Abstract
Successful regeneration of cartilage tissue at a clinical scale has been a tremendous challenge in the past decades. Microcarriers (MCs), usually used for cell and drug delivery, have been studied broadly across a wide range of medical fields, especially the cartilage tissue engineering (TE). Notably, microcarrier systems provide an attractive method for regulating cell phenotype and microtissue maturations, they also serve as powerful injectable carriers and are combined with new technologies for cartilage regeneration. In this review, we introduced the typical methods to fabricate various types of microcarriers and discussed the appropriate materials for microcarriers. Furthermore, we highlighted recent progress of applications and general design principle for microcarriers. Finally, we summarized the current challenges and promising prospects of microcarrier-based systems for medical applications. Overall, this review provides comprehensive and systematic guidelines for the rational design and applications of microcarriers in cartilage TE.
Keywords: Cartilage tissue engineering, Cartilage regeneration, Microcarriers, Cargo delivery, Bioprinting
Graphical abstract
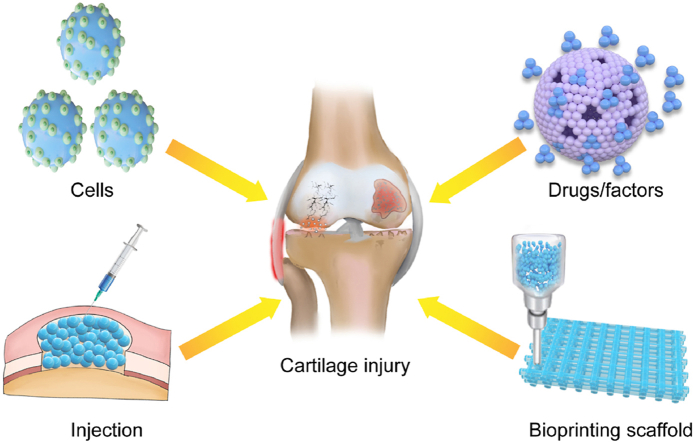
Microcarriers applied in joints for the repair of cartilage defects and cartilage regeneration. Microcarriers are three-dimensional culture platform that have been applied as scaffolds in biological and biomedical applications such as cell culture, expansion, delivery, modeling for biological studies, and medical implants, and can be combined with injection and bioprinting techniques for potential clinical application.
Highlights
-
•
This review summarized fabrication techniques and cartilage repaired application of microcarriers.
-
•
The appropriate materials and design principle for microcarriers in cartilage tissue engineering are discussed.
-
•
Promising future perspectives and challenges in microcarriers fields are outlined.
Abbreviations
- 3D
Three-dimensional
- ADSCs
Adipose-derived stem cells
- BMSCs
Bone marrow-derived mesenchymal stem cells
- BMP
Bone morphogenetic proteins
- ECM
Extracellular matrix
- EMM
Electromagnetic manipulation
- GAG
Glycosaminoglycan
- GelMA
Gelatin-methacryloyl
- HA
Hyaluronic acid
- iPSC
Induced pluripotent stem cell
- IGF
Insulin-like growth factor
- MCs
Microcarriers
- MSC
Mesenchymal stem cell
- MA
Methacrylic anhydride
- OPMs
Open-porous PLGA microspheres
- PLGA
Poly Lactic-co-Glycolic Acid
- PCL:
Poly (ε-caprolactone)
- PLA
Poly (lactic acid)
- PLLA
Poly (l-Lactide)
- RGD
Arginine-glycine-aspartic acid
- SF
Silk fibroin
- TNF-α:
Tumor necrosis factor-α
- TE
Tissue engineering
- TGF-β:
Transforming growth factor beta
1. Introduction
Tremendous progress has been achieved in the field of cartilage tissue engineering (TE), which mainly involves transplanting cell-scaffold complexes into a cartilage defect site to repair and improve the structure and function of the damaged tissue [[1], [2], [3]]. Here we review the advancements and enlightenments of microcarriers in cartilage regeneration from the perspective of cartilage TE development, and envision directions for future development. The aim of TE is to develop tissue and organ substitutes that can be transplanted into the diseased or injured counterparts to maintain, or recover their in vivo functions [4]. Based on the classic cartilage TE theory [5], in author's opinions, the key points for successful regeneration include the following: first, renewable sources of function cells and/or signaling factors. Second, scaffold with tunable desired properties (mechanical, chemical and biological properties). Third, implanted substitutes that can easily integrate into the host native tissues with immunocompatibility and biocompatibility. During the past decade, we have witnessed advanced progress in the field of cartilage TE, embodied by the following changes: (i) sufficient seed cell selection for applications (mesenchymal stem cells, induced pluripotent stem cells) [[6], [7], [8]]; (ii) precise patterning of biomaterials and novel biomaterials with advanced chemistries (more efficient and versatile biomaterial conjugations) [1,9,10]; (iii) active modulation of cellular biological functions and behaviors via structure and properties (e.g., stiffness, viscoelasticity, porosity and degradability) of biomaterials [[11], [12], [13]]; (iv) combination of biological drugs and factors are combined to improve bioavailability and bioactivity [[14], [15], [16]]; (v) rapid development of biofabrication technologies including programmed self-assembly and three-dimensional (3D) bioprinting [[17], [18], [19], [20], [21], [22], [23]].
Cartilage consists of a dense extracellular matrix and embedded chondrocytes (the only cell type in the cartilage). According to the composition of the matrix especially the fibers, cartilage can be classified into three main types: hyaline cartilage, elastic cartilage and fibrocartilage. This review mainly discusses knee articular cartilage, which is the most common type of cartilage in the human body and has a semitransparent appearance. As a tough and durable supporting connective tissue, articular cartilage plays supportive and protective roles in the musculoskeletal system. The main components of articular cartilage are water (60–85% of total wet weight), collagen type II (15–22% of dry weight), proteoglycan (15–40% of dry weight), and chondrocytes (2–5% of total cartilage volume) [24,25]. From its surface to its lowest depth, articular cartilage consists of the superficial/tangential zone, the middle/transitional zone and the deep/radial zone. In the superficial zone, type II collagen fibrils are parallel to the articular surface with flattened shape chondrocytes, and this layout confers cartilage with high-tensile stiffness and strength because of the low content of proteoglycans and the low permeability. This zone maintains the function of joint lubrication [26]. Notably, the collagen fibrils are thick and randomly oriented, and the cells are rounder, in the meddle zone, which provides the first defense in resisting compressive forces. In the deep zone, the collagen tough fibrils are perpendicular to the cartilage surface and contain hydroxyapatite, and the interstitial chondrocytes are aligned with collagen fibers [2].
The high stiffness of cartilage is attributed to the viscoelastic and poroelastic dissipation of the tissue networks [27,28]. Abundant strong collagen fibers interpenetrate with proteoglycan macromolecules in the articular cartilage, which provides viscoelasticity and poroelasticity for mechanical dissipation [29,30]. The viscoelasticity of articular cartilage is mainly due to its rearrangement of aggrecan and the reconfiguration of collagen, whereas the poroelasticity is mainly associated with the interstitial fluid movement. Furthermore, articular cartilage is superficially lubricated and presents the most efficiently-lubricated surface known in nature. Such low friction is indeed essential for cartilage well-being. Lubrication minimizes the cartilage degradation associated with osteoarthritis by reducing shear stress on the mechanotransductive, cartilage-embedded chondrocytes [31]. Despite so many protective mechanisms, degenerated articular cartilage, an avascular and aneural tissue, has a very limited capacity for regeneration after damage. Additionally, the hypocellular structure (chondrocytes and stem cells) may also underlie an intrinsic inability to repair [32]. Without appropriate and timely intervention, the chondral defect may extend deep into the subchondral bone [2]. Understanding such composition, structure and characteristics of articular cartilage, therefore, is of major importance to slow or even reverse its breakdown by cartilage TE.
Microcarriers are generally described as microparticles made from natural or synthetic materials with sizes ranging from 1 μm to 1000 μm, that are widely used in drug/cell delivery, regenerative medicine, and TE [33,34]. Moreover, microcarriers have been applied as microspherical scaffolds in biological and biomedical applications such as cell culture, expansion, delivery, modeling for biological studies, biosensor, and medical implants [[35], [36], [37], [38]]. To date, a variety of biomaterials have been developed to fabricate microcarriers using various techniques and methods, such as bioactive inorganic materials, natural/synthetic polymers, and their composites [[39], [40], [41]]. A significant advantage of microcarriers versus bulk scaffolds, hydrogels, or films is that they offer a large specific surface area for cell growth, facilitation of adhesions and maintenance of cell differentiation phenotypes. For example, a study compared cell pellets, collagen hydrogel bulk and collagen hydrogel MCs with regard to the chondrogenic phenotype and matrix synthesis of mesenchymal stem cells (MSCs) in vitro, it demonstrated the advantages of collagen hydrogel MCs microenvironments compared with those of collagen hydrogel bulk and pellets via an improved mimicking of the natural MSC proliferation process and enhanced mass exchange [42]. Microcarriers can be divided into solid and porous microcarriers according to the surface properties. In the solid MCs, cells only adhere on the surface of microcarriers as a monolayer, which limits the amplification of cell numbers, whereas porous microcarriers can offer a larger specific surface area and greater volume [43,44]. Furthermore, the open pore structure facilitates interactions between cells, excretion of metabolic waste, and exchange of nutrients.
Recently, microcarriers have been developed with distinct techniques as candidate materials for cartilage regeneration and have become research focus. The unique histological characteristics of articular cartilage result in its limited self-repair ability, to summarize: (i) cartilage is an avascular and aneural tissue with hypocellular structure (progenitor cells and chondrocytes); (ii) cartilage possesses a dense extracellular matrix (ECM) that limits the ability of chondrocytes to migrate quickly and gather at the damaged site for self-repair; and (iii) the wet and dynamic mechanical environment affects the therapeutic efficacy of drugs and implants in the joint cavity [[45], [46], [47]]. Therefore, the repair of damaged or degenerated cartilage tissue caused by arthritis, injuries, and many other types of damage remains a challenging obstacle in clinical medicine. A sufficient quantity of seeded cells is essential for cartilage regeneration, furthermore, few cell expansion methods are without problems of differentiation and/or loss of potency, that can be met by microcarriers. A study showed that monolayer expanded chondrocytes lost their native morphology within 1 week. Conversely, the use of 3D microcarriers can lead to large cellular yields, preserving of the chondrogenic phenotype for synthesis of cartilaginous tissue in 3 weeks of expansion [48]. Microcarriers can also be used as injectable carriers to directly deliver cells to the defect site, combinated with hydrogel scaffolds, or embedded in bioinks for 3D bioprinting. With such variety, microcarriers have been widely used in cartilage TE.
In this review, we concisely outlined the corresponding advantages and fabrication methods of various types of microcarriers that can be customized to fulfil different needs of cartilage regeneration. We then highlighted the recent progress in application of microcarriers in cartilage TE, including the carriers for seed cells or cargo, combination biological scaffold, injectable/bioprinting microcarriers, and bioresponsive microcarriers (Fig. 1). Finally, we summarized the current challenges and promising directions of microcarrier-based systems for cartilage TE. We hope this review can provide comprehensive information and inspire new thoughts to researchers.
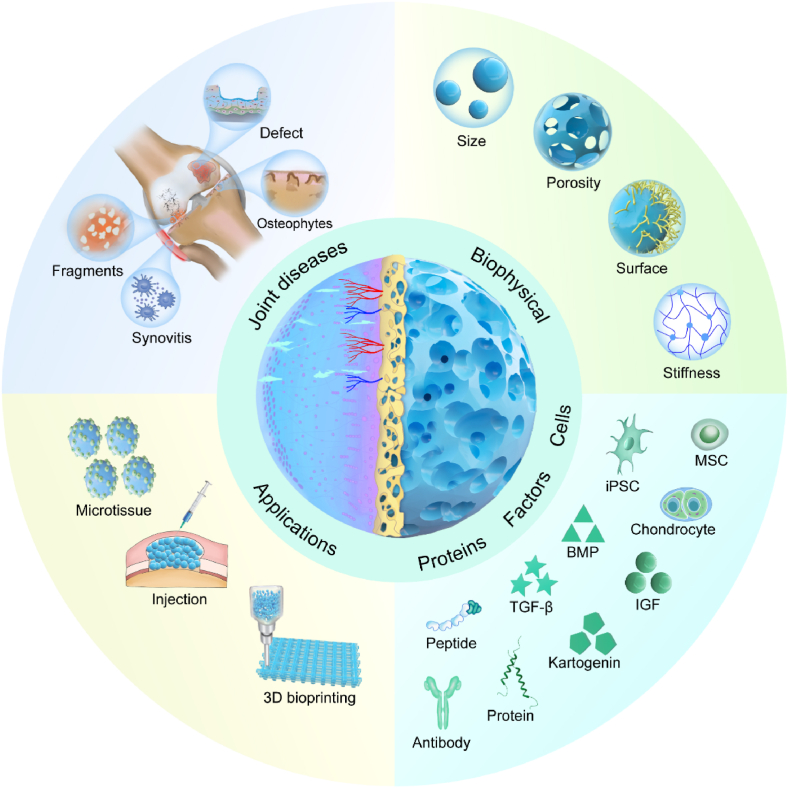
Fig. 1.
A schematic illustration of the microcarrier-based therapeutical platforms, utilizing various methodologies for numerous applications in joint diseases. MSC: Mesenchymal stem cells; iPSC: induced pluripotent stem cell; BMP: bone morphogenetic proteins; TGF-β: transforming growth factor beta; IGF:insulin-like growth factor.
2. Fabrication techniques of microcarriers
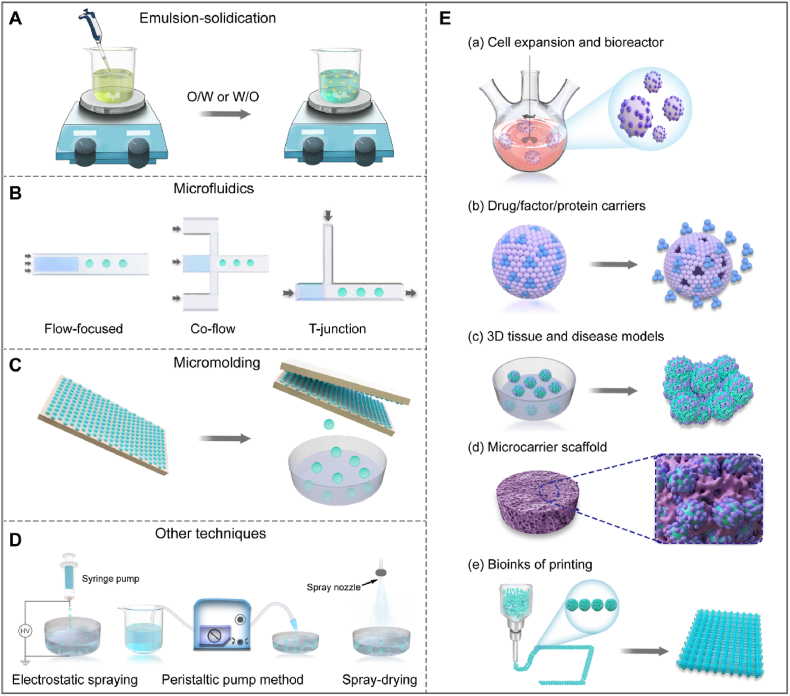
Microcarriers, which have recently attracted much attention in TE, are a class of microspherical scaffolds with biofunctional capabilities in cell culture, delivery and expansion. To date, several methods have been developed for the fabrication of microcarriers. Here we focus on several promising techniques, including emulsion-solidication, microfluidics, the mold method, and more (Table 1). It is extremely important to choose the appropriate preparation methods, balancing polymerization conditions and devices as well as low price, and efficiency to meet different needs. Fig. 2A–D shows typical examples of these microcarriers preparation strategies.
Table 1.
Selected processing techniques for the fabrication of microcarriers.
| Technique | Advantages | Disadvantages | Refs. |
|---|---|---|---|
| Emulsion-solidication | Easily scaled-up, simple and convenient, low cost, | Limited to low viscosity solutions, suffers from a wide particle size distribution. | [37,[49], [50], [51]] |
| Microfluidics | Well adapted to produce monodispersed particles with narrow distribution of particle size | Low production rate, costly and tedious device preparation | [[56], [57], [58],62,63,66] |
| Mold methods | Easily scaled-up, simple and convenient, low cost | Low production rate, costly and tedious device preparation | [68,69] |
| Spray-drying | Easily scaled-up, low cost | Limited polymer range | [73,74] |
| Electrostatic spraying | Small particle size | Very low production rate | [76] |
Fig. 2.
Schematic diagram of fabrication techniques and application of microcarriers. A: emulsion-solidication, O/W: oil-in water; W/O: water-in-oil; B: microfluidics. C: Mold method. D: other techniques, including electrostatic spraying, peristatic pump, and spray-drying. E: The application of microcarriers, including large-scale cell culture (a); drugs/factors delivery platform (b); microtissue construction in vitro (c); combination with scaffolds (d); and injectable and three-dimensional (3D) bioprinting microcarriers (e).
2.1. Emulsion-solidication
The emulsion-solidification technique is the most commonly employed method to fabricate microcarriers from different polymers, various natural biopolymers, including chitosan, alginate, collagen, hyaluronic acid, and gelatin, and synthetic materials such as poly (ethylene glycol), inorganic ceramics, and Poly Lactic-co-Glycolic Acid (PLGA) [[49], [50], [51], [52]]. Polymers usually are classified into two types according to their solubility: oil-soluble polymers and water-soluble polymers. This technique has been described in the literature [37,38,53]. Briefly, the polymer is made into an emulsion of O/W (oil-in water), W/O (water-in-oil), W1/O/W2 (water-in-oil-in-water) or O/W/O (oil-in-water-in-oil) using different emulsification processes (Fig. 2A). The solvent then is removed via different routes depending on the solvent properties for solidification of the polymer to obtain microcarriers; simultaneously, MCs also may be synthesized via physical or chemical cross-linking [54]. In this approach, the stirring speed and emulsifying speed, significantly affects the particle size distribution of the microcarriers (ranging from nanometer to millimeter). Sprio et al. described biomimetic hybrid MCs made of collagen type I-like peptide matrix mineralized with Fe2+/Fe3+ doping hydroxyapatite by emulsification of the hybrid slurries in the presence of citrate ions with a surface functionalization and dispersion ability [55]. The emulsification method is suitable for large-scale production of microcarriers, but limitations exist. The method suffers from a wide particle size distribution, so it is necessary to screen out MCs with the same particle size. In addition, irritating chemical solvents are used in the emulsification process, hence such solvent should be taken into consideration with regard to the cell compatibility.
2.2. Microfluidics
Microfluidics, inspired by an extrusion-solidification technique, perfectly overcome the problem of large particle size caused by injection needles. The microfluidics technique is based on the operation, and control of micro-fluids at the micro-scale using micro-pipes [[56], [57], [58]]. In these techniques, an aqueous polymer solution and typically a nonpolar oil or other fluids are co-extruded to produce consistently-sized droplets [59]. Therefore, the microfluidic device produces MCs with high monodispersity and a controllable size and shape, the operation is simple and superior over conventional emulsification techniques. The production rate of microcarriers can be adjusted by controlling the size of the orifice of the microfluidic channel, the viscosity of the immiscible phases, the hydrophilicity or hydrophobicity of the channel surface, and the velocity ratio of the continuous phase to discrete phase [60]. Currently, three channel designs exist in microfluidics to prepare microcarriers: T-junction [61], co-flow [62], and flow-focused geometries (Fig. 2B) [63,64]. The microfluidics-based procedure typically involves two steps, the formation of emulsion droplets and the solidification of emulsion droplets.
By coupling to photo-crosslinking, the microfluidics can generate cell-laden microparticles with varying sizes and shapes. Lee et al. fabricated macrophage-laden droplets containing methacrylic gelatin using a double a microfluidic flow-focusing device, which was developed a co-culture tissue model to study the mutual effects between macrophages in different stages of differentiation and the surrounding cells [65]. Furthermore, drugs and cells can be loaded at the same time to achieve a sustained release effect. For example, Zhao et al. presented a strategy of microfluidics-assisted technology that entrapped cells and growth factors to generate photo-crosslinkable gelatin (GelMA) MCs [66]. Similarly, photopolymerizable hydrogels from methacrylated laminarin were also been proposed as an enabling platform combination of microfluidics technology [67]. Additionally, microcarriers with superior sophisticated structure can be prepared by microfluidics to mimic the cell adhesive microenvironment (e.g., stem cell niche) [57]. The preparation of polymer MCs by microfluidics is influenced by many factors, including the properties of the fluids and materials, the geometric size and shape of micro-channels, and absolute ratio of liquid velocity to flow rate. Microfluidics can be well adapted to produce monodispersed particles with narrow distribution of particle size; however, the microcarrier production rate is very slow.
2.3. Mold method
In 2014, Liu et al. integrated microfabrication technology with cryogel preparation to develop a microcryogel array chip containing arrayed microscale PEG-derived cryogels with predefined sizes and shapes (Fig. 2C) [68]. The microscale and macroporosity of the novel microcryogels allowed automatic and homogeneous loading of cellular niche components on the array chip using a simple scraping approach. The array chip was generally fabricated with a mold that had many microunits and photo-lithography methods, and the microcarriers with desired shapes were stripped using a matched ejector chip or other techniques [69,70]. However, the application of mold methods may be limited by their low yield and cumbersome steps.
2.4. Other techniques
In addition to the above-mentioned widely used techniques, many other techniques are available, such as the spray-solidification technique [71,72], electrostatic spraying [[73], [74], [75]], and the use of electrostatic microdroplets [76]. Recently, Costantini et al. reported a highly efficient method, pulsed electrodripping, to form porous microbeads with tailorable dimensions, and modeled the process to predict the size of the template droplets [56]. Moreover, Zhang et al. designed a simple and low-cost acid-dissolved/alkali-solidified self-sphering shaping for rapid and facile production of chitosan/graphene oxide hybrid MCs via a peristatic pump method (Fig. 2D) [77]. Generally, most fabrication processes need organic solvents, photoinitiators, chemical crosslinkers, ultraviolet irradiation, and/or cytotoxic reagents, which may be hindered to their medical application. Herein, Tang and coworker presented a simple, flexible, biocompatible technique using gas-shearing strategy to fabricating multifaced MCs [78,79].
The specific surface area and the available cell concentration of solid microcarriers are low. The cells can be easily damaged by dynamic factors such as stirring, collision between MCs, and flow shear force. Macroporous microcarriers can be used widely in a stirred tank biochemical reactor. In the emulsification process, porous microcarriers can be prepared by combinatining with porogens, such as sodium chloride, bicarbonate, ammonium, sodium bicarbonate, gelatin and water [80,81]. The porogen is usually removed by filtration [81], gas-foaming [53,82], or other means according to the porogen properties to desynthesize the porous microcarriers. Huang et al. reported the formation of highly porous chitosan MCs via an emulsion-based, thermally induced phase separation without the use of toxic crosslinkers and chemical porogenic agents other than ice [39]. Furthermore, a novel strategy was presented by Zhang and coworkers that the porogen could be uniformly distributed using microfluidic technique, and subsequent removal lead to the formation of porous architectures [44,83,84]. In summary, the microstructures of microcarriers can be controlled by altering the preparation conditions during the emulsification stage. Functionally, Microcarriers are widely used in medical regeneration, including use in cell expansion/bioreactors, cargo delivery, micro-tissue and disease models, scaffold combinations, and injectable/bioprinting (Fig. 2E). Collectively, each of these preparation methods has its own features and requirements, and each polymer material has its own specific properties and characteristics. Specific requirements should be considered when selecting any of these techniques to fabricate microcarriers for the application of TE.
3. Appropriate materials for microcarriers in cartilage TE
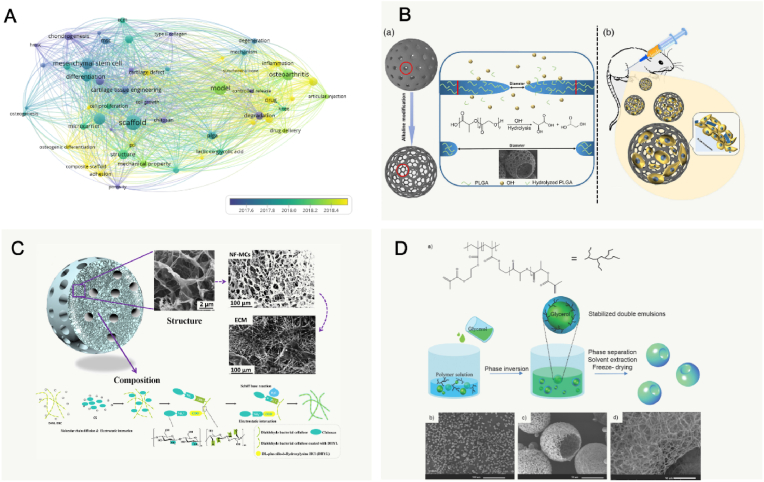
Choosing appropriate materials with desirable physical and chemical properties for fabrication of microcarriers is crucial in microcarrier cultures, because the porosity, mechanical strength, size, density, and shape highly affects the cell phenotypes. Notably, the visualized co-occurrence networks of keywords (VOSviewer version 1.6) indicated that research focus has changed from cells loading systems to applications such as tissue engineering, drug delivery, injectable and controllable degradation in recent years (Fig. 3A). Various sources of materials, including the synthetic polymers, polysaccharides, proteins, and an acellular matrix, can be made into microcarriers (Table 2). Details are described in the following sections.
Fig. 3.
Appropriate composition and structure of microcarriers for cartilage tissue engineering. A: Summary of the published articles of microcarriers of keywords evolution over time in cartilage TE (2015–2021). Its research focus changes (keywords of articles) from cells loading systems in the earlier period to applications such as tissue engineering, drug delivery, injectable and controllable degradation etc. in recent years. B: Schematic illustration for the fabrication and application of open-porous PLGA MCs in cartilage regeneration. Reproduced with permission [86]. 2021, Wiley Periodicals LLC. C: Schematic illustration of nanofibrous microcarriers were designed to structurally and functionally mimic extracellular matrix. Reproduced with permission [166]. 2018, Elsevier Ltd. D: Schematic illustration the emulsification and phase separation techniques to fabricate functional nanofibrous hollow MCs (a) and SEM graphs (b) of functional nanofibrous hollow MCs fabricated from poly (l-lactic acid)-graft-poly (hydroxyethyl methacrylate)-acrylic. Reproduced with permission [167]. 2014, WILEY‐VCH Verlag GmbH & Co. KGaA, Weinheim.
Table 2.
Biocompatible polymer used in microcarriers for cartilage tissue engineering.
| Polymer type | Polymer name | Targeted diseases | Fabrication techniques | Advantages | Limitations | Refs. |
|---|---|---|---|---|---|---|
| Synthetic polymers Polysaccharides |
PLGA | Osteochondral defect Osteoarthritis Acute arthritis |
|
|
|
[[85], [86], [87], [88],92,[212], [213], [214], [215], [216], [217], [218], [219], [220], [221], [222], [223], [224]] |
| PCL | Endochondral defect Osteochondral Defects |
|
|
|
[93,95,225,226] | |
| PLA | Osteochondral defect Cartilage defects Osteoarthritis Degeneration of articular cartilage |
|
|
|
[144,167,[227], [228], [229], [230], [231]] | |
| Alginate | Intra-articular injection Osteochondral defect Osteoarthritis |
|
|
|
[[232], [233], [234], [235], [236], [237], [238]] | |
| Hyaluronic Acid | Cartilage defects Osteoarthritis |
|
|
|
[95,128,239,240] | |
| Chitosan | Cartilage defects Osteoarthritis |
|
|
|
[[241], [242], [243], [244]] | |
| Agarose | Cartilage defects |
|
|
|
[151,164,245] | |
| Cellulose | Osteochondral defect |
|
|
|
[166] | |
| Fibrin | Osteoarthritis |
|
|
|
[246] | |
| Proteins | Gelatin | Osteoarthritis Cartilage defects |
|
|
|
[123,181,183,[247], [248], [249]] |
| Collagen | Articular cartilage defects Osteoarthritis Osteochondral defect |
|
|
|
[168,169] | |
| Silk fibroin | Osteochondral defect Cartilage defects osteoarthritis |
|
|
|
[250] | |
| ECM-based materials | – | Cartilage defects |
|
|
|
[197,199,200,251,252] |
3.1. Synthetic polymers
Synthetic polymers, such as poly PLGA [[85], [86], [87], [88], [89], [90], [91], [92]], poly (ε-caprolactone) (PCL) [[93], [94], [95], [96], [97]], and poly (lactic acid) (PLA) [[98], [99], [100]] are promising materials for cartilage TE. Generally, synthetic polymers can be processed via many techniques with good physical, mechanical, and chemical properties that can be modified to improve the parameters of the microcarriers. Most of these polymers are biocompatible as they can degrade into components that are metabolizable in the body.
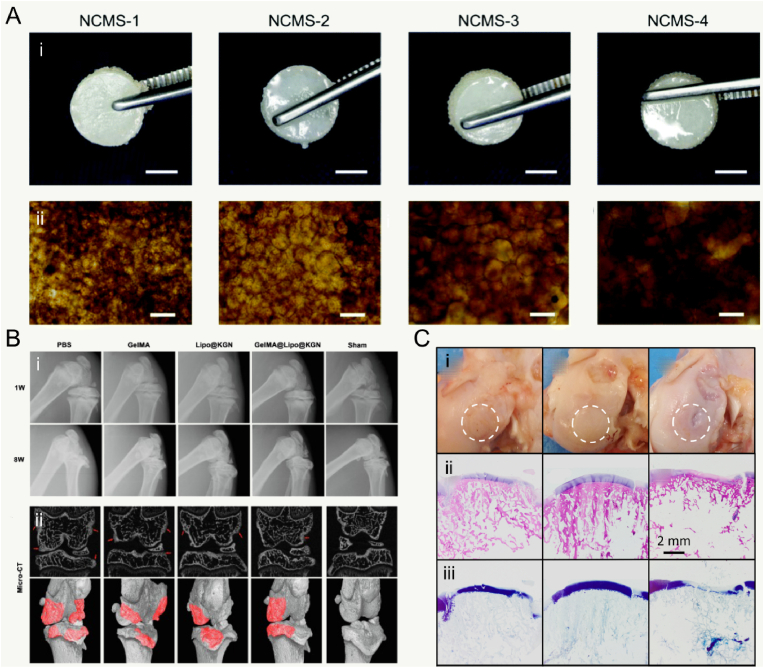
PLGA is a reliable and high-performance copolymer owing to its biodegradability and biocompatibility. The PLGA matrices ultimately degrade into lactic acid and glycolic acids by hydrolysis of the ester linkages, and the acids are then eliminated as carbon dioxide and water [101]. A suitable degradation that matches the rate neotissue formation is necessary for tissue regeneration. A recent study demonstrated that PLGA-rapamycin MCs could be used successfully not only for sustained release of rapamycin (more than 3 months) but also be used as cell carriers for cellular therapy [35]. Another study developed an acellular agarose hydrogel carrier with embedded dexamethasone-loaded PLGA MCs to provide sustained release for at least 99 days, which indicated a better histological score compared with an osteochondral autograft transfer in a pre-clinical canine model [88]. Apart from the superior biocompatibility of PLGA, surface structures of opened pores could enhance metabolic activities and improve tissue regeneration. Qu et al. modified traditional porous PLGA MCs with NaOH to obtain open-porous PLGA MCs (OPMs), in which pore sizes could be large enough for cellular infiltration and migration into the inner space (Fig. 3B) [86]. Together, MCs made of PLGA have been extensively used as cell and/or drug carriers in cartilage TE with excellent performance as a result of their larger pores, controllable degradation, and increased surface roughness. However, it has been reported that the acidic degradation of PLGA can reduce the local pH low enough to create an autocatalytic environment [102].
PCL is also known as a robust biocompatible and biodegradable material. It is one of the most commonly used polymers for cartilage repair and has initial mechanical stability tailored to mechanical properties [103]. Remarkably, chondrogenic and osteogenic cells have been successfully co-cultured on porous PCL scaffolds. However, chondrocyte seeding onto macroporous PCL results in uneven cell distribution, cell loss, and initial dedifferentiation due to the monolayer-like cell attachment [104]. Lam et al. reported that porous PCL microcarriers coated with extracellular matrices can be used to efficiently expand a variety of MSC lines in a cost-effective manner while maintaining surface markers expression and differentiation capability [97]. Osteochondral defects involve multiple tissues, and a biphasic scaffold model that combines a cartilaginous phase and a bony phase is the most common strategy for cartilage TE. A novel strategy for constructing the hydroxyapatite (HA)/PCL MCs using a selective laser sintering technique, demonstrated that the multilayer scaffolds could induce articular cartilage formation by accelerating the early subchondral bone regeneration [97]. The PCL polymer has been widely used in cartilage TE; however, its application is limited by hydrophobicity and the lack of active functional groups.
PLA is approved by the U.S. Food and Drug Administration as a biodegradable polyester that is used to produce MCs, and it has been proposed as a support matrix for cartilage TE [105]. Notably, PLA possesses chirality, which enable the mid-chain residues to exist in three enantiomeric states, l-Lactide, d-Lactide, and meso-lactide. The most widely used PLA is the poly (l-Lactide) (PLLA) [106]. Using star-shaped PLA technology, Liu et al. have fabricated nanofibrous biodegradable hollow MCs as injectable chondrocyte carriers for knee cartilage repair. The self-assembled MCs were designed to mimic the structure of collagen fibers in the ECM, and the study showed that MCs are an excellent cell carrier for chondrocytes to facilitate high-quality hyaline cartilage regeneration [107]. An advantage of PLA-based biomaterials is their ability of well-established processing technologies with the appropriate mechanical properties such as injection molding and 3D printing [108]. For example, it was shown recently by Ghosh et al. that PLA MCs contained decellularized cartilage matrix, and they successfully fabricated a hybrid PCL filament containing MC encapsulating ALP enzyme as a surrogate via melt extrusion [98].
In summary, the synthetic polymers discussed in this section have been widely studied for cartilage TE. Despite the high mechanical strength of these polymers, most are biologically inert. Thus, increasing works combined natural biological macromolecules with synthetic polymers to simultaneously provide good mechanical strength and support biological functions.
3.2. Polysaccharides
Alginates, as anionic natural polysaccharides, are derivated from bacteria or the cell walls of brown marine algae and possess biocompatibility, solubility and hydrophilicity, but do not readily degrade in-vivo [109]. As per the degradation rate consideration, one study showed that the degradation rate of alginate microbeads can be controlled by incorporating alginate-lyase in the hydrogel at 4 °C [110]. Consequently, the controlled degradation MCs could affect cell release rates and growth factor production [110]. Another experiment with alginate demonstrated that decreasing alginate molecular weight can also promote the degradation of alginate MCs [111]. Despite these credentials, alginates biofunctionality is challenged by the absence of arginine-glycine-aspartic acid (RGD) molecules, which affects interactions with proteins and cells (e.g., adhesion of cells). In 2014, RGD-modified alginate MCs were prepared by Woo et al. using an emulsion method, and results indicated that the MCs formed an aggregate in the presence of chondrocytes and effectively regenerated cartilage tissues in vivo [112]. The application of alginate is limited by its mechanical strength. A recent research study using a novel strategy showed that calcium alginate Janus MCs were made with MSCs in one compartment and iron oxide magnetic nanoparticles or drug-loading ability in the other compartment, which showed proper formation of calcium alginate and displayed mechanical integrity lasting up to 30 days, targeting was controlled using an electromagnetic manipulation (EMM) device [113]. Alginate can be used in various biofabrication techniques, including molding, spraying, and 3D bioprinting due to the ability of physically crosslinks via divalent cations (e.g., Ca2+) [114,115]. Despite these successes, alginate hydrogels have some limitations. For example, the alginate microcarrier with physical crosslink lacks long-term stability. Furthermore, alginate lacks a cell adhesive site and cellular interaction ability, resulting in alginate MCs that are generally modified with cell adhesion peptide, polymer modifiers, and oppositely charged polysaccharides [116,117].
Hyaluronic acid (HA), as the most abundant glycosaminoglycan (GAG) in native cartilage, is a linear biomacromolecule and the component of articular cartilage in the extracellular matrix (ECM), and is composed of repeating units of β−1,4-d-glucuronic acid-β−1,3-N-acetyl-Dglucosamine residues that can maintain cartilage homeostasis [118]. HA is involved in some key cellular processes of chondrocytes (including morphogenesis and proliferation) and has stimulatory effects on chondrocyte metabolism in vitro, which could significantly increase the synthesis of collagen type II, hydroxyproline, and glycosaminoglycan [[119], [120], [121]]. HA is also well known for interacting with specific receptors such as CD44, to regulate signal transduction, cell migration, and differentiation [122]. Therefore, tissue-mimetic pellets composed of chondrocytes and HA-graft-amphiphilic gelatin microcapsules can serve as biomimetic chondrocyte ECM environments with targeting on CD44 receptors, and these MCs can stimulate chondrogenesis and sulfated glycosaminoglycan synthesis [123]. The injectability of hydrogels broadens the clinical applications for drug delivery and regenerative cell therapies. HA has abundant carboxyl and hydroxyl groups that can be modified by various chemical reactions to control the mechanical properties of HA, including degradation resistance and elasticity [124]. Additionally, the multifunctionality (e.g., self-healing and shear-thinning properties) of HA can be obtained by introducing biofunctional molecules or reactive moieties to provide ease of injectability [125]. HA-based hydrogel particulates have been developed by incorporating bioactive ceramic nanoparticles to enhance the structural stability of HA under enzymatic degradation. However, a high injection force resulting from the presence of nanosized ceramic fillers and nonuniform shapes and sizes of gel granules is unavoidable [126]. To address this challenge, the injectable HA-based hybrid hydrogel MCs with nanosized calcium phosphate were fabricated using a W/O emulsion process and in situ precipitation process [127]. In clinical practice, uncross-linked HA is generally mixed as a lubricant. A more recent study developed an inverse opal-structured MC scaffold for osteoarthritis treatment, and used the HA microcarrier as a lubricant vehicle to deliver drugs when the temperature increased in the joint cavity during exercise or osteoarthritis [128]. In addition, HA can be modified by chemical reaction of carboxylic group and photo-crosslinkable functional groups such as methacrylate and glycidyl methacrylate. As a result, a variety of HA-based microcarriers can be fabricated towards controllable biodegradability and improve mechanical properties.
Chitosan is a naturally linear polysaccharide composed of N-glucosamine and N-acetylglucosamine units from the shells of shrimp and other crustaceans and the molecule cationic was obtained from the amino/acetamido group in chitosan [129]. Chitosan has been known as a promising candidate material in cartilage TE due to its excellent biological functions, including antibacterial properties, biodegradable, affordability, lack of immunogenicity, and biocompatibility that promote cell adhesion, proliferation, and differentiation [[130], [131], [132], [133]]. Moreover, Chitosan is a repeating glucosamine unit, which is an essential ingredient for the synthesis of glycoproteins [134,135]. For example, Sheehy et al. reported that chitosan constructs accumulated the highest levels of sulfated glycosaminoglycan and collagen compared with alginate and fibrin [136]. In addition, chitosan has a hydrophilic surface that it may maintain and attract fluid and cells to defective sites [137]. Ma and coworker prepared chitosan microcarriers that impregnated with soybean protein isolate, their outcomes show that composite microcarriers better-supported cell adhesion and proliferation than chitosan microcarriers [138]. Several chitosan-based scaffolds have demonstrated good results because of the structural similarities between sGAG in the articular cartilage and chitosan. These scaffolds can provide a suitable microenvironment for chondrocytes to maintain the correct phenotype, to sustain chondrogenesis, and to repair cartilage tissue defects [[139], [140], [141]]. On the whole, the porous structures in chitosan can be prepared by freeze-drying acidic solutions or gels. Specifically, Lu et al. fabricated porous chitosan microcarriers with sizes ranging from 180 μm to 280 μm using an emulsion-solidification technique combined with freeze-drying [142].
Nevertheless, the mechanical stability of the chitosan scaffold is inadequate hindering the production of MCs with stable structures and controlled sizes, thus, the scaffold cannot easily be applied in clinical cases. To address this limitation, an appropriate strategy for combining the chitosan hydrogel and solid-state bio-matrix could overtop the inadequate mechanical stability of chitosan [132,143]. Subsequently, a novel kind of porous PLGA/chitosan polyelectrolyte complex MC was developed by electrostatic interaction using the emulsion-solidification technique combined with freeze-drying for its simple operation and easy scalability [144]. Another study that attempted to address the poor mechanical properties of chitosan, involved preparation of size-controllable chitosan/PEGDA hydrogel MCs using a water-in-oil approach after photo-crosslinking and physical-crosslinking, results showed that these cell-laden MCs were self-assembled into a 3D cartilage-like scaffold [145]. Furthermore, many studies have indicated that the physical properties of chitosan were mainly affected by the molecular weight, the sequence of the acetamido/amino groups, and the purity of the product [146,147]. The poor solubility in water, the low cellular interaction, and the allergenicity of chitosan may limit extensive translation of chitosan into clinical use [148,149].
Agarose is extracted from marine red algae composed of alternating units of 3,6-anhydro-α-l-galactopyranosyl and β-d-galactopyranosyl units, which is a thermosetting hydrogel that undergoes gelation in response to a reduction in temperature [150,151]. Specially, agarose forms a gel when cooled to below an upper critical solution temperature (UCST), and this process is related to the agarose molecule twisting [152]. Various factors such as concentration, molecular weight, and lateral groups significantly affect the melting and gelling temperature [153]. Therefore. The agarose polymer can be transformed into microcarriers by extrusion of droplets hardened when the temperature is lower than UCST. Furthermore, other preparing methods (e.g., water-in-oil emulsion, microfluidics) are also followed by a reduction in the temperature to allow gelation of the agarose droplets. For example, Sakai et al. developed agarose microcapsules with a single hollow core templated by alginate microcarriers, and subsequently the vascular endothelial cells grew and formed embryoid body-like spherical tissue in the core [154]. Moreover, agarose is also successfully used as a biocompatible substrate combined with ceramic contents for augmentation, such as bioactive ceramics or glasses [155]. Composite microspheres were prepared for the first time by agarose enforcement with combination of biphasic calcium phosphate and calcium sulfate dehydrate [156]. In more recent study, Zhao et al. prepared agarose microcarriers with a controllable pore structure by varying agarose types and crosslinking degrees. Various agarose could tailor the gel formation of microspheres matrix and thus affect the final pore structures [157]. Agarose hydrogel, along with proper biocompatibility and biodegradability, can offer a suitable microenvironment for chondrocytes, and stabilize the chondrocyte phenotype and enhanced the proteoglycan and glycosaminoglycans precipitation [158]. Particularly, low concentration agarose increased the deposition of the extracellular matrix, which improved the mechanical properties [159]. Therefore, the distinctive advantage of agarose hydrogels is the encapsulation of chondrocytes which enables 3D culture maintaining the cellular phenotype or morphology. Collectively, agarose and its composites presented a conspicuous role in the cartilage TE due to the excellent characteristics, such as mechanical properties, biocompatibility, nontoxicity, and high cell interaction.
Cellulose is a linear chain polysaccharide consisting of d-glucose units. Notably, bacterial nanocellulose is nanofibrillar material that combines high flexibility and tensile strength, and it has a nano-network structure similar to the collagen fibrils in tissue ECM. Cellulose has various advantages as a nanoscale structure and is available in different formats for cartilage regeneration [[160], [161], [162], [163]]. Moreover, the production of cellulose is more economically practicable due to the reasonable cost, yet approaches are application-dependent and vastly diverse. For example, porous bacterial cellulose scaffolds, as described by Yin et al., were prepared by cultivating Acetobacter xylinum in the presence of agarose microparticles, which could control the physical dimensions of the pore network [164]. However, an intrinsic limitation of TE is the compact structure with the 0.02–10 μm in the fibril network, resulting in slow biodegradation and limiting cell penetration and migration [162,165]. To address these concerns, bacterial nanocellulose has been modified by chemical and physical methods, including modification of chemical structure and functionalities, changes in porosity, crystallinity, and fiber density, respectively [161]. In a more recent study, Wang et al. fabricated the bionic nanofibrous microcarriers to mimic collagen microfibers, and hydroxylysine and chitosan by crosslinking dialdehyde bacterial cellulose through electrostatic interactions (Fig. 3C). The biodegradation rate as well as mechanical properties and porosity could be regulated by the orthogonal design [166]. Collectively, fibrous microcarriers may represent real progress in the development of biomimetic MCs. However, it is a challenge to control fiber diameter and micro-nano structure as well as pore geometry to affect cell attachment and cell–cell interactions.
Overall, polysaccharides have been used in the field of cartilage TE because of their high biocompatibility and resemblance to the glycan constituent of the ECM. However, the presently available polysaccharide microcarriers exhibit limitations, including the lack of active functional groups, the low mechanical strength, and fast degradation rate. Therefore, a reasonable strategy involves combining these polysaccharides with another category of polymers.
3.3. Protein
Collagen is a type of ECM protein that has low immunogenicity in native cartilage and can support the proliferation and maturation of chondrocytes. One study that established 3D collagen MCs, demonstrated the phenotypic changes of primary human osteoarthritic chondrocytes in collagen MCs when exposed to a few external factors [168]. In another study, Yu et al. prepared type I collagen MCs seeded with chondrocytes for 14 days, which showed the formation of cartilage particulates in vitro [169]. Notably, another study found that type II collagen could convert auricular chondrocytes into articular cartilage after dedifferentiation by a two-step protocol [170]. Collagen reduce the risk of immune response by ECM formation, and can be used as a coating material for microcarriers to improve cell adhesion. In natural ECM, collagen nanofibers are found to improve stem cell attachment, proliferation, and differentiation along various lineages. Zhang et al. successfully synthesized a novel functionalized graft copolymer that can self-assemble into functional nanofibrous hollow MCs (Fig. 3D). The results showed the nanofibrous structure could enhance the efficacy of GF signals in stem cell differentiation [167]. Moreover, collagen can be combined with other polymers to form hybrid hydrogel scaffolds that have enhanced properties compared with those of the individual components. For instance, collagen-coated PLA microcarriers have been successfully fabricated, the results indicated that the mechanical properties of scaffold were substantially enhanced, and formed cell attachment [171]. Type I collagen is widely employed as scaffolds products for cartilage TE in clinical treatment. Nonetheless, the pure type I collagen suffers its limited biological activity and weak mechanical properties. Compared with type I collagen, type II collagen shows better chondrogenic performance. However, type II collagen is not an ideal polymer for cartilage TE due to its arthritogenic potency [172].
Gelatin, as a hydrolyzed form of collagen, is famously used for various medical diseases due to its biocompatibility, proteolytic degradability, and controlled delivery system advantages [173,174]. The gelatin microcarriers can provide a surface for cellular adhesion and proliferation, while simultaneously allowing control over the release of drugs or biological agents [175]. The biodegradability of gelatin microcarriers can be achieved by collagenase. For instance, Ng et al. fabricated dissolvable gelatin-based microcarriers for MSC expansion [176]. Another study showed that the gelatin microcarriers could enhance the efficiency of chondrogenesis in bone marrow stromal cells (BMSCs) in vitro [177]. In term of safety, gelatin microcarriers were seemed to be non-cytotoxic and noninflammatory because of low immunogenic properties. In terms of efficacy, the gelatin microcarriers can maintain drug levels in the plasma for more than 48h [178]. Generally, the content and the degree of crosslinking of gelatin could influence the biodegradation of gelatin microcarrier, resulting in the rate of drug release. These studies demonstrated that the controlled release of biologics is vital to ensuring success in tissue regeneration. Besides, methacrylic anhydride (MA) can be easily modified with gelatin to form photo-crosslinkable GelMA. Several studies have prepared GelMA MCs via ultraviolet cross-linking [[179], [180], [181], [182], [183]]. The gelatin MC has been endorsed for its potential in cartilage TE applications. However, the major disadvantage of poor mechanical properties limits its application for medical purposes [184]. Taken together, the gelatin microcarrier not only can provide a platform for cellular and drug delivery, but also serve as a building block to form a more complex tissue construct.
Silk fibroin (SF), as a natural fibrous protein, exhibits non-toxicity, excellent biocompatibility and biodegradability, self-assembly, and mechanical stability for the development of microcarriers [185,186]. The silk of silkworm cocoons consists of fibroin (a semicrystalline fibrillar protein) and sericin (a water-soluble glue-like protein) [187]. Singh et al. fabricated an agarose/silk fibroin hydrogel via minimal secretion of tumor necrosis factor-α (TNF-α) by murine macrophages. The results demonstrated that silk is an alternative biomaterial with good immunocompatibility for cartilage TE. Pure silk fibroin-based microcarriers were fabricated by Wang et al. using a high voltage electrostatic field [188]. Fang et al. prepared silk fibroin porous microcarriers containing strontium for injectable bone TE [189]. In addition, various biopolymers have been combined with silk fibroin to improve its mechanical and biological properties. For instance, Perteghella et al. innovatively developed composite microcarriers containing SF and alginate to realize cell delivery [190]. Conventionally, the SF of composite microcarriers is induced into the β-sheet conformation, which could provide a more stable, insoluble homogeneous structure by immersion with ethanol [[190], [191], [192]]. Furthermore, the SF could be combined with positively charged polymers, such as chitosan and collagen, to provide an adhesive microenvironment [193,194]. It is worth noting that pure SF is difficult to degrade in vivo, and its poor thermal stability also limits its biological application. Although few studies about SF-based microcarriers have been reported for cartilage TE, this type of biomaterial has a great potential biomedical application prospect. Interestingly, there is still no study to develop serin-based microcarriers owing to its water solubility and weak mechanical properties [195].
Proteins are denatured by high temperature, physical stress, or exposure to strong organic solvents. Furthermore, protein-based microcarriers may be hampered by its weak mechanical strength, and shrinkage, which could be optimized through blending with other materials. Considering the aforementioned works, proteins served as essential polymers for the next generation of cartilage TE due to their excellent biocompatibility and biodegradability.
3.4. ECM-based materials
Polymers derived from the cartilage ECM are widely used in cartilage tissue regeneration, because they can provide a highly biocompatible environment for proliferation of chondrocytes and MSCs. In a previous study, cartilage ECM/peptide coating microcarriers could improve MSC expression of CXCR4, and trigger MSC migration from microcarriers for cell therapy [196]. Yin et al. proposed a novel cell carrier derived from natural cartilage ECM, which can support proliferation of MSCs and facilitate their chondrogenic differentiation without the exogenous growth factors [197]. ECM not only offers a complex 3D microenvironment for the survival, organization and differentiation of the cells, but also accelerates the formation of tissue-engineered cartilage because of simulating the native osteochondral tissue. In addition, ECM plays a key role in the transmission of mechanical forces, growth factor release and signaling [198]. The decellularized extracellular matrix (dECM) can be termed by all cells and genetic material while maintaining the physical and biochemical characteristics. Sivandzade et al. introduced porous injectable microcarriers composed of dECM of cartilage tissue, which could be a potential candidate to be used in cartilage tissue engineering applications [199]. A more recent study, the bionic cartilage acellular matrix MCs were prepared for chondrogenic differentiation of bone marrow cells and combination with microfracture [200]. These study support that the ECM proteins from cartilage acellular matric may provide favorable conditions for cells toward chondrogenesis. However, the disadvantages of using dECM are also obvious, like weak mechanical properties rapid in vivo degradation, limited source. Hence, standardized decellularization procedures and guidelines primarily need to be provided in the future.
As mentioned previously, various synthetic and natural biopolymers have been used for the fabrication of microcarriers. Synthetic materials provide high reproducibility and tailorability. However, synthetic biopolymers exhibit disadvantages that include poor hydrophilicity, cytocompatibility, and low biodegradability. Although natural biopolymers have excellent biocompatibility, there are also limitations such as poor mechanical properties, low cell adhesiveness and cellular interaction, uncontrollable degradation, and the inconsistency of source batches. Therefore, selecting a desirable biomaterial in microcarrier development to that will improve the biological properties. The ideal biomaterial will provide microcarriers that are similar to native cartilage in composition and structure.
3.5. Surface biological modification of microcarriers
The interaction between the microcarrier surface, surrounding medium, and cells are critical for the cell cultures, and cell attachment involves interaction between cell adhesion molecules and various substrates on the surface of the microcarrier [201]. There are a number of adhesion factors, such as fibroblast growth factor, bone morphogenetic protein, endothelium, glass fibronectin, which need first be absorbed before the adhesion of cells to the MC surfaces [202]. Notably, some protein like collagen, laminin, and fibronectin, can also improve the cell attachment on microcarriers [203]. For example, Lu et al. modified the surface of porous carboxymethyl chitosan microcarriers with collagen for application in cartilage TE [204]. Moreover, the introduction of collagen can enhance the proliferation, and differentiation of cells in MCs. Surface modification of MCs with short peptide sequences or growth factors is an attractive approach to guiding the spatially and temporally complex multicellular processes. Dandekar et al. illustrated the procedure for expansion of human BMSCs on collagen-I-based recombinant peptide-based microcarriers [205]. The growth factors paly crucial roles in situ cell recruitment and attachment. Gelatin microspheres have been used as a method of releasing TGF-β1, which is a secreted protein to create 3D pieces of cartilage tissue [206]. The growth factor TGF-β3 has also been explored for cartilage regeneration, other growth factors, such as bone morphogenetic protein (BMP)-2, have been utilized in similar degradable microspheres to stimulate chondrocyte development [207]. One can add surface moieties to the MCs that will improve cell attachment by tailoring the surface chemistry or functional molecular. Functional groups such as hydroxyl, carboxyl, and amino groups can be introduced to the surface modification to obtain hydrophilicity and positive surface charge [208]. Besides, surface charge and hydrophilicity can be achieved by incorporating chemical groups, e.g., amino groups (-NH2) or carboxyl groups (-COOH), which can also significantly affect cell attachment and behavior [209]. And several studies have shown that a better attachment on positively charged compared to negatively charged microcarriers [210,211]. However, the charge on the surface of the microcarrier will affect the dispersion between the MCs and easily adsorb surrounding impurities. The ability of adhesion and proliferation of cells was clearly improved by modifying the surface hydrophilicity. It is well-established that slightly hydrophilic surfaces lead to better cell attachment than hydrophobic (>90°) and superhydrophobic (>150° contact angle) surfaces [211]. Therefore, these functional molecules are a common route to improve cell adhesion, proliferation, and differentiation. However, these bioactive moieties are often expensive, and the process of chemically linking them to microcarriers is very complicated. It is worth considering that cell-surface, protein-cell interactions as well as protein-surface should be carefully investigated.
4. Microcarriers design application in cartilage TE
In cartilage TE, two main ways have been proposed for the regeneration of cartilage (including at the osteochondral interface and full-thickness). One method involves preparation of complex scaffolds to mimic the architectural features, mechanical properties, and biological functions of native cartilage tissues. The other approach is to develop the appropriate biomaterials that serve as a temporary 3D microenvironment for chondrogenic cell growth, proliferation, and differentiation to generate cartilage tissue [253]. To produce desirable engineered cartilage, we still need to prioritize three elements of TE: cells, scaffolds, and growth factors [254,255]. MCs are mainly applied as the form of hydrogels or sponge scaffolds in drug delivery or cell carriage. In this section, we summarize advantages of microcarriers as cell/drug/factor-laden delivery platform, and in combination with biological scaffolds to achieve efficient chondrogenesis for cartilage regeneration. Furthermore, we highlight recent advances in biomanufacturing technologies (e.g., 3D bioprinting) for the fabrication of MC-based scaffolds to mimic the native cartilage counterpart. Understanding these methods affect MC and scaffold properties, thereby serving as a valuable guide for the design of microcarriers or MC-based scaffold for diverse clinical needs.
4.1. Delivery vehicles and scaffold for cargo/cells
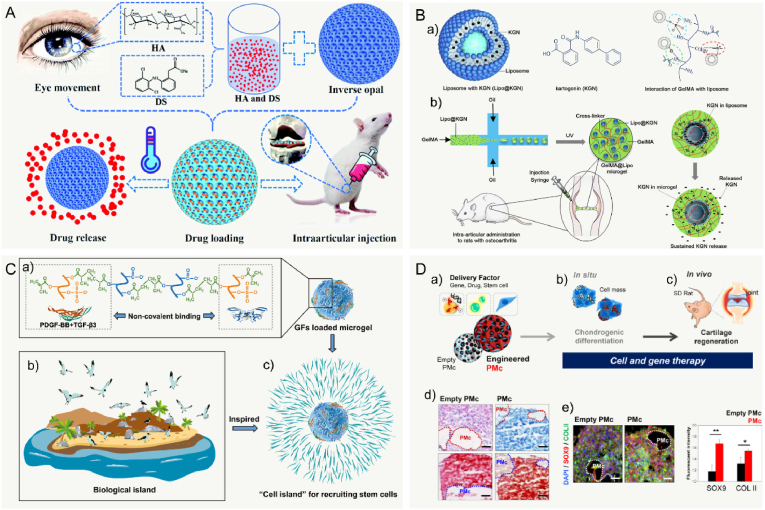
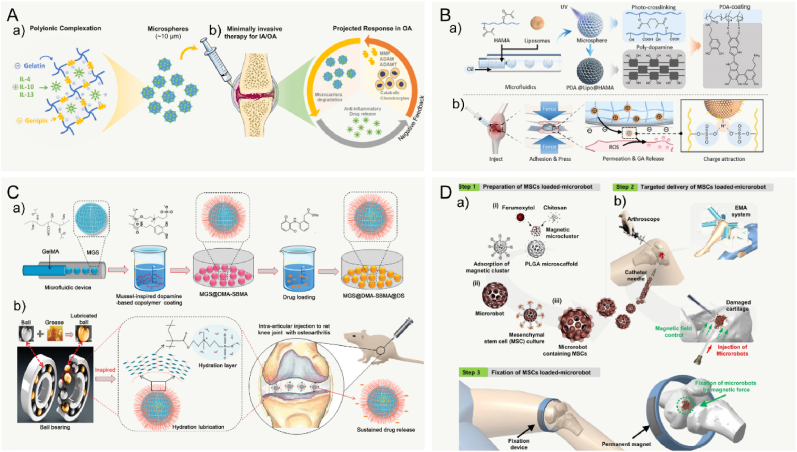
Many clinical therapeutics like intra-articular drug injection have been widely used for treating joint diseases. But some shortcomings still need to be overcome. First, drugs need to be administered frequently and repeatedly to reach the therapeutic dose, which may cause the increasing of drug level. Second, some existing drug delivery systems released the drugs rapidly and exceed the therapeutic concentration and the maximum safe level. In past decades, various microcarriers have been developed for controlled drug delivery to reduce dosing frequency and to improve the therapeutic effects by minimally invasive Injection. In general, drug delivery microcarriers are often prepared by natural or synthetic polymers. The cargo (e.g., drug and factor) is encapsulated in MCs to increase the bioavailability and provide a long release period with constant drug plasma concentration [256]. Moreover, Controllable MC porosity and pore structure are important properties. In one recent study, Yang et al. prepared the smart microcarriers that could shrink or swell to release the drugs with pathological response switches for treating osteoarthritis [128] (Fig. 4A). Therefore, the release rate of the delivered drugs can be precisely regulated by controlling the porosity of the MCs. Similarly, Han et al. successfully developed lubricating MCs encapsulated with an anti-inflammatory drug of diclofenac sodium [181]. The result showed that these MCs possessed lubrication and controllable drug release for the treatment of osteoarthritis. With regard of releasing drug for a long period, the Immobilizing liposomes, interacted with GelMA microgels by the physical network, were designed to protect Kartogenin against rapid clearance in the joint cavity [183]. These microgels could extend Kartogenin release for over five weeks (Fig. 4B). The flowability and flexibility of microcarriers allow them to disperse adequately in the solution and fulfill the requirement of joint loading. Additionally, more researchers believe that the functional microcarriers should be combined with bioactive factors to promote the growth and differentiation of seeded cells. In more recent study, inspired by the recruiting of seabirds home to nesting, Lei et al. fabricated cell island” microgels that encapsulated platelet-derived growth factor-BB and transforming growth factor-beta3 to recruit stem cells [257] (Fig. 4C). Collectively, microcarriers, as a carrier for drug delivery, can be prepared to provide excellent encapsulation performance and advanced controlled release performance.
Fig. 4.
Microcarriers served as cargo/cells delivery platform in application of cartilage TE. A: Schematic diagram of the generation of the bio-inspired lubricant drug delivery particle derived from HA with pathological-state responsive switches for the treatment of osteoarthritis. HA: hyaluronic acid and DS: diclofenac sodium. Reproduced with permission [128]. 2020, the Royal Society of Chemistry. B: Schematic of kartogenin-loaded liposomes, chemical structures of kartogenin, and non-covalent interactions between GelMA and liposomes, the kartogenin-loaded GelMA@Lipo hybrid microgels were used for treatment in a rat osteoarthritis via intraarticular injection. Reproduced with permission [183]. 2020, Elsevier B.V. C: Inspired by the phenomenon where islands can recruit seabirds for nesting, the “cell island” microgels were employed for recruiting the stem cells. The injectable porous microgel was fabricated by photopolymerization of methacrylated hyaluronic acid and heparin (HAMA@HepMA) blend pregel droplets generated via microfluidic technology. Subsequently, PDGF-BB and TGF-β3 were non-covalently incorporated within the microgels by binding heparin. Reproduced with permission [257]. 2021, Wiley‐VCH GmbH. D: Brief illustration of the preparation and analysis of histology by Alcian blue staining and Safranin-O staining of stem cells mixed with pocket-type microcarrier. Scale bar: 500 μm. Reproduced with permission [258]. 2021, The Authors.
The cell delivery efficiency can be adjusted by controlling the structure and pore size of the microcarrier. For instance, Kim et al. prepared pocket-type biodegradable PLGA microcarriers with pores larger than 30 μm for use in cell delivery [258]. The results in this study indicated that the pocket-type MCs produced differentiation of stem cells in combination with containing SOX9 pDNA (Fig. 4D). Various stem cells, such as adipose-derived stem cells (ADSCs), embryonic stem cells, and bone marrow-derived mesenchymal stem cells (BMSCs), have been demonstrated pre-clinical or clinical efficacy to improve the outcomes of cartilage repair [259,260]. However, limitations based on MSC therapeutic strategies still exist. In particular, results of clinical study have shown fibrous cartilage formation in repaired joint defects implanted with MSCs. The most advanced cartilage TE is the matrices seeded with chondrocytes, which might tend to lose their phenotype, with dedifferentiation after long term culture in vitro, producing fibroblastic type I and III collagens [261,262]. Understanding the suitable microenvironment for cell-cell and cell-matrix interactions is essential [51]. In one study, researchers investigated the influence of microenvironment on chondrocytes by comparing the collagen hydrogel in bulk and microspherical forms, the results suggested that chondrocyte phenotype could be maintained in MCs at an early stage of the in vitro culture [263]. Zhou et al. reported chitosan microcarriers with an ECM-mimicking nanofibrous structure that could easily develop a macroscopic 3D geometrically shaped cartilage-like composite [264]. Furthermore, the chitosan microcarriers provided researchers with bottom-up cell-carrier components for repairing cartilage defects (Fig. 5A).
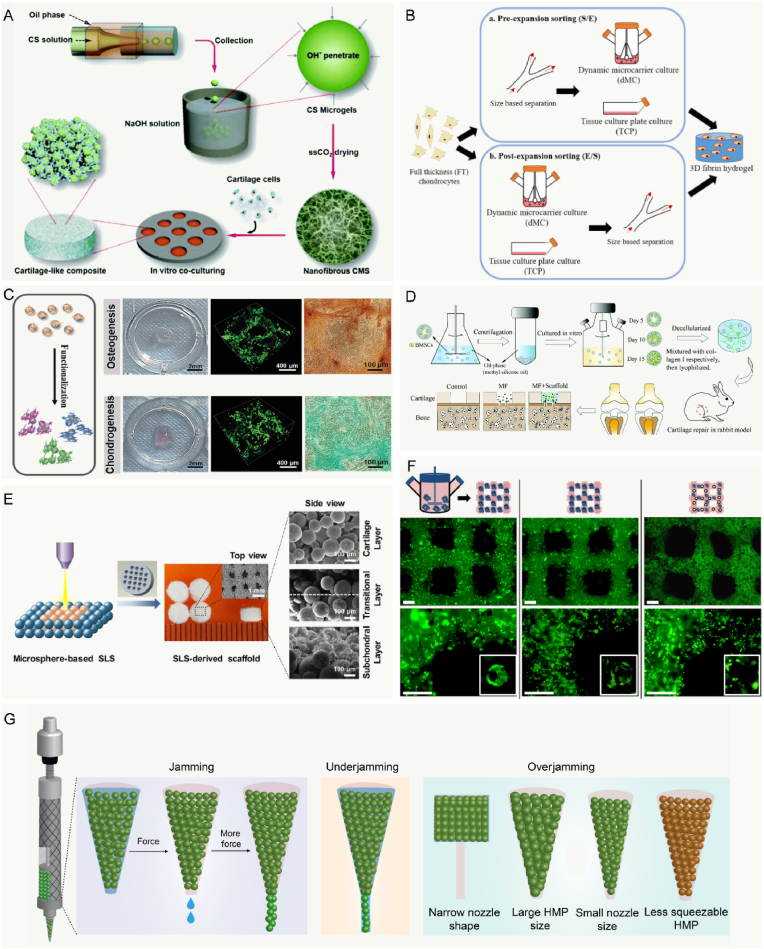
Fig. 5.
Combination microcarriers with technologies application for cartilage regeneration. A: Schematic representation of the nanofibrous chitosanwith an ECM-mimicking nanofibrous structure based on physical hydrogels of chitosan through the direct alkaline induced gelation of chitosan MCs emulsions. Reproduced with permission [264]. 2016, The Royal Society of Chemistry. B: Illustration of expansion and sorting strategy. Sorted small and medium/large were expanded for 1 passage in tissue culture plate, then further expanded in dynamic microcarrier condition or TCP for 2 passages. Reproduced with permission [267]. 2019, Elsevier Ltd. C: Schematic diagram of the printed GelMA MCs for macrotissues construction through a “bottom-up” method, and macroscopic images of the osteogenic macrotissue and chondrogenic macrotissue. Reproduced with permission [268]. 2020, WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. D: Preparation and in vivo implantation of bionic cartilage acellular matrix MCs scaffolds in comparison with microfracture. Reproduced with permission [200]. 2021, The Royal Society of Chemistry. E: The PCL MCs and hydroxyapatite/PCL composite MCs were used as building blocks to fabricate bio-inspired multilayer scaffolds via selective laser sintering technique. Reproduced with permission [95]. 2017, Elsevier Ltd. F: Immunofluorescence staining for actin cytoskeleton (green) on bioprinted GelMA-Gellan Gum hydrogels with encapsulated cells and microcarriers. Scale bar: 500 μm. Reproduced with permission [301]. 2014, IOP Publishing Ltd. G: Schematic of the HMP extrusion process under varying conditions. Jamming: Interstitial water was extruded first, and HMPs were packed closer before yielding to flow. Underjamming: Due to less resistance, HMPs were extruded with interstitial water in these scenarios. Overjamming: Due to more resistance, HMPs were not extruded until rupture of the beads. Reproduced with permission [302]. Copyright 2021, the Authors.
Scaffold with microcarriers, including MC-incorporating scaffolds and MC-based scaffolds, can serve as TE treatment strategy. Microcarriers can be assembled into MC-based scaffolds in three main packing strategies: random packing, directed assembly, and rapid prototyping [265]. As described previous studies, articular cartilage is composed of three layers that have distinct characteristics in terms of cell density, cell shape, collagen organization and ECM content [266]. The shearing force and compressional loading could be withstood by the zonal property of articular cartilage with biphasic mechanical properties. Therefore, restoration in the zonal hierarchy of natural articular cartilage is extremely important. Lee et al. has developed a sorting protocol in expanding chondrocytes and showed that post-expansion size-based sorting can be applied on microcarriers-expanded chondrocytes, generating enriched zonal subpopulations that form phenotypically distinct cartilage constructs in the 3D hydrogel, these constructs could support the stratified zonal repair of articular cartilage (Fig. 5B) [267]. The interaction between the biologics and microcarriers can be achieved by physical encapsulation, physical or chemical immobilization, and electrostatic interaction [128,182,212]. In addition, the adjacent ECM that produced by cells on the microcarriers can be able to assemble and stick MCs together. Traditionally, the majority of studies have followed a top-down approach by taking a bulk material and degrading sections of it to create pores scaffolds. Recently, hADSC-loaded GelMA microcarriers were induced to form osteogenic and chondrogenic microtissues through a “bottom-up” method, which presented the capability to achieve the construction of macrotissues [268] (Fig. 5C). Microfracture surgery is still considered as the gold standard method for articular cartilage repair due to its cost-effectiveness, minimally invasive nature, and technical simplicity, which could trigger the release of BMSCs [269]. There are tremendous challenges in developing a scaffold with homogenous materials to work with microfracture for researchers. Recently, Liu and coworkers have fabricated a biofunctionalized scaffold with cartilage acellular matrix microcarriers to improve the outcome of microfracture surgery [200]. This study showed that the cell-free MCs scaffolds exhibited enhanced both articular cartilage regeneration and subchondral bone repair (Fig. 5D). Overall, biological scaffold, as a temporary 3D construct to fill the osteochondral defect, can offer mechanical support, promote cell infiltration, and encapsulate drugs/factors to form an appropriate microenvironment for cartilage regeneration.
4.2. Scale-up cell expansion using microcarriers
Cell therapeutic strategies have been proven to be a well-tolerated, safe therapy for a variety of indications, which need a target production lot size of ∼100B cells [270]. Microcarriers have a 3D culture area that could rapidly expand cells for a sufficient amount and maintain the phenotype of cells, which have been used commonly in cell-line production with oscillating and multiplate bioreactors [271]. Therefore, a robust suspension bioreactor process that can be scaled-up is crucial to meet this demand for clinical manufacturing of seeded cells [272]. Furthermore, during the bioreactor manufacturing process, cell quality and phenotype may be influenced by the key parameters of bioreactor, such as oxygen concentration (pO2), temperature, cell concentration, pH, agitation and pressure (Table 3). Oxygen is one of the most critical nutrients for cell expansion, and is continuously added into the bioreactor via the sparger. Most mammalian cell expansion are performed with a dissolved oxygen of around 20–50% of the saturation with air. It is important to maintain a homogenous constant temperature in the bioreactor. When the temperature is above 38 °C, cellular viability was significantly reduced. The pH is usually set between 7 and 7.5 and the pH can be controlled by diluted base, diluted acid and CO2 [273]. It is needed to get a homogenous distribution of the culture, temperature, pH, nutrients, and oxygen in the bioreactor and prevent settling, but on the other hand, high-speed agitation has demonstrated significantly higher shear stress, which is the most severe potential damage to cells [274]. It may be the case that a small percentage of cells (e.g., chondrocytes, endothelial cells) need a high shear stress, but the average shear stress of most bioreactor remains low [275]. Collectively, these key parameters are very important for the designing of bioreactor that aims to successfully manufacture a large number of adherent cells.
Table 3.
Key parameters of scale-up bioreactors.
| Key parameters | Recommended range | Refs. |
|---|---|---|
| pO2 | Dissolved oxygen of 20–50% of the saturation with air. | [[280], [281], [282]] |
| Temperature | 30 °C–37 °C, it must be tightly controlled to within about 1 °C. | [282,283] |
| Cell concentration | 104–107 cells/cm3, it needs to reach the minimum inoculation density. | [284,285] |
| pH | 7.0–7.5, it is maintained in the range naturally with correct buffers. | [286,287] |
| Agitation | 10–150 rpm, it depends on the type of cell and bioreactor. | [[288], [289], [290]] |
| Hydrostatic pressure | 30–90 mmHg, cell production can be enhanced by the moderate hydrostatic pressure. | [291] |
This way of culturing cells raises additional challenges on the bioprocessing side. These challenges include the separation of cells and MCs, establishment of the minimum agitation level required, optimization of the feeding regime and the optimization of the gassing strategy [276]. The main strategies to separate cells from microcarriers are enzymatic dissociation combined with high stirring speed or using non continuous mixing [277]. Moreover, the use of magnetic microcarriers to separate cells is also an alternative way during culture harvest. Monitoring seed cell state after expansion whilst maintaining cell quality presents a key process step. Quality control tests include e purity, viability, genetic stability and, immunophenotype characterization [278]. Recently, Gong et al. reported a novel microfluidic approach that label-free and continuous-flow monitoring of single microcarrier using co-planar Field's metal electrodes and can be integrated into bioreactors for long-term [279]. Taken together, by employing bioreactors and microcarriers, it is expected that production costs would decrease due to improved process monitoring and quality control leading to better consistency and process efficiency, and enabling economies of scale.
4.3. Injectable and 3D bioprinting microcarriers
In general, the direct transplantation of cells sometimes may be ineffective. Because transplanted cells may be killed by the variable mechanical pressure, limited nutrient supplies, and shearing force [292]. It is estimated that only 1–20% of transplanted cells survive or remain at the site of injection, limiting the cell therapeutic potential [293]. Microcarriers have proven to be useful method as delivery vehicles through injectable methods due to their small size and spherical shape [207]. Furthermore, the injectable microcarriers could improve the delivery of cells with excellent properties, like the physical support. In addition, they can be performed in a minimally invasive fashion at the site of the defect and easily conform to any shapes, especially in joint diseases. This malleability provides a 3D platform for releasing agents, cell proliferation, and increasing lubricity [294]. In these MCs systems, viscosity decreases when shear strain is increased, allowing the possibility that MCs could flow during the injection [295,296]. Due to the limitations of injectable techniques, the issues of layer separation and weak interface bonding frequently exist. Therefore, Du et al. prepared a bio-inspired multilayer scaffold with PCL microcarriers for osteochondral repair using an advanced selective laser sintering (SLS) [95]. Compared with the powder form used in conventional SLS strategies, MC-based SLS technique is utilized to enhance micro-scale porosity during the sintering process (Fig. 5E).
Microcarrier-based osteochondral and cartilage constructs with microarchitectures can be achieved with biomanufacturing technologies (e.g., 3D printing). Various bioprinting techniques were developed, the most preferred deposition technique is extrusion because of its convenience, flexibility, precision, and high levels of cell compatibility [297,298]. However, challenges in fabrication of microcarrier-based inks include sufficient cells with high cell viability, the ability of extrusion molding, the stability and resolution of printed constructs and restrictions. In bioprinting, these inks should have controlled rheological properties (e.g., shear-thinning) that can be stabilized after deposition with both nontoxic and compatible [22,299]. Furthermore, the filamentous inks need rapidly stabilize to preserve fidelity of the printed structure from a reservoir onto a print surface [300]. Few studies have reported on bio-inks based on microcarriers so far, especially application of cartilage repair. In 2014, Levato et al. fabricated bilayer osteochondral models using microcarrier-laden bioink for bone and osteochondral constructs [301]. In this study, 3D bioprinting of cell-laden microcarriers showed the powerful ability to create a precisely-designed 3D architecture with high cell concentration and viability. Moreover, these PLA microcarriers provide a mechanical reinforcement to the inks (Fig. 5F). The result in this study also demonstrated that it is not advisable to solely use MSCs in MCs-based hydrogels for the cartilage region, since they can easily differentiate towards osteoblastic lineage.
As a whole, the large aggregates can easily block the injection nozzle [301]. Microcarriers flow is largely different from the liquid flow of common continuous bioinks during 3D printing. Unlike traditional hydrogel boinks, MCs-based inks are usually packed closely together in a jammed state, they look like a solid, hence their rheological properties need to be adjusted by chemical or physical modification and a secondary crosslinking is often required. Besides, the wall of the syringe and nozzle provide resistance and confine the MC-inks flow because of the considerable size of microcarriers. And then MCs may present closer packing to remove the aqueous solutions in the interstitial spaces, further deforming, squeezing, or sometimes rupturing themselves before yielding to flow. Moreover, the physicochemical properties of MCs-inks, like the size and modulus, can influence the dissipation process (Fig. 5G). The research results of Xin et al. revealed a large enough opening was required for smooth printing of MCs bioinks, but the shape and size of the syringe and nozzle as well as the size and polydispersity of the HMPs must also be considered [302]. Furthermore, the jamming process within the syringes also affected the printing stability and cytocompatibility. For instance, Highley et al. developed jammed microgels inks from norbornene-modified hyaluronic acid with shear-thinning behavior and short-term stability, and cell viability within the microgel was generally high (≈70%) [303]. Notably, it is worthwhile to consider that the post-cross-linking should be tested between the microcarrier. Nevertheless, the tissue defects usually feature curved surfaces or even more intricate geometries, where the mismatch of the shapes may be further worsened by the possible deformation of the local tissues [304,305]. Therefore, in situ MCs based-bioprinting address this dilemma to reconstruction of defective tissues in a clinical setting by handheld approaches. Taken together, extrusion-based bioprinting technology has progressed substantially and has paved the way during the last decade for bioprinting of cells and microtissues. The microcarriers could be performed via the extrusion-based bioprinting technology, while the orifice could easily be blocked.
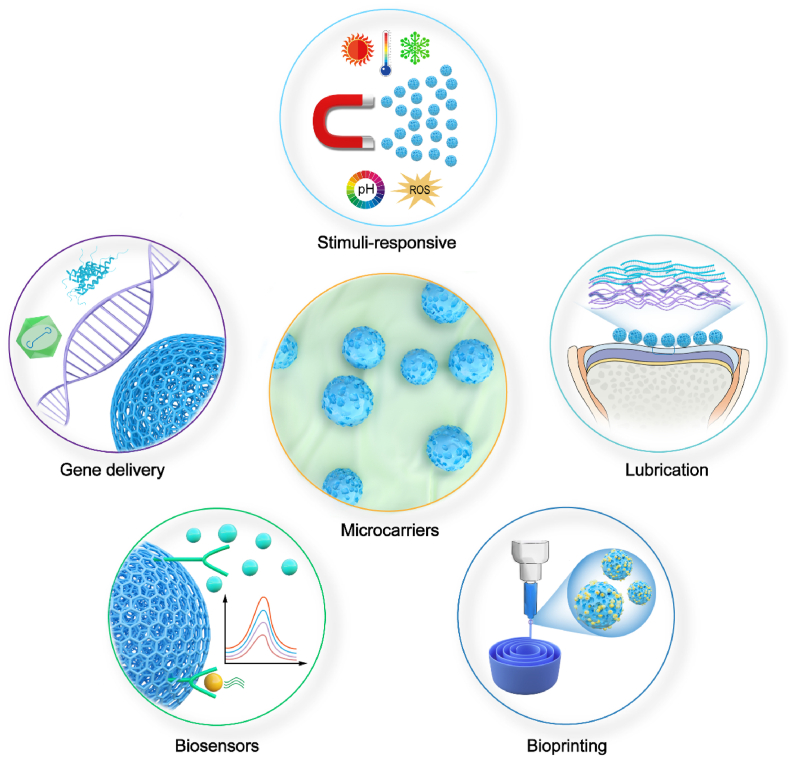
4.4. Stimuli-responsive microcarriers
Recently, stimuli responsive microcarriers have attracted particular interest, which offer great advantages for its nanostructured features and rapid transitions by the small alterations in the environment. These smart hydrogels can undergo reversible transitions of chemical/physical properties in response to various external factors such as physical stimuli like the magnetic field, temperature, light, and mechanical force, and the chemical or biochemical stimuli including the pH, solvent composition, or chemical triggers like reactive oxygen species and inflammatory environment [306]. The drug-releases process in stimuli responsive microcarrier is activated by external environmental changes, which not only reduces drug waste and improve drug utilization, but also improves the safety of treatment as well.
Thermosensitive polymers generally undergo so-gel transition upon temperature alteration, causing changes in the configuration, solubility, and hydrophilic–hydrophobic balance [307]. The hydrogel will become soluble when water molecules form hydrogen bonds with polar groups during critically low solution temperatures, polymer chains shrink and become hydrophobic and insoluble above the lower critical solution temperature. For example, Hydroxypropyl cellulose, a water-soluble non-ionic polymer, has an adjustable range lower critical solution temperature value in the temperature range of 41–45 °C in water because of the existence of hydrophilic and hydrophobic groups [308]. Recently, Işiklan et al. have prepared temperature-responsive chitosan/hydroxypropyl cellulose blend nanospheres for sustainable flurbiprofen release [309]. In 2021, Bulut et al. introduced temperature-responsive hydroxypropyl cellulose-g-polyacrylamide and chitosan MCs containing amoxicillin trihydrate to ensure the sustained release of the drug by increasing graft yield [310]. In another study, Yang et al. introduced poly (N-isopropylacrylamide) microcarriers that could swell once the temperature increased, and then encapsulated drugs can be released when temperature rises in the joint cavity during exercise or osteoarthritis. Thus, the smart microcarrier has the ability of intelligently releasing [128]. The advantage of temperature-triggered microcarriers is the ease of fabrication with no need for external cross-linking agents [311,312]. However, the temperature, and pH of the polymer solution should be suitable for clinical applications [313,314].
Articular cartilage-related diseases often lead to changes in the cartilage microenvironment, including the secretion of inflammatory cytokines, oxidative stress, and an acidified environment. In one study, researchers designed a net gelatin MC with negative charge to sequester cationic anti-inflammatory cytokines that controlled by the crosslinking density. For example, Park et al. designed gelatin microcarriers that are responsive to proteolytic enzymes typically expressed in arthritic flares, resulting in on-demand and spatiotemporally controlled release of anti-inflammatory cytokines [315]. These MCs are responsive to proteolytic enzymes and spatiotemporally controlled release of anti-inflammatory cytokines for cartilage repair (Fig. 6A) [248]. The cartilage also faces the problem of drug lymph due to the dense structure and the high-density negative charge. The high-density ECM was formed by the collagen fiber network with 60–200 nm pore size, and the negative electrostatic barrier was attributed to abundant negatively charged chondroitin sulfate mucopolysaccharide chains [316,317]. Feng et al. introduced an adhesive hydrogel MC characterized with positively charged nanostructure that allowed it to penetrate deeply into the cartilage with charge guidance (Fig. 6B) [318]. Articular cartilage lesions are often accompanied by the appearance of an acidic environment. Therefore, pH-responsive hydrogels, consisting of polymers with acidic or basic groups, display promising properties for biomedical applications. The mechanism of pH-responsive hydrogels generally involves dissociation and association with hydrogen ions depending on the pH level. Such as some poly acids that are deprotonated at neutral and basic pH and protonated at acidic pH [319]. Although several studies have reported on the use of pH-responsive microcarriers, these microcarriers remain rarely used in cartilage TE [[320], [321], [322]].
Fig. 6.
Responsive microcarriers specifically designed for articular cartilage injury. A: The microcarriers with the inflammatory response for delivery of the anti-inflammatory cytokines in osteoarthritis. Reproduced with permission [248]. 2019, Wiley Periodicals, Inc. B: Schematic illustration of charge-guided micro/nano-hydrogel MCs with ROS-responsive drug release for treating OA. Reproduced with permission [318]. 2021, Wiley-VCH GmbH. C: The design of ball bearing-inspired superlubricated MCs for synergetic treatment toward osteoarthritis based on enhanced hydration lubrication and sustained drug release. Reproduced with permission [247]. 2020, Wiley‐VCH GmbH. D: The MSC–based medical magnetic microrobot delivery system consists of a microrobot body capable of supporting MSCs, an electromagnetic actuation system, and a magnet for fixation of the microrobot to the damaged cartilage. Reproduced with permission [328]. 2020, The American Association for the Advancement of Science.
Since articular cartilage is constantly under mechanical forces within the microenvironment, and mechanical forces play a key role in regulating cartilage development and chondrocyte phenotypes, such as the cell growth and differentiation [323]. Moreover, a range of mechanical forces can ultimately affect the conformation of hydrogels, and the mechanical force can cause the hydrogel self-assembly, and stimulate polymer matrices easily to release drug/growth factors in responsive to mechanically stressed environments [324,325]. For instance, Yang et al. fabricated highly monodispersed photo-crosslinkable GelMA MCs containing poly sulfobetaine methacrylate brush, which can reduce the wear between the sliding cartilage surfaces as well as achieve the sustained release of anti-inflammatory drugs (Fig. 6C) [247]. Furthermore, mechanical loading may support proliferation and differentiation of stem cells for cartilage TE application.
To the best of our knowledge, microcarriers with ferromagnetic domains or magnetization have been initially developed and explored for magnetic responsiveness and/or drug delivery and invasive surgery applications using magnetic guidance to target the lesion sites [180,[326], [327], [328]]. Targeted cell delivery is a promising technique to enhance the low targeting efficiency of cells via magnetization in the cartilage TE. Go et al. reported findings using a magnetic adipose-derived MSC-based microcarrier composed of PLGA for knee cartilage regeneration, and they also developed a microrobot system consists an electromagnetic actuation system and a magnet for fixation (Fig. 6D) [328]. More recently, a magnetic implant system for stem cell-based knee cartilage repair, which consists of magnetic microcarriers, a portable magnet array device, and a paramagnetic implant, has been fabricated [327]. Magnetic responsive drug delivery to and targeting of the injury site can greatly improve the therapeutic effect in the joint cavity. Ideally, the magnetic carrier system consists of the cell-loaded magnetic MCs with biocompatibility and biodegradation and an external magnetic field generator. With regard magnetic carrier system, the magnetic actuation is usually developed by electromagnetic actuation using external magnetic field (controlling multiple coils) to target the desired position. One reasonable way for MCs to possess magnetization is to embed magnetic components such as hardmagnetic, soft-magnetic, or super-paramagnetic particles in the polymer matrices [329]. It should be noted that some magnetic metals such as cobalt and nickel can cause substantial acute toxicity [12,330,331]. Additionally, magnetic particles should be corrosive in the aqueous environments; therefore, the magnetic particles can be coated with protective layers (e.g., silica layers) to enhance their chemical stability [332,333]. In summary, the potential toxicity and the metabolism of the magnetic particles, as well as the large volume and limited workspace of the magnetic targeting devices, remain challenging issues associated with future microcarrier systems development.
4.5. Evaluation of microcarrier system for cartilage TE
A crucial prerequisite for the successful application of microcarriers is the careful evaluation of characterization by techniques. In fact, few histological scoring systems are available to evaluate of cartilage TE. The assessment of cartilage regeneration and repair mainly analyzes the structure and composition of the new tissue through histology and imaging, including matrix-staining metachromasia, tissue morphology, immunohistochemical staining, chondrocytes clusters, and glycosaminoglycan content and collagen [334]. The morphology of cartilaginous tissue can be adequately preserved. Zhou et al. reported the chitosan MCs with an ECM-mimicking nanofibrous structure based on physical hydrogels of chitosan [264]. In this study, macroscopic semitransparent 3D cake-like composites constructed by these MCs were obtained and bright field microscopy images show the dense cartilage-like composite consisted of chondrocytes after 2 weeks of agitated incubation (Fig. 7A).
Fig. 7.
Histological characteristics of microcarriers used for cartilage regeneration. A: the semitransparent cartilage-like composite tissue contained chondrocytes and nanofibrous chitosan MCs in digital photographs (i) and bright field microscopy images (ii). Scale bar: 2 mm (i), 400 μm (ii). Reproduced with permission [264]. 2016 The Royal Society of Chemistry. B: The knee osteoarthritis was treated by liposomes-anchored microgels at week 1, and 8 post-surgeries in X-ray (i) and micro-CT images (ii). Reproduced with permission [183]. 2020 Elsevier B.V. C: An acellular agarose hydrogel carrier with embedded DEX-loaded poly (lactic-co-glycolic) acid MCs was developed to provide sustained release. Gross image (i), H&E staining (ii), toluidine blue (iii). Scale bar: 2 mm. Reproduced with permission [88]. 2019, Elsevier Ltd.
The development of in vitro TE techniques also extends knowledge of cartilage structure and composition. For example, Yang et al. proposed a novel strategy for preparing liposomes-anchored microgels that could achieve controllability of extended drug delivery for treatments of osteoarthritis [183]. The joint spaces of osteoarthritic models were evaluated using X-ray imaging and the osteophyte formation and subchondral bone changes were detected using Micro-CT (Fig. 7B). Another radiographic measurement, like magnetic resonance imaging, is also an alternative technique analyzing for cartilage quality. Generally, hematoxylin-eosin staining (H&E) can provide a general overview of cell distribution and tissue organization. The high-quality cartilage demonstrated that the intense Safranin-O staining and high amount of matrix as well as chondrocyte-like cells in the tissue. Additionally, the proteoglycan deposition can be evident from toluidine blue staining (Fig. 7C). Evaluation of cartilage quality through histological analysis significantly contributes to the assessment of the extent of cartilage damage or the success of cartilage regeneration. When evaluating cartilage characteristics, choosing the appropriate histological scoring system is important for analysis of cartilage pathology, evaluation of results of in vivo treatment, and determination of additional TE research needs. Different histological scoring systems exist for each of these categories.
5. Principle for cartilage regeneration using microcarriers
An optimal cell-loaded microcarrier delivery system is critical to ultimately achieve the promise of cartilage TE. The ideal biomaterial-assisted cell delivery system needs to satisfy the following requirements [68]: (i) simple ingredient with economical and cost-effective design along with high microcarrier production rate; (ii) efficient and reproducible loading of the functional ingredients (matrix, drug or bioactive factors); (iii) good biocompatibility, ease of processing into microcarriers, long-term biological stability, and controllable biodegradability that match to the growth rate of the osteochondral tissue; (iv) targeted delivery and long-term retention at the therapeutic site; (v) enhanced integration with the lesion tissue and therapeutic efficacy with degradable properties; and (vi) ease and readiness for adaption to existing clinical practices for cell therapy.
A salient feature of articular cartilage is their capability of healing after mild injury, and the self-healing can provide cartilage with damage mitigation and long-term robustness. However, this property mostly relies on the function of chondrocytes and MSCs, and engineering materials whether to form new cross-link and/or interactions in the vicinity of damaged regions [335]. Self-healing enables materials to restore their morphology and mechanical properties after defects, which have been developed using multiple crosslinking mechanisms with large deformation capabilities. The commonly used for healing process is weak physical cross-links (hydrogen bonds, metal coordinations, ionic bonds and hydrophobic interactions) and dynamic covalent cross-links (reversible cross-links) [[335], [336], [337]]. In addition, the self-healing capability can also be achieved via interdiffusion of polymer chains to form entangled chains that span the crack surfaces [338].
There are some critical properties that should be carefully taken into consideration if the clinical success of the technology is aimed. A big challenge in cartilage TE is to achieve mechanical properties similar to native cartilage. Articular cartilage displays intensive mechanical properties such as high fracture energy (toughness)≥1000 J/m2, and stiffness≥1 MPa [339]. The Young's moduli for cartilage are in range of 10–20 kPa [340]. In addition, chondrocytes are surrounded by a pericellular matrix with a stiffness of ∼25–200 kPa in vivo, but most existing 3D microenvironmental stiffness (<5 kPa) for cell encapsulation is lower than cartilage ECM [341]. Mechanical properties of microcarriers, such as stiffness, elasticity, topography and roughness are important, which may be commonly controlled by the polymer concentration and degree of cross-linking. These properties not only to determine the durability and stability of microcarriers during the production and implantation, but also affect cell adhesion and phenotypes such as differentiation, proliferation, and migration. For instance, Donald et al. described primary chondrocytes maintained high viability in environment of high-stiffness (∼35 kPa) agarose gels [245]. In more recent study, researchers fabricated PLLA porous MCs blending SF and gelatin with remarkably mechanical properties that promote chondrogenesis [231]. Besides, mechanical properties of MCs were found positively correlating with chondrogenic differentiation, it demonstrated that the mechanical property negatively correlates with type I collagen and total collagen [342]. Current microcarrier technologies typically provide a stiffer substrate, which may have detrimental effects on cell phenotype and function [343]. However, extensive studies indicated that the proliferation and matrix formation of chondrocytes could be limited by the elastic stress [344,345]. Lee et al. exhibited that fast stress relaxation could provide a microenvironment that is more conducive to cartilage matrix formation by chondrocytes [13]. Additionally, recent studies have found that viscoelastic hydrogels could promote spreading and proliferation of adherent cells [346,347]. Furthermore, the spectrum of stiff to soft substrates can alter MSC surface markers, with MSCs lineage markers primed to neurogenic following growth on low-stiffness substrates, myogenic on medium-stiffness substrates and osteogenic on stiff substrates. There are several detailed evaluations of mechanical properties by characterization techniques. Traditional methods mainly provide information data on the mechanical properties of microcarriers through compression experiments, such as uniaxial compression tests of a rheometer [348]. Besides, extensive studies use atomic force microscopy and/or nanoindentation to evaluate the surface modulus of microcarriers [[349], [350], [351]]. Another effective way is to subject the sample to a shear-flow, for example, a novel microfluidic method measures the elastic properties of a population of MCs was presented by Wang and coworkers [352]. The Young's modulus of normal articular cartilage is about 6 MPa, the prepared material prefers to achieve the mechanical structural parameters. Although the effect of stiffness on cell differentiation pathways are well known, there is a gap in the literature regarding microcarrier surface effect on MSC secretome, and thus more researches need to be performed [343]. The small size of the microcarriers could be beneficial in medical application, especially for intra-articular injection. Microcarriers with an inner diameter of 100–300 μm are accepted by most researchers and used in cartilage repair [292,353]. Besides, the immune reaction was much lower when employing smaller MCs comparing with bigger size MCs. In overall, the diameter of the microcapsules should not exceed 400 μm.
Understanding the components and structure of cartilage tissue is essential for the design and fabrication of cartilage TE-scaffolds. The bionic principle that mimics the structure and composition of native cartilage should be the first consideration. A successful microcarrier design depends on the interaction between the carrier surface and cells. Generally, the 3D architecture could provide transient support to cells for enabling their growth and differentiation, and this support is a crucial determinant of neocartilage formation. It is difficult to determine what micro-architecture features could affect cartilage regeneration, while pore orientation/aspect ratio seems to be a strong architectural feature that may influence cartilage formation/integration. In addition, the anisotropic multizonal pore architecture mimics the collagen of native articular cartilage, and the anisotropic architecture may promote the integration of these constructs in osteochondral defects. Therefore, microcarriers should have interconnected open pore structures (high porosity) with a large surface area. Additionally, change the surface hydrophilicity and charge characteristics via chemical modification (e.g., copolymerization and grafting methods) and surface modification method (e.g., immobilization of collagen, polylysine and RGD) to improve cell adhesion. Since articular cartilage involves the interface with bone, the implanting scaffold needs to consider the combination with subchondral bone and has the stratification to provide appropriate cushioning. In addition, the degradation rate of microcarrier scaffolds should match the regeneration speed of cartilage tissue.
Finally, mechanical properties of MCs are crucial to the cartilage function, and greatly affect the above-mentioned applications. Despite a number of research on improving the sufficient mechanical properties and certain functional properties on microcarriers, these systems still encounter serious challenges within the vibrant and mechanically demanding environment. In summary, besides biocompatibility and low content of toxic, porosity, mouldability, injectability, degradability, controllable swelling, and intensive mechanical properties as well as accretion with the adjoining native cartilage are attributes to consideration of design the microcarriers for cartilage TE.
6. Perspectives and challenges
6.1. Promising future directions
Generally, cartilage may not self-repair due to lack of blood vessels, nerves. At present, the synthesis and application of microcarriers are still in its infancy and still impeded by several limitations. However, smart microcarriers should be integrated more functions and be used in various field for cartilage regeneration, such as stimuli-responsive options, gene delivery, lubrication, biosensors, and bioprinting (Fig. 8). If these aspects are thoroughly studied, it will open a new chapter and confirm their clinical transformation ability for cartilage TE in future.
Fig. 8.
Schematic diagram of promising future directions for microcarriers in cartilage TE, including application of stimuli-responsive options, gene delivery, lubrication, biosensors, and bioprinting.
Smart or stimuli-responsive microcarriers are novel class of materials used for tissue engineering and drug delivery, which may be used to free the cell or drugs from their carrier to the local injury sites depend on a variety of stimuli, including temperature, reactive oxygen species, pH, light, oxidative stress, and magnet fields. After cartilage injury, the surrounding microenvironment will change significantly, such as high oxidative stress level, weak acidity and inflammation environment. For example, Poly (N-isopropyl acrylamide) (pNIPAM) can make a reversible conformational transition an expanded coil to a compact globule with temperature increasing in an aqueous environment. Thus, cell adhesion and detachment may be alternated upon the temperature. The stimuli can be internal or external, like the pH vary in different severity of OA. Similarly, magnetic nanoparticles can be incorporated into microcarriers for cell and drug delivery through the application of a magnetic field [354]. Moreover, multi-stage responsiveness is emerged in a complex physiological environment. Even though, while several different stimuli responsive microcarriers are continuously being developed, the potential to reach a successful implementation is excellent.
MCs-mediated delivery systems of small molecules are composites of a biodegradable polymer that can be formulated to encapsulate drugs or factors. Similarly, vehicles for gene delivery from microcarriers may have the potential to overcome extracellular barriers that limit gene therapy. Their function is home into the local tissue injury sites with maintaining the gene. In general, the microcarriers cannot be readily internalized for cells, but retain within the tissue to release DNA. Moreover, the transfection on a 3D construct may extend transgene expression due to the distribution of microcarriers in a 3D space. Articular joints need to withstand millions of motion cycles, but some implanted hydrogels have shown early loosening and surface wear [355]. Native articular cartilage has a smooth and shiny surface with friction coefficients as low as 0.001 that can provide the joints connections with load transmission capabilities during a range of motions, and there are few microcarriers with lubrication function applied to cartilage repair [128,181]. Theoretically, developing microcarriers with appropriate tribological properties requires a detailed understanding of the governing lubrication mechanisms under a range of loading and motion conditions. The interfacial friction from polymeric interactions should be highly considered in tribology, and the friction coefficient of hydrogel systems has been related to the probability of chain–chain interactions [356]. Recently, inspired by the super lubricated surface of ice that consists of a contiguous and ultrathin layer of bound water, Yang et al. developed methacrylate anhydride-hyaluronic acid drug delivery MCs with satisfying strength and enhanced lubrication from microfluidic electrospray, these MCs could form a hydrated lubricating layer by modifying with the positively (N+(CH3)3) and negatively (PO4−) charged chemical groups [357]. The local water content in the contact of hydrogels has also been found to affect friction forces. Therefore, hydrogel with high water content has been proposed for improvement of lubrication for mimicking the excellent lubrication mechanism of natural synovial joint. With the support of friction theory, low-friction microcarriers need to be developed.
Biosensor in cartilage TE have not yet been used by microcarriers, but the minimal invasiveness is suitable for the joint cavity injection. Through application of MC-based biosensors, it can bring great possibilities in the field. Smart protein-based biosensor for protein conformation detection using silk MCs have been recently reviewed by Zhang and coworkers [36]. These microcarriers need to be biocompatible, sterilizable and nontoxic. Moreover, the inactivation and loss of the recognition element must be considered, especially with regard to enzyme-based biosensors [358]. The microcarriers-based 3D printing has gained much attention. The 3D printing is a pioneering technology that enables the recapitulation of complex tissues with high process flexibility and versatility. Since the development of bioprinting technology can provides the precise dimensional architecture and biological multifunction to mimic the native cartilage tissues, microcarriers based inks still face extremely challenges, because microcarriers based inks lack suitable printability, shape fidelity, and weak mechanical strength. And the mechanisms of microcarriers jamming within printing nozzles and yielding to flow remain underexplored. Usually, an ideal bio-ink should meet the cell compatibility, mechanical property, and integrated printability for cartilage TE. In term of microcarriers based cell inks, the properties of inks, such as physicochemical characteristics, rheological, and mechanical, could been well modified by various strategies and advanced techniques.
With the development of delivery strategy, especially the targeted delivery systems, a wide range of inorganic materials has been proposed and studied. Bioactive glasses were originally designed to be an inert material for biocompatibility. Subsequently, mesoporous bioactive glasses were served to being using for highly efficient drug delivery application in various forms, such as micro- or nano-particles, hierarchical scaffolds, and fibers [359]. Silica-based mesoporous bioactive glasses are recognized as most promising candidates for the drug delivery with the functionalizing silanol group [360]. All in all, bioactive glasses can be used as highly versatile and tailorable platforms for the controlled delivery of drug. Nanoparticles vectors, with large surface area and small size, unmatched flexibility and diversity. Carbon nanomaterials have become increasingly popular in the material society because of the strong plasticity of carbon [361]. For instance, fullerene is one of the pioneer classes of carbon-based nanoparticles for targeted delivery with a specific geometry, size and surface characteristics, possess suitable properties for interaction with the cellular environment [362,363]. It is composed entirely of carbon with various shapes (e.g., spherical, ellipsoidal, cylindrical and tubular) [364]. In addition, fullerenes possess obvious electrophilicity. Researchers found that fullerenes with positive charge were used to delivery small molecules owing to their low cost and high efficacy [365]. Furthermore, the use of noncovalently adsorbed drugs and fullerenes have been reported; therefore, they could present acceptable and efficient transdermal drug delivery at room temperature [366]. With the specific structure and modified group used to increase delivery efficiency, there is no doubt that fullerene-based delivery systems offer many opportunities in disease treatment. However, it is immensely vital to understanding the molecular mechanisms behind the effect of released therapeutical agent from these inorganic materials to develop safer and more-effective delivery strategies. Few studies reported the combined application of microcarriers and nanoparticles. Incorporating microcarriers and natural polymers to avoid cytotoxicity, these inorganic materials may provide new insights into TE.
6.2. Future challenges
In the past decade, considerable progress has been made in cartilage TE, which offers the opportunity for the translation from the bench to the bedside in the near future. The translation of microcarrier engineered products, in terms of 3D cell culture, cells that mainly adhere and grow on the outermost surface or the external pore, resulting in insufficient and multidirectional cell–cell interactions, low differentiation efficiency and poor tissue adhesion, which hinders the formation of high-quality hyaline type cartilage [367,368]. Meanwhile, the microcarrier delivery cell platform does not guarantee the accurate shipment of cells and formation of neocartilage tissue around the injury site. In addition, synovial fluid may make it difficult for cells to adhere and may affect cell viability. Mimicking the in vivo environment for the cell culture system on microcarriers will still be challenging. Forming a cavity inside the MCs is one option; however, the problem of low nutrient exchange efficiency needs to be further solved. Combined with macroscopic scaffolds, the constructed micro-tissue is more soluble in native organs and tissues than 2D culture.
Despite the success in preclinical studies, use of microcarriers therapeutics in clinical application require careful consideration; specifically, several critical issues such as (i) production methods, characterization, and quantification for microcarrier bank, (ii) A high-quality transportation system from laboratories to hospital, (iii) safety profile assessments for clinical application. Microcarrier bank is a system that includes process preparation, cell culture and cryopreservation, and quality assessment. The generation of MC banks for cell and gene therapy, must comply with current good manufacturing practice regulations. The quality of microcarriers and cell lines use as therapeutic materials, must be also assessed. Several critical issues, including sufficient cells source, standard manufacturing practices, new reasonably priced chemically defined xeno-free media, and quality inspection, must be overcome in future (left panel of Fig. 9). Specially, manufacturing procedures should be validated according to Good Manufacturing Practices and, for this reason, an assessment of materials’ product specifications is required during the whole process. Additionally, an efficient large scale cell harvesting method and the interrelations between cell expansion and efficiency of differentiation on microcarriers is very important [369]. Moreover, development of biodegradable microcarriers can be advantageous as the scaffold for in vivo transplantation.
Fig. 9.
Key issues of microcarriers-based tissue engineering from bench to bed-side. Left panel: a standardized microcarrier cell bank needs to be built, which was hindered by the cell source, MCs fabrication, standard procedures, xeno-free media, and quality control for the MC-based systems. Middle panel: a high-quality transportation system and cell cryopreservation and recovery system are essential to meet current clinic needs. Left panel: The clinical application of microcarriers requires a combination of multiple disciplines and technologies, and many problems need to be solved urgently, such as the safety issue, quality assessment, tumorigenicity and the immune rejection of seed cells.
A high-quality transportation system is essential for effective and safe application of microcarrier-based therapeutics from laboratories to hospital. The transportation system consists of efficient cryopreservation, a freeze-thaw process, and a packaging process (middle panel of Fig. 9). The frozen cell transportation is an ideal method of cell transportation and represents an important way to achieve long-term storage of cells, tissues, organs, and other biological materials by using a very low temperature [370]. The formation, growth, and recrystallization of ice crystals are crucial factors for cryopreserved samples in the process of cryopreservation with a variety of chemical and physical damage [371]. Hence, it is fundamental and crucial to control, restriction, and elimination of ice crystals.
It's well known that the ice-crystal formation is inevitable regardless of slow or rapid freezing. It is critical to control and inhibition of the ice-crystal formation to weaken the effects of ice injury. The ice-inhibition molecules, including cryoprotectants, antifreeze proteins, synthetic polymers, nanomaterials, and hydrogels, provide great opportunities to improve cryopreservation [372]. In addition, the unique biological structure can also suppress ice and reduce cryoinjury. For example, the core-shell structure as a barrier to prevent cells direct contact with ice. In addition to the freezing process, it is well known that the thawing efficiency is also a crucial factor in the survival of cryopreserved cells [373]. One key question is how to achieve fast and uniform recovery during the thawing process via external physical field technologies (e.g., magnetic warming). Furthermore, the tubes usually used for frozen transportation are not completely sealed receptacles, the transported materials should withstand some external environmental factors, including temperature changes, micro-organisms, shock, and ultraviolet radiation. Hence, a standard packaging procedure should be obtained to meet clinical needs.
The first marketed product sustained-release injectable microcarrier (Lupron depot) could be traced back to 1985. Currently, about 11 FDA-approved sustained release microcarriers are available in the market [374]. PLGA and PLA are most used as synthetic polymers for microcarriers because of biodegradable and biocompatible. For example, the FX006, a novel extended-release PLGA MC containing the triamcinolone acetonide, which was proved to provide increased therapeutic benefit by intraarticular Injection for patients with knee osteoarthritis [[375], [376], [377]]. The in vivo use of cells-seeded (e.g., MSC or iPSC) microcarriers for clinical application has not yet been described; however, these studies are likely imminent. Currently, the clinically use cell-based microcarriers faces potential risks. The following several aspects are considered to the most important (right panel of Fig. 9). First, significant cell doses of clinical grade are needed for cellular therapies that need biocompatibility, it relates not only the interaction between the biomaterials and the host, but also the interaction between the biomaterials and the encapsulated cells. Second, the versatility of differentiation capability to become multiple cell types in the body. Third, the safety and quality of cells must be systematically assessed, bacteriological tests should be carefully performed during the various phases of production and at harvest. Fourth, rejection of the body and immune regulation function should be required understanding of the biological characteristic of MC. The clinical transformation of microcarriers requires the cooperation of many disciplines, including biomaterials, immunology, tissue engineering, molecular biology, transplantation biology, and stem cell biology. As an immunomodulator, although MSCs exhibit immunoprivileged/immunoreactive properties and can be used in allogeneic therapies, the microcarriers implanted in the body will lead to the immune response. Microcarriers size and morphology could influence the immune response against. Bigger size and rough surfaces of MCs may elicit immunological reaction when implanted [378]. In addition to the biomaterial's chemical properties, a clean surface, controlled geometry and could decrease the acute inflammatory response and minimize fibrosis formation [379]. Moreover, the efficient purification process of biomaterial's components is considered to be a key element for implantation purposes, which include purification of endotoxins, polyphenols and certain proteins. The surgical implantation method is seemed to be an additional parameter that influences the host reaction or biocompatibility, the surgical implantation mainly activates a non-specific response against implanted devices. The use of transient immunosuppressive protocols has been proposed to overcome this obstacle. Fifth, low microcarrier-derived particulate levels are advisable to consideration in the original process development. In fact, these four aspects are combined and overlapped. Based on the continuing study of dynamic bioreactors operated with microcarriers, certain challenges remain, further scale-up to produce cells for cell therapy or TE is possible.
7. Conclusions
During the past several decades, the development of MCs in the regeneration of cartilage has gained considerable progress. In this review, we summarized the progress and development trends of microcarriers for cartilage TE during the past 6 years. We described the main preparation techniques and advantages of microcarriers. In addition, we also introduced the application of microcarriers in cartilage TE. Finally, we identified the design principles as well as promising directions and challenges for cartilage regeneration using microcarriers. All these achievements provide an important foundation for widening and developing the application of porous microcarriers. We sincerely hope that this review will assist interested readers to understand application direction of microcarriers in cartilage TE, and improve understanding for researchers who intend to enter this field. The main conclusions are as follows:
-
(1)
Several microcarrier preparation technologies provide possibilities for the design of various microcarriers that meet the needs in cartilage TE. The fabrication of injectable, well-controlled, polymer-based porous, and controlled degradation microcarriers with cost-effective is the main development trend for its use in cartilage regeneration. The novel structure design and superior properties like the stimuli-responsive MCs is the promising future trends.
-
(2)
Microcarriers are generally applied in the form of sponge scaffolds in cargo delivery. And the combination biofabrication methods and emerging technology are advanced approach in fabrication of bio-mimicking, heterogeneous, and complex tissue structures. Nevertheless, clinical applications of MCs may be limited by the safety and regulatory processes.
-
(3)
The main design principle of microcarriers is mimicking of natural cartilage in composition and structure. Cellulose is advantageous with respect to biocompatibility and similarities with nanostructures of the ECM, but its biodegradation is difficult. In authors' opinion, the cartilage ECM is a good alternative candidate. Porous microcarriers with fibrous architectures could be regarded as simulation of the physiological microenvironment of natural cartilage. Porous microcarriers offer larger specific surface areas and higher volumes for cell adhesion, proliferation, and growth over nonporous microcarriers. However, the porosity can easily affect the physical properties, mechanical properties, and degradation rate. Also, these properties can be conveniently tailored by controlling the synthetic methods and conditions to meet the need for biomaterials in cartilage TE.
In summary, the microcarrier platform provides a potential treatment strategy for cartilage TE caused by diseases and trauma. Many crucial challenges still exist, and microcarriers technologies and the associated process need to be modified to tailored the requirements of cell types of interest, and the clinical application. However, microcarriers will undoubtedly produce great achievements as biomedicine, biotechnology and materials science develop.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors thank Shuo Wang and Shen Ji for helpful discussion. This work was supported by the National Natural Science Foundation of China (Grant No. 81773091), the Natural Science Foundation of Beijing Municipality (Grant No. 7212020), Science and Technology Planning Project of Beijing Municipal Education Commission (Grant No. KM202110025013), the Beijing Municipal Excellent Talents Project (Grant No. 2020A43), Strategic Priority Research Program of Chinese Academy of Sciences (Grant No. XDA16020802), CAS Engineering Laboratory for Intelligent Organ Manufacturing (Grant No. KFJ-PTXM-039), and the National Natural Science Foundation of China (Grant No. 82001848).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Qi Gu, Email: qgu@ioz.ac.cn.
Ming-Zhu Zhang, Email: mingzhuzhang@mail.ccmu.edu.cn.
References
- 1.Wei W., Ma Y., Yao X., Zhou W., Wang X., Li C., et al. Advanced hydrogels for the repair of cartilage defects and regeneration. Bioact Mater. 2021;6(4):998–1011. doi: 10.1016/j.bioactmat.2020.09.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wei W., Dai H. Articular cartilage and osteochondral tissue engineering techniques: recent advances and challenges. Bioact Mater. 2021;6(12):4830–4855. doi: 10.1016/j.bioactmat.2021.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Do Visscher, Lee H., van Zuijlen P.P.M., Helder M.N., Atala A., Yoo J.J., et al. A photo-crosslinkable cartilage-derived extracellular matrix bioink for auricular cartilage tissue engineering. Acta Biomater. 2021;121:193–203. doi: 10.1016/j.actbio.2020.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Khademhosseini A., Langer R. A decade of progress in tissue engineering. Nat. Protoc. 2016;11(10):1775–1781. doi: 10.1038/nprot.2016.123. [DOI] [PubMed] [Google Scholar]
- 5.Koons G.L., Diba M., Mikos A.G. Materials design for bone-tissue engineering. Nat. Rev. Mater. 2020;5(8):584–603. doi: 10.1038/s41578-020-0204-2. [DOI] [Google Scholar]
- 6.Lee M.S., Stebbins M.J., Jiao H., Huang H.C., Leiferman E.M., Walczak B.E., et al. Comparative evaluation of isogenic mesodermal and ectomesodermal chondrocytes from human iPSCs for cartilage regeneration. Sci. Adv. 2021;7(21) doi: 10.1126/sciadv.abf0907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sun J., Xing F., Zou M., Gong M., Li L., Xiang Z. Comparison of chondrogenesis-related biological behaviors between human urine-derived stem cells and human bone marrow mesenchymal stem cells from the same individual. Stem Cell Res. Ther. 2021;12(1):1–19. doi: 10.1186/s13287-021-02370-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nguyen D., Hägg D.A., Forsman A., Ekholm J., Nimkingratana P., Brantsing C., et al. Cartilage tissue engineering by the 3D bioprinting of iPS cells in a nanocellulose/alginate bioink. Sci. Rep. 2017;7(1):1–10. doi: 10.1038/s41598-017-00690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hall G.N., Tam W.L., Andrikopoulos K.S., Casas-Fraile L., Voyiatzis G.A., Geris L., et al. Patterned, organoid-based cartilaginous implants exhibit zone specific functionality forming osteochondral-like tissues in vivo. Biomaterials. 2021;273:120820. doi: 10.1016/j.biomaterials.2021.120820. [DOI] [PubMed] [Google Scholar]
- 10.DF Duarte Campos W Drescher, Rath B., Tingart M., Fischer H. Supporting biomaterials for articular cartilage repair. Cartilage. 2012;3(3):205–221. doi: 10.1177/1947603512444722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Huang G., Li F., Zhao X., Ma Y., Li Y., Lin M., et al. Functional and biomimetic materials for engineering of the three-dimensional cell microenvironment. Chem. Rev. 2017;117(20):12764–12850. doi: 10.1021/acs.chemrev.7b00094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yasa I.C., Tabak A.F., Yasa O., Ceylan H., Sitti M. 3D‐Printed microrobotic transporters with recapitulated stem cell niche for programmable and active cell delivery. Adv. Funct. Mater. 2019;29(17):1808992. doi: 10.1002/adfm.201808992. [DOI] [Google Scholar]
- 13.Lee H.P., Gu L., Mooney D.J., Levenston M.E., Chaudhuri O. Mechanical confinement regulates cartilage matrix formation by chondrocytes. Nat. Mater. 2017;16(12):1243–1251. doi: 10.1038/nmat4993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seo B.B., Kwon Y., Kim J., Hong K.H., Kim S.E., Song H.R., et al. Injectable polymeric nanoparticle hydrogel system for long-term anti-inflammatory effect to treat osteoarthritis. Bioact Mater. 2022;7:14–25. doi: 10.1016/j.bioactmat.2021.05.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu X., Chen Y., Mao A.S., Xuan C., Wang Z., Gao H., et al. Molecular recognition-directed site-specific release of stem cell differentiation inducers for enhanced joint repair. Biomaterials. 2020;232:119644. doi: 10.1016/j.biomaterials.2019.119644. [DOI] [PubMed] [Google Scholar]
- 16.Kwon H., Brown W.E., Lee C.A., Wang D., Paschos N., Hu J.C., et al. Surgical and tissue engineering strategies for articular cartilage and meniscus repair. Nat. Rev. Rheumatol. 2019;15(9):550–570. doi: 10.1038/s41584-019-0255-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.AC Daly FE Freeman, Gonzalez-Fernandez T., Critchley S.E., Nulty J., DJ Kelly. 3D bioprinting for cartilage and osteochondral tissue engineering. Adv Healthc Mater. 2017;6(22):1700298. doi: 10.1002/adhm.201700298. [DOI] [PubMed] [Google Scholar]
- 18.O'Connell G., Garcia J., Amir J. 3D bioprinting: new directions in articular cartilage tissue engineering. ACS Biomater. Sci. Eng. 2017;3(11):2657–2668. doi: 10.1021/acsbiomaterials.6b00587. [DOI] [PubMed] [Google Scholar]
- 19.Shi W., Sun M., Hu X., Ren B., Cheng J., Li C., et al. Structurally and functionally optimized silk-fibroin-gelatin scaffold using 3D printing to repair cartilage injury in vitro and in vivo. Adv. Mater. 2017;29(29):1701089. doi: 10.1002/adma.201701089. [DOI] [PubMed] [Google Scholar]
- 20.Gao F., Xu Z., Liang Q., Li H., Peng L., Wu M., et al. Osteochondral regeneration with 3D-printed biodegradable high-strength supramolecular polymer reinforced-gelatin hydrogel scaffolds. Adv. Sci. 2019;6(15):1900867. doi: 10.1002/advs.201900867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Melo B.A.G., Jodat Y.A., Mehrotra S., MA Calabrese, Kamperman T., Mandal B.B., et al. 3D printed cartilage‐like tissue constructs with spatially controlled mechanical properties. Adv. Funct. Mater. 2019;29(51):1906330. doi: 10.1002/adfm.201906330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mandrycky C., Wang Z., Kim K., Kim D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016;34(4):422–434. doi: 10.1016/j.biotechadv.2015.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Feng Q., Li Q., Wen H., Chen J., Liang M., Huang H., et al. Injection and self‐assembly of bioinspired stem cell‐laden gelatin/hyaluronic acid hybrid microgels promote cartilage repair in vivo. Adv. Funct. Mater. 2019;29(50):1906690. doi: 10.1002/adfm.201906690. [DOI] [Google Scholar]
- 24.Mow V.C., Ratcliffe A., Poole A.R. Cartilage and diarthrodial joints as paradigms for hierarchical materials and structures. Biomaterials. 1992;13(2):67–97. doi: 10.1016/0142-9612(92)90001-5. [DOI] [PubMed] [Google Scholar]
- 25.Chen F.H., Rousche K.T., Tuan R.S. Technology Insight: adult stem cells in cartilage regeneration and tissue engineering. Nat. Clin. Pract. Rheumatol. 2006;2(7):373–382. doi: 10.1038/ncprheum0216. [DOI] [PubMed] [Google Scholar]
- 26.Malda J., Ke Benders, Klein T.J., de Grauw J.C., Kik M.J., Hutmacher D.W., et al. Comparative study of depth-dependent characteristics of equine and human osteochondral tissue from the medial and lateral femoral condyles. Osteoarthritis Cartilage. 2012;20(10):1147–1151. doi: 10.1016/j.joca.2012.06.005. [DOI] [PubMed] [Google Scholar]
- 27.Culliton K.N., Speirs A.D. Sliding contact accelerates solute transport into the cartilage surface compared to axial loading. Osteoarthritis Cartilage. 2021;29(9) doi: 10.1016/j.joca.2021.05.060. [DOI] [PubMed] [Google Scholar]
- 28.McNary S.M., Athanasiou K.A., Reddi A.H. Engineering lubrication in articular cartilage. Tissue Eng. B Rev. 2012;18(2):88–100. doi: 10.1089/ten.TEB.2011.0394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mow V.C., Holmes M.H., Lai W.M. Fluid transport and mechanical properties of articular cartilage: a review. J. Biomech. 1984;17(5):377–394. doi: 10.1016/0021-9290(84)90031-9. [DOI] [PubMed] [Google Scholar]
- 30.Han L., Frank E.H., Greene J.J., Lee H.Y., Hung H.H., Grodzinsky A.J., et al. Time-dependent nanomechanics of cartilage. Biophys. J. 2011;100(7):1846–1854. doi: 10.1016/j.bpj.2011.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lin W., Klein J. Recent progress in cartilage lubrication. Adv. Mater. 2021;33(18) doi: 10.1002/adma.202005513. [DOI] [PubMed] [Google Scholar]
- 32.Berthiaume F., Maguire T.J., Yarmush M.L. Tissue engineering and regenerative medicine: history, progress, and challenges. Ann. Rev. Chem. Biomol. Eng. 2011;2:403–430. doi: 10.1146/annurev-chembioeng-061010-114257. [DOI] [PubMed] [Google Scholar]
- 33.AL van Wezel Growth of cell-strains and primary cells on micro-carriers in homogeneous culture. Nature. 1967;216(5110):64–65. doi: 10.1038/216064a0. [DOI] [PubMed] [Google Scholar]
- 34.Meier R.P., Mahou R., Morel P., Meyer J., Montanari E., Muller Y.D., et al. Microencapsulated human mesenchymal stem cells decrease liver fibrosis in mice. J. Hepatol. 2015;62(3):634–641. doi: 10.1016/j.jhep.2014.10.030. [DOI] [PubMed] [Google Scholar]
- 35.Zhu C.C., Yang H.B., Shen L., Zheng Z.Y., Zhao S.C., Li Q.G., et al. Microfluidic preparation of PLGA microspheres as cell carriers with sustainable Rapa release. J. Biomater. Sci. Polym. Ed. 2021;30(9):737–755. doi: 10.1080/09205063.2019.1602930. [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y., Zhang C., Fan Y., Liu Z., Hu F., Zhao Y.S. Smart protein-based biolasers: an alternative way to protein conformation detection. ACS Appl. Mater. Interfaces. 2021;13(16):19187–19192. doi: 10.1021/acsami.0c22270. [DOI] [PubMed] [Google Scholar]
- 37.Yuan Z., Yuan X., Zhao Y., Cai Q., Wang Y., Luo R., et al. Injectable GelMA cryogel microspheres for modularized cell delivery and potential vascularized bone regeneration. Small. 2021;17(11) doi: 10.1002/smll.202006596. [DOI] [PubMed] [Google Scholar]
- 38.Vilabril S., Nadine S., Neves C., Correia C.R., Freire M.G., Coutinho J.A.P., et al. One-step all-aqueous interfacial assembly of robust membranes for long-term encapsulation and culture of adherent stem/stromal cells. Adv Healthc Mater. 2021;10(10) doi: 10.1002/adhm.202100266. [DOI] [PubMed] [Google Scholar]
- 39.Huang L., Xiao L., Jung Poudel A., Li J., Zhou P., Gauthier M., et al. Porous chitosan microspheres as microcarriers for 3D cell culture. Carbohydr. Polym. 2018;202:611–620. doi: 10.1016/j.carbpol.2018.09.021. [DOI] [PubMed] [Google Scholar]
- 40.Li J., Lam A.T., Toh J.P., Reuveny S., Oh S.K., Birch W.R. Tunable volumetric density and porous structure of spherical poly-ε-caprolactone microcarriers, as applied in human mesenchymal stem cell expansion. Langmuir. 2017;33(12):3068–3079. doi: 10.1021/acs.langmuir.7b00125. [DOI] [PubMed] [Google Scholar]
- 41.Park J.S., Hong S.J., Kim H.Y., Yu H.S., Lee Y.I., Kim C.H., et al. Evacuated calcium phosphate spherical microcarriers for bone regeneration. Tissue Eng. 2010;16(5):1681–1691. doi: 10.1089/ten.TEA.2009.0624. [DOI] [PubMed] [Google Scholar]
- 42.Liu J., Yu C., Chen Y.F., Cai H.X., Lin H., Sun Y., et al. Fast fabrication of stable cartilage-like tissue using collagen hydrogel microsphere culture. J. Mater. Chem. B. 2017;5(46):9130–9140. doi: 10.1039/c7tb02535a. [DOI] [PubMed] [Google Scholar]
- 43.Kuang R., Zhang Z., Jin X., Hu J., Gupte M.J., Ni L., et al. Nanofibrous spongy microspheres enhance odontogenic differentiation of human dental pulp stem cells. Adv Healthc Mater. 2015;4(13):1993–2000. doi: 10.1002/adhm.201500308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kankala R.K., Zhao J., Liu C.G., Song X.J., Yang D.Y., Zhu K., et al. Highly porous microcarriers for minimally invasive in situ skeletal muscle cell delivery. Small. 2019;15(25) doi: 10.1002/smll.201901397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tew S., Redman S., Kwan A., Walker E., Khan I., Dowthwaite G., et al. Differences in repair responses between immature and mature cartilage. Clin. Orthop. Relat. Res. 2001;(391 Suppl):S142–S152. doi: 10.1097/00003086-200110001-00014. [DOI] [PubMed] [Google Scholar]
- 46.Service R.F. Tissue engineering. Coming soon to a knee near you: cartilage like your very own. Science. 2008;322(5907):1460–1461. doi: 10.1126/science.322.5907.1460. [DOI] [PubMed] [Google Scholar]
- 47.Rahman R.A., Radzi MAzA., Sukri N.M., Nazir N.M., Sha’ban M. Tissue engineering of articular cartilage: from bench to bed-side. Tissue Eng. Regen. Med. 2015;12(1):1–11. doi: 10.1007/s13770-014-9044-8. [DOI] [Google Scholar]
- 48.Surrao D.C., Khan A.A., McGregor A.J., Amsden B.G., Waldman S.D. Can microcarrier-expanded chondrocytes synthesize cartilaginous tissue in vitro? Tissue Eng. 2011;17(15–16):1959–1967. doi: 10.1089/ten.TEA.2010.0434. [DOI] [PubMed] [Google Scholar]
- 49.Gerecht S., Burdick J.A., Ferreira L.S., Townsend S.A., Langer R., Vunjak-Novakovic G. Hyaluronic acid hydrogel for controlled self-renewal and differentiation of human embryonic stem cells. Proc. Natl. Acad. Sci. U. S. A. 2007;104(27):11298–11303. doi: 10.1073/pnas.0703723104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Moshaverinia A., Xu X., Chen C., Akiyama K., Snead M.L., Shi S. Dental mesenchymal stem cells encapsulated in an alginate hydrogel co-delivery microencapsulation system for cartilage regeneration. Acta Biomater. 2013;9(12):9343–9350. doi: 10.1016/j.actbio.2013.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Chan B.P., Hui T.Y., Yeung C.W., Li J., Mo I., Chan G.C. Self-assembled collagen-human mesenchymal stem cell microspheres for regenerative medicine. Biomaterials. 2007;28(31):4652–4666. doi: 10.1016/j.biomaterials.2007.07.041. [DOI] [PubMed] [Google Scholar]
- 52.Nichol J.W., Koshy S.T., Bae H., Hwang C.M., Yamanlar S., Khademhosseini A. Cell-laden microengineered gelatin methacrylate hydrogels. Biomaterials. 2010;31(21):5536–5544. doi: 10.1016/j.biomaterials.2010.03.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Choi S.W., YC Yeh, Zhang Y., Sung H.W., Xia Y. Uniform beads with controllable pore sizes for biomedical applications. Small. 2010;6(14):1492–1498. doi: 10.1002/smll.201000544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zhou Z.H., Wu W., Fang J.J., Yin J.B. Polymer-based porous microcarriers as cell delivery systems for applications in bone and cartilage tissue engineering. Int. Mater. Rev. 2021;66(2):77–113. doi: 10.1080/09506608.2020.1724705. [DOI] [Google Scholar]
- 55.Fernandes Patrício T.M., Panseri S., Montesi M., Iafisco M., Sandri M., Tampieri A., et al. Superparamagnetic hybrid microspheres affecting osteoblasts behaviour. Mater. Sci. Eng. C Mater. Biol. Appl. 2019;96:234–247. doi: 10.1016/j.msec.2018.11.014. [DOI] [PubMed] [Google Scholar]
- 56.Costantini M., Guzowski J., Żuk P.J., Mozetic P., De Panfilis S., Jaroszewicz J., et al. Electric field assisted microfluidic platform for generation of tailorable porous microbeads as cell carriers for tissue engineering. Adv. Funct. Mater. 2018;28(20):1800874. doi: 10.1002/adfm.201804527. [DOI] [Google Scholar]
- 57.Wang J., Cheng Y., Yu Y., Fu F., Chen Z., Zhao Y., et al. Microfluidic generation of porous microcarriers for three-dimensional cell culture. ACS Appl. Mater. Interfaces. 2015;7(49):27035–27039. doi: 10.1021/acsami.5b10442. [DOI] [PubMed] [Google Scholar]
- 58.Hao N., Nie Y., Zhang J.X. Microfluidic synthesis of functional inorganic micro-/nanoparticles and applications in biomedical engineering. Int. Mater. Rev. 2018;63(8):461–487. doi: 10.1080/09506608.2018.1434452. [DOI] [Google Scholar]
- 59.Nweke C.E., Stegemann J.P. Modular microcarrier technologies for cell-based bone regeneration. J. Mater. Chem. B. 2020;8(18):3972–3984. doi: 10.1039/d0tb00116c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Shang L., Cheng Y., Zhao Y. Emerging droplet microfluidics. Chem. Rev. 2017;117(12):7964–8040. doi: 10.1021/acs.chemrev.6b00848. [DOI] [PubMed] [Google Scholar]
- 61.Tan W.H., Takeuchi S. Monodisperse alginate hydrogel microbeads for cell encapsulation. Adv. Mater. 2007;19(18):2696–2701. doi: 10.1002/adfm.201906690. [DOI] [Google Scholar]
- 62.Nisisako T., Torii T., Takahashi T., Takizawa Y. Synthesis of monodisperse bicolored janus particles with electrical anisotropy using a microfluidic Co‐Flow system. Adv. Mater. 2006;18(9):1152–1156. doi: 10.1002/adma.200502431. [DOI] [Google Scholar]
- 63.Nie Z., Park J.I., Li W., Bon S.A., Kumacheva E. An "inside-out" microfluidic approach to monodisperse emulsions stabilized by solid particles. J. Am. Chem. Soc. 2008;130(49):16508–16509. doi: 10.1021/ja807764m. [DOI] [PubMed] [Google Scholar]
- 64.Yobas L., Martens S., Ong W.L., Ranganathan N. High-performance flow-focusing geometry for spontaneous generation of monodispersed droplets. Lab Chip. 2006;6(8):1073–1079. doi: 10.1039/b602240e. [DOI] [PubMed] [Google Scholar]
- 65.Lee D., Lee K., Cha C. Microfluidics‐assisted fabrication of microtissues with tunable physical properties for developing an in vitro multiplex tissue model. Adv. Biosyst. 2018;2(12):1800236. doi: 10.1002/adbi.201800236. [DOI] [Google Scholar]
- 66.Zhao X., Liu S., Yildirimer L., Zhao H., Ding R., Wang H., et al. Injectable stem cell‐laden photocrosslinkable microspheres fabricated using microfluidics for rapid generation of osteogenic tissue constructs. Adv. Funct. Mater. 2016;26(17):2809–2819. doi: 10.1002/adfm.201504943. [DOI] [Google Scholar]
- 67.Martins C.R., Custódio C.A., Mano J.F. Multifunctional laminarin microparticles for cell adhesion and expansion. Carbohydr. Polym. 2018;202:91–98. doi: 10.1016/j.carbpol.2018.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu W., Li Y., Zeng Y., Zhang X., Wang J., Xie L., et al. Microcryogels as injectable 3-D cellular microniches for site-directed and augmented cell delivery. Acta Biomater. 2014;10(5):1864–1875. doi: 10.1016/j.actbio.2013.12.008. [DOI] [PubMed] [Google Scholar]
- 69.Li X., Zhang X., Zhao S., Wang J., Liu G., Du Y. Micro-scaffold array chip for upgrading cell-based high-throughput drug testing to 3D using benchtop equipment. Lab Chip. 2014;14(3):471–481. doi: 10.1039/c3lc51103k. [DOI] [PubMed] [Google Scholar]
- 70.Xu F., Inci F., Mullick O., Gurkan U.A., Sung Y., Kavaz D., et al. Release of magnetic nanoparticles from cell-encapsulating biodegradable nanobiomaterials. ACS Nano. 2012;6(8):6640–6649. doi: 10.1021/nn300902w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Straub J.A., DE Chickering, Church C.C., Shah B., Hanlon T., Bernstein H. Porous PLGA microparticles: AI-700, an intravenously administered ultrasound contrast agent for use in echocardiography. J. Contr. Release. 2005;108(1):21–32. doi: 10.1016/j.jconrel.2005.07.020. [DOI] [PubMed] [Google Scholar]
- 72.Cai H., Sharma S., Liu W., Mu W., Liu W., Zhang X., et al. Aerogel microspheres from natural cellulose nanofibrils and their application as cell culture scaffold. Biomacromolecules. 2014;15(7):2540–2547. doi: 10.1021/bm5003976. [DOI] [PubMed] [Google Scholar]
- 73.Ding L., Lee T., Wang C.H. Fabrication of monodispersed Taxol-loaded particles using electrohydrodynamic atomization. J. Contr. Release. 2005;102(2):395–413. doi: 10.1016/j.jconrel.2004.10.011. [DOI] [PubMed] [Google Scholar]
- 74.Reyderman L., Stavchansky S. Electrostatic spraying and its use in drug delivery—cholesterol microspheres. Int. J. Pharm. 1995;124(1):75–85. doi: 10.1016/0378-5173(95)00078-W. [DOI] [Google Scholar]
- 75.Xu Y.C., Peng J., Richards G., Lu S.B., Eglin D. Optimization of electrospray fabrication of stem cell-embedded alginate-gelatin microspheres and their assembly in 3D-printed poly(epsilon-caprolactone) scaffold for cartilage tissue engineering. J. Orthop. Trans. 2019;18:128–141. doi: 10.1016/j.jot.2019.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zhang Q., Yang T., Zhang R., Liang X., Wang G., Tian Y., et al. Platelet lysate functionalized gelatin methacrylate microspheres for improving angiogenesis in endodontic regeneration. Acta Biomater. 2021;136:441–455. doi: 10.1016/j.actbio.2021.09.024. [DOI] [PubMed] [Google Scholar]
- 77.Zhang S., Ma B., Wang S., Duan J., Qiu J., Li D., et al. Mass-production of fluorescent chitosan/graphene oxide hybrid microspheres for in vitro 3D expansion of human umbilical cord mesenchymal stem cells. Chem. Eng. J. 2018;331:675–684. doi: 10.1016/j.cej.2017.09.014. [DOI] [Google Scholar]
- 78.Tang G., Chen L., Lian L., Li F., Ravanbakhsh H., Wang M., et al. Designable dual-power micromotors fabricated from a biocompatible gas-shearing strategy. Chem. Eng. J. 2021;407:127187. doi: 10.1016/j.cej.2020.127187. [DOI] [Google Scholar]
- 79.Tang G., Chen L., Wang Z., Gao S., Qu Q., Xiong R., et al. Faithful fabrication of biocompatible multicompartmental memomicrospheres for digitally color-tunable barcoding. Small. 2020;16(24) doi: 10.1002/smll.201907586. [DOI] [PubMed] [Google Scholar]
- 80.Lee J., Oh Y.J., Lee S.K., Lee K.Y. Facile control of porous structures of polymer microspheres using an osmotic agent for pulmonary delivery. J. Contr. Release. 2010;146(1):61–67. doi: 10.1016/j.jconrel.2010.05.026. [DOI] [PubMed] [Google Scholar]
- 81.Huang C.C., Wei H.J., YC Yeh, Wang J.J., Lin W.W., Lee T.Y., et al. Injectable PLGA porous beads cellularized by hAFSCs for cellular cardiomyoplasty. Biomaterials. 2012;33(16):4069–4077. doi: 10.1016/j.biomaterials.2012.02.024. [DOI] [PubMed] [Google Scholar]
- 82.Kim T.K., Yoon J.J., Lee D.S., Park T.G. Gas foamed open porous biodegradable polymeric microspheres. Biomaterials. 2006;27(2):152–159. doi: 10.1016/j.biomaterials.2005.05.081. [DOI] [PubMed] [Google Scholar]
- 83.Wang Y., Kankala R.K., Zhang J., Hao L., Zhu K., Wang S., et al. Modeling endothelialized hepatic tumor microtissues for drug screening. Adv. Sci. 2020;7(21):2002002. doi: 10.1002/advs.202002002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Wang Y., Kankala R.K., Cai Y.Y., Tang H.X., Zhu K., Zhang J.T., et al. Minimally invasive co-injection of modular micro-muscular and micro-vascular tissues improves in situ skeletal muscle regeneration. Biomaterials. 2021;277:121072. doi: 10.1016/j.biomaterials.2021.121072. [DOI] [PubMed] [Google Scholar]
- 85.Zhao Y.H., Zhao X.G., Zhang R., Huang Y., Li Y.J., Shan M.H., et al. Cartilage extracellular matrix scaffold with kartogenin-encapsulated PLGA microspheres for cartilage regeneration. Front. Bioeng. Biotechnol. 2020;8:600103. doi: 10.3389/fbioe.2020.600103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Qu M., Liao X., Jiang N., Sun W., Xiao W., Zhou X., et al. Injectable open-porous PLGA microspheres as cell carriers for cartilage regeneration. J. Biomed. Mater. Res. 2021;109(11) doi: 10.1002/jbm.a.37196. [DOI] [PubMed] [Google Scholar]
- 87.Wang L.J., Che K.K., Liu Y. Pharmacokinetics, distribution and efficacy of triptolide PLGA microspheres after intra-articular injection in a rat rheumatoid arthritis model. Xenobiotica. 2021;51(6):703–715. doi: 10.1080/00498254.2021.1923860. [DOI] [PubMed] [Google Scholar]
- 88.Stefani R.M., Lee A.J., Tan A.R., Halder S.S., Hu Y.Z., Guo X.E., et al. Sustained low-dose dexamethasone delivery via a PLGA microsphere-embedded agarose implant for enhanced osteochondral repair. Acta Biomater. 2020;102:326–340. doi: 10.1016/j.actbio.2019.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Dhanabalan K.M., Gupta V.K., Agarwal R. Rapamycin-PLGA microparticles prevent senescence, sustain cartilage matrix production under stress and exhibit prolonged retention in mouse joints. Biomater. Sci. 2020;8(15):4308–4321. doi: 10.1039/d0bm00596g. [DOI] [PubMed] [Google Scholar]
- 90.Liu G.M., Li Y.L., Yang S.T., Zhao Y.A., Lu T.C., Jia W.Y., et al. DOPA-IGF-1 coated HA/PLGA microspheres promoting proliferation and osteoclastic differentiation of rabbit bone mesenchymal stem cells. Chem. Res. Chin. Univ. 2019;35(3):514–520. doi: 10.1007/s40242-019-9007-7. [DOI] [Google Scholar]
- 91.Sun X.M., Wang J.H., Wang Y.Y., Zhang Q.Q. Collagen-based porous scaffolds containing PLGA microspheres for controlled kartogenin release in cartilage tissue engineering. Artificial Cells Nanomed. Biotechnol. 2018;46(8):1957–1966. doi: 10.1080/21691401.2017.1397000. [DOI] [PubMed] [Google Scholar]
- 92.Goto N., Okazaki K., Akasaki Y., Ishihara K., Murakami K., Koyano K., et al. Single intra-articular injection of fluvastatin-PLGA microspheres reduces cartilage degradation in rabbits with experimental osteoarthritis. J. Orthop. Res. 2017;35(11):2465–2475. doi: 10.1002/jor.23562. [DOI] [PubMed] [Google Scholar]
- 93.Aydin O., Korkusuz F., Korkusuz P., Tezcaner A., Bilgic E., Yaprakci V., et al. In vitro and in vivo evaluation of doxycycline-chondroitin sulfate/PCLmicrospheres for intraarticular treatment of osteoarthritis. J. Biomed. Mater. Res. B Appl. Biomater. 2015;103(6):1238–1248. doi: 10.1002/jbm.b.33303. [DOI] [PubMed] [Google Scholar]
- 94.Filova E., Jakubcova B., Danilova I., Kostakova E.K., Jarosikova T., Chernyavskiy O., et al. Polycaprolactone foam functionalized with chitosan microparticles - a suitable scaffold for cartilage regeneration. Physiol. Res. 2016;65(1):121–131. doi: 10.33549/physiolres.932998. [DOI] [PubMed] [Google Scholar]
- 95.Du Y.Y., Liu H.M., Yang Q., Wang S., Wang J.L., Ma J., et al. Selective laser sintering scaffold with hierarchical architecture and gradient composition for osteochondral repair in rabbits. Biomaterials. 2017;137:37–48. doi: 10.1016/j.biomaterials.2017.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Jin G.Z., Kim H.W. Porous microcarrier-enabled three-dimensional culture of chondrocytes for cartilage engineering: a feasibility study. Tissue Eng. Regen. Med. 2016;13(3):235–241. doi: 10.1007/s13770-016-0038-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Lam A.T., Li J., Toh J.P., Sim E.J., Chen A.K., Chan J.K., et al. Biodegradable poly-ε-caprolactone microcarriers for efficient production of human mesenchymal stromal cells and secreted cytokines in batch and fed-batch bioreactors. Cytotherapy. 2017;19(3):419–432. doi: 10.1016/j.jcyt.2016.11.009. [DOI] [PubMed] [Google Scholar]
- 98.Ghosh P., Gruber S.M.S., Lin C.Y., Whitlock P.W. Microspheres containing decellularized cartilage induce chondrogenesis in vitro and remain functional after incorporation within a poly(caprolactone) filament useful for fabricating a 3D scaffold. Biofabrication. 2018;10(2) doi: 10.1088/1758-5090/aaa637. [DOI] [PubMed] [Google Scholar]
- 99.Gavenis K., Heussen N., Hofman M., Andereya S., Schneider U., Schmidt-Rohlfing B. Cell-free repair of small cartilage defects in the Goettinger minipig: the effects of BMP-7 continuously released by poly(lactic-co-glycolid acid) microspheres. J. Biomater. Appl. 2014;28(7):1008–1015. doi: 10.1177/0885328213491440. [DOI] [PubMed] [Google Scholar]
- 100.Correia C.R., Reis R.L., Mano J.F. Nanostructured capsules for cartilage tissue engineering. Methods Mol. Biol. 2015;1340:181–189. doi: 10.1007/978-1-4939-2938-2_13. [DOI] [PubMed] [Google Scholar]
- 101.Paik J., Duggan S.T., Keam S.J. Triamcinolone acetonide extended-release: a review in osteoarthritis pain of the knee. Drugs. 2019;79(4):455–462. doi: 10.1007/s40265-019-01083-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Zolnik B.S., Burgess D.J. Effect of acidic pH on PLGA microsphere degradation and release. J. Contr. Release. 2007;122(3):338–344. doi: 10.1016/j.jconrel.2007.05.034. [DOI] [PubMed] [Google Scholar]
- 103.Mintz B.R., Cooper J.A., Jr. Hybrid hyaluronic acid hydrogel/poly(ϵ-caprolactone) scaffold provides mechanically favorable platform for cartilage tissue engineering studies. J. Biomed. Mater. Res. 2014;102(9):2918–2926. doi: 10.1002/jbm.a.34957. [DOI] [PubMed] [Google Scholar]
- 104.Schagemann J.C., Chung H.W., Mrosek E.H., Stone J.J., Fitzsimmons J.S., O'Driscoll S.W., et al. Poly-epsilon-caprolactone/gel hybrid scaffolds for cartilage tissue engineering. J. Biomed. Mater. Res. 2010;93(2):454–463. doi: 10.1002/jbm.a.32521. [DOI] [PubMed] [Google Scholar]
- 105.Ko J.Y., Choi Y.J., Jeong G.J., Im G.I. Sulforaphane-PLGA microspheres for the intra-articular treatment of osteoarthritis. Biomaterials. 2013;34(21):5359–5368. doi: 10.1016/j.biomaterials.2013.03.066. [DOI] [PubMed] [Google Scholar]
- 106.Madhavan Nampoothiri K., Nair N.R., John R.P. An overview of the recent developments in polylactide (PLA) research. Bioresour. Technol. 2010;101(22):8493–8501. doi: 10.1016/j.biortech.2010.05.092. [DOI] [PubMed] [Google Scholar]
- 107.Liu X., Jin X., Ma P.X. Nanofibrous hollow microspheres self-assembled from star-shaped polymers as injectable cell carriers for knee repair. Nat. Mater. 2011;10(5):398–406. doi: 10.1038/nmat2999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Narayanan G., Vernekar V.N., EL Kuyinu, Laurencin C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016;107:247–276. doi: 10.1016/j.addr.2016.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Rastogi P., Kandasubramanian B. Review of alginate-based hydrogel bioprinting for application in tissue engineering. Biofabrication. 2019;11(4) doi: 10.1088/1758-5090/ab331e. [DOI] [PubMed] [Google Scholar]
- 110.Leslie S.K., Cohen D.J., Sedlaczek J., Pinsker E.J., Boyan B.D., Schwartz Z. Controlled release of rat adipose-derived stem cells from alginate microbeads. Biomaterials. 2013;34(33):8172–8184. doi: 10.1016/j.biomaterials.2013.07.017. [DOI] [PubMed] [Google Scholar]
- 111.Lee C.S., Nicolini A.M., Watkins E.A., Burnsed O.A., Boyan B.D., Schwartz Z. Adipose stem cell microbeads as production sources for chondrogenic growth factors. J. Stem Cells Regen. Med. 2014;10(2):38–48. doi: 10.46582/jsrm.1002007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Woo E., Park H., Lee K.Y. Shear reversible cell/microsphere aggregate as an injectable for tissue regeneration. Macromol. Biosci. 2014;14(5):740–748. doi: 10.1002/mabi.201300365. [DOI] [PubMed] [Google Scholar]
- 113.Thomas R.G., Unnithan A.R., Moon M.J., Surendran S.P., Batgerel T., Park C.H., et al. Electromagnetic manipulation enabled calcium alginate Janus microsphere for targeted delivery of mesenchymal stem cells. Int. J. Biol. Macromol. 2018;110:465–471. doi: 10.1016/j.ijbiomac.2018.01.003. [DOI] [PubMed] [Google Scholar]
- 114.Ky Lee DJ Mooney. Alginate: properties and biomedical applications. Prog. Polym. Sci. 2012;37(1):106–126. doi: 10.1016/j.progpolymsci.2011.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Ivanovska J., Zehnder T., Lennert P., Sarker B., Boccaccini A.R., Hartmann A., et al. Biofabrication of 3D alginate-based hydrogel for cancer research: comparison of cell spreading, viability, and adhesion characteristics of colorectal HCT116 tumor cells. Tissue Eng. C Methods. 2016;22(7):708–715. doi: 10.1089/ten.TEC.2015.0452. [DOI] [PubMed] [Google Scholar]
- 116.Alsberg E., Anderson K.W., Albeiruti A., Franceschi R.T., Mooney D.J. Cell-interactive alginate hydrogels for bone tissue engineering. J. Dent. Res. 2001;80(11):2025–2029. doi: 10.1177/00220345010800111501. [DOI] [PubMed] [Google Scholar]
- 117.Hashimoto T., Suzuki Y., Tanihara M., Kakimaru Y., Suzuki K. Development of alginate wound dressings linked with hybrid peptides derived from laminin and elastin. Biomaterials. 2004;25(7–8):1407–1414. doi: 10.1016/j.biomaterials.2003.07.004. [DOI] [PubMed] [Google Scholar]
- 118.Antich C., de Vicente J., Jiménez G., Chocarro C., Carrillo E., Montañez E., et al. Bio-inspired hydrogel composed of hyaluronic acid and alginate as a potential bioink for 3D bioprinting of articular cartilage engineering constructs. Acta Biomater. 2020;106:114–123. doi: 10.1016/j.actbio.2020.01.046. [DOI] [PubMed] [Google Scholar]
- 119.Chung C., Mesa J., Randolph M.A., Yaremchuk M., Burdick J.A. Influence of gel properties on neocartilage formation by auricular chondrocytes photoencapsulated in hyaluronic acid networks. J. Biomed. Mater. Res. 2006;77(3):518–525. doi: 10.1002/jbm.a.30660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Barbucci R., Lamponi S., Borzacchiello A., Ambrosio L., Fini M., Torricelli P., et al. Hyaluronic acid hydrogel in the treatment of osteoarthritis. Biomaterials. 2002;23(23):4503–4513. doi: 10.1016/s0142-9612(02)00194-1. [DOI] [PubMed] [Google Scholar]
- 121.Kang J.Y., Chung C.W., Sung J.H., Park B.S., Choi J.Y., Lee S.J., et al. Novel porous matrix of hyaluronic acid for the three-dimensional culture of chondrocytes. Int. J. Pharm. 2009;369(1–2):114–120. doi: 10.1016/j.ijpharm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 122.Choi K.Y., Han H.S., Lee E.S., Shin J.M., Almquist B.D., Lee D.S., et al. Hyaluronic acid-based activatable nanomaterials for stimuli-responsive imaging and therapeutics: beyond CD44-mediated drug delivery. Adv. Mater. 2019;31(34) doi: 10.1002/adma.201803549. [DOI] [PubMed] [Google Scholar]
- 123.Hou K.T., Liu T.Y., Chiang M.Y., Chen C.Y., Chang S.J., Chen S.Y. Cartilage tissue-mimetic pellets with multifunctional magnetic hyaluronic acid-graft-amphiphilic gelatin microcapsules for chondrogenic stimulation. Polymers. 2020;12(4):785. doi: 10.3390/polym12040785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Beck E.C., Barragan M., Libeer T.B., Kieweg S.L., Converse G.L., Hopkins R.A., et al. Chondroinduction from naturally derived cartilage matrix: a comparison between devitalized and decellularized cartilage encapsulated in hydrogel pastes. Tissue Eng. 2016;22(7–8):665–679. doi: 10.1089/ten.tea.2015.0546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Rodell C.B., Kaminski A.L., Burdick J.A. Rational design of network properties in guest-host assembled and shear-thinning hyaluronic acid hydrogels. Biomacromolecules. 2013;14(11):4125–4134. doi: 10.1021/bm401280z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Jeong S.H., Koh Y.H., Kim S.W., Park J.U., He Kim, Song J. Strong and biostable hyaluronic acid-calcium phosphate nanocomposite hydrogel via in situ precipitation process. Biomacromolecules. 2016;17(3):841–851. doi: 10.1021/acs.biomac.5b01557. [DOI] [PubMed] [Google Scholar]
- 127.Seong Y.J., Lin G., Kim B.J., He Kim, Kim S., Jeong S.H. Hyaluronic acid-based hybrid hydrogel microspheres with enhanced structural stability and high injectability. ACS Omega. 2019;4(9):13834–13844. doi: 10.1021/acsomega.9b01475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Yang L., Liu Y., Shou X., Ni D., Kong T., Zhao Y. Bio-inspired lubricant drug delivery particles for the treatment of osteoarthritis. Nanoscale. 2020;12(32):17093–17102. doi: 10.1039/d0nr04013d. [DOI] [PubMed] [Google Scholar]
- 129.Islam M.M., Shahruzzaman M., Biswas S., Sakib M Nurus, Tu Rashid. Chitosan based bioactive materials in tissue engineering applications-A review. Bioact Mater. 2020;5(1):164–183. doi: 10.1016/j.bioactmat.2020.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lahiji A., Sohrabi A., Hungerford D.S., Frondoza C.G. Chitosan supports the expression of extracellular matrix proteins in human osteoblasts and chondrocytes. J. Biomed. Mater. Res. 2000;51(4):586–595. doi: 10.1002/1097-4636(20000915)51:4<586::aid-jbm6>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- 131.Choi B., Kim S., Lin B., Wu B.M., Lee M. Cartilaginous extracellular matrix-modified chitosan hydrogels for cartilage tissue engineering. ACS Appl. Mater. Interfaces. 2014;6(22):20110–20121. doi: 10.1021/am505723k. [DOI] [PubMed] [Google Scholar]
- 132.Oryan A., Sahvieh S. Effectiveness of chitosan scaffold in skin, bone and cartilage healing. Int. J. Biol. Macromol. 2017;104(Pt A):1003–1011. doi: 10.1016/j.ijbiomac.2017.06.124. [DOI] [PubMed] [Google Scholar]
- 133.Man Z., Hu X., Liu Z., Huang H., Meng Q., Zhang X., et al. Transplantation of allogenic chondrocytes with chitosan hydrogel-demineralized bone matrix hybrid scaffold to repair rabbit cartilage injury. Biomaterials. 2016;108:157–167. doi: 10.1016/j.biomaterials.2016.09.002. [DOI] [PubMed] [Google Scholar]
- 134.Bellich B., D'Agostino I., Semeraro S., Gamini A., Cesàro A. The good, the bad and the ugly" of chitosans. Mar. Drugs. 2016;14(5):99. doi: 10.3390/md14050099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Bottegoni C., Muzzarelli R.A., Giovannini F., Busilacchi A., Gigante A. Oral chondroprotection with nutraceuticals made of chondroitin sulphate plus glucosamine sulphate in osteoarthritis. Carbohydr. Polym. 2014;109:126–138. doi: 10.1016/j.carbpol.2014.03.033. [DOI] [PubMed] [Google Scholar]
- 136.Sheehy E.J., Mesallati T., Vinardell T., Kelly D.J. Engineering cartilage or endochondral bone: a comparison of different naturally derived hydrogels. Acta Biomater. 2015;13:245–253. doi: 10.1016/j.actbio.2014.11.031. [DOI] [PubMed] [Google Scholar]
- 137.Costa-Pinto A.R., Reis R.L., Neves N.M. Scaffolds based bone tissue engineering: the role of chitosan. Tissue Eng. B Rev. 2011;17(5):331–347. doi: 10.1089/ten.teb.2010.0704. [DOI] [PubMed] [Google Scholar]
- 138.Ma M., He W., Liu X., Zheng Y., Peng J., Xie Y., et al. Soybean protein isolate/chitosan composite microcarriers for expansion and osteogenic differentiation of stem cells. Compos. B Eng. 2021;230:109533. doi: 10.1016/j.compositesb.2021.109533. [DOI] [Google Scholar]
- 139.Reed S., Wu B.M. Biological and mechanical characterization of chitosan-alginate scaffolds for growth factor delivery and chondrogenesis. J. Biomed. Mater. Res. B Appl. Biomater. 2017;105(2):272–282. doi: 10.1002/jbm.b.33544. [DOI] [PubMed] [Google Scholar]
- 140.Sadeghianmaryan A., Naghieh S., Alizadeh Sardroud H., Yazdanpanah Z., Soltani Y Afzal, Sernaglia J., et al. Extrusion-based printing of chitosan scaffolds and their in vitro characterization for cartilage tissue engineering. Int. J. Biol. Macromol. 2020;164:3179–3192. doi: 10.1016/j.ijbiomac.2020.08.180. [DOI] [PubMed] [Google Scholar]
- 141.Huang H., Zhang X., Hu X., Dai L., Zhu J., Man Z., et al. Directing chondrogenic differentiation of mesenchymal stem cells with a solid-supported chitosan thermogel for cartilage tissue engineering. Biomed. Mater. 2014;9(3) doi: 10.1088/1748-6041/9/3/035008. [DOI] [PubMed] [Google Scholar]
- 142.Lu G., Zhu L., Kong L., Zhang L., Gong Y., Zhao N., et al. Porous chitosan microcarriers for large scale cultivation of cells for tissue engineering: fabrication and evaluation. Tsinghua Sci. Technol. 2006;11(4):427–432. doi: 10.1016/S1007-0214(06)70212-7. [DOI] [Google Scholar]
- 143.Nguyen L.H., Kudva A.K., Guckert N.L., Linse K.D., Roy K. Unique biomaterial compositions direct bone marrow stem cells into specific chondrocytic phenotypes corresponding to the various zones of articular cartilage. Biomaterials. 2011;32(5):1327–1338. doi: 10.1016/j.biomaterials.2010.10.009. [DOI] [PubMed] [Google Scholar]
- 144.Fang J., Zhang Y., Yan S., Liu Z., He S., Cui L., et al. Poly(L-glutamic acid)/chitosan polyelectrolyte complex porous microspheres as cell microcarriers for cartilage regeneration. Acta Biomater. 2014;10(1):276–288. doi: 10.1016/j.actbio.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 145.Lin L., Wang Y.F., Wang L., Pan J.Y., Xu Y.C., Li S.Y., et al. Injectable microfluidic hydrogel microspheres based on chitosan and poly(ethylene glycol) diacrylate (PEGDA) as chondrocyte carriers. RSC Adv. 2020;10(65):39662–39672. doi: 10.1039/d0ra07318k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Shoueir K.R., El-Desouky N., Rashad M.M., Ahmed M.K., Janowska I., El-Kemary M. Chitosan based-nanoparticles and nanocapsules: overview, physicochemical features, applications of a nanofibrous scaffold, and bioprinting. Int. J. Biol. Macromol. 2021;167:1176–1197. doi: 10.1016/j.ijbiomac.2020.11.072. [DOI] [PubMed] [Google Scholar]
- 147.Kim I.Y., Seo S.J., Moon H.S., Yoo M.K., Park I.Y., Kim B.C., et al. Chitosan and its derivatives for tissue engineering applications. Biotechnol. Adv. 2008;26(1):1–21. doi: 10.1016/j.biotechadv.2007.07.009. [DOI] [PubMed] [Google Scholar]
- 148.Ahsan S.M., Thomas M., Reddy K.K., Sooraparaju S.G., Asthana A., Bhatnagar I. Chitosan as biomaterial in drug delivery and tissue engineering. Int. J. Biol. Macromol. 2018;110:97–109. doi: 10.1016/j.ijbiomac.2017.08.140. [DOI] [PubMed] [Google Scholar]
- 149.Waibel K.H., Haney B., Moore M., Whisman B., Gomez R. Safety of chitosan bandages in shellfish allergic patients. Mil. Med. 2011;176(10):1153–1156. doi: 10.7205/milmed-d-11-00150. [DOI] [PubMed] [Google Scholar]
- 150.Ak Brun-Graeppi C Richard, Bessodes M., Scherman D., Merten O.W. Cell microcarriers and microcapsules of stimuli-responsive polymers. J. Contr. Release. 2011;149(3):209–224. doi: 10.1016/j.jconrel.2010.09.023. [DOI] [PubMed] [Google Scholar]
- 151.MA Salati, Khazai J., Tahmuri A.M., Samadi A., Taghizadeh A., Taghizadeh M., et al. Agarose-based biomaterials: opportunities and challenges in cartilage tissue engineering. Polymers. 2020;12(5) doi: 10.3390/polym12051150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Zarrintaj P., Manouchehri S., Ahmadi Z., Saeb M.R., Urbanska A.M., Kaplan D.L., et al. Agarose-based biomaterials for tissue engineering. Carbohydr. Polym. 2018;187:66–84. doi: 10.1016/j.carbpol.2018.01.060. [DOI] [PubMed] [Google Scholar]
- 153.MA Szychlinska, D'Amora U., Ravalli S., Ambrosio L., Di Rosa M., Musumeci G. Functional biomolecule delivery systems and bioengineering in cartilage regeneration. Curr. Pharmaceut. Biotechnol. 2019;20(1):32–46. doi: 10.2174/1389201020666190206202048. [DOI] [PubMed] [Google Scholar]
- 154.Sakai S., Hashimoto I., Kawakami K. Production of cell-enclosing hollow-core agarose microcapsules via jetting in water-immiscible liquid paraffin and formation of embryoid body-like spherical tissues from mouse ES cells enclosed within these microcapsules. Biotechnol. Bioeng. 2008;99(1):235–243. doi: 10.1002/bit.21624. [DOI] [PubMed] [Google Scholar]
- 155.Suzawa Y., Funaki T., Watanabe J., Iwai S., Yura Y., Nakano T., et al. Regenerative behavior of biomineral/agarose composite gels as bone grafting materials in rat cranial defects. J. Biomed. Mater. Res. 2010;93(3):965–975. doi: 10.1002/jbm.a.32518. [DOI] [PubMed] [Google Scholar]
- 156.Hasan M.L., Padalhin A.R., Kim B., Lee B.T. Preparation and evaluation of BCP-CSD-agarose composite microsphere for bone tissue engineering. J. Biomed. Mater. Res. B Appl. Biomater. 2019;107(7):2263–2272. doi: 10.1002/jbm.b.34318. [DOI] [PubMed] [Google Scholar]
- 157.Zhao L., Huang Y., Zhu K., Miao Z., Zhao J., Che X.J., et al. Manipulation of pore structure during manufacture of agarose microspheres for bioseparation. Eng. Life Sci. 2020;20(11):504–513. doi: 10.1002/elsc.202000023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Cigan A.D., Roach B.L., Nims R.J., Tan A.R., Albro M.B., Stoker A.M., et al. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J. Biomech. 2016;49(9):1909–1917. doi: 10.1016/j.jbiomech.2016.04.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Kock L.M., Geraedts J., Ito K., van Donkelaar C.C. Low agarose concentration and TGF-β3 distribute extracellular matrix in tissue-engineered cartilage. Tissue Eng. 2013;19(13–14):1621–1631. doi: 10.1089/ten.TEA.2012.0541. [DOI] [PubMed] [Google Scholar]
- 160.Chinta M.L., Velidandi A., Pabbathi N.P.P., Dahariya S., Parcha S.R. Assessment of properties, applications and limitations of scaffolds based on cellulose and its derivatives for cartilage tissue engineering: a review. Int. J. Biol. Macromol. 2021;175:495–515. doi: 10.1016/j.ijbiomac.2021.01.196. [DOI] [PubMed] [Google Scholar]
- 161.Gorgieva S., Trček J. Bacterial cellulose: production, modification and perspectives in biomedical applications. Nanomaterials. 2019;9(10):1352. doi: 10.3390/nano9101352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Czaja W.K., Young D.J., Kawecki M., Brown R.M., Jr. The future prospects of microbial cellulose in biomedical applications. Biomacromolecules. 2007;8(1):1–12. doi: 10.1021/bm060620d. [DOI] [PubMed] [Google Scholar]
- 163.Nimeskern L., Ávila H Martínez, Sundberg J., Gatenholm P., Müller R., Stok K.S. Mechanical evaluation of bacterial nanocellulose as an implant material for ear cartilage replacement. J. Mech. Behav. Biomed. Mater. 2013;22:12–21. doi: 10.1016/j.jmbbm.2013.03.005. [DOI] [PubMed] [Google Scholar]
- 164.Yin N., Stilwell M.D., Santos T.M.A., Wang H.P., Weibel D.B. Agarose particle-templated porous bacterial cellulose and its application in cartilage growth in vitro. Acta Biomater. 2015;12:129–138. doi: 10.1016/j.actbio.2014.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Rk Lau AC Kwok, Chan W.K., Zhang T.Y., Wong J.T. Mechanical characterization of cellulosic thecal plates in dinoflagellates by nanoindentation. J. Nanosci. Nanotechnol. 2007;7(2):452–457. doi: 10.1166/JNN.2007.18041. [DOI] [PubMed] [Google Scholar]
- 166.Wang Y., Yuan X., Yu K., Meng H., Zheng Y., Peng J., et al. Fabrication of nanofibrous microcarriers mimicking extracellular matrix for functional microtissue formation and cartilage regeneration. Biomaterials. 2018;171:118–132. doi: 10.1016/j.biomaterials.2018.04.033. [DOI] [PubMed] [Google Scholar]
- 167.Zhang Z.P., Gupte M.J., Jin X.B., Ma P.X. Injectable peptide decorated functional nanofibrous hollow microspheres to direct stem cell differentiation and tissue regeneration. Adv. Funct. Mater. 2015;25(3):350–360. doi: 10.1002/adfm.201402618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Yeung P., Cheng K.H., Yan C.H., Chan B.P. Collagen microsphere based 3D culture system for human osteoarthritis chondrocytes (hOACs) Sci. Rep. 2019;9(1):12453. doi: 10.1038/s41598-019-47946-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Yu C., Liu J., Lu G.G., Xie Y.X., Sun Y., Wang Q.G., et al. Repair of osteochondral defects in a rabbit model with artificial cartilage particulates derived from cultured collagen-chondrocyte microspheres. J. Mater. Chem. B. 2018;6(31):5164–5173. doi: 10.1039/c8tb01185k. [DOI] [PubMed] [Google Scholar]
- 170.Wong C.C., Chen C.H., Chiu L.H., Tsuang Y.H., Bai M.Y., Chung R.J., et al. Facilitating in vivo articular cartilage repair by tissue-engineered cartilage grafts produced from auricular chondrocytes. Am. J. Sports Med. 2018;46(3):713–727. doi: 10.1177/0363546517741306. [DOI] [PubMed] [Google Scholar]
- 171.Hong Y., Gong Y., Gao C., Shen J. Collagen-coated polylactide microcarriers/chitosan hydrogel composite: injectable scaffold for cartilage regeneration. J. Biomed. Mater. Res. 2008;85(3):628–637. doi: 10.1002/jbm.a.31603. [DOI] [PubMed] [Google Scholar]
- 172.Irawan V., Sung T.C., Higuchi A., Ikoma T. Collagen scaffolds in cartilage tissue engineering and relevant approaches for future development. Tissue Eng. Regen. Med. 2018;15(6):673–697. doi: 10.1007/s13770-018-0135-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Han L., Xu J., Lu X., Gan D., Wang Z., Wang K., et al. Biohybrid methacrylated gelatin/polyacrylamide hydrogels for cartilage repair. J. Mater. Chem. B. 2017;5(4):731–741. doi: 10.1039/c6tb02348g. [DOI] [PubMed] [Google Scholar]
- 174.Song K., Li L., Li W., Zhu Y., Jiao Z., Lim M., et al. Three-dimensional dynamic fabrication of engineered cartilage based on chitosan/gelatin hybrid hydrogel scaffold in a spinner flask with a special designed steel frame. Mater. Sci. Eng. C Mater. Biol. Appl. 2015;55:384–392. doi: 10.1016/j.msec.2015.05.062. [DOI] [PubMed] [Google Scholar]
- 175.Sulaiman S.B., Idrus R.B.H., Hwei N.M. Gelatin microsphere for cartilage tissue engineering: current and future strategies. Polymers. 2020;12(10):2404. doi: 10.3390/polym12102404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Ng E.X., Wang M., Neo S.H., Tee C.A., Chen C.H., Van Vliet K.J. Dissolvable gelatin-based microcarriers generated through droplet microfluidics for expansion and culture of mesenchymal stromal cells. Biotechnol. J. 2021;16(3) doi: 10.1002/biot.202000048. [DOI] [PubMed] [Google Scholar]
- 177.Sulaiman S., Chowdhury S.R., Fauzi M.B., Rani R.A., Yahaya N.H.M., Tabata Y., et al. 3D culture of MSCs on a gelatin microsphere in a dynamic culture system enhances chondrogenesis. Int. J. Mol. Sci. 2020;21(8) doi: 10.3390/ijms21082688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Lu Y., Zhang G., Sun D., Zhong Y. Preparation and evaluation of biodegradable flubiprofen gelatin micro-spheres for intra-articular administration. J. Microencapsul. 2007;24(6):515–524. doi: 10.1080/02652040701433479. [DOI] [PubMed] [Google Scholar]
- 179.Jiang J., Liu A.M., Chen C.L., Tang J.J., Fan H.J., Sun J., et al. An efficient two-step preparation of photocrosslinked gelatin microspheres as cell carriers to support MC3T3-E1 cells osteogenic performance. Colloids Surf. B Biointerfaces. 2020;188:110798. doi: 10.1016/j.colsurfb.2020.110798. [DOI] [PubMed] [Google Scholar]
- 180.Zhao Z., Li G., Ruan H., Chen K., Cai Z., Lu G., et al. Capturing magnesium ions via microfluidic hydrogel microspheres for promoting cancellous bone regeneration. ACS Nano. 2021;15(8):13041–13054. doi: 10.1021/acsnano.1c02147. [DOI] [PubMed] [Google Scholar]
- 181.Han Y., Yang J., Zhao W., Wang H., Sun Y., Chen Y., et al. Biomimetic injectable hydrogel microspheres with enhanced lubrication and controllable drug release for the treatment of osteoarthritis. Bioact Mater. 2021;6(10):3596–3607. doi: 10.1016/j.bioactmat.2021.03.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Bian J., Cai F., Chen H., Tang Z., Xi K., Tang J., et al. Modulation of local overactive inflammation via injectable hydrogel microspheres. Nano Lett. 2021;21(6):2690–2698. doi: 10.1021/acs.nanolett.0c04713. [DOI] [PubMed] [Google Scholar]
- 183.Yang J., Zhu Y., Wang F., Deng L., Xu X., Cui W. Microfluidic liposomes-anchored microgels as extended delivery platform for treatment of osteoarthritis. Chem. Eng. J. 2020;400:126004. doi: 10.1016/j.cej.2020.126004. [DOI] [Google Scholar]
- 184.Wang L.S., Du C., Toh W.S., Wan A.C., Gao S.J., Kurisawa M. Modulation of chondrocyte functions and stiffness-dependent cartilage repair using an injectable enzymatically crosslinked hydrogel with tunable mechanical properties. Biomaterials. 2014;35(7):2207–2217. doi: 10.1016/j.biomaterials.2013.11.070. [DOI] [PubMed] [Google Scholar]
- 185.Zhou Y., Liang K., Zhao S., Zhang C., Li J., Yang H., et al. Photopolymerized maleilated chitosan/methacrylated silk fibroin micro/nanocomposite hydrogels as potential scaffolds for cartilage tissue engineering. Int. J. Biol. Macromol. 2018;108:383–390. doi: 10.1016/j.ijbiomac.2017.12.032. [DOI] [PubMed] [Google Scholar]
- 186.Verma D., Gulati N., Kaul S., Mukherjee S., Nagaich U. Protein based nanostructures for drug delivery. J. Pharm. (Lahore) 2018:9285854. doi: 10.1155/2018/9285854. 2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Rockwood D.N., Preda R.C., Yücel T., Wang X., Ml Lovett DL Kaplan. Materials fabrication from Bombyx mori silk fibroin. Nat. Protoc. 2011;6(10):1612–1631. doi: 10.1038/nprot.2011.379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Qu J., Wang L., Hu Y., Wang L., You R., Li M. Vol. 4. 2013. pp. 84–90. (Preparation of silk fibroin microspheres and its cytocompatibility). 1. [DOI] [Google Scholar]
- 189.Fang J., Wang D., Hu F., Li X., Zou X., Xie J., et al. Strontium mineralized silk fibroin porous microcarriers with enhanced osteogenesis as injectable bone tissue engineering vehicles. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;128:112354. doi: 10.1016/j.msec.2021.112354. [DOI] [PubMed] [Google Scholar]
- 190.Perteghella S., Martella E., de Girolamo L., Orfei C Perucca, Pierini M., Fumagalli V., et al. Fabrication of innovative silk/alginate microcarriers for mesenchymal stem cell delivery and tissue regeneration. Int. J. Mol. Sci. 2017;18(9):1829. doi: 10.3390/ijms18091829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Orfei C Perucca, Talò G., Viganò M., Perteghella S., Lugano G., Fontana F Fabro, et al. Silk/fibroin microcarriers for mesenchymal stem cell delivery: optimization of cell seeding by the design of experiment. Pharmaceutics. 2018;10(4):200. doi: 10.3390/pharmaceutics10040200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Duchi S., Piccinini F., Pierini M., Bevilacqua A., Torre M.L., Lucarelli E., et al. A new holistic 3D non-invasive analysis of cellular distribution and motility on fibroin-alginate microcarriers using light sheet fluorescent microscopy. PLoS One. 2017;12(8) doi: 10.1371/journal.pone.0183336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Chung T.-W., Chang C.-H., Ho C.-W. Incorporating chitosan (CS) and TPP into silk fibroin (SF) in fabricating spray-dried microparticles prolongs the release of a hydrophilic drug. J. Taiwan Inst. Chem. Eng. 2011;42(4):592–597. doi: 10.1016/j.jtice.2010.11.003. [DOI] [Google Scholar]
- 194.Yang M.C., Wang S.S., Chou N.K., Chi N.H., Huang Y.Y., Chang Y.L., et al. The cardiomyogenic differentiation of rat mesenchymal stem cells on silk fibroin-polysaccharide cardiac patches in vitro. Biomaterials. 2009;30(22):3757–3765. doi: 10.1016/j.biomaterials.2009.03.057. [DOI] [PubMed] [Google Scholar]
- 195.Veiga A., Castro F., Rocha F., Oliveira A. Silk-based microcarriers: current developments and future perspectives. IET Nanobiotechnol. 2020;14(8):645–653. doi: 10.1049/iet-nbt.2020.0058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Levato R., Planell J.A., Mateos-Timoneda M.A., Engel E. Role of ECM/peptide coatings on SDF-1 alpha triggered mesenchymal stromal cell migration from microcarriers for cell therapy. Acta Biomater. 2015;18:59–67. doi: 10.1016/j.actbio.2015.02.008. [DOI] [PubMed] [Google Scholar]
- 197.Yin H., Wang Y., Sun Z., Sun X., Xu Y., Li P., et al. Induction of mesenchymal stem cell chondrogenic differentiation and functional cartilage microtissue formation for in vivo cartilage regeneration by cartilage extracellular matrix-derived particles. Acta Biomater. 2016;33:96–109. doi: 10.1016/j.actbio.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 198.Brown B.N., Badylak S.F. Extracellular matrix as an inductive scaffold for functional tissue reconstruction. Transl. Res. 2014;163(4):268–285. doi: 10.1016/j.trsl.2013.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Sivandzade F., Mashayekhan S. Design and fabrication of injectable microcarriers composed of acellular cartilage matrix and chitosan. J. Biomater. Sci. Polym. Ed. 2018;29(6):683–700. doi: 10.1080/09205063.2018.1433422. [DOI] [PubMed] [Google Scholar]
- 200.Liu J., Lu Y., Xing F., Liang J., Wang Q.G., Fan Y.J., et al. Cell-free scaffolds functionalized with bionic cartilage acellular matrix microspheres to enhance the microfracture treatment of articular cartilage defects. J. Mater. Chem. B. 2021;9(6):1686–1697. doi: 10.1039/d0tb02616f. [DOI] [PubMed] [Google Scholar]
- 201.Seetharaman S., Etienne-Manneville S. Cytoskeletal crosstalk in cell migration. Trends Cell Biol. 2020;30(9):720–735. doi: 10.1016/j.tcb.2020.06.004. [DOI] [PubMed] [Google Scholar]
- 202.Wilson C.J., Clegg R.E., DI Leavesley, Pearcy M.J. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1–2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 203.Ruoslahti E. Arg-Gly-Asp: a versatile cell recognition signal. Cell. 1986;144:517–518. doi: 10.1016/0092-8674(86)90259-X. [DOI] [PubMed] [Google Scholar]
- 204.Lu G., Sheng B., Wei Y., Wang G., Zhang L., Ao Q., et al. Collagen nanofiber-covered porous biodegradable carboxymethyl chitosan microcarriers for tissue engineering cartilage. Eur. Polym. J. 2008;44(9):2820–2829. [Google Scholar]
- 205.M Suarez Muñoz D Confalonieri, Walles H., van Dongen E., Dandekar G. Recombinant collagen I peptide microcarriers for cell expansion and their potential use as cell delivery system in a bioreactor model. JoVE. 2018;132 doi: 10.3791/57363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Kudva A.K., Dikina A.D., Luyten F.P., Alsberg E., Patterson J. Gelatin microspheres releasing transforming growth factor drive in vitro chondrogenesis of human periosteum derived cells in micromass culture. Acta Biomater. 2019;90:287–299. doi: 10.1016/j.actbio.2019.03.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Kulchar R.J., Denzer B.R., Chavre B.M., Takegami M., Patterson J. A review of the use of microparticles for cartilage tissue engineering. Int. J. Mol. Sci. 2021;22(19):10292. doi: 10.3390/ijms221910292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Shi X., Cui L., Sun H., Jiang N., Heng L., Zhuang X., et al. Promoting cell growth on porous PLA microspheres through simple degradation methods. Polym. Degrad. Stabil. 2019;161:319–325. doi: 10.1016/j.polymdegradstab.2019.01.003. [DOI] [Google Scholar]
- 209.Derakhti S., Safiabadi-Tali S.H., Amoabediny G., Sheikhpour M. Attachment and detachment strategies in microcarrier-based cell culture technology: a comprehensive review. Mater. Sci. Eng. C. 2019;103:109782. doi: 10.1016/j.msec.2019.109782. [DOI] [PubMed] [Google Scholar]
- 210.Guo S., Zhu X., Li M., Shi L., Ong J.L., Jańczewski D., et al. Parallel control over surface charge and wettability using polyelectrolyte architecture: effect on protein adsorption and cell adhesion. ACS Appl. Mater. Interfaces. 2016;8(44):30552–30563. doi: 10.1021/acsami.6b09481. [DOI] [PubMed] [Google Scholar]
- 211.Bodiou V., Moutsatsou P., Post M.J. Microcarriers for upscaling cultured meat production. Front Nutr. 2020;7:10. doi: 10.3389/fnut.2020.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Lin S.J., Chan Y.C., Su Z.C., Yeh W.L., Lai P.L., Chu I.M. Growth factor-loaded microspheres in mPEG-polypeptide hydrogel system for articular cartilage repair. J. Biomed. Mater. Res. 2021;109(12):2516–2526. doi: 10.1002/jbm.a.37246. [DOI] [PubMed] [Google Scholar]
- 213.Rudnik-Jansen I., Schrijver K., Woike N., Tellegen A., Versteeg S., Emans P., et al. Intra-articular injection of triamcinolone acetonide releasing biomaterial microspheres inhibits pain and inflammation in an acute arthritis model. Drug Deliv. 2019;26(1):226–236. doi: 10.1080/10717544.2019.1568625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Liu P.J., Gu L.L., Ren L.Y., Chen J.J., Li T., Wang X., et al. Intra-articular injection of etoricoxib-loaded PLGA-PEG-PLGA triblock copolymeric nanoparticles attenuates osteoarthritis progression. Am. J. Tourism Res. 2019;11(11):6775–6789. [PMC free article] [PubMed] [Google Scholar]
- 215.Chen H.L., He D.W., Zhu Y., Yu W.Y., Ramalingam M., Wu Z.W. In situ osteochondral regeneration by controlled release of stromal cell-derived factor-1 chemokine from injectable biomaterials: a preclinical evaluation in animal model. J. Biomater. Tissue Eng. 2019;9(7):958–967. doi: 10.1166/jbt.2019.2096. [DOI] [Google Scholar]
- 216.Zhang X.P., Shi Y., Zhang Z.Y., Yang Z.L., Huang G.H. Intra-articular delivery of tetramethylpyrazine microspheres with enhanced articular cavity retention for treating osteoarthritis. Asian J. Pharm. Sci. 2018;13(3):229–238. doi: 10.1016/j.ajps.2017.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 217.Sun Q., Zhang L., Xu T., Ying J., Xia B., Jing H., et al. Combined use of adipose derived stem cells and TGF-3 microspheres promotes articular cartilage regeneration in vivo. Biotech. Histochem. 2018;93(3):168–176. doi: 10.1080/10520295.2017.1401663. [DOI] [PubMed] [Google Scholar]
- 218.Bodick N., Williamson T., Strand V., Senter B., Kelley S., Boyce R., et al. Local effects following single and repeat intra-articular injections of triamcinolone acetonide extended-release: results from three nonclinical toxicity studies in dogs. Rheumatol. Therapy. 2018;5(2):475–498. doi: 10.1007/s40744-018-0125-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Vayas R., Reyes R., Rodriguez-Evora M., del Rosario C., Delgado A., Evora C. Evaluation of the effectiveness of a bMSC and BMP-2 polymeric trilayer system in cartilage repair. Biomed. Mater. 2017;12(4) doi: 10.1088/1748-605X/aa6f1c. [DOI] [PubMed] [Google Scholar]
- 220.Gui X.E., Li Q.Q., Yu J.T., Jiang Z.H. Injectable controlled release of SDF-1-loaded microspheres reduces cartilage degradation in a rabbit experimental osteoarthritis model. J. Biomater. Tissue Eng. 2017;7(11):1177–1183. doi: 10.1166/jbt.2017.1671. [DOI] [Google Scholar]
- 221.Wang J.H., Yang Q., Cheng N.M., Tao X.J., Zhang Z.H., Sun X.M., et al. Collagen/silk fibroin composite scaffold incorporated with PLGA microsphere for cartilage repair. Mater. Sci. Eng. C Mater. Biol. Appl. 2016;61:705–711. doi: 10.1016/j.msec.2015.12.097. [DOI] [PubMed] [Google Scholar]
- 222.Morille M., Toupet K., Montero-Menei C.N., Jorgensen C., Noel D. PLGA-based microcarriers induce mesenchymal stem cell chondrogenesis and stimulate cartilage repair in osteoarthritis. Biomaterials. 2016;88:60–69. doi: 10.1016/j.biomaterials.2016.02.022. [DOI] [PubMed] [Google Scholar]
- 223.Kim C., Jeon O.H., Kim D.H., Chae J.J., Shores L., Bernstein N., et al. Local delivery of a carbohydrate analog for reducing arthritic inflammation and rebuilding cartilage. Biomaterials. 2016;83:93–101. doi: 10.1016/j.biomaterials.2015.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Kumar A., Bendele A.M., Blanks R.C., Bodick N. Sustained efficacy of a single intra-articular dose of FX006 in a rat model of repeated localized knee arthritis. Osteoarthritis Cartilage. 2015;23(1):151–160. doi: 10.1016/j.joca.2014.09.019. [DOI] [PubMed] [Google Scholar]
- 225.Lam A., Reuveny S., Li J., Hui J., Birch W., Oh S. Human early mesenchymal stromal cells delivered on biodegradable polycaprolactone based microcarriers result in improved cartilage formation. Cytotherapy. 2020;22(5):S97. doi: 10.1016/j.jcyt.2020.03.169. [DOI] [Google Scholar]
- 226.Filova E., Tonar Z., Lukasova V., Buzgo M., Litvinec A., Rampichova M., et al. Hydrogel containing anti-CD44-labeled microparticles, guide bone tissue formation in osteochondral defects in rabbits. Nanomaterials. 2020;10(8):1504. doi: 10.3390/nano10081504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Li T., Liu B.Z., Jiang Y.H., Lou Y.Y., Chen K., Zhang D. L-polylactic acid porous microspheres enhance the mechanical properties and in vivo stability of degummed silk/silk fibroin/gelatin scaffold. Biomed. Mater. 2021;16(1) doi: 10.1088/1748-605X/abca11. [DOI] [PubMed] [Google Scholar]
- 228.Salgado C., Guenee L., Cerny R., Allemann E., Jordan O. Nano wet milled celecoxib extended release microparticles for local management of chronic inflammation. Int. J. Pharm. 2020;589:119783. doi: 10.1016/j.ijpharm.2020.119783. [DOI] [PubMed] [Google Scholar]
- 229.Abou-ElNour M., Soliman M.E., Skouras A., Casettari L., Geneidi A.S., Ishak R.A.H. Microparticles-in-Thermoresponsive/Bioadhesive hydrogels as a novel integrated platform for effective intra-articular delivery of triamcinolone acetonide. Mol. Pharm. 2020;17(6):1963–1978. doi: 10.1021/acs.molpharmaceut.0c00126. [DOI] [PubMed] [Google Scholar]
- 230.Liu R., Chen Y., Liu L.L., Gong Y., Wang M.B., Li S.J., et al. Long-term delivery of rhIGF-1 from biodegradable poly(lactic acid)/hydroxyapatite@Eudragit double-layer microspheres for prevention of bone loss and articular degeneration in C57BL/6 mice. J. Mater. Chem. B. 2018;6(19):3085–3095. doi: 10.1039/c8tb00324f. [DOI] [PubMed] [Google Scholar]
- 231.Zeng Y., Li X.K., Liu X., Yang Y.Z., Zhou Z.M., Fan J.C., et al. PLLA porous microsphere-reinforced silk-based scaffolds for auricular cartilage regeneration. ACS Omega. 2021;6(4):3372–3383. doi: 10.1021/acsomega.0c05890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 232.Han L., Xu N.W., Lv S.W., Yin J.J., Zheng D., Li X. Enhanced in vitro and in vivo efficacy of alginate/silk protein/hyaluronic acid with polypeptide microsphere delivery for tissue regeneration of articular cartilage. J. Biomed. Nanotechnol. 2021;17(5):901–909. doi: 10.1166/jbn.2021.3071. [DOI] [PubMed] [Google Scholar]
- 233.Khatab S., Leijs M.J., van Buul G., Haeck J., Kops N., Nieboer M., et al. MSC encapsulation in alginate microcapsules prolongs survival after intra-articular injection, a longitudinal in vivo cell and bead integrity tracking study. Cell Biol. Toxicol. 2020;36(6):553–570. doi: 10.1007/s10565-020-09532-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Bozkurt M., Asik M.D., Gursoy S., Turk M., Karahan S., Gumuskaya B., et al. Autologous stem cell-derived chondrocyte implantation with bio-targeted microspheres for the treatment of osteochondral defects. J. Orthop. Surg. Res. 2019;14(1):394. doi: 10.1186/s13018-019-1434-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Leslie S.K., Cohen D.J., Hyzy S.L., Dosier C.R., Nicolini A., Sedlaczek J., et al. Microencapsulated rabbit adipose stem cells initiate tissue regeneration in a rabbit ear defect model. J. Tissue Eng. Regen. Med. 2018;12(7):1742–1753. doi: 10.1002/term.2702. [DOI] [PubMed] [Google Scholar]
- 236.Kim B.J., Arai Y., Choi B., Park S., Ahn J., Han I.B., et al. Restoration of articular osteochondral defects in rat by a bi-layered hyaluronic acid hydrogel plug with TUDCA-PLGA microsphere. J. Ind. Eng. Chem. 2018;61:295–303. doi: 10.1016/j.jiec.2017.12.027. [DOI] [Google Scholar]
- 237.Choi S., Kim J.H., Ha J., Jeong B.I., Jung Y.C., Lee G.S., et al. Intra-articular injection of alginate-microencapsulated adipose tissue-derived mesenchymal stem cells for the treatment of osteoarthritis in rabbits. Stem Cell. Int. 2018;2018:2791632. doi: 10.1155/2018/2791632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Tsukuda Y., Onodera T., Ito M., Izumisawa Y., Kasahara Y., Igarashi T., et al. Therapeutic effects of intra-articular ultra-purified low endotoxin alginate administration on an experimental canine osteoarthritis model. J. Biomed. Mater. Res. 2015;103(11):3441–3448. doi: 10.1002/jbm.a.35490. [DOI] [PubMed] [Google Scholar]
- 239.Wu H.C., Shen L., Zhu Z., Luo X.S., Zhai Y.S., Hua X.B., et al. A cell-free therapy for articular cartilage repair based on synergistic delivery of SDF-1 & KGN with HA injectable scaffold. Chem. Eng. J. 2020;393:124649. doi: 10.1016/j.cej.2020.124649. [DOI] [Google Scholar]
- 240.Wang J.H., Wang Y.Y., Sun X.M., Liu D.H., Huang C.G., Wu J.L., et al. Biomimetic cartilage scaffold with orientated porous structure of two factors for cartilage repair of knee osteoarthritis. Artificial Cells Nanomed. Biotechnol. 2019;47(1):1710–1721. doi: 10.1080/21691401.2019.1607866. [DOI] [PubMed] [Google Scholar]
- 241.Naghizadeh Z., Karkhaneh A., Nokhbatolfoghahaei H., Farzad-Mohajeri S., Rezai-Rad M., Dehghan M.M., et al. Cartilage regeneration with dual-drug-releasing injectable hydrogel/microparticle system: in vitro and in vivo study. J. Cell. Physiol. 2021;236(3):2194–2204. doi: 10.1002/jcp.30006. [DOI] [PubMed] [Google Scholar]
- 242.Chen P.F., Xia C., Mei S., Wang J.Y., Shan Z., Lin X.F., et al. Intra-articular delivery of sinomenium encapsulated by chitosan microspheres and photo-crosslinked GelMA hydrogel ameliorates osteoarthritis by effectively regulating autophagy. Biomaterials. 2016;81:1–13. doi: 10.1016/j.biomaterials.2015.12.006. [DOI] [PubMed] [Google Scholar]
- 243.Zhu S.A., Lu P., Liu H.H., Chen P.F., Wu Y., Wang Y.Y., et al. Inhibition of Rac1 activity by controlled release of NSC23766 from chitosan microspheres effectively ameliorates osteoarthritis development in vivo. Ann. Rheum. Dis. 2015;74(1):285–293. doi: 10.1136/annrheumdis-2013-203901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Sun Y., Chi X., Meng H., Ma M., Wang J., Feng Z., et al. Polylysine-decorated macroporous microcarriers laden with adipose-derived stem cells promote nerve regeneration in vivo. Bioact Mater. 2021;6(11):3987–3998. doi: 10.1016/j.bioactmat.2021.03.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Zignego D.L., Jutila A.A., Gelbke M.K., Gannon D.M., June R.K. The mechanical microenvironment of high concentration agarose for applying deformation to primary chondrocytes. J. Biomech. 2014;47(9):2143–2148. doi: 10.1016/j.jbiomech.2013.10.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Kuznetsov S.A., Hailu-Lazmi A., Cherman N., de Castro L.F., Robey P.G., Gorodetsky R. In vivo formation of stable hyaline cartilage by naive human bone marrow stromal cells with modified fibrin microbeads. Stem Cells Trans. Med. 2019;8(6):586–592. doi: 10.1002/sctm.18-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Yang J.L., Han Y., Lin J.W., Zhu Y., Wang F., Deng L.F., et al. Ball-bearing-inspired polyampholyte-modified microspheres as bio-lubricants attenuate osteoarthritis. Small. 2020;16(44):2004519. doi: 10.1002/smll.202004519. [DOI] [PubMed] [Google Scholar]
- 248.Park E., Hart M.L., Rolauffs B., Stegemann J.P., Annamalai R.T. Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J. Biomed. Mater. Res. 2020;108(3):722–733. doi: 10.1002/jbm.a.36852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Dang P.N., Solorio L.D., Alsberg E. Driving cartilage formation in high-density human adipose-derived stem cell aggregate and sheet constructs without exogenous growth factor delivery. Tissue Eng. 2014;20(23–24):3163–3175. doi: 10.1089/ten.tea.2012.0551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Ratanavaraporn J., Soontornvipart K., Shuangshoti S., Shuangshoti S., Damrongsakkul S. Localized delivery of curcumin from injectable gelatin/Thai silk fibroin microspheres for anti-inflammatory treatment of osteoarthritis in a rat model. Inflammopharmacology. 2017;25(2):211–221. doi: 10.1007/s10787-017-0318-3. [DOI] [PubMed] [Google Scholar]
- 251.Yin H.Y., Wang Y., Sun X., Cui G.H., Sun Z., Chen P., et al. Functional tissue-engineered microtissue derived from cartilage extracellular matrix for articular cartilage regeneration. Acta Biomater. 2018;77:127–141. doi: 10.1016/j.actbio.2018.07.031. [DOI] [PubMed] [Google Scholar]
- 252.Hv Almeida, Eswaramoorthy R., Cunniffe G.M., Buckley C.T., O'Brien F.J., Kelly D. Fibrin hydrogels functionalized with cartilage extracellular matrix and incorporating freshly isolated stromal cells as an injectable for cartilage regeneration. Acta Biomater. 2016;36:55–62. doi: 10.1016/j.actbio.2016.03.008. [DOI] [PubMed] [Google Scholar]
- 253.Yang J., Zhang Y.S., Yue K., Khademhosseini A. Cell-laden hydrogels for osteochondral and cartilage tissue engineering. Acta Biomater. 2017;57:1–25. doi: 10.1016/j.actbio.2017.01.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 254.Rim N.G., Shin C.S., Shin H. Current approaches to electrospun nanofibers for tissue engineering. Biomed. Mater. 2013;8(1) doi: 10.1088/1748-6041/8/1/014102. [DOI] [PubMed] [Google Scholar]
- 255.Bruder S.P., Fox B.S. Tissue engineering of bone. Cell based strategies. Clin. Orthop. Relat. Res. 1999;367(Suppl):S68–S83. doi: 10.1097/00003086-199910001-00008. [DOI] [PubMed] [Google Scholar]
- 256.Butreddy A., Gaddam R.P., Kommineni N., Dudhipala N., Voshavar C. PLGA/PLA-Based long-acting injectable depot microspheres in clinical use: production and characterization overview for protein/peptide delivery. Int. J. Mol. Sci. 2021;22(16):8884. doi: 10.3390/ijms22168884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 257.Lei Y., Wang Y., Shen J., Cai Z., Zeng Y., Zhao P., et al. Stem cell‐recruiting injectable microgels for repairing osteoarthritis. Adv. Funct. Mater. 2021;34(48):2105084. doi: 10.1002/adfm.202105084. [DOI] [Google Scholar]
- 258.Kim H.J., Park J.M., Lee S., Hong S.J., Park J.I., Lee M.S., et al. In situ pocket-type microcarrier (PMc) as a therapeutic composite: regeneration of cartilage with stem cells, genes, and drugs. J. Contr. Release. 2020;332:337–345. doi: 10.1016/j.jconrel.2020.08.057. [DOI] [PubMed] [Google Scholar]
- 259.Griffith L.A., Arnold K.M., Sengers B.G., Tare R.S., Houghton F.D. A scaffold-free approach to cartilage tissue generation using human embryonic stem cells. Sci. Rep. 2021;11(1):18921. doi: 10.1038/s41598-021-97934-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Makris E.A., Gomoll A.H., Malizos K.N., Hu J.C., Athanasiou K.A. Repair and tissue engineering techniques for articular cartilage. Nat. Rev. Rheumatol. 2015;11(1):21–34. doi: 10.1038/nrrheum.2014.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Charlier E., Deroyer C., Ciregia F., Malaise O., Neuville S., Plener Z., et al. Chondrocyte dedifferentiation and osteoarthritis (OA) Biochem. Pharmacol. 2019;165:49–65. doi: 10.1016/j.bcp.2019.02.036. [DOI] [PubMed] [Google Scholar]
- 262.van Susante J.L., Buma P., van Osch G.J., Versleyen D., van der Kraan P.M., van der Berg W.B., et al. Culture of chondrocytes in alginate and collagen carrier gels. Acta Orthop. Scand. 1995;66(6):549–556. doi: 10.3109/17453679509002314. [DOI] [PubMed] [Google Scholar]
- 263.Liu J., Lin H., Li X.P., Fan Y.J., Zhang X.D. Chondrocytes behaviors within type I collagen microspheres and bulk hydrogels: an in vitro study. RSC Adv. 2015;5(67):54446–54453. doi: 10.1039/c5ra04496k. [DOI] [Google Scholar]
- 264.Zhou Y., Gao H.L., Shen L.L., Pan Z., Mao L.B., Wu T., et al. Chitosan microspheres with an extracellular matrix-mimicking nanofibrous structure as cell-carrier building blocks for bottom-up cartilage tissue engineering. Nanoscale. 2016;8(1):309–317. doi: 10.1039/c5nr06876b. [DOI] [PubMed] [Google Scholar]
- 265.Gupta V., Khan Y., Berkland C.J., Laurencin C.T., Detamore M.S. Microsphere-based scaffolds in regenerative engineering. Annu. Rev. Biomed. Eng. 2017;19:135–161. doi: 10.1146/annurev-bioeng-071516-044712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Hayes A.J., Hall A., Brown L., Tubo R., Caterson B. Macromolecular organization and in vitro growth characteristics of scaffold-free neocartilage grafts. J. Histochem. Cytochem. 2007;55(8):853–866. doi: 10.1369/jhc.7A7210.2007. [DOI] [PubMed] [Google Scholar]
- 267.Tee C.A., Yang Z., Yin L., Wu Y.N., Han J., Lee E.H. Improved zonal chondrocyte production protocol integrating size-based inertial spiral microchannel separation and dynamic microcarrier culture for. clinical application. Biomaterials. 2019;220 doi: 10.1016/j.biomaterials.2019.119409. 119409. [DOI] [PubMed] [Google Scholar]
- 268.He Q., Liao Y., Zhang J., Yao X., Zhou W., Hong Y., et al. All-in-One" gel system for whole procedure of stem-cell amplification and tissue engineering. Small. 2020;16(16) doi: 10.1002/smll.201906539. [DOI] [PubMed] [Google Scholar]
- 269.Frehner F., Benthien J.P. Microfracture: state of the art in cartilage surgery? Cartilage. 2018;9(4):339–345. doi: 10.1177/1947603517700956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 270.Olsen T.R., Ng K.S., Lock L.T., Ahsan T., Rowley J.A. Peak MSC-are we there yet? Front. Med. 2018;5:178. doi: 10.3389/fmed.2018.00178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Bellani C.F., Ajeian J., Duffy L., Miotto M., Groenewegen L., Connon C.J. Scale-up technologies for the manufacture of adherent cells. Front Nutr. 2020;7:575146. doi: 10.3389/fnut.2020.575146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 272.Lembong J., Kirian R., Takacs J.D., Olsen T.R., Lock L.T., Rowley J.A., et al. Bioreactor parameters for microcarrier-based human MSC expansion under xeno-free conditions in a vertical-wheel system. Bioengineering. 2020;7(3) doi: 10.3390/bioengineering7030073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Yan X., Zhang K., Yang Y., Deng D., Lyu C., Xu H., et al. Dispersible and dissolvable porous microcarrier tablets enable efficient large-scale human mesenchymal stem cell expansion. Tissue Eng. C Methods. 2020;26(5):263–275. doi: 10.1089/ten.TEC.2020.0039. [DOI] [PubMed] [Google Scholar]
- 274.Tsai A.-C., Pacak C.A. Bioprocessing of human mesenchymal stem cells: from planar culture to microcarrier-based bioreactors. Bioengineering. 2021;8(7):96. doi: 10.3390/bioengineering8070096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 275.Jossen V., Schirmer C., Mostafa Sindi D., Eibl R., Kraume M., Pörtner R., et al. Theoretical and practical issues that are relevant when scaling up hMSC microcarrier production processes. Stem Cell. Int. 2016;2016:4760414. doi: 10.1155/2016/4760414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Couto P.S., Rotondi M.C., Bersenev A., Hewitt C.J., Nienow A.W., Verter F., et al. Expansion of human mesenchymal stem/stromal cells (hMSCs) in bioreactors using microcarriers: lessons learnt and what the future holds. Biotechnol. Adv. 2020;45:107636. doi: 10.1016/j.biotechadv.2020.107636. [DOI] [PubMed] [Google Scholar]
- 277.Nienow A.W., Rafiq Q.A., Coopman K., Hewitt C.J. A potentially scalable method for the harvesting of hMSCs from microcarriers. Biochem. Eng. J. 2014;85:79–88. doi: 10.1016/j.bej.2014.02.005. [DOI] [Google Scholar]
- 278.Sun C., Yue J., He N., Liu Y., Zhang X., Zhang Y. Fundamental principles of stem cell banking. Adv. Exp. Med. Biol. 2016;951:31–45. doi: 10.1007/978-3-319-45457-3_3. [DOI] [PubMed] [Google Scholar]
- 279.Gong L., Petchakup C., Shi P., Tan P.L., Tan L.P., Tay C.Y., et al. Direct and label-free cell status monitoring of spheroids and microcarriers using microfluidic impedance cytometry. Small. 2021;17(21) doi: 10.1002/smll.202007500. [DOI] [PubMed] [Google Scholar]
- 280.Xing Z., Kenty B.M., Li Z.J., Lee S.S. Scale-up analysis for a CHO cell culture process in large-scale bioreactors. Biotechnol. Bioeng. 2009;103(4):733–746. doi: 10.1002/bit.22287. [DOI] [PubMed] [Google Scholar]
- 281.Sieblist C., Hägeholz O., Aehle M., Jenzsch M., Pohlscheidt M., Lübbert A. Insights into large-scale cell-culture reactors: II. Gas-phase mixing and CO₂ stripping. Biotechnol. J. 2011;6(12):1547–1556. doi: 10.1002/biot.201100153. [DOI] [PubMed] [Google Scholar]
- 282.Xing Z., Lewis A.M., Borys M.C., Li Z.J. A carbon dioxide stripping model for mammalian cell culture in manufacturing scale bioreactors. Biotechnol. Bioeng. 2017;114(6):1184–1194. doi: 10.1002/bit.26232. [DOI] [PubMed] [Google Scholar]
- 283.Xu J., Tang P., Yongky A., Drew B., Borys M.C., Liu S., et al. Systematic development of temperature shift strategies for Chinese hamster ovary cells based on short duration cultures and kinetic modeling. mAbs. 2019;11(1):191–204. doi: 10.1080/19420862.2018.1525262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Tapia F., Vázquez-Ramírez D., Genzel Y., Reichl U. Bioreactors for high cell density and continuous multi-stage cultivations: options for process intensification in cell culture-based viral vaccine production. Appl. Microbiol. Biotechnol. 2016;100(5):2121–2132. doi: 10.1007/s00253-015-7267-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Kwon T., Prentice H., Oliveira J., Madziva N., Warkiani M.E., Hamel J.P., et al. Microfluidic cell retention device for perfusion of mammalian suspension culture. Sci. Rep. 2017;7(1):6703. doi: 10.1038/s41598-017-06949-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 286.Xu S., Chen H. High-density mammalian cell cultures in stirred-tank bioreactor without external pH control. J. Biotechnol. 2016;231:149–159. doi: 10.1016/j.jbiotec.2016.06.019. [DOI] [PubMed] [Google Scholar]
- 287.Ahleboot Z., Khorshidtalab M., Motahari P., Mahboudi R., Arjmand R., Mokarizadeh A., et al. Designing a strategy for pH control to improve CHO cell productivity in bioreactor. Avicenna J. Med. Biotechnol. (AJMB) 2021;13(3):123–130. doi: 10.18502/ajmb.v13i3.6365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 288.Zhou Y., Han L.R., He H.W., Sang B., Yu D.L., Feng J.T., et al. Effects of agitation, aeration and temperature on production of a novel glycoprotein GP-1 by streptomyces kanasenisi ZX01 and scale-up based on volumetric oxygen transfer coefficient. Molecules. 2018;23(1) doi: 10.3390/molecules23010125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Heathman T.R., Nienow A.W., Rafiq Q.A., Coopman K., Kara B., Hewitt C.J. Agitation and aeration of stirred-bioreactors for the microcarrier culture of human mesenchymal stem cells and potential implications for large-scale bioprocess development. Biochem. Eng. J. 2018;136:9–17. doi: 10.1016/j.bej.2018.04.011. [DOI] [Google Scholar]
- 290.Nienow A.W., Hewitt C.J., Heathman T.R.J., Glyn V.A.M., Fonte G.N., Hanga M.P., et al. Agitation conditions for the culture and detachment of hMSCs from microcarriers in multiple bioreactor platforms. Biochem. Eng. J. 2016;108:24–29. doi: 10.1016/j.bej.2015.08.003. [DOI] [Google Scholar]
- 291.Shang M., Kwon T., Hamel J.P., Lim C.T., Bl Khoo J Han. Investigating the influence of physiologically relevant hydrostatic pressure on CHO cell batch culture. Sci. Rep. 2021;11(1):162. doi: 10.1038/s41598-020-80576-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Zhao Z., Wang Z., Li G., Cai Z., Wu J., Wang L., et al. Injectable microfluidic hydrogel microspheres for cell and drug delivery. Adv. Funct. Mater. 2021;31(31):2103339. doi: 10.1002/adfm.202103339. [DOI] [Google Scholar]
- 293.O Levy R Kuai, Siren E.M.J., Bhere D., Milton Y., Nissar N., et al. Shattering barriers toward clinically meaningful MSC therapies. Sci. Adv. 2020;6(30) doi: 10.1126/sciadv.aba6884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 294.Lin X., Tsao C.T., Kyomoto M., Zhang M. Injectable natural polymer hydrogels for treatment of knee osteoarthritis. Adv Healthc Mater. 2021 doi: 10.1002/adhm.202101479. [DOI] [PubMed] [Google Scholar]
- 295.Nakielski P., Rinoldi C., Pruchniewski M., Pawlowska S., Gazinska M., Strojny B., et al. Laser-assisted fabrication of injectable nanofibrous cell carriers. Small. 2021 doi: 10.1002/smll.202104971. [DOI] [PubMed] [Google Scholar]
- 296.Jiang L.-B., Ding S.-L., Ding W., Su D.-H., Zhang F.-X., Zhang T.-W., et al. Injectable sericin based nanocomposite hydrogel for multi-modal imaging-guided immunomodulatory bone regeneration. Chem. Eng. J. 2021;418 doi: 10.1016/j.cej.2021.129323. [DOI] [Google Scholar]
- 297.Cheng W., Zhang J., Liu J., Yu Z. Granular hydrogels for 3D bioprinting applications. View. 2020;1(3) doi: 10.1002/VIW.20200060. [DOI] [Google Scholar]
- 298.Zhang Y.S., Haghiashtiani G., Hübscher T., Kelly D.J., Lee J.M., Lutolf M., et al. 3D extrusion bioprinting. Nat. Rev. Methods Primers. 2021;1(1):75. doi: 10.1038/s43586-021-00073-8. [DOI] [Google Scholar]
- 299.Malda J., Visser J., Melchels F.P., Jüngst T., Hennink W.E., Dhert W.J., et al. 25th anniversary article: engineering hydrogels for biofabrication. Adv. Mater. 2013;25(36):5011–5028. doi: 10.1002/adma.201302042. [DOI] [PubMed] [Google Scholar]
- 300.Ribeiro A., Blokzijl M.M., Levato R., Visser C.W., Castilho M., Hennink W.E., et al. Assessing bioink shape fidelity to aid material development in 3D bioprinting. Biofabrication. 2017;10(1) doi: 10.1088/1758-5090/aa90e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Levato R., Visser J., Planell J.A., Engel E., Malda J., Mateos-Timoneda M.A. Biofabrication of tissue constructs by 3D bioprinting of cell-laden microcarriers. Biofabrication. 2014;6(3) doi: 10.1088/1758-5082/6/3/035020. [DOI] [PubMed] [Google Scholar]
- 302.Xin S., Deo K.A., Dai J., Pandian N.K.R., Chimene D., Moebius R.M., et al. Generalizing hydrogel microparticles into a new class of bioinks for extrusion bioprinting. Sci. Adv. 2021;7(42) doi: 10.1126/sciadv.abk3087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 303.Highley C.B., Song K.H., Daly A.C., Burdick J.A. Jammed microgel inks for 3D printing applications. Adv. Sci. 2019;6(1):1801076. doi: 10.1002/advs.201801076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 304.Li H., Cheng F., Orgill D.P., Yao J., Zhang Y.S. Handheld bioprinting strategies for in situ wound dressing. Essays Biochem. 2021;65(3):533–543. doi: 10.1042/ebc20200098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Ying G., Manríquez J., Wu D., Zhang J., Jiang N., Maharjan S., et al. An open-source handheld extruder loaded with pore-forming bioink for in situ wound dressing. Mater Today Bio. 2020;8:100074. doi: 10.1016/j.mtbio.2020.100074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 306.Eslahi N., Abdorahim M., Simchi A. Smart polymeric hydrogels for cartilage tissue engineering: a review on the chemistry and biological functions. Biomacromolecules. 2016;17(11):3441–3463. doi: 10.1021/acs.biomac.6b01235. [DOI] [PubMed] [Google Scholar]
- 307.Onaca O., Enea R., Hughes D.W., Meier W. Stimuli-responsive polymersomes as nanocarriers for drug and gene delivery. Macromol. Biosci. 2009;9(2):129–139. doi: 10.1002/mabi.200800248. [DOI] [PubMed] [Google Scholar]
- 308.Bagheri M., Shateri S., Niknejad H., Entezami A.A. Thermosensitive biotinylated hydroxypropyl cellulose-based polymer micelles as a nano-carrier for cancer-targeted drug delivery. J. Polym. Res. 2014;21(10):1–15. doi: 10.1007/s10965-014-0567-4. [DOI] [Google Scholar]
- 309.Işıklan N., Erol Ü H. Design and evaluation of temperature-responsive chitosan/hydroxypropyl cellulose blend nanospheres for sustainable flurbiprofen release. Int. J. Biol. Macromol. 2020;159:751–762. doi: 10.1016/j.ijbiomac.2020.05.071. [DOI] [PubMed] [Google Scholar]
- 310.Bulut E., Turhan Y. Synthesis and characterization of temperature-sensitive microspheres based on acrylamide grafted hydroxypropyl cellulose and chitosan for the controlled release of amoxicillin trihydrate. Int. J. Biol. Macromol. 2021;191 doi: 10.1016/j.ijbiomac.2021.09.193. [DOI] [PubMed] [Google Scholar]
- 311.Zhang H., Liu Y., Chen C., Cui W., Zhang C., Ye F., et al. Responsive drug-delivery microcarriers based on the silk fibroin inverse opal scaffolds for controllable drug release. Applied Materials Today. 2020;19:100540. doi: 10.1016/j.apmt.2019.100540. [DOI] [Google Scholar]
- 312.Fan L., Zhang X., Liu X., Sun B., Li L., Zhao Y. Responsive hydrogel microcarrier-integrated microneedles for versatile and controllable drug delivery. Adv Healthc Mater. 2021;10(9) doi: 10.1002/adhm.202002249. [DOI] [PubMed] [Google Scholar]
- 313.HJ Moon MH Park, Joo M.K., Jeong B. Temperature-responsive compounds as in situ gelling biomedical materials. Chem. Soc. Rev. 2012;41(14):4860–4883. doi: 10.1039/C2CS35078E. [DOI] [PubMed] [Google Scholar]
- 314.Balakrishnan B., Jayakrishnan A. Self-cross-linking biopolymers as injectable in situ forming biodegradable scaffolds. Biomaterials. 2005;26(18):3941–3951. doi: 10.1016/j.biomaterials.2004.10.005. [DOI] [PubMed] [Google Scholar]
- 315.Park E., Hart M.L., Rolauffs B., Stegemann J.P., TA R Bioresponsive microspheres for on-demand delivery of anti-inflammatory cytokines for articular cartilage repair. J. Biomed. Mater. Res. 2020;108(3):722–733. doi: 10.1002/jbm.a.36852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 316.Ng L., Grodzinsky A.J., Patwari P., Sandy J., Plaas A., Ortiz C. Individual cartilage aggrecan macromolecules and their constituent glycosaminoglycans visualized via atomic force microscopy. J. Struct. Biol. 2003;143(3):242–257. doi: 10.1016/j.jsb.2003.08.006. [DOI] [PubMed] [Google Scholar]
- 317.Evans C.H. Drug delivery to chondrocytes. Osteoarthritis Cartilage. 2016;24(1):1–3. doi: 10.1016/j.joca.2015.08.012. [DOI] [PubMed] [Google Scholar]
- 318.Lin F., Wang Z., Xiang L., Deng L., Cui W. Charge‐guided micro/nano‐hydrogel microsphere for penetrating cartilage matrix. Adv. Funct. Mater. 2021:2107678. doi: 10.1002/adfm.202107678. [DOI] [Google Scholar]
- 319.Elliott J.E., Macdonald M., Nie J., Bowman C.N. Structure and swelling of poly (acrylic acid) hydrogels: effect of pH, ionic strength, and dilution on the crosslinked polymer structure. Polymer. 2004;45(5):1503–1510. doi: 10.1016/j.polymer.2003.12.040. [DOI] [Google Scholar]
- 320.Işıklan N., Tokmak Ş. Development of thermo/pH-responsive chitosan coated pectin-graft-poly(N,N-diethyl acrylamide) microcarriers. Carbohydr. Polym. 2019;218:112–125. doi: 10.1016/j.carbpol.2019.04.068. [DOI] [PubMed] [Google Scholar]
- 321.Almeida E., Bellettini I.C., Garcia F.P., Farinácio M.T., Nakamura C.V., Rubira A.F., et al. Curcumin-loaded dual pH- and thermo-responsive magnetic microcarriers based on pectin maleate for drug delivery. Carbohydr. Polym. 2017;171:259–266. doi: 10.1016/j.carbpol.2017.05.034. [DOI] [PubMed] [Google Scholar]
- 322.Chou P.M., Khiew P.S., Brown P.D., Hu B. Development of thermally responsive PolyNIPAm microcarrier for application of cell culturing-Part I: a feasibility study. Polymers. 2021;13(16):2629. doi: 10.3390/polym13162629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 323.O'Conor C.J., Case N., Guilak F. Mechanical regulation of chondrogenesis. Stem Cell Res. Ther. 2013;4(4):61. doi: 10.1186/scrt211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 324.Lee K.Y., Peters M.C., Anderson K.W., Mooney D.J. Controlled growth factor release from synthetic extracellular matrices. Nature. 2000;408(6815):998–1000. doi: 10.1038/35050141. [DOI] [PubMed] [Google Scholar]
- 325.Zhang Y., Yu J., Bomba H.N., Zhu Y., Gu Z. Mechanical force-triggered drug delivery. Chem. Rev. 2016;116(19):12536–12563. doi: 10.1021/acs.chemrev.6b00369. [DOI] [PubMed] [Google Scholar]
- 326.Zhang X., Yang J., Cheng B., Zhao S., Li Y., Kang H., et al. Magnetic nanocarriers as a therapeutic drug delivery strategy for promoting pain-related motor functions in a rat model of cartilage transplantation. J. Mater. Sci. Mater. Med. 2021;32(4):37. doi: 10.1007/s10856-021-06508-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 327.Go G., Yoo A., Kim S., Seon J.K., Kim C.S., Park J.O., et al. Magnetization-switchable implant system to target delivery of stem cell-loaded bioactive polymeric microcarriers. Adv Healthc Mater. 2021;10(19) doi: 10.1002/adhm.202100068. [DOI] [PubMed] [Google Scholar]
- 328.Go G., Jeong S.G., Yoo A., Han J., Kang B., Kim S., et al. Human adipose-derived mesenchymal stem cell-based medical microrobot system for knee cartilage regeneration in vivo. Sci Robot. 2020;5(38) doi: 10.1126/scirobotics.aay6626. [DOI] [PubMed] [Google Scholar]
- 329.Hu W., Lum G.Z., Mastrangeli M., Sitti M. Small-scale soft-bodied robot with multimodal locomotion. Nature. 2018;554(7690):81–85. doi: 10.1038/nature25443. [DOI] [PubMed] [Google Scholar]
- 330.Chen X.-Z., Liu J.-H., Dong M., Müller L., Chatzipirpiridis G., Hu C., et al. Magnetically driven piezoelectric soft microswimmers for neuron-like cell delivery and neuronal differentiation. Materials Horizons. 2019;6(7):1512–1516. doi: 10.1039/C9MH00279K. [DOI] [Google Scholar]
- 331.Jeon S., Kim S., Ha S., Lee S., Kim E., Kim S.Y., et al. Magnetically actuated microrobots as a platform for stem cell transplantation. Sci Robot. 2019;4(30):eaav4317. doi: 10.1126/scirobotics.aav4317. [DOI] [PubMed] [Google Scholar]
- 332.Burhannuddin N.L., Nordin N.A., Mazlan S.A., Aziz S.A.A., Choi S.-B., Kuwano N., et al. Effects of corrosion rate of the magnetic particles on the field-dependent material characteristics of silicone based magnetorheological elastomers. Smart Mater. Struct. 2020;29(8) doi: 10.1088/1361-665x/ab972c. [DOI] [Google Scholar]
- 333.Kim Y., Parada G.A., Liu S., Zhao X. Ferromagnetic soft continuum robots. Sci Robot. 2019;4(33):eaax7329. doi: 10.1126/scirobotics.aax7329. [DOI] [PubMed] [Google Scholar]
- 334.Rutgers M., van Pelt M.J., Dhert W.J., Creemers L.B., Saris D.B. Evaluation of histological scoring systems for tissue-engineered, repaired and osteoarthritic cartilage. Osteoarthritis Cartilage. 2010;18(1):12–23. doi: 10.1016/j.joca.2009.08.009. [DOI] [PubMed] [Google Scholar]
- 335.Blaiszik B.J., Kramer S.L., Olugebefola S.C., Moore J.S., Sottos N.R., White S.R. Self-healing polymers and composites. Annu. Rev. Mater. Res. 2010;40:179–211. doi: 10.1146/annurev-matsci-070909-104532. [DOI] [Google Scholar]
- 336.Han L., Yan L., Wang K., Fang L., Zhang H., Tang Y., et al. Tough, self-healable and tissue-adhesive hydrogel with tunable multifunctionality. NPG Asia Mater. 2017;9(4) doi: 10.1038/am.2017.33. e372. [DOI] [Google Scholar]
- 337.Zhang H., Wang C., Zhu G., Zacharia N.S. Self-healing of bulk polyelectrolyte complex material as a function of pH and salt. ACS Appl. Mater. Interfaces. 2016;8(39):26258–26265. doi: 10.1021/acsami.6b06776. [DOI] [PubMed] [Google Scholar]
- 338.Zhao X., Chen X., Yuk H., Lin S., Liu X., Parada G. Soft materials by design: unconventional polymer networks give extreme properties. Chem. Rev. 2021;121(8):4309–4372. doi: 10.1021/acs.chemrev.0c01088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Hafezi M., Khorasani S Nouri, Zare M., Neisiany R Esmaeely, Davoodi P. Advanced hydrogels for cartilage tissue engineering: recent progress and future directions. Polymers. 2021;13(23) doi: 10.3390/polym13234199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Nguyen B.V., Wang Q.G., Kuiper N.J., El Haj A.J., Thomas C.R., Zhang Z. Biomechanical properties of single chondrocytes and chondrons determined by micromanipulation and finite-element modelling. J. R. Soc. Interface. 2010;7(53):1723–1733. doi: 10.1098/rsif.2010.0207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 341.Darling E.M., Wilusz R.E., Bolognesi M.P., Zauscher S., Guilak F. Spatial mapping of the biomechanical properties of the pericellular matrix of articular cartilage measured in situ via atomic force microscopy. Biophys. J. 2010;98(12):2848–2856. doi: 10.1016/j.bpj.2010.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 342.Li C.H., Chik T.K., Ngan A.H., Chan S.C., Shum D.K., Chan B.P. Correlation between compositional and mechanical properties of human mesenchymal stem cell-collagen microspheres during chondrogenic differentiation. Tissue Eng. 2011;17(5–6):777–788. doi: 10.1089/ten.TEA.2010.0078. [DOI] [PubMed] [Google Scholar]
- 343.Cherian D.S., Bhuvan T., Meagher L., Heng T.S.P. Biological considerations in scaling up therapeutic cell manufacturing. Front. Pharmacol. 2020;11:654. doi: 10.3389/fphar.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Bryant S.J., Anseth K.S. Hydrogel properties influence ECM production by chondrocytes photoencapsulated in poly(ethylene glycol) hydrogels. J. Biomed. Mater. Res. 2002;59(1):63–72. doi: 10.1002/jbm.1217. [DOI] [PubMed] [Google Scholar]
- 345.Mouw J.K., Case N.D., Guldberg R.E., Plaas A.H., Levenston M.E. Variations in matrix composition and GAG fine structure among scaffolds for cartilage tissue engineering. Osteoarthritis Cartilage. 2005;13(9):828–836. doi: 10.1016/j.joca.2005.04.020. [DOI] [PubMed] [Google Scholar]
- 346.Chaudhuri O., Gu L., Klumpers D., Darnell M., Bencherif S.A., Weaver J.C., et al. Hydrogels with tunable stress relaxation regulate stem cell fate and activity. Nat. Mater. 2016;15(3):326–334. doi: 10.1038/nmat4489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.McKinnon D.D., Domaille D.W., Cha J.N., Anseth K.S. Biophysically defined and cytocompatible covalently adaptable networks as viscoelastic 3D cell culture systems. Adv. Mater. 2014;26(6):865–872. doi: 10.1002/adma.201303680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 348.Pasut E., Toffanin R., Voinovich D., Pedersini C., Murano E., Grassi M. Mechanical and diffusive properties of homogeneous alginate gels in form of particles and cylinders. J. Biomed. Mater. Res. 2008;87(3):808–818. doi: 10.1002/jbm.a.31680. [DOI] [PubMed] [Google Scholar]
- 349.Kumachev A., Tumarkin E., Walker G.C., Kumacheva E. Characterization of the mechanical properties of microgels acting as cellular microenvironments. Soft Matter. 2013;9(10):2959–2965. doi: 10.1039/C3SM27400D. [DOI] [Google Scholar]
- 350.Luetchford K.A., Chaudhuri J.B., De Bank P.A. Silk fibroin/gelatin microcarriers as scaffolds for bone tissue engineering. Mater. Sci. Eng. C Mater. Biol. Appl. 2020;106:110116. doi: 10.1016/j.msec.2019.110116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 351.Dias A.D., Elicson J.M., Murphy W.L. Microcarriers with synthetic hydrogel surfaces for stem cell expansion. Adv Healthc Mater. 2017;6(16) doi: 10.1002/adhm.201700072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 352.Wang X.-Y., Merlo A., Dupont C., V Salsac A.-, Barthes-Biesel D. A microfluidic methodology to identify the mechanical properties of capsules: comparison with a microrheometric approach. Flowline. 2021;1:E8. doi: 10.1017/flo.2021.8. [DOI] [Google Scholar]
- 353.Hernández R.M., Orive G., Murua A., Pedraz J.L. Microcapsules and microcarriers for in situ cell delivery. Adv. Drug Deliv. Rev. 2010;62(7–8):711–730. doi: 10.1016/j.addr.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 354.Municoy S., Echazú MI Álvarez, Antezana P.E., Galdopórpora J.M., Olivetti C., Mebert A.M., et al. Stimuli-responsive materials for tissue engineering and drug delivery. Int. J. Mol. Sci. 2020;21(13):4724. doi: 10.3390/ijms21134724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 355.Porte E., Cann P., Masen M. A lubrication replenishment theory for hydrogels. Soft Matter. 2020;16(45):10290–10300. doi: 10.1039/d0sm01236j. [DOI] [PubMed] [Google Scholar]
- 356.Dunn A.C., Sawyer W.G., Angelini T.E. Gemini interfaces in aqueous lubrication with hydrogels. Tribol. Lett. 2014;54(1):59–66. doi: 10.1007/s11249-014-0308-1. [DOI] [Google Scholar]
- 357.Yang L., Sun L., Zhang H., Bian F., Zhao Y. Ice-inspired lubricated drug delivery particles from microfluidic electrospray for osteoarthritis treatment. ACS Nano. 2021 doi: 10.1021/acsnano.1c09325. [DOI] [PubMed] [Google Scholar]
- 358.Zuncheddu D., E Della Bella, Schwab A., Petta D., Rocchitta G., Generelli S., et al. Quality control methods in musculoskeletal tissue engineering: from imaging to biosensors. Bone Res. 2021;9(1):46. doi: 10.1038/s41413-021-00167-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 359.Baino F., Fiorilli S., Vitale-Brovarone C. Bioactive glass-based materials with hierarchical porosity for medical applications: review of recent advances. Acta Biomater. 2016;42:18–32. doi: 10.1016/j.actbio.2016.06.033. [DOI] [PubMed] [Google Scholar]
- 360.Kargozar S., Baino F., Hamzehlou S., Hill R.G., Mozafari M. Bioactive glasses entering the mainstream. Drug Discov. Today. 2018;23(10):1700–1704. doi: 10.1016/j.drudis.2018.05.027. [DOI] [PubMed] [Google Scholar]
- 361.Georgakilas V., Perman J.A., Tucek J., Zboril R. Broad family of carbon nanoallotropes: classification, chemistry, and applications of fullerenes, carbon dots, nanotubes, graphene, nanodiamonds, and combined superstructures. Chem. Rev. 2015;115(11):4744–4822. doi: 10.1021/cr500304f. [DOI] [PubMed] [Google Scholar]
- 362.Kazemzadeh H., Mozafari M. Fullerene-based delivery systems. Drug Discov. Today. 2019;24(3):898–905. doi: 10.1016/j.drudis.2019.01.013. [DOI] [PubMed] [Google Scholar]
- 363.Goodarzi S., Da Ros T., Conde J., Sefat F., Mozafari M. Fullerene: biomedical engineers get to revisit an old friend. Mater. Today. 2017;20(8):460–480. doi: 10.1016/j.mattod.2017.03.017. [DOI] [Google Scholar]
- 364.Ye L., Kollie L., Liu X., Guo W., Ying X., Zhu J., et al. Antitumor activity and potential mechanism of novel fullerene derivative nanoparticles. Molecules. 2021;26(11) doi: 10.3390/molecules26113252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 365.Wang J., Xie L., Wang T., Wu F., Meng J., Liu J., et al. Visible light-switched cytosol release of siRNA by amphiphilic fullerene derivative to enhance RNAi efficacy in vitro and in vivo. Acta Biomater. 2017;59:158–169. doi: 10.1016/j.actbio.2017.05.031. [DOI] [PubMed] [Google Scholar]
- 366.Wang Y., Wei X., Zhang R., Wu Y., Farid M.U., Huang H. Comparison of chemical, ultrasonic and thermal regeneration of carbon nanotubes for acetaminophen, ibuprofen, and triclosan adsorption. RSC Adv. 2017;7(83):52719–52728. doi: 10.1039/c7ra08812d. [DOI] [Google Scholar]
- 367.Li F., Truong V.X., Fisch P., Levinson C., Glattauer V., Zenobi-Wong M., et al. Cartilage tissue formation through assembly of microgels containing mesenchymal stem cells. Acta Biomater. 2018;77:48–62. doi: 10.1016/j.actbio.2018.07.015. [DOI] [PubMed] [Google Scholar]
- 368.Huang L., Abdalla A.M.E., Xiao L., Yang G. Biopolymer-based microcarriers for three-dimensional cell culture and engineered tissue formation. Int. J. Mol. Sci. 2020;21(5) doi: 10.3390/ijms21051895. 1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 369.Chen A.K., Reuveny S., Oh S.K. Application of human mesenchymal and pluripotent stem cell microcarrier cultures in cellular therapy: achievements and future direction. Biotechnol. Adv. 2013;31(7):1032–1046. doi: 10.1016/j.biotechadv.2013.03.006. [DOI] [PubMed] [Google Scholar]
- 370.Mazur P. Cryobiology: the freezing of biological systems. Science. 1970;168(3934):939–949. doi: 10.1126/science.168.3934.939. [DOI] [PubMed] [Google Scholar]
- 371.Akhoondi M., Oldenhof H., Sieme H., Wolkers W.F. Freezing-induced cellular and membrane dehydration in the presence of cryoprotective agents. Mol. Membr. Biol. 2012;29(6):197–206. doi: 10.3109/09687688.2012.699106. [DOI] [PubMed] [Google Scholar]
- 372.Chang T., Zhao G. Ice inhibition for cryopreservation: materials, strategies, and challenges. Adv. Sci. 2021;8(6):2002425. doi: 10.1002/advs.202002425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 373.Ravanbakhsh H., Luo Z., Zhang X., Maharjan S., Mirkarimi H.S., Tang G., et al. Freeform cell-laden cryobioprinting for shelf-ready tissue fabrication and storage. Matter. 2021 doi: 10.1016/j.matt.2021.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 374.Zhong H., Chan G., Hu Y., Hu H., Ouyang D. A comprehensive map of FDA-approved pharmaceutical products. Pharmaceutics. 2018;10(4):263. doi: 10.3390/pharmaceutics10040263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 375.Conaghan P.G., Cohen S.B., Berenbaum F., Lufkin J., Johnson J.R., Bodick N. Brief report: a phase IIb trial of a novel extended-release microsphere formulation of triamcinolone acetonide for intraarticular injection in knee osteoarthritis. Arthritis Rheumatol. 2018;70(2):204–211. doi: 10.1002/art.40364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 376.Conaghan P.G., Hunter D.J., Cohen S.B., Kraus V.B., Berenbaum F., Lieberman J.R., et al. Effects of a single intra-articular injection of a microsphere formulation of triamcinolone acetonide on knee osteoarthritis pain: a double-blinded, randomized, placebo-controlled, multinational study. J Bone Joint Surg Am. 2018;100(8):666–677. doi: 10.2106/jbjs.17.00154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 377.Kraus V.B., Conaghan P.G., HA Aazami, Mehra P., Kivitz A.J., Lufkin J., et al. Synovial and systemic pharmacokinetics (PK) of triamcinolone acetonide (TA) following intra-articular (IA) injection of an extended-release microsphere-based formulation (FX006) or standard crystalline suspension in patients with knee osteoarthritis (OA) Osteoarthritis Cartilage. 2018;26(1):34–42. doi: 10.1016/j.joca.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 378.Sakai S., C Mu K Kawabata, Hashimoto I., Kawakami K. Biocompatibility of subsieve-size capsules versus conventional-size microcapsules. J. Biomed. Mater. Res. 2006;78(2):394–398. doi: 10.1002/jbm.a.30676. [DOI] [PubMed] [Google Scholar]
- 379.Zhang X., He H., Yen C., Ho W., Lee L.J. A biodegradable, immunoprotective, dual nanoporous capsule for cell-based therapies. Biomaterials. 2008;29(31):4253–4259. doi: 10.1016/j.biomaterials.2008.07.032. [DOI] [PubMed] [Google Scholar]