Abstract
Background
Tuberculosis (TB) is one of the leading causes of death worldwide. Radiology has an important role in the diagnosis of both drug-sensitive (DS) and rifampicin-resistant (RR) pulmonary TB (PTB). This study aimed to compare the chest x-ray (CXR) patterns of microbiologically confirmed DS and RR PTB cases stratified by HIV serostatus in Uganda.
Methods
We conducted a hospital-based retrospective study at the Mulago National Referral Hospital (MNRH) TB wards. All participants had a microbiologically confirmed diagnosis of PTB. CXR findings extracted included infiltrates, consolidation, cavity, fibrosis, bronchiectasis, atelectasis, and other non-lung parenchymal findings. All films were examined by two independent radiologists blinded to the clinical diagnosis.
Results
We analyzed CXR findings of 165 participants: 139 DS- and 26 RR-TB cases. The majority (n = 118, 71.7%) of the participants were seronegative for HIV. Overall, 5/165 (3%) participants had normal CXR. There was no statistically significant difference in the proportion of participants with consolidations (74.8% versus 88.5%; p = 0.203), bronchopneumonic opacities (56.1% versus 42.3%, p = 0.207) and cavities (38.1% versus 46.2%, p = 0.514), across drug susceptibility status (DS versus RR TB). Among HIV-infected participants, consolidations were predominantly in the middle lung zone in the DS TB group and in the lower lung zone in the RR TB group (42.5% versus 12.8%, p = 0.66). HIV-infected participants with RR TB had statistically significantly larger cavity sizes compared to their HIV uninfected counterparts with RR TB (7.7 ± 6.8 cm versus 4.2 ± 1.3 cm, p = 0.004).
Conclusions
We observed that a vast majority of participants had similar CXR changes, irrespective of drug susceptibility status. However, HIV-infected RR PTB had larger cavities.
The diagnostic utility of cavity sizes for the differentiation of HIV-infected and non-infected RR TB could be investigated further.
Abbreviations: PTB, Pulmonary Tuberculosis; HIV, Human Immunodeficiency Virus; DS-TB, Drug sensitive tuberculosis; RIF, Resistance to rifampicin; RR-TB, Rifampicin-resistant tuberculosis; MDR, Multidrug resistant tuberculosis; MNRH, Mulago national referral hospital; MTB, Mycobacterium tuberculosis; WHO, World Health Organization
Keywords: Chest radiograph, Pulmonary tuberculosis, Rifampicin-resistant, Drug-sensitive
1. Background
Tuberculosis (TB) is one of the leading causes of mortality globally [1]. In 2019, an estimated 10 million people were diagnosed with TB globally [1]. In its 2020 Global TB report, WHO reported 1,408,000 deaths and 467,000 rifampicin-resistant (RR) TB [2].
Chest X-ray (CXR) is the primary radiologic evaluation of suspected or proven pulmonary TB (PTB) [3]. When combined with clinical symptoms and signs, CXR has a high sensitivity in the diagnosis of PTB [4]. However, several factors have been shown to affect CXR findings in patients with PTB; namely, human immunodeficiency virus (HIV) infection and the degree of immunosuppression, previous treatment for PTB, and microbiological profile, that is drug-sensitive (DS) or RR-TB [5]. Though some studies suggest that DS and RR-TB present differently on the CXR in terms of morphology, size, and location of the lesions, a detailed description of the difference in CXR findings between DS- and RR-TB is not widely published [5], [6].
Among immunocompetent patients in Indonesia, patients with RR TB had CXR features predominantly of consolidations, infiltrates, cavities, fibrosis, bronchiectasis, calcifications, nodes, with accompanying extrapulmonary lesions such as effusions and empyema [5]. The lesions were larger in size usually more than one. On the other hand, DS-TB patients presented with majorly infiltrates and lesions which were generally smaller in size [5].
Among HIV-infected multi-drug resistant (MDR) TB patients in South Africa, CXR features of hilar and mediastinal lymphadenopathy, consolidations, volume loss, bronchiectasis, and pleural effusions were the commonest findings in decreasing frequency [7].
Even though CXR are widely available and do play a central role in the screening, triaging, and diagnosis of PTB [8], [9], in sub-Saharan Africa where the burden of DS and RR TB, as well as HIV, is disproportionately high, literature is scarce on similarities and differences in radiological features of DS and RR TB patients. Despite wide availability of the GeneXpert machine in Uganda for the detection of rifampicin resistance, currently, culture-based drug susceptibility testing for full profiling of susceptibility pattern to anti-tuberculous agents is not readily available to all patients in our setting and for those who get the opportunity, it takes 2 to 8 weeks to get the results. In this study, therefore, we sought to address this critical knowledge gap by describing CXR findings of DS TB and RR TB patients and stratified findings by HIV status.
2. Methods
2.1. Study design and setting
This was a hospital-based retrospective comparative study conducted at the TB ward of Mulago National Referral Hospital (MNRH), Kampala, Ugandan, between 1st January 2018 and 31st December 2018. Mulago National Referral Hospital is in Mulago hill road 2 km from Kampala city center. The TB ward in the hospital runs both inpatient and outpatient clinics for patients being managed for TB. It is the largest RR TB referral and treatment center serving about 10 districts in central Uganda and is considered a center of excellence. In 2018, 137 and 713 patients were diagnosed with RR-TB and DS-TB respectively which was 1:5.
2.2. Study population
Records of all patients aged 15 years and above that had been diagnosed with PTB by GeneXpert, also who had baseline frontal chest radiograph and were managed from TB ward were retrieved. Patients with poor quality radiographs and additional recorded respiratory co-morbidities such as chronic pulmonary obstructive disease, pulmonary cancer, interstitial lung disease, heart failure and sarcoidosis were excluded. Patients younger than 15 years of age were also excluded since bedaquiline, which is used in the management of MDR TB in Uganda is contraindicated in these patients. Moreover, patients below the age of < 15 are considered as pediatric age group in Uganda (MNRH).
2.3. Sample size
Sample size formula by Kelsey et al., 1996 [10].
And
| N2 = rN1 |
where
N1 = Number of patients with RR-TB.
N2 = Number of patients with DS-TB.
= Standard normal deviate for two-tailed test based on alpha level of 5% (95% Confidence interval) = 1.96.
= Standard normal deviate for one-tailed test based on beta level of 20% (80% power) = 0.84.
r = ratio of N2 to N1 = 5.
2.4. Size of lesion
p1 = Proportion of patients with RR-TB who have large lesions = 96% [5].
p2 = Proportion of patients with DS-TB who have large lesions = 27% [5].
= and = 1 -.
N1 = 5, N2 = 5x5 Therefore final sample size for lesions = 5 + 25 = 30.
2.5. Morphology and location
p1 = Proportion of patients with RR-TB who have upper right lung infiltrate = 36.6%.
p2 = Proportion of patients with DS-TB who have upper right lung infiltrate = 66.7%.
N1 = 25, N2 = 5x25 Therefore final sample size = 25 + 125 = 150.
The larger of the two sample sizes (n = 150) will be the sample size of the study.
Assuming a non-response rate of 10%, Final sample size = 150/0.9 = 167 participants.
During the calendar year 2018, 137 patients were diagnosed as having RR-TB and 713 with DS-TB. The ratio is 1:5, which was used to select a final sample of participants with the same ratio. Using the above sample size formula, we arrived at a final sample of 28 RR-TB and 139 DS-TB cases.
2.6. Study procedure
The first RR-TB and DS-TB files to be enrolled in the study were selected randomly from the first five RR-TB and five DS-TB records using ballot papers. After enrolling the first patient file for each group, every other 5th DS-TB and RR-TB file was recruited into their respective groups. Records that were excluded were replaced by the next consecutive eligible record. Using a structured data collection tool bio-demographic and relevant history were obtained (Appendix 1).
The baseline CXR were then read independently in a systematic manner by the principal investigator and two senior radiologists and findings were recorded. An Ewen-Janus viewing box Model D94405 Landau was used. The readers of the chest radiographs were blinded to the clinical information including the drug sensitivity, as such only study numbers were used to identify images. The principal investigator read all the CXR. One of the senior radiologists was consulted in the presence of any lack of clarity. Then the second senior radiologist was used as a tie-breaker, if there was any disagreement between the principal investigator and the former senior radiologist. The CXR were reviewed for the presence of the following pathologies using a structured data collecting tool (Appendix 2), hilar/mediastinal lymphadenopathy, bronchopneumonic opacification, segmental/lobar consolidation, cavities, miliary opacification, pleural effusion, bronchiectasis, atelectasis, fibrotic bands, and pneumothorax. A chest radiograph lacking all the above features was normal. The location of the lesion was described by side and zone. The zones were; upper (between 1st and 2nd anterior ribs), middle (between 2nd and 4th anterior ribs) and lower (between the 4th and last anterior rib). The widest dimension of a lesion was taken as its size. Measurement was done using a HACO ruler that is factory calibrated.
2.7. Statistical analysis
Data was entered into EPIDATA version 4.4.2 and thereafter exported to SPPSS version 26 for analysis. Patient characteristics were summarized using mean and standard deviation for normally distributed continuous variables or median and range for skewed continuous variables. Categorical variables were summarized using frequencies and proportions. Normality was tested using the Shapiro wilk test and normal distribution probability plots. The outcome of the study was CXR findings. The primary independent variable was drug sensitivity. The potential confounders in this study were age, sex, immunological status, and history of PTB treatment. To describe CXR findings of pulmonary DS-TB and RR-TB among patients in Mulago Hospital, we used frequencies and proportion. The CXR findings were grouped into three categories namely Morphology, size, number of zones affected and location: each with several indicator variables. To compare the proportions of CXR findings in patients with RR-TB and DS-TB we used Chi square test for cell counts above 5 or Fischer exact test for cells counts less than 5. Continuous variables such as age were categorized as per existing literature to increase clinical significance. Mean for independent data were compared using the independent t-test. Statistical significance was set at p < 0.05.
2.8. Radiological diagnostic definitions
Baseline CXR was a CXR obtained at the time of diagnosis of PTB. Hilar/mediastinal lymphadenopathy was described as nodular masses in the hilum or mediastinum. Bronchopneumonic opacification was described as multiple, ill-defined, confluent, nodular opacities in the lung fields. Segmental/lobar consolidation was described as a homogeneous increase in pulmonary parenchymal attenuation that obscures the margins of vessels and airway walls. Cavities were described as gas-filled spaces, seen as a lucency or low-attenuation area within pulmonary consolidation. Miliary opacification was described as profuse, tiny, discrete, rounded pulmonary opacities (≤3 mm in diameter) that are generally uniform in size and diffusely distributed throughout the lungs. Pleural effusion was described as pleural opacification with a meniscus sign. Bronchiectasis was described as thickened and dilated peripheral bronchioles. Atelectasis was described as signs suggestive of lobar or segmental collapse. Fibrotic bands were described as thick linear bands more than 3 mm. Pneumothorax was described as an area devoid of air bronchograms in the apical areas of an erect CXR.
3. Results
3.1. Characteristics of the study participants
A total of 165 participants, 139 DS and 26 RR-TB of which 106 (63.9%) males were recruited in the study. Two participants were excluded, one due to missing essential information and another due inadequate CXR. Overall, 118 (71.7%) participants were HIV uninfected. Of the 118, 103 (87.3%) had DS and 15 (12.7%) RR TB. Overall, 37 (22.4%) participants had a history of previous TB treatment with 9 defaulting and 3 failing on treatment. The proportion of participants with previous history of PTB was higher among RR TB compared to DS TB group (46.2% versus 18%, p = 0.04). Table 1 summarizes the baseline characteristics of the study participants.
Table 1.
Baseline demographic Characteristics of 165 participants.
| Baseline characteristics | All | Drug sensitivity | P-value | |
| DS TB(n=139), % | DR TB (n=26), % | |||
| Gender | ||||
| Female | 59 (36.1) | 49 (35.3) | 10 (38.5) | 0.8 |
| Male | 106 (63.9) | 90 (64.7) | 16 (61.5) | |
| Age , median (range), years | 30 (15-65) | 30 (15-65) | 33 (20-65) | 0.103 |
| HIV serostatus | ||||
| Negative | 118 (71.7) | 103 (74.1) | 15 (57.7) | 0.099 |
| Positive | 47 (28.3) | 36 (25.8) | 11 (42.3) | |
| CD4 counts, median (range) | 218 (2 – 896) | 208.5 (2-896) | 218.0 (61 -783) | 0.268 |
| HIV positive | ||||
| History of smoking | ||||
| Yes | 36 (21.7) | 31 (22.3) | 5 (19.2) | 1 |
| No | 129 (78.3) | 108 (77.6) | 21 (80.8) | |
| History of PTB | ||||
| Yes | 37 (22.3) | 25 (18.0) | 12 (46.2) | 0.04 |
| No | 128 (77.7) | 114 (82.0) | 14 (53.8) | |
| Treatment outcomes (n=37) | ||||
| Failure | 3 (8.1) | 0 (0.0) | 3 (25.0) | 0.037 |
| Completed | 7 (18.9) | 4 (16.0) | 3 (25.0) | |
| Defaulted | 9 (24.3) | 7 (28.0) | 2 (16.7) | |
| Cure | 18 (48.8) | 14 (56.0) | 4 (33.3) | |
| Extrapulmonary TB | ||||
| Yes | 14 (8.4) | 14 (10.1) | 0 (0.0) | 0.13 |
| No | 151 (91.6) | 125 (90.0) | 26 (100.0) | |
| Current or previous alcohol use | ||||
| Yes | 91 (54.8) | 71 (51.1) | 20 (76.9) | 0.018 |
| No | 74 (45.2) | 68 (49.0) | 6 (23.1) | |
3.2. Chest X-ray findings
Five (3%) participants, 3 DS and 2 RR TB, had normal CXR. All the five participants were symptomatic and the two showed resistance to rifampicin (RIF) in Xpert MTB/RIF assay. The most dominant feature of active PTB was consolidations (77%, n = 127). There was no statistically significant difference in the proportion of consolidation among RR compared to DS TB participants (88.5% versus 74.8%, p = 0.2). The participants with miliary pattern opacities on CXR were 3 (1.8%) and all of them were from DS-TB group (Table 2).
Table 2.
Chest X-ray Findings of 165 participants.
| Radiological findings (N = 165) | Totaln (%) |
Drug sensitivity |
P-value | |
|---|---|---|---|---|
| DS-TB (%) | DR-TB (%) | |||
| Normal | 5 (3.0) | 3 (2.2) | 2 (7.7) | 0.177 |
| Bronchopneumonic process | 89 (53.3) | 78 (56.1) | 11 (42.3) | 0.207 |
| Consolidation | 127 (77.0) | 104 (74.8) | 23 (88.5) | 0.203 |
| Cavities | 65 (39.4) | 53 (38.1) | 12 (46.2) | 0.514 |
| Miliary | 3 (1.8) | 3 (2.2) | 0 (0.0) | >0.999 |
| Bronchiectasis | 50 (30.3) | 44 (31.7) | 6 (23.1) | 0.488 |
| Atelectasis | 17 (10.3) | 13 (9.4) | 4 (15.4) | 0.314 |
| Fibrotic bands | 50 (30.3) | 43 (30.9) | 7 (26.9) | 0.818 |
Participants with DS TB had consolidation as the commonest (104, 74.8%) CXR finding of active PTB followed by bronchopneumonic opacities (78, 56.1%) and cavities (53, 38.1%). A similar proportion of HIV uninfected compared to HIV infected had cavities (41.7 versus 27.8%, p = 0.165) (Table 3).
Table 3.
Chest X-ray Findings in DS TB stratified by HIV status.
| Radiological findings (N = 139) | Totaln (%) |
HIV status |
P-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Bronchopneumonic process | 78 (56.1) | 55 (53.4) | 23 (63.9) | 0.331 |
| Consolidation | 104 (74.8) | 77 (74.8) | 27 (75.0) | >0.999 |
| Cavities | 53 (38.1) | 43 (41.7)) | 10 (27.8) | 0.165 |
| Bronchiectasis | 44 (31.7) | 29 (28.2) | 15 (41.7) | 0.149 |
| Atelectasis | 13 (9.4) | 11 (10.7) | 2 (5.6) | 0.514 |
| Fibrotic bands | 43 (30.9) | 32 (31.1) | 11 (30.6) | >0.999 |
Of the 26 participants with RR TB, 23 (88.5%) had consolidations followed by cavities and bronchopneumonic opacities respectively. Stratified by HIV status, there was no statistically significant difference in the proportion of participants with cavities (53.3% for HIV negative versus 36.4% for HIV positive, p = 0.45) (Table 4).
Table 4.
Chest X-ray Finding in DR TB stratified by HIV status.
| Radiological findings (N = 26) | Total n (%) | HIV status |
P-value | |
|---|---|---|---|---|
| Negative | Positive | |||
| Bronchopneumonic process | 11 (42.3) | 7 (46.7) | 4 (36.4) | 0.701 |
| Consolidation | 23 (88.5) | 14 (93.3) | 9 (81.8) | 0.556 |
| Cavities | 12 (46.2) | 8 (53.3) | 4 (36.4) | 0.453 |
| Bronchiectasis | 6 (23.1) | 5 (33.3) | 1 (9.1) | 0.197 |
| Atelectasis | 4 (15.4) | 1 (6.7) | 3 (27.3) | 0.279 |
| Fibrotic bands | 7 (26.9) | 6 (40.0) | 1 (9.1) | 0.178 |
3.3. Location and number of lesions
Among HIV uninfected participants, consolidations were predominantly in the upper lung zones (47.1% in DS versus 9.2% in RR TB, p = 0.38) and middle lung zones (48.7% in DS Vs 7.6% in RR TB, p = 0.27). The bronchopneumonic opacifications were predominantly in the middle lung zones (37% in DS versus 5.9% in RR p = 0.59).
Of the HIV infected participants, the consolidations were predominantly in the middle lung zone (42.5%) in the DS TB group and in the lower lung zone (12.8%) in the RR TB group (p = 0.48). Bronchopneumonic opacities were mostly seen in the middle and lower lung zones (34% versus 32%, p = 0.46) in the DS TB group and in the three lung zones unilaterally in RR TB group.
The average number of zones affected by signs of active disease was comparable between the DS TB and RR TB groups (2.7 versus 3, p = 0,46).
Among the HIV infected participants, cavity sizes were statistically significantly large in those with RR TB compared to HIV uninfected participants with RR TB (7.7 ± 6.8 cm versus 4.2 ± 1.3 cm, p = 0.004). There was no statistically significant difference in the mean consolidation sizes (p = 0.8) and the mean number of cavities (p = 0.4) among HIV uninfected participants with RR TB compared to HIV infected participants with RR TB. The commonest extrapulmonary findings were pleural effusion followed by fibrosis and lymphadenopathy with no statistically significant difference between the DS and RR TB groups (Table 5).
Table 5.
Extrapulmonary findings.
| HIV negative n=118 |
HIV positive n=47 |
|||||
| X-ray features | DS TB n(%) | DR TB n(%) | p-value | DS TB n(%) | DR TB n(%) | p-value |
| Lymphadenopathy | 3 (2.5) | 1 (0.8) | 0.52 | 1 (2.1) | 4 (8.5) | 0.43 |
| Unilateral | 2 (1.7) | 1 (0.8) | 1 (2.1) | 1 (2.1) | ||
| Bilateral | 1 (0.8) | 0 (0) | 0 (0) | 3 (6.4) | ||
| Pleural effusion | 21 (17.6) | 3 (2.5) | 0.86 | 4 (8.5) | 5 (10.6) | 0.22 |
| Unilateral | 19 (16.0) | 3 (2.5) | 3 (6.4) | 3 (6.4) | ||
| Bilateral | 2 (1.7) | 0 (0) | 1 (2.1) | 2 (4.3) | ||
| Fibrosis | 6 (5.0) | 2 (1.7) | 0.4 | 0 (0) | 4 (8.5) | 0.33 |
| Unilateral | 5 (4.2) | 2 (1.7) | 0 (0) | 4 (8.5) | ||
| Bilateral | 1 (0.8) | 0 (0) | 0 (0) | 0 (0) | ||
CXR of HIV uninfected and HIV infected participants are demonstrated in Fig. 1 and Fig. 2 respectively.
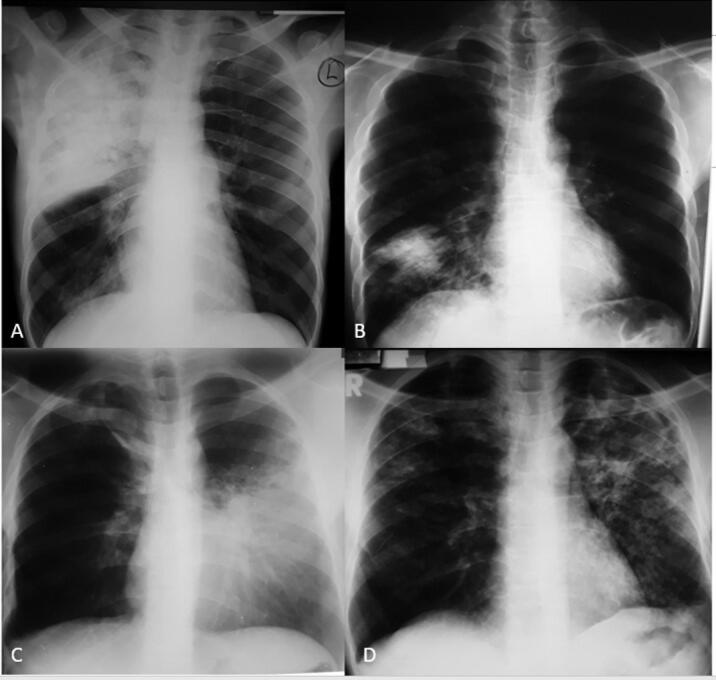
Fig. 1.
Frontal chest radiograph of HIV seronegative participants A) Of a 38 year old male patient with DS TB showing a consolidation involving the right upper and middle lung zones. B) Of a 26-year-old patient with DS TB showing consolidation in the right lower lung zone. C) Of a 26-year-old patient with a consolidation involving the left middle and lower lung zones. D) Of a 34 year old with DS TB showing multiple left upper lobe cavities, bronchopneumonic opacities in the whole left lung and right upper and middle lung zones. There are also bilateral areas of bronchiectasis.
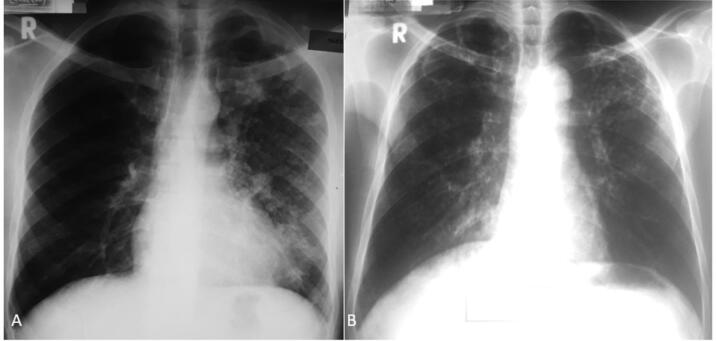
Fig. 2.
Frontal chest radiograph of HIV seropositive participants A) Of a 39-year-old patient with DS TB showing multiple cavities in the left upper and middle lung zones and bronchopneumonic opacities involving the whole left lung field. B) Of a 42 year old patient with DS TB with bilateral upper lung zone cavities, bilateral upper and middle lung zone bronchiectasis and bilateral upper and middle lung zone bronchopneumonic opacities.
4. Discussion
In this retrospective study aimed at comparing the CXR patterns of DS and RR TB participants, we found no difference in radiological presentation. The commonest CXR findings in DS TB was consolidation. The distribution of consolidative lesions in both HIV negative and HIV positive participants was more in the upper and middle zone compared to the lower zones. Mean consolidation size was higher among HIV-positive participants when compared to HIV seronegative participants but was not statistically significant. The lack of difference in the radiological presentation was in keeping with a study that was done in Indonesia and another study from India among MDR TB patients [5], [11]. The commonest CXR features among the RR TB group were consolidations in the upper lung zones.
Due to acquired mutations of mycobacterium during treatment [12], previous history of treatment was significantly higher among participants diagnosed with RR TB 12(46.2%) compared to 25(18%) among DS TB. A meta-analysis done in Ethiopia showed similar results [12].
Few studies have been done that compares CXR finding of DS TB and RR TB, with particular focus to the findings in RR TB [5], [13]. Moreover, less has been done comparing finding among adults with HIV coinfection [14].
4.1. DS TB
Bronchopneumonic opacities were the second commonest CXR finding with predominantly middle and lower lung zones distribution in both HIV seronegative and HIV seropositive participants. Bronchopneumonic opacities followed by consolidation and cavities were the commonest findings among HIV seronegative participants in Indonesia [5]. Similar findings were found in an HIV seropositive group in South Africa [5], [15]. However, in the south African study cavities were more dominant than infiltrates among the HIV-negative patients [15].
Among the HIV-negative group, the majority had upper zone consolidations followed by middle zone and lower zone. Moreover, consolidations followed by cavities and bronchopneumonic opacities were the commonest finding among both HIV positive and HIV seronegative groups. This was in keeping with a study done among HIV seropositive participants where the commonest CXR findings were bronchopneumonic infiltrates and consolidations [5], [16]. In HIV seropositive participants the majority of the lesions were in the lower lung zone. The mean consolidation size was bigger in HIV seronegative participants than in the HIV seropositive participants. The lower lung zone distribution among HIV seropositive participants was also seen in a study done by similar to a study done by Padyana et al. [16].
Cavities were the least common feature of active disease occurring most in the middle lung zones in both the HIV seronegative and HIV seropositive participants with RR TB. In a metanalysis done by Xiáng J and colleagues, the prevalence of cavities among HIV positive patients was as low as 9% which is similar to low prevalence in this study [13]. However, among immunocompetent patient cavitary disease was the commonest finding in most studies [5], [13]. In the pathophysiology of PTB consolidations and bronchopneumonic opacities precede cavities [17]. Therefore, the participants could have presented early before formation of cavities.
4.2. RR TB
The cavity sizes were also larger among HIV-seropositive patients than among HIV-seronegative in the RR TB group. The difference in cavities sizes between the groups is unusual since cavities are not common in immunosuppressed individuals. And different studies demonstrated the opposite of what we found in this study [18], [19], [20], [21].
The average number of zones with active disease in RR TB group was 3. Chuchottaworn and colleagues found that 59.8% of the patients with MDR had involvement of more than two lung zones [22].
This study showed that consolidations and bronchopneumonic opacities formed the largest proportions of CXR findings in both RR TB and DS TB with cavities formed least common findings. This study showed no significant difference in proportions of the different lesions between RR TB and DS TB. In a study that used chest computed tomography, all the different lesions of active PTB were not significantly different between the two groups except for a higher proportion of cavities amongst the RR TB group (p = 0.007) [23]. Most authors stipulate those cavities facilitate development of resistance to antimicrobial treatment as the lining of the cavity prevents penetration of drugs and the high bacterial titers in the cavity facilitate the development of resistance [23]. It is not clear if primary infection with MDR causes cavity formation. In some studies, patients with no previous history of MDR infection had a fewer proportion of cavities [13]. This could explain why cavities were not so dominant in this population since only three patients had a history of failed treatment.
Majority of findings were unilateral in both RR TB and DS TB with no significant difference between the two groups. Some studies demonstrated that there was a difference in distribution with DS TB showing a more bilateral distribution and was attributed to delayed diagnosis and initiation of treatment allowing for more spread of disease to involve both lungs [5], [13].
The limitation of the study was that it was a retrospective study looking at records as such some records were incomplete. This was a hospital-based study and only patients with CXR were included in the study, henceforth it introduced a selection bias.
5. Conclusion
The study found no statistically significant difference in chest x-ray patterns of PTB between RR TB and DS TB among immunocompetent patients. Among HIV-positive patients, cavity sizes were significantly larger in RR TB compared to DS TB. Our study found that CXR couldn’t differentiate RR TB from DS TB. Hence clinicians should not bother using CXR as a screening tool for RR TB.
6. Ethics approval and consent to participate
Waiver of informed consent was obtained. Ethical approval was obtained from Makerere University School of Medicine Research and Ethics committee and administrative clearance was sought from Mulago National Referral Hospital.
7. Consent for publication
Not applicable.
8. Availability of data and materials
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Funding
No funding.
Author contributions
All authors made a significant contribution to the study, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
CRediT authorship contribution statement
Anthony Oriekot: Conceptualization, Funding acquisition, Methodology, Investigation, Project administration, Resources, Writing – original draft. Senai Goitom Sereke: Investigation, Writing – original draft, Writing – review & editing. Felix Bongomin: Software, Formal analysis, Data curation, Writing – review & editing. Samuel Bugeza: Methodology, Investigation, Resources, Validation. Zeridah Muyinda: Methodology, Supervision, Validation, Visualization.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
We would like to acknowledge the TB ward staffs for actively supporting the process of mobilization of study participants’ files and data collection.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jctube.2022.100312.
Contributor Information
Anthony Oriekot, Email: anthonyoriekot@gmail.com.
Senai Goitom Sereke, Email: nayhersen@gmail.com.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Fukunaga R., Glaziou P., Harris J.B., Date A., Floyd K., Kasaeva T. Epidemiology of tuberculosis and progress toward meeting global targets – worldwide, 2019. MMWR Morb Mortal Wkly Rep. 2021;70(12):427–430. doi: 10.15585/mmwr.mm7012a4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.World Health Organization. Global tuberculosis report 2020: executive summary [Internet]. Geneva: World Health Organization; 2020 [cited 2022 Mar 19]. 11 p. Available from: https://apps.who.int/iris/handle/10665/337538.
- 3.Ubaidi BAA, Ubaidi BAA. The Radiological Diagnosis of Pulmonary Tuberculosis (TB) in Primary Care. [cited 2021 Aug 22]; Available from: https://clinmedjournals.org/articles/jfmdp/journal-of-family-medicine-and-disease-prevention-jfmdp-4-073.php?jid=jfmdp.
- 4.Liu CH, Li L, Chen Z, Wang Q, Hu YL, Zhu B, et al. Characteristics and treatment outcomes of patients with MDR and XDR tuberculosis in a TB referral hospital in Beijing: a 13-year experience. PLoS One. 2011 Apr 29;6(4):e19399. [DOI] [PMC free article] [PubMed]
- 5.Icksan A.G., Napitupulu M.R.S., Nawas M.A., Nurwidya F. Chest X-ray findings comparison between multi-drug-resistant tuberculosis and drug-sensitive tuberculosis. J Nat Sci Biol Med. 2018;9(1):42–46. doi: 10.4103/jnsbm.JNSBM_79_17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Solsona Peiró J., de Souza Galvão M.L., Altet Gómez M.N. Inactive fibrotic lesions versus pulmonary tuberculosis with negative bacteriology. Arch Bronconeumol. 2014;50(11):484–489. doi: 10.1016/j.arbres.2013.07.009. [DOI] [PubMed] [Google Scholar]
- 7.Brust JCM, Berman AR, Zalta B, Haramati LB, Ning Y, Heo M, et al. Chest radiograph findings and time to culture conversion in patients with multidrug-resistant tuberculosis and HIV in Tugela Ferry, South Africa. PLoS One. 2013;8(9):e73975. [DOI] [PMC free article] [PubMed]
- 8.Story A., Aldridge R.W., Abubakar I., Stagg H.R., Lipman M., Watson J.M., et al. Active case finding for pulmonary tuberculosis using mobile digital chest radiography: an observational study. Int J Tuberc Lung Dis. 2012;16(11):1461–1467. doi: 10.5588/ijtld.11.0773. [DOI] [PubMed] [Google Scholar]
- 9.World Health Organization. Regional Office for Europe, European Observatory on Health Systems and Policies, Sagan A, McDaid D, Rajan S, Farrington J, et al. Screening: when is it appropriate and how can we get it right? [Internet]. Copenhagen: World Health Organization. Regional Office for Europe; 2020 [cited 2021 Dec 29]. (Health Systems and Policy Analysis: policy brief, 35). Available from: https://apps.who.int/iris/handle/10665/330810. [PubMed]
- 10.Jennifer L K, Alice S. W, Alfred S. E, W. Douglas T. Methods in Observational Epidemiology. Second Edition. Oxford, New York: Oxford University Press; 1996. 448 p. (Monographs in Epidemiology and Biostatistics).
- 11.Bhattacharyya S.K., Barma P., Bhattacharyya R. A study of X-ray chest patterns in multidrug-resistant tuberculosis (MDR-TB) between HIV reactive and HIV non-reactive patients in a tertiary hilly medical centre. jemds. 2019;8(6):389–393. [Google Scholar]
- 12.Girum T., Muktar E., Lentiro K., Wondiye H., Shewangizaw M. Epidemiology of multidrug-resistant tuberculosis (MDR-TB) in Ethiopia: a systematic review and meta-analysis of the prevalence, determinants and treatment outcome. Trop Dis Travel Med Vaccines. 2018;4:5. doi: 10.1186/s40794-018-0065-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wáng Y.X.J., Chung M.J., Skrahin A., Rosenthal A., Gabrielian A., Tartakovsky M. Radiological signs associated with pulmonary multi-drug resistant tuberculosis: an analysis of published evidences. Quant Imaging Med Surg. 2018;8(2):161–173. doi: 10.21037/qims.2018.03.06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manikkam S., Archary M., Bobat R. Chest X-ray patterns of pulmonary multidrug-resistant tuberculosis in children in a high HIV-prevalence setting. South African Journal of Radiology. 2016;20(1):6. [Google Scholar]
- 15.Kistan J, Laher F, Otwombe K, Panchia R, Mawaka N, Lebina L, et al. Pulmonary TB: varying radiological presentations in individuals with HIV in Soweto, South Africa. Trans R Soc Trop Med Hyg. 2017 Mar;111(3):132–6. [DOI] [PMC free article] [PubMed]
- 16.Padyana M., Bhat R.V., Dinesha M., Nawaz A. HIV-tuberculosis: a study of chest X-ray patterns in relation to CD4 count. N Am J Med Sci. 2012;4(5):221–225. doi: 10.4103/1947-2714.95904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bhalla A.S., Goyal A., Guleria R., Gupta A.K. Chest tuberculosis: radiological review and imaging recommendations. Indian J Radiol Imaging. 2015;25(3):213–225. doi: 10.4103/0971-3026.161431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kisembo H.N., Boon S.D., Davis J.L., Okello R., Worodria W., Cattamanchi A., et al. Chest radiographic findings of pulmonary tuberculosis in severely immunocompromised patients with the human immunodeficiency virus. Br J Radiol. 2012;85(1014):e130–e139. doi: 10.1259/bjr/70704099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ravimohan S, Kornfeld H, Weissman D, Bisson GP. Tuberculosis and lung damage: from epidemiology to pathophysiology. European Respiratory Review [Internet]. 2018 Mar 31 [cited 2021 Aug 20];27(147). Available from: https://err.ersjournals.com/content/27/147/170077. [DOI] [PMC free article] [PubMed]
- 20.Buregyeya E., Criel B., Nuwaha F., Colebunders R. Delays in diagnosis and treatment of pulmonary tuberculosis in Wakiso and Mukono districts, Uganda. BMC Public Health. 2014;14(1):586. doi: 10.1186/1471-2458-14-586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sendagire I, Schim Van der Loeff M, Mubiru M, Konde-Lule J, Cobelens F. Long delays and missed opportunities in diagnosing smear-positive pulmonary tuberculosis in Kampala, Uganda: a cross-sectional study. PLoS One. 2010 Dec 29;5(12):e14459. [DOI] [PMC free article] [PubMed]
- 22.Chuchottaworn C, Thanachartwet V, Sangsayunh P, Than TZM, Sahassananda D, Surabotsophon M, et al. Risk factors for multidrug-resistant tuberculosis among patients with pulmonary tuberculosis at the Central Chest Institute of Thailand. PLoS One. 2015;10(10):e0139986. [DOI] [PMC free article] [PubMed]
- 23.Kim S.H., Min J.H., Lee J.Y. Radiological findings of primary multidrug-resistant pulmonary tuberculosis in HIV-seronegative patients. Hong Kong J Radiol. 2014;17(1):4–8. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.