Abstract
The conclusions of the EFSA following the peer review of the initial risk assessments carried out by the competent authorities of the rapporteur Member State, France, and co‐rapporteur Member State, Spain, for the pesticide active substance heptamaloxyloglucan and the considerations as regards the inclusion of the substance in Annex IV of Regulation (EC) No 396/2005 are reported. The context of the peer review was that required by Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The conclusions were reached on the basis of the evaluation of the representative use of heptamaloxyloglucan as a plant elicitor on grapevines for protection against frost damage (field use). The reliable end points, appropriate for use in regulatory risk assessment, are presented. Missing information identified as being required by the regulatory framework is listed. Concerns are not identified.
Keywords: heptamaloxyloglucan, peer review, risk assessment, pesticide, elicitor, protection against frost damage
Summary
Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659, lays down the procedure for the renewal of the approval of active substances submitted under Article 14 of Regulation (EC) No 1107/2009. The list of those substances is established in Commission Implementing Regulation (EU) No 686/2012 as amended by Commission Implementing Regulation (EU) No 2016/183. Heptamaloxyloglucan is one of the active substances listed in that Regulation.
In accordance with Article 1 of Regulation (EU) No 844/2012, the rapporteur Member State (RMS), France, and co‐rapporteur Member State (co‐RMS), Spain, received an application from ELICITYL SA for the renewal of approval of the active substance heptamaloxyloglucan.
An initial evaluation of the dossier on heptamaloxyloglucan was provided by the RMS in the renewal assessment report (RAR), and subsequently, a peer review of the pesticide risk assessment on the RMS evaluation was conducted by EFSA in accordance with Article 13 of Commission Implementing Regulation (EU) No 844/2012, as amended by Commission Implementing Regulation (EU) No 2018/1659. The following conclusions are derived.
The use of heptamaloxyloglucan according to the representative use as a plant elicitor applied on field grapevine crops using tractor‐mounted sprayer, as proposed at EU level, results in a sufficient plant elicitor efficacy for protection against frost damage.
The assessment of the data package revealed no issues that could not be finalised or that need to be included as critical areas of concern with respect to the section identity, physical–chemical and technical properties of the active substance and the representative formulation and analytical methods.
In the area of mammalian toxicology, there were no issues that could not be finalised or that needed to be included as critical areas of concern.
In the area of residues, the use of heptamaloxyloglucan as plant protection product is not expected to result in an increase of glycans or xyloglucans naturally present in grapes. With regard to the five assessment criteria according to Commission guidance SANCO/11188/2013 rev. 2 (European Commission, 2015) for potential inclusion of substances in Annex IV of Regulation (EC) No 396/2005, two criteria were considered to be met for heptamaloxyloglucan.
Heptamaloxyloglucan is present naturally as a component of plants and soil, and the data available on environmental fate and behaviour were sufficient to carry out the required environmental exposure assessments at EU level for the representative use assessed.
In the area of ecotoxicology, a low risk to all groups of non‐target organisms was concluded for the representative use of heptamaloxyloglucan.
Heptamaloxyloglucan does not meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605.
Background
Commission Implementing Regulation (EU) No 844/2012 1 , as amended by Commission Implementing Regulation (EU) No 2018/1659 2 (hereinafter referred to as ‘the Regulation’), lays down the provisions for the procedure of the renewal of the approval of active substances, submitted under Article 14 of Regulation (EC) No 1107/2009. 3 This regulates for the European Food Safety Authority (EFSA) the procedure for organising the consultation of Member States, the applicant(s) and the public on the initial evaluation provided by the rapporteur Member State (RMS) and/or co‐rapporteur Member State (co‐RMS) in the renewal assessment report (RAR), and the organisation of an expert consultation where appropriate.
In accordance with Article 13 of the Regulation, unless formally informed by the European Commission that a conclusion is not necessary, EFSA is required to adopt a conclusion on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009 within 5 months from the end of the period provided for the submission of written comments, subject to an extension of an additional 3 months where additional information is required to be submitted by the applicant(s) in accordance with Article 13(3). Furthermore, in accordance with Article 13(3a), where the information available in the dossier is not sufficient to conclude the assessment on whether the approval criteria for endocrine disruption are met, additional information can be requested to be submitted in a period of minimum 3 months, not exceeding 30 months, depending on the type of information requested.
In accordance with Article 1 of the Regulation, the RMS, France, and co‐RMS, Spain, received an application from ELICITYL SA for the renewal of approval of the active substance heptamaloxyloglucan. Complying with Article 8 of the Regulation, the RMS checked the completeness of the dossier and informed the applicant, the co‐RMS (Spain), the European Commission and EFSA about the admissibility.
The RMS provided its initial evaluation of the dossier on heptamaloxyloglucan in the RAR, which was received by EFSA on 29 September 2020 (France, 2020, 1980).
In accordance with Article 12 of the Regulation, EFSA distributed the RAR to the Member States and the applicant, ELICITYL SA, for consultation and comments on 17 March 2021. EFSA also provided comments. In addition, EFSA conducted a public consultation on the RAR. EFSA collated and forwarded all comments received to the European Commission on 19 May 2021. At the same time, the collated comments were forwarded to the RMS for compilation and evaluation in the format of reporting table. In addition, the applicant was invited to respond to the comments received. The comments and the applicant’s response were evaluated by the RMS in column 3.
The need for expert consultation and the necessity for additional information to be submitted by the applicant in accordance with Article 13(3) of the Regulation were considered in a telephone conference between EFSA and the RMS France on 29 June 2021. On the basis of the comments received, the applicant’s response to the comments and the RMS’s evaluation thereof, it was concluded that additional information should be requested from the applicant, and that EFSA should conduct an expert consultation in the areas of mammalian toxicology and ecotoxicology.
The outcome of the telephone conference, together with EFSA’s further consideration of the comments, is reflected in the conclusions set out in column 4 of the reporting tables. All points that were identified as unresolved at the end of the comment evaluation phase and which required further consideration, including those issues to be considered in an expert consultation, were compiled by EFSA in the format of an evaluation table.
The conclusions arising from the consideration by EFSA, and as appropriate by the RMS, of the points identified in the evaluation table, together with the outcome of the expert consultation and the written consultation on the assessment of additional information, where these took place, were reported in the final column of the evaluation table.
A final consultation on the conclusions arising from the peer review of the risk assessment and on the considerations for inclusion of the substance in Annex IV of Regulation (EC) No 396/2005 took place with Member States via a written procedure in January–February 2022.
This conclusion report summarises the outcome of the peer review of the risk assessment of the active substance and the representative formulation, evaluated on the basis of the representative use of heptamaloxyloglucan as a plant elicitor on grapevines for protection against frost damage (field use), as proposed by the applicant. In accordance with Article 12(2) of Regulation (EC) No 1107/2009, risk mitigation options identified in the RAR and considered during the peer review, if any, are presented in the conclusion.
A list of the relevant end points for the active substance and the formulation is provided in Appendix B. In addition, the considerations as regards the cut‐off criteria for heptamaloxyloglucan according to Annex II of Regulation (EC) No 1107/2009 are summarised in Appendix A.
A key supporting document to this conclusion is the peer review report (EFSA, 2022), which is a compilation of the documentation developed to evaluate and address all issues raised in the peer review, from the initial commenting phase to the conclusion. The peer review report comprises the following documents, in which all views expressed during the course of the peer review, including minority views, where applicable, can be found:
the comments received on the RAR;
the reporting table (29 June 2021);
the evaluation table (11 February 2022);
the reports of the scientific consultation with Member State experts;
the comments received on the assessment of the additional information;
the comments received on the draft EFSA conclusion.
Given the importance of the RAR, including its revisions (France, 2000a, 2022), and the peer review report, both documents are considered as background documents to this conclusion and thus are made publicly available.
It is recommended that this conclusion and its background documents would not be accepted to support any registration outside the EU for which the applicant has not demonstrated that it has regulatory access to the information on which this conclusion report is based.
The active substance and the formulated product
Heptamaloxyloglucan is the given common name for α‐L‐fucopyranosyl‐(1 → 2)‐β‐d‐galactopyranosyl‐(1 → 2)‐α‐d‐xylopyranosyl‐(1 → 6)‐[α‐d‐xylopyranosyl‐(1 → 6)‐β‐d‐glucopyranosyl‐(1 → 4)]‐β‐d‐glucopyranosyl‐(1 → 4)‐d‐glucitol (IUPAC). It should be noted that there is no ISO common name for this substance; the name ‘heptamaloxyloglucan’ has been used in the literature, but it has no official status.
The representative formulated product for the evaluation was ’PEL101GV’, a lyophilisate (freeze‐dried) cake containing 780 g/kg heptamaloxyloglucan. ’PEL101GV’ appears as a white solid block that can break into shiny crumbs of different sizes and shapes after shaking and it cannot be assigned to any of the formulation codes. The codes which are the closest to the formulation are water‐soluble powder (SP), water‐soluble granule (SG) or water‐soluble tablet (ST) as ‘PEL101GV’ is a solid to be used for dissolution in water. However, the representative formulation is neither a powder nor a granule or a tablet; therefore, it is labelled as ’XX’.
The representative use evaluated comprises field applications by spraying with tractor‐mounted equipment as a plant elicitor on grapevines for protection against frost damage. Full details of the good agricultural practice (GAP) can be found in the list of end points in Appendix B.
Data were submitted to conclude that the use of heptamaloxyloglucan according to the representative use proposed at EU level results in a sufficient plant elicitor efficacy for protection against frost damage, following the guidance document SANCO/2012/11251‐rev. 4 (European Commission, 2014).
Conclusions of the evaluation
1. Identity, physical/chemical/technical properties and methods of analysis
The following guidance documents were followed in the production of this conclusion (European Commission, 2000a, 2022,1977, 2000b, 2001,2010).
The proposed specifications for heptamaloxyloglucan were based on batch data from reanalysis of the batches used during its first evaluation and on recent quality control (QC) data from industrial scale production, all analysed with the same analytical method using an improved equipment. The proposed minimum purity of the active substance as manufactured is 780 g/kg. Patulin was considered as a relevant impurity with maximum specification of 50 µg/kg (see Section 2). It is noted that the manufacturing process of heptamaloxyloglucan has not been changed since its first evaluation; however, based on the improved analytical equipment used for the reanalysis of the batches, an update of the reference specification is proposed. The batches used in the ecotoxicological assessment fully support the original reference and the newly proposed reference specification (see Section 5). The batches used in the mammalian toxicity studies are not representative of the reference specification, however, given the nature of these impurities, the batches can be considered as supporting the original and the updated reference specification (see Section 2). An FAO specification does not exist for heptamaloxyloglucan.
The main data regarding the identity of heptamaloxyloglucan and its physical and chemical properties are given in Appendix B.
Analytical methods for the generation of pre‐approval data required for the risk assessment were not provided, as risk assessment studies were not submitted. Methods of analysis are available for the determination of the active substance in the technical material and in the representative formulation. A data gap was identified for validation data to demonstrate the specificity of the analytical method ISO 8128‐1:1993 for the determination of the relevant impurity patulin in the starting material used for heptamaloxyloglucan manufacturing, in the technical heptamaloxyloglucan and in the representative formulation (see Section 10). Methods for the analysis of residues in food and feed of plant origin, animal products, in body fluids and body tissues and environmental compartments are not required as residue definitions were not set.
2. Mammalian toxicity
The following guidance documents were followed in the production of this conclusion (EFSA PPR Panel, 2012; European Commission, 2012; EFSA, 2014).
The toxicological profile of the active substance heptamaloxyloglucan was discussed at the Pesticides Peer Review Experts’ Teleconference TC 64 in November 2021.
The batches used in the mammalian toxicity studies are not representative of both the current and the newly proposed reference specifications. In the batches used in the Ames test and in the mutagenicity test in mammalian cell, several impurities are not present at a sufficient level to support the reference specifications. However, given the nature of these impurities, the batches can be considered as acceptable. The only toxicologically relevant impurity which might be present in the starting material is the mycotoxin patulin. Although not classified by ECHA, the mycotoxin patulin is considered to be genotoxic according to the WHO (WHO, 2018 4 ). It should be noted that the limit set in the technical material is 50 µg/kg which is in accordance with the limits set in fruit juices and drinks by Commission Regulation (EC) No 1425/2003 5 . Furthermore, according to the data available, patulin is not expected to be present in the technical material due to the type of processing used during the manufacturing process and, in the absence of microbial contamination, the impurity is unlikely to be present after storage and after application. Therefore, as the only toxicologically relevant impurity is not expected to be formed, the toxicological specifications are considered to be covered. Due to the genotoxicity potential of patulin, a particular attention should be paid in the event of a contamination from patulin‐producing microbes during the manufacturing process.
Heptamaloxyloglucan is naturally present in food of plant origin, as it is extracted from apples. No toxicokinetic (ADME) studies have been submitted for heptamaloxyloglucan. Based on literature review, and in view of heptamaloxyloglucan’s chemical structure (xyloglucan‐derived oligosaccharide) and its physical properties (Kow < 10‐4 and molecular weight > 1,000 g/mol), this active substance is not expected to be absorbed in the gastrointestinal tract as an unchanged molecule, or to bioaccumulate. A fraction of the ingested active substance can undergo hydrolysis and fermentation in the gastrointestinal tract releasing glucidic monomer units (glucose, fucose, xylose and galactose), which are naturally occurring in food, and are not classified in any hazard class according to the CLP criteria (Regulation (EC) No 1272/2008 6 ).
Heptamaloxyloglucan has low acute toxicity via the oral and dermal routes in rats, as well as low short‐term oral toxicity. No acute inhalation toxicity data/information are available for heptamaloxyloglucan or any suitable analogue. Heptamaloxyloglucan is applied as a plant protection product by spraying, which is a condition requiring acute inhalation toxicity assessment according to Regulation (EU) No 283/2013 7 ; in the absence of a robust justification for study waiving, a data gap for inhalation toxicity has been identified (see Section 10), although the physico‐chemical properties of the substance do not support inhalation systemic toxicity as a likely outcome.
Heptamaloxyloglucan is neither a skin or eye irritant, nor a skin sensitiser. It is not mutagenic in bacterial and mammalian cells. No data/information or in silico predictions addressing the aneugenic/clastogenic potential of heptamaloxyloglucan have been provided (data gap; see Section 10). The low level of additional exposure from the use as plant protection product in comparison to the background exposure to oligosaccharides has been demonstrated, and in order to avoid unnecessary experiments in mammals, no new vertebrate study is requested.
No adverse effects are expected to be elicited by heptamaloxyloglucan in long‐term, reproductive and neurotoxicity studies based on the absence of mutagenicity, acute, short‐term or target organ toxicity. Overall, no additional animal studies would be needed.
As regards human data, no clinical case or poisoning incident has been reported with heptamaloxyloglucan during any phase of development, production or use of the active substance.
In line with the previous evaluation of heptamaloxyloglucan (European Commission, 2009), no reference values, i.e. acceptable daily intake (ADI), (acute) acceptable operator exposure level (A)(AOEL) or acute reference dose (ARfD), are considered needed, because of the nature of this active substance (low toxicological concern), its higher natural occurrence levels in edible plants compared to the potential contribution from the use as plant protection product (see Section 3), and based on the limited toxicological evidence available.
Consequently, non‐dietary risk assessment for operators, workers, bystanders and residents could not be conducted by comparison to exposure modelling. Nevertheless, as the compound is of low toxicity and is a normal part of the diet, no adverse effects are anticipated.
Based on the physico‐chemical properties of heptamaloxyloglucan, exposure via the dermal route is considered as not relevant (e.g. for workers re‐entering the treated crops area after application). If compared with the estimated background levels of dietary exposure, the relative contribution of non‐dietary exposure (mainly by inhalation) resulting from the use of heptamaloxyloglucan as a plant protection product would be very limited. This approach was considered pragmatic in the absence of information on acute inhalation toxicity.
3. Residues
Due to the nature of the active substance, its proposed representative use and toxicological profile, neither metabolism studies in plants or livestock nor supervised residue trials and feeding studies are presented and they are not required. Grapevines, the only representative use is a permanent crop. Consequently, studies investigating the nature and the quantity of residues in rotational crops are not necessary.
Heptamaloxyloglucan is prepared from dry apple pomace, which is an edible plant part naturally containing this substance at low levels.
Heptamaloxyloglucan is a signal molecule naturally occurring at low levels in plant tissues which is not expected to exhibit toxic effects. Due to its low toxicity, no ADI and no ARfD are proposed for heptamaloxyloglucan (see Section 2). Consequently, it is not necessary to propose residue definitions for the active substance heptamaloxyloglucan in plant or animal commodities. For the above reasons, it is also not necessary to estimate the potential and actual exposure through diet and other sources for heptamaloxyloglucan.
Furthermore, considering the low intended use levels (maximum application rate of 4 × 0.437 g/ha), the early application (up to BBCH 16) and the high water solubility of the active substance (558 g/L), heptamaloxyloglucan is likely to disappear from leaves surface as the last application occurs more than 4 months before harvest. Therefore, the use of heptamaloxyloglucan in the plant protection product is not expected to result in an increase of glycans or xyloglucans naturally present in grapes.
With regard to the five assessment criteria according to Commission guidance SANCO/11188/2013 rev. 2 (European Commission, 2015) for potential inclusion of substances in Annex IV of Regulation (EC) No 396/2005, i.e. approval as basic substance (criterion I), listed in Annex I of Regulation (EC) No 396/2005 (criterion II), having no identified hazardous properties (criterion III), natural exposure is higher than the one linked to the use as plant protection product (criterion IV) and consumer exposure is not expected considering the representative uses (criterion V)), two criteria were considered to be met for heptamaloxyloglucan for the following reasons:
The combination of the low total application rate, together with the high water solubility and the early application growth stage (BBCH 16) are unlikely to result in consumer exposure (criterion V) and hence results in lower exposure than from natural sources (criterion IV). Considering criterion III, toxicological reference values are not required for heptamaloxyloglucan. However, it is noted that fulfilment of criterion III is subject to the data gap in Section 2 addressing the aneugenic/clastogenic potential of heptamaloxyloglucan. Criteria I and II are not met.
4. Environmental fate and behaviour
Specific guideline studies on the route and rate of degradation of heptamaloxyloglucan were not submitted, but the published scientific literature provided demonstrated that heptamaloxyloglucan, which is a xyloglucan‐derived oligosaccharide, can be easily degraded in soil under natural conditions. Degradation products are shorter xyloglucans and monomeric sugars, i.e. d‐glucopyranose, d‐glucitol, d‐xylopyranose, d‐galactopyranose and l‐fucopyranose, that are metabolised by a wide range of microorganisms and are part of the natural organic matter found in soil. It is considered that the amount of heptamaloxyloglucan added to the soil from the intended use (maximum 4 × 0.437 g a.s./ha) can be considered trivial compared to the total loading of oligosaccharides naturally existing in the soil biomass.
Although no experimental DT50 in soil was made available, the estimated values of log Kow < 0 and the established readily biodegradability, demonstrated in a modified Sturm test using a sewage sludge inoculum (OECD 301B; OECD, 2017, 1992), allowed to estimate a conservative half‐life for bulk soil based on results from standardised biodegradation test in line with ECHA (2017, 1992) guidance. According to the value resulting from this guidance, heptamaloxyloglucan exhibited moderate persistence in soil.
Heptamaloxyloglucan is part of the organic matter of the soil, and therefore, calculation of a Koc is not considered relevant due to the nature of the active substance. However, it was agreed that the risk assessment for the requested use could be completed without such quantitative information, assuming that soil adsorption would be expected to be very low.
Heptamaloxyloglucan was shown to be stable under sterile hydrolysis conditions and would be considered photochemically stable in aqueous solution due to its low molecular absorption at wavelengths > 290 nm.
Experimental information from guideline studies on the fate and behaviour of heptamaloxyloglucan in natural water sediment system was not provided. However, from its ready biodegradability, half‐lives in water and sediments can be estimated in line with ECHA (2017, 1992) guidance as exhibiting low and moderate persistence in water and sediment, respectively. It is considered that the amount of heptamaloxyloglucan that may reach natural water systems from the representative use will be minor compared to the total loading of oligosaccharides that will result from biota, in particular from plant cell wall materials rich in xyloglucans. Therefore, it was accepted that the risk assessment for the requested use could be completed without further experimental evidence.
The necessary surface water and sediment exposure assessments (predicted environmental concentrations (PEC) calculations) were carried out for heptamaloxyloglucan using the FOCUS (FOCUS, 2001) Step 1 (version 3.2 of the FOCUS Step 1–2 calculator) based on a single maximum annual total application therefore using conservative assumptions.
For the intended use and considering the fact that heptamaloxyloglucan is naturally present in the environment and undergoes degradation in ubiquitous substances by soil microorganisms already in superficial layers, it is not expected to reach groundwater. For this reason, PEC groundwater calculations were not provided and are not considered necessary. Heptamaloxyloglucan and its degradation products would not reach deeper soil layers such that groundwater concentrations would exceed 0.1 µg/L, even though heptamaloxyloglucan would be expected to have low soil adsorption.
No degradation products of concern are expected to be formed according to heptamaloxyloglucan structure. Consequently, data on its impact on water treatment procedures are not necessary. Nevertheless, a submitted study shows that a pretreatment with NaOH followed by ozone‐altered cellulose and hemicellulose‐promoting subsequent enzymatic hydrolysis of degraded products, including heptamaloxyloglucan.
The PEC in soil (based on a maximum annual total dose), surface water and sediment covering the representative use assessed can be found in Appendix B of this conclusion. A key to the wording used to describe the persistence and mobility of the compound assessed, relating these words to the numerical DT and Koc endpoints values estimated, can be found in Appendix C of this conclusion.
5. Ecotoxicology
The risk assessment was based on the following documents European Commission (2002a,b), SETAC (1977, 2000b, 2001), EFSA (2009, 2013) and EFSA PPR Panel (2013).
The batches used in the ecotoxicity studies were considered representative of the original and the newly proposed technical specification. 8
No ecotoxicity data were available for birds. However, a low acute and long‐term risk was concluded by considering that (i) heptamaloxyloglucan is a natural component of birds' diet, (ii) low environmental levels are expected following its representative use (maximum application rate of 4 × 0.437 g a.s./ha) and (iii) the lack of dietary toxicity to broilers of compounds closely related to heptamaloxyloglucan as shown in relevant peer‐reviewed publications. Acute and short‐term toxicity studies were available for mammals with the active substance. A low acute and reproductive risk was indicated at the screening step. The risk assessment from exposure to contaminated water and via secondary poisoning for earthworm‐ and fish‐eating birds and mammals was not triggered.
For aquatic organisms, acute toxicity data with the technical heptamaloxyloglucan were submitted for fish (Oncorhynchus mykiss), aquatic invertebrates (Daphnia magna) and algae (Scenedesmus subcapitata). A quantitative tier 1 risk assessment indicated low risk for all taxa at PECsw of FOCUS Step 1 for the representative use of heptamaloxyloglucan.
Acute (oral and contact) toxicity data for honeybees were available with technical heptamaloxyloglucan. A low acute risk to bees via oral and contact exposure was indicated following the SANCO Guidance on Terrestrial ecotoxicology (European Commission, 2002a) and the EFSA Bee Guidance (EFSA, 2013) schemes. In the absence of chronic toxicity data, the chronic risk to honeybees was addressed by considering (i) the natural exposure of bees to carbohydrates in plant parts; (ii) the low levels of additional exposure to natural background levels following the representative use in grapes; (iii) carbohydrates usually represent a source of energy for honey bees; and (iv) the outcome of an indicative risk assessment based on EFSA (2013) using the endpoint from a peer‐reviewed study with galactose on honeybee adults 9 based on the worst‐case assumption that heptamaloxyloglucan was degraded completely to galactose. On this basis, a low chronic risk to honeybee adults and larvae was concluded for the representative use of heptamaloxyloglucan. Although a quantitative assessment was not presented, the risk from exposure to contaminated water is expected to be low. No studies were submitted for bumblebees and solitary bees.
No studies on the toxicity of heptamaloxyloglucan to non‐target arthropods other than bees were submitted. A low risk was concluded considering (i) the nature of heptamaloxyloglucan (xyloglucan is extracted from apple), (ii) its non‐toxic mode of action (it is a plant elicitor to protect vine from freezing) and (iii) its natural occurrence in plants and limited additional exposure to natural background levels.
Ecotoxicity studies were not submitted for any group of soil organisms. Using evidence from the literature, it was demonstrated that heptamaloxyloglucan is degraded by microorganism species into monomeric sugars. In addition, low amounts of residues are expected to reach the soil following the representative use of heptamaloxyloglucan. On this basis, the risk to earthworms, soil meso‐ and macrofauna other than earthworms and to soil microorganisms was concluded low.
To address the risk to non‐target terrestrial plants, a vegetative vigour study on three plant species (red cover, wheat and mustard) was available for the representative formulation. Based on the limitations identified, 10 the study was considered as supportive information. On the basis of the outcome of the study (no adverse effects up to 20.0 g a.s./ha were observed), the nature and non‐toxic mode of action of the substance and the low levels of exposure following the representative use, a low risk to non‐target terrestrial plants was concluded.
A low risk was indicated for the organisms involved in biological methods for sewage treatment for the representative use of heptamaloxyloglucan.
6. Endocrine disruption properties
With regard to the assessment of the endocrine disruption potential of heptamaloxyloglucan for humans and non‐target organisms according to the ECHA/EFSA guidance (2018), although a full (eco)toxicological data set was not available to assess the endocrine‐disrupting properties, this does not appear scientifically necessary by considering the following justifications:
Heptamaloxyloglucan is not absorbed as unchanged molecule and has no bioaccumulation potential;
It has a non‐toxic mode of action;
Carbohydrates, compounds similar to heptamaloxyloglucan are largely distributed in soil and fruits;
The absorbable metabolites of heptamaloxyloglucan are monosaccharides (glucose, fucose, xylose and galactose). They are naturally present in metabolic pathways of animals and humans and are known to be devoid of toxicity except when ingested in very large quantities;
No indication of oestrogen, androgen, thyroid and steroidogenesis (EATS) mediated adversity were observed in the available (eco)toxicological studies;
The level of exposure to heptamaloxyloglucan following application as a plant protection product is considered low in comparison to the natural background via food consumption.
Considering the above, it can be concluded that heptamaloxyloglucan does not meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II to Regulation (EC) No 1107/2009, as amended by Commission Regulation (EU) 2018/605. 11
7. Overview of the risk assessment of compounds listed in residue definitions triggering assessment of effects data for the environmental compartments (Tables 1, 2, 3–4)
Table 1.
Soil
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Heptamaloxyloglucan | Low risk to soil organisms |
Table 2.
Groundwater a
| Compound (name and/or code) |
> 0.1 μg/L at 1 m depth for the representative uses b Step 2 |
Biological (pesticidal) activity/relevance Step 3a. |
Hazard identified Steps 3b. and 3c. |
Consumer RA triggered Steps 4 and 5 |
Human health relevance |
|---|---|---|---|---|---|
| Heptamaloxyloglucan | No | Yes | – | – | Yes |
Assessment according to European Commission guidance of the relevance of groundwater metabolites (2003).
FOCUS scenarios or relevant lysimeter.
Table 3.
Surface water and sediment
| Compound (name and/or code) | Ecotoxicology |
|---|---|
| Heptamaloxyloglucan | Low risk to aquatic organisms |
Table 4.
Air
| Compound (name and/or code) | Toxicology |
|---|---|
| Heptamaloxyloglucan | Not available (data gap) |
8. Particular conditions proposed to be taken into account by risk managers
Risk mitigation measures (RMMs) identified following consideration of Member State (MS) and/or applicant’s proposal(s) during the peer review, if any, are presented in this section. These measures applicable for human health and/or the environment leading to a reduction of exposure levels of operators, workers, bystanders/residents, environmental compartments and/or non‐target organisms for the representative uses are listed below. The list may also cover any RMMs as appropriate, leading to an acceptable level of risks for the respective non‐target organisms.
It is noted that final decisions on the need of RMMs to ensure the safe use of the plant protection product containing the concerned active substance will be taken by risk managers during the decision‐making phase. Consideration of the validity and appropriateness of the RMMs remains the responsibility of MSs at product authorisation, taking into account their specific agricultural, plant health and environmental conditions at national level.
No particular conditions are proposed for the representative use evaluated.
9. Concerns and related data gaps
9.1. Issues that could not be finalised
An issue is listed as ‘could not be finalised’ if there is not enough information available to perform an assessment, even at the lowest tier level, for one or more of the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011 12 and if the issue is of such importance that it could, when finalised, become a concern (which would also be listed as a critical area of concern if it is of relevance to all representative uses).
An issue is also listed as ‘could not be finalised’ if the available information is considered insufficient to conclude on whether the active substance can be expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following issues or assessments that could not be finalised have been identified, together with the reasons including the associated data gaps where relevant, which are reported directly under the specific issue to which they are related:
Issues that could not be finalised and associated data gaps were not identified.
9.2. Critical areas of concern
An issue is listed as a critical area of concern if there is enough information available to perform an assessment for the representative uses in line with the uniform principles in accordance with Article 29(6) of Regulation (EC) No 1107/2009 and as set out in Commission Regulation (EU) No 546/2011, and if this assessment does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if the assessment at a higher tier level could not be finalised due to lack of information, and if the assessment performed at the lower tier level does not permit the conclusion that, for at least one of the representative uses, it may be expected that a plant protection product containing the active substance will not have any harmful effect on human or animal health or on groundwater, or any unacceptable influence on the environment.
An issue is also listed as a critical area of concern if, in the light of current scientific and technical knowledge using guidance documents available at the time of application, the active substance is not expected to meet the approval criteria provided for in Article 4 of Regulation (EC) No 1107/2009.
The following critical areas of concern are identified, together with any associated data gaps, where relevant, which are reported directly under the specific critical area of concern to which they are related:
Critical areas of concern were not identified.
9.3. Overview of the concerns identified for each representative use considered (Table 5)
Table 5.
Overview of concerns reflecting the issues not finalised, critical areas of concerns and the risks identified that may be applicable for some but not for all uses or risk assessment scenarios
| Representative use | Vine | |
|---|---|---|
|
Field use Foliar spraying using an air pressured system |
||
| Operator risk | Risk identified | |
| Assessment not finalised | ||
| Worker risk | Risk identified | |
| Assessment not finalised | ||
| Resident/bystander risk | Risk identified | |
| Assessment not finalised | ||
| Consumer risk | Risk identified | |
| Assessment not finalised | ||
| Risk to wild non‐target terrestrial vertebrates | Risk identified | |
| Assessment not finalised | ||
| Risk to wild non‐target terrestrial organisms other than vertebrates | Risk identified | |
| Assessment not finalised | ||
| Risk to aquatic organisms | Risk identified | |
| Assessment not finalised | ||
| Groundwater exposure to active substance | Legal parametric value breached | |
| Assessment not finalised | ||
| Groundwater exposure to metabolites | Legal parametric value breached a | |
| Parametric value of 10 µg/L b breached | ||
| Assessment not finalised | ||
When the consideration for classification made in the context of this evaluation under Regulation (EC) No 1107/2009 is confirmed under Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008.
Value for non‐relevant metabolites prescribed in SANCO/221/2000‐rev. 10 final, European Commission (2003).
(If a particular condition proposed to be taken into account to manage an identified risk, as listed in Section 8, has been evaluated as being effective, then ‘risk identified’ is not indicated in Table 5.)
10. List of other outstanding issues
Remaining data gaps not leading to critical areas of concern or issues not finalised but considered necessary to comply with the data requirements, and which are relevant for some or all of the representative uses assessed at EU level. Although not critical, these data gaps may lead to uncertainties in the assessment and are considered relevant.
These data gaps refer only to the representative uses assessed and are listed in the order of the sections.
Validation data to demonstrate the specificity of the analytical method ISO 8128‐1:1993 for the determination of the relevant impurity patulin in the starting material used for heptamaloxyloglucan manufacturing, in the technical heptamaloxyloglucan and in the representative formulation (relevant for the representative use, see Section 1).
Acute inhalation toxicity assessment was not available for heptamaloxyloglucan (relevant for the representative use evaluated; see Section 2), although the physico‐chemical properties of the substance do not support inhalation systemic toxicity as a likely outcome.
The aneugenic/clastogenic potential of heptamaloxyloglucan was not addressed (relevant for the representative use evaluated; see Section 2). Nonetheless, the low level of exposure from the use of the plant protection product in comparison to the natural exposure to oligosaccharides is not expected to lead to health concerns.
Abbreviations
- 1/n
slope of Freundlich isotherm
- Λ
wavelength
- ε
decadic molar extinction coefficient
- a.s.
active substance
- ADI
acceptable daily intake
- AOEL
acceptable operator exposure level
- ARfD
acute reference dose
- DT50
period required for 50% dissipation (define method of estimation)
- DT90
period required for 90% dissipation (define method of estimation)
- FAO
Food and Agriculture Organization of the United Nations
- FOCUS
Forum for the Co‐ordination of Pesticide Fate Models and their Use
- GAP
Good Agricultural Practice
- ISO
International Organization for Standardization
- IUPAC
International Union of Pure and Applied Chemistry
- iv
intravenous
- mm
millimetre (also used for mean measured concentrations)
- OECD
Organisation for Economic Co‐operation and Development
- Pa
pascal
- r2
coefficient of determination
- RAC
regulatory acceptable concentration
- RAR
Renewal Assessment Report
- SC
suspension concentrate
- SFO
single first‐order
- SMILES
simplified molecular‐input line‐entry system
- WHO
World Health Organization
Appendix A – Consideration of cut‐off criteria for heptamaloxyloglucan according to Annex II of Regulation (EC) No 1107/2009 of the European Parliament and of the Council
| Properties | Conclusion a | |
|---|---|---|
| CMR | Carcinogenicity (C) | Heptamaloxyloglucan is not considered to be mutagenic, carcinogenic or toxic for reproduction according to points 3.6.2, 3.6.3 and 3.6.4 of Annex II of Regulation (EC) 1107/2009. |
| Mutagenicity (M) | ||
| Toxic for Reproduction (R) | ||
| Endocrine‐disrupting properties | Heptamaloxyloglucan is not considered to meet the criteria for endocrine disruption for humans and non‐target organisms according to points 3.6.5 and 3.8.2 of Annex II of Regulation No 1107/2009, as amended by Commission Regulation (EU) 2018/605. | |
| POP | Persistence | Heptamaloxyloglucan is not considered to be a persistent organic pollutant (POP) according to point 3.7.1 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
| Long‐range transport | ||
| PBT | Persistence | Heptamaloxyloglucan is not considered to be a persistent, bioaccumulative and toxic (PBT) substance according to point 3.7.2 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
| Toxicity | ||
| vPvB | Persistence | Heptamaloxyloglucan is not considered to be a very persistent, very bioaccumulative substance according to point 3.7.3 of Annex II of Regulation (EC) 1107/2009. |
| Bioaccumulation | ||
Origin of data to be included where applicable (e.g. EFSA, ECHA RAC, Regulation).
Appendix B – List of end points for the active substance and the representative formulation
Appendix B can be found in the online version of this output (‘Supporting information’ section): https://doi.org/10.2903/j.efsa.2022.7210
Appendix C – Wording EFSA used in Section 4 of this conclusion, in relation to DT and Koc ‘classes’ exhibited by each compound assessed
| Wording | DT50 normalised to 20°C for laboratory incubations 13 or not normalised DT50 for field studies (SFO equivalent, when biphasic, the DT90 was divided by 3.32 to estimate the DT50 when deciding on the wording to use) |
|---|---|
| Very low persistence | < 1 day |
| Low persistence | 1–< 10 days |
| Moderate persistence | 10–< 60 days |
| Medium persistence | 60–< 100 days |
| High persistence | 100 days–< 1 year |
| Very high persistence | A year or more |
Note these classes and descriptions are unrelated to any persistence class associated with the active substance cut‐off criteria in Annex II of Regulation (EC) No 1107/2009. For consideration made in relation to Annex II, see Appendix A.
| Wording | Koc (either KFoc or Kdoc) mL/g |
|---|---|
| Very high mobility | 0–50 |
| High mobility | 51–150 |
| Medium mobility | 151–500 |
| Low mobility | 501–2,000 |
| Slight mobility | 2,001–5,000 |
| Immobile | > 5,000 |
Appendix D – Used compound codes
| Code/trivial name a | IUPAC name/SMILES notation/InChiKey b | Structural formula c |
|---|---|---|
| Heptamaloxyloglucan |
α‐l‐fucopyranosyl‐(1 → 2)‐β‐d‐galactopyranosyl‐(1 → 2)‐α‐d‐xylopyranosyl‐(1 → 6)‐[α‐d‐xylopyranosyl‐(1 → 6)‐β‐d‐glucopyranosyl‐(1 → 4)]‐β‐d‐glucopyranosyl‐(1 → 4)‐d‐glucitol OC[C@H](O)[C@@H](O)[C@H](O[C@@H]1O[C@H](CO[C@H]2OC[C@@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@H](CO)[C@H](O)[C@H](O)[C@H]2O[C@@H]2O[C@@H](C)[C@@H](O)[C@@H](O)[C@@H]2O)[C@@H](O[C@@H]2O[C@H](CO[C@H]3OC[C@@H](O)[C@H](O)[C@H]3O)[C@@H](O)[C@H](O)[C@H]2O)[C@H](O)[C@H]1O)[C@H](O)CO RAUODYOTTYNEJP‐WTQIIJSDSA‐N |

|
| Patulin |
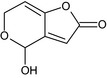
4‐hydroxy‐4H‐furo[3,2‐c]pyran‐2(6H)‐one O=C1C=C2C(=CCOC2O)O1 ZRWPUFFVAOMMNM‐UHFFFAOYSA‐N |
|
| Glucose |
d‐glucose O[C@H]([C@@H](O)C=O)[C@H](O)[C@H](O)CO GZCGUPFRVQAUEE‐SLPGGIOYSA‐N |

|
| Fucose |
6‐deoxy‐l‐galactose O[C@@H](C)[C@@H](O)[C@@H](O)[C@H](O)C=O PNNNRSAQSRJVSB‐KCDKBNATSA‐N |

|
| Xylose |
d‐xylose O[C@@H](C=O)[C@@H](O)[C@H](O)CO PYMYPHUHKUWMLA‐VPENINKCSA‐N l‐xylose O[C@H](C=O)[C@H](O)[C@@H](O)CO PYMYPHUHKUWMLA‐WISUUJSJSA‐N |
|
| Galactose |
d‐galactose O[C@H]([C@@H](O)C=O)[C@@H](O)[C@H](O)CO GZCGUPFRVQAUEE‐KCDKBNATSA‐N l‐galactose O[C@@H]([C@H](O)C=O)[C@H](O)[C@@H](O)CO GZCGUPFRVQAUEE‐DPYQTVNSSA‐N |
|
| d‐glucopyranose |
d‐glucopyranose O[C@H]1[C@H](O)[C@@H](CO)OC(O)[C@@H]1O WQZGKKKJIJFFOK‐GASJEMHNSA‐N |

|
| d‐glucitol |
d‐glucitol O[C@H]([C@H](O)CO)[C@H](O)[C@@H](O)CO FBPFZTCFMRRESA‐JGWLITMVSA‐N |

|
| d‐xylopyranose |
d‐xylopyranose O[C@H]1[C@H](O)COC(O)[C@@H]1O SRBFZHDQGSBBOR‐IOVATXLUSA‐N |

|
| d‐galactopyranose |
d‐galactopyranose O[C@H]1[C@@H](O)[C@@H](CO)OC(O)[C@@H]1O WQZGKKKJIJFFOK‐SVZMEOIVSA‐N |

|
| l‐fucopyranose |
6‐deoxy‐l‐galactopyranose O[C@@H]1[C@H](O)[C@H](C)OC(O)[C@H]1O SHZGCJCMOBCMKK‐DHVFOXMCSA‐N |

|
The metabolite name in bold is the name used in the conclusion.
ACD/Name 2020.2.1 ACD/Labs 2020 Release (File version N15E41, Build 116563, 15 June 2020).
ACD/ChemSketch 2020.2.1 ACD/Labs 2020 Release (File version C25H41, Build 121153, 22 March 2021).
Supporting information
List of end points for the active substance and the representative formulation
Suggested citation: EFSA (European Food Safety Authority) , Alvarez F, Arena M, Auteri D, Castoldi AF, Chiusolo A, Colagiorgi A, Colas M, Crivellente F, De Lentdecker C, Egsmose M, Fait G, Gouliarmou V, Ferilli F, Ippolito A, Istace F, Jarrah S, Kardassi D, Kienzler A, Lava R, Linguadoca A, Lythgo C, Magrans O, Mangas I, Miron I, Molnar T, Padovani L, Parra Morte JM, Serafimova R, Sharp R, Szentes C, Terron A, Theobald A, Tiramani M and Villamar‐Bouza L, 2022. Conclusion on the peer review of the pesticide risk assessment of the active substance heptamaloxyloglucan. EFSA Journal 2022;20(3):7210, 22 pp. 10.2903/j.efsa.2022.7210
Requestor: European Commission
Question number: EFSA‐Q‐2017‐00547
Note: This scientific output, approved on 28 February 2022, supersedes the previous output published on 6 August 2009 (EFSA, 2009).
Declarations of interest: The declarations of interest of all scientific experts active in EFSA’s work are available at https://ess.efsa.europa.eu/doi/doiweb/doisearch.
Acknowledgments: EFSA wishes to thank the rapporteur Member State, France, for the preparatory work on this scientific output.
Approved: 28 February 2022
Notes
Commission Implementing Regulation (EU) No 844/2012 of 18 September 2012 setting out the provisions necessary for the implementation of the renewal procedure for active substances, as provided for in Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 252, 19.9.2012, p. 26–32.
Commission Implementing Regulation (EU) No 2018/1659 of 7 November 2018 amending Implementing Regulation (EU) No 844/2012 in view of the scientific criteria for the determination of endocrine disrupting properties introduced by Regulation (EU) 2018/605.
Regulation (EC) No 1107/2009 of 21 October 2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market and repealing Council Directives 79/117/EEC and 91/414/EEC. OJ L 309, 24.11.2009, p. 1–50.
Commission Regulation (EC) No 1425/2003 of 11 August 2003 amending Regulation (EC) No 466/2001 as regards patulin. OJ L 203, 12.8.2003, p. 1–3.
Regulation (EC) No 1272/2008 of the European Parliament and of the Council of 16 December 2008 on classification, labelling and packaging of substances and mixtures, amending and repealing Directives 67/548/EEC and 1999/45/EC, and amending Regulation (EC) No 1907/2006. OJ L 353, 31.12.2008, p. 1–1355.
Commission Regulation (EU) No 283/2013 of 1 March 2013 setting out the data requirements for active substances, in accordance with Regulation (EC) No 1107/2009 of the European Parliament and of the Council concerning the placing of plant protection products on the market. OJ L 93, 3.4.2013, p. 1–84.
Only acute toxicity studies with aquatic organisms and honeybees were available.
Barker RJ, 1977. Some carbohydrates found in pollen and pollen substitutes are toxic to honeybees. The Journal of Nutrition, 107, 1859–1862. https://doi.org/10.1093/jn/107.10.1859
The fulfilment of the validity criteria for seedling emergence and survival in the control groups outlined in the OECD 227 Test Guideline could not be confirmed. Furthermore, no analytical verification of the applied rates was performed as recommended in OECD 227.
Commission Regulation (EU) 2018/605 of 19 April 2018 amending Annex II to Regulation (EC) No 1107/2009 by setting out scientific criteria for the determination of endocrine‐disrupting properties. OJ L 101, 20.4.2018, p. 33–36.
Commission Regulation (EU) No 546/2011 of 10 June 2011 implementing Regulation (EC) No 1107/2009 of the European Parliament and of the Council as regards uniform principles for evaluation and authorisation of plant protection products. OJ L 155, 11.6.2011, p. 127–175.
For laboratory soil incubations normalisation was also to field capacity soil moisture (pF2/10 kPa). For laboratory sediment water system incubations, the whole system DT values were used.
References
- Barker RJ, 1977. Some carbohydrates found in pollen and pollen substitutes are toxic to honey bees. The Journal of Nutrition, 107, 1859–1862. 10.1093/jn/107.10.1859 [DOI] [PubMed] [Google Scholar]
- ECHA (European Chemical Agency) , 2017. Guidance on Biocidal Products Regulation: Volume IV Environment ‐ Assessment and Evaluation (Parts B+C), October 2017. Reference: ECHA‐17‐G‐23‐EN; Cat. Number: ED‐01‐17‐897‐EN‐N; ISBN: 978‐92‐9020‐151‐9. 10.2823/033935 [DOI]
- ECHA (European Chemicals Agency) and EFSA (European Food Safety Authority) with the technical support of the Joint Research Centre (JRC), Andersson N, Arena M, Auteri D, Barmaz S, Grignard E, Kienzler A, Lepper P, Lostia AM, Munn S, Parra Morte JM, Pellizzato F, Tarazona J, Terron A and Van der Linden S, 2018. Guidance for the identification of endocrine disruptors in the context of Regulations (EU) No 528/2012 and (EC) No 1107/2009. EFSA Journal 2018;16(6):5311,135 pp. 10.2903/j.efsa.2018.5311. ECHA‐18‐G‐01‐EN. [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2009. Guidance on Risk Assessment for Birds and Mammals on request from EFSA. EFSA Journal 2009;7(12):1438, 358 pp. 10.2903/j.efsa.2009.1438 [DOI] [Google Scholar]
- EFSA (European Food Safety Authority) , 2013. EFSA Guidance Document on the risk assessment of plant protection products on bees (Apis mellifera, Bombus spp. and solitary bees). EFSA Journal 2013;11(7):3295, 268 pp. 10.2903/j.efsa.2013.3295 [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2014. Guidance on the assessment of exposure of operators, workers, residents and bystanders in risk assessment for plant protection products. EFSA Journal 2014;12(10):3874, 55 pp. 10.2903/j.efsa.2014.3874 Available online: www.efsa.europa.eu/efsajournal [DOI] [PMC free article] [PubMed] [Google Scholar]
- EFSA (European Food Safety Authority) , 2022. Peer review report to the conclusion regarding the peer review of the pesticide risk assessment of the active substance heptamaloxyloglucan. Available online: www.efsa.europa.eu [DOI] [PMC free article] [PubMed]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) , 2012. Guidance on dermal absorption. EFSA Journal 2012;10(4):2665, 30 pp. 10.2903/j.efsa.2012.2665 [DOI] [Google Scholar]
- EFSA PPR Panel (EFSA Panel on Plant Protection Products and their Residues) 2013. Guidance on tiered risk assessment for plant protection products for aquatic organisms in edge‐of‐field surface waters. EFSA Journal 2013;11(7):3290, 186 pp. 10.2903/j.efsa.2013.3290 [DOI] [Google Scholar]
- European Commission , 2000a. Residues: guidance for generating and reporting methods of analysis in support of pre‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3029/99‐rev. 4, 11 July 2000.
- European Commission , 2000b. Technical material and preparations: guidance for generating and reporting methods of analysis in support of pre‐ and post‐registration data requirements for Annex II (Part A, Section 4) and Annex III (Part A, Section 5) of Directive 91/414. SANCO/3030/99‐rev. 4, 11 July 2000.
- European Commission , 2002a. Guidance Document on Terrestrial Ecotoxicology Under Council Directive 91/414/EEC. SANCO/10329/2002‐rev. 2 final, 17 October 2002.
- European Commission , 2002b. Guidance Document on Aquatic Ecotoxicology Under Council Directive 91/414/EEC. SANCO/3268/2001‐rev. 4 final, 17 October 2002.
- European Commission , 2003. Guidance Document on Assessment of the Relevance of Metabolites in Groundwater of Substances Regulated under Council. Directive 91/414/EEC. SANCO/221/2000‐rev. 10 final, 25 February 2003
- European Commission , 2009. Review report for the active substance heptamaloxyloglucan. Finalised in the Standing Committee on the Food Chain and Animal Health at its meeting on 27 November 2009 in view of the inclusion of heptamaloxyloglucan in Annex I of Council Directive 91/414/EEC. SANCO/10502/09 ‐ final, 27 November 2009.
- European Commission , 2010. Guidance Document on residue analytical methods. SANCO/825/00‐rev. 8.1, 16 November 2010.
- European Commission , 2012. Guidance document on the assessment of the equivalence of technical materials of substances regulated under Regulation (EC). No 1107/2009. SANCO/10597/2003‐rev. 10.1, 13 July 2012.
- European Commission , 2014. Guidance document on the renewal of approval of active substances to be assessed in compliance with Regulation (EU) No 844/2012. SANCO/2012/11251‐rev. 4, 12 December 2014.
- European Commission , 2015. Guidance document on criteria for the inclusion of active substances into Annex IV of Regulation (EC) No 396/2005. SANCO/11188/2013, Rev. 2, 14 September 2015.
- FOCUS (Forum for the Co‐ordination of Pesticide Fate Models and their Use) , 2001. FOCUS surface water scenarios in the EU evaluation process under 91/414/EEC. Report of the FOCUS Working Group on Surface Water Scenarios. EC Document Reference SANCO/4802/2001‐rev. 2, 245 pp., as updated by Generic guidance for FOCUS surface water scenarios, v. 1.4, May 2015.
- France , 2020. Renewal Assessment Report (RAR) on the active substance heptamaloxyloglucan prepared by the rapporteur Member State France, in the framework of Commission Implementing Regulation (EU). No 844/2012, September 2020. Available online: www.efsa.europa.eu
- France , 2022. Revised Renewal Assessment Report (RAR) on heptamaloxyloglucan prepared by the rapporteur Member State France in the framework of Commission Implementing Regulation (EU). No 844/2012, January 2022. Available online: www.efsa.europa.eu
- McCall PJ, Laskowski DA, Swann RL and Dishburger HJ, 1980. Measurements of sorption coefficients of organic chemicals and their use in environmental fate analysis. In: Test Protocols for Environmental Fate and Movement of Toxicants. In: Proceedings of the 94th Annual Meeting of the American Association of Official Analytical Chemists (AOAC)Oct 21–22, Washington, DC, pp. 89–109. [Google Scholar]
- OECD (Organisation for Economic Co‐operation and Development) , 1992. Test No. 301: Ready Biodegradability, OECD Guidelines for the Testing of Chemicals, Section 3, OECD Publishing, Paris. Available online: www.oecd.org [Google Scholar]
- SETAC (Society of Environmental Toxicology and Chemistry) , Candolfi MP, Barrett KL, Campbell PJ, Forster R, Grandy N, Huet MC, Lewis G, Oomen PA, Schmuck R and Vogt H (eds.), 2001. Guidance document on regulatory testing and risk assessment procedures for plant protection products with non‐target arthropods. ESCORT 2 workshop.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
List of end points for the active substance and the representative formulation




