Abstract
Objective:
Balance and mobility function worsen with age, more so for those with underlying chronic diseases. We recently found that asymptomatic carotid artery stenosis (ACAS) restricts blood flow to the brain and might also contribute to balance and mobility impairment. In the present study, we tested the hypothesis that ACAS is a modifiable risk factor for balance and mobility impairment. Our goal was to assess the effect of restoring blood flow to the brain by carotid revascularization on the balance and mobility of patients with high-grade ACAS (≥70% diameter-reducing stenosis).
Methods:
Twenty adults (age, 67.0 ± 9.4 years) undergoing carotid endarterectomy for high-grade stenosis were enrolled. Balance and mobility assessments were performed before and 6 weeks after revascularization. These included the Short Physical Performance Battery, the Berg Balance Scale, the Four Square Step Test, the Dynamic Gait Index (DGI), the Timed Up and Go test, gait speed, the Mini-Balance Evaluation Systems Test (Mini-BESTest), and the Walking While Talking complex test.
Results:
Consistent with our previous findings, patients demonstrated reduced scores on the Short Physical Performance Battery, Berg Balance Scale, DGI, and Timed Up and Go test and in gait speed. Depending on the outcome measure, 25% to 90% of the patients had scored in the impaired range at baseline. After surgery, significant improvements were observed in the outcome measures that combined walking with dynamic movements, including the DGI (P = .02) and Mini-BESTest (P = .002). The proportion of patients with Mini-BESTest scores indicating a high fall risk had decreased significantly from 90% (n = 18) at baseline to 40% (n = 8) after surgery (P = .02). We used Pearson’s correlations to examine the relationship between balance and mobility before surgery and the change after surgery. Patients with lower baseline DGI and Mini-BESTest scores demonstrated the most improvement after surgery (r = −0.59, P = .006; and r = −0.70, P = .001, respectively).
Conclusions:
Carotid revascularization improved patients’ balance and mobility, especially for measures that combine walking and dynamic movements. The greatest improvements were observed for the patients who had been most impaired at baseline.
Keywords: Asymptomatic carotid artery stenosis, Balance, Mobility, Revascularization
One in four older adults aged ≥65 years will fall each year.1 Falls and fall-related injuries are a significant public health concern because of their frequency, the associated loss of functional independence, and the costs of treatment.2,3 In addition, 10% to 20% of falls are associated with serious injuries such as fractures or head trauma and can result in death.4 Nonfatal fall-related injury results in an estimated $31.3 billion in treatment-related expenses.3 Although the cause of falls is multifactorial, declines in balance and mobility are major contributors.5 Balance and mobility dysfunction are significant predictors of falls, disability, institutionalization, and death.6,7 Several disease states contribute to the decline in function. However, some of the impairments could be reversible and, therefore, offer an opportunity to prevent or reduce fall-related morbidity.8 Thus, understanding the mechanisms that underlie the decline in function is essential for developing interventions that will improve balance and mobility and decrease the incidence of resultant falls.
Asymptomatic carotid artery stenosis (ACAS) affects ~10% of older adults.9 Evidence has suggested that 40% to 50% of older adults with ACAS have cognitive dysfunction.10,11 We have also found that individuals with ACAS have a greater decline in mobility and report higher fall rates than older adults without carotid stenosis.10,12 Our studies have demonstrated that ACAS is associated with cerebral hypoperfusion.10 Thus, it is plausible that flow restriction secondary to luminal narrowing from the carotid plaque will lead to impaired cerebral perfusion with resultant cognitive and mobility declines. Furthermore, improvement in middle cerebral artery flow after carotid revascularization has been associated with improved cognitive performance.13 Given that cognitive and mobility function are inherently linked, we believe that similar improvement in mobility function could be seen with carotid revascularization. With the strong association between cognitive and mobility function,14,15 we hypothesized that balance and mobility performance would improve 6 weeks after revascularization in individuals with high-grade ACAS of ≥70%.
METHODS
A total of 110 patients scheduled to undergo carotid endarterectomy were screened. Of the 110 patients, 48 meet the inclusion and exclusion criteria, and 20 agreed to participate in the present study. The patients were recruited from the Baltimore Veterans Affairs Medical Center and the University of Maryland Medical Center. Balance and mobility function was assessed in all patients using objective, standardized, and validated tests 1 month before and 6 weeks after surgery. The university institutional review board approved the present study, and the included patients provided written informed consent. All patients were confirmed to be receiving appropriate antiplatelet and lipid-lowering therapy, with adequate blood pressure and blood glucose control before surgery. Most of the recruited patients had been receiving appropriate risk factor modification therapy, as outlined, before enrollment. Thus, minimal changes in medical management were required. All consecutive patients were screened, and those who had fulfilled the inclusion and exclusion criteria and agreed to participate were enrolled. The study exclusion criteria included a history of stroke or transient ischemic attack, occlusion of the index carotid artery, occlusion or high-grade stenosis of the opposite (nonindex) carotid artery, severe medical illness that would interfere with the evaluations, and carotid revascularization not planned.
The order of the balance and mobility tests was the same before and after surgery, with scoring performed within 24 hours. The total testing time varied from 30 to 45 minutes among the patients owing to interindividual variability in the completion time of those tests without time limits. The study tests included the Short Physical Performance Battery (SPPB), Berg Balance Scale (BBS), Four Square Step Test (FSST), Dynamic Gait Index (DGI), Timed Up and Go (TUG) test, Mini-Balance Evaluation Systems Test (Mini-BESTest), and Walking While Talking complex (WWTc) test. The patients’ gait speed was also assessed. The SPPB comprises three items that assess gait velocity, static balance, and lower extremity strength.6 Each item within the battery is scored on a scale of 0 to 4, with a total maximum score of 12. The short-form BBS measures the ability to maintain balance during a series of predetermined positions that alter the base of support.16 The individual items within the test are scored on a scale of 0 to 4, with a total maximum score of 28. The four-item DGI assesses the ability to modify balance while walking in the presence of external demands (walking on a level surface, changes in walking speed, walking with horizontal head turns, and walking with vertical head turns).17 The scale ranges from 0 to 3, with a total maximum score of 12. A higher score on the SPPB, BBS, and DGI indicates better balance. The TUG test assesses functional mobility by evaluating the time taken for the patient to rise from a chair, walk 3 m, turn around, return to the chair, and sit down.18 A lower time indicates better performance. The Mini-BESTest, a comprehensive measure that assesses anticipatory postural adjustments, reactive postural control, sensory orientation, and dynamic gait, has an item scale that ranges from 0 to 3, with a maximum score of 28 and higher scores indicating better balance performance.19 The WWTc assesses a patient’s ability to walk with divided attention and was performed at a usual walking pace with and without a cognitive task. The patients were instructed to walk 20 ft, turn around, and walk back 20 ft to the starting point at their usual walking pace.20 The patients performed two trials, one without a cognitive task and one with a cognitive task. For the cognitive task, patients were asked to recite alternating letters of the alphabet starting at the letter “n.” The WWTc time to complete the task is presented. WWTc errors represent the number of incorrect responses while reciting alternating letters (ie, reciting two consecutive letters in the alphabet). A faster walking time and fewer errors indicate better performance with the WWTc.
Each of these outcome measures has established impaired score thresholds (age-related normative values or established thresholds predictive of falls or mobility impairment). The fall risk cutoff for the DGI is a score <10.17 For the Mini BESTest, the cutoff is a score of ≤ 23 for those aged 60 to 69 years and ≤22 for those aged 70 to 79 years.21 The cutoff for the FSST is >15 seconds for someone who has already experienced multiple falls.22 Individuals with a gait speed of <1.0 m are considered more likely to fall and to have a greater risk of adverse health-related outcomes.23,24 A time of >33 seconds for the WWTc has 95.6% specificity for identifying individuals more likely to fall.20 The average number of errors for the WWTc is two.25 The normative cutoff values reported for older adults for the short-form BBS score is 20.61, with lower scores indicating poor balance.16 The cutoff for the TUG test is 8 seconds for those aged 60 to 69 years and 9 seconds for those aged 70 to 79 years.26 For the cutoff scores with two values according to age (ie, the Mini-BESTest and TUG test), we used the reported values for the oldest age group. Finally, individuals are considered to have a disability in mobility when the SPPB score is ≤10.27
Statistical analysis.
The data are reported as the mean ± standard deviation, median, 25th and 75th percentiles, and minimum and maximum values and were analyzed using SPSS for Windows, version 22.0 (IBM Corp, Armonk, NY). Dependent Student’s t tests were used to evaluate the differences between the baseline (preoperative) and postoperative results for the balance and mobility measures (ie, SPPB, BBS, FSST, DGI, TUG test, Mini-BESTest, WWTc time, WWTc errors). Box and whisker plots of the clinical outcome measures are presented for the balance and mobility measures at baseline and 6 weeks postoperatively. Histograms were created of the baseline and post-operative values to indicate the number of individuals who scored in the impaired range (ie, considered to have a risk of falling or impaired mobility or with scores less than the age-related normative values). McNemar’s test was used to compare the frequency of patients impaired at baseline against the frequency of patients impaired postoperatively. A two-tailed P value of <.05 was interpreted as indicating statistical significance.
RESULTS
Baseline clinical features.
The mean age of the patients was 67.0 ± 9.4 years. Almost one half were women (45%), and more were African American (30%) than in the U.S. distribution (Table I). Data for the WWTc and WWTc errors were missing for one patient who could not speak. None of the patients had vertebral artery occlusive disease. No patient died or experienced stroke or transient ischemic attack, cranial nerve injury, or bleeding or required revision surgery. The median length of hospital stay was 2 days.
Table I.
Clinical patient characteristics (n = 20)
| Characteristic | Mean ± SD or No. (%) |
|---|---|
| Age, years | 67.0 ± 9.4 |
| Female sex | 9 (45.0) |
| African-American race | 6 (30.0) |
| Coronary artery disease | 10 (50.0) |
| Diabetes mellitus | 11 (55.0) |
| Hypertension | 19 (95.0) |
| Dyslipidemia | 18 (90.0) |
| Smoking | 14 (70.0) |
| Stenosis, right | 12 (60.0) |
| Peak systolic velocity, cm/s | 437.0 ± 142.0 |
SD, Standard deviation.
Baseline mobility scores and frequency of impairment.
The mean scores for each mobility function measure were plotted to determine their frequency and distribution (Fig 1). Before surgery, all the participants, except for three, had had a gait speed greater than the value classified as a risk of frailty (>0.80 m/s). The reported age-related normative scores or established thresholds predictive of falls were used to quantify the proportion of patients who had performed at an impaired level at baseline.16,17,21–28 The distribution of the scores and the frequency of patients with impairment varied across the tests. However, most of the tests identified a subgroup of individuals with impaired mobility or a high risk of falling. At baseline, no patients with impairment were identified using the FFST and WWTc time (Fig 1 and Table II). For the remainder of the tests, 25.0% to 90.0% of the patients were classified as impaired at baseline, depending on the test (Fig 1). The Mini-BESTest demonstrated the greatest frequency of impaired patients, with 90% classified as having a risk of falling compared with the DGI, which classified 35% as having a risk of falling.
Fig 1.
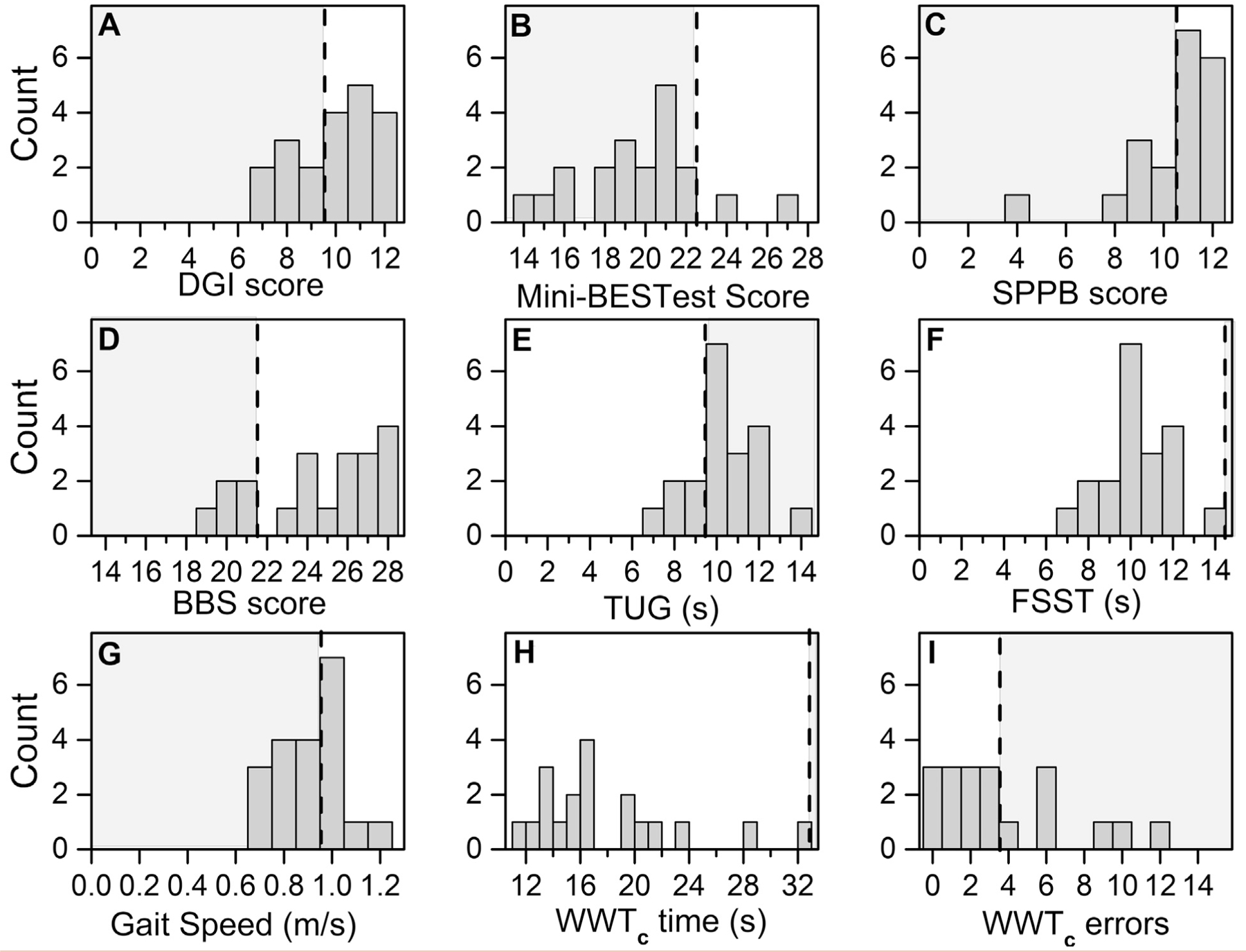
Frequency graphs of mobility function assessments at baseline for patients with high-grade asymptomatic carotid artery stenosis (ACAS). A, Dynamic Gait Index (DGI) score. B, Mini-Balance Evaluation Systems Test (Mini- BESTest) score. C, Short Physical Performance Battery (SPPB) score. D, Berg Balance Scale (BBS) score. E, Timed Up and Go (TUG) time. F, Four Square Step Test (FSST) time. G, Gait speed. H, Walking While Talking complex (WWTc) time. I, WWTc errors. The gray area demarcated by the black dotted line represents the values below the age-related normative scores (BBS, TUG), indicating mobility disability (SPPB), or indicating a high fall risk (DGI, Mini-BESTest, FSST, gait speed, WWTc time and errors).
Table II.
Proportion of patients with impairment on balance and mobility measures (n = 20)
| Balance and mobility measure | Patients with impairment, % |
P valuea | |
|---|---|---|---|
| Baseline | Postoperatively | ||
| Dynamic Gait Index | 35.0 | 20.0 | .38 |
| Mini-BESTest | 90.0 | 40.0 | .02 |
| Short Physical Performance Battery | 35.0 | 30.0 | .9 |
| Short-form Berg Balance Scale | 25.0 | 0.0 | .06 |
| Timed Up and Go test | 75.0 | 65.0 | .53 |
| Four Square Step Test | 0.0 | 0.0 | 1.0 |
| Gait speed | 55.0 | 70.0 | .45 |
| Walking While Talking complex | |||
| Time | 0.0 | 0.0 | 1.0 |
| Errors | 45.0 | 40.0 | .9 |
Mini-BESTest, Balance Evaluation Systems Test.
Using the McNemar test.
Change in functional status after revascularization.
The DGI (P = .021) and Mini-BESTest (P = .002) scores improved significantly after carotid artery revascularization (Fig 2). The SPPB (P < .28) and BBS (P < .43) scores improved numerically; however, the differences did not reach statistical significance. Next, we computed the proportion of patients who had performed at an impaired level at 6 weeks postoperatively using the same reported normative scores to classify impairment at baseline (Fig 3).16,17,21–28 As at baseline, the distribution of scores and frequency of patients with impairment varied across the tests. After surgery, almost all the test scores demonstrated a decrease in the proportion of patients classified as having impairment (range, 0%−70%). Compared with baseline, the proportion of patients with impairment after surgery was significantly reduced using the Mini-BESTest (baseline, 90%; postoperatively, 40%; P = .02; Table II). Only the gait speed showed an increase in the proportion of patients with impairment post-operatively (baseline, 55%; postoperatively, 70%). No other balance and mobility outcome measures were significant.
Fig 2.
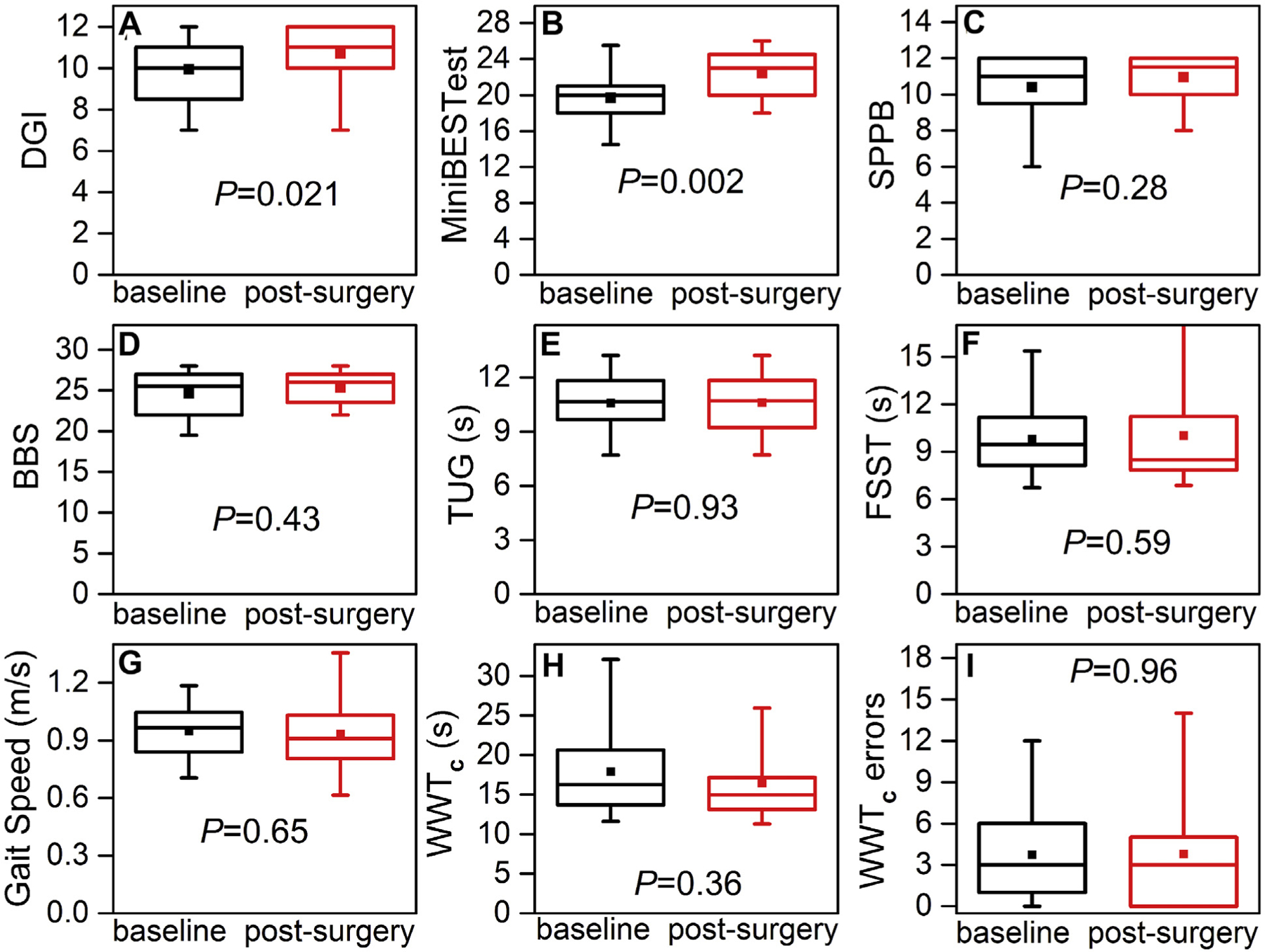
Boxplots of mobility function assessments for patients with high-grade asymptomatic carotid artery stenosis (ACAS) at baseline (black boxes) and 6 weeks after carotid endarterectomy (red boxes). A, Dynamic Gait Index (DGI). B, Mini-Balance Evaluation Systems Test (Mini-BESTest). C, Short Physical Performance Battery (SPPB). D, Berg Balance Scale (BBS). E, Timed Up and Go (TUG) test. F, Four Square Step Test (FSST). G, Gait speed. H, Walking While Talking complex (WWTc) time. I, WWTc errors. Horizontal line in box indicates the median; top line of box, upper quartile (75th percentile); bottom line of box, lower quartile (25th percentile); filled square, mean; and vertical lines (ends of whiskers), minimum and maximum values. P values computed using paired t tests.
Fig 3.
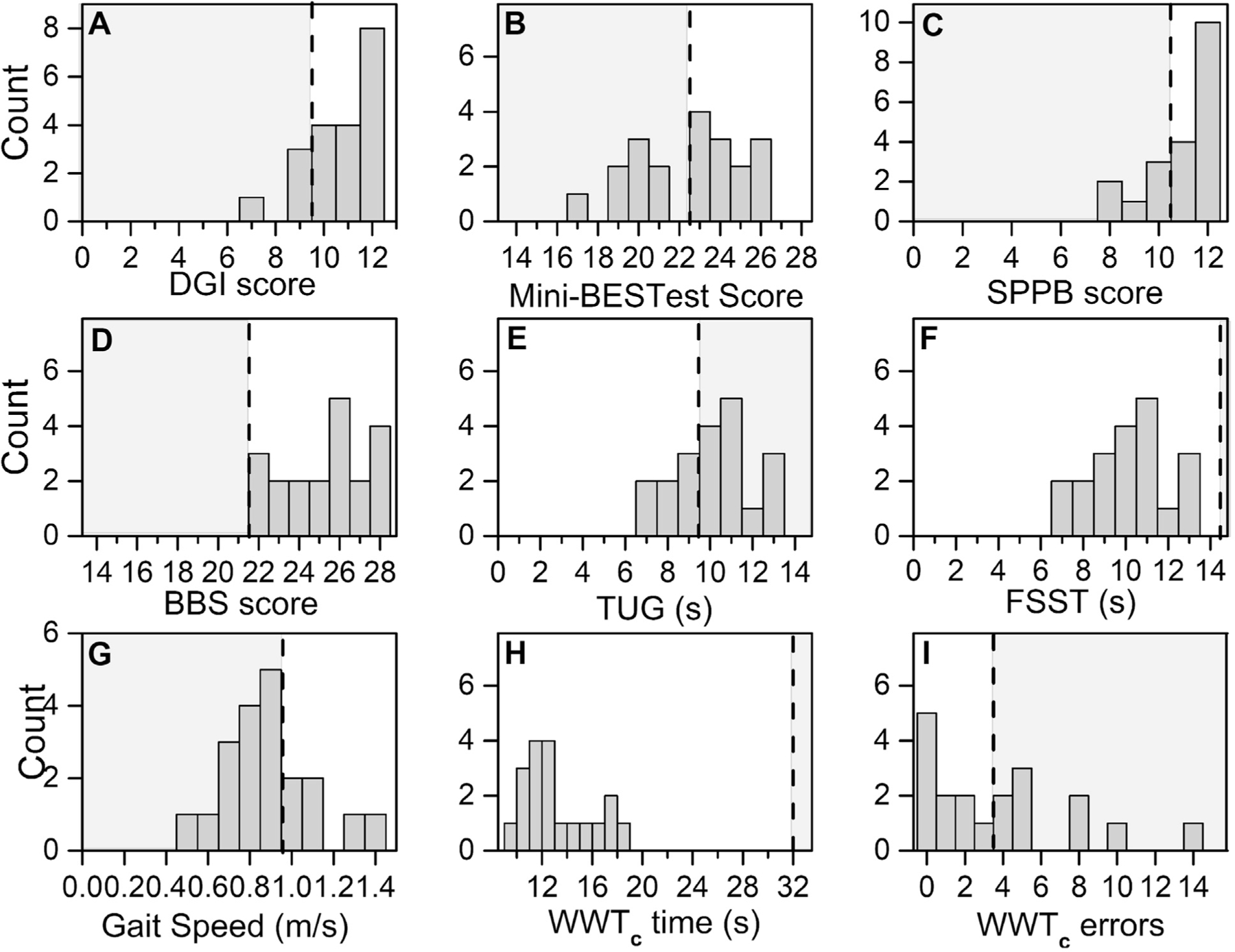
Frequency graphs of mobility function assessments after carotid endarterectomy for patients with high-grade asymptomatic carotid artery stenosis (ACAS). A, Dynamic Gait Index (DGI) score. B, Mini-Balance Evaluation Systems Test (Mini-BESTest) score. C, Short Physical Performance Battery (SPPB) score. D, Berg Balance Scale (BBS) score. E, Timed Up and Go (TUG) time. F, Four Square Step Test (FSST) time. G, Gait speed. H, Walking While Talking complex (WWTc) time. I, WWTc errors. The gray area demarcated by the black dotted line represents the values below age-related normative scores (BBS, TUG), indicating mobility disability (SPPB), or indicating a high fall risk (DGI, Mini-BESTest, FSST, gait speed, WWTc time and errors).
DISCUSSION
To the best of our knowledge, we have demonstrated for the first time that carotid artery revascularization can be beneficial for balance and mobility dysfunction in patients with high-grade (≥70% diameter-reducing) ACAS at 6 weeks after revascularization. Improvements were more significant for the tests involving walking with external demands. This is noteworthy because these tests reflect real-world tasks essential to maintaining independent community mobility. Thus, we have shown that some aspects of balance and mobility performance will improve for patients with ACAS within as few as 6 weeks after revascularization.
Most studies of patients with ACAS have focused on impaired cognition. Given the interdependency of mobility and cognitive function,14,15 which reflects the shared central neural mechanisms, we investigated the effects of ACAS on balance and mobility. The multifactorial processes necessary for balance, mobility, and cognitive function are controlled by similar regions of the brain (ie, frontal and prefrontal lobe-related networks).29 Because similar areas of the brain are used, it is no surprise that individuals with cognitive impairment will also perform poorly on mobility tests.30 We also found that individuals with ACAS and impaired balance and mobility had lower cognitive scores.12 However, not all cognitive domains are equally associated with mobility. Superior performance on balance and mobility tests parallels better performance on executive function, memory, and processing speed assessments.31 Executive function, attention, and fine motor coordination have also been reported to improve after carotid artery revascularization.32–34 Further studies are needed to understand the interconnectedness between mobility and cognitive impairment and the mechanisms that link the two processes in those with ACAS. This will assist in the development of interventions to mitigate these impairments, which is essential for alleviating the burden of disability.
Cerebral perfusion is a likely mechanism through which balance and mobility improvements were mediated after carotid artery revascularization. Compelling evidence is available to suggest that cognitive function is associated with cerebral perfusion.10,35–37 In longitudinal studies, reduced cerebral blood flow has been associated with worsening gait speed, memory, and executive function in those with diabetes.38 Evidence has suggested that patients with ACAS will have cerebral hypoperfusion, which normalizes after revascularization.39,40 Given the relationship between mobility and cognition, and the changes in cognition and cerebral perfusion after surgery,41 it is reasonable that a similar mechanism would lead to changes in balance and mobility. Further research is necessary to explore the exact mechanism that revascularization targets to improve balance and mobility.
Other mechanisms that could negatively affect balance and mobility for patients with ACAS include inflammation, silent brain infarction, and intracranial atherosclerosis.42,43 Elevated inflammatory markers in adults with chronic obstructive pulmonary disease, congestive heart failure, and high cardiovascular risk have been associated with impaired mobility.44 Systemic inflammation impairs cerebral vasoregulation and accelerates declines in executive function, activities of daily living, and gait in older adults with diabetes.45 Interleukin-6 and C-reactive protein are associated with decreased functional connectivity in the frontal and parietal regions in older adults with diabetes.46 More importantly, interleukin-6 is associated with a lower blood oxygen level-dependent signal in the left middle frontal gyrus when working memory demand increases.46 This suggests that systemic inflammation might contribute to impaired balance and mobility. Chronic inflammation results in sarcopenia and muscle weakness, which is known to affect balance and mobility negatively.47 However, it is unlikely that a reversal of sarcopenia and muscle weakness would occur as rapidly as 6 weeks after surgery without active and intense physical therapy. A more direct effect of inflammation on cerebral microglia can also result in mobility declines, although this mechanism is thought to be mediated through chronic cerebral hypoperfusion.48,49 Thus, both inflammation and perfusion might interact to contribute to declines in mobility function. Fixed silent brain infarctions and intracranial atherosclerotic lesions are also known to be associated with cognitive decline50 and mobility function declines.51 Silent infarctions, reported in ~20% of those with ACAS, predominately affects the basal ganglia,52 which, along with the cerebral cortex and cerebellum, are important neural pathways for balance and mobility.53 Silent infarctions have also been associated with an abnormal gait.54 However, these non-modifiable risk factors would not be expected to respond to carotid artery revascularization.
The Mini-BESTest classified 90% of the study patients into an impaired (fall risk) category. The Mini-BESTest measures multiple aspects of balance control, including anticipatory control, reactive control, sensory orientation, and dynamic gait, and might be more sensitive to detecting a fall risk. Our results suggest that more sensitive balance and mobility outcome measures that specifically target the expected impairments in those with ACAS will be essential for identifying patients with impaired performance at baseline and those patients more likely to benefit from therapeutic interventions. We used the same measures in our previous study and found they were sensitive enough to detect group differences among patients with moderate-grade vs high-grade vs no stenosis. In that study, we found that patients with stenosis who demonstrated cognitive and mobility declines were 2.86 times more likely to fall compared with adults without stenosis.12 Also, the patients with the greatest impairment in balance and mobility were more likely to show the greatest improvements at 6 weeks after surgery. Most patients scored well on the SPPB at baseline and, therefore, did not demonstrate a measurable change after surgery. High SPPB scores indicate a higher functioning patient population that is less likely to be limited in activities of daily living (ADLs) or have a risk of frailty. Although we did not assess ADLs or frailty, our patients’ gait speed (range, 0.7–1.2 m/s) did not place them at risk of frailty (<0.80 m/s)55 or dependency in ADLs (<0.55 m/s), except for three patients.56 Balance and mobility function are components of several frailty indexes currently being evaluated for use as risk stratification tools before surgery and in general clinical care. Our results have indicated that some of the frailty scores might actually improve after carotid revascularization. It is evident that not all balance and mobility measures in the present study were impaired. Potentially, these measures (ie, FSST and WWTc time) were not altered, might require longer postoperatively to show changes, or were not sensitive enough to detect an impairment at baseline. Thus, we would not have expected to find improvements in those measures after surgery.
The absence of a control group was a limitation, although withholding surgery to create such a group would have been unethical. Owing to the relatively small number of patients included and the selection criteria, a treatment selection bias could have been present. The patients enrolled in the present study might have had a greater propensity to experience increases in balance and mobility function and those not selected might have been be less likely to show such changes. Potentially, confounders could have been present, such as the interactions with the research staff, that might underlie the balance and mobility improvements. However, the balance and mobility tests administered in the present study are commonly used and have been reported to have excellent testeretest reliability, ranging from 0.84 to 0.97, in older adults, indicating the stability of scores for intervals as brief as 1 to 4 weeks.16,26,57,58 A large sample size would be necessary to definitely ascertain the benefits of carotid revascularization on balance and mobility dysfunction.
CONCLUSIONS
Preserving mobility and balance function is vital for ADLs and for maintaining injury-free independence for older adults. Thus, approaches that can effectively mitigate declines in mobility and balance function are critically important for the aging population. It is becoming increasingly apparent that ACAS is associated with declines in mobility and balance. The results from the present study suggest benefits to balance and mobility dysfunction can be attenuated by 6 weeks after carotid artery surgery. This could indicate that ACAS is a modifiable risk factor for mobility decline in the elderly. Those individuals with the greatest impairments in balance and mobility showed the largest improvements in function after surgery. The next step is to determine why some balance and mobility measures improved after surgery and other domains did not change. Finally, understanding the underlying mechanisms that drive the changes in function are necessary for developing other therapeutic approaches for those patients not recommended for surgery.
ARTICLE HIGHLIGHTS.
Type of Research: A repeated measure design
Key Findings: Individuals with asymptomatic carotid stenosis with diameter reduction ≥70% performed better on some balance and mobility measures after revascularization.
Take Home Message: Asymptomatic carotid artery stenosis is associated with impaired mobility and cognition and a greater fall risk.
Acknowledgments
The present study was supported by a Veterans Affairs Merit Award (grant CX001621); the Geriatric Research, Education, and Clinical Center, Baltimore Veterans Affairs Medical Center; and the National Institute on Aging (grant P30 AG028747).
Footnotes
Author conflict of interest: none.
Accepted for presentation at the 2020 Vascular Annual Meeting of the Society for Vascular Surgery, Toronto, Ontario, Canada, June 17–20, 2020 (conference cancelled). Presented virtually at the 2020 Vascular Annual Meeting of the Society for Vascular Surgery, June 20-July 31, 2020.
The editors and reviewers of this article have no relevant financial relationships to disclose per the JVS policy that requires reviewers to decline review of any manuscript for which they may have a conflict of interest.
REFERENCES
- 1.Bergen G, Stevens MR, Burns ER. Falls and fall injuries among adults aged ≥65 years–United States, 2014. Morb Mortal Wkly Rep 2016;65: 938–83. [DOI] [PubMed] [Google Scholar]
- 2.Stevens JA, Olson S. Reducing falls and resulting hip fractures among older women. MMWR Recomm Rep 2000;49:3–12. [PubMed] [Google Scholar]
- 3.Burns ER, Stevens JA, Lee R. The direct costs of fatal and non-fatal falls among older adults–United States. J Safety Res 2016;58:99–103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Alexander BH, Rivara F, Wolf ME. The cost and frequency of hospitalization for fall-related injuries in older adults. Am J Public Health 1992;82:5–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Onder G, Penninx BWJH, Lapuerta P, Fried LP, Ostir GV, Guralnik JM, et al. Change in physical performance over time in older women: the Women’s Health and Aging study. J Gerontol A Biol Sci Med Sci 2002;57:289–93. [DOI] [PubMed] [Google Scholar]
- 6.Guralnik JM, Simonsick E, Ferrucci L, Glynn R, Berkman L, Blazer D, et al. A short physical performance battery assessing lower extremity function: association with self-reported disability and prediction of mortality and nursing home admission. J Gerontol 1994;49:M85–94. [DOI] [PubMed] [Google Scholar]
- 7.Deandrea S, Lucenteforte E, Bravi F, Foschi R, La Vecchia C, Negri E. Risk factors for falls in community-dwelling older people: a systematic review and meta-analysis. Epidemiology 2010;21:658–68. [DOI] [PubMed] [Google Scholar]
- 8.Guralnik JM, LaCroix AZ, Abbott RD, Berkman L, Satterfield S, Evans DA, et al. Maintaining mobility in late life. I. Demographic characteristics and chronic conditions. Am J Epidemiol 1993;137: 845–57. [DOI] [PubMed] [Google Scholar]
- 9.de Weerd M, Greving JP, de Jong AWF, Buskens E, Bots ML. Prevalence of asymptomatic carotid artery stenosis according to age and sex: systematic review and metaregression analysis. Stroke 2009;40: 1105–13. [DOI] [PubMed] [Google Scholar]
- 10.Lal BK, Dux MC, Sikdar S, Goldstein C, Khan AA, Yokemick J, et al. Asymptomatic carotid stenosis is associated with cognitive impairment. J Vasc Surg 2017;66:1083–92. [DOI] [PubMed] [Google Scholar]
- 11.Johnston SC, O’Meara ES, Manolio TA, Lefkowitz D, O’Leary DH, Goldstein S, et al. Cognitive impairment and decline are associated with carotid artery disease in patients without clinically evident cerebrovascular disease. Ann Intern Med 2004;140:237–47. [DOI] [PubMed] [Google Scholar]
- 12.Gray VL, Goldberg AP, Rogers MW, Anthony L, Terrin ML, Guralnik JM, et al. Asymptomatic carotid stenosis is associated with mobility and cognitive dysfunction and heightens falls in older adults. J Vasc Surg 2020;71:1930–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Whooley JL, David BC, Woo HH, Hoh BL, Raftery KB, Siddiqui AH, et al. Carotid revascularization and its effect on cognitive function: a prospective nonrandomized multicenter clinical study. J Stroke Cerebrovasc Dis 2020;29:104702. [DOI] [PubMed] [Google Scholar]
- 14.Montero-Odasso M, Verghese J, Beauchet O, Hausdorff JM. Gait and cognition: a complementary approach to understanding brain function and the risk of falling. J Am Geriatr Soc 2012;60:2127–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Handing EP, Chen H, Rejeski WJ, Rosso AL, Balachandran AT, King AC, et al. Cognitive function as a predictor of major mobility disability in older adults: results from the LIFE study. Innov Aging 2019;3:igz010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Karthikeyan G, Sheikh SG, Chippala P. Test-retest reliability of short form of berg balance scale in elderly people. Glo Adv Res J Med Med Sci 2012;1:139–44. [Google Scholar]
- 17.Marchetti GF, Whitney SL. Construction and validation of the 4-item dynamic gait index. Phys Ther 2006;86:1651–60. [DOI] [PubMed] [Google Scholar]
- 18.Podsiadlo D, Richardson S. The timed “Up & Go”: a test of basic functional mobility for frail elderly persons. J Am Geriatr Soc 1991;39: 142–8. [DOI] [PubMed] [Google Scholar]
- 19.Franchignoni F, Horak F, Godi M, Nardone A, Giordano A. Using psychometric techniques to improve the Balance Evaluation Systems Test: the mini-BESTest. J Rehabil Med 2010;42:323–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Verghese J, Buschke H, Viola L, Katz M, Hall C, Kuslansky G. Validity of divided attention tasks in predicting falls in older individuals: a preliminary study. J Am Geriatr Soc 2002;50:1572–6. [DOI] [PubMed] [Google Scholar]
- 21.Magnani PE, Genovez MB, Porto JM, Fernanda N, Zanellato G, Alvarenga IC, et al. Use of the BESTest and the Mini-BESTest for fall risk prediction in community-dwelling older adults between 60 and 102 years of age. J Geriatr Phys Ther 2020;43:179–84. [DOI] [PubMed] [Google Scholar]
- 22.Dite W, Temple VA. A clinical test of stepping and change of direction to identify multiple falling older adults. Arch Phys Med Rehabil 2002;83:1566–71. [DOI] [PubMed] [Google Scholar]
- 23.Kyrdalen IL, Ormstad H. Associations between gait speed and well-known fall risk factors among community-dwelling older adults. Physiother Res Int 2019;24:e1743. [DOI] [PubMed] [Google Scholar]
- 24.Cesari M, Kritchevsky SB, Penninx BWHJ, Nicklas BJ, Simonsick EM, Newman AB, et al. Prognostic value of usual gait speed in well-functioning older peopledresults from the health, aging and body composition study. J Am Geriatr Soc 2005;53:1675–80. [DOI] [PubMed] [Google Scholar]
- 25.Verghese J, Kuslansky G, Holtzer R, Katz M, Xue X. Walking while talking: effect of task prioritization. Arch Phys Med Rehabil 2007;88: 50–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Steffen TM, Hacker TA, Mollinger L. Age- and gender-related test performance in community-dwelling elderly people: six-minute walk test, Berg balance scale, timed up & go test and gait speeds. Phys Ther 2002;82:128–37. [DOI] [PubMed] [Google Scholar]
- 27.Vasunilashorn S, Coppin AK, Patel KV, Lauretani F, Ferrucci L, Bandinelli S, et al. Use of the Short Physical Performance Battery score to predict loss of ability to walk 400 meters: analysis from the InCHIANTI study. J Gerontol Med Sci 2009;64A:223–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shumway-Cook A, Brauer S, Woollacott M. Predicting the probability for falls in community-dwelling older adults using the timed up & go test. Phys Ther 2000;80:896–903. [PubMed] [Google Scholar]
- 29.Ezzati A, Katz MJ, Lipton ML, Lipton RB, Verghese J. The association of brain structure with gait velocity in older adults: a quantitative volumetric analysis of brain MRI. Neuroradiology 2015;57:851–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.McGough EL, Kelly VE, Logsdon RG, McCurry SM, Cochrane BB, Engel JM, et al. Associations between physical performance and executive function in older adults with mild cognitive impairment: gait speed and the timed “up & go” test. Phys Ther 2011;91:1198–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Demnitz N, Esser P, Dawes H, Valkanova V, Johansen-Berg H, Ebmeier KP, et al. A systematic review and meta-analysis of cross-sectional studies examining the relationship between mobility and cognition in healthy older adults. Gait Posture 2016;50:164–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wang Q, Zhou M, Zhou Y, Ji J, Raithel D, Qiao T. Effects of carotid endarterectomy on cerebral reperfusion and cognitive function in patients with high grade carotid stenosis: a perfusion weighted magnetic resonance imaging study. Eur J Vascular Endovasc Surg 2015;50:5–12. [DOI] [PubMed] [Google Scholar]
- 33.Wapp M, Everts R, Burren Y, Kellner-Weldon F, El-Koussy M, Wiest R, et al. Cognitive improvement in patients with carotid stenosis is independent of treatment type. Swiss Med Wkly 2015;145:w14226. [DOI] [PubMed] [Google Scholar]
- 34.Pucite E, Krievina I, Miglane E, Erts R, Krievins D, Millers A. Changes in cognition, depression and quality of life after carotid stenosis treatment. Curr Neurovasc Res 2019;16:47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang T, Xiao F, Wu G, Fang J, Sun Z, Feng H, et al. Impairments in brain perfusion, metabolites, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients: an integrated MRI study. Neural Plasticity 2017;2017:8738714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ghogawala Z, Amin-Hanjani S, Curran J, Ciarleglio M, Berenstein A, Stabile L, et al. The effect of carotid endarterectomy on cerebral blood flow and cognitive function. J Stroke Cerebrovasc 2013;22: 1029–37. [DOI] [PubMed] [Google Scholar]
- 37.Lal BK, Younes M, Cruz G, Kapadia I, Jamil Z, Pappas PJ. Cognitive changes after surgery vs stenting for carotid artery stenosis. J Vasc Surg 2011;54:691–8. [DOI] [PubMed] [Google Scholar]
- 38.Dai W, Duan W, Alfaro FJ, Gavrieli A, Kourtelidis F, Novak V. The resting perfusion pattern associates with functional decline in type 2 diabetes. Neurobiol Aging 2017;60:192–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Schröder J, Heinze M, Günther M, Cheng B, Nickel A, Schröder T, et al. Dynamics of brain perfusion and cognitive performance in revascularization of carotid artery stenosis. Neuroimage Clin 2019;22:101779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang T, Sun D, Liu Y, Mei B, Li H, Zhang S, et al. The impact of carotid artery stenting on cerebral perfusion, functional connectivity, and cognition in severe asymptomatic carotid stenosis patients. Front Neurol 2017;8:1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cheng Y, Wang YJ, Yan JC, Zhou R, Zhou HD. Effects of carotid artery stenting on cognitive function in patients with mild cognitive impairment and carotid stenosis. Exp Ther Med 2013;5:1019–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cesari M, Penninx BWJH, Pahor M, Lauretani F, Corsi AM, Williams GR, et al. Inflammatory markers and physical performance in older persons: the InCHIANTI study. J Gerontol A Biol Sci Med Sci 2004;59: 242–8. [DOI] [PubMed] [Google Scholar]
- 43.Poredos P, Spirkoska A, Lezaic L, Mijovski MB, Jezovnik MK. Patients with an inflamed atherosclerotic plaque have increased levels of circulating inflammatory markers. J Atheroscler Thromb 2017;24: 39–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Brinkley TE, Leng X, Miller ME, Kitzman DW, Pahor M, Berry MJ, et al. Chronic inflammation is associated with low physical function in older adults across multiple comorbidities. J Gerontol A Biol Sci Med Sci 2009;64:455–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chung C, Pimentel DA, Alfaro FJ, Lioutas V, Novak V, Israel B, et al. Lower cerebral vasoreactivity as a predictor of gait speed decline in type 2 diabetes mellitus. J Neurol 2018;265:2267–76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dev SI, Moore RC, Soontornniyomkij B, Achim CL, Jeste DV, Eyler LT. Peripheral inflammation related to lower fMRI activation during a working memory task and resting functional connectivity among older adults: a preliminary study. Int J Geriatr Psychiatry 2017;32:341–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ferrucci L, Penninx BWJH, Volpato S, Harris TB, Bandeen-Roche K, Balfour J, et al. Change in muscle strength explains accelerated decline of physical function in older women with high interleukin-6 serum levels. J Am Geriatr Soc 2002;50:1947–54. [DOI] [PubMed] [Google Scholar]
- 48.Kawamoto Y, Akiguchi I, Tomimoto H, Shirakashi Y, Honjo Y, Budka H. Upregulated expression of 14–3-3 proteins in astrocytes from human cerebrovascular ischemic lesions. Stroke 2006;37:830–5. [DOI] [PubMed] [Google Scholar]
- 49.Saggu R, Schumacher T, Gerich F, Rakers C, Tai K, Delekate A, et al. Astroglial NF-kB contributes to white matter damage and cognitive impairment in a mouse model of vascular dementia. Acta Neuropathol Commun 2016;4:76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.de Groot JC, de Leeuw F-E, Oudkerk M, van Gijn J, Hoffman A, Jolles J, et al. Cerebral white matter lesions and cognitive function: the Rotterdam scan study. Ann Neurol 2000;47:145–51. [DOI] [PubMed] [Google Scholar]
- 51.Guo X, Skoog I, Matousek M, Larsson L, Palsson S, Sundh V, et al. A population-based study on motor performance and white matter lesions in older women. J Am Geriatr Soc 2000;48:967–70. [DOI] [PubMed] [Google Scholar]
- 52.Kakkos SK, Sabetai M, Tegos T, Stevens J, Thomas D, Griffin M. Silent embolic infarcts on computed tomography brain scans and risk of ipsilateral hemispheric events in patients with asymptomatic internal carotid artery stenosis. J Vasc Surg 2009;49:902–9. [DOI] [PubMed] [Google Scholar]
- 53.Boisgontier MP, Cheval B, Chalavi S, van Ruitenbeek P, Leunissen I, Levin O, et al. Individual differences in brainstem and basal ganglia structure predict postural control and balance loss in young and older adults. Neurobiol Aging 2017;50:47–59. [DOI] [PubMed] [Google Scholar]
- 54.Brott T, Tomsick T, Feinberg W, Johnson C, Biller J, Broderick J, et al. Baseline silent cerebral infarction in the Asymptomatic Carotid Atherosclerosis Study. Stroke 1994;25:1122–9. [DOI] [PubMed] [Google Scholar]
- 55.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci 2001;56:M146–56. [DOI] [PubMed] [Google Scholar]
- 56.Potter JM, Evans AL, Duncan G. Gait speed and activities of daily living function in geriatric patients. Arch Phys Med Rehabil 1995;76: 997–9. [DOI] [PubMed] [Google Scholar]
- 57.Anson E, Thompson E, Ma L, Jeka J. Reliability and fall risk detection for the BESTest and mini-BESTest in older adults. J Geriatr Phys Ther 2019;42:81–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Studenski S, Perera S, Wallace D, Chandler JM, Duncan PW, Rooney E, et al. Physical performance measures in the clinical setting. J Am Geriatr Soc 2003;51:314–22. [DOI] [PubMed] [Google Scholar]


