Abstract
Peptide drugs play an important role in diabetes mellitus treatment. Oral administration of peptide drugs is a promising strategy for diabetes mellitus because of its convenience and high patient compliance compared to parenteral administration routes. However, there are a series of formidable unfavorable conditions present in the gastrointestinal (GI) tract after oral administration, which result in the low oral bioavailability of these peptide drugs. To overcome these challenges, various nanoparticles (NPs) have been developed to improve the oral absorption of peptide drugs due to their unique in vivo properties and high design flexibility. This review discusses the unfavorable conditions present in the GI tract and provides the corresponding strategies to overcome these challenges. The review provides a comprehensive overview on the NPs that have been constructed for oral peptide drug delivery in diabetes mellitus treatment. Finally, we will discuss the rational application and give some suggestions that can be utilized for the development of oral peptide drug NPs. Our aim is to provide a systemic and comprehensive review of oral peptide drug NPs that can overcome the challenges in GI tract for efficient treatment of diabetes mellitus.
Keywords: Oral nanoparticles, Peptide drugs, Gastrointestinal tract, Diabetes mellitus, Oral bioavailability
Graphical abstract
Highlights
•Oral administration of peptide drugs is a promising strategy for diabetes mellitus treatment
•A series of formidable unfavorable conditions in gastrointestinal tract result in the low oral bioavailability of peptide drugs
•Nanoparticles can improve the oral bioavailability of peptide drugs
1. Introduction
Diabetes mellitus is a group of chronic metabolic syndrome characterized by hyperglycemia, which has reached epidemic dimensions around the world [[1], [2], [3]]. Diabetes mellitus can cause a series of complications including limb amputation, blindness, kidney failure and cardiovascular disease [[4], [5], [6]]. These complications seriously threaten the life of diabetes mellitus patients. Diabetes mellitus can be mainly divided into type 1 diabetes mellitus (T1DM) (∼5%) and type 2 diabetes mellitus (T2DM) (∼95%) according to the pathology features [[7], [8], [9]]. The pathology of T1DM is insulin deficiency own to the apoptosis and loss of insulin-secreting pancreatic β-cells that are destroyed by the T and B cells of the autoimmune system [[10], [11], [12], [13], [14], [15], [16]]. So far, a cure for T1DM is almost not available. T2DM is a complex metabolic disorder featured by insulin resistance in liver and muscle tissues, and excessive hepatic glucose production associated with inappropriately high level of glucagon [[17], [18], [19], [20]]. Chronic hyperglycemia further impairs pancreatic β-cells, which induces the deterioration of the disease [[21], [22], [23]].
Peptide drugs play an important role in different classes of diabetes mellitus treatment due to their excellent therapeutic efficacy and selectivity [24]. Insulin, 51 amino acids, can control glucose homeostasis by stimulating glucose uptake in the insulin-responsive tissues such as skeletal muscles and myocardium, and suppressing glucagon secretion from pancreatic α-cells [25,26]. T1DM and advanced T2DM patients are dependent on multiple daily insulin injections to control the blood glucose level in clinical [[27], [28], [29], [30], [31]]. Glucagon-like peptide 1 (GLP-1) is one of the most effective drugs for the treatment of T2DM [[32], [33], [34]]. GLP-1 increases the insulin secretion from pancreatic β-cells and decreases the glucagon release from pancreatic α-cells [35,36]. In addition, GLP-1 can stimulate the proliferation of pancreatic β-cells and slow the progression of T2DM. Moreover, its effect is glucose concentration-dependent, and the effect is lost when the glucose concentration is below 77 mg/dL. This unique feature can avoid the risk of hypoglycemia. However, GLP-1 is easily degraded by dipeptidyl peptidase-4 (DDP-4), and its clinical application is impeded due to its short half-life in blood (<2 min) [37,38]. There have been many efforts to develop GLP-1 receptor agonists (GLP-1RA). In recent years, GLP-1RA such as exenatide, liraglutide and semaglutide, have received considerable attention for T2DM treatment [[39], [40], [41], [42]]. Compared with the native GLP-1, the GLP-1RA are resistance to the degradation of DDP-4 and have a longer plasma half-life [43]. As the native GLP-1, GLP-1RA can bind to the GLP-1 receptors expressed on pancreatic β-cells, and induce the secretion of insulin in a glucose-dependent manner [44].
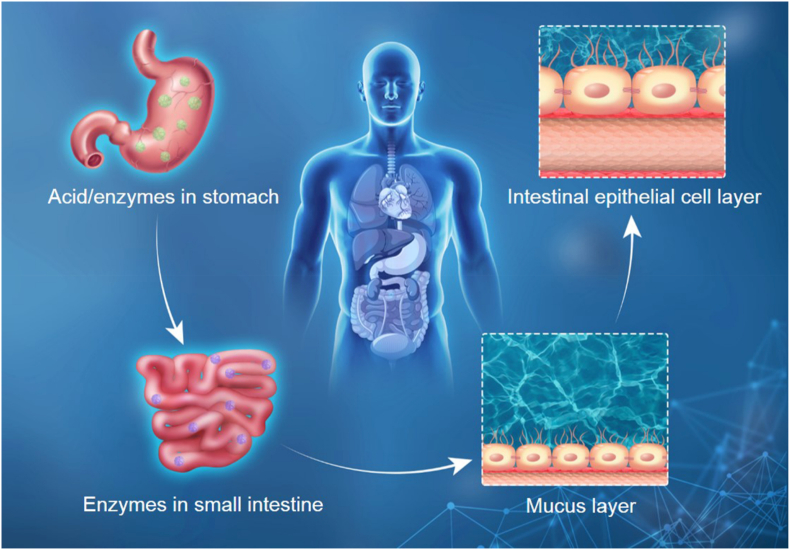
However, most of these peptide drugs are frequently injected via subcutaneous administration for diabetes mellitus therapy in clinical. Unfortunately, subcutaneous injection is invasive [45,46]. The pain, side effects and injection site infection result in the low patient compliance and safety issues of subcutaneous injection [47,48]. Especially, subcutaneous injection of insulin may lead to inadequate diabetes control including hypoglycemia and hyperinsulinemia, which is detrimental and even lethal to diabetes mellitus patients [49,50]. There are some alternative routes for peptide drugs administration, including oral, pulmonary and transdermal et al. [51]. In comparison, oral administration is a promising strategy in treating various diseases because of its convenience and high patient compliance compared to parenteral administration routes [[52], [53], [54]]. Oral administration is a simple and repeatable administration route [55]. Additionally, insulin secreted from pancreatic β-cells gets access to systematic circulation via the portal vein, and GLP-1 is directly secreted by intestinal L-cells [56,57]. Oral administration of insulin or GLP-1RA can imitate the dynamics of the endogenous insulin or GLP-1, and provides better glucose homeostasis. However, the peptide drugs have poor chemical and physical stability against external factors. The poor oral absorption of peptide drugs into blood circulation limits their further clinical application as there are a series of formidable unfavorable conditions present in the gastrointestinal (GI) tract after oral administration [58,59]. As shown in Fig. 1, these mainly include ultra-acidic pH in the stomach, enzymatic degradation in the GI tract, intestinal mucus layer and intestinal epithelial cell layer [60,61]. Among them, their inherent low permeability across the epithelium is the key issue. These unfavorable conditions result in low oral bioavailability (<1%) and high dosage requirement of peptide drugs, which substantially increase the cost of treatment. Therefore, the premise of oral peptide drugs application for diabetes mellitus treatment is to overcome these challenges.
Fig. 1.
The unfavorable conditions in GI tract that oral peptide drug encountered for diabetes mellitus treatment.
Nanoparticle (NP)-based drug delivery systems are regarded as a promising platform to improve the oral absorption of peptide drugs due to their unique in vivo properties and high design flexibility [62,63]. NPs can load peptide drugs and enhance their stability in the GI tract. Additionally, NPs can facilitate the peptide drugs across the mucus layer and intestinal epithelial cell layer to enhance their oral absorption into blood circulation. So far, many efforts have been devoted to develop oral peptide drug NPs for diabetes mellitus therapy. Various NPs have been explored as oral formulations for the treatment of diabetes mellitus. In this review, we will discuss the unfavorable conditions present in the GI tract after oral administration in detail and provide the strategies that can be used to overcome these challenges. Furthermore, we will make efforts to provide a comprehensive overview on the NPs that have been constructed for oral peptide drug delivery. We will highlight the unique properties of each kind of oral peptide drug NPs for diabetes mellitus treatment. Finally, we will discuss the rational application and comment on the potential of translation of these oral NPs. We will give some suggestions that can be utilized for the development of new oral peptide drug NPs. The review will also provide an outlook for this field. Our aim is to provide a systemic and comprehensive review of oral peptide drug NPs that can overcome the challenges in GI tract for efficient diabetes mellitus treatment.
2. Strategies of oral peptide drug NPs to overcome the challenges in gastrointestinal tract
The oral delivery of insulin was firstly studied in the year of 1923 [64]. In September 2019, semaglutide tablet of Novo Nordisk (Rybelsus®) entered the marked. Although this is a big breakthrough in oral peptide drugs for diabetes mellitus treatment, the oral bioavailability of Rybelsus® is only 0.4%–1% after oral administration. The low oral bioavailability of peptide drugs is still a challenge for their clinical application. There are a series of unfavorable conditions in GI tract that affect the oral bioavailability of peptide drugs [[65], [66], [67]]. These unfavorable conditions can restrict the access of toxins and microbial pathogens. However, these chemical and physical conditions also reduce the oral absorption of peptide drug delivery systems [68]. Various strategies have been proposed to overcome these challenges.
The first environment the peptide drugs encounter after oral administration is the degradation environment in the stomach and small intestine. The stomach has an ultra-acidic pH (pH 1–3) and various proteolytic enzymes such as pepsin and cathepsin [69]. The harsh microenvironment in the stomach can destroy the structure of peptide drugs, leading to inactivation and degradation of the peptide drugs. The proteolytic enzymes are present in the lumen of the small intestine, which can also degrade the peptide drugs. The premise of protecting the peptide drugs from degradation in the stomach and small intestine is that the NPs are acid and enzyme-resistant. Additionally, oral peptide drug delivery systems encounter a sudden pH change from the stomach to the small intestine (pH 6.5–8.0), which would induce the dissociation of NPs and result in the leakage of peptide drugs from the oral delivery systems [70]. Hence, the excellent GI stability of NPs is crucial for the success of oral peptide drug delivery. Different enteric polymer materials have been extensively employed in the capsules and/or coating of oral peptide drug NPs. The enteric polymer materials can retain the integrity of oral peptide drug NPs in the stomach. In the more neutral pH environment of the small intestine, the enteric polymer materials are dissolve and achieve controlled release of oral peptide drug NPs. pH-Sensitive poly(methacrylic acid-co-ethyl acrylate) enteric coating, Eudragit L100-55, dissolves at a pH > 5.5 [71]. Therefore, the Eudragit L100-55 is stable in the stomach environment and releases the payloads in the neutral environment of the small intestine. Oral administration of Eudragit L100-55 coated insulin-loaded nanocomplex composed of N-(2-hydroxy)-propyl-3-trimethylammonium chloride modified chitosan (CS)/sodium tripolyphosphate (TPP) showed stronger fluorescence intensity in the small intestine, especially in the ileum segment [72]. The results suggested that the enteric encapsulation could protect the loaded insulin from denaturation and acidic degradation in the stomach environment, and then achieve controlled release in the small intestine.
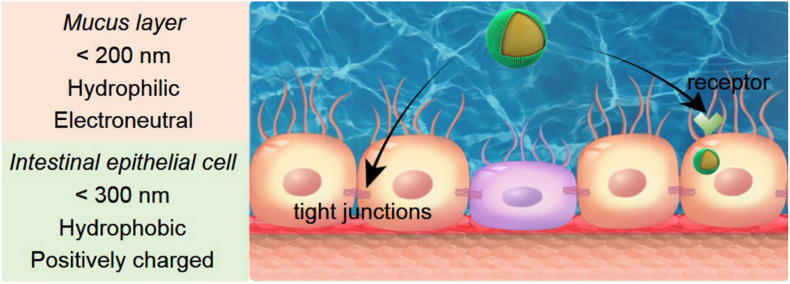
The second barrier is the large GI mucus layer that is continuously secreted by goblet cells. Mucus is composed of cell-associated mucins and glycoproteins [73]. The mucus layer lies adjacent to the absorptive enterocytes and serves as a lubricant for ingested nutrients [74]. The access of the oral peptide drug NPs to the intestinal epithelial cell layer is largely determined by their penetration ability of the mucus layer. The continuously secreted mucus, with a thickness between 37 and 170 μm, efficiently traps larger molecules and pathogens, especially those with cationic property. The mucus then rapidly clears them from penetrating the intestinal epithelial cell layer [75]. Therefore, particles with a positive charge and/or a hydrophobic surface are difficult to cross the mucus layer. Mucus Penetrating Particles (MPP) have been developed to solve this problem. These MPP have hydrophilic and neutral surface. As hydrophilic and electrically neutral polyethylene glycol (PEG) can hinder the positive charge of the drug delivery systems, PEG is always used as a coating on the surface of MPP for rapid diffusion through the mucus layer [76]. However, PEG would reduce the interaction of NPs with epithelial cells. Nowadays, some dissociable "muco-inert" coating materials such as poly(N-(2-hydroxypropyl) methacrylamide) (pHPMA) have been synthesized for mucus permeation [77]. Additionally, NPs were expected to transport primarily through low viscosity pores within the elastic in mucus. The mess spacing ranges from about 10 to 200 nm [78]. Therefore, oral peptide drug NPs with the diameter within 200 nm can achieve easy diffusion.
Intestinal epithelial cell layer is one of the key players of intestinal absorption. The peptide drugs can be absorbed into the blood only after crossing the intestinal epithelial cell layer. The lipid-bilayer cell membranes of the intestinal epithelial cell layer prevent foreign particles from entering into blood. The particles with high molecular weights and hydrophilicity are more difficult to cross the intestinal epithelial cell layer. The transport of hydrophilic macromolecules through intestinal epithelium relies on the paracellular route. However, the paracellular route is restricted by the presence of tight junctions (TJs) that are important components of the intestinal epithelial cell layer [79]. TJs are composed of a complex combination of transmembrane integral proteins, and are located at the luminal aspect of adjacent epithelial cells [80,81]. Only molecules less than 1 nm in hydrodynamic radius are permitted to permeate the TJs. Therefore, TJs form a barrier that prevents the passage of oral peptide drug delivery systems.
There are several pathways that have been proposed to be involved in the transport of free peptide drugs or NPs [82]. These pathways mainly include paracellular transport and receptor-mediated transcytosis [[83], [84], [85]]. Many intestinal permeation enhancers have been explored to facilitate the peptide drugs across the intestinal epithelial cell layer via the paracellular route [[86], [87], [88]]. These absorption enhancers facilitate the paracellular transport by opening the TJs. Over 250 permeation enhancers have improved intestinal permeability. Salcaprozate sodium (SNAC) and sodium caprate (C10) are the two leading intestinal permeation enhancers in oral peptide drug formulations in clinical trials. There is a relatively poor translation of permeation enhancer-based delivery systems for oral peptides. The clinical evaluation has largely been limited to a small group of permeation enhancers that can be formulated into solid-dose formulations and have well safety in man [87,89].
Another strategy is the receptor-mediated endocytosis of NPs to cross the epithelial layer via transcytosis. Ligand functionalization increases membrane affinity and enhances cellular uptake via specific receptor recognition. As shown in Table 1, several ligands can be utilized to facilitate the transport of NPs. Vitamin B12 absorption occurs via receptor-mediated endocytosis after binding its gut transporter [90]. The apical sodium-dependent bile acid transporter (ASBT), the foremost transporter in the distal ileum, can transport the bile acid with a capacity approximated to be 12–18 g/day in human [91]. The neonatal Fc receptor (FcRn) is expressed in the apical region of epithelial cells in the small intestine and diffusely throughout the colon [92]. FcRn interacts with the Fc portion of IgG with a high affinity in acidic pH (pH < 6.5), but not at pH around 7.4. The environment of duodenum and portions of the jejunum is acidic, where the Fc fragments can bind to the FcRn. Additionally, CSKSSDYQC (CSK) peptide has been identified to specifically recognize goblet cells, which is the second large cell population on the epithelium [93,94]. The CSK peptide facilitates the M13 bacteriophages across the intestinal epithelium through its goblet cell-targeting ability [95]. Folic acid can increase the transport of NPs across the intestinal epithelial cell layer by targeting the folate receptors expressed on the apical side mucosa of the intestinal epithelial cell layer [96,97]. Transferrin can mediate receptor-mediated transcytosis across the intestinal epithelium [98]. Butyrate can bind to monocarboxylate transporter-1 (MCT-1), and mediate the transcytosis of NPs to increase their transepithelial transport efficiency [99]. In addition, zwitterions with the structure similar to biological substances have the target ability to the epithelial cell layer. Carboxybetaine (CB) and sulfobetaine (SB) with similar structure to betaine can bind with the proton-assisted amino acid transporter 1 (PAT1) [100,101]. Phosphorylcholine (PC) has high affinity to the intestinal peptide transporter PEPT1 due to its similar structure to di/tripeptides [102]. The cut-off size for NPs to be taken up by epithelial cells has been reported as 300 nm. Therefore, NPs with a size below 300 nm are expected to have better intestinal absorption.
Table 1.
Summary of ligand-receptor based targeting approaches for crossing the intestinal epithelial cell layer.
| Targeting ligand | Receptor | Ref. |
|---|---|---|
| Vitamine B12 | VitB12-IF-IFR (intrinsic factor receptor) | [90] |
| Bile acid | Apical sodium-dependent bile acid transporter | [91] |
| Fc portion of IgG | Neonatal Fc receptor | [92] |
| CSKSSDYQC peptide | Specific receptor on the goblet cells | [93,94] |
| Folic acid | Folate receptor | [96,97] |
| Transferrin | Transferrin receptor | [98] |
| Butyrate | Monocarboxylate transporter-1 | [99] |
| Carboxybetaine/sulfobetaine | Proton-assisted amino acid transporter 1 | [100,101] |
| Phosphorylcholine | Intestinal peptide transporter PEPT1 | [102] |
Overcoming these challenges is essential for enhancing the oral absorption of peptide drugs for diabetes mellitus treatment. As shown in Fig. 2, it should be noted that the requirements of oral peptide drug NPs for crossing the mucus layer and intestinal epithelial cell layer are different, or even contradictory. Hydrophilic and electroneutral surface is required for mucus permeation. However, the hydrophilic and electroneutral surface might decrease the cellular uptake of NPs by the intestinal epithelial cells. NPs with hydrophobic and positively charged surface are more favorable for crossing the intestinal epithelial cells. Therefore, the development of oral peptide drug NPs is always struggled to deal with these dilemmas [103].
Fig. 2.
Favorable physicochemical properties and strategies for oral peptide drug NPs across the mucus layer and intestinal epithelial cell layer.
3. Advances in oral peptide drug NPs for diabetes mellitus treatment
The poor oral bioavailability of peptide drugs is mainly due to their poor stability in the GI physiological environment and low epithelial permeability. Overcoming these problems is essential for enhancing the bioavailability of orally administered peptide drugs. Extensive efforts have been explored to construct oral NPs that can encapsulate peptide drugs. These oral NPs have been designed to overcome the challenges in the GI tract and enhance the oral bioavailability of peptide drugs [104]. Oral peptide drug NPs can be prepared from a variety of materials. As shown in Fig. 3, these oral peptide drug NPs mainly include lipid NPs, polymer NPs, mesoporous silica NPs (MSNs) and metal-organic frameworks (MOFs) [105,106].
Fig. 3.
Illustrative examples of representative kinds of oral peptide drug NPs for diabetes mellitus treatment.
3.1. Lipid NPs
Lipid NPs are delivery systems based on lipid molecules, which can effectively encapsulate hydrophilic and hydrophobic drugs [[107], [108], [109]]. Liposomes have been extensively investigated as drug delivery systems due to their good drug loading ability and biocompatibility [110,111]. Liposomes are spherical, which are composed of hydrophobic lipid bilayers outside and an aqueous space inside. The hydrophilic peptide drugs can be encapsulated in the aqueous space inside the liposomes, which can protect them from the severe enzymatic degradation environment [112]. More importantly, the liposomes with similar bilayer structure of the cell membrane can be absorbed more efficiently by the intestinal cells. The liposomes can be divided into cationic liposomes (CLs), anionic liposomes and neutral liposomes. Among them, neutral liposomes are favorable for mucus penetration due to their hydrophilic and electroneutral surface. However, neutral liposomes have weak interaction with the intestinal epithelial cells.
To deal with this dilemma, target ligands are always modified on the surface of neutral liposomes to promote their ability of crossing the intestinal epithelial cell layer. Yu and co-workers reported a glucose-responsive oral insulin liposome for postprandial glycemic regulation (Table 2) [92]. The glucose-responsive oral insulin liposomes are composed of a glucose-responsive phenylboronic acid (PBA) conjugated hyaluronic acid (HA-PBA) shell and Fc receptor targeted neutral liposomes core loaded with insulin. The encapsulation efficiency and loading content of insulin in liposomes were 20.7% and 17.1%, respectively. The HA-PBA coated the liposomes core through the boronate ester formulation between PBA and catechol groups on the liposome surface. The HA-Fc-Liposomes had an average diameter of approximately 94 nm and a zeta potential of −28.1 mV. The HA-PBA coating could protect the insulin from digesting in the GI tract. Under elevated postprandial glucose concentration in the intestine, the HA shells detached due to the competitive binding of glucose with PBA. The exposed Fc groups then promoted the intestinal absorption of insulin-loaded liposomes for diabetes therapy. In streptozotocin (STZ)-induced type 1 diabetic mice, the green fluorescence of FITC-labeled insulin was obviously distributed in the villi from the mice treated with HA-Fc-Liposomes + glucose after 2 h oral administration. In comparison, there was little fluorescence signal found in the section of mice treated with liposomes with crosslinked HA shell (HA_CL-Fc-liposomes) that could not detach the HA shell to expose the Fc groups. Correspondingly, mice treated with HA-Fc-Liposomes presented a higher plasma insulin concentration and lower blood glucose level than HA_CL-Fc-liposomes.
Table 2.
Summary of NPs used for oral peptide drug delivery in diabetes mellitus treatment.
| Formulation | Delivery system | Drug/In vivo model/Dose | Delivery mechanism | Ref. |
|---|---|---|---|---|
| Lipid NPs | IgG Fc fragment-modified liposome with PBA conjugated HA shell | Insulin/STZ-induced T1DM mice/10 U/kg | HA detached due to the binding of glucose with PBA under elevated postprandial glucose level, exposing Fc that promoted the intestinal absorption of liposomes. | [92] |
| BSA absorbed CLs | Insulin/STZ-induced T1DM mice/75 U/kg | BSA was hydrolyzed by enzymes to expose CLs in mucus layer. | [115] | |
| GCA-decorated CLs | Exendin-4/High fat diet-STZ induced T2DM rats/300 μg/kg | GCA facilitated the transport of NPs across the intestinal epithelial cell layer. | [116] | |
| PLGA core with DSPE-PEG and PLGA-PLR shell | Insulin/STZ-induced T1DM rats/50 U/kg | PEG assisted the NPs across the mucus, and the PLR CPP mediated the transepithelial transport. | [117] | |
| PLGA core with DSPE-PEG-butyrate shell | Insulin/STZ-induced T1DM mice/50 U/kg | Butyrate avoided extra entanglement with mucin and had access to receptor on epithelial cells to facilitate endocytosis. | [118] | |
| DSPE-PCB encapsulated Zn-insulin complex | Insulin/STZ-induced T1DM mice/20 U/kg | PCB enabled micelles penetration through the mucus and cross the intestinal epithelial cell layer mediated by PAT1. | [100] | |
| DLPC on the surface of PLA-based NPs | Insulin/STZ-induced T1DM rats/50 U/kg | DLPC facilitated the mucus permeation and had a high affinity to the PEPT1. | [102] | |
| Polymer NPs | CS/γPGA | Insulin/STZ-induced T1DM rats/30 U/kg | CS increased the residence time of NPs in the intestine and acted as an intestinal permeation enhancer. | [138] |
| Alginate/CS NPs loading Cp1-11 peptide/insulin | Insulin/STZ-induced T1DM rats/50 U/kg | CS increased the residence time of NPs in the intestine and acted as an intestinal permeation enhancer. | [142] | |
| IgG Fc fragment-modified PLA-PEG NPs | Insulin/Wild-type mice/1.1 U/kg | Fc fragments bound to FcRn at the apical surface of absorptive epithelial cells. | [144] | |
| CPP/insulin nanocomplex core with pHPMA coating | Insulin/STZ-induced T1DM rats/75 U/kg | pHPMA facilitated mucus permeation. | [77] | |
| Zwitterionic PCB | Insulin/STZ-induced T1DM rats/50 U/kg | PCB/insulin particles induced the TJs open of intestinal epithelium | [145] | |
| CS-CPP modified GLP-1 loaded PLGA NPs | GLP-1/Nicotinamide-STZ induced T2DM rats/300 μg/kg | CS and CPP increased the intestinal permeation | [147] | |
| Insulin and TMC core with pHPMA coating | Insulin/STZ-induced T1DM rats/50 U/kg | pHPMA facilitated mucus permeation and TMC open the TJs between epithelial cells | [148] | |
| Synthetic polymer PC6 coating on CS NPs | Insulin/STZ-induced T1DM rats/50 U/kg | PC6 facilitated NPs across the mucus and triggered the TJs opening by covalently bind to the cysteine-rich receptors | [149] | |
| Polymerized UDCA | Insulin/NOD mice/285 mIU/kg | pUDCA NPs functioned as a high-avidity bile-acid receptor agonist. | [150] | |
| MSNs | Insulin-loaded MSN modified with PLA-PEG-CPP | Insulin/STZ-induced T1DM rats/80 U/kg | PEG and CPP achieved hydrophilic and electroneutral interaction with mucus. | [157] |
| Insulin-loaded MSN with cationic CPP5 and anionic glutaric anhydride | Insulin/STZ-induced T1DM rats/100 U/kg | The hydrophilic and electroneutral NPs showed lower binding to mucin and faster penetration of the mucus layer. | [158] | |
| CS-conjugated UnTHCPSi NPs with l-cysteine | Insulin/STZ-induced T1DM rats/50 U/kg | Thiol groups of cysteine formed disulfide linkage with the mucin glycoproteins and enhanced the mucoadhesion. | [159] | |
| Insulin-loaded UnTHCPSi NPs encapsulated into a lignin matrix with Fc fragment of IgG | Insulin | Lignins could resist the harsh acidic conditions and Fc fragments bound to FcRn to increase insulin permeation across the intestine. | [161] | |
| CS and CPP modified GLP-1 loaded MSN and DPP4 inhibitor encapsulated in HPMC-AS | GLP-1 | Enteric HPMC-AS polymer protected the insulin from premature release and degradation in the stomach | [162] | |
| GLP-1 loaded CSUn NPs and DPP4 inhibitor incorporated in HPMC-AS | GLP-1/Nicotinamid-STZ induced T2DM rats/250 μg/kg | Enteric HPMC-AS polymer protected the insulin from premature release and degradation in the stomach | [163,164] | |
| MOFs | Acidic-resistant Zr6-based MOF, NU-1000 | Insulin | The one-dimensional pores of NU-1000 allowed insulin encapsulation and excluded pepsin | [167] |
| Insulin-loaded MIL-100 NPs with sodium dodecyl sulfate embedded into mPEG-b-PLLA microspheres | Insulin/STZ-induced T1DM rats/50 U/kg | mPEG-b-PLLA microspheres were resist in the acidic stomach and the hydrophobic modification of sodium dodecyl sulfate promoted the cellular uptake and intestinal absorption. | [168] | |
| Exendin-4-loaded NH2-MIL101 MOF NPs with hydrogel coating of NIPAM and MPDMSA | Exendin-4/High fat diet-STZ induced T2DM rats/500 μg/kg | NIPAM promoted cellular uptake and MPDMSA was benefit for mucus penetration and cellular uptake mediated by the PAT1. | [101] |
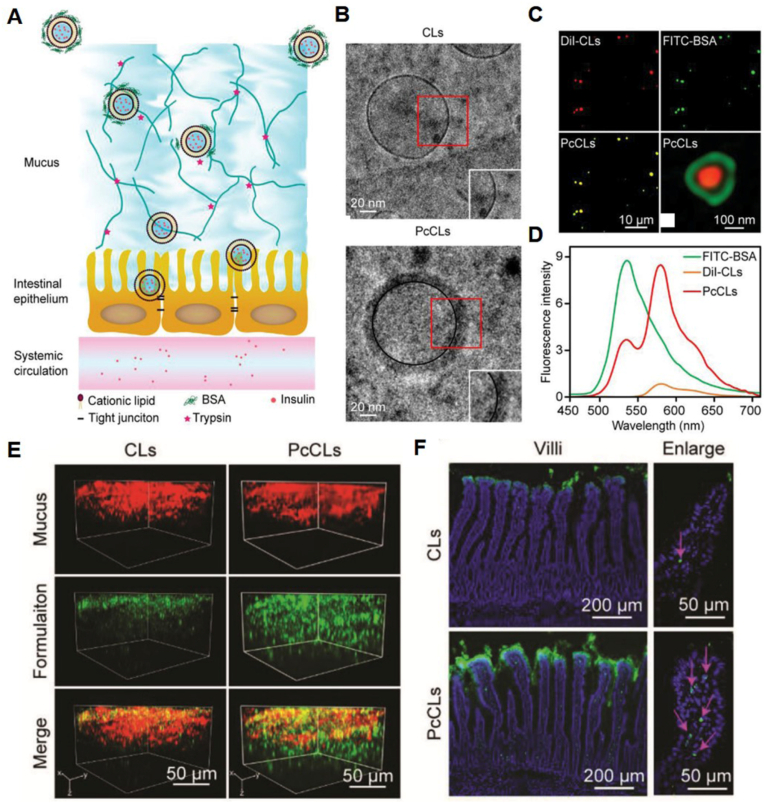
In comparison, CLs can be trapped by the negatively charged mucin [113,114]. However, CLs are benefit for crossing the intestinal epithelial cell layer due to their good cellular uptake. To solve this problem, CLs are coated with negatively charged materials to hide the positive charges. Wang et al. developed protein corona liposomes (PcCLs) for oral insulin delivery (Fig. 4A) [115]. CLs with the component of egg yolk lecithin, cholesterol and 1,2-dioleoyl-3-trimethylammonium-propane (DOTAP) were prepared by thin-film hydration method. Bovine serum albumin (BSA) adsorbed to the surface of the CLs to form PcCLs (Fig. 4B–D). After BSA absorbing, the diameter of PcCLs increased from 144.4 nm to 194.9 nm, and the zeta potential decreased from +41.3% to −10.93%. Insulin was loaded by electroporation method. The encapsulation efficiency and loading capacity of insulin for PcCLs were 28.7 ± 5.1% and 1.5 ± 0.4%, respectively. Compared with native CLs, the PcCLs showed a significantly greater mucus-penetrating velocity (Fig. 4E). The BSA protein corona could be gradually hydrolyzed by enzymes when the PcCLs crossed the mucus layer. The exposed CLs then interacted with the underlying intestinal epithelium to improve the transepithelial transport (Fig. 4F). In STZ-induced T1DM rats, the in vivo antidiabetic experiment showed that the PcCLs groups effectively increased the oral bioavailability of insulin (11.9%), which was 2.21-fold higher than that of CLs without BSA.
Fig. 4.
(A) Schematic diagram for the process of the transport of the PcCLs through the mucus layer and epithelial cell layer. (B) Cryogenic transmission electron microscopy (cryo-TEM) images of CLs and PcCLs. (C) Visualization of the double-labeled PcCLs using fluorescence microscopy and simulated emission depletion (STED) microscopy. (D) Emission spectra of FITC-BSA, Dil-CLs and double-labeled PcCLs at an excitation of 420 nm. (E) 3D images of the mucus penetration of the CLs and PcCLs. Green: DiO-labeled formuation. Red: mucus stained with Alexa Fluro 555-wheat germ albumin. (F) The distribution of the CLs and PcCLs in rat intestinal villi. Green: DiO-labeled formuation. Blue: intestinal villi nuclei stained with 2-(4-amidinophenyl)-6-indolecarbamidine dihydrochloride (DAPI). Red arrow indicated the point of absorption. Reprinted from Ref. [115] with permission.
To further improve the intestinal absorption of CLs, target ligands were modified on the coating molecules. The coating molecules with target ligands were then absorbed onto the CLs to overcome their intrinsic deficiencies. Suzuki and co-workers developed chondroitin sulfate-g-glycocholic acid (CSG)-coated and exendin-4-loaded CLs (EL-CSG) for oral administration of exendin-4 to treat T2DM [116]. CLs comprised of DOTAP and 1,2-dioleoyl-sn-glycero-3-phosphocholine (DOPC) lipids were prepared to encapsulate exendin-4. Glycocholic acid, the richest component in human bile salts, is a promising candidate for target transport of NPs across the intestinal epithelial cell layer. EL-CSG were prepared by electrostatic interaction by mixing cationic liposomes with anionic CSG complex. The size of EL-CSG was 230 nm, which was larger than the CLs before modification (154 nm). The zeta potential of EL-CSG changed from +57 mV to −31 mV after coating with anionic CSG. The loading efficiency of exendin-4 was 74.2%, and the loading content was 1.2% for EL-CSG. In Sprague-Dawley rats, a single oral dose (200 μg/kg) of EL-CSG exhibited an enhanced oral bioavailability (19.5%), while it was 4.1% for EL-CS.
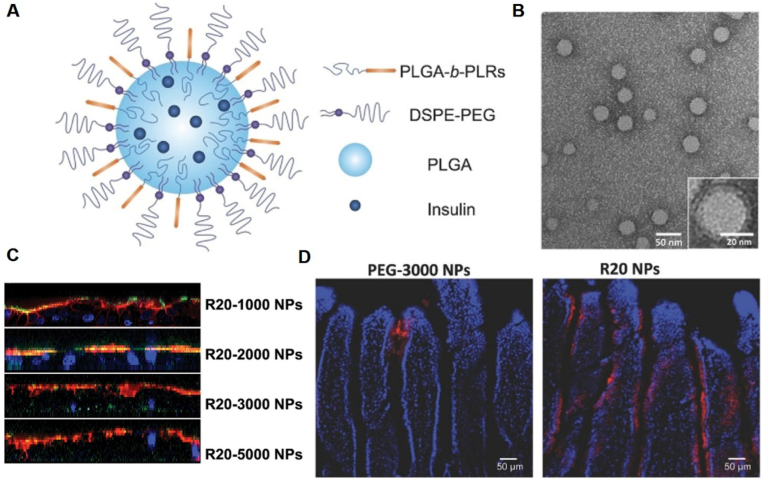
Lipid-based micelles consist of a hydrophobic core and monolayer lipid, and can also be used for peptide drug delivery. Different from liposomes, peptide drugs are encapsulated in the hydrophobic core by co-precipitation. Zhu et al. reported a unique sub-50 nm NP platform that possessed two key surface components, 1,2-distearoyl-sn-glycero-3-phosphoethanolamine (DSPE)-PEG and poly(D,L-lactide-co-glycolide) (PLGA)-polyarginine (PLR) block copolymer (Fig. 5A and B) [117]. Insulin dissolved in dimethyl sulfoxide (DMSO) was co-precipitated with the polymers. The NPs had mean particle size in the range of 40.3–46.8 nm and zeta potential of 7.0–26.9 mV. The encapsulation efficiency was over 50%, and the NPs exhibited sustained release of insulin in phosphate buffer saline (PBS) environment. The "muco-inert" PEG shield assisted the NPs across the mucus (Fig. 5C), and the PLR cell penetrating peptide (CPP) could mediate the cell binding and transepithelial transport (Fig. 5D). In STZ-induced T1DM mice, the pharmacological bioavailability of R20 NPs could reach 12.2%, relative to that of subcutaneous injection. Wu and co-workers reported bioinspired butyrate functionalized PEGylated lipid-based micelles [118]. The micelles with PLGA core and DSPE-PEG-butyrate could load insulin for oral delivery. Butyrate, the largest proportion of metabolites short chain fatty acids, could avoid extra entanglement with mucin by remaining hydrophilicity of NP surface, and has access to receptor on epithelial cells, which facilitated endocytosis of NPs. The diameter of insulin-loaded butyrate-PEG NPs was around 90.8 nm, and the zeta potential was −9.89 mV. The loading efficiency and loading content of insulin were 57.47% and 4.98%, respectively. Insulin-loaded butyrate-PEG NPs generated an oral bioavailability of 9.28%, which was higher than that of PEG NPs (3.23%) in STZ-induced type 1 diabetic rats.
Fig. 5.
(A) Schematic diagram of NP structure. (B) Transmission electron microscopy image of NPs. (C) Vertical distribution of NPs (green) on E12 cell monolayer; mucus were stained with Rho-UEA-I (red), and cell nuclei were stained with DAPI (blue). (D) Representative fluorescence images of 12 mm sections of mouse intestine after administration of fluorescently labeled PEG3000 or R20 NPs (red) by oral gavage. Cell nuclei were stained with DAPI (blue). Reprinted from Ref. [117] with permission.
Additionally, metal ions such as zinc ion (Zn2+) can chelate peptide drugs and achieve high drug loading, while maintaining the small size of delivery systems [119]. Zn-insulin is more resistant to enzymatic degradation by chymotrypsin and does not undergo fibrillation. Zn-insulin can be converted to free insulin by Zn2+ dilution. Recently, it has been found that zwitterionic polymers are promising for oral peptide drug delivery for diabetes mellitus treatment. Han and co-workers developed zwitterionic polycarboxybetaine (PCB) polymer modified lipid DSPE-PCB of 5,000 Da molecular weight for insulin oral delivery [100]. Zn-insulin complexes were loaded into DSPE-PCB micelle with a hydrodynamic size below 30 nm. The encapsulation efficiency for insulin could reach over 98%. Zwitterions are electrically neutral that composed of equal cationic groups and anionic groups [[120], [121], [122]]. Micelles with PCB possess zwitterionic and hydrophilic surfaces as capsid viruses. The DSPE-PCB micelles diffused in mucus nearly one order of magnitude faster than PEG particles of a comparable size, which enabled drug penetration through the mucus. Moreover, due to the recognition between PCB and PAT1, DSPE-PCB were able to address the epithelial cell layer. The freeze-drying process of the DSPE-PCB/insulin formulation did not deteriorated the in vivo absorption performance. Directly encapsulated into a Eudragit L100-55 enteric-coating porcine gelatin capsule after lyophilization, the oral bioavailability of the DSPE-PCB/insulin capsule was as high as 42.6%. The components and preparation process of DSPE-PCB/insulin formulation were simple, and the DSPE-PCB/insulin formulation could cleverly overcome the GI physiological barriers. Therefore, the DSPE-PCB/insulin formulation had the potential to be a practical solution for oral peptide drug delivery in diabetes mellitus treatment.
Some researchers have tried to find the neutral lipids that might have strong interaction with the intestinal epithelial cell layer. Interestingly, Shan, et al. reported a simple zwitterionics-based NP delivery platform with the dilauroylphosphatidylcholine (DLPC) coating on the surface of poly(lactic acid) (PLA)-based NPs [102]. The insulin-loaded DLPC NPs had a size of 107.5 nm and nearly neutral charge surface. The insulin encapsulation efficiency was 29.6% with the loading content of 4.6%. DLPC with a hydrophilic zwitterions PC headgroup, as a "muco-inert" material, facilitated the mucus permeation. In addition, the hydrophilic head of DLPC has a high affinity to the intestinal peptide transporter PEPT1. Compared with PEGylated NPs, the DLPC NPs significantly improved (4.5-fold) the cellular uptake. The oral bioavailability was 4.76% for the insulin-loaded DLPC NPs in STZ-induced type 1 diabetic rats, which was 6.89-fold higher than that of free oral insulin and 2.07-fold higher than that of poly(vinyl alcohol) coated PLA NPs. The zwitterions lipids might provide a new opportunity for oral peptide drug delivery in diabetes mellitus treatment.
Although lipid-based NPs can improved the oral bioavailability of peptide drugs for diabetes mellitus treatment, from the existing studies, we could see that the encapsulation efficiency of peptide drugs in lipid-based NPs was relative low, especially liposomes. The low encapsulation efficiency would increase the cost for treatment. In addition, the poor stability of lipid-based NPs in biological fluids and during storage is always disturbing, which would hinder their clinical application.
3.2. Polymer NPs
NPs prepared from a variety of polymers have been explored for oral peptide drug delivery in diabetes mellitus treatment due to their good biocompatibility, degradation ability and diverse chemistry [[123], [124], [125], [126]]. These polymers could be divided into natural polymers and synthetic polymers according to their sources [127]. The natural polymers for oral peptide drug delivery mainly include chitosan (CS), alginate, dextran and gelatin. CS, a polysaccharide derived from the naturally occurring chitin, is non-toxic and soft tissue compatible [128]. CS with different biodegradability and charge density at physiological pH can be obtained by varying its molecular weight and degree of deacetylation [129]. CS has been intensively studied for oral peptide drug delivery due to its unique properties. CS can adhere to the mucosal and cellular surface, thereby increasing the residence time of the delivery systems at the absorption site [130,131]. Moreover, CS has the unique feature of transiently opening the TJs between intestinal epithelial cells, subsequently increasing the permeation of peptide drugs via the paracellular pathway [132,133]. Therefore, CS can act as an intestinal permeation enhancer that facilitates the peptide drugs efficiently overcome the intestinal epithelial cell layer. It has been found that the positively charged amino groups on the CS backbone had interaction with negatively charged macromolecules such as integrin αγβ3 on the cell membrane, which could lead to the redistribution of claudin-4 (CLDN4) from the cell membrane to the cytosol, where the CLDN4 was degraded in lysosomes [[134], [135], [136]]. The degradation of CLDN4 resulted in an increase in paracellular permeability. Furthermore, the TJs could be recovered after CS treatment due to the synthesis of CLDN4.
Numerous studies have demonstrated the potential use of CS as an intestinal permeation enhancer for the oral delivery of peptide drugs. In addition, CS has a number of free amine groups and can easily form ionic cross-linkage with multivalent anions [137]. Sung et al. described pH-responsive CS/poly(γ-glutamic acid) (CS/γPGA) NPs for oral delivery of insulin [138]. γPGA is a water-soluble anion peptide that is biodegradable and nontoxic [139]. With an excessive CS, the NPs had a diameter of approximately 250 nm and a zeta potential of 25 mV. The insulin loading efficiency and content were 75% and 15%, respectively. The NPs with CS could adhere to and infiltrate the mucus layer. The NPs then approached the epithelial surface, especially luminal surface in the duodenum (pH 6.0–6.6). The CS NPs opened the epithelial TJs and increased the paracellular permeability of insulin. The oral bioavailability was about 15% for CS/γPGA NPs. The CS NPs remained intact in the pH range of 2.0–7.2. However, the NPs could be disintegrated at lower pH in the stomach [140]. Experiments found that the oral bioavailability could reach 21% after filled the free-dried NPs in Eudragit L100-55 enteric-coated capsules. Additionally, they found that the CS NPs were suitable for oral delivery of exendin-4. The oral bioavailability could reach approximately 15% after oral administration of the capsules containing exendin-4-loaded CS NPs. Therefore, the CS NPs would provide an efficient platform for oral peptide drug delivery in diabetes mellitus therapy.
CS has also been used to combine with other biodegradable and biocompatible nature polymers such as alginate and dextran to increase the encapsulation efficiency of peptide drugs, prolong the residence time of NPs in the small intestine and enhance their cellular permeability. Alginate is a polysaccharide derived from brown seaweeds and is an unbranched copolymer consisting of alternating blocks of 1–4 linked α-l-guluronic acid (G-block) and β-d-mannuronic acid (M-block) residues [141]. Chen and co-works have constructed CS/alginate NPs as carriers for the oral delivery of the Cp1-11 peptide/insulin complex (CILN) [142]. Cp1-11 peptide (EAEDLQGVE) formed a fold with insulin via a supramolecular interaction, making the insulin dimer disaggregate and dramatically improving the activity and bioavailability of insulin. The strong electrostatic interaction between alginate and CS led to shrinking and gel formation at low pH, and swelling into a core-shell structure of CILN. The diameter of CILN was 237.2 nm. The insulin loading efficiency was 90.43%, and the loading content was 28.06%. The pH-responsive CILN protected insulin against degradation in the GI tract. In STZ-induced diabetic rats, the CILN led to much higher oral bioavailability (15.62%) than free insulin (0.08%).
In addition, NPs formed from synthetic polymers such as PLGA, poly(ε-caprolactone) (PCL) and PLA have been increasingly used for oral peptide drug delivery in diabetes mellitus treatment due to their unique properties [143]. Compared with natural polymers, the structure of synthetic polymers is more diverse. Additionally, the strong mechanical strength gives the synthetic polymers high stability. Pridgen et al. prepared Fc-targeted PLA-PEG NPs for oral delivery of insulin [144]. After dissolving insulin and PLA-PEG-MAL in DMSO, the insulin-loaded NPs were prepared by nanoprecipitation with a mean hydrodynamic diameter of 57 nm and an insulin load of 0.5% (w/w). Polyclonal IgG Fc fragments were covalently conjugated to the NPs using MAL-thiol chemistry. Oral administered Fc-targeted PLA-PEG NPs crossed the intestinal epithelium and reached the systemic circulation with a mean absorption efficiency of 13.7%*h compared with only 1.2%*h for non-targeted NPs in mice. Targeted NPs with insulin exhibited a prolonged hypoglycemic response in wide-type mice at a clinically relevant insulin dose of 1.1 U/kg.
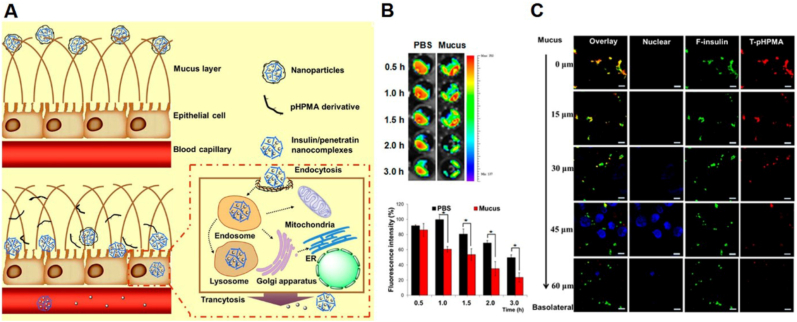
Another synthetic polymer is pHPMA that is always used in oral peptide drug delivery for the treatment of diabetes mellitus. pHPMA can be a dissociable "mucus-inert" coating material, that facilitates mucus permeation. Shan et al. constructed self-assembled NPs composed of insulin and CPP that coated with pHPMA derivatives (Fig. 6A) [77]. The size of pHPMA coated NPs was approximately 175 nm. NPs exhibited insulin encapsulation efficiency above 80% and loading content above 40%. Although CPP can facilitate cellular internalization of NPs, their cationic property hinders their permeation in mucus. The pHPMA coating overcame this drawback and could gradually detach from the NPs during their permeation through the mucus to expose the CPP (Fig. 6B and C). The NPs exhibited 20-fold high absorption than free insulin on mucus-secreting epithelial cells. The orally administered NPs generated a prominent hypoglycemic response and an increase of the serum insulin concentration in STZ-induced diabetic rats. The oral bioavailability of pHPMA NPs-1 was 3.02%, which was 2.08-fold higher than that of CPP/insulin nanocomplex.
Fig. 6.
(A) Schematic illustration of the pHPMA NPs across the mucus layer and intestinal epithelial cells. (B) Fluorescence resonance energy transfer (FRET) intensity of particles prepared with F-insulin and T-pHPMA after incubation in PBS or mucus for different times. The fluorescent intensity of tetraethyl rhodamine isothiocyanate (TRITC) represented the structural integrity of particles. (C) Confocal laser scanning microscopy (CLSM) images of the distribution of F-insulin and T-pHPMA in NPs on E12 cell monolayer from apical to basolateral side. Reprinted from Ref. [77] with permission.
Zwitterionic PCB, with cationic quaternary ammonium group and anionic carboxyl acid group alone, is neutral at pH 7.4 and cationic in acidic environment. In our recent study, we found that charge-switchable zwitterionic PCB could load insulin through the electrostatic interaction in pH 5.0 [145]. The encapsulation efficiency of insulin was above 86%, and the diameter of PCB122/insulin particles was about 80 nm. The zeta potential of particles was decreased with the increased of pH value simulating stomach, duodenum, and the body fluid at the intercellular spaces between epithelial cells. The PCB/insulin particles with negatively charged carboxyl acid groups on the surface, could induce the open of the TJs of intestinal epithelium in the endocytosis-mediated lysosomal degradation pathway. This effect increased the intestinal permeability of insulin that sustained release from the particles at pH 7.4. In STZ-induced type 1 diabetic rats, oral administration of PCB122/insulin particles, especially in capsules, significantly enhanced the oral bioavailability of insulin (27.0%). Additionally, the open effect of the particles on the TJs of intestinal epithelium was reversible, and there was no endotoxin and pathological change during treatment.
Both natural polymers and synthetic polymers have their own advantages. By combing natural polymers with synthetic polymers, superior oral peptide drug delivery systems would be constructed with enhanced oral bioavailability and therapeutic efficacy on diabetes mellitus. For instance, PLGA can achieve controlled release of peptide drugs and protect them from enzymatic degradation in the GI tract [146]. However, the penetration of PLGA NPs through the mucus and epithelial cell layer is limited due to their negatively charged surface. Cationic polymers or CPP could be modified on the surface of PLGA NPs to increase the intestinal permeation. Araújo and co-workers prepared GLP-1 loaded PLGA NPs that were functionalized with CS and CPP [147]. The conjugation of CS and CPP increased the size of NPs from 174 ± 5 to 351 ± 4 nm. The CS and CPP conferred a positive charge of NPs from −20 ± 2 to 40 ± 0 mV. The modification had almost no influence on the association efficiency of GLP-1 (approximately 70%). They then assembled GLP-1 and DPP4 inhibitor (iDPP4) dual-delivery system by encapsulating GLP-1 loaded PLGA NPs and iDPP4 within an enteric hydroxypropylmethylcellulose acetylsuccinate (HPMC-AS) polymer through microfluidics system. The H-PLGA particles presented size around 60 ± 7 μm. In STZ-nicotinamide induced T2DM rats, the blood glucose levels were decreased by 44%, and were constant for another 4 h after oral administration of the dual-delivery H-PLGA particles.
Additionally, it has been found that the mucoadhesive property of CS might limit their access to the epithelial surface. The dissociable synthetic polymer pHPMA can be used to overcome the drawback of the CS-based oral peptide drug delivery system. Liu et al. reported an insulin-loaded oral NP platform that possessed a core composed of insulin and trimethyl chitosan (TMC) with pHPMA derivative coating [148]. The size of P-T-NPs was 163.1 nm with a low polydispersity index (PDI) of 0.160 after the pHPMA coating. The zeta potential was nearly neutral charge (−3.35 mV). The loading efficiency and loading content of insulin for P-T-NPs were 54.1% and 26.5%, respectively. The P-T-NPs exhibited excellent mucus permeability due to the pHPMA modification. The pHPMA molecules started to dissociate from the P-T-NPs in mucus, and the TMC facilitated transepithelial transport via the paracellular pathway. In STZ-induced diabetic rats, the P-T-NPs exhibited an oral bioavailability of 8.56%, which was 2.8-fold higher than that of uncoated TMC-based NPs. Zhou and co-workers constructed PC6/CS NPs that synthetic polymer poly(acrylic acid)-cysteine-6-mercaptonicotinic acid (PC6) was coated on chitosan NPs [149]. The size of PC6/CS NPs was 233 nm with a PDI of 0.31. The zeta potential decreased from 32 mV to 12 mV for the PC6/CS NPs. The encapsulation efficiency and loading content were 80.5% and 3.49%, respectively. The PC6 with reactive thiol groups could covalently bind to the cysteine-rich receptors such as EGFR and IGFR, and trigger the TJs opening. Additionally, it could interact with mucin by disulfide formation in the mucus and facilitate the CS NPs across the mucus. PC6/CS NPs showed an oral bioavailability of 16.22%, which had a significant difference compared to CS NPs. Therefore, the combination of natural and synthetic polymers would be a promising strategy for the development of oral peptide drug NPs in diabetes mellitus therapy.
Additionally, some monomers with biological activity can be polymerized as effector therapeutic systems. Polymerization can amplify the effector function of monomers, and the polymers can be used to encapsulate and control the release of peptide drugs. Bile-acid monomer ursodeoxycholic acid (UDCA) can impact insulin sensitivity and lower insulin resistance in T2DM. Lee et al. polymerized UDCA (pUDCA) and formulated it into NPs for the oral delivery of insulin [150]. NPs with spherical morphology were with an average diameter of about 344.3 nm and a zeta potential of about −24.9 mV. Loading efficiency of insulin was 0.02 μg/mg particles. The pUDCA NPs functioned as a protective insulin carrier and a high-avidity bile-acid receptor agonist. The pUDCA NPs increased the intestinal absorption of insulin and polarized intestinal macrophages towards the M2 phenotype. The pUDCA NPs preferentially accumulated in the pancreas of the mice, binding to the islet-cell bile acid membrane receptor TGR5 with high avidity and activating the secretion of glucagon-like peptide and of endogenous insulin. The pUDCA NPs could also reverse inflammation, restore metabolic functions and extend animal survival. The pUDCA NPs could restore blood glucose level in mice and pigs with established T1DM. The metabolic and immunomodulatory functions of pUDCA NPs may offer translational opportunities for the prevention and treatment of T1DM.
The encapsulation efficiency of peptide drugs in polymer NPs is obviously high, and the diverse chemistry makes polymers have multiple functions. However, the polymer NPs are easily disaggregated upon dilution in biological fluids, which would result in the burst and early release of peptide drugs. The early release of peptide drugs in GI tract would reduce their oral bioavailability and hypoglycemic effect. If this occurs in blood for insulin, this would cause the adverse reaction of hypoglycemia.
3.3. MSNs
NPs composed of inorganic molecules have also been explored as oral peptide drug delivery for the treatment of diabetes mellitus. MSNs have recently been studied as a delivery system for a variety of drugs due to their excellent physicochemical stability and good biocompatibility [151,152]. MSNs have large internal surface area and pore volumes with adjustable diameters (2–50 nm), and they can incorporate peptide drugs with high payloads [153]. Drugs can be simply loaded inside the mesopores via physical absorption or electrostatic interaction. Araújo and co-workers constructed three different kinds of NPs with PLGA, lipid Witepsol E85 and porous silicon, respectively [154]. They found that the undecylenic acid modified thermally hydrocarbonized porous silicon (UnTHCPSi) NPs exhibited the highest encapsulation efficiency (85.0 ± 0.6%) and loading content (17.00 ± 0.05%) of GLP-1 after physical absorption.
However, the MSNs alone cannot completely overcome the multiple GI challenges after oral administration. Therefore, the MSNs are always tailored with organic materials due to their tunable surface modification [155]. Different organic materials can be modified on the surface of MSNs with silanol groups. The decoration of the organic materials on the inner or outer surface of the MSNs can impart some features to overcome unfavorable conditions in the GI tract for successful oral peptide drug delivery [156]. To enhance the penetration of the intestinal mucus layer and the intestinal epithelium, polymers, CPP and/or target ligands could be modified on the surface of MSNs. Tan et al. reported insulin-loaded MSNs modified with a hydrophilic block polymer PLA-PEG-CPP (INS@MSN@PLA-PEG-CPP) [157]. The INS@MSN@PLA-PEG-CPP with a hydrodynamic particle size of about 333.9 nm had a zeta potential of 0.8 mV. The insulin encapsulation efficiency was approximately 81.0%. The sequential PEG and CPP modification achieved hydrophilic and electroneutral interaction with mucus, which promoted cellular uptake against mucus trapping and increased in vivo pharmacological availability by 14.2-folds than that of the oral insulin solution. Zhang and co-workers fabricated insulin-loaded MSNs with modification of cationic CPP5 (KLPVM peptide) and anionic glutaric anhydride (MSN-NH2@COOH/CPP5) [158]. The insulin encapsulation efficiency was above 80%, and the loading content was approximately 18%. The hydrophilic and electroneutral NPs showed dramatically lower binding to mucin and faster penetration of the mucus layer than the positively charged MSN@NH2. In STZ-induced type 1 diabetic rats, insulin-loaded MSN-NH2@COOH/CPP5 had the highest oral bioavailability of 2.48% after intrajejunal administration, which was 2.1-fold higher than that of free insulin. Shrestha and co-workers synthesized CS-conjugated UnTHCPSi NPs that were further modified with l-cysteine (CYS-CSUn NPs) [159]. Surface modification with CS led to significant increase in interaction of porous silicon with Caco-2/TH-29 cell co-culture monolayers, and significantly improved the permeation of insulin [160]. The thiol groups presented on the surface of CYS-CSUn NPs would form disulfide linkage with the mucin glycoproteins and enhance the mucoadhesion. The NPs had comparable size and zeta potential with the unmodified NPs (229 ± 15 nm, +26 ± 6 mV). The insulin encapsulation efficiency and loading content were approximately 60% and 20%, respectively. The CYS-CSUn could significantly enhance the permeability of insulin, and the oral bioavailability of CYS-CSUn was 5.1%, which was 2.03-fold higher than oral insulin solution.
Furthermore, considering the harsh environment of the stomach, pH-sensitive polymers are always used to protect the peptide drugs from the degradation in the stomach. Martins and co-workers constructed neonatal Fc receptor-targeted pH-responsive lignin matrix encapsulated UnTHCPSi NPs for oral insulin delivery (insulin-loaded UnTHCPSi@LNPs-Fc) [161]. The insulin-loaded UnTHCPSi@LNPs-Fc had a negative surface charge of −21 ± 1 mV. The loading efficiency and loading content of insulin for UnTHCPSi@LNPs-Fc were 7.8% and 2.1%, respectively. Lignins could resist the harsh acidic conditions and protect the insulin from premature release and degradation in the stomach. Compared to the non-functionalized NPs, the Fc-functionalized NPs induced an increase in the permeation of insulin across a Caco-2/TH29-MTX co-culture model.
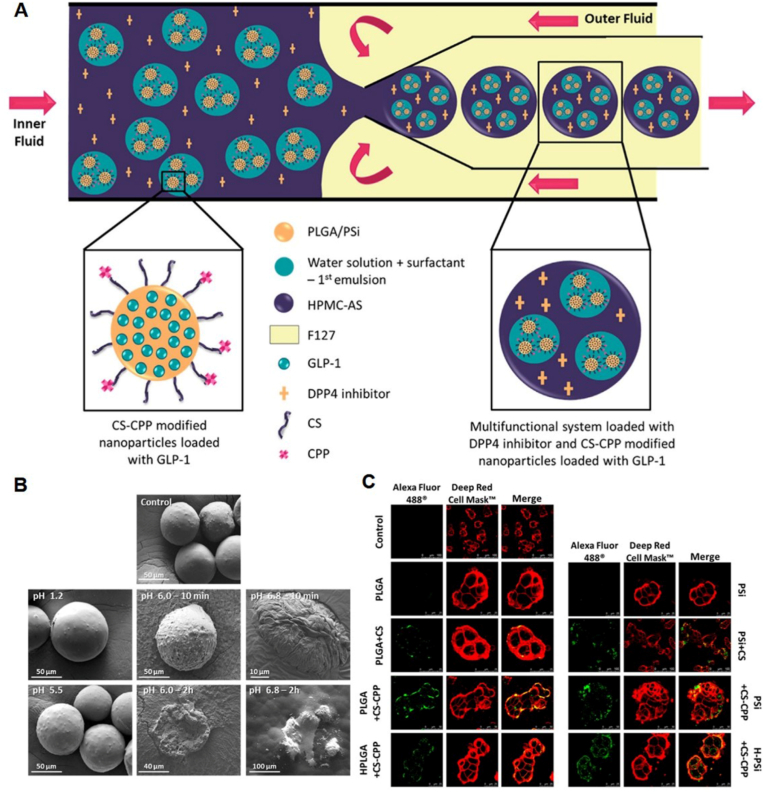
Araújo and co-workers constructed HPMC-AS coated multifunctional system loaded with DPP4 enzymatic inhibitor and CS-polyarginine R9 CPP modified porous silicon NPs loaded with GLP-1 (PSi + CS-CPP) (Fig. 7A) [162]. The PSi + CS-CPP NPs had a size of 320.0 ± 9.8 nm with a PDI of 0.33 ± 0.02. The zeta potential was 19.1 ± 1.0 mV. The encapsulation efficiency and loading content of GLP-1 for PSi + CS-CPP NPs were 75.0 ± 0.5% and 7.50 ± 0.03%, respectively, which were higher than that of PLGA + CS-CPP NPs. The PSi + CS-CPP NPs were further encapsulated in an enteric HPMC-AS polymer using microfluidics technique. The produced particles (H–PSi + CS-CPP) had a spherical shape with size around 59.44 ± 8.01 μm. The enteric polymer inhibited the degradation of CS and CPP in the harsh stomach, and could release the NPs only in intestinal conditions (Fig. 7B). With the assistant of CS and CPP, the MSNs showed stronger interaction with the intestinal cells than the unmodified NPs (Fig. 7C). They also incorporated GLP-1 loaded CSUn NPs in the matrix of enteric HPMC-AS polymer (H-CSUn NPs) for the co-delivery of GLP-1 and DPP4 inhibitor using a single step aerosol flow reactor technology [163,164]. Different from H–PSi + CS-CPP, the size of HPMC-AS coating particles was approximately 400 nm. No detectable amounts of GLP-1 were released at pH 1.2, and a burst drug release of ∼37% was observed at pH 6.8 due to the encapsulation and protection of HPMC-AS. Therefore, the tailored MSNs have good clinical potential for oral peptide drug delivery for the treatment of diabetes mellitus.
Fig. 7.
(A) Schematic representation of the microfluidics approach used to produce the pH-responsive systems, coloaded with GLP-1 and DPP4 inhibitor. (B) Scanning electron microscopy (SEM) images showed the dissolution behavior of the CS-CPP NPs encapsulated in the HPMA-AS polymer at different pH condition. (C) Interaction between the different NPs and the Caco-2/HT29-MTX coculture after incubation for 3 h at 37 °C. CLSM images of the cell membranes stained in red by CellMask Red, and the NPs in green conjugated with Alexa Fluor 488. Reprinted from Ref. [162] with permission.
Due to the large internal surface area and pore volumes, the encapsulation efficiency of peptide drugs in MSNs is always high. However, the ability of MSNs across the unfavorable conditions in GI tract is limited, and MSNs are always needed further modification with functional excipients. In addition, MSNs are not biodegradable. Therefore, the clearance and immune response need more attention.
3.4. MOFs
MOFs composed of metal nodes connected by organic ligands are a class of porous materials with uniform crystalline structures. The pore sizes and functionalities of the MOFs can be modulated through the change of organic ligands and metal-containing units [165]. MOFs have a high surface area and water stability. MOFs have been studied for a variety of applications, including membrane separations, catalysis and gas storage due to their structural advantages. Recently, nanoscale MOFs have received wide interest as oral peptide drug delivery systems due to their high drug loading capacity and biodegradability under specific conditions [166]. Peptide drugs can be immobilized in MOFs through either de novo or post-synthetic methods. However, traditional MOFs are always rapidly degraded in a strongly acidic environment, which would result in the degradation of peptide drugs in the stomach environment.
Fortunately, variable topological structures and the versatilities for the diverse chemical can help MOFs fit the harsh environment in the GI tract. Chen et al. reported an acidic-resistant Zr6-based MOF, NU-1000, as an oral insulin carrier [167]. The one-dimensional pores of NU-1000 (mesopores with size ∼30 Å and micropores with size ∼12 Å in diameters) were ideally sized to allow insulin encapsulation, and could also exclude pepsin to limit the proteolysis. The NU-1000 exhibited a loading capacity of 40 wt%. The confinement within the pores could inhibit excessive insulin unfolding and significantly reduce degradation. The NU-1000 could slowly be degraded and release the encapsulated insulin in the bloodstream with high phosphate ions.
In addition, surface modification is another way that can achieve the acidic stability of MOFs. Zhou and co-works synthesized MIL-100 MOF NPs via microwave-mediated reactions between FeCl3 · H2O and 1,3,5-benzenetricarboxylic acid (BTC) [168]. The MIL-100 MOF NPs with a relatively large pore size and large surface area possessed high insulin loading capacity. The insulin-loaded MIL-100 NPs were hydrophobic surface modified with sodium dodecyl sulfate (Ins@MIL100/SDS) to promote their cellular uptake and intestinal absorption. The Ins@MIL100/SDS NPs had a hydrodynamic diameter of 132.8 nm and a zeta potential of −18.33 mV. To further enhance their acid stability, MIL-100 NPs were embedded into biodegradable methoxy PEG-block-poly(l-lactide) (mPEG-b-PLLA) microspheres (Ins@MIL100/SDS@MS) for oral insulin delivery. The Ins@MIL100/SDS@MS exhibited a spherical morphology with sizes of 3–5 μm. The insulin loading efficiency was 77.1% with a loading content of 4.6%. The mPEG-b-PLLA microspheres showed considerable resistance in the acidic environment of the stomach and could release Ins@MIL100/SDS in the intestine. The Ins@MIL100/SDS could transport the insulin across the mucosa and intestinal epithelial cell layer for T1DM treatment. In vivo pharmacodynamics and pharmacokinetics assessment in type 1 diabetic rat models showed that oral administration of Ins@MIL100/SDS@MS caused a remarkably enhanced effect in reducing blood glucose level for over 6 h compared with the oral administration of free insulin or Ins@MIL100/SDS.
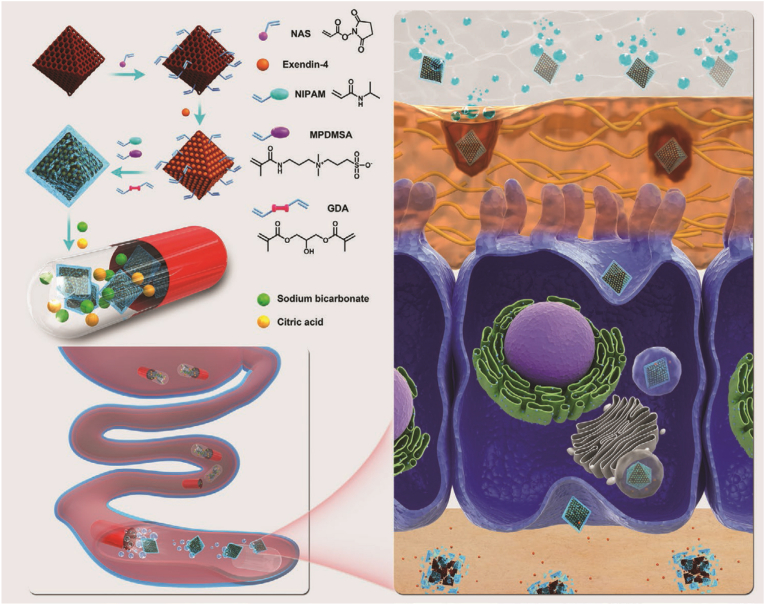
To protect against GI degradation and promote penetration across the intestinal mucosa, Zhou, et al. also developed a pH-triggered self-unpacking capsule encapsulating zwitterionic hydrogel-coated MOF NPs [101]. As shown in Fig. 8, N-isopropylacrylamide (NIPAM) and a zwitterionic comonomer, [3-(methacryloylamino)propyl]dimethyl(3-sulfopropyl)ammonium hydroxide inner salt (MPDMSA) were introduced in the hydrogel coating of exendin-4-loaded NH2-MIL101 MOF NPs via free-radical copolymerization (Ex@MIL101@Gel±). The particles had a size of above 150 nm with a slightly negative charge. The loading efficiency and loading content of exendin-4 for the Ex@MIL101@Gel± were 87.0 ± 1.7% and 0.402 ± 0.005 g/g, respectively. NIPAM could promote cellular uptake due to its hydrophobicity. MPDMSA was benefit for mucus penetration due to its zwitterionic nature, and it also contributed to cellular uptake mediated by the PAT1. To endow the system with reliable protection in the gastric environment, Eudragit L100-55 gelatin capsule was used to encapsulate the Ex@MIL101@Gel±, along with a bubble-generating mixture of sodium bicarbonate and citric acid (Ex@MIL101@Gel±@Cap). After the dissolution of Eudragit L100-55 coating, sodium bicarbonate and citric acid could rapidly react to generate abundant CO2, which triggered the sudden unpacking of the capsules and Ex@MIL101@Gel±. The jejunum injected Ex@MIL101@Gel ± showed the highest fluorescence intensity in the intestine that lasted over 4 h. After oral administration into high fat diet and STZ induced T2DM rats, the Ex@MIL101@Gel±@Cap prolonged increases in both plasma exendin-4 and insulin levels, while orally administered capsules containing free exendin-4 or MIL101@Gel±@Cap showed negligible variation.
Fig. 8.
Illustration of pH-triggered self-unpacking capsule containing Ex@MIL101@Ge ± NPs for oral exendin-4 delivery. Reprinted from Ref. [101] with permission.
The development of oral peptide NP-based on MOFs is still in an early-stage, and there is no formulation in clinical trial. Although MOFs has numerous advantages in oral peptide delivery, there are still some questions. The ability of MOFs overcoming the challenges in GI tract is limited, and they are always needed further modification. The stability of MOFs in biological fluids should be further studied as the premature or burst release of peptide drugs would cause seriously side effect in diabetes mellitus treatment. Lastly, the biodegradation and biosafety of MOFs is concerned and needed comprehensive evaluation.
4. Conclusions and future directions
Oral peptide drug NPs for diabetes mellitus treatment is a highly attractive and promising area. NPs can overcome the challenges for oral delivery, and lead to the development of platforms improving the bioavailability of peptide drugs. Despite numerous efforts have been devoted to explore oral peptide drug NPs, the success of oral delivery of peptide drugs remains to be difficult. There have only a few of clinical trials of oral peptide drug NPs for diabetes mellitus treatment. Oshadi Drug Administration has developed an oral Oshadi carrier (Oshadi Icp) for the delivery of insulin and proinsulin C-peptide. The insulin and proinsulin C-peptide are non-covalently associated with silica NPs (NCT01973920). Diasome has developed an oral HDV-I that uses liposomes with hepatic targeting for insulin delivery (NCT00814294). This is meaningful and inspires us to develop more effective oral peptide drug NPs for diabetes mellitus therapy.
However, most of the studied oral peptide drug NPs formulation are hard or failed for clinical translation. The microenvironment of GI tract is complex, and the standard delivery formulations are always incorporated with a series of functional excipients. The combination of different kinds of materials to overcome the challenges in GI tract would make the oral NPs too complex, which is not benefit for the clinical translation. The DSPE-PCB micelles in Han's work might be a good example [100]. The zwitterionic PCB could overcome the multiple challenges in GI tract, and the components of the NPs were simple. Importantly, the oral bioavailability of insulin was obviously higher than other NPs. Therefore, it is important to explore multifunctional biomedical materials for oral administration. Most studies only measured the blood glucose level after treatment in rodent. The oral bioavailability, the key critical parameter, is missed. In addition, the oral bioavailability and hypoglycemic effect would be different in rodent and human. This is one of the key factors that most existing oral peptide drug NPs are failed in clinical translation. Therefore, hypoglycemic effect and oral bioavailability could be measured on different kinds of animal models. Last but not least, the biosafety of the oral peptide drug NPs is another important index. The biosafety includes the local biosafety caused by direct interaction of NPs with intestinal cells and the systemic biosafety caused by their interaction with different tissues [169,170]. However, the biosafety study is always negligible in most studies. More attention should be paid on the biosafety study in further as it is very important for clinical translation.
Besides the NPs reviewed above, other kinds of NPs also have great potential for oral peptide drug delivery in diabetes mellitus treatment. Exosomes, especially milk exosomes, would be a potential carrier for oral peptide drugs [171]. Milk exosomes have good stability and low immunogenicity. Different from the exosomes derived from cells, the milk exosomes have a rich source, which is beneficial for clinical production. In addition, it has been found that the milk exosomes have some advantages in oral delivery for diabetes mellitus treatment. The bovine milk exosomes are stable in low pH, and can protect their microRNA [172,173]. Porcine milk exosomes could promote the proliferation of intestinal epithelial cells [174]. Immune-related microRNA are abundant in breast milk exosomes, and the milk exosomes might have an immunoregulation effect [175]. Therefore, more attention should be paid to milk exosomes for oral peptide drug delivery in diabetes mellitus treatment.
Furthermore, it should be noted that diabetes mellitus would cause a host of complications, such as diabetic cardiovascular complication, diabetic neuropathy, diabetic retinopathy and diabetic nephropathy. These diabetic complications are fatal to the diabetes mellitus patients. Therefore, it is important to treat the diabetic complications while regulating the blood. Oral NPs with the function of diabetic complication treatment and blood regulation simultaneous would be a new direction, and this raises new and higher requirement for the oral peptide drug NPs. Despite numerous efforts have been devoted in exploring the oral peptide drug NPs, more studies are also required to successfully put the NPs in clinical field.
Credit author statement
Yan Li: Conceptualization, Investigation, Writing-original draft, Funding acquisition. Wen Zhang: Validation. Ruichen Zhao: Software. Xin Zhang: Conceptualization, Supervision, Writing-review & editing, Funding acquisition.
Declaration of competing interest
The authors declare no conflict of interest.
Acknowledgements
This work was financially supported by the National Natural Science Foundation of China (32071391, 21905283, 31771095, 21875254, 52073287 and 22075289), the Fundamental Research Funds for the Central Universities (06500230), and the Beijing Nova Program (Z201100006820140).
Footnotes
Peer review under responsibility of KeAi Communications Co., Ltd.
Contributor Information
Yan Li, Email: liyan310@ustb.edu.cn.
Xin Zhang, Email: xzhang@ipe.ac.cn.
References
- 1.Xiao Y., Sun H., Du J. Sugar-breathing glycopolymersomes for regulating glucose level. J. Am. Chem. Soc. 2017;139(22):7640–7647. doi: 10.1021/jacs.7b03219. [DOI] [PubMed] [Google Scholar]
- 2.Wong C.Y., Al-Salami H., Dass C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Contr. Release. 2017;264:247–275. doi: 10.1016/j.jconrel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 3.Volpatti L.R., Facklam A.L., Cortinas A.B., Lu Y.C., Matranga M.A., Maclsaac C., Hill M.C., Langer R., Anderson D.G. Microgel encapsulated nanoparticles for glucose-responsive insulin delivery. Biomaterials. 2021;267:120458. doi: 10.1016/j.biomaterials.2020.120458. [DOI] [PubMed] [Google Scholar]
- 4.Wang J., Ye Y., Yu J., Kahkoska A.R., Zhang X., Wang C., Sun W., Corder R.D., Chen Z., Khan S.A., Buse J.B., Gu Z. Core-shell microneedle gel for self-regulated insulin delivery. ACS Nano. 2018;12(3):2466–2473. doi: 10.1021/acsnano.7b08152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niu L., Xu Y.C., Dai Z., Tang H.Q. Gene therapy for type 1 diabetes mellitus in rats by gastrointestinal administration of chitosan nanoparticles containing human insulin gene. World J. Gastroenterol. 2008;14(26):4209–4215. doi: 10.3748/wjg.14.4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wang C., Ye Y., Sun W., Yu J., Wang J., Lawrence D.S., Buse J.B., Gu Z. Red blood cells for glucose-responsive insulin delivery. Adv. Mater. 2017;29(18):1606617. doi: 10.1002/adma.201606617. [DOI] [PubMed] [Google Scholar]
- 7.Vetere A., Choudhary A., Burns S.M., Wagner B.K. Targeting the pancreatic β-cell to treat diabetes. Nat. Rev. Drug Discov. 2014;13(4):278–289. doi: 10.1038/nrd4231. [DOI] [PubMed] [Google Scholar]
- 8.Tauschmann M., Hovorka R. Technology in the management of type 1 diabetes mellitus-current status and future prospects. Nat. Rev. Endocrinol. 2018;14(8):464–475. doi: 10.1038/s41574-018-0044-y. [DOI] [PubMed] [Google Scholar]
- 9.Shrestha N., Araújo F., Sarmento B., Hirvonen J., Sanntos H.A. Gene-based therapy for type 1 diabetes mellitus: viral and nonviral vectors. Diabetes Manag. 2014;4(4):367–380. [Google Scholar]
- 10.Santin I., Eizirik D.L. Candidate genes for type 1 diabetes modulate pancreatic islet inflammation and β-cell apoptosis. Diabetes Obes. Metabol. 2013;15:71–81. doi: 10.1111/dom.12162. [DOI] [PubMed] [Google Scholar]
- 11.An D., Chiu A., Flanders J.A., Song W., Shou D., Lu Y.C., Grunnet L.G., Winkel L., Ingvorsen C., Christophersen N.S., Fels J.J., Sand F.W., Ji Y., Qi L., Pardo Y., Luo D., Silberstein M., Fan J., Ma M. Designing a retrievable and scalable cell encapsulation device for potential treatment of type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 2018;115(2):263–272. doi: 10.1073/pnas.1708806115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaïdane H., Sauter P., Sane F., Goffard A., Gharbi J., Hober D. Enteroviruses and type 1 diabetes: towards a better understanding of the relationship. Rev. Med. Virol. 2010;20(5):265–280. doi: 10.1002/rmv.647. [DOI] [PubMed] [Google Scholar]
- 13.Hober D., Sauter P. Pathogenesis of type 1 diabetes mellitus: interplay between enterovirus and host. Nat. Rev. Endocrinol. 2010;6(5):279–289. doi: 10.1038/nrendo.2010.27. [DOI] [PubMed] [Google Scholar]
- 14.So M., Elso C.M., Tresoldi E., Pakusch M., Pathiraja V., Wentworth J.M., Harrison L.C., Krishnamurthy B., Thomas H.E., Rodda C., Cameron F.J., McMahon J., Kay T.H., Mannering S.I. Proinsulin C-peptide is an autoantigen in people with type 1 diabetes. Proc. Natl. Acad. Sci. U.S.A. 2018;115(42):10732–10737. doi: 10.1073/pnas.1809208115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ruan Q., Wang T., Kameswaran V., Wei Q., Johnson D.S., Matschinsky F., Shi W., Chen Y.H. The microRNA-21-PDCD4 axis prevents type 1 diabetes by blocking pancreatic β cell death. Proc. Natl. Acad. Sci. U.S.A. 2011;108(29):12303–12305. doi: 10.1073/pnas.1101450108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eizirik D.L., Colli M.L., Ortis F. The role of inflammation in insulitis and β-cell loss in type 1 diabetes. Nat. Rev. Endocrinol. 2009;5(4):219–226. doi: 10.1038/nrendo.2009.21. [DOI] [PubMed] [Google Scholar]
- 17.Zhang J., Zhou J., Zhang T., Niu Z., Wang J., Guo J., Li Z., Zhang Z. Facile fabrication of an amentoflavone-loaded micelle system for oral delivery to improve bioavailability and hypoglycemic effects in KKAy mice. ACS Appl. Mater. Interfaces. 2019;11(13):12904–12913. doi: 10.1021/acsami.9b03275. [DOI] [PubMed] [Google Scholar]
- 18.Lopes M., Aniceto D., Abrantes M., Simões S., Branco F., Vitória I., Botelho M.F., Seica R., Veiga F., Ribeiro A. In vivo biodistribution of antihyperglycemic biopolymer-based nanoparticles for the treatment of type 1 and type 2 diabetes. Eur. J. Pharm. Biopharm. 2017;113:88–96. doi: 10.1016/j.ejpb.2016.11.037. [DOI] [PubMed] [Google Scholar]
- 19.Tsao C., Zhang P., Yuan Z., Dong D., Wu K., Niu L., McMullen P., Luozhong S., Hung H.C., Cheng Y.H., Jiang S. Zwitterionic polymer conjugated glucagon-like peptide-1 for prolonged glycemic control. Bioconjugate Chem. 2020;31(7):1812–1819. doi: 10.1021/acs.bioconjchem.0c00286. [DOI] [PubMed] [Google Scholar]
- 20.Stumvoll M., Goldstein B.J., van Haeften T.W. Type 2 diabetes: principles of pathogenesis and therapy. Lancet. 2005;365(9467):1333–1346. doi: 10.1016/S0140-6736(05)61032-X. [DOI] [PubMed] [Google Scholar]
- 21.Haythorne E., Rohm M., van de Bunt M., Brereton M.F., Tarasov A.I., Blacker T.S., Sachse G., dos Santos M.S., Exposito R.T., Davis S., Baba O., Fischer R., Duchen M.R., Rorsman P., MacRae J.I., Ashcroft F.M. Diabetes causes marked inhibition of mitochondrial metabolism in pancreatic β-cells. Nat. Commun. 2019;10(1):2474. doi: 10.1038/s41467-019-10189-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cnop M., Welsh N., Jonas J.C., Jörns A., Lenzen S., Eizirik D.L. Mechanisms of pancreatic β-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54:S97–S107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 23.Aguayo-Mazzucato C., Bonner-Weir S. Pancreatic β cell regeneration as a possible therapy for diabetes. Cell Metabol. 2018;27(1):57–67. doi: 10.1016/j.cmet.2017.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brayden D.J., Hill T.A., Fairlie D.P., Maher S., Mrsny R.J. Systemic delivery of peptides by the oral route: formulation and medicinal chemistry approaches. Adv. Drug Deliv. Rev. 2020;157:2–36. doi: 10.1016/j.addr.2020.05.007. [DOI] [PubMed] [Google Scholar]
- 25.Wong C.Y., Al-Salami H., Dass C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Contr. Release. 2017;264:247–275. doi: 10.1016/j.jconrel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 26.Yu J., Qian C., Zhang Y., Cui Z., Zhu Y., Shen Q., Ligler F.S., Buse J.B., Gu Z. Hypoxia and H2O2 dual-sensitive vesicles for enhanced glucose-responsive insulin delivery. Nano Lett. 2017;17(2):733–739. doi: 10.1021/acs.nanolett.6b03848. [DOI] [PubMed] [Google Scholar]
- 27.Hu X., Yu J., Qian C., Lu Y., Kahkoska A.R., Xie Z., Jing X., Buse J.B., Gu Z. H2O2-responsive vesicles integrated with transcutaneous patches for glucose-mediated insulin delivery. ACS Nano. 2017;11(1):613–620. doi: 10.1021/acsnano.6b06892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wang J., Wang Z., Yu J., Kahkoska A.R., Buse J.B., Gu Z. Glucose-responsive insulin and delivery systems: innovation and translation. Adv. Mater. 2020;32(13):1902004. doi: 10.1002/adma.201902004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo F.Q., Chen G., Xu W., Zhou D., Li J.X., Huang Y.C., Lin R., Gu Z., Du J.Z. Microneedle-array patch with pH-sensitive formulation for glucose-responsive insulin delivery. Nano Res. 2021;14:2689–2696. [Google Scholar]
- 30.GhavamiNejad A., Li J., Lu B., Zhou L., Lam L., Giacca A., Wu X.Y. Glucose-responsive composite microneedle patch for hypoglycemia-triggered delivery of native glucagon. Adv. Mater. 2019;31:1901051. doi: 10.1002/adma.201901051. [DOI] [PubMed] [Google Scholar]
- 31.Lim Z.W., Ping Y., Miserez A. Glucose-responsive peptide coacervates with high encapsulation efficiency for controlled release of insulin. Bioconjugate Chem. 2018;29(7):2176–2180. doi: 10.1021/acs.bioconjchem.8b00369. [DOI] [PubMed] [Google Scholar]
- 32.He Z., Hu Y., Gui Z., Zhou Y., Nie T., Zhu J., Liu Z., Chen K., Liu L., Leong K.W., Cao P., Chen Y., Mao H.Q. Sustained release of exendin-4 from tannic acid/Fe (III) nanoparticles prolongs blood glycemic control in a mouse model of type II diabetes. J. Contr. Release. 2019;301:119–128. doi: 10.1016/j.jconrel.2019.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Shatil Shahriar S.M., An J.M., Hasan M.N., Surwase S.S., Kim Y.C., Lee D.Y., Cho S., Lee Y. Plasmid DNA nanoparticles for nonviral oral gene therapy. Nano Lett. 2021;21(11):4666–4675. doi: 10.1021/acs.nanolett.1c00832. [DOI] [PubMed] [Google Scholar]
- 34.Araújo F., Fonte P., Santos H.A., Sarmento B. Oral delivery of glucagon-like peptide-1 and analogs: alternatives for diabetes control? J. Diabetes Sci. Technol. 2012;6(6):1486–1497. doi: 10.1177/193229681200600630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Araújo F., Shrestha N., Shahbazi M.A., Fonte P., Mäkilä E.M., Salonen J.J., Hirvonen J.T., Granja P.L., Santos H.A., Sarmento B. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials. 2014;35(33):9199–9207. doi: 10.1016/j.biomaterials.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 36.Lakkireddy H.R., Urmann M., Besenius M., Werner U., Haack T., Brun P., Alié J., Illel B., Hortala L., Vogel R., Bazile D. Oral delivery of diabetes peptides-comparing standard formulations incorporating functional excipients and nanotechnologies in the translational context. Adv. Drug Deliv. Rev. 2016;106:196–222. doi: 10.1016/j.addr.2016.02.011. [DOI] [PubMed] [Google Scholar]
- 37.Christou G.A., Katsiki N., Blundell J., Fruhbeck G., Kiortsis D.N. Semaglutide as a promising antibesity drug. Obes. Rev. 2019;20(6):805–815. doi: 10.1111/obr.12839. [DOI] [PubMed] [Google Scholar]
- 38.Craddy P., Palin H.J., Ian Johnson K. Comparative effectiveness of dipeptidylpeptidase-4 inhibitors in type 2 diabetes: a systematic review and mixed treatment comparison. Diabetes Ther. 2014;5(1):1–41. doi: 10.1007/s13300-014-0061-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chen W., Wang G., Yung B.C., Liu G., Qian Z., Chen X. Long-acting release formulation of exendin-4 based on biomimetic mineralization for type 2 diabetes therapy. ACS Nano. 2017;11(5):5062–5069. doi: 10.1021/acsnano.7b01809. [DOI] [PubMed] [Google Scholar]
- 40.Bao X., Qian K., Yao P. Oral delivery of exenatide-loaded hybrid zein nanoparticles for stable blood glucose control and β-cell repair of type 2 diabetes mice. J. Nanobiotechnol. 2020;18(1):67. doi: 10.1186/s12951-020-00619-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Qi Y., Simakova A., Ganson N.J., Li X., Luginbuhl K.M., Ozer I., Liu W., Hershfield M.S., Matyjaszewski K., Chilkoti A. A brush-polymer/exendin-4 conjugate reduces blood glucose levels for up to five days and eliminates poly(ethylene glycol) antigenicity. Nat. Biomed. Eng. 2016;1 doi: 10.1038/s41551-016-0002. 0002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lau J., Bloch P., Schäffer L., Pettersson I., Spetzler J., Kofoed J., Madsen K., Knudsen L.B., McGuire J., Steensgaard D.B., Strauss H.M., Gram D.X., Knudsen S.M., Nielsen F.S., Thygesen P., Reedtz-Runge S., Kruse T. Discovery of the once-weekly glucagon-like peptide-1 (GLP-1) analogue semaglutide. J. Med. Chem. 2015;58(18):7370–7380. doi: 10.1021/acs.jmedchem.5b00726. [DOI] [PubMed] [Google Scholar]
- 43.Alavi S.E., Cabot P.J., Moyle P.M. Glucagon-like peptide-1 receptor agonists and strategies to improve their efficiency. Mol. Pharm. 2019;16(6):2278–2295. doi: 10.1021/acs.molpharmaceut.9b00308. [DOI] [PubMed] [Google Scholar]
- 44.Knudsen L.B., Lau J. The discovery and development of liraglutide and semaglutide. Front. Endocrinol. 2019;10:155. doi: 10.3389/fendo.2019.00155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhao L., Ding J., Xiao C., He P., Tang Z., Pang X., Zhuang X., Chen X. Glucose-sensitive polypeptide micelles for self-regulated insulin release at physiological pH. J. Mater. Chem. 2012;22:12319–12328. [Google Scholar]
- 46.Chen Z., Wang J., Sun W., Archibong E., Kahkoska A.R., Zhang X., Lu Y., Ligler F.S., Buse J.B., Gu Z. Synthetic beta cells for fusion-mediated dynamic insulin secretion. Nat. Chem. Biol. 2018;14(1):86–93. doi: 10.1038/nchembio.2511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh S., Kushwah V., Agrawal A.K., Jain S. Insulin- and quercetin-loaded liquid crystalline nanoparticles: implications on oral bioavailability, antidiabetic and antioxidant efficacy. Nanomedicine. 2018;13(5):521–537. doi: 10.2217/nnm-2017-0278. [DOI] [PubMed] [Google Scholar]
- 48.Gu Z., Dang T.T., Ma M., Tang B., Cheng H., Jiang S., Dong Y., Zhang Y., Anderson D.G. Glucose-responsive microgels integrated with enzyme nanocapsules for closed-loop insulin delivery. ACS Nano. 2013;7(8):6758–6766. doi: 10.1021/nn401617u. [DOI] [PubMed] [Google Scholar]
- 49.Wang J., Yu J., Zhang Y., Zhang X., Kahkoska A.R., Chen G., Wang Z., Sun W., Cai L., Chen Z., Qian C., Shen Q., Khademhosseini A., Buse J.B., Gu Z. Charge-switchable polymeric complex for glucose-responsive insulin delivery in mice and pigs. Sci. Adv. 2019;5(7) doi: 10.1126/sciadv.aaw4357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Turksoy K., Bayrak E.S., Quinn L., Littlejohn E., Rollins D., Cinar A. Hypoglycemia early alarm systems based on multivariable models. Ind. Eng. Chem. Res. 2013;52(35):12329–12336. doi: 10.1021/ie3034015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dombu C., Carpentier R., Betbeder D. Influence of surface charge and inner composition of nanoparticles on intracellular delivery of proteins in airway epithelial cells. Biomaterials. 2012;33(35):9117–9126. doi: 10.1016/j.biomaterials.2012.08.064. [DOI] [PubMed] [Google Scholar]
- 52.Zhao Q., Zhang S., Wu F., Li D., Zhang X., Chen W., Xing B. Rational design of nanogels for overcoming the biological barriers in various administration routes. Angew. Chem. Int. Ed. 2021;60(27):14760–14778. doi: 10.1002/anie.201911048. [DOI] [PubMed] [Google Scholar]
- 53.Sheng J., He H., Han L., Qin J., Chen S., Ru G., Li R., Yang P., Wang J., Yang V.C. Enhancing insulin oral absorption by using mucoadhesive nanoparticles loaded with LMWP-linked insulin conjugates. J. Contr. Release. 2016;233:181–190. doi: 10.1016/j.jconrel.2016.05.015. [DOI] [PubMed] [Google Scholar]
- 54.Forbes D.C., Peppas N.A. Oral delivery of small RNA and DNA. J. Contr. Release. 2012;162(2):438–445. doi: 10.1016/j.jconrel.2012.06.037. [DOI] [PubMed] [Google Scholar]
- 55.Zhu Q., Talton J., Zhang G., Cunningham T., Wang Z., Waters R.C., Kirk J., Eppler B., Klinman D.M., Sui Y., Gagnon S., Belyakov I.M., Mumper R.J., Berzofsky J.A. Large intestine-targeted, nanoparticle-releasing oral vaccine to control genitorectal viral infection. Nat. Med. 2012;18(8):1291–1296. doi: 10.1038/nm.2866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hristov D., McCartney F., Beirne J., Mahon E., Reid S., Bhattacharjee S., Penarier G., Werner U., Bazile D., Brayden D.J. Silica-coated nanoparticles with a core of zinc, L-arginine and a peptide designed for oral delivery. ACS Appl. Mater. Interfaces. 2020;12(1):1257–1269. doi: 10.1021/acsami.9b16104. [DOI] [PubMed] [Google Scholar]
- 57.Araújo F., Shrestha N., Granja P.L., Hirvonen J., Santos H.A., Sarmento B. Antihyperglycemic potential of incretins orally delivered via nano and microsystems and subsequent glucoregulatory effects. Curr. Pharmaceut. Biotechnol. 2014;15(7):609–619. doi: 10.2174/1389201015666140915150312. [DOI] [PubMed] [Google Scholar]
- 58.Kong X.D., Moriya J., Carle V., Pojer F., Abriata L.A., Deyle K., Heinis C. De novo development of proteolytically resistant therapeutic peptides for oral administration. Nat. Biomed. Eng. 2020;4(5):560–571. doi: 10.1038/s41551-020-0556-3. [DOI] [PubMed] [Google Scholar]
- 59.Li L., Jiang G., Yu W., Liu D., Chen H., Liu Y., Tong Z., Kong X., Yao J. Preparation of chitosan-based multifunctional nanocarriers overcoming multiple barriers for oral delivery of insulin. Mater. Sci. Eng. C. 2017;70:278–286. doi: 10.1016/j.msec.2016.08.083. [DOI] [PubMed] [Google Scholar]
- 60.Xiao Y., Tang Z., Wang J., Liu C., Kong N., Farokhzad O.C., Tao W. Oral insulin delivery platforms: strategies to address the biological barriers. Angew. Chem. Int. Ed. 2020;59(45):19787–19795. doi: 10.1002/anie.202008879. [DOI] [PubMed] [Google Scholar]
- 61.Drucker D.J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov. 2020;19(4):277–289. doi: 10.1038/s41573-019-0053-0. [DOI] [PubMed] [Google Scholar]
- 62.Sharma G., Sharma A.R., Nam J.S., Doss G.P.C., Lee S.S., Chakraborty C. Nanoparticle based insulin delivery system: the next generation efficient therapy for type 1 diabetes. J. Nanobiotechnol. 2015;13:74. doi: 10.1186/s12951-015-0136-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Italiya K.S., Basak M., Mazumdar S., Sahel D.K., Shrivastava R., Chitkara D., Mittal A. Scalable self-assembling micellar system for enhanced oral bioavailability and efficacy of lisofylline for treatment of type-I diabetes. Mol. Pharm. 2019;16(12):4954–4967. doi: 10.1021/acs.molpharmaceut.9b00833. [DOI] [PubMed] [Google Scholar]
- 64.Zelikin A.N., Ehrhardt C., Healy A.M. Materials and methods for delivery of biological drugs. Nat. Chem. 2016;8(11):997–1007. doi: 10.1038/nchem.2629. [DOI] [PubMed] [Google Scholar]
- 65.Sadeghi S., Lee W.K., Kong S.N., Shetty A., Drum C.L. Oral administration of protein nanoparticles: an emerging route to disease treatment. Pharmacol. Res. 2020;158:104685. doi: 10.1016/j.phrs.2020.104685. [DOI] [PubMed] [Google Scholar]
- 66.Brown T.D., Whitehead K.A., Mitragotri S. Materials for oral delivery of proteins and peptides. Nat. Rev. Mater. 2020;5:127–148. [Google Scholar]
- 67.Yin L., Song Z., Qu Q., Kim K.H., Zheng N., Yao C., Chaudhury I., Tang H., Gabrielson N.P., Uckun F.M., Cheng J. Supramolecular self-assembled nanoparticles mediate oral delivery of therapeutic TNF-α siRNA against systemic inflammation. Angew. Chem. Int. Ed. 2013;52(22):5757–5761. doi: 10.1002/anie.201209991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Liu L., Zhang Y., Yu S., Zhang Z., He C., Chen X. pH- and amylase-responsive carboxymethyl starch/poly(2-isobutyl-acrylic acid) hybrid microgels as effective enteric carriers for oral insulin delivery. Biomacromolecules. 2018;19(6):2123–2136. doi: 10.1021/acs.biomac.8b00215. [DOI] [PubMed] [Google Scholar]
- 69.Gupta R., Badhe Y., Mitragotri S., Rai B. Permeation of nanoparticles across the intestinal lipid membrane: dependence on shape and surface chemistry studied through molecular simulations. Nanoscale. 2020;12(11):6318–6333. doi: 10.1039/c9nr09947f. [DOI] [PubMed] [Google Scholar]
- 70.Hu Q., Luo Y. Recent advances of polysaccharide-based nanoparticles for oral insulin delivery. Int. J. Biol. Macromol. 2018;120:775–782. doi: 10.1016/j.ijbiomac.2018.08.152. [DOI] [PubMed] [Google Scholar]
- 71.Banerjee A., Qi J., Gogoi R., Wong J., Mitragotri S. Role of nanoparticle size, shape and surface chemistry in oral drug delivery. J. Contr. Release. 2016;238:176–185. doi: 10.1016/j.jconrel.2016.07.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Sun L., Liu Z., Tian H., Le Z., Liu L., Leong K.W., Mao H.Q., Chen Y. Scalable manufacturing of enteric encapsulation systems for site-specific oral insulin delivery. Biomacromolecules. 2019;20(1):528–538. doi: 10.1021/acs.biomac.8b01530. [DOI] [PubMed] [Google Scholar]
- 73.Leong K.W., Sung H.W. Nanoparticle- and biomaterials-mediated oral delivery for drug, gene, and immunotherapy. Adv. Drug Deliv. Rev. 2013;65(6):757–758. doi: 10.1016/j.addr.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 74.Ensign L.M., Cone R., Hanes J. Oral drug delivery with polymeric nanoparticles: the gastrointestinal mucus barriers. Adv. Drug Deliv. Rev. 2012;64(6):557–570. doi: 10.1016/j.addr.2011.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.de Sousa I.P., Steiner C., Schmutzler M., Wilcox M.D., Veldhuis G.J., Pearson J.P., Huck C.W., Salvenmoser W., Bernkop-Schnürch A. Mucus permeating carriers: formulation and characterization of highly densely charged nanoparticles. Eur. J. Pharm. Biopharm. 2015;97:273–279. doi: 10.1016/j.ejpb.2014.12.024. [DOI] [PubMed] [Google Scholar]
- 76.Ruiz-Gatón L., Espuelas S., Larrañeta E., Reviakine I., Yate L.A., Irache J.M. Pegylated poly(anhydride) nanoparticles for oral delivery of docetaxel. Eur. J. Pharmaceut. Sci. 2018;118:165–175. doi: 10.1016/j.ejps.2018.03.028. [DOI] [PubMed] [Google Scholar]
- 77.Shan W., Zhu X., Liu M., Li L., Zhong J., Sun W., Zhang Z., Huang Y. Overcoming the diffusion barrier of mucus and absorption barrier of epithelium by self-assembled nanoparticles for oral delivery of insulin. ACS Nano. 2015;9(3):2345–2356. doi: 10.1021/acsnano.5b00028. [DOI] [PubMed] [Google Scholar]
- 78.Lai S.K., Wang Y.Y., Hanes J. Mucus-penetrating nanoparticles for drug and gene delivery to mucosal tissues. Adv. Drug Deliv. Rev. 2009;61(2):158–171. doi: 10.1016/j.addr.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Lee B., Moon K.M., Kim C.Y. Tight junction in the intestinal epithelium: its association with diseases and regulation of phytochemicals. J. Immunol. Res. 2018;2018:2645465. doi: 10.1155/2018/2645465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Garcia-Hernandez V., Quiros M., Nusrat A. Intestinal epithelial claudins: expression and regulation in homeostasis and inflammation. Ann. N. Y. Acad. Sci. 2017;1397(1):66–79. doi: 10.1111/nyas.13360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cong X., Zhang Y., Li J., Mei M., Ding C., Xiang R.L., Zhang L.W., Wang Y., Wu L.L., Yu G.Y. Claudin-4 is required for modulation of paracellular permeability by muscarinic acetylcholine receptor in epithelial cells. J. Cell Sci. 2015;128(12):2271–2286. doi: 10.1242/jcs.165878. [DOI] [PubMed] [Google Scholar]
- 82.McCartney F., Rosa M., Brayden D.J. Evaluation of sucrose laurate as an intestinal permeation enhancer for macromolecules: ex vivo and in vivo studies. Pharmaceutics. 2019;11(11):565. doi: 10.3390/pharmaceutics11110565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Yun Y., Cho Y.W., Park K. Nanoparticles for oral delivery: targeted nanoparticles with peptidic ligands for oral protein delivery. Adv. Drug Deliv. Rev. 2013;65(6):822–832. doi: 10.1016/j.addr.2012.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Zhang Y., Zhang L., Li Y., Sun S., Tan H. Different contributions of clathrin- and caveolae-mediated endocytosis of vascular endothelial cadherin to lipopolysaccharide-induced vascular hyperpermeability. PLoS One. 2014;9(9) doi: 10.1371/journal.pone.0106328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Pereira C., Araújo F., Granja P.L., Santos H.A., Sarmento B. Targeting membrane transporters and receptors as a mean to optimize orally delivered biotechnological based drugs through nanoparticle delivery systems. Curr. Pharmaceut. Biotechnol. 2014;15(7):650–658. doi: 10.2174/1389201015666140915152330. [DOI] [PubMed] [Google Scholar]
- 86.Li X., Uehara S., Sawangrat K., Morishita M., Kusamori K., Katsumi H., Sakane T., Yamamoto A. Improvement of intestinal absorption of curcumin by cyclodextrins and the mechanisms underlying absorption enhancement. Int. J. Pharm. 2018;535(1–2):340–349. doi: 10.1016/j.ijpharm.2017.11.032. [DOI] [PubMed] [Google Scholar]
- 87.Maher S., Mrsny R.J., Brayden D.J. Intestinal permeation enhancers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016;6106:277–319. doi: 10.1016/j.addr.2016.06.005. [DOI] [PubMed] [Google Scholar]
- 88.McCartney F., Jannin V., Chevrier S., Boulghobra H., Hristov D.R., Ritter N., Miolane C., Chavant Y., Demarne F., Brayden D.J. Labrasol® is an efficacious intestinal permeation enhancer across rat intestine: ex vivo and in vivo rat studies. J. Contr. Release. 2019;310:115–126. doi: 10.1016/j.jconrel.2019.08.008. [DOI] [PubMed] [Google Scholar]
- 89.Twarog C., Liu K., O′Brien P.J., Dawson K.A., Fattal E., Illel B., Brayden D.J. A head-to-head Caco-2 assay comparison of the mechanisms of action of the intestinal permeation enhancer: SNCA and sodium caprate (C10) Eur. J. Pharm. Biopharm. 2020;152:95–107. doi: 10.1016/j.ejpb.2020.04.023. [DOI] [PubMed] [Google Scholar]
- 90.Verma A., Sharma S., Gupta P.K., Singh A., Teja B.V., Dwivedi P., Gupta G.K., Trivedi R., Mishra P.R. Vitamin B12 functionalized layer by layer calcium phosphate nanoparticles: a mucoadhesive and pH responsive carrier for improved oral delivery of insulin. Acta Biomater. 2016;31:288–300. doi: 10.1016/j.actbio.2015.12.017. [DOI] [PubMed] [Google Scholar]
- 91.Kim K.S., Suzuki K., Cho H., Youn Y.S., Bae Y.H. Oral nanoparticles exhibit specific high-efficiency intestinal uptake and lymphatic transport. ACS Nano. 2018;12(9):8893–8900. doi: 10.1021/acsnano.8b04315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Yu J., Zhang Y., Wang J., Wen D., Kahkoska A.R., Buse J.B., Gu Z. Glucose-responsive oral insulin delivery for postprandial glycemic regulation. Nano Res. 2019;12:1539–1545. [Google Scholar]
- 93.Zhang P., Xu Y., Zhu X., Huang Y. Goblet cell targeting nanoparticle containing drug-loaded micelle cores for oral delivery of insulin. Int. J. Pham. 2015;496(2):993–1005. doi: 10.1016/j.ijpharm.2015.10.078. [DOI] [PubMed] [Google Scholar]
- 94.Yin M., Song Y., Guo S., Zhang X., Sun K., Li Y., Shi Y. Intelligent escape system for the oral delivery of liraglutide: a perfect match for gastrointestinal barriers. Mol. Pharm. 2020;17(6):1899–1909. doi: 10.1021/acs.molpharmaceut.9b01307. [DOI] [PubMed] [Google Scholar]
- 95.Song Y., Shi Y., Zhang L., Hu H., Zhang C., Yin M., Chu L., Yan X., Zhao M., Zhang X., Mu H., Sun K. Synthesis of CSK-DEX-PLGA nanoparticles for the oral delivery of exenatide to improve its mucus penetration and intestinal absorption. Mol. Pharm. 2019;16(2):518–532. doi: 10.1021/acs.molpharmaceut.8b00809. [DOI] [PubMed] [Google Scholar]
- 96.Roger E., Kalscheuer S., Kirtane A., Guru B.R., Grill A.E., Whittum-Hudson J., Panyam J. Folic acid-functionalized nanoparticles for enhanced oral drug delivery. Mol. Pharm. 2012;9(7):2103–2110. doi: 10.1021/mp2005388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Wan L., Wang X., Zhu W., Zhang C., Song A., Sun C., Jiang T., Wang S. Folate-polyethyleneimine functionalized mesoporous carbon nanoparticles for enhancing oral bioavailability of paclitaxel. Int. J. Pharm. 2015;484(1–2):207–217. doi: 10.1016/j.ijpharm.2015.02.054. [DOI] [PubMed] [Google Scholar]
- 98.Kavimandan N.J., Losi E., Peppas N.A. Novel delivery system based on complexation hydrogels as delivery vehicles for insulin-transferrin conjugates. Biomaterials. 2006;27(20):3846–3854. doi: 10.1016/j.biomaterials.2006.02.026. [DOI] [PubMed] [Google Scholar]
- 99.Wu L., Bai Y., Liu M., Li L., Shan W., Zhang Z., Huang Y. Transport mechanisms of butyrate modified nanoparticles: insight into “easy entry, hard transcytosis” of active targeting system in oral administration. Mol. Pharm. 2018;15(9):4273–4283. doi: 10.1021/acs.molpharmaceut.8b00713. [DOI] [PubMed] [Google Scholar]
- 100.Han X., Lu Y., Xie J., Zhang E., Zhu H., Du H., Wang K., Song B., Yang C., Shi Y., Cao Z. Zwitterionic micelles efficiently deliver oral insulin without opening tight junctions. Nat. Nanotechnol. 2020;15(7):605–614. doi: 10.1038/s41565-020-0693-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou Y., Chen Z., Zhao D., Li D., He C., Chen X. A pH-triggered self-unpacking capsule containing zwitterionic hydrogel-coated MOF nanoparticles for efficient oral exendin-4 delivery. Adv. Mater. 2021;33(32) doi: 10.1002/adma.202102044. [DOI] [PubMed] [Google Scholar]
- 102.Shan W., Zhu X., Tao W., Cui Y., Liu M., Wu L., Li L., Zheng Y. Enhanced oral delivery of protein drugs using zwitterion-functionalized nanoparticles to overcome both the diffusion and absorption barriers. ACS Appl. Mater. Interfaces. 2016;8(38):25444–25453. doi: 10.1021/acsami.6b08183. [DOI] [PubMed] [Google Scholar]
- 103.Wu J., Zheng Y., Liu M., Shan W., Zhang Z., Huang Y. Biomimetic viruslike and charge reversible nanoparticles to sequentially overcome mucus and epithelial barriers for oral insulin delivery. ACS Appl. Mater. Interfaces. 2018;10(12):9916–9928. doi: 10.1021/acsami.7b16524. [DOI] [PubMed] [Google Scholar]
- 104.Gamboa J.M., Leong K.W. In vitro and in vivo models for the study of oral delivery of nanoparticles. Adv. Drug Deliv. Rev. 2013;65(6):800–810. doi: 10.1016/j.addr.2013.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Wang R., Zhou L., Wang W., Li X., Zhang F. In vivo gastrointestinal drug-release monitoring through second near-infrared window fluorescent bioimaging with orally delivered microcarriers. Nat. Commun. 2017;8:14702. doi: 10.1038/ncomms14702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Qin X., Yu C., Wei J., Li L., Zhang C., Wu Q., Liu J., Yao S.Q., Huang W. Rational design of nanocarriers for intracellular protein delivery. Adv. Mater. 2019;31(46) doi: 10.1002/adma.201902791. [DOI] [PubMed] [Google Scholar]
- 107.Das S., Chaudhury A. Recent advances in lipid nanoparticle formulations with solid matrix for oral drug delivery. AAPS PharmSciTech. 2011;12(1):62–76. doi: 10.1208/s12249-010-9563-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Wang T., Luo Y. Fabrication strategies and supramolecular interactions of polymer-lipid complex nanoparticles as oral delivery systems. Nano Res. 2021;14(12):4487–4501. [Google Scholar]
- 109.Niu Z., Conejos-Sánchez I., Griffin B.T., O'Driscoll C.M., Alonso M.J. Lipid-based nanocarriers for oral peptide delivery. Adv. Drug Deliv. Rev. 2016;106:337–354. doi: 10.1016/j.addr.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 110.He H., Lu Y., Qi J., Zhu Q., Chen Z., Wu W. Adapting liposomes for oral drug delivery. Acta Pharm. Sin. B. 2019;9(1):36–48. doi: 10.1016/j.apsb.2018.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Talegaonkar S., Bhattacharyya A. Potential of lipid nanoparticles (SLNs and NLCs) in enhancing oral bioavailability of drugs with poor intestinal permeability. AAPS PharmSciTech. 2019;20(3):121. doi: 10.1208/s12249-019-1337-8. [DOI] [PubMed] [Google Scholar]
- 112.Chen C., Zhu X., Dou Y., Xu J., Zhang J., Fan T., Du J., Liu K., Deng Y., Zhao L., Huang Y. Exendin-4 loaded nanoparticles with a lipid shell and aqueous core containing micelles for enhanced intestinal absorption. J. Biomed. Nanotechnol. 2015;11(5):865–876. doi: 10.1166/jbn.2015.1971. [DOI] [PubMed] [Google Scholar]
- 113.Nie T., He Z., Zhou Y., Zhu J., Chen K., Liu L., Leong K.W., Mao H.Q., Chen Y. Surface coating approach to overcome mucosal entrapment of DNA nanoparticles for oral gene delivery of glucagon-like peptide 1. ACS Appl. Mater. Interfaces. 2019;11(33):29593–29603. doi: 10.1021/acsami.9b10294. [DOI] [PubMed] [Google Scholar]
- 114.Li X., Guo S., Zhu C., Zhu Q., Gan Y., Rantanen J., Rahbek U.L., Hovgaard L., Yang M. Intestinal mucosa permeability following oral insulin delivery using core shell corona nanolipoparticles. Biomaterials. 2013;34(37):9678–9687. doi: 10.1016/j.biomaterials.2013.08.048. [DOI] [PubMed] [Google Scholar]
- 115.Wang A., Yang T., Fan W., Yang Y., Zhu Q., Guo S., Zhu C., Yuan Y., Zhang T., Gan Y. Protein corona liposomes achieve efficient oral insulin delivery by overcoming mucus and epithelial barriers. Adv. Healthc. Mater. 2019;8(12) doi: 10.1002/adhm.201801123. [DOI] [PubMed] [Google Scholar]
- 116.Suzuki K., Kim K.S., Bae Y.H. Long-term oral administration of exendin-4 to control type 2 diabetes in a rat model. J. Contr. Release. 2019;294:259–267. doi: 10.1016/j.jconrel.2018.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu X., Wu J., Shan W., Zhou Z., Liu M., Huang Y. Sub-50 nm nanoparticles with biomimetic surfaces to sequentially overcome the mucosal diffusion barrier and the epithelial absorption barrier. Adv. Funct. Mater. 2016;26(16):2728–2738. [Google Scholar]
- 118.Wu L., Liu M., Shan W., Zhu X., Li L., Zhang Z., Huang Y. Bioinspired butyrate-functionalized nanovehicles for targeted oral delivery of biomacromolecular drugs. J. Contr. Release. 2017;262:273–283. doi: 10.1016/j.jconrel.2017.07.045. [DOI] [PubMed] [Google Scholar]
- 119.Ito S., Torii Y., Chikamatsu S., Harada T., Yamaguchi S., Ogata S., Sonoda K., Wakayama T., Masuda T., Ohtsuki S. Oral coadministration of Zn-insulin with D-form small intestine-permeable cyclic peptide enhances its blood glucose-lowering effect in mice. Mol. Pharm. 2021;18(4):1593–1603. doi: 10.1021/acs.molpharmaceut.0c01010. [DOI] [PubMed] [Google Scholar]
- 120.Li Y., Cheng Q., Jiang Q., Huang Y., Liu H., Zhao Y., Cao W., Ma G., Dai F., Liang X., Liang Z., Zhang X. Enhanced endosomal/lysosomal escape by distearoyl phosphoethanolamine-polycarboxybetaine lipid for systemic delivery of siRNA. J. Contr. Release. 2014;176:104–114. doi: 10.1016/j.jconrel.2013.12.007. [DOI] [PubMed] [Google Scholar]
- 121.Li Y., Li Y., Ji W., Lu Z., Liu L., Shi Y., Ma G., Zhang X. Positively charged polyprodrug amphiphiles with enhanced drug loading and reactive oxygen species-responsive release ability for traceable synergistic therapy. J. Am. Chem. Soc. 2018;140(11):4164–4171. doi: 10.1021/jacs.8b01641. [DOI] [PubMed] [Google Scholar]
- 122.Liu S., Jiang S. Zwitterionic polymer-protein conjugates reduce polymer-specific antibody response. Nano Today. 2016;11(3):285–291. [Google Scholar]
- 123.He C., Yin L., Tang C., Yin C. Size-dependent absorption mechanism of polymeric nanoparticles for oral delivery of protein drugs. Biomaterials. 2012;33(33):8569–8578. doi: 10.1016/j.biomaterials.2012.07.063. [DOI] [PubMed] [Google Scholar]
- 124.Palacio J., Agudelo N.A., Lopez B.L. PEGylation of PLA nanoparticles to improve mucus-penetration and colloidal stability for oral delivery systems. Curr. Opin. Chem. Eng. 2016;11:14–19. [Google Scholar]
- 125.Ojer P., de Cerain A.L., Areses P., Peñuelas I., Irache J.M. Toxicity studies of poly(anhydride) nanoparticles as carriers for oral drug delivery. Pharm. Res. (N. Y.) 2012;29(9):2615–2627. doi: 10.1007/s11095-012-0791-8. [DOI] [PubMed] [Google Scholar]
- 126.Volpatti L.R., Matranga M.A., Cortinas A.B., Delcassian D., Daniel K.B., Langer R., Anderson D.G. Glucose-responsive nanoparticles for rapid and extended self-regulated insulin delivery. ACS Nano. 2020;14(1):488–497. doi: 10.1021/acsnano.9b06395. [DOI] [PubMed] [Google Scholar]
- 127.Mansoor S., Kondiah P.P.D., Choonara Y.E., Pillay V. Polymer-based nanoparticles strategies for insulin delivery. Polymers. 2019;11(9):1380. doi: 10.3390/polym11091380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.He Z., Santos J.L., Tian H., Huang H., Hu Y., Liu L., Leong K.W., Chen Y., Mao H.Q. Scalable fabrication of size-controlled chitosan nanoparticles for oral delivery of insulin. Biomaterials. 2017;130:28–41. doi: 10.1016/j.biomaterials.2017.03.028. [DOI] [PubMed] [Google Scholar]
- 129.Lin P.Y., Chiu Y.L., Huang J.H., Chuang E.Y., Mi F.L., Lin K.J., Juang J.H., Sung H.W., Leong K.W. Oral nonviral gene delivery for chronic protein replacement therapy. Adv. Sci. 2018;5(8):1701079. doi: 10.1002/advs.201701079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Lopes M., Shrestha N., Correia A., Shahbazi M.A., Sarmento B., Hirvonen J., Veiga F., Seiça R., Ribeiro A., Santos H.A. Dual chitosan/albumin-coated alginate/dextran sulfate nanoparticles for enhanced oral delivery of insulin. J. Contr. Release. 2016;232:29–41. doi: 10.1016/j.jconrel.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 131.Seyam S., Nordin N.A., Alfatama M. Recent progress of chitosan and chitosan derivatives-based nanoparticles: pharmaceutical perspectives of oral insulin delivery. Pharmaceuticals. 2020;13(10):307. doi: 10.3390/ph13100307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lin Y.H., Mi F.L., Chen C.T., Chang W.C., Peng S.F., Liang H.F., Sung H.W. Preparation and characterization of nanoparticles shelled with chitosan for oral insulin delivery. Biomacromolecules. 2007;8(1):146–152. doi: 10.1021/bm0607776. [DOI] [PubMed] [Google Scholar]
- 133.Chuang E.Y., Nguyen G.T., Su F.Y., Lin K.J., Chen C.T., Mi F.L., Yen T.C., Juang J.H., Sung H.W. Combination therapy via oral co-administration of insulin- and exendin-4 loaded nanoparticles to treat type 2 diabetic rats undergoing OGTT. Biomaterials. 2013;34(32):7994–8001. doi: 10.1016/j.biomaterials.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 134.Yeh T.H., Hsu L.W., Tseng M.T., Lee P.L., Sonjae K., Ho Y.C., Sung H.W. Mechanism and consequence of chitosan-mediated reversible epithelial tight junction opening. Biomaterials. 2011;32(26):6164–6173. doi: 10.1016/j.biomaterials.2011.03.056. [DOI] [PubMed] [Google Scholar]
- 135.Hsu L.W., Ho Y.C., Chuang E.Y., Chen C.T., Juang J.H., Su F.Y., Hwang S.M., Sung H.W. Effects of pH on molecular mechanisms of chitosan-integrin interactions and resulting tight-junction disruptions. Biomaterials. 2013;34(3):784–793. doi: 10.1016/j.biomaterials.2012.09.082. [DOI] [PubMed] [Google Scholar]
- 136.Hsu L.W., Lee P.L., Chen C.T., Mi F.L., Juang J.H., Hwang S.M., Ho Y.C., Sung H.W. Elucidating the signaling mechanism of an epithelial tight-junction opening induced by chitosan. Biomaterials. 2012;33(26):6254–6263. doi: 10.1016/j.biomaterials.2012.05.013. [DOI] [PubMed] [Google Scholar]
- 137.Zare M., Mohammadi Samani S., Sobhani Z. Enhanced intestinal permeation of doxorubicin using chitosan nanoparticles. Adv. Pharmaceut. Bull. 2018;8(3):411–417. doi: 10.15171/apb.2018.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Sung H.W., Sonaje K., Liao Z.X., Hsu L.W., Chuang E.Y. pH-responsive nanoparticles shelled with chitosan for oral delivery of insulin from mechanism to therapeutic applications. Acc. Chem. Res. 2012;45(4):619–629. doi: 10.1021/ar200234q. [DOI] [PubMed] [Google Scholar]
- 139.Nguyen H.N., Wey S.P., Juang J.H., Sonaje K., Ho Y.C., Chuang E.Y., Hsu C.W., Yen T.C., Lin K.J., Sung H.W. The glucose-lowering potential of exendin-4 orally delivered via a pH-sensitive nanoparticle vehicle and effects on subsequent insulin secretion in vivo. Biomaterials. 2011;32(10):2673–2682. doi: 10.1016/j.biomaterials.2010.12.044. [DOI] [PubMed] [Google Scholar]
- 140.Sonaje K., Chen Y.J., Chen H.L., Wey S.P., Juang J.H., Nguyen H.N., Hsu C.W., Lin K.J., Sung H.W. Enteric-coated capsules filled with freeze-dried chitosan/poly(γ-glutamic acid) nanoparticles for oral insulin delivery. Biomaterials. 2010;31(12):3384–3394. doi: 10.1016/j.biomaterials.2010.01.042. [DOI] [PubMed] [Google Scholar]
- 141.Alfatama M., Lim L.Y., Wong T.W. Alginate-C18 conjugate nanoparticles loaded in tripolyphosphate-cross-linked chitosan-oleic acid conjugate-coated calcium alginate beads as oral insulin carrier. Mol. Pharm. 2018;15(8):3369–3382. doi: 10.1021/acs.molpharmaceut.8b00391. [DOI] [PubMed] [Google Scholar]
- 142.Chen X., Ren Y., Feng Y., Xu X., Tan H., Li J. Cp1-11 peptide/insulin complex loaded pH-responsive nanoparticles with enhanced oral bioactivity. Int. J. Pharm. 2019;562:23–30. doi: 10.1016/j.ijpharm.2019.03.020. [DOI] [PubMed] [Google Scholar]
- 143.Chopra S., Bertrand N., Lim J.M., Wang A., Farokhzad O.C., Karnik R. Design of insulin-loaded nanoparticles enabled by multistep control of nanoprecipitation and zinc chelation. ACS Appl. Mater. Interfaces. 2017;9(13):11440–11450. doi: 10.1021/acsami.6b16854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Pridgen E.M., Alexis F., Kuo T.T., Levy-Nissenbaum E., Karnik R., Blumberg R.S., Langer R., Farokhzad O.C. Transepithelial transport of Fc-targeted nanoparticles by the neonatal Fc receptor for oral delivery. Sci. Transl. Med. 2013;5(213):213ra167. doi: 10.1126/scitranslmed.3007049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Li Y., Ji W., Peng H., Zhao R., Zhang T., Lu Z., Yang J., Liu R., Zhang X. Charge-switchable zwitterionic polycarboxybetaine particle as an intestinal permeation enhancer for efficient oral insulin delivery. Theranostics. 2021;11(9):4452–4466. doi: 10.7150/thno.54176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Sheng J., Han L., Qin J., Ru G., Li R., Wu L., Cui D., Yang P., He Y., Wang J. N-trimethyl chitosan chloride-coated PLGA nanoparticles overcoming multiple barriers to oral insulin absorption. ACS Appl. Mater. Interfaces. 2015;7(28):15430–15441. doi: 10.1021/acsami.5b03555. [DOI] [PubMed] [Google Scholar]
- 147.Araújo F., Shrestha N., Gomes M.J., Herranz-Blanco B., Liu D., Hirvonen J.J., Granja P.L., Santos H.A., Sarmento B. In vivo dual-delivery of glucagon like peptide-1 (GLP-1) and dipeptidyl peptidase-4 (DPP4) inhibitor through composites prepared by microfluidics for diabetes therapy. Nanoscale. 2016;8(20):10706–10713. doi: 10.1039/c6nr00294c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Liu M., Zhang J., Zhu X., Shan W., Li L., Zhong J., Zhang Z., Huang Y. Efficient mucus permeation and tight junction opening by dissociable “mucus-inert” agent coated trimethyl chitosan nanoparticles for oral insulin delivery. J. Contr. Release. 2016;222:67–77. doi: 10.1016/j.jconrel.2015.12.008. [DOI] [PubMed] [Google Scholar]
- 149.Zhou S., Deng H., Zhang Y., Wu P., He B., Dai W., Zhang H., Zhang Q., Zhao R., Wang X. Thiolated nanoparticles overcome the mucus barrier and epithelial barrier for oral delivery of insulin. Mol. Pharm. 2020;17(1):239–250. doi: 10.1021/acs.molpharmaceut.9b00971. [DOI] [PubMed] [Google Scholar]
- 150.Lee J.S., Han P., Chaudhury R., Khan S., Bickerton S., McHugh M.D., Park H.B., Siefert A.L., Rea G., Carballido J.M., Horwitz D.A., Criscione J., Perica K., Samstein R., Ragheb R., Kim D., Fahmy T.M. Metabolic and immunomodulatory control of type 1 diabetes via orally delivered bile-acid-polymer nanocarriers of insulin or rapamycin. Nat. Biomed. Eng. 2021;5(9):983–997. doi: 10.1038/s41551-021-00791-0. [DOI] [PubMed] [Google Scholar]
- 151.Griffin B.T., Guo J., Presas E., Donovan M.D., Alonso M.J., O'Driscoll C.M. Pharmacokinetic, pharmacodynamics and biodistribution following oral administration of nanocarriers containing peptide and protein drugs. Adv. Drug Deliv. Rev. 2016;106:367–380. doi: 10.1016/j.addr.2016.06.006. [DOI] [PubMed] [Google Scholar]
- 152.Tan X., Zhang Y., Wang Q., Ren T., Gou J., Guo W., Yin T., He H., Zhang Y., Tang X. Cell-penetrating peptide together with PEG-modified mesostructured silica nanoparticles promotes mucous permeation and oral delivery of therapeutic proteins and peptides. Biomater. Sci. 2019;7(7):2934–2950. doi: 10.1039/c9bm00274j. [DOI] [PubMed] [Google Scholar]
- 153.Wong C.Y., Al-Salami H., Dass C.R. Potential of insulin nanoparticle formulations for oral delivery and diabetes treatment. J. Contr. Release. 2017;264:247–275. doi: 10.1016/j.jconrel.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 154.Araújo F., Shrestha N., Shahbazi M.A., Fonte P., Mäkilä E.M., Salonen J.J., Hirvonen J.T., Granja P.L., Santos H.A., Sarmento B. The impact of nanoparticles on the mucosal translocation and transport of GLP-1 across the intestinal epithelium. Biomaterials. 2014;35(33):9199–9207. doi: 10.1016/j.biomaterials.2014.07.026. [DOI] [PubMed] [Google Scholar]
- 155.Martins J.P., Liu D., Fontana F., Ferreira M.P.A., Correia A., Valentino S., Kemell M., Moslova K., Mäkilä E., Salonen J., Hirvonen J., Sarmento B., Santos H.A. Microfluidic nanoassembly of bioengineered chitosan-modified FcRn-targeted porous silicon nanoparticles @ hypromellose acetate succinate for oral delivery of antidiabetic peptides. ACS Appl. Mater. Interfaces. 2018;10(51):44354–44367. doi: 10.1021/acsami.8b20821. [DOI] [PubMed] [Google Scholar]
- 156.Argyo C., Weiss V., Bräuchle C., Bein T. Multifunctional mesoporous silica nanoparticles as a universal platform for drug delivery. Chem. Mater. 2014;26:435–451. [Google Scholar]
- 157.Tan X., Yin N., Liu Z., Sun R., Gou J., Yin T., Zhang Y., He H., Tang X. Hydrophilic and electroneutral nanoparticles to overcome mucus trapping and enhance oral delivery of insulin. Mol. Pharm. 2020;17(9):3177–3191. doi: 10.1021/acs.molpharmaceut.0c00223. [DOI] [PubMed] [Google Scholar]
- 158.Zhang Y., Xiong M., Ni X., Wang J., Rong H., Su Y., Yu S., Mohammad I.S., Leung S.S.Y., Hu H. Virus-mimicking mesoporous silica nanoparticles with an electrically neutral and hydrophilic surface to improve the oral absorption of insulin by breaking through dual barriers of the mucus layer and the intestinal epithelium. ACS Appl. Mater. Interfaces. 2021;13(15):18077–18088. doi: 10.1021/acsami.1c00580. [DOI] [PubMed] [Google Scholar]
- 159.Shrestha N., Araújo F., Shahbazi M.A., Mäkilä F., Gomes M.J., Herranz-Blanco B., Lindgren R., Granroth S., Kukk E., Salonen J., Hirvonen J., Sarmento B., Santos H.A. Thiolation and cell-penetrating peptide surface functionalization of porous silicon nanoparticles for oral delivery of insulin. Adv. Funct. Mater. 2016;26(20):3405–3416. [Google Scholar]
- 160.Shrestha N., Shahbazi M.A., Araújo F., Zhang H., Mäkilä E.M., Kauppila J., Sarmento B., Salonen J.J., Hirvonen J.T., Santos H.A. Chitosan-modified porous silicon microparticles for enhanced permeability of insulin across intestinal cell monolayers. Biomaterials. 2014;35(25):7172–7179. doi: 10.1016/j.biomaterials.2014.04.104. [DOI] [PubMed] [Google Scholar]
- 161.Martins J.P., Figueiredo P., Wang S., Espo E., Celi E., Martins B., Kemell M., Moslova K., Mäkilä E., Salonen J., Kostiainen M.A., Celia C., Cerullo V., Viitala T., Sarmento B., Hirvonen J., Santos H.A. Neonatal Fc receptor-targeted lignin-encapsulated porous silicon nanoparticles for enhanced cellular interactions and insulin permeation across the intestinal epithelium. Bioact. Mater. 2022;9:299–315. doi: 10.1016/j.bioactmat.2021.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Araújo F., Shrestha N., Shahbazi M.A., Liu D., Herranz-Blanco B., Mäkilä E.M., Salonen J.J., Hirvonen J.T., Granja P.L., Sarmento B., Santos H.A. Microfluidic assembly of a multifunctional tailorable composite system designed for site specific combined oral delivery of peptide drugs. ACS Nano. 2015;9(8):8291–8302. doi: 10.1021/acsnano.5b02762. [DOI] [PubMed] [Google Scholar]
- 163.Shrestha N., Araújo F., Shahbazi M.A., Mäkilä E., Gomes M.J., Airavaara M., Kauppinen E.I., Raula J., Salonen J., Hirvonen J., Sarmento B., Santos H.A. Oral hypoglycaemic effect of GLP-1 and DPP4 inhibitor based nanocomposites in a diabetic animal model. J. Contr. Release. 2016;232:113–119. doi: 10.1016/j.jconrel.2016.04.024. [DOI] [PubMed] [Google Scholar]
- 164.Shrestha N., Shahbazi M.A., Araújo F., Mäkilä E., Raula J., Kauppinen E.I., Salonen J., Sarmento B., Hirvonen J., Santos H.A. Multistage pH-responsive mucoadhesive nanocarriers prepared by aerosol flow reactor technology: a controlled dual protein-drug delivery system. Biomaterials. 2015;68:9–20. doi: 10.1016/j.biomaterials.2015.07.045. [DOI] [PubMed] [Google Scholar]
- 165.Wang S., Morris W., Liu Y., McGuirk C.M., Zhou Y., Hupp J.T., Farha O.K., Mirkin C.A. Surface-specific functionalization of nanoscale metal-organic frameworks. Angew. Chem. Int. Ed. 2015;54(49):14738–14742. doi: 10.1002/anie.201506888. [DOI] [PubMed] [Google Scholar]
- 166.Wu M.X., Yang Y.W. Metal-organic framework (MOF)-based drug/cargo delivery and cancer therapy. Adv. Mater. 2017;29(23):1606134. doi: 10.1002/adma.201606134. [DOI] [PubMed] [Google Scholar]
- 167.Chen Y., Li P., Modica J.A., Drout R.J., Farha O.K. Acid-resistant mesoporous metal-organic framework toward oral insulin delivery: protein encapsulation, protection, and release. J. Am. Chem. Soc. 2018;140(17):5678–5681. doi: 10.1021/jacs.8b02089. [DOI] [PubMed] [Google Scholar]
- 168.Zhou Y., Liu L., Cao Y., Yu S., He C., Chen X. A nanocomposite vehicle based on metal-organic framework nanoparticle incorporated biodegradable microspheres for enhanced oral insulin delivery. ACS Appl. Mater. Interfaces. 2020;12(20):22581–22592. doi: 10.1021/acsami.0c04303. [DOI] [PubMed] [Google Scholar]
- 169.Araújo F., Shrestha N., Granja P.L., Hirvonen J., Santos H.A., Sarmento B. Safety and toxicity concerns of orally delivered nanoparticles as drug carriers, Expert Opin. Drug Metab. Toxicol. 2014;11(3):381–389. doi: 10.1517/17425255.2015.992781. [DOI] [PubMed] [Google Scholar]
- 170.Araújo F., das Neves J., Martins J.P., Granja P.L., Santos H.A., Sarmento B. Functionalized materials for multistage platforms in the oral delivery of biopharmaceuticals. Prog. Mater. Sci. 2017;89:306–344. [Google Scholar]
- 171.Adriano B., Cotto N.M., Chauhan N., Jaggi M., Chauhan S.C., Yallapu M.M. Milk exosomes: nature's abundant nanoplatform for theranostic applications. Bioact. Mater. 2021;6(8):2479–2490. doi: 10.1016/j.bioactmat.2021.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Izumi H., Kosaka N., Shimizu T., Sekine K., Ochiya T., Takase M. Bovine milk contains micorRNA and messenger RNA that are stable under degradative condition. J. Dairy Sci. 2012;95(9):4831–4841. doi: 10.3168/jds.2012-5489. [DOI] [PubMed] [Google Scholar]
- 173.Zhong J., Xia B., Shan S., Zheng A., Zhang S., Chen J., Liang X.J. High-quality milk exosomes as oral drug delivery system. Biomaterials. 2021;277:121126. doi: 10.1016/j.biomaterials.2021.121126. [DOI] [PubMed] [Google Scholar]
- 174.Chen T., Xie M.Y., Sun J.J., Ye R.S., Cheng X., Sun R.P., Wei L.M., Li M., Lin D.L., Jiang Q.Y., Xi Q.Y., Zhang Y.L. Porcine milk-derived exosomes promote proliferation of intestinal epithelial cells. Sci. Rep. 2016;6:33862. doi: 10.1038/srep33862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Zhou Q., Li M., Wang X., Li Q., Wang T., Zhu Q., Zhou X., Wang X., Gao X., Li X. Immune-related microRNAs are abundant in breast milk exosomes. Int. J. Biol. Sci. 2012;8(1):118–123. doi: 10.7150/ijbs.8.118. [DOI] [PMC free article] [PubMed] [Google Scholar]