol. Perchloric acid, 5, 5′-disulfanediylbis (2-nitrobenzoic acid) (DTNB), ethylenediaminetetraacetic acid (EDTA), reduced nicotinamide adenine dinucleotide phosphate (NADPH), 2-nitro-5-thiobenzoic acid (TNB), glutathione reductase (GR), and thiobarbituric acid (TBA). The bicinchoninic acid protein assay kit was procured from Thermo Fisher Scientific, South Africa.
2.2. Preparation of A. difformis extract
A. difformis leaves were harvested from Abeokuta, Ogun state in Nigeria. The plant parts were harvested and authenticated (LUH6623) and a specimen was kept at the herbarium in the University of Lagos in Nigeria. Aqueous extract of A. difformis were prepared from the leaves of the plant via cold extraction method using distilled water as the solvent. The leaves were first dried in the shade for fourteen days at 28 ± 2 °C and then blended with a blender. The powder obtained was soaked into distilled water at a ratio of 1:10. The solution was filtered using the vacuum filtration method and the extracts were lyophilized. The final extracts were stored at -20 °C until the commencement of the study where it was given to rats orally.
2.3. Ethical considerations
The university's research ethics committee approved ethical clearance (CPUT/HW-REC 2016/A4). Furthermore, animal ethical approval (REF. 04/17) was obtained from the South African Medical Research Council's Ethics Committee for Research on Animals, where the study was conducted, and the criteria for animal research were satisfactorily followed. The Faculty of Health and Wellness Sciences Research Ethical Committee (REC) (ethics number: CPUT/AEC 2019/04) approved the use of reproductive organs, specifically the testes and epididymis, prior to the start of the study.
2.4. Animal’s study
Male Wistar rats (8 weeks old) weighing 180 ± 10 g were obtained from the animal facility at Stellenbosch University (Tygerberg campus) and maintained at the South African Medical Research Council under Primate Unit & Delft Animal Centre (PUDAC). The rats were kept under standard laboratory conditions, with an ambient temperature of 22 to 28 °C and a humidity of 45 to 55 %, and were placed in cages with 5 rats per cage to allow for free movement. The cages had a plastic base, a stainless steel canopy, and a feeder that also served as a storage area for the water bottle. Every day, the beds were made with mashed sterilized maize cob. At a 12 h dark/12 h light cycle, the rats were fed ad libitum with standard rat chow (SRC) and water. The rats were acclimatized for 3–4 weeks before the start of the experiment. The study included feeding (through a flexible feeding tube) and treatment administration while strictly adhering to compliance and animal care guidelines. The animals were handled with humane care and abnormal behaviours were observed during treatment (Alabi et al., 2020).
2.5. Treatment and induction of diabetes
Insulin resistance was induced by 10% fructose (Wilson and Islam, 2012). Rats were placed on 10% fructose ad libitum for 2 weeks. After 18 h of overnight fasting, the animals were injected once intraperitoneally with freshly prepared 40 mg/kg body weight STZ (Jaiswal et al., 2013). The STZ was dissolved in 0.1 M cold citrate buffer, pH 4.5 (Jaiswal et al., 2013). Blood was collected from the tails of the rats to measure blood glucose levels using a glucometer (Accu-check, Roche, Germany). Rats with blood glucose value ≥ 324 mg/dl three days STZ injection were considered diabetic. Treatment with A. difformis and the standard drug commenced after confirmation of diabetes, and that was considered day 1 of treatment. Rats were weighed every week and percentage change was calculated between the initial body weight and the final body weight measured after 10 weeks.
2.6. Study design
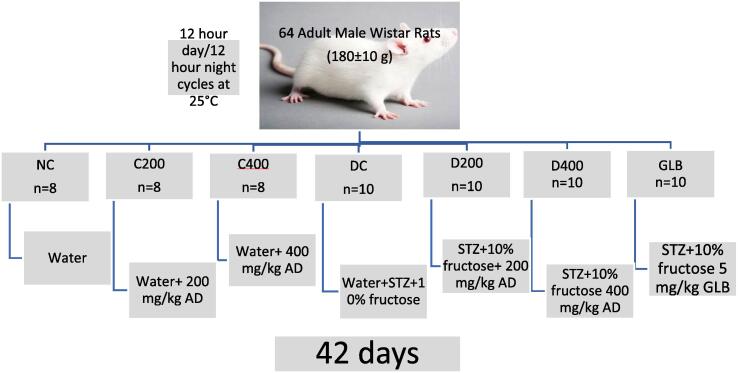
Sixty-four (64) male Wistar rats were procured for this study. The rats were randomly divided into 7 groups with 8 rats in each group of non-diabetic (normal) rats, and 10 rats per group of diabetic rats (Flecknell, 2002). Water was used as a vehicle to dissolve A. difformis and fructose, while citrate dissolved STZ. Induction was carried out after 14 days of 10% fructose administration (for the diabetic rats) and treatment followed 5 days after the induction, lasting for 42 days. Rats were grouped according to the following design: Group 1, the normal control group (NC), received SRC and the vehicle, water. Group 2, the normal treated group (C200), was treated with an aqueous extract of A. difformis at a 200 mg/kg dose (Oghale and Idu, 2016, Olanlokun et al., 2017). Group 3, the normal treated group (C400) was treated with a 400 mg/kg dose of aqueous extract of AD. Group 4, the diabetic control (DC), was treated with water, the vehicle for dissolving extract and standard drug. Group 5, the diabetic treated group (D200) was treated with a 200 mg/kg dose of aqueous extract of A. difformis. Group 6, the diabetic treated group (D400) was treated with a 400 mg/kg dose of AD. Group 7, the diabetic glibenclamide group (GLB) was treated with the standard anti-diabetic drug, glibenclamide (5 mg/kg) (Fig. 1).
Fig. 1.
Induction and treatment of the different groups over 42 days.
2.7. Tissue preparation
After 10 weeks, all animals were euthanized with the inhalation of 2% isoflurane per oxygen (1 L/min flow rate) followed by cardiac puncture. On dissection, the testes and epididymis were carefully removed from each animal and immediately weighed. The rest of the testes and epididymis were stored in the freezer for further biochemical analysis. Testes and epididymis tissue from individual rats were weighed (100 mg) in a 1 ml Eppendorf tube and 1 ml of phosphate-buffered saline (PBS) was added onto the tissue. The tissue and buffer were transferred to a glass cylinder and homogenized by a homogenizer for 15 sec. Homogenates were transferred to the Eppendorf tubes and stored in a −80 °C freezer for further assays.
2.8. Thiobarbituric acid reactive species (TBARS)
TBARS was determined as described by Buege and Aust, 1978, Esterbauer and Cheeseman, 1990. Lipid peroxidation was measured in testes and epididymis by adding 12 µl of TBA (prepared by adding 0.11 TBA in 0.1 NaOH), 12 µl of BHT (prepared by adding 4 mM BHT in 10 ml absolute ethanol) followed by 100 µl of phosphoric acid (684 µl phosphoric acid in 50 ml distilled water) into 100 µl of each homogenate sample. This was placed in a water bath at 90 °C for 45 min. An aliquot of 1000 µl butanol and 100 µl of saturated salt were added to the same Eppendorf tubes after 15 min of cooling. Butanol was used as a blank. A pipette was used to load 300 µl of butanol and the testes and epididymis samples were loaded in the wells of a 96-well plate in triplicates, with the first three well containing only butanol and the rest containing the testes and epididymis samples. The plate was read in a Multiskan Spectrum plate (Thermo Fisher Scientific, Waltham, MA, USA) reader at 532 nm (Buege and Aust, 1978, Esterbauer and Cheeseman, 1990).
2.9. FRAP assay
FRAP assay was carried out to calculate the amount of reduced Fe2+ formed by the donation of electrons by antioxidants in the testes and epididymis tissue, from oxidized Fe3+ according to the method outlined by Esterbauer and Cheesemans (1990). The antioxidant, L-ascorbic acid was employed as the standard reference. Fifty milliliters (50 ml) of the FRAP reagent was prepared by adding 30 ml of the acetate buffer (pH 3.6), 3 ml of FeCl3 reagent, 3 ml of 2, 4, 6-tripyridyl-s-triazine (TPTZ) reagent, and 6 ml of distilled water. FeCl3 reagent was prepared by mixing 0.053 g of FeCl3 with 10 ml of distilled water in a 15 ml plastic tube, and TPTZ was prepared by mixing 0.0093 g of TPTZ in 15 ml of 0.1 M HCl in a 15 ml plastic tube. A stock solution of ascorbic acid (100 mg/l) was diluted into a series of dilutions. Ten microliters (10 µl) of each sample (testes and epididymis) and the different concentrations of the vitamin C standard were loaded in triplicates in a 96-well plate, and 300 µl of FRAP reagent was added in each well. The plate was incubated for 30 min to allow the reaction to occur. The plate was read in a plate reader at 593 nm (Esterbauer & Cheesemans, 1990).
2.10. DPPH assay
The antioxidant activity of the sample was compared to the activity of Trolox as a standard in this study, following the method of Esterbauer and Cheesemans (1990). A serial dilution of the standard was prepared from a Trolox stock solution (1000 mg/l). A pipette was used to load 25 µl of both the testes and epididymis samples and standard in triplicates in the well of a 96-well plate and 275 µl of DPPH (0.4 mg/ml) was added in the wells and incubated for 30 min to allow the reaction to occur. The plate was read at 593 nm after 30 min in a plate reader (Esterbauer & Cheesemans, 1990).
2.11. Protein determination
The testes and epididymis protein content were measured, and antioxidant enzyme activities such as superoxide dismutase (SOD), catalase (CAT), and content (GSH) were calculated relative to the protein concentration. A dilution series was prepared from the original concentration of 2000 mg/l of bovine serum albumin (BSA). This was used to set up a standard curve for the determination of protein concentration in the samples. Using a pipette, 25 µl of the standard and testes and epididymis sample homogenates were loaded in a 96-well plate in triplicates. Reagent A/B was prepared by mixing bicinchoninic acid solution (Reagent A) and copper sulphate pentahydrate 4% solution (Reagent B) at a 50:1 ratio. A micropipette was used to load 200 µl of Reagent A/B into each well loaded with the samples and the standards. The plate was incubated for 30 min and read in a plate reader at 562 nm.
2.12. SOD activity determination
SOD activity was estimated by calculating the percentage inhibition of auto-oxidation of 6-hydroxydopamine (6-OHD) by superoxide free radicals. SOD activity was determined using the established protocol of Brannan et al. (1981). DETAPAC was used to inhibit any cycle formation of other free radicals. A 6-OHD solution was freshly prepared by adding 4 mg of 6-OHD in 10 ml of distilled water and 50 µl of perchloric acid in a 15 ml plastic tube. DETAPAC was prepared by adding 2 mg of DETAPAC in 50 ml of the PBS buffer. A mixture of oxidised 6-OHD and DETAPAC forms a pink/orange colour. A volume of 10 µl of each sample (testes and epididymis) was loaded in wells in triplicates and 15 µl of 6-OHD was transferred to each well. 170 µl of DETAPAC was then added to each well and the plate was read at 490 nm in a plate reader (Ellerby & Bredesden, 2000).
2.13. Catalase activity determination
The dissociation of H2O2 due to the activity of the catalase was determined by performing a catalase assay as described by Brannan et al. (1981). The preparation of H2O2 reagent was carried out by transferring 34 µl of H2O2 to 10 ml of the PBS buffer. PBS buffer (170 µl) was added to each well of the 96-well plate, and 10 µl of samples (testes and epididymis) was added to each well containing the PBS buffer·H2O2 reagent (75 µl) was transferred to each well and the plate was read in a plate reader at 532 nm (Brannan et al., 1981).
2.14. Glutathione assay
GSH was used as a standard in this assay and performed according to the method outlined by Ellerby and Bredesden (2000). PBS buffer, EDTA, pH 7.5, was used for this assay. NADPH solution was prepared by adding 12 ml of buffer in NADPH (0.3 mM). DTNB (0.3 mM) was prepared by adding 0.006 g DTNB in 50 ml of the PBS buffer. A GR solution was freshly prepared by adding 80 µl of GR and 5 ml of the PBS buffer in a 15 ml plastic tube. GSH (standard) of 3 mM was prepared by adding 0.046 g GSH in 50 ml of the PBS buffer. 50 µl of the testes and epididymis samples and standard were plated and 50 µl of DTNB was added to the wells. A volume of 50 µl of GR solution was also added to the wells. 50 µl of NADPH solution was added prior to the reading of the plate. The plate was read in a plate reader at 412 nm (Ellerby & Bredesden, 2000).
2.15. Statistical analysis
Graph Pad Prism version 5 software was explored to analyze the results. Data were expressed as mean ± SD. Normality and equality of variance in the data were tested using Levene’s test. The differences in the means between the groups were estimated by using a one-way analysis of variance (ANOVA). Group differences were tested by using the Kruskal-Wallis test. A probability of P < 0.05 was considered significant.
3. Results
3.1. Body weights and relative testes and epididymis weights
After 10 weeks, a decrease in body weight was observed in all rats in the diabetic groups (Fig. 2A). When compared to the normal control group, there was 24% weight loss in the diabetic control rats. The diabetic rats that received a 200 mg/kg dosage of A. difformis had a significantly (p < 0.05) increased weight (15.5%) compared to the diabetic untreated rats. The weight was improved more with the increase of dosage of treatment, as depicted by an increase in body weight (17.7%) of the diabetic rats treated with 400 mg/kg A. difformis extract, compared to the diabetic untreated rats. Diabetic rats that received glibenclamide had a 12.9% significant (p < 0.05) increase in body weight than diabetic untreated rats. Contrary to the observation in the control group, the diabetic control group's relative testes weight increased considerably (p < 0.05) (Fig. 2B). However, compared to the diabetic control group, the relative testes weight of the diabetic groups treated with both doses of A. difformis (200 mg/kg and 400 mg/kg) remained significantly higher. In diabetic rats treated with glibenclamide, no significant difference in relative testes weight was observed as compared to diabetic control rats. The diabetic control rats' epididymis weight was significantly reduced (p < 0.05) compared to the normal control rats' epididymis weight, which was significantly increased (p0.05) with both doses of A. difformis extract treatment (Fig. 2C). Similarly, glibenclamide increased the weight of the epididymis of rats significantly (p < 0.05).
Fig. 2.
“Effect of A. difformis on A. body weight of the diabetic rats, B. relative testes weight and C. relative epididymis weight. Mean values ± SD of body weight change is indicated by points and that of relative organ weights by bars. The difference in letters on bars indicate significance (p < 0.05). Group names abbreviated above are, Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with Glibenclamide (GLB)”.
3.2. Blood glucose levels
Blood glucose increased throughout the 10 weeks in the diabetic groups, while those of control groups remained largely unchanged as illustrated in Fig. 3. When compared to the normal control groups, the diabetic control group had a significantly (p < 0.05) higher blood glucose increase. As compared to the untreated diabetic group, the diabetic group given A. difformis extract had a significant (p < 0.05) decrease in blood glucose.
Fig. 3.
“Blood glucose levels over 10 weeks of treatment. The effect of A. difformis extract on blood glucose of diabetic rats is illustrated. Mean values ± SD of body weight change is indicated by points and that of relative organ weights by bars. The difference in letters on bars indicate significance (p < 0.05). Group names abbreviated above are, Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with Glibenclamide (GLB)”.
3.3. TBARS determination
The amount of MDA produced in the diabetic rats' testes increased significantly (p < 0.05) as compared to the control groups. In the diabetic groups treated with 200 mg/kg and 400 mg/kg A. difformis, there was a decrease in MDA production in the testes compared to the untreated diabetic group (p < 0.05), as shown in Fig. 4. MDA generation, on the other hand, in the 400 mg/kg A. difformis treated group increased drastically as compared to the group given 200 mg/kg A. difformis. There was no significant difference between the generation of MDA in the testes of the normal control groups and the group that received 200 mg/kg of A. difformis. When glibenclamide was given to the diabetic rats, their testes produced considerably lower MDA than untreated diabetic rat testes (p > 0.05). The epididymis production of MDA did not differ significantly (p > 0.05) between the normal control groups and the untreated diabetic group, as shown in Fig. 4B. In the epididymis, there was no significant difference in MDA levels between the untreated diabetic and treated diabetic groups (p > 0.05). The levels of MDA in the epididymis did not differ significantly (p > 0.05) across the diabetic-treated groups.
Fig. 4.
“The effect of administration of A. difformis on oxidative stress. MDA level in the testes compared within groups (A. representing testes and B. epididymis). The difference in letters on bars indicate significance (p < 0.05). Group names abbreviated above are: Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with glibenclamide (GLB)”.
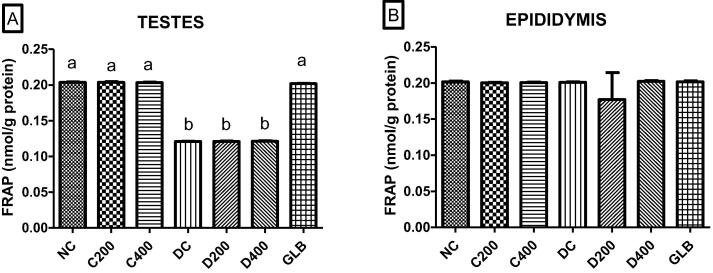
3.4. FRAP assay
The antioxidant capacity measured using FRAP in the rat testes and epididymis is illustrated in Fig. 5A and B, respectively. There was no difference in antioxidant capacity between the different normal control groups of both the testes and epididymis (p > 0.05). There was a substantial decrease in the reduction of Fe2+ in the testes of diabetic untreated rats compared to the normal control groups (p < 0.05). Similarly, the testes of the diabetic group treated with 200 mg/kg and 400 mg/kg, respectively, displayed a lower antioxidant capacity compared to that of the normal control groups (p < 0.05). There was no noticeable difference in antioxidant capacity between the untreated diabetic group and both groups treated with doses of A. difformis (200 mg/kg and 400 mg/kg, respectively) in the testes (p > 0.05). Conversely, there was an increase in Fe2+ reduction capacity in the testes of the rats treated with glibenclamide compared to the diabetic untreated group (p < 0.05). There is no difference in the Fe2+ reducing capacity amongst the normal control groups and between the normal control groups and the untreated diabetic group in the rat epididymis (p > 0.05). There no noticeable difference in the antioxidant capacity were evident between the untreated diabetic group and all the treated diabetic groups of the rat epididymis (p > 0.05).
Fig. 5.
“The effect of administration of A. difformis on FRAP scavenging capacity in the A. testes and B. epididymis compared within groups. The difference in letters on bars indicate significance levels (p < 0.05). Group names abbreviated above are, Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with glibenclamide (GLB)”.
3.5. DPPH assay
Fig. 6A and B depict the antioxidant activity using DPPH in rat testes and epididymis, respectively. There was no significant difference (p > 0.05) in the tissue radical scavenging capacity amongst the normal control groups of both the testes and epididymis. No significant difference in antioxidant capacity was evident in the untreated diabetic group compared to the normal control groups of the testes, as depicted in Fig. 6A. Similarly, the antioxidant capacity of the epididymis tissue in the untreated diabetic group exhibited no significant difference (p > 0.05) when compared to the normal control groups. A. difformis extract administration of both doses (200 mg/kg and 400 mg/kg) had no significant difference (p > 0.05) on radical scavenging capacity of the untreated diabetic groups of both the testes and epididymis. This was depicted in Fig. 6A and B, where no significant differences were evident in the diabetic groups treated with A. difformis compared to the untreated diabetic groups in the testes and epididymis. In addition, there were no significant differences (p > 0.05) in the radical scavenging capacity between the diabetic control group and that of the diabetic group treated with glibenclamide, in both the testes and the epididymis. The antioxidant capacity was no significant difference (p > 0.05) compared amongst all the diabetic treatment groups in both the testes and the epididymis.
Fig. 6.
“The effect of administration of A. difformis extract on scavenging capacity (DPPH) in A. testes and B. epididymis. Scavenging capacity in the organs compared within groups. The absence of letters on bars indicates insignificance (p > 0.05). Group names abbreviated above are, Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and diabetic-treated with glibenclamide (GLB)”.
3.6. SOD assay
SOD activity in rat testes and epididymis is shown in Fig. 7A and B, respectively. No significant difference was evident amongst the normal control groups. In the untreated diabetes group, SOD activity in the testes was considerably (p > 0.05) lower than in the normal control rats. In the testes, both the diabetic groups treated with 200 mg/kg and 400 mg/kg dosages of A. difformis had significantly increased SOD activity than the untreated diabetic group (p > 0.05). There was no significant difference in SOD activity between the treatment group treated with 200 mg/kg of A. difformis in the testes and the treatment group treated with 400 mg/kg of A. difformis in the testes (p > 0.05). The diabetic group treated with glibenclamide had significantly higher SOD activity in the testes (p > 0.05) than the diabetic group not treated. As indicated in Fig. 7B, there was no significant difference in SOD activity between the normal control groups in the epididymis (p > 0.05). In the epididymis, no significant difference in SOD activity was found between the normal control groups and the untreated diabetic group (p > 0.05). In the epididymis, no significant difference in SOD activity was found between the normal control groups and the untreated diabetic group (p > 0.05). There was no significant difference between the untreated diabetic group as compared to the group treated with glibenclamide or A. difformis (p > 0.05). The activity of SOD in the testes and epididymis did not differ significantly (p > 0.05) among all diabetic treatment groups.
Fig. 7.
“SOD activity in A. testes and B. epididymis compared within groups. The effect of administration of A. difformis and diabetic drug on SOD activity. Difference in letters on the bars represents significance (p < 0.05), and the absence of letters shows insignificance (p > 0.05). Group names abbreviated above are, Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with glibenclamide (GLB)”.
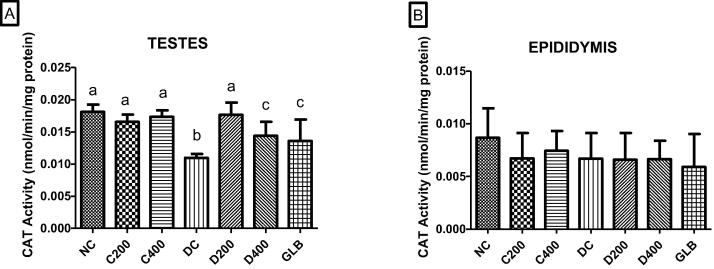
3.7. Catalase activity assay
Fig. 8A and B show the effect of A. difformis on catalase in normal and diabetic rat testes and epididymis. In rat testes from normal control and A. difformis-treated control groups, there were no significant variations in CAT activity (p > 0.05). However, compared to CAT activity in the testes of normal control rats, a decrease in CAT activity was observed in the diabetic control (p < 0.05). As represented in Fig. 8A, the administration of A. difformis extracts dramatically raised CAT activity in the testes of diabetic rats, with a concomitant increase in CAT activity in both A. difformis treatment groups compared to the untreated diabetic group. The CAT activity in the testes of the glibenclamide-treated diabetes group was observed to be higher than in the untreated diabetic group (p < 0.05). In the 200 mg/kg A. difformis dose group, CAT activity in testes was considerably higher than in the 400 mg/kg dose group (p < 0.05). The testes of the 400 mg/kg A. difformis treatment group and the glibenclamide treatment group showed no difference in CAT activity (p > 0.05). Furthermore, when compared to the group treated with 200 mg/kg A. difformis, the CAT activity in the testes of the glibenclamide group was noticeably lower (p < 0.05). There was no difference in CAT activity between the normal and A. difformis treatment groups in the epididymis. In the epididymis tissue, there was no difference in CAT activity between the normal control groups and the untreated diabetic groups (p > 0.05). In the epididymis, there were no difference between the untreated diabetic group and all of the diabetic treatment groups (p > 0.05). In the epididymis, the activity of CAT was no difference observed among all diabetic treatment groups (p > 0.05).
Fig. 8.
“CAT activity in A. testes and B. epididymis compared within groups. The effect of A. difformis extract on CAT activity of a diabetic model. Difference in letters on the bars represents significance (p < 0.05), and the absence of letters shows insignificance (p > 0.05). Group names abbreviated above are Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with glibenclamide (GLB)”.
3.8. Reduced glutathione assay
The findings of glutathione quantification in rat testes and epididymis are shown in Fig. 9A and B. There was no difference in GSH content between the normal control groups in the testes (p > 0.05). In the epididymis, there was no difference in GSH content between the normal control groups (p > 0.05). The GSH content in the untreated diabetic groups was not difference from the normal control groups in both the testes and the epididymis (p > 0.05). GSH content in the testes and epididymis were unaffected by A. difformis extract. As demonstrated in Fig. 9A and B, there was no difference in GSH content in the testes and epididymis between the untreated diabetic groups and the groups treated with A. difformis (p > 0.05). There was no difference in GSH content between the untreated diabetic group and the glibenclamide-treated diabetic group in both the testes and the epididymis (p > 0.05). All of the diabetic treated groups had similar levels of GSH in the testes and epididymis (p > 0.05).
Fig. 9.
“The effect of A. difformis extract and on GSH concentration in A. testes and B. epididymis. GSH concentration compared within groups. The absence of letters on bars indicates insignificance (p > 0.05). Group names abbreviated above are, Normal control (NC), Control-treated with 200 mg/kg dosage (C200), Control-treated with 400 mg/kg (C400), Diabetic control (DC), Diabetic-treated with 200 mg/kg (D200), Diabetic-treated with 400 mg/kg (D400) and Diabetic-treated with Glibenclamide (GLB)”.
4. Discussion
Hyperglycemia is associated with oxidative stress in the testes, which consequently leads to reproductive dysfunction (Kong et al., 2020). The increase in blood glucose causes an increase in the production of free radicals through the electron transport chain of the mitochondria, during glucose oxidation (Nna et al., 2019). STZ-induced partial loss of pancreatic beta cells, together with insulin resistance induced by a 10% fructose dosage, has been linked to T2DM symptoms and consequences (Goboza et al., 2019, Oyenihi et al., 2020). In the present study, blood glucose ≥ 324 mg/dl was indicative of diabetes. The increase in blood glucose (final glucose level ≥ 324 mg/dl) of the diabetic rats after 10 weeks, confirmed the establishment of a diabetic model in this study. In addition, diabetic symptoms such as frequent urination, polyphagia, increased thirst, elevated blood glucose levels, and weight loss were exhibited in this study.
The significantly lower final blood glucose level of the 200 mg/kg and 400 mg/kg A. difformis treated diabetic rats compared to the blood glucose level of the untreated diabetic rats suggests the hypoglycemic effects of A. difformis. This finding correlates with other studies that reported the probable effect of A. difformis in reducing the level of blood glucose (Alabi et al., 2021, Ovuakporie-uvo and Idu, 2015). The evident decrease in final blood glucose level in rats treated with glibenclamide compared to that of the untreated diabetic group confirms the hypoglycemic effect of glibenclamide (Liu et al., 2018, Rambiritch et al., 2014). However, this study shows a possibly higher hypoglycemic effect of A. difformis compared to glibenclamide.
Several studies have recorded evident weight loss in a diabetic model (Long et al., 2018, Olson et al., 2016). Although weight loss between 5% and 10% have been stated to be one of the interventions to curb complications associated with T2DM (Feldman et al., 2017), extreme weight loss may implicate compromised metabolism (Ige et al., 2011) and may lead to further complications (Yang et al., 2016, Olson et al., 2016), which is seen and depicted in this study. The non-significant change in bodyweight between the untreated diabetic rats and the treated diabetic rats suggests that A. difformis may not have a beneficial effect on the body weight of a diabetic model. This finding supports that of a diabetic study conducted by Ovuakporie-uvo and Idu (2015), where A. difformis showed no effect on body weight. Relative organ weight can be used as a parameter to investigate the effect of diabetes following plant supplementation (Ovuakporie-uvo and Idu, 2015). The weight and size of an organ are associated with the availability of functional units and the integrity of the organ (Baffoe et al., 2021). Studies have reported a diabetes-induced increase in relative testes weight due to hypertrophy (Ghafari et al., 2011, Ricci et al., 2009).
The result on the relative testes’ weight obtained in this study agrees with that reported by Ricci and colleagues, and this was not affected by both the plant extract and Glibenclamide treatments. The significantly lower relative epididymis weights of untreated diabetic rats compared to the normal control support the other studies that reported atrophy observed in the epididymis due to hyperglycemia (Korejo et al., 2016, Long et al., 2018). Atrophy of the epididymis can lead to its constriction, causing apoptosis and consequently, a reduction in the stored sperm cells (Korejo et al., 2016). The level of MDA is increased with diabetes, implying an increase in oxidative stress with hyperglycemia (Ayeleso et al., 2014, Sankaranarayanan and Kalaivani, 2020). The increase in MDA (oxidative stress) in the diabetic testes could have been caused by both the production of ROS that accompanied the established hyperglycemia, and the decrease in antioxidant activity (Alsenosy et al., 2019, Sefidgar et al., 2019). These findings support the other studies that have reported the association between DM and oxidative stress (Nna et al., 2019, Oguntibeju et al., 2020, Oyenihi et al., 2020). Alabi and colleagues (2020) reported the possible significance of A. difformis to extract in ameliorating oxidative stress through the Nrf2 pathway. In their study, it was reported that NrF2 was increased with diabetes and normalized when A. difformis was used as treatment, reducing oxidative stress. In support of these findings, this study depicted that the level of MDA in testes of rats treated with 200 mg/kg extract was significantly reduced, suggesting the effect of the extract in ameliorating oxidative stress in the testes, and possibly its positive effect in the improvement of male fertility.
Interestingly, the increase in the dose of A. difformis extracts to 400 mg/kg had a lower ameliorative effect on the testes of diabetic rats compared to that of the control. The 400 mg/kg dose could have possibly induced toxicity leading to higher oxidative stress (Ataman & Idu, 2015). Glibenclamide has also been associated with elevated oxidative stress by other studies (Liu et al., 2018). Previous studies found a higher level of oxidative stress in the epididymis of untreated diabetic rats compared to normal control rats (Nna et al., 2019, Ostovan et al., 2017). In contrast to these findings, there was no significant difference in MDA levels between the diabetic control group and the normal control groups' epididymis tissues. In this study, the antioxidants in the epididymis were not compromised and could be responsible for the scavenged free radicals and the non-significant change in MDA production. Several previous studies have revealed the antioxidant capacity and the availability of phytochemicals such as saponins, tannins, and flavonoids in A. difformis (Aliyu et al., 2013, Oghale and Idu, 2016). Interestingly, the phytochemical profiles of the studied plant had been previously carried out in our research group and reported in the study of Alabi et al. (2020) where phloridzin, rutin, quercetin, and kaempferol were identified as the predominant compounds in the crude extract. The presence of these compounds might be responsible for the biological activities demonstrated by the plant. As a result, the significant decrease in the reduction of Fe2+ in the testes of diabetic rats compared to the control groups in this study corroborates with the report of Oyenihi et al. (2020) that measured the FRAP capacity of the testes and deduced that antioxidant power was reduced when DM was induced. The antioxidant power may be compromised by oxidative stress (Erukainure, 2019, Pieme et al., 2017), further confirmed in the present study. This study showed a significant increase in FRAP in the testes of the diabetic rats treated with glibenclamide. The direct action of glibenclamide in scavenging free radicals was reported by Oguntibeju et al. (2020). This may suggest that the mechanism of scavenging free radicals by glibenclamide in the testes targets the reduction of Fe2+ in free radicals. Due to the unchanged oxidative status in the epididymis, there was no significant difference in Fe2+ reduction capacity and DPPH antioxidant capacity between all groups in the epididymis.
ROS is produced in the testes to aid in the production of sperms (Khorramabadi et al., 2018). To counteract the continuous production of ROS, the testes contain antioxidant enzymes (Tian et al., 2020). Findings from this present study revealed that both CAT and SOD activity was decreased in the testes of diabetic rats compared to the CAT activity in the testes of the normal control groups. These findings correlate with the results of other studies where oxidative stress was induced in the testes of rats and all these parameters were reduced (Fan et al., 2020, Nna et al., 2019). In other studies reported, reduction in antioxidant enzymes and oxidative stress was associated with protein glycation (Dzięgielewska-Gęsiak et al., 2019, Ghelani et al., 2018). Protein glycation was reported to cause the inactivation of antioxidant enzymes due to the alteration of amino acids present in the active sites of these antioxidant enzymes (Tavares et al., 2019). This phenomenon decreases the activity of CAT and SOD; it also contributes to the further elevation of oxidative stress (Tavares et al., 2019). However, GSH concentration remained significantly unaffected throughout the different groups of the testes in this study. This could imply that the level of GSH was not affected by glycation or the increase in oxidative stress. It could also suggest that the antioxidant enzymes were affected by oxidative stress independently, opposing the idea of focussing on NrF2 as the main factor affected.
Expression of CAT, SOD, and GSH in the epididymis of the diabetic group was not altered. This however corroborates with the finding in this study, that the production of MDA remained unaffected in the epididymis of the untreated diabetic group. This further suggests the association between oxidative stress and antioxidant enzyme activation (Pieme et al., 2017). In the study documented by Nna and colleagues (2019), reduction of antioxidant enzymes and increase in TBARS in the epididymis implied that germ cells were still exposed to oxidative stress even in storage. This study suggests that the damage of the germ cells could occur mainly in the testes, possibly due to the weight increase in the testes which could imply an increase in cell number or size, producing even more ROS. Treatment with 200 mg/kg and 400 mg/kg dosage of aqueous extract of A. difformis in the present study showed a significant increase in both CAT and SOD in the testes of diabetic rats. This could imply that the plant alleviates the deactivation of these enzymes caused by oxidative stress in the testes. The extract could also possibly have ROS scavengers (Martha et al., 2020, Oghale and Idu, 2016, Sri Harsha and Lavelli, 2019), which possibly reduced oxidative stress, thereby leaving the antioxidant system uncompromised.
In the study by Mohasseb and colleagues (2011), a marker of protein glycation (HbA1c) was decreased with the administration of antioxidants in the reproductive organs of rats, and it was concluded that antioxidants could ameliorate glycation. With the finding that A. difformis contains phytochemicals with antioxidant potential, it is fair to suggest that deactivation of some antioxidant enzymes could have been ameliorated with the administration of the extract, leading to an increase in these antioxidant enzymes. This study revealed the benefits of glibenclamide in increasing the activation of CAT and SOD. Interestingly, CAT activity is higher in the 200 mg/kg dosage of A. difformis group compared to the 400 mg/kg dose group, supporting the dependency of the effect of A. difformis on its dosage (Olanlokun et al., 2017).
5. Conclusion
The diabetic model demonstrated in this study successfully mimicked the characteristics of T2DM, that is, reduced body weight gain, hyperglycemia, tissue oxidative stress, insulin dysfunction, and resistance. Hyperglycemia and oxidative stress both synergistically lead to the impairment of the testes and epididymis. Results from this study have shown the benefits of extract A. difformis in ameliorating oxidative stress. This study has successfully answered the research question and has concluded that treatment with A. difformis boosted the antioxidant system and reduced diabetes-induced free radicals in testes. The above findings suggest A. difformis extract as a therapeutic agent against male infertility associated with DM, although more studies on its toxicity and molecular mechanisms to restore male infertility A. difformis in diabetic rats are worthy of investigation in further studies.
CRediT authorship contribution statement
Murendeni Nethengwe: Methodology, Investigation, Software, Formal analysis, Writing – original draft, Visualization, Validation, Data curation. Kunle Okaiyeto: Writing – review & editing. Oluwafemi O. Oguntibeju: Conceptualization, Methodology, Supervision, Project administration, Resources, Writing – review & editing. Nicole L. Brooks: Conceptualization, Methodology, Supervision, Project administration, Resources, Writing – review & editing.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
This work was funded by the National Research Foundation (NRF), South Africa.
Footnotes
Peer review under responsibility of King Saud University.
References
- Ahmed H.A. Anchomanes difformis: A multipurpose Phytomedicine. Int. Org. Sci. Res. J. Pharm. Biol. Sci. 2018;13(2):62–65. doi: 10.9790/3008-1302036265. [DOI] [Google Scholar]
- Alabi T.D., Chegou N.N., Brooks N.L., Oguntibeju O.O. Effects of Anchomanes difformis on inflammation, apoptosis, and organ toxicity in STZ-induced diabetic cardiomyopathy. Biomedicines. 2020;8(2):1–22. doi: 10.3390/biomedicines8020029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alabi T.D., Brooks N.L., Oguntibeju O.O. Leaf extracts of anchomanes difformis ameliorated kidney and pancreatic damage in type 2 diabetes. Plants. 2021;10(2):1–26. doi: 10.3390/plants10020300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aliyu A.B., Ibrahim M.A., Musa A.M., Musa A.O., Kiplimo J.J., Oyewale A.O. Free radical scavenging and total antioxidant capacity of root extracts of Anchomanes difformis Engl. (Araceae). Acta Pol. Pharm-Drug Res. 2013;70(1):115–121. [PubMed] [Google Scholar]
- Alsenosy A.W.A., El-Far A.H., Sadek K.M., Ibrahim S.A., Atta M.S., Sayed-Ahmed A., Al Jaouni S.K., Mousa S.A. Graviola (Annona muricata) attenuates behavioural alterations and testicular oxidative stress induced by streptozotocin in diabetic rats. PLoS ONE. 2019;14(9):1–18. doi: 10.1371/journal.pone.0222410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asadi N., Bahmani M., Kheradmand A., Rafieian-Kopaei M. The impact of oxidative stress on testicular function and the role of antioxidants in improving it: A review. J. Clin. Diagn. Res. 2017;11(5):1–5. doi: 10.7860/JCDR/2017/23927.9886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ataman E.J., Idu M. Renal effects of Anchomanes difformis crude extract in wistar rats. Avicenna J. Phytomed. 2015;5(1):17–25. [PMC free article] [PubMed] [Google Scholar]
- Ayeleso A., Brooks N., Oguntibeju O.O. Modulation of antioxidant status in streptozotocin-induced diabetic male wistar rats following intake of red palm oil and/or rooibos. Asian Pac. J. Trop. Med. 2014;7(7):536–544. doi: 10.1016/S1995-7645(14)60090-0. [DOI] [PubMed] [Google Scholar]
- Baffoe M., Koffuor G., Baffour-Awuah A., Sallah L. Assessment of reproductive toxicity of hydroethanolic root extracts of caesalpinia benthamiana, sphenocentrum jollyanum, and paullinia pinnata. J. Experimental Pharmacol. 2021;13:223–234. doi: 10.2147/JEP.S283557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brannan T.S., Maker H.O., Raess I.P. Regional distribution of catalase in adult rat brain. J. Neurochem. 1981;86:307–309. doi: 10.1111/j.1471-4159.1981.tb02411.x. [DOI] [PubMed] [Google Scholar]
- Buege J.A., Aust S.D. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- Cho N.H., Shaw J.E., Karuranga S., Huang Y., da Rocha Fernandes J.D., Ohlrogge A.W., Malanda B. IDF Diabetes Atlas: Global estimates of diabetes prevalence for 2017 and projections for 2045. Diabetes Res. Clin. Pract. 2018;138:271–281. doi: 10.1016/j.diabres.2018.02.023. [DOI] [PubMed] [Google Scholar]
- Dzięgielewska-Gęsiak S., Płóciniczak A., Wilemska-Kucharzewska K., Kokot T., Muc-Wierzgoń M., Wysocka E. The relationship between plasma lipids, oxidant – antioxidant status, and glycated proteins in individuals at risk for atherosclerosis. Clin. Interv. Aging. 2019;14:789–796. doi: 10.2147/CIA.S196016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellerby L.M., Bredesden D.E. Measurement of cellular oxidation, reactive oxygen species, and antioxidant enzymes during apoptosis. Methods Enzymol. 2000;322:413–421. doi: 10.1016/s0076-6879(00)22040-5. [DOI] [PubMed] [Google Scholar]
- Erukainure O.L. Flowers of Clerodendrum volubile modulates redox homeostasis and suppresses DNA fragmentation in Fe2+ − induced oxidative hepatic and pancreatic injuries; and inhibits carbohydrate catabolic enzymes linked to type 2 diabetes. J. Diabetes Metab. Disord. 2019;18(1):513–524. doi: 10.1007/s40200-019-00458-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esterbauer H., Cheeseman K.H. Determination of aldehydic lipid peroxidation product: malondialdehyde and 4-hydroxynonenol. Methods Enzymol. 1990;186(1):407–421. doi: 10.1016/0076-6879(90)86134-h. [DOI] [PubMed] [Google Scholar]
- Fan D., Li L., Li Z., Zhang Y., Ma X., Wu L., Zhang H., Guo F. Biosynthesis of selenium nanoparticles and their protective, antioxidative effects in streptozotocin induced diabetic rats. Sci. Technol. Adv. Mater. 2020;21(1):505–514. doi: 10.1080/14686996.2020.1788907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman A.L., Griffin S.J., Ahern A.L., Long G.H., Weinehall L., Fhärm E., Norberg M., Wennberg P. Impact of weight maintenance and loss on diabetes risk and burden: a population-based study in 33,184 participants. BMC Public Health. 2017;17(1):1–10. doi: 10.1186/s12889-017-4081-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flecknell P. Replacement, reduction and refinement. ALTEX : Alternativen zu Tierexperimenten. 2002;19(2):73–78. [PubMed] [Google Scholar]
- Ghafari S., Kabiri B.B., Golalipour M.J. Effect of Urtica dioica L. (Urticaceae) on testicular tissue in STZ-induced diabetic rat. Pak. J. Biol. Sci. 2011;14(16):798–804. doi: 10.3923/pjbs.2011.798.804. [DOI] [PubMed] [Google Scholar]
- Ghelani H., Razmovski-naumovski V., Pragada R.R. Attenuation of glucose-induced myoglobin glycation and the formation of advanced glycation end products (AGEs) by (R)-α-lipoic acid in vitro. Biomoleules. 2018;8(9):1–13. doi: 10.3390/biom8010009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goboza M., Aboua Y.G., Chegou N., Oguntibeju O.O. Vindoline effectively ameliorated diabetes-induced hepatotoxicity by docking oxidative stress, inflammation and hypertriglyceridemia in type 2 diabetes-induced male Wistar rats. Biomed. Pharmacother. 2019;112(1):1–11. doi: 10.1016/j.biopha.2019.108638. [DOI] [PubMed] [Google Scholar]
- Jaiswal D., Rai P.K., Mehta S., Chatterji S., Shukla S., Kumar D. Role of A. difformis in the regulation of diabetes-induced oxidative stress. Asian Pac. J. Trop. Med. 2013;6(6):426–432. doi: 10.1016/S1995-7645(13)60068-1. [DOI] [PubMed] [Google Scholar]
- Johnson A., Cheng S.C., Tsou D., Kong Z.L. Attenuation of reproductive dysfunction in diabetic male rats with timber cultured Antrodia cinnamomea ethanol extract. Biomed. Pharmacother. 2019;112(1):1–13. doi: 10.1016/j.biopha.2019.108684. [DOI] [PubMed] [Google Scholar]
- Ige S.F., Akhigbe R.E., Edeogho O., Ajao F.O., Owolabi O.Q., Oyekunle O.S., Ajayi A.F. Hepatoprotective activities of allium cepa in cadmium-treated rats. Int. J. Pharm. Pharm. Sci. 2011;3(5):60–63. [Google Scholar]
- Khaki A., Khaki A.A., Hajhosseini L., Golzar F.S., Ainehchi N. The anti-oxidant effects of ginger and cinnamon on spermatogenesis dys-function of diabetes rats. Afr. J. Tradit. Complement. Altern. Med. 2014;11(4):1–8. doi: 10.4314/ajtcam.v11i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khorramabadi K.M., Talebi A.R., Sarcheshmeh A.A., Mirjalili A. Protective effect of vitamin E on oxidative stress and sperm apoptosis in diabetic Mice. Int. J. Reprod. Biomed. 2018;17(2):127–134. doi: 10.18502/ijrm.v17i2.3990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kong Z.L., He J.L., Sudirman S., Kuo M.T., Miao S., Chang K.L.B., Tsou D. Nanoparticles of antroquinonol-rich extract from solid-state-cultured antrodia cinnamomea improve reproductive function in diabetic male rats. International Journal of Nanomedicine. 2020;15:4191–4203. doi: 10.2147/IJN.S252885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korejo N.A., Wei Q.W., Shah A.H., Shi F.X. Effects of concomitant diabetes mellitus and hyperthyroidism on testicular and epididymal histoarchitecture and steroidogenesis in male animals. J. Zhejiang Univ. Sci. B. 2016;17(11):850–863. doi: 10.1631/jzus.B1600136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu M., Wang M., Lv J., Wei J., Wan J. Glibenclamide exacerbates adriamycin-induced cardiotoxicity by activating oxidative stress-induced endoplasmic reticulum stress in rats. Exp. Ther. Med. 2018;15:3425–3431. doi: 10.3892/etm.2018.5862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Long L., Qiu H., Cai B., Chen N., Lu X., Zheng S., Ye X., Li Y. Hyperglycemia induced testicular damage in type 2 diabetes mellitus rats exhibiting microcirculation impairments associated with vascular endothelial growth factor decreased via PI3K/Akt pathway. Oncotarget. 2018;9(4):1–6. doi: 10.18632/oncotarget.23915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martha R., Gutierrez P., Velazquez E.G. Glucopyranoside flavonoids isolated from leaves of Spinacea oleracia (spinach) inhibit the formation of advanced glycation end products (AGEs) and aldose reductase activity (RLAR) Biomed. Pharmacother. 2020;128:1–7. doi: 10.1016/j.biopha.2020.110299. [DOI] [PubMed] [Google Scholar]
- Mohasseb M., Ebied S., Yehia M.A.H., Hussein N. Testicular oxidative damage and role of combined antioxidant supplementation in experimental diabetic rats. J. Physiol. Biochem. 2011;67(2):185–194. doi: 10.1007/s13105-010-0062-2. [DOI] [PubMed] [Google Scholar]
- Nna V.U., Bakar A.B.A., Ahmad A., Eleazu C.O., Mohamed M. Oxidative stress, NF-κB-mediated inflammation and apoptosis in the testes of streptozotocin–induced diabetic rats: Combined protective effects of malaysian propolis and metformin. Antioxidants. 2019;8(10):1–23. doi: 10.3390/antiox8100465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nna V.U., Bakar A.B.A., Mohamed M. Diabetes mellitus-induced male reproductive impairment: The role of natural products: A review. J. Appl. Pharm. Sci. 2017;7(9):233–242. [Google Scholar]
- Oghale O.U., Idu M. Phytochemistry, anti-asthmatic and antioxidant activities of Anchomanes difformis (Blume) Engl. leaf extract. Asian Pac. J Trop. Biomed. 2016;6(3):225–231. [Google Scholar]
- Oguntibeju O.O., Aboua Y., Kachepe P. Heliyon possible therapeutic effects of vindoline on testicular and epididymal function in diabetes-induced oxidative stress male Wistar rats. Heliyon. 2020;6 doi: 10.1016/j.heliyon.2020.e03817. 1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olanlokun J., Babarinde C., Olorunsogo O. Toxicity of Anchomanes difformis, An Antimalarial Herb in Murine Models. Eur. J. Med. Plants. 2017;20(3):1–13. [Google Scholar]
- Olson S.H., Xu Y., Herzog K., Saldia A., DeFilippis E.M., Li P., Allen J.P., O’Reilly E.M., Kurtz R.C. Weight loss, diabetes, fatigue, and depression preceding pancreatic cancer. Physiol. Behav. 2016;45(7):986–991. doi: 10.1097/MPA.0000000000000590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostovan F., Sc M., Gol A., Javadi A. Investigating the effects of Citrullus colocynthis pulp on oxidative stress in testes and epididymis in streptozotocin-induced diabetic male rats. Int. J. Reprod. Biomed. 2017;15(1):41–48. [PMC free article] [PubMed] [Google Scholar]
- Ovuakporie-uvo O., Idu M. Eff ect of the aqueous leaf extract of Anchomanes difformis on the glucose level and organ/body weight ratio of Wistar rats. J. Med. Herbs Ethnomed. 2015;1(1):64–67. [Google Scholar]
- Oyenihi A.B., Opperman M., Alabi T.D., Mpahleni B., Masola B. Centella asiatica alleviates diabetes-induced changes in fatty acid profile and oxidative damage in rat testis. Andrologia. 2020;52(10):1–12. doi: 10.1111/and.13751. [DOI] [PubMed] [Google Scholar]
- Pieme C.A., Tatangmo J.A., Simo G., Cabral P., Nya B., Jocelyne V., Moor A., Moukette B.M., Nzufo F.T., Legrand B., Nono N., Sobngwi E. Relationship between hyperglycemia, antioxidant capacity and some enzymatic and non-enzymatic antioxidants in African patients with type 2 diabetes. BMC Res. Notes. 2017;10(141):1–7. doi: 10.1186/s13104-017-2463-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rambiritch V., Maharaj B., Naidoo P. Glibenclamide in patients with poorly controlled type 2 diabetes: a 12-week, prospective, single-center, open-label, dose-escalation study. Clin. Pharmacol.: Adv. Appl. 2014;6:63–69. doi: 10.2147/CPAA.S54809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricci G., Catizone A., Esposito R., Pisanti F.A., Vietri M.T., Galdieri M. Diabetic rat testes: Morphological and functional alterations. Andrologia. 2009;41(6):361–368. doi: 10.1111/j.1439-0272.2009.00937.x. [DOI] [PubMed] [Google Scholar]
- Sankaranarayanan C., Kalaivani K. Isopulegol mitigates hyperglycemia mediated oxidative and endoplasmic reticulum stress in HFD/STZ induced diabetic rats. Arch. Med. Res. 2020;51:204–214. doi: 10.1016/j.arcmed.2020.02.001. [DOI] [PubMed] [Google Scholar]
- Sefidgar S.M., Ahmadi-hamedani M., Javan A.J. Effect of crocin on biochemical parameters, oxidative / antioxidative profiles, sperm characteristics and testicular histopathology in streptozotocin-induced diabetic rats. Avicenna J. Phytomedicine. 2019;9(4):347–361. [PMC free article] [PubMed] [Google Scholar]
- Silva E., Almeida H., Castro J.P. (In)Fertility and Oxidative Stress : New Insights into Novel Redox Mechanisms Controlling Fundamental Reproductive Processes. Oxid. Med. Cell. Longev. 2020;2020:2–3. doi: 10.1155/2020/4674896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sri Harsha P.S.C., Lavelli V. Use of grape pomace phenolics to counteract endogenous and exogenous formation of advanced glycation end-products. Nutrients. 2019;11:1–16. doi: 10.3390/nu11081917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tavares A.M., Silva J.H., Bensusan C.D.O., Claudia A., Ferreira F., Pinto L., Matos D.L., Luiz K., Araujo D., Cardoso-weide L.D.C., Fernandes G., Id T. Altered superoxide dismutase-1 activity and intercellular adhesion molecule 1 (ICAM-1) levels in patients with type 2 diabetes mellitus. Plos One. 2019;14(5):1–10. doi: 10.1371/journal.pone.0216256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tchicaillat-Landou M., Petit J., Gaiani C., Miabangana E.S., Kimbonguila A., Nzikou J.M., Scher J., Matos L. Ethnobotanical study of medicinal plants used by traditional healers for the treatment of oxidative stress-related diseases in the Congo Basin. J. Herbal Med. 2018;13:76–90. [Google Scholar]
- Temidayo S.O., Stefan S.P. Diabetes mellitus and male infertility. Asian Pac. J. Reprod. 2017;7(1):6–14. [Google Scholar]
- Tian Y., Song W., Xu D., Chen X., Li X., Zhao Y. Review article autophagy induced by ROS aggravates testis oxidative damage in diabetes via breaking the feedforward loop linking p62 and Nrf2. Oxid. Med. Cell. Longev. 2020;2020:1–9. doi: 10.1155/2020/7156579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson R.D., Islam M. Fructose-fed streptozotocin-injected rat: an alternative model for type 2 diabetes. Pharmacological reports. 2012;64(1):129–139. doi: 10.1016/s1734-1140(12)70739-9. [DOI] [PubMed] [Google Scholar]
- Yang S., Wang S., Yang B., Zheng J., Cai Y., Yang Z. Weight loss before a diagnosis of type 2 diabetes mellitus is a risk factor for diabetes complications. Medicine. 2016;95(49):1–6. doi: 10.1097/MD.0000000000005618. [DOI] [PMC free article] [PubMed] [Google Scholar]