Abstract
Background:
Alemtuzumab efficacy and safety was demonstrated in CARE-MS I and extension studies (CAMMS03409; TOPAZ).
Objective:
Evaluate serum neurofilament light chain (sNfL) in CARE-MS I patients and highly active disease (HAD) subgroup, over 7 and 2 years for alemtuzumab and subcutaneous interferon beta-1a (SC IFNB-1a), respectively.
Methods:
Patients received SC IFNB-1a 44 µg 3×/week or alemtuzumab 12 mg/day at baseline and month 12, with further as-needed 3-day courses. sNfL was measured using single-molecule array (Simoa™). HAD definition was ⩾2 relapses in year before randomization and ⩾1 baseline gadolinium-enhancing lesion.
Results:
Baseline median sNfL levels were similar in alemtuzumab (n = 354) and SC IFNB-1a–treated (n = 159) patients (31.7 vs 31.4 pg/mL), but decreased with alemtuzumab versus SC IFNB-1a until year 2 (Y2; 13.2 vs 18.7 pg/mL; p < 0.0001); 12.7 pg/mL for alemtuzumab at Y7. Alemtuzumab-treated patients had sNfL at/below healthy control median at Y2 (72% vs 47%; p < 0.0001); 73% for alemtuzumab at Y7. HAD patients (n = 102) had higher baseline sNfL (49.4 pg/mL) versus overall population; alemtuzumab HAD patients attained similar levels (Y2, 12.8 pg/mL; Y7, 12.7 pg/mL; 75% were at/below control median at Y7).
Conclusion:
Alemtuzumab was superior to SC IFNB-1a in reducing sNfL, with levels in alemtuzumab patients remaining stable through Y7.
ClinicalTrials.gov identifier:
Keywords: Alemtuzumab, biomarkers, clinical trials randomized controlled, highly active disease, NfL/neurofilament light chain, multiple sclerosis
Introduction
Neurofilaments are cytoskeletal proteins whose release into CSF and blood is a quantitative measure of neuronal injury. 1 Serum neurofilament light chain (sNfL) in MS has been validated as a biomarker for disease activity, therapy response, and as a predictor of disease worsening.2–6 Increased sNfL levels may indicate subclinical disease activity and suboptimal treatment response;2,5–11 however, few studies have examined the impact of high-efficacy disease-modifying therapy (DMT) on sNfL levels in head-to-head randomized controlled trials.9,10
In the 2-year phase 3 CARE-MS I trial, alemtuzumab (LEMTRADA®; Sanofi Genzyme, Cambridge, MA, USA) improved relapse, MRI, and patient-reported outcomes in treatment-naive patients with relapsing–remitting MS (RRMS) versus subcutaneous interferon beta-1a (SC IFNB-1a). 12 In two consecutive extension studies (CAMMS03409 and TOPAZ), these pharmacodynamic effects were followed over an additional 7 years.13–16 In clinical trials and post-marketing, the alemtuzumab safety profile includes infusion-associated reactions, more frequent infections and potential for opportunistic infections, secondary autoimmunity (i.e. thyroid disorders, immune thrombocytopenia, nephropathies, autoimmune cytopenias, autoimmune hepatitis, and other less common autoimmune events), acute acalculous cholecystitis, and cardiovascular and pulmonary events possibly related to infusion.12,14,16–21
These studies enabled longitudinal analysis of the alemtuzumab effect on sNfL over 7 years. We examined (a) whether highly active disease (HAD) patients had higher sNfL levels than the overall cohort, (b) whether the higher clinical efficacy of alemtuzumab versus SC IFNB-1a, both in HAD and the overall population, is reflected by a stronger decrease of sNfL levels over time, and (c) how NfL levels correlate with long-term clinical efficacy.
Patients and methods
Patients and procedures
The study design for the rater-blinded, active-controlled, head-to-head, phase 3 CARE-MS I trial and the open-label CARE-MS extension has been published previously.12,16 Treatment-naive patients with active RRMS (disease duration of ⩽5 years, ⩾2 relapses in the previous 2 years and ⩾1 in the previous year, Expanded Disability Status Scale (EDSS) scores of ⩽3.0) were randomly allocated in a 2:1 ratio to either alemtuzumab 12 mg/day on 5 consecutive days at baseline and on 3 consecutive days 12 months later, or SC IFNB-1a 44 µg 3 times per week. In the extension, patients who completed the core study could receive additional alemtuzumab courses (12 mg/day on 3 consecutive days ⩾12 months after the previous course) as needed for disease activity (⩾1 protocol-defined relapse, and/or ⩾2 unique lesions defined as either new/enlarging T2 hyperintense, and/or gadolinium (Gd)-enhancing brain and/or spinal cord lesions on MRI) at the discretion of the investigator. Other approved DMT was also permitted. Patients completing the 4-year CARE-MS extension study could enroll in the subsequent, ongoing, 5-year TOPAZ extension study and receive additional alemtuzumab 12 mg/day on 3 consecutive days ⩾12 months after the previous course at the discretion of the investigator (no disease criteria), or receive another DMT at any time. 15
Protocol approval, registration, and patient consent
CARE-MS I, CAMMS03409, and TOPAZ are registered with ClinicalTrials.gov (NCT00530348; NCT00930553; NCT02255656). Patients provided written informed consent, and all procedures were approved by local institutional ethics review boards of participating sites. 12
Sample collection and analysis
sNfL levels were assessed using a single-molecule array (Simoa®; Quanterix, Lexington, MA, USA) assay. 22 Healthy control samples were collected at the University of Basel (Basel, Switzerland) and this analysis has been published previously. 22 Samples from CARE-MS I patients were collected at core study baseline and at 3- or 6-month intervals and analyzed in the overall study population as well as the subgroup of patients with HAD at baseline. HAD was defined as ⩾2 relapses in the year before randomization and ⩾1 Gd-enhancing lesion at core study baseline. Details of the MRI methods are provided in the Supplementary Material.
Statistical analyses
sNfL values below the lower limit of quantification (LLOQ; 1.28 pg/mL) were imputed as LLOQ/2 in 1.2% (86/7100) of alemtuzumab samples and 0.7% (5/721) of SC IFNB-1a samples.
For correlation analyses between baseline sNfL versus baseline patient or disease characteristics, sample data were pooled from both treatment arms. The relationships between baseline sNfL levels and baseline Gd-enhancing lesion count, T2 hyperintense lesion volume, and T1 hypointense lesion volume were analyzed by regression analyses conducted with log-transformed sNfL levels as the dependent variable; estimates were back-transformed to the original scale and therefore represent multiplicative effects on the geometric mean of sNfL. Multivariate analyses of the relationship between baseline sNfL and baseline clinical or MRI parameters were adjusted for baseline characteristics reported previously to affect sNfL levels. 22 For clinical characteristics, analyses considered baseline age, sex, EDSS score, and prior relapse within 60 days of study start as covariates. For MRI characteristics, analyses considered baseline age, sex, Gd-enhancing lesion count, and T2 hyperintense lesion volume as covariates.
Analyses of sNfL levels during treatment with alemtuzumab or SC IFNB-1a were performed in patients who had ⩾1 post-baseline measurement. p-Values evaluating treatment group differences in sNfL at baseline were derived using the Wilcoxon rank sum test. p-Values evaluating treatment group differences in sNfL post-baseline were derived using rank analysis of covariance adjusted for age and baseline sNfL level. p-Values evaluating sNfL change from baseline were derived using the Wilcoxon signed rank test.
The distribution of sNfL in healthy controls and its association with age was modeled using the Generalized Additive Model of Location, Scale, and Shape. 23 The model utilized 341 observations, including 254 healthy controls at baseline and 87 at 1 year follow-up to derive age-dependent percentiles. 22 Each CARE-MS I patient sample was dichotomized into levels above or below the respective age-matched healthy control median or the 80th percentile to obtain an age-independent measure of sNfL. Proportions of alemtuzumab- and SC IFNB-1a–treated patients with sNfL levels at or below the healthy control median or 80th percentile were compared using the chi-square test. Odds ratio estimates of sNfL levels equal to or less than that of healthy control median and p-values were based on repeated logistic regression with exchangeable covariance structure, adjusted for treatment, visit, a treatment-by-visit interaction term, baseline EDSS score, prior relapse within 60 days, and baseline sNfL.
Data availability statement
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.
Results
Availability of samples for sNfL analysis
Baseline samples from 513 alemtuzumab- or SC IFNB-1a–treated CARE-MS I patients were available for analysis, representing 91% of the CARE-MS I population receiving any study drug. Of 354 alemtuzumab-treated patients, 276 remained on study through year 7, and 78 either discontinued the study (n = 54) or did not enroll in the CARE-MS extension (n = 17) or TOPAZ (n = 7). Reasons for discontinuation included withdrawal of consent (n = 28), patient lost to follow-up (n = 8), other (n = 7), physician decision (n = 5), death (n = 4), lack of efficacy (n = 1), and study terminated by sponsor (n = 1). Of the 276 patients completing year 7, samples for sNfL analysis were available at year 7 for 110 patients. The other 166 patients did not have samples collected during the specific time window that was required for sNfL analysis. This proportion with missing samples increased during year 7 (Supplementary eTable).
Correlation of baseline sNfL levels with baseline clinical and MRI characteristics
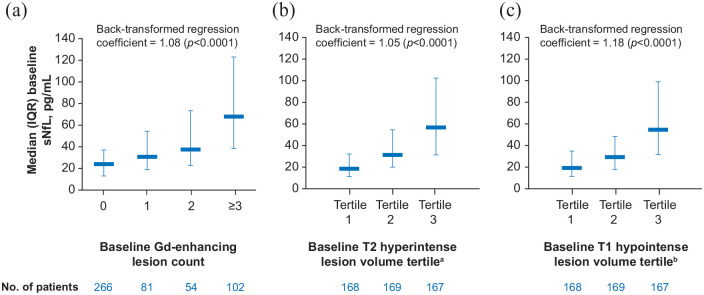
Patients in the sNfL analysis sample had a mean disease duration of approximately 2 years and a mean EDSS score of 2.0; all had relapse activity within the past year per protocol, and almost half had Gd-enhancing lesions at baseline (Table 1). Age range was 18−53 years. Median (interquartile range (IQR)) sNfL level at baseline was 31.5 (17.2–61.1) pg/mL. Higher baseline sNfL levels were associated with higher baseline Gd-enhancing lesion count, higher baseline T2 hyperintense lesion volume, and higher baseline T1 hypointense lesion volume (Figure 1).
Table 1.
Baseline characteristics of CARE-MS I patients with sNfL samples in this analysis.
| Parameter | Overall CARE-MS I population | HAD subgroup | ||
|---|---|---|---|---|
| Alemtuzumab (n = 354) | SC IFNB-1a (n = 159) | Alemtuzumab (n = 102) | SC IFNB-1a (n = 53) | |
| Age, years | 32.9 (8.0) | 33.0 (8.3) | 32.3 (8.0) | 29.7 (7.3) |
| Female, n (%) | 232 (65.5) | 108 (67.9) | 68 (66.7) | 36 (67.9) |
| White, n (%) | 332 (93.8) | 154 (96.9) | 93 (91.2) | 51 (96.2) |
| EDSS score | 2.0 (0.8) | 2.0 (0.8) | 2.0 (0.8) | 2.1 (0.9) |
| Years since initial relapse | 2.1 (1.4) | 2.0 (1.4) | 1.7 (1.4) | 2.0 (1.5) |
| No. of relapses in prior 1 year | 1.8 (0.8) | 1.8 (0.8) | 2.3 (0.6) | 2.3 (0.6) |
| No. of relapses in prior 2 years | 2.5 (0.8) | 2.4 (0.8) | 2.8 (0.9) | 2.7 (0.9) |
| Patients with Gd-enhancing lesions, n (%) | 162 (46.4) a | 77 (49.4) b | 102 (100) | 53 (100) |
| Gd-enhancing lesion count | 2.3 (5.2) a | 2.1 (4.6) b | 5.5 (7.2) | 4.6 (5.2) |
| T2 hyperintense lesion volume, cm3 | 7.4 (9.0) a | 7.1 (10.0) c | 9.9 (10.3) | 11.4 (13.6) |
| T1 hypointense lesion volume, cm3 | 1.2 (2.1) a | 1.2 (2.4) c | 1.5 (2.1) | 2.3 (3.7) |
| Brain parenchymal fraction | 0.82 (0.02) a | 0.82 (0.02) d | 0.82 (0.02) | 0.82 (0.02) |
EDSS: Expanded Disability Status Scale; Gd: gadolinium; HAD: highly active disease; SC IFNB-1a: subcutaneous interferon beta-1a; SD, standard deviation; sNfL: serum neurofilament light chain.
All values are mean (SD) unless specified otherwise.
n = 349.
n = 156.
n = 157.
n = 158.
Figure 1.
Unadjusted analyses showing the relationship between sNfL levels and (a) Gd-enhancing lesion count, (b) T2 hyperintense lesion volume, and (c) T1 hypointense lesion volume at baseline.
Gd: gadolinium; IQR: interquartile range; sNfL: serum neurofilament light chain.
aT2 hyperintense lesion volume Tertile 1: ⩽1.997 cm3; Tertile 2: >1.997 and ⩽6.520 cm3; Tertile 3: >6.520 cm3.
bT1 hypointense lesion volume Tertile 1: ⩽0.117 cm3; Tertile 2: >0.117 and ⩽0.804 cm3; Tertile 3: >0.804 cm3.
In a multivariate model, baseline EDSS score (confidence interval) (β = 1.19 (1.06–1.33) p = 0.0033), time since last relapse (β = 0.43 (0.28–0.65) p < 0.0001), and the number of Gd-enhancing lesions (β = 1.04 (1.02–1.06) p < 0.0001), as well as the T2 hyperintense lesion volume (β = 1.03 (1.02–1.04) p < 0.0001) were associated with baseline sNfL levels across the CARE-MS I population (Table 2).
Table 2.
Association between baseline sNfL with baseline patient characteristics: multivariate regression analysis.
| Parameter | Regression coefficient | 95% CI | Percent increase in sNfL per unit parameter change a | p-Value | Other covariates |
|---|---|---|---|---|---|
| Age | 0.99 | 0.98–1.00 | 0.12 | Sex, race, baseline age, baseline EDSS, disease duration, time since last relapse | |
| Sex | 0.91 | 0.76–1.10 | 0.34 | ||
| Race | 0.86 | 0.58–1.27 | 0.44 | ||
| EDSS score | 1.19 | 1.06–1.33 | 19% (per 1-point EDSS score increase) | 0.0033 | |
| Disease duration | 0.98 | 0.91–1.04 | 0.48 | ||
| Time since last relapse prior to study entry | 0.43 | 0.28–0.65 | 57% (per year more proximal to last relapse) | <0.0001 | |
| Gd-enhancing lesion count | 1.04 | 1.02–1.06 | 4% (per increase of 1 Gd-enhancing lesion) | <0.0001 | Baseline age, baseline Gd-enhancing lesion count, baseline T2 hyperintense lesion volume, log-transformed baseline BPF |
| T2 hyperintense lesion volume | 1.03 | 1.02–1.04 | 3% (per 1 cm3 increase in T2 hyperintense lesion volume) | <0.0001 | |
| BPF | 1.50 | −1.67, 4.66 | 0.35 |
BPF: brain parenchymal fraction; CI: confidence interval; EDSS: Expanded Disability Status Scale; Gd: gadolinium; sNfL: serum neurofilament light chain.
Computed as (back-transformed regression coefficient−1) × 100%.
sNfL levels after alemtuzumab or SC IFNB-1a treatment
A total of 7100 samples (core study: 1667, extension study: 5433) from 354 alemtuzumab-treated patients, and 721 samples from 159 SC IFNB-1a–treated patients (core study only) were available for analysis. Baseline characteristics were balanced between groups (Table 1). The HAD subgroup consisted of 102 alemtuzumab-treated patients (2140 samples) and 53 SC IFNB-1a–treated patients (245 samples). Throughout the core and extension studies, 58% (190 of 329 who entered the extension) of alemtuzumab-treated patients with available sNfL data received no additional dose or other DMT after the initial standard two courses. Five patients received other DMT only, 127 received additional alemtuzumab only, and 7 received both. sNfL levels from month 24 to the end of follow-up were higher in these 139 patients receiving other DMTs or additional courses of alemtuzumab (median (IQR) 17.2 (10.8–27.5) pg/mL) than in the 190 patients who did not receive any further treatment (14.3 (9.5–21.0) pg/mL; p < 0.0001).
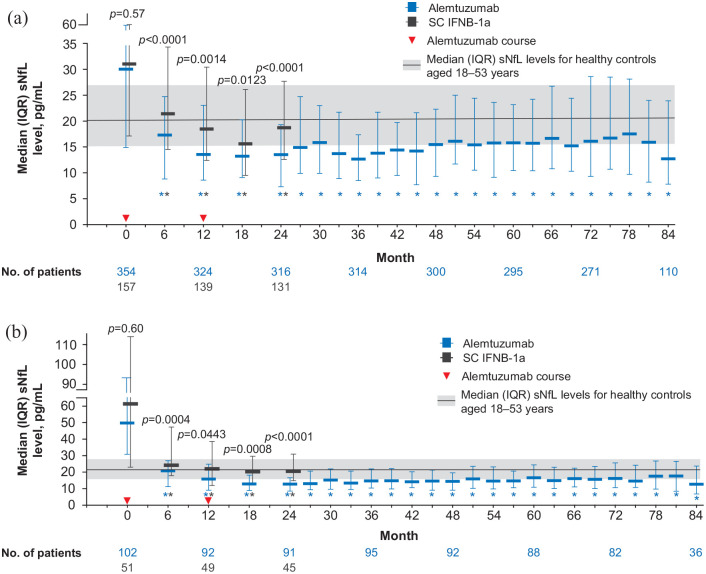
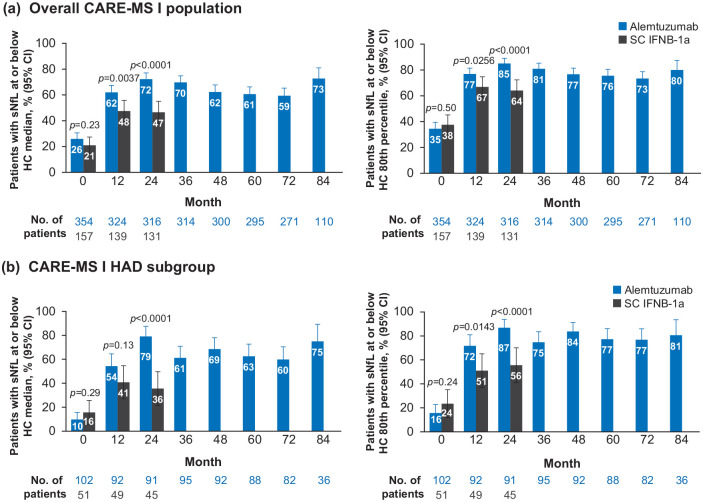
Median (IQR) sNfL levels were similar in alemtuzumab-treated patients and SC IFNB-1a–treated patients at baseline (31.7 (17.1–60.4) pg/mL vs 31.4 (17.5–61.1) pg/mL; p = 0.57; Figure 2(a)); after 6 months of treatment, median (IQR) sNfL levels were significantly lower with alemtuzumab versus SC IFNB-1a (17.2 (9.7–24.7) vs 21.4 (14.4–33.9) pg/mL; p < 0.0001). This reduction of sNfL by both drugs continued in the following months of therapy, but the gap between alemtuzumab and SC IFNB-1a remained at month 12 (median, 14.2 (IQR, 8.9–22.9) vs 17.7 (11.9–29.2) pg/mL; p = 0.0014) and month 18 (13.2 (8.4–18.8) vs 15.6 (9.5–24.7) pg/mL; p = 0.0123), while at the end of the core study, sNfL remained stable at low levels in the alemtuzumab group, but re-increased in the SC IFNB-1a group (month 24–13.2 (8.6–19.5) vs 18.7 (12.6–27.7) pg/mL; p < 0.0001); this re-increase in the latter treatment arm was significant (difference between month 18 vs month 24: p = 0.0045). Significantly more alemtuzumab-treated patients had sNfL levels at or below the median level for age-matched healthy controls at month 24 after treatment versus SC IFNB-1a (72% vs 47%; p < 0.0001; Figure 3(a)). Similarly, higher proportions of alemtuzumab-treated patients achieved sNfL levels at or below the 80th percentile for healthy controls at month 24 (85% vs 64%; p < 0.0001). The odds of achieving an sNfL level at or below the healthy control median and at or below the healthy control 80th percentile favored alemtuzumab over SC IFNB-1a (Table 3).
Figure 2.
sNfL levels over time in patients treated with alemtuzumab or SC IFNB-1a in (a) the overall CARE-MS I population and (b) the CARE-MS I HAD subgroup.
ANCOVA: analysis of covariance; HAD: highly active disease; IQR: interquartile range; SC IFNB-1a: subcutaneous interferon beta-1a; sNfL: serum neurofilament light chain.
Plot shows unadjusted sNfL values. p-Values indicate comparison between treatment groups (baseline: Wilcoxon rank sum test; post-baseline: rank ANCOVA adjusted for age and baseline sNfL).
*p < 0.0001 for comparison of baseline with each post-baseline time point (Wilcoxon signed rank test). sNfL values below the lower limit of quantification were imputed; this represented 86/7100 (1.2%) alemtuzumab samples and 5/721 (0.7%) SC IFNB-1a samples. Solid line and shaded area represent median and IQR (20.6 pg/mL (15.6−27.1) sNfL level for healthy controls aged 18–53 years (the age range of CARE-MS I patients).
Figure 3.
Proportions of patients with sNfL level at or below the median level for age-matched healthy controls (left panels) and at or below the 80th percentile of age-matched healthy controls (right panels) for (a) the overall CARE-MS I population and (b) the CARE-MS I HAD subgroup.
CI: confidence interval; HAD: highly active disease; HC: healthy controls; SC IFNB-1a: subcutaneous interferon beta-1a; sNfL: serum neurofilament light chain.
p-Values indicate comparison between treatment groups and are based on chi-square test.
Table 3.
Odds ratio comparing proportion of alemtuzumab-treated patients with sNfL levels at or below healthy control median or 80th percentile thresholds versus SC IFNB-1a–treated patients.
| Population | Month | Odds ratio a (95% CI) | p-Value |
|---|---|---|---|
| Overall CARE-MS I | |||
| Healthy control median | 12 | 1.81 (1.20–2.74) | 0.0046 |
| 24 | 2.85 (1.84–4.43) | <0.0001 | |
| Healthy control 80th percentile | 12 | 1.68 (1.06–2.67) | 0.0270 |
| 24 | 3.01 (1.76–5.17) | <0.0001 | |
| HAD CARE-MS I | |||
| Healthy control median | 12 | 1.63 (0.79–3.36) | 0.19 |
| 24 | 6.08 (2.55–14.50) | <0.0001 | |
| Healthy control 80th percentile | 12 | 2.42 (1.11–5.26) | 0.0263 |
| 24 | 4.57 (1.71–12.23) | 0.0024 | |
CI: confidence interval; HAD: highly active disease; SC IFNB-1a: subcutaneous interferon beta-1a; sNfL: serum neurofilament light chain.
Odds ratio of sNfL level less than or equal to that of healthy control median or healthy control 80th percentile, adjusted for age and baseline sNfL.
Baseline sNfL levels were higher in HAD patients compared with the overall population, but alemtuzumab treatment reduced sNfL to levels similar to those of healthy controls, and as observed in the overall population (Figure 2(b)). At baseline, HAD patients in the alemtuzumab arm had numerically lower median (IQR) sNfL levels versus those in the SC IFNB-1a arm (49.4 (30.4–92.3) vs 61.0 (22.6–111.7) pg/mL; p = 0.60); and significantly lower sNfL levels were seen with alemtuzumab versus SC IFNB-1a post-treatment (month 6: 20.8 (11.2–26.9) vs 24.2 (17.8–47.3) pg/mL (p = 0.0004); month 12: 15.8 (9.0–24.8) vs 22.0 (11.9. 38.6) pg/mL (p = 0.0443); month 18: 12.9 (8.9–18.2) vs 20.3 (12.2–29.6) pg/mL (p = 0.0008); month 24: 12.8 (8.5–16.6) vs 20.5 (14.8–30.9) pg/mL (p < 0.0001)). More HAD patients had sNfL levels at or below the median level or the 80th percentile level for age-matched healthy controls at month 24 with alemtuzumab versus SC IFNB-1a (median: 79% vs 36% (p < 0.0001); 80th percentile: 87% vs 56% (p < 0.0001); Figure 3(b)), and the odds of achieving these thresholds in the HAD subgroup favored alemtuzumab over SC IFNB-1a (Table 3).
During the extension studies, sNfL levels in alemtuzumab-treated patients remained stable at each time point through month 84, at which point 73% of alemtuzumab-treated patients (n = 110) had sNfL levels at or below the healthy control median and 80% were below the healthy control 80th percentile. Similarly, sNfL levels remained stable through year 7 in the HAD subgroup; proportions of patients meeting the median and 80th percentile thresholds were 75% and 81%, respectively (Figures 2 and 3).
A sensitivity analysis was performed in patients in the alemtuzumab group who had available sNfL data at month 84 (n = 110), or at month 24 for patients in the SC IFNB-1a group (n = 131; “completers”). Results were similar to that of the overall population, with significant between-group differences at months 6 (p < 0.0001), 12 (p = 0.001), and 24 (p < 0.0001). sNfL levels in the alemtuzumab-treated completer population throughout the extension study were similar to those in the overall population (data not shown).
Discussion
Our results show that alemtuzumab was more effective in reducing sNfL in RRMS patients than SC IFNB-1a. They parallel the significant reductions in relapses (relative reduction 55%; p < 0.0001) and patients with Gd-enhancing lesions (19% for SC IFNB-1a vs 7% for alemtuzumab; p < 0.0001) and new or enlarging T2 hyperintense lesions (58% vs 48%; p = 0.04) observed with alemtuzumab over 2 years. 12 Moreover, alemtuzumab therapy allowed patients to reach sNfL levels similar to those seen in age-matched healthy controls. Interestingly, in the SC IFNB-1a group, a significant increase in median sNfL levels was observed between months 18 and 24 of the core study, indicative of a return of disease activity. More important in view of long-term course of disability is that levels of sNfL were maintained close to those observed physiologically over 7 years in the majority of patients treated with alemtuzumab in the core study or those who received additional alemtuzumab or other DMT as needed in case of recurrent disease activity. These results of sNfL are congruent with the 7-year clinical and MRI data published previously from the long-term extensions of CARE-MS I, in which relapse rates remained low (0.12–0.19 per patient per year), 74% of alemtuzumab-treated patients remained free from 6-month confirmed disability worsening, and in each year 66%–77% were free of MRI lesion activity (Gd-enhancing and/or new/enlarging T2 hyperintense lesions).13,15
Conceptually, current results provide additional evidence that sNfL levels reflect subclinical disease activity (focal lesion formation or brain-diffuse neurodegenerative processes),7,11,24 which is more prominent in the SC IFNB-1a group. The effect of alemtuzumab on sNfL supports a stronger reduction in neurodegenerative processes compared with SC IFNB-1a, and is in accordance with previous studies wherein patients who were clinically stable (without relapse or MRI lesion activity) had less brain volume loss with alemtuzumab treatment than with SC IFNB-1a. 25
The stable reduction in sNfL at the population level over 7 years post-alemtuzumab facilitates interpretation of the clinical data from the long-term extension studies, in which there was no comparator arm. As correlations between sNfL and brain volume loss have been established, 2 the sustained low sNfL levels at the population level lend context to the modest degree of brain volume loss observed in the extension studies13,14,16 and provide orthogonal evidence for an overall stabilization of pathogenic processes under alemtuzumab treatment.
Stability in sNfL levels over time in alemtuzumab-treated patients was also achieved in the subgroup with HAD. This group, which represented less than one-third of CARE-MS I patients, had higher baseline median sNfL and greater scatter in their sNfL values compared with the overall CARE-MS I cohort. This is not surprising given their greater MRI lesion burden and disease activity at baseline. However, treatment with alemtuzumab brought sNfL down within the range of age-matched healthy controls, with the levels in alemtuzumab patients remaining stable through year 7. As in the overall CARE-MS I population, sNfL reduction in the HAD population corresponded with clinical and MRI stability reported previously for the extension study. 26 The congruency of sNfL data in the HAD subgroup with the overall CARE-MS I population demonstrates the efficacy of alemtuzumab to attenuate pathogenic processes over the short- and long-term in RRMS patients with more acute clinical or MRI disease features.
Current results allow conclusions only at the population level, so they do not address the real-world utility of sNfL as a biomarker in individual patients. However, data from a pilot study in alemtuzumab-treated patients showed a temporal relationship between sNfL spikes and relapse or MRI lesion activity, with periods of concurrent clinical MRI and sNfL stability in individual patients, suggesting a potential role for sNfL in personalized disease management. 7 A second limitation of this study is the lack of comparative long-term sNfL data in the SC IFNB-1a group. Given the re-increase of sNfL levels observed in SC IFNB-1a–treated patients between month 18 and 24 of the core study, it would be highly relevant to see how such re-increase develops longer term and how it correlates with clinical and MRI progression. Few data on sNfL from clinical trials of other MS drugs beyond the standard 2 year core trials are available,9,10 so it remains unclear how the long-term effect of alemtuzumab on sNfL would compare with other DMTs; this would be of specific interest for other highly effective treatments such as ocrelizumab or natalizumab. In addition, the sample size for sNfL analysis decreased to 110 patients by year 7, although this was primarily due to the increasing number of samples that were not collected during the required time window, rather than patients discontinuing the study. Finally, CARE-MS I patients were studied early in their disease course, at a time during which MS disease is believed to be more responsive to therapy. Whether patients at a more advanced disease stage would have a comparable or less robust sNfL response to alemtuzumab is unknown. Analysis of samples from CARE-MS II patients who were treatment-experienced and had a higher EDSS score and longer disease duration at baseline would enable such questions to be addressed.
Our results show that alemtuzumab treatment effectively reduced sNfL levels in treatment-naive CARE-MS I patients, and sNfL levels in these patients remained stable over 7 years. The sustained reduction of sNfL over time is in line with the clinical and MRI efficacy of alemtuzumab. The observed difference to SC IFNB-1a supports the concept that sNfL is a sensitive marker of subclinical disease activity, and hence may be in the future a therapy-monitoring tool on the individual level.
Supplemental Material
Supplemental material, sj-docx-1-msj-10.1177_13524585211032348 for Sustained reduction of serum neurofilament light chain over 7 years by alemtuzumab in early relapsing–remitting MS by Jens Kuhle, Nadia Daizadeh, Pascal Benkert, Aleksandra Maceski, Christian Barro, Zuzanna Michalak, Maria Pia Sormani, Jean Godin, Srinivas Shankara, Tarek A Samad, Alan Jacobs, Luke Chung, Nora Rӧsch, Carina Kaiser, Colin P Mitchell, David Leppert, Evis Havari and Ludwig Kappos in Multiple Sclerosis Journal
Acknowledgments
The authors and Sanofi thank the patients for their participation in the CARE-MS I, CAMMS03409, and TOPAZ studies, as well as the Steering Committees and the investigators. Critical review of the manuscript was provided by Darren P Baker, PhD, and Ericka Bueno, PhD, of Sanofi. Editorial support was provided by Valerie P Zediak, PhD, of Eloquent Scientific Solutions, and was funded by Sanofi.
Footnotes
Author’s Note: Statistical analysis was carried out by Nadia Daizadeh, PhD (Sanofi, Cambridge, MA, USA).
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: J.K. reports his institution (University Hospital Basel) received and used exclusively for research support: consulting fees from Biogen, Novartis, Protagen AG, Roche, and Teva, speaker fees from Biogen, Genzyme, Novartis, Roche, and Swiss MS Society, travel expenses from Merck Serono, Novartis, and Roche, and grants from Bayer AG, Biogen, Celgene, Genzyme, ECTRIMS Research Fellowship Program, Merck, Novartis, Roche, Swiss MS Society, Swiss National Research Foundation (320030_160221), and University of Basel. P.B., A.M., and Z.M. have nothing to disclose. C.B. reports travel support from Novartis and Teva. M.P.S. reports consulting fees from Biogen, Celgene, GeNeuro, MedDay, Merck, Novartis, Roche, Sanofi Genzyme, and Teva. N.D., J.G., E.H., C.K., C.P.M., N.R., and S.S. are employees of Sanofi. L.C., A.J., and T.A.S. were employees of Sanofi during study conduct and analysis. D.L. reports personal fees from Novartis, Orion, Roche, and Sanofi; he is chief medical officer at GeNeuro. L.K. reports his institution (University Hospital Basel) received in the last 3 years the following, which was used exclusively for research support: steering committee, advisory board, consultancy fees, and support of educational activities from: Actelion, Allergan, Almirall, Baxalta, Bayer, Biogen, Celgene, CSL Behring, Desitin, EXCEMED, Eisai, F. Hoffmann-La Roche, Genzyme, Japan Tobacco, Merck, Minoryx, Novartis, Pfizer, Sanofi-Aventis, Santhera, and Teva; license fees for Neurostatus-UHB products; the Research of the MS Center in Basel has been supported by grants from Bayer, Biogen, European Union, Innosuisse, Novartis, Roche Research Foundations, Swiss MS Society, and Swiss National Research Foundation.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Editorial support for the development of this paper was funded by Sanofi. The CARE-MS I, CAMMS03409, and TOPAZ studies were funded by Sanofi and Bayer HealthCare Pharmaceuticals. Funding for analysis of samples for sNfL was provided by Sanofi.
Previous Presentation of the Data: Some of the data included in this manuscript were presented previously at the 71st Annual Meeting of the American Academy of Neurology (AAN), 4–10 May, 2019, Philadelphia, PA, USA (Kuhle et al., P3.2-045); 2019 Annual Meeting of the Consortium of Multiple Sclerosis Centers (CMSC), May 28–June 1, 2019, Seattle, WA, USA (Havari et al., NIB01); 5th Congress of the European Academy of Neurology (EAN), 29 June–2 July, 2019, Oslo, Norway (Kuhle et al., EPR2077); 35th Congress of the European Committee for Treatment and Research in Multiple Sclerosis (ECTRIMS), 11–13 September, 2019, Stockholm, Sweden (Kuhle et al., P583); and Americas Committee for Treatment and Research in Multiple Sclerosis (ACTRIMS) Forum, 27-29 February, 2020, West Palm Beach, FL, USA (Kuhle et al., P017).
ORCID iDs: Pascal Benkert  https://orcid.org/0000-0001-6525-8174
https://orcid.org/0000-0001-6525-8174
Maria Pia Sormani  https://orcid.org/0000-0001-6892-104X
https://orcid.org/0000-0001-6892-104X
Ludwig Kappos  https://orcid.org/0000-0003-4175-5509
https://orcid.org/0000-0003-4175-5509
Supplemental Material: Supplemental material for this article is available online.
Contributor Information
Jens Kuhle, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Medicine, Biomedicine, and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Nadia Daizadeh, Sanofi, Cambridge, MA, USA.
Pascal Benkert, Clinical Trial Unit Basel, Department of Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Aleksandra Maceski, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Medicine, Biomedicine, and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Christian Barro, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel, University of Basel, Basel, Switzerland Current affiliation: Ann Romney Center for Neurologic Diseases, Brigham & Women’s Hospital, Harvard Medical School, Boston, MA, USA.
Zuzanna Michalak, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), University Hospital Basel, University of Basel, Basel, Switzerland Current affiliation: F. Hoffmann-La Roche Ltd., Basel, Switzerland.
Maria Pia Sormani, Department of Health Sciences, University of Genoa and IRCCS Ospedale Policlinico San Martino, Genoa, Italy.
Jean Godin, Sanofi, Cambridge, MA, USA.
Srinivas Shankara, Sanofi, Framingham, MA, USA.
Tarek A Samad, Sanofi, Framingham, MA, USA; Current affiliation: Immunitas Therapeutics, Inc., Cambridge, MA, USA.
Alan Jacobs, Sanofi, Cambridge, MA, USA; Current affiliation: Immunovant, New York, NY, USA.
Luke Chung, Sanofi, Cambridge, MA, USA; Current affiliation: Immune-Onc Therapeutics, Palo Alto, CA, USA.
Nora Rӧsch, Sanofi, Cambridge, MA, USA.
Carina Kaiser, Sanofi, Baar, Switzerland.
Colin P Mitchell, Sanofi, Cambridge, MA, USA.
David Leppert, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Medicine, Biomedicine, and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
Evis Havari, Sanofi, Framingham, MA, USA.
Ludwig Kappos, Neurologic Clinic and Policlinic, MS Center and Research Center for Clinical Neuroimmunology and Neuroscience Basel (RC2NB), Departments of Medicine, Biomedicine, and Clinical Research, University Hospital Basel, University of Basel, Basel, Switzerland.
References
- 1. Gafson AR, Barthelemy NR, Bomont P, et al. Neurofilaments: Neurobiological foundations for biomarker applications. Brain 2020; 143(7): 1975–1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Barro C, Benkert P, Disanto G, et al. Serum neurofilament as a predictor of disease worsening and brain and spinal cord atrophy in multiple sclerosis. Brain 2018; 141(8): 2382–2391. [DOI] [PubMed] [Google Scholar]
- 3. Chitnis T, Gonzalez C, Healy BC, et al. Neurofilament light chain serum levels correlate with 10-year MRI outcomes in multiple sclerosis. Ann Clin Transl Neurol 2018; 5(12): 1478–1491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Delcoigne B, Manouchehrinia A, Barro C, et al. Blood neurofilament light levels segregate treatment effects in multiple sclerosis. Neurology 2020; 94(11): e1201–e1212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuhle J, Nourbakhsh B, Grant D, et al. Serum neurofilament is associated with progression of brain atrophy and disability in early MS. Neurology 2017; 88(9): 826–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Piehl F, Kockum I, Khademi M, et al. Plasma neurofilament light chain levels in patients with MS switching from injectable therapies to fingolimod. Mult Scler 2018; 24(8): 1046–1054. [DOI] [PubMed] [Google Scholar]
- 7. Akgun K, Kretschmann N, Haase R, et al. Profiling individual clinical responses by high-frequency serum neurofilament assessment in MS. Neurol Neuroimmunol Neuroinflamm 2019; 6(3): e555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kuhle J, Barro C, Disanto G, et al. Serum neurofilament light chain in early relapsing remitting MS is increased and correlates with CSF levels and with MRI measures of disease severity. Mult Scler 2016; 22(12): 1550–1559. [DOI] [PubMed] [Google Scholar]
- 9. Kuhle J, Kropshofer H, Barro C, et al. Siponimod reduces neurofilament light chain blood levels in secondary progressive multiple sclerosis patients. Neurology 2018; 90(Suppl. 15): S8.006. [Google Scholar]
- 10. Kuhle J, Kropshofer H, Haering DA, et al. Blood neurofilament light chain as a biomarker of MS disease activity and treatment response. Neurology 2019; 92(10): e1007–e1015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Novakova L, Zetterberg H, Sundstrom P, et al. Monitoring disease activity in multiple sclerosis using serum neurofilament light protein. Neurology 2017; 89(22): 2230–2237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Cohen JA, Coles AJ, Arnold DL, et al. Alemtuzumab versus interferon beta 1a as first-line treatment for patients with relapsing-remitting multiple sclerosis: A randomised controlled phase 3 trial. Lancet 2012; 380(9856): 1819–1828. [DOI] [PubMed] [Google Scholar]
- 13. Arnold DL, Barnett M, Comi G, et al. Durable reduction in MRI disease activity and slowing of brain volume loss with alemtuzumab in patients with active RRMS: 7-year follow-up of CARE-MS I patients (TOPAZ study). Mult Scler 2017; 23(Suppl. 3): P1189. [Google Scholar]
- 14. Coles AJ, Arnold DL, Bass A, et al. Efficacy and safety of alemtuzumab over 6 years: final results of the 4-year CARE-MS extension trial. Ther Adv Neurol Dis. Epub ahead of print 1 January 2021. DOI: 10.1177/1756286420982134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Coles AJ, Boyko AN, De Seze J, et al. Alemtuzumab durably improves clinical outcomes in patients with active RRMS in the absence of continuous treatment: 7-year follow-up of CARE-MS I patients (TOPAZ study). Mult Scler 2017; 23(Suppl. 3): P1188. [Google Scholar]
- 16. Havrdova E, Arnold DL, Cohen JA, et al. Alemtuzumab CARE-MS I 5-year follow-up: Durable efficacy in the absence of continuous MS therapy. Neurology 2017; 89(11): 1107–1116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cuker A, Bass AD, Nadj C, et al. Immune thrombocytopenia in alemtuzumab-treated MS patients: Incidence, detection, and management. Mult Scler 2020; 26(1): 48–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Sanofi Genzyme. LEMTRADA® (alemtuzumab) [prescribing information]. Cambridge, MA: Sanofi Genzyme. [Google Scholar]
- 19. Phelps R, Winston JA, Wynn D, et al. Incidence, management, and outcomes of autoimmune nephropathies following alemtuzumab treatment in patients with multiple sclerosis. Mult Scler 2019; 25(9): 1273–1288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Singer BA, Alroughani R, Broadley S, et al. Improved clinical and MRI disease activity outcomes, including slowing of brain volume loss, in alemtuzumab-treated RRMS patients: 8-year follow-up of CARE-MS II (TOPAZ study). Neurology 2019; 92(Suppl. 15): P3.2–058. [Google Scholar]
- 21. Wray S, Havrdova E, Snydman DR, et al. Infection risk with alemtuzumab decreases over time: Pooled analysis of 6-year data from the CAMMS223, CARE-MS I, and CARE-MS II studies and the CAMMS03409 extension study. Mult Scler 2018; 25(12): 1605–1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Disanto G, Barro C, Benkert P, et al. Serum neurofilament light: A biomarker of neuronal damage in multiple sclerosis. Ann Neurol 2017; 81(6): 857–870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Rigby RA, Stasinopoulos DM. Smooth centile curves for skew and kurtotic data modelled using the Box-Cox power exponential distribution. Stat Med 2004; 23(19): 3053–3076. [DOI] [PubMed] [Google Scholar]
- 24. Dalla Costa G, Martinelli V, Sangalli F, et al. Prognostic value of serum neurofilaments in patients with clinically isolated syndromes. Neurology 2019; 92(7): e733–e741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Schippling S, Amato MP, Arnold DL, et al. Alemtuzumab reduces brain atrophy in patients with neither relapses nor MRI disease activity: A pooled CARE-MS I and II analysis. In: 5th Congress of the European Academy of Neurology, Oslo, June 29–July 2, 2019. [Google Scholar]
- 26. Limmroth V, Achiron A, Bass AD, et al. Alemtuzumab efficacy and safety were maintained over 8 years in RRMS patients with highly active disease from CARE-MS I: TOPAZ study follow-up. Mult Scler 2019; 25: P1012. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-msj-10.1177_13524585211032348 for Sustained reduction of serum neurofilament light chain over 7 years by alemtuzumab in early relapsing–remitting MS by Jens Kuhle, Nadia Daizadeh, Pascal Benkert, Aleksandra Maceski, Christian Barro, Zuzanna Michalak, Maria Pia Sormani, Jean Godin, Srinivas Shankara, Tarek A Samad, Alan Jacobs, Luke Chung, Nora Rӧsch, Carina Kaiser, Colin P Mitchell, David Leppert, Evis Havari and Ludwig Kappos in Multiple Sclerosis Journal
Data Availability Statement
Qualified researchers may request access to patient-level data and related study documents, including the clinical study report, study protocol with any amendments, blank case report form, statistical analysis plan, and dataset specifications. Patient-level data will be anonymized and study documents will be redacted to protect the privacy of trial participants. Further details on Sanofi’s data-sharing criteria, eligible studies, and process for requesting access can be found at https://www.clinicalstudydatarequest.com.