Abstract
Accumulating evidence suggests that the detrimental effect of nicotine and tobacco smoke on the central nervous system (CNS) is caused by the neurotoxic role of nicotine on blood-brain barrier (BBB) permeability, nicotinic acetylcholine receptor expression, and the dopaminergic system. The ultimate consequence of these nicotine associated neurotoxicities can lead to cerebrovascular dysfunction, altered behavioral outcomes (hyperactivity and cognitive dysfunction) as well as future drug abuse and addiction. The severity of these detrimental effects can be associated with several biological determinants. Sex and age are two important biological determinants which can affect the pharmacokinetics and pharmacodynamics of several systemically available substances, including nicotine. With regard to sex, the availability of gonadal hormone is impacted by the pregnancy status and menstrual cycle resulting in altered metabolism rate of nicotine. Additionally, the observed lower smoking cessation rate in females compared to males is a consequence of differential effects of sex on pharmacokinetics and pharmacodynamics of nicotine. Similarly, age-dependent alterations in the pharmacokinetics and pharmacodynamics of nicotine have also been observed. One such example is related to severe vulnerability of adolescence towards addiction and long-term behavioral changes which may continue through adulthood. Considering the possible neurotoxic effects of nicotine on the central nervous system and the deterministic role of sex as well as age on these neurotoxic effects of smoking, it has become important to consider sex and age to study nicotine induced neurotoxicity and development of treatment strategies for combating possible harmful effects of nicotine. In the future, understanding the role of sex and age on the neurotoxic actions of nicotine can facilitate the individualization and optimization of treatment(s) to mitigate nicotine induced neurotoxicity as well as smoking cessation therapy. Unfortunately, however, no such comprehensive study is available which has considered both the sex- and age-dependent neurotoxicity of nicotine, as of today. Hence, the overreaching goal of this review article is to analyze and summarize the impact of sex and age on pharmacokinetics and pharmacodynamics of nicotine and possible neurotoxic consequences associated with nicotine in order to emphasize the importance of including these biological factors for such studies.
Keywords: nicotine, pharmacokinetics, pharmacodynamics, sex, age, neurotoxicity
Graphical Abstract
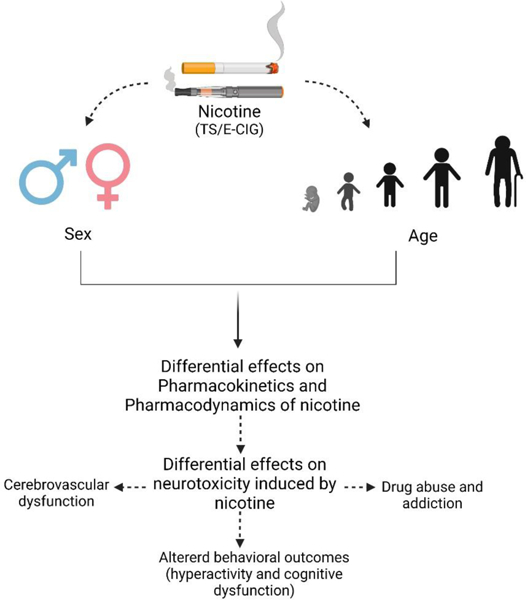
1. Introduction:
Tobacco smoking has been identified as one of the major health concerns for several decades. Smoking is a major contributor to both long term disability and harm to several organs of the body causing various diseases including but not limited to cancer, stroke, heart disease, lung disease, diabetes, and chronic obstructive pulmonary disease (COPD) [1] . It remains the leading cause of preventable death in the US [2]. More than 16 million Americans are suffering from a disease caused by smoking [1] and each year more than 7 million people die worldwide due to smoking [3] . If global smoking patterns continue at this rate, it is assumed that more than 8 million people will die yearly from diseases associated with tobacco smoke by 2030 [1]. Fortunately, in the US, the number of smokers declined in the 1970s and 1980s, remained comparatively stable during 1990s, and then declined further through the early 2000s. Interestingly, this decline in smoking was greater among men compared to women therefore, there is less difference between men and women regarding prevalence of smoking. Several factors have been observed to be associated with these sex-difference associated smoking rates, which includes lower rates of smoking cessation as well as post smoking cessation relapse in women compared to men [4].
Although the rates of cigarette smoking has declined over the past 50 years in the United States [5], alternatives like electronic cigarette (e-cig) products have gained rapid popularity among all age groups and sexes. Recent data demonstrates an alarming, consistent growth in female smokers. Surprisingly, young, and even pregnant women, are using e-cigs at a greater extent these days, considering it as a safer alternative to tobacco smoke. However, e-cigs also contains nicotine as a main constituent as well as other, potentially harmful components which may affect the cerebrovascular and cardiovascular systems and lung [6].
Several studies suggest that men and women differ in their smoking behaviors. It has been observed that women smoke fewer cigarettes per day and have a tendency of using cigarettes with lower nicotine content. Women also do not inhale cigarettes as deeply as men [7]. Interestingly, women may smoke for different reasons than men, including mood and stress management [8]. However, it is vague whether these differences in smoking behaviors are due to high sensitivity of women to nicotine, or they find the sensations associated with smoking less rewarding, or social factors contributing to the difference. Additionally, some research also suggests that women may experience more stress and anxiety as a result of nicotine withdrawal compared to men [9]. Risk of death from smoking-related lung cancer, COPD, heart disease, and stroke continues to increase among women, approaching rates for men [10]. According to data collected from 2005 to 2009, each year about 201,000 women die due to smoking related factors whereas the number is 278,000 for men [4]. Some health hazards associated with smoking, including blood clots, heart attack, or stroke, have been reported to increase in women who use oral contraceptives [11].
Along with sex, age is another determinant of smoking and nicotine action. According to Centers for Disease Control and Prevention (CDC), currently the greatest rates of e-cig and tobacco smoking are observed in person between 25–44 years and 45–64 years respectively whereas the lowest cigarette smoking is observed in 18–24 aged people [12]. About 9 of every 10 adults who smoke cigarettes daily first try smoking by age 18, and 99% first try smoking by age 26 which represents that tobacco use is initiated and established primarily during adolescence stage. It is speculated that if the rate of cigarette smoking among youth continues to increase at the current rate, 5.6 million of Americans below 18 years will die early from a smoking-related illness [13]. Alarmingly, the e-cig vaping rate among adolescent and youth has been increasing rapidly. About 1 out of 20 middle school students (4.7%) and 1 of every 5 high school students (19.6%) reported in 2020 that they used e-cigarettes in the past 30 days [13]. It is also interesting that many young people are now using multiple tobacco products which may increase the risk for developing nicotine dependence and might be more likely to continue using tobacco into adulthood [13].Several studies have demonstrated that age and sex are important determinants for evaluating nicotine action and toxicity. Physiological differences between male and female along with age differences may alter the pharmacokinetics and pharmacological effects of nicotine. Unpublished data from our research group has also demonstrated sex- and age-difference effects of nicotine on blood-brain barrier (BBB) integrity, cognitive function, and locomotor activity. However, there is a lack of study related to differences in nicotine action in both pharmacokinetics and pharmacodynamics perspective considering both sexes and different ages. Considering the impact of nicotine on human health and the role of age and sex on nicotine action, the aim of this review article is to summarize and discuss the effect of these intrinsic factors on nicotine pharmacokinetics and pharmacodynamics. We believe this comprehensive review will help the readers to understand the importance of considering sex and age dependent action of nicotine for the development of biologically relevant therapies for treating cerebrovascular and neurological dysfunctions.
2. Epidemiological data on tobacco/e-cig users based on age and sex:
Although smokers are aware of the toxic effects of nicotine, the use of nicotine through tobacco and e-cig remains a substantial cause of preventable deaths [14]. When comparing e-cig use to tobacco smoke use, the nicotine consumption regarding both users is comparable [15]. Worldwide, 30% of all men and 7% of all women are tobacco smokers which makes it around 1 billion in total number [14]. Currently in the US, 15.3% of men and 12.7% of women are tobacco cigarette users [16]. Men are nearly twice as likely as women to be an e-cig user. In 2018, 4.3% of men and 2.3% of women were current e-cig users [17]. Although a larger number of men are smokers, women have a harder time quitting and are more vulnerable to dependencies. Overall smoking cessation is declining, but the decline of women smokers is less pronounced than that of men [18]. For users, nicotine is a risk factor for a variety of health issues including cancer, lung disease, stroke, heart disease, and vascular diseases. In addition, use can reduce fertility in both men and women and can harbor dangerous effects on the fetus [14]. Although pregnant women are aware of the harmful effects smoking can have on the fetus, e-cig use among pregnant women ranges from 0.6% to 15% [19]. CDC data shows that around 7.0% of women use e-cigs around the time of pregnancy and 1.4% of women use them during the last 3 months of pregnancy [20]. The amount of women who smoke tobacco cigarettes during pregnancy remains comparable, amounting to 7.2% during pregnancy [21]. Table 1 summarizes the rate of tobacco smoking and e-cig vaping between male and female.
Table 1:
Sex-difference in tobacco smoking and e-cig vaping rate
In 2019, around 50.6 million adults nationwide admitted to using tobacco products. Cigarette use among adults was around 14.0% and e-cig use was around 4.5% [12]. Current data shows that those nationwide, 8.0% of adults aged 18–24, 16.7% of adults aged 25–44, 17.0% of adults aged 45–64, and 8.2% of adults aged 65 and older are regular cigarette users [16]. E-Cig use is highest among those aged 18–24 amounting to 9.3% [12]. E-Cigs are commonly used in youth, as 3.6 million youth used e-cig products in the past 30 days [22]. Evidence shows that adolescents are vulnerable to nicotine dependency which can lead to other deficits later in life [23]. In addition, nicotine use among adolescents increases the likelihood to participate in risky behaviors such as sexual activity and violence [24]. In 2020, an estimated 1 in 5 (19.6%) high school students and 1 in 20 (4.7%) middle school students were e-cig users [25]. There has been a continual decline in cigarette use but a 10% increase in e-cig use among adolescents [25, 26]. Table 2 summarizes the rate of tobacco smoking and e-cig vaping among different age groups.
Table 2:
Age-difference in tobacco smoking and e-cig vaping rate
These data clearly indicate that how nicotine containing product usage (tobacco and e-cig) is immersing into different age groups and both sexes at distinct rates and requires serious consideration.
3. The neurovascular system:
The neurovascular unit (NVU) is a dynamic structure in which multicellular interaction of blood vessels and the brain is responsible for the integrity of the vascular and nervous systems in the body [27]. Neurons, astrocytes, pericytes, microglia, endothelial cells, and extracellular matrix are all integral components of the NVU. Working together, the neurovascular unit maintains a selective blood-brain barrier (BBB), cerebral homeostasis, and cerebral blood flow. The BBB acts as an interface between the brain and periphery, regulating major ions, solutes, and nutrients across the brain and blood and prevents movement of harmful molecules to the brain [28]. The homeostatic function of the BBB occurs at the brain microvascular endothelium level, where brain endothelial cells are the primary anatomical unit that maintains selective permeability. However, the brain endothelial cells do not form the intrinsic barrier solely by themselves but from interaction with other components of the unit [29, 30]. The mechanism of linkage of neurons and the cerebral vessel is often regarded as neurovascular coupling. This crucial association occurs with the release of glutamate through the activation of neurons. Glutamate further activates astrocytes, pericytes, and other vasoactive mediators, regulating cerebral blood flow [31]. Astrocytes, major glial cells of the NVU, have foot processes across the blood-brain barrier and form a complex network surrounding the capillaries [32, 33]. The close association between these cells further helps in the induction of the barrier properties. Pericytes are discontinuously distributed across the cerebral capillary, and the endothelial cells are enclosed by and contribute to the formation of the extracellular matrix, also called the basal lamina. Pericytes are essential in angiogenesis and vesicular transport of essential molecules across the BBB [34]. Moreover, the vascular pericytic coverage correlates with the inter-endothelial junction tightness across the barrier. The microglia serve as immunocompetent cells that, together with astrocytes and extracellular matrix, release vasoactive agents and cytokines, which further establish tight junction formation and barrier permeability [34].
The molecular characteristics of the brain microvascular endothelium that ensures ‘barrier’ like function is due to endothelial cells connected by adherens junction (AJs) and then sealed up by tight junction proteins (TJs) [35]. AJs constitute cadherin proteins that bridge the intercellular cleft, and these proteins are scaffolded with the cell cytoplasm by alpha, beta, and gamma catenin. Cadherins are essential for the formation of AJs and maintenance of the structure and integrity of the BBB. The TJs constitute another group of proteins, claudins and occludin, that bridge junctional adhesion molecules and the intercellular cleft. The claudins and occludin are linked further by regulatory scaffolding proteins ZO-1, ZO-2, ZO-3, and cingulin to intracellular actin and the cytoskeleton [31]. The TJs are responsible for the strict restriction of ions and solutes through paracellular diffusional pathways, resulting in high electrical trans endothelial resistance of around 1800 Ω cm2 across the BBB [36]. The components of BBB and associated function clearly indicate the importance of a healthy neurovascular unit to avoid cerebrovascular dysfunction [37].
Nicotinic acetylcholine receptor:
As a major pharmacologically active chemical in tobacco smoke, nicotine exerts its biological effects and action through binding to nicotinic acetylcholine receptors (nAchRs) [38]. Neuronal nAchRs are pentameric transmembrane cation channels which belong to the superfamily of ligand-gated ion channels that include the GABA, 5-HT and glycine receptors and consist of α2–10 and β2–4 subunits. Endogenous (acetylcholine) or exogenous (nicotine) agonists bind to nAChR and open an intrinsic ion channel in the receptor, resulting in the flow of cations (Na+, Ca2+, and K+) through the cell membrane and induce a wide variety of biological responses [38]. The composition and neuroanatomical localization of the receptor regulate different pharmacologic and biologic actions. nAChRs are expressed in brain regions which regulate a variety of behaviors. β2 nAChRs and α7 nAChRs are the most common subtypes in the CNS which are expressed in the amygdala, dorsal striatum, and thalamus but with neuroanatomical overlap in the ventral tegmental area (VTA), cortex, hippocampus, and basal ganglia [39–41]. These brain regions control sensory transmission, learning and memory function, emotion, and reward. The α6β2 nAChRs are selectively expressed in catecholaminergic nuclei and enriched in the mesolimbic DA system, which is believed to be associated with addiction. The α3β4 nAChRs have reasonable expression in the CNS but are highly expressed in the medial habenula (mHb) to interpeduncular nucleus (IPN) pathway with a small subset of these receptors containing the α5 (i.e. α3α5β4) [42–44]. The mHb-IPN pathway controls the mesolimbic system and is highly involved in smoking phenotype. Also, the α3 and β4 nAChRs form nAChRs in the ganglion, raising attention to possible side effects associated with peripheral nervous system that could result from drug targeting of α3β4 nAChRs. A small number of α3β2 nAChRs in the habenula and IPN may be considered important for smoking phenotype, however there are currently limited tools to assess this [45]. These studies demonstrate the role of nicotinic receptors in different biological responses which could be altered by nicotine exposure.
4. Sex-difference and age-dependent effect on BBB integrity and disruption:
4.1. Role of sex on BBB disruption:
The BBB disruption can either be a cause or an effect of neurodegeneration, which is yet to be elucidated. However, based on disease prevalence between male and female patients, sex possibly plays a crucial role in the BBB disruption associated with several diseases including Alzheimer’s disease, Parkinson’s disease, multiple sclerosis, and motor neuron disease, which demonstrate clear sexual dimorphisms [46].
Several studies have been conducted using induced pluripotent stem cell (iPSC) lines from both male and female subjects induced to brain microvascular endothelial cells (BMECs). These experiments suggest that iPSC-derived BMECs from pre-menopausal women have reduced permeability, and increased barrier strength, compared to iPSC-derived BMECs from men. Lippmann et al. reported that DF19–9–11T cell line from male demonstrated a significantly lower trans-endothelial electrical resistance (TEER) value (777 ± 112) relative to the female IMR90–4 cell line (1450 ± 140). However, male DF19–9–11T cells showed higher platelet endothelial cell adhesion molecule-1 (PECAM-1 or CD31) expression compared to the female IMR90–4 cells (75% vs 68%) [47]. Expression and association of PECAM-1 in BMEC tight junction integrity [48] indicates an increase in other tight junction proteins possibly resulting in higher TEER values in the female IMR90–4 BMEC. Sex differences in BBB permeability are also evident in the SAMP8 mouse model of accelerated aging [49]. Increased mRNA expression of tight junction proteins (claudin-1, claudin-5, claudin-12, occludin, ZO-1), junction adhesion molecule A (JAMA), major facilitator superfamily domain containing 2, and brain-derived neurotrophic factor (BDNF) has been observed in female mice compared to male [49]. This study indicates sex-different expression of tight junction proteins which could affect brain endothelial cell interaction and transient changes in BBB permeability. Sex dependent BBB characteristics should be further studied in future capitalizing on different in vitro and in vivo models [46] to more precisely determine the role of sex as a biological determinant of neurovascular function in brain diseases.
Sex differences in shear stress responses may also impact susceptibility of neurodegenerative disease. Cerebral vasodilation is important for glucose supply to metabolically active brain areas by increasing local blood flow [50]. A human brachial artery study suggested that the endothelium of pre-menopausal women may have more sensitivity to shear stress, leading to increased vasodilation compared to similar aged men and post-menopausal women [51]. Gracilis muscle arterioles isolated from female rats showed increased flow-mediated dilation compared to male counterparts, which decreased wall shear stress and shear stress-induced endothelial damage [52]. Decreased arterial [53] and capillary pressure [54] was also observed in pre-menopausal women compared to men. Although these studies did not consider brain, it has been suggested that women may have increased vasodilation in response to shear stress in the cerebral vasculature as well [46].
Sex difference may also impact vasodilation through endothelial-derived hyperpolarization (EDH). In EDH, stimulation of G-protein coupled receptor (GPCR) increases intracellular calcium and hyperpolarizes the cell membrane. This hyperpolarization is believed to be transmitted through gap junctions to smooth muscle cells, resulting in dilation of the blood vessels, thereby increase cerebral vascular perfusion. A recent study related to EDH aging has reported reduced GPCR function in male mice compared to age-matched female mice, leading to a decreased EDH response [55]. On the contrary, increased EDH has been shown in female rat following ovariectomy which was reversed with estrogen supplementation, indicating that estrogen may decrease the EDH response. Interestingly, the EDH response is attenuated by NO [56], which increases with estrogen [57]. It is apparent that more studies are needed to confirm direct relationships between EDH and female sex hormones [46].
These abovementioned studies on sex-different BBB disruption clearly indicate the importance of inclusion of both sexes in preclinical and clinical studies to design proper treatment strategies associated for BBB-disrupted cerebrovascular diseases.
4.2. Role of age on BBB disruption:
The age-associated decline of the neurological and cognitive functions becomes a serious challenge for developed countries which have large number of aged populations. Multiple investigations have focused on morphologic and biochemical differences in the aging brain. There is no specific age range to define physiological aging, however it can be defined as a deterioration of physiological processes without cognitive dysfunction and dementia concerning brain and BBB [58]. Several studies have reported that aging has significant impact on essential components of the BBB and is associated with impairment and disruption of the BBB contributing to several neurodegenerative disorders. Table 3 summarizes the effects of aging on BBB components.
Table 3:
Effect of physiological aging on components of blood-brain barrier (BBB)
| Component of BBB | Properties | Reference |
|---|---|---|
| Endothelial cells (ECs) | Capillary wall thickness: increased in human and decreased in rats and monkeys | [59–62] |
| Number of ECs: decreased in human | ||
| Number of mitochondria: decreased in rodents and human | ||
| Tight junctions (TJs) | Expression: decreased in mice | [63, 64] |
| Basal lamina | Thickness: increased in human | [65, 66] |
| Concentration of laminin: decreased in human | ||
| Concentration of argin and collagen IV: increased in human | ||
| Astrocytes | Proliferation: increased in human | [67–70] |
| GFAP expression: increased in human and rodents | ||
| Pericytes | Number: loss of pericytes in human | [60, 61, 71, 72] |
| Ultrastructural changes: vesicular and lipofuscin-like inclusions, increased size of mitochondria, foamy transformation in rodents | ||
| Neurons | Neurogenesis: impaired in mouse | [73–76] |
| Apoptosis: increased in rats | ||
| Synaptic plasticity: deteriorated in rats | ||
| Neuronal damage in rodents | ||
| Deficit in long term potentiation in rats | ||
| Microglia | Changes to amoeboid morphology in human | [77, 78] |
| Production of neurotoxic mediators such as nitric oxide and peroxide and proinflammatory cytokines (i.e., TNF-α), proteases and complement components in rodent and human model. |
Neurodegeneration is considered as an essential component of age-related pathology. It can be defined as progressive loss of neuronal structure and function resulting in neuronal cell death. Most of the neurodegenerative diseases, including Alzheimer’s disease (AD), Parkinson’s disease (PD), multiple sclerosis (MS), and pharmaco-resistant epilepsy, have been found to begin in mid-life. These diseases can be characterized by motor and/or cognitive dysfunction that progressively deteriorates with age and may reduce life expectancy [58]. Thus, it is evident that age can play a significant role on BBB disruption and associated cerebrovascular dysfunction.
5. Effect of nicotine and TS on cerebrovascular disorders and critical role of sex and age:
Nicotine is the key constituent of tobacco smoke (TS) and also present in e-cig products. TS is one of the leading causes of preventable death and is accountable for over 480,000 deaths each year in the US [79]. Tobacco smoke is a varied mixture of more than 4700 toxic chemicals that are carcinogenic and mutagenic. Nicotine, carbon monoxide, and oxidants such as free radicals and nitrogen oxides are a few of the many constituents of TS that are considered both cardiotoxic and neurotoxic [80]. TS has been associated with worsening ischemic stroke, traumatic brain injury, and other neurological diseases such as AD, MS, and Neuro-AIDS [81–85]. Preclinical studies attribute TS to vascular endothelial dysfunction in a causative and dose-dependent manner related to acute or chronic nicotine exposure, ROS content of TS, and oxidative stress-driven inflammation [86–88]. On the other hand, e-cig vaping contains significantly less harmful constituents than TS, but a study has been linked to similar oxidative stress-driven inflammatory potential [89]. Additionally, side-by-side experiments involving e-cig and TS use showed a similar risk of blood coagulation [90]. Nevertheless, the negative effect of nicotine containing e-cigs on various neurological disease states has not been explored as much compared to TS.
5.1. Ischemic stroke (IS):
Ischemic stroke is characterized by a transient or permanent block of blood flow to the brain due to occlusion of a major cerebral artery by a clot or an embolus [91]. It remains the fifth leading cause of death in the US, and TS is a well-known risk factor [92]. In a study using an experimental stroke model, nicotine exacerbated ischemic reperfusion injury by increasing brain edema formation [93]. Additionally, in both in vitro and in vivo stroke settings, researchers found that there was a decrease in Na+/K+/2Cl− co-transporter activity at the BBB level after nicotine or tobacco smoke exposure [94–96]. Likewise, apart from single nicotine exposure, studies have shown e-cig vaping causes cellular oxidative stress by nuclear translocation and increased expression of Nrf2, a major regulator of anti-oxidative defense mechanism, loss of BBB integrity, and cellular inflammation [97–99]. Furthermore, these studies demonstrated a decreased level of thrombomodulin, an anticoagulant factor, and an increased level of TNF-α, a pro-inflammatory cytokine, in animals exposed to TS and e-cig vapors. Lastly, similar stroke outcome assessed by neurological evaluations was observed in both e-cig and TS exposed animals. Thus, e-cig vaping could be as dangerous as TS concerning negative effects on the BBB and cerebrovascular system and could promote post-ischemic brain injury.
Sex and age have been identified as complex and interactive determinants on ischemic stroke risk and pathophysiology. Preclinical studies of sex differences show that female animals are less prone to ischemic stroke than male animals [100]. Interestingly, estrogens have shown to be highly neuroprotective in the context of ischemic stroke because of their anti-atherogenic effect in the vasculature and subsequent regulation of adipogenesis [101]. Moreover, in ovariectomized animals, the advantage of the female group being less prone seems to disappear, thus supporting the role of estrogen in ameliorating ischemic stroke in the female population [102, 103]. However, age reverses this in female mice, as shown by a study that found that middle-aged (16 months) female mice had larger brain infarct and poor recovery compared to male animals [104]. It is believed that this factor is attributable to the loss of estrogen in the female population at this age. Furthermore, exogenous 17β-estradiol has been shown to reverse the worsened outcome in female animals [105]. Studies show that male animals have higher ischemia-induced microglial activation in neonatal and young adult life stages than female animals [106]. Similarly, in vitro studies show that primary brain endothelial cells derived from male mice are more sensitive to oxygen-glucose deprivation (OGD) than female cells [107]. Astrocytes, vital for BBB integrity, also follow a similar trend to other cells to be more sensitive to ischemia in male animals. A study showed that female astrocytes are resistant to OGD-induced ischemic damage, and that too depends on estrogens [108].
Age is one of the significant nonmodifiable risk factors for ischemic stroke. Elderly stroke patients have higher morbidity and mortality rate and poorer functional recovery compared to young patients [109]. Interestingly, it has been reported that sex-different effects in ischemic stroke epidemiology depend on patient age as the influence of sex on stroke risk and outcome changes across the lifespan [109]. In childhood and early stage of adulthood, men experience higher IS incidence and poorer functional outcomes compared to women [110, 111] while in middle age, IS rates begin to increase in females, associated with the onset of menopause and loss of female sex hormones [112]. After middle age, continuous increases in stroke rates have been observed in women, while some of the studies reported on the higher stroke incidence in elderly women (age >85 years) compared to elderly men [110, 111]. Moreover, adolescents experience acute ischemic stroke (AIS) with an annual incidence ranging from 0.54 to 2.4 per 100,000 which results in devastating consequences including psychomotor development, disrupted quality of life, future work inability, and familial and financial costs. Several factors have been identified which might play an important role in stroke genesis in adolescence for instance growth spurts inducing the rapid and important anatomic evolution of heart and neck vessels, hormonal changes, and exposure to lifestyle or environmental factors such as use of tobacco, drugs, or oral contraceptives [113]. Hence, the occurrence of stroke in adolescents’ and young adults has a disproportionately huge physical, social, and economic impact by leaving stroke victims disabled before their most productive years [114].
All these abovementioned studies indicate that age and sex influence ischemic stroke risk and outcome, but in a complex manner and, in future, it is important to explore sex and age different effects on nicotine induced IS for developing effective and personalized therapeutics
5.2. Traumatic brain injury (TBI):
Traumatic brain injury or TBI is one of the most common causes of death and disability related to cerebrovascular and neurologic complications [115]. Each year, around 69 million people suffer from TBI around the world and the majority of TBIs are mild (81%) to moderate (11%) [116]. Chronic smoking is a premorbid risk factor that will impact the severity of TBI and retard post-traumatic recovery. Both preclinical and clinical studies have shown that TBI with premorbid TS exposure results in exacerbated post-traumatic neuroinflammation and cerebrovascular conditions compared to non-smokers [117, 118]. The likely mechanism includes the generation of ROS-driven oxidative stress leading to membrane damage and tissue necrosis. Similarly, findings from another preclinical TBI study showed that animals exposed to TS had worse post-traumatic motor activity than TBI alone [83]. Downregulation of Nrf2 in TS pre-exposed TBI animals was also observed in same study. Further study needs to be conducted to assess the effect of e-cig or vaping on TBI as no study is available as of today.
Interestingly, sex and age have been identified as important determinants to measure TBI outcome. Several studies have reported less post-TBI delayed injury as well as better functional outcomes in younger females compared to older females which is probably due to the neuroprotective effects of sex hormones in pre-menopausal women [119, 120]. Higher level of interleukin-6 (IL-6), tumor necrosis factor (TNF α) and chemokine ligand 2 have been found in female mice compared to male mice. However, anti-inflammatory cytokine, interleukin-10 (IL-10) was found to be higher in male, not in female mice [121]. Although the neuroprotective effects of sex hormones have been observed in experimental animal models, several clinical trial studies utilizing female sex hormones showed contradictory results [122, 123]. Women have been reported to be more vulnerable to post-TBI brain damage and mortality rate compared to men [124]. Women showed 1.28 times higher post-TBI fatality rates and 1.57 times more likely to suffer from acute or/and chronic post-traumatic symptoms like severe disability relative to men [125]. Moreover, microglia and astrocytes induced inflammatory responses after TBI have been observed higher in women than men [126]. A large randomized controlled trial on TBI patients around world suggested that women experienced deteriorated short and long term post-TBI outcomes [127]. In addition, better post-TBI outcomes have been observed in postmenopausal women compared to age-matched men, whereas pre-menopausal women have shown the same outcomes as age-matched men [128]. Thus, clinical and preclinical studies on sex and age effects on post-TBI vulnerability are still conflicting due to the heterogeneous mode of injury and population.
In addition, age should also be considered in measuring the differences related to TBI pathophysiology, post-TBI outcomes and recovery. Studies have shown that the response of the developing brain to TBI differs from the response of the mature, adult brain. There are critical developmental trajectories in the young brain, whereby injury can lead to long term functional abnormalities [129]. Children have shown better recovery rates compared to older patients due to their greater degree of neuroplasticity [130]. Post-TBI mortality rate has been found higher (24%) in elderly patients whereas it is only 12.8% in other age groups. Although elderly patients suffer comparatively less severe head injuries compared to non-elderly patients, however they have shown slower recovery rates and tend to experience more distress [131]. Moreover, TBI among adolescents have been identified as a significant public health concern globally in the past 15 years. A recent study has demonstrated that 15 to 19 years old adolescents have some of the highest emergency department visits, hospitalizations, and deaths related to TBIs compared to other age groups. About 20% and 22% of adolescents sustain a TBI in their lifetime in US and Canada respectively, and as many as 5.5% have multiple injuries [132]. Age-dependent effect has been also observed in post-TBI rehabilitation therapy. Although age does not affect the degree of functional improvement obtained during inpatient rehabilitation after TBI [133], however several studies have shown that older TBI patients experience poorer rehabilitation outcomes than younger patients [134, 135] suggesting the significance of age consideration on post-TBI rehabilitation strategy.
As TBI has been increasing rapidly among women [136]and the post-TBI outcome differ between men and women, it has become more important to understand the complex interplay between sex and age in the pathophysiology of post-TBI for finding an effective therapy for both sexes.
5.3. Alzheimer’s disease (AD) and multiple sclerosis (MS):
TS is associated with an increased risk for the prevalence of various types of dementia, specifically AD and vascular dementia [81]. AD is pathologically characterized by selective loss of nAchR and elevated deposition of amyloid-β. Studies have suggested that smoking enhances amyloid-β deposition and has more severe neuropathological variation than non-smokers [137]. Cerebral amyloid angiopathy is one of the variations that might be attributable to smoking enhanced amyloid-β deposition. A research study has reported increased amyloid-β buildup contributes to reduced tight junction proteins and increased expression of matrix metalloproteinases (MMP), which eventually leads to BBB dysfunction [81]. Amyloid-β is often co-localized to the α7 subunit of nAchR. On its elevation, it binds to the subunit with high affinity and inhibits neuroprotective activity leading to synaptic degeneration [138].
Clinical and preclinical studies have shown that females have an increased risk of AD compared to males [139–141]. This might be attributable to age-related hormonal imbalances, the risk from other diseases such as diabetes, depression, and cardiovascular diseases, and sex differences in brain anatomy and brain glucose utilization [142, 143]. The female hormone estrogen has a sharp decline after menopause in women while testosterone levels slowly decline in males in advanced age, which may contribute to the sex dependent pathology observed in AD. In addition, estrogens have been shown to increase neurogenesis in the dentate gyrus and CA1 of the hippocampus that contributes to learning and memory [144]. Studies have also reported that estrogens reduce Aβ levels by promoting their clearance by stimulating microglial phagocytosis and degradation [145, 146]. Similarly, depletion of progesterone and estradiol resulted in increased levels of Aβ in transgenic mouse models of AD [147]. These studies suggest the potential role of sex hormone and possible sex-different effects on Alzheimer’s disease.
Aging is the most crucial known risk factor for developing AD. Studies have shown that the number of people with AD doubles about every 5 years beyond age 65 and around one-third of all people aged 85 and above may have AD. Alzheimer’s disease can be divided into two categories: early onset and late onset. Early-onset AD occurs between 30 to mid-60 years and represents less than 10 percent of all people with AD whereas late onset represents symptoms which occur at mid-60. Several age-related changes in the brain have been identified which may contribute to Alzheimer’s disease including but not limited to atrophy, inflammation, vascular damage, ROS production and disruption in energy production in cells [148].
Likewise, multiple sclerosis or MS, an autoimmune neurodegenerative disorder, has a deteriorative prognosis in the smoking population [84]. Increased serum MMP-9 concentration is considered a hallmark in MS. Interestingly, TS has been reported to be associated with an increased level of serum MMP-9. MMP-9 degrades the extracellular matrix and helps migrate autoreactive immune cells into the CNS through the BBB [149].
Several studies suggested that sex-difference affects the susceptibility and progression of multiple sclerosis. Higher prevalence and better prognosis have been observed in women compared to men
This sex dimorphism may be explained by the potential role of sex hormones and sex chromosome on immune system, brain damage and repair mechanisms [150, 151]. Preclinical and clinical studies as well as MRI evidence confirms a pathogenetic link between sex hormones and MS, suggesting the sex-different effects of hormones in multiple sclerosis pathophysiology and therapy [150]. Hormonal fluctuations in menstrual cycles, pregnancy, exogenous sex steroids intake have been found to be associated with MS exacerbations. Studies have reported the association between pregnancy, specifically the late stage of pregnancy and decreased clinical symptoms or relapse rate in MS [152–155]. This is observed mainly in the third trimester when estrogen and progesterone levers are increased (up to 20 times than normal) [156]. Based on the potential role of sex hormones in MS, it has been suggested that steroid supplementation may show beneficial impact on MS due to its immunoregulatory, anti-inflammatory and neuroprotective properties [151] although further investigations need to be conducted for proper treatment and management.
In addition, age is considered as a major determinant of onset of MS progression. Accumulating evidence suggests that MS prognosis depends on age at some extent regardless of initial, exacerbating or remitting or progressive disease course [157–160]. Many age-dependent changes have been identified to affect the brain which can collectively affect neuronal viability as well as MS vulnerability. These changes include, but not limited to, increased iron accumulation, oxidative stress, and mitochondrial injury, decreased trophic support from the peri-plaque environment, reduced remyelination, and elevated production of inflammatory molecules such as pro-inflammatory cytokines [161–165]. Interestingly pediatric and adolescent MS have been reported in several studies from all over the world. MS onset during childhood accounts for 5% of total MS patients in the US [166]. A relapsing-remitting MS occurs in more than 97% of MS patients with onset before the age of 18 years which is an important factor to be considered [167].
5.4. Neuro-AIDS:
TS exacerbates neuro-AIDS, a group of neurological disorders resulting from human immunodeficiency virus (HIV) infection in the central nervous system, in which cytokines and toxins are released by the infected brain endothelial cells and give rise to BBB breakdown and neurological pathogenesis. Studies have reported that oxidative stress induced by TS results in disease progression [85]. In addition, nicotine augments viral replication by activating cellular gene progression and inducing oxidative stress in macrophages by CYP2A6 metabolism [168, 169]. Nevertheless, more studies are needed to explore the exact mechanism through which nicotine or other components of TS mediate neuro-AIDS progression.
Clinical and preclinical studies reporting the effect of sex as a biological determinant in magnitude and pattern of neuro-AIDS are inconsistent [170]. Early clinical reports observed no sex differences in the progression of neurocognitive deficits [171]. However, recent clinical studies have reported that HIV-1 seropositive women had impaired learning and memory, verbal fluency, and information processing capability than HIV-1 seropositive men [172]. Additionally, female animals with HIV-1 transgene showed worse locomotor activity, objection recognition memory, and temporal processing compared to male animals [173, 174]. In contrast, a study reported that female transgenic animals showed decreased anxiety-like behavior and increased forelimb grip test compared to male animals [175].
In elderly healthy people, immune activation and inflammation play a vital role in the gradual decrease of the immune system’s functiona lity [176]. However, aging with HIV infection has been shown to exacerbate immune activation and inflammation pathways, leading to neurocognitive impairments [177]. Microglia, the main resident immune cells, switch to an activated state after the viral invasion [178]. Furthermore, they interact with the infiltrating immune cells such as macrophages and T and B lymphocytes in the CNS and trigger neuroinflammation. Other possible consequences include poor adherence to medication in the elderly and expression of apolipoprotein -E4 [179, 180]. Also, excess hyperphosphorylated tau, alpha-synuclein, and amyloid have been reported in relatively aged HIV-positive patients [181, 182]. Moreover, children can acquire HIV-1 through mother during the antenatal, perinatal, or breastfeeding periods and develop neuro-AIDS. With the survival of children in adolescence and adulthood, there is an increasing concern regarding neurocognitive disorders, psychosocial development, and psychiatric disorder. Perinatally HIV infected adolescents have been reported to face difficulties in decision making and risk taking [183]. Further studies are warranted to understand the exact mechanisms underlying age and sex differences in neuro-AIDS.
These studies clearly explain the effect of nicotine on cerebrovascular dysfunction as well as neurodegenerative disorders and describe the role of biological determinants (sex and age) on these diseases. Extensive studies need to be conducted to evaluate the sex and age-dependent effects on nicotine induced cerebrovascular and neurodegenerative disorders for designing better treatment strategy in these populations.
6. Pharmacokinetics and pharmacodynamics of nicotine:
6.1. Nicotine pharmacokinetics:
Pharmacokinetics refer to the fate of a drug or compound after entering the body involving absorption, distribution, metabolism and excretion of that drug or compound. Pharmacokinetics of nicotine depends on physicochemical properties, route of administration as well as the physiological system. Different routes of administration are available for nicotine self-administration including inhalation, ingestion, and intra-nasal, buccal, and transdermal absorption. The most common route of self-administration is inhalation in a form of tobacco products, for instance, cigarette, electronic cigarette, hookah, cigars. Moreover chewing, snuff, and dissolvable lozenges containing nicotine are available [184].
Absorption:
Nicotine is rapidly absorbed into mucosa, respiratory tissues and skin because of its water and lipid solubility property. Oral nicotine products are buffered to alkaline pH that helps absorption through the mucous membrane lining the mouth [185]. As stomach has very low pH (highly acidic), less nicotine is absorbed in stomach [186] but rapidly absorbed in the small intestine and undergoes first pass metabolism which results in decreased bioavailability [187]. Nicotine bioavailability also depends on route of administration. Bioavailability of nicotine is around 80–90% via smoking while 51–56% through nicotine inhaler [185]. Alkalinity or acidity of smoke from cigarette can affect the rate and extent of nicotine absorption probably due to presence of non-protonated, free-base nicotine in tobacco smoke which promotes better nicotine transfer into the respiratory epithelium [188]. In addition, nicotine in e-cig liquid can exist as free base or protonated (salt form). Protonated or salt form of nicotine makes the inhalation less aversive compared to free base nicotine ( e.g. Juul) yielding high nicotine concentration and increased bioavailability in a small puff volume [189]
Distribution:
After reaching to the small airways and alveoli of the lung, nicotine transports readily into arterial and venous circulation. Nicotine reaches the arterial circulation very rapidly, within 7–24 s after the first puff inhalation [190] and maximal venous blood plasma concentration ranges from 15 to 30 ng/ml within 5 minutes [186]. It has been reported that within 5–7 s of the start of an initial puff of cigarette smoke, nicotine reaches the brain and the maximal nicotine concentration in brain was found within 3–5 minutes [191, 192]. Nicotine distributes throughout the body with a steady-state distribution volume ranging between 2.2 and 3.3 L/kg [186, 193]. Nicotine concentrates in different parts of the body, mostly in the lung, liver, kidney, spleen, and brain. It can freely cross the placental barrier and can transfer into breast milk and cervical fluid as well [194]. The distribution half-life of nicotine is approximately 9 min [195].
Metabolism:
Nicotine metabolism primarily occurs in liver, but lung and kidney are also involved in biotransformation of nicotine [186]. Around 80% of nicotine is metabolized into major metabolite cotinine involving two steps process. In the first step, nicotine is hydrolyzed to 5-hydroxycotinine by CYP450 2A6 enzyme resulting in nicotine-iminium ion. In the second step, aldehyde oxidase enzyme produces cotinine [193, 194]. Around 4–7% of nicotine is metabolized to nicotine N′-oxide by flavin-containing monooxygenase 3 enzyme [186] and roughly 3–5% of nicotine is transformed to nicotine glucuronide via glucuronidation reaction catalyzed by uridine diphosphate-glucuronosyltransferase enzyme [193]. In addition, a small fraction of nicotine (0.4–0.8%) is metabolized into nornicotine probably by CYP450 enzyme [186]. Nicotine metabolism could be impacted by several factors including but not limited to sex, age, genetic polymorphism, race, ethnicity, diet, certain health conditions and use of certain medications [186]. Age and sex dependent effect on nicotine metabolism has been explained extensively in the later part of this paper.
Excretion:
The elimination half-life of nicotine ranges from 100 to 150 minutes. Around 15% of nicotine is excreted in the urine as unchanged involving glomerular filtration and tubular secretion. The rate of renal clearance is about 35–90 ml/min. Moreover, a small amount of nicotine is excreted through feces and sweat. Metabolites of nicotine including nornicotine, cotinine, nicotine-n-oxide, nicotine glucuronide are also excreted in urine. Hukkanen et al reported that approximately 10–15% of nicotine and its metabolites are excreted in urine as 4-oxo-4-(3-pyridyl)butanoic acid and 4-hydroxy-4-(3-pyridyl)butanoic acid [186].
6.2. Nicotine pharmacodynamics:
Nicotine is structurally similar to endogenous neurotransmitter acetylcholine (Ach) and binds to nicotinic acetylcholine receptors (nAchRs).The nAchRs containing α4 and β2 subunits are the most abundant and particularly the β2 containing nAchR mediates reinforcing effects of nicotine [196, 197]. The β2 subunit is crucial for nicotine-mediated DA release and behavioral responses including nicotine self-administration, conditioned reinforcement, conditioned place preference (CPP), and locomotor activity [197]. Consistent upregulation of β2 nAchRs in brain by nicotine has been observed in some seminal preclinical and postmortem human studies [198]. Higher β2 nAchR in the cortex and striatum has been found in smokers when they imaged at 7–9 days of smoking abstinence compared to non-smokers [199, 200].
Nicotine-induced actions at nAChRs in the brain can be either excitatory or inhibitory depending on the site of action. The neurotransmitters dopamine (DA), glutamate, and GABA mediate nicotine’s effects through a complex network of projections between brain regions key in reward-seeking behavior, learning and memory, emotion, and daily function [184]. Long term exposure to nicotine from tobacco smoke use is associated with physiological dependence and tolerance due to receptor desensitization [201]. In receptor desensitization, agonist-dependent confirmational change from active to inactive site happens and the receptor loses its sensitivity to agonist. It has been reported that day-long continued nicotine exposure at low concentration results in strong, deep desensitization of primarily the β2-type nAchRs [202]. On contrary, the α7 nAChRs do not get desensitized with the low concentrations of plasma nicotine in smokers due to their low affinity for nicotine [203]. This chronic desensitization results in nAchRs dysregulation which plays a pivotal role in development and maintenance of nicotine addiction [202]. Upregulation of nAChR number has been observed in the brain autopsy reports of smokers [204].
Moreover, abrupt removal of agonist stimulation at nAChRs leads to reduced level of neurotransmitter release [194] and alteredhomeostasis. Smoking cessation in long-term tobacco users often demonstrates mild but uncomfortable withdrawal syndrome including but not limited to restlessness, irritability, insomnia, anxiety, depression, loss of appetite and cognitive dysfunction at its worst within the first week of abstinence, however it eventually disappears over a period of weeks [184].
The mesolimbic DA system, including the DA neurons of the ventral tegmental area (VTA) is a target for addictive drugs, projecting to the nucleus accumbens (NAc) of the ventral striatum [205–208]. A single dose of nicotine induces synaptic plasticity that increases the activity of VTA DA neurons [205, 207, 209, 210]. Hence, dopamine (DA) and dopaminergic pathways play a crucial role in reinforcing effects of nicotine. It has been reported that β2-nAChRs on DA neurons gets activated by acute nicotine administration which results in DA release within the mesolimbic DA system [211]. The extent of ventral striatal DA release is associated with the pleasurable response to nicotine in humans [212]. On the other hand, selective lesions of DA projections to the nucleus accumbens (NAc) or nAChR antagonists administration into the ventral tegmental area (VTA) blocks nicotine-induced behavior [211] suggesting the pivotal role of DA system on nicotine action. Moreover, DA D2/D3 receptor availability as well as increased DA released has been measured in in-vivo using PET imaging [213].
7. Factors impacting nicotine pharmacokinetics and pharmacodynamics:
Several factors have been identified affecting nicotine pharmacokinetics, specifically metabolism and nicotine pharmacodynamics. Physiological factors (diet, meal, sex, age), use of medications (inducer, inhibitor), smoking behavior, race and ethnic difference, genetic polymorphism, and certain medical condition impact metabolism and clearance of nicotine [185]. Among these abovementioned factors, sex and age play a crucial role in nicotine pharmacokinetics and pharmacodynamics.
8. Role of sex on nicotine pharmacokinetics and pharmacodynamics:
8.1. Role of sex on nicotine pharmacokinetics:
Sex differences have been observed in several studies related to body weight, plasma volume, plasma protein levels, gastric emptying time, cytochrome P450 enzyme activity, drug transporter, renal and excretion activity which ultimately affect nicotine pharmacokinetics [214]. Nicotine brain levels were found to rise faster in female CD rats compared to males following nicotine injection, prominently in the cortex suggesting that female rats are more sensitive to nicotine than male rats [215, 216]. Urinary nicotine metabolite profile also revealed sex differences between male and female rats [216, 217]. Lower plasma cotinine levels were observed in female rats compared to males as higher urinary recoveries of nicotine and higher urinary output of nicotine metabolites were observed in females and males respectively, suggesting a decreased nicotine metabolism rate and larger volume of distribution in females. Moreover, faster nicotine metabolism was reported in male rats following an acute intravenous (IV) injection [218]. However, chronic nicotine administration showed contradictory results in some studies. Chronic nicotine administration by subcutaneous (SC) injection for 15 days (0.6 mg/kg) demonstrated the same level of cotinine in male and female Sprague-Dawley rats although lower levels of nicotine were measured in females compared to males, indicating the shorter half-life of nicotine in females [219]. Future controlled studies are needed to determine the impact of sex on nicotine brain bioavailability and PK to understand the apparent sex differences observed in nicotine addiction.
Role of hormones:
Nicotine is metabolized by cytochrome P450 enzymes, predominantly CYP 2A6. The higher the activity of CYP2A6, the faster nicotine will be metabolized, which is related to high nicotine dependance [220]. Activities of CYP2A6 and CYP2B6 have been found higher in female compared to male, demonstrating the potential role of female sex hormones on nicotine metabolism. This hypothesis has been tested to determine the effects of pregnancy, contraceptives, and menopause on nicotine metabolism
Pregnancy:
Accelerated metabolism of nicotine and cotinine was observed in pregnant smokers [221]. Following infusion of deuterium-labeled nicotine and cotinine in 10 healthy pregnant smokers, both renal and non-renal clearance of nicotine and cotinine were measured from blood and urine samples. Increased clearance of nicotine and cotinine (60% and 140%) and shorter half-life of cotinine were found during the second half of pregnancy compared to that observed postpartum. These data suggest that increased smoking might be expected during pregnancy to compensate for elevated nicotine elimination. However, no change was observed regarding nicotine intake between pregnancy and postpartum stage suggesting either that smoking during pregnancy is not influenced by nicotine elimination or that other factors also contribute to smoking rates during pregnancy [221]. Another study, which included pregnant smokers (who could not quit heavy smoking during/after the first trimester), demonstrated a pharmacokinetic predisposition to a higher rate of nicotine metabolism [222]. Future studies are needed to determine the influence of pregnancy on nicotine metabolism which could influence preferences for certain tobacco products and usage patterns.
Use of oral contraceptives:
Although prevalence of oral contraceptive use is high in premenopausal women, less studies exist testing the relation between oral contraceptives and nicotine. Some studies have reported on oral contraceptive mediated nicotine metabolism, indicating the potential role of estrogen levels on faster metabolism of nicotine [223–225]. The role of oral contraceptives on nicotine metabolism has been observed in a study involving two hundred and seventy-eight volunteers. Nicotine and cotinine metabolism were faster in women taking oral contraceptives compared to the control group who did not take oral contraceptives. Also, among oral contraceptive taking volunteers, accelerated rates of nicotine metabolism were observed in the group who took combined, or only estrogen oral contraceptive compared to only progesterone oral contraceptive users [225]. It has been suggested that higher rates of nicotine metabolism could be associated with intense smoking [225, 226], more rewarding effect [227], greater withdrawal symptoms [228] as well as worse smoking cessation outcomes [229, 230]. These outcomes propose that use of oral contraceptives may increase nicotine metabolism which may result in increased dependency on nicotine and, therefore, have more difficulties achieving abstinence.
Menstrual Cycle:
Endogenous female hormones can regulate nicotine metabolism suggesting the role of menopausal stage on nicotine metabolism. Benowitz et al conducted a study on premenopausal (n= 145), menopausal (n=25) and postmenopausal (n=13) women compared to men. Premenopausal women showed faster metabolism of nicotine compared to men however, men did not show a significant difference compared to menopausal and post-menopausal women. Also, nicotine clearance in premenopausal women did not significantly differ from those in menopausal or postmenopausal women. However, cotinine clearance was found higher in premenopausal women compared to menopausal women [225]. Although this study did not consider the phase of menstrual cycle, another study reported that nicotine or cotinine clearance does not get affected by phase of the menstrual cycle [231]. Moreover, menstrual cycle phase did not affect the hemodynamic response to nicotine, activation of the hypothalamic pituitary adrenal axis or catecholamine secretion response following IV nicotine infusion. In the luteal phase of menstrual cycle, norepinephrine levels were found to be higher however, this did not seem to influence neuroendocrine response to IV nicotine. Hence it has been suggested that different physiological levels of ovarian hormones across the phases of menstrual cycle are not enough to explain the notable increase in women smoking during luteal phase. On the contrary, presence of higher amounts of steroid hormones during pregnancy and in hormone replacement therapy may increase the rate of nicotine metabolism [231]. Another study reported that heavy use of tobacco smoking can reverse the advantageous use of oral estrogens for hot flashes, probably due to elevated clearance of estrogen because of heavy smoking. As higher dose of estrogen can increase breast cancer risk, therefore oral estrogen dose should not be increased to increase the estrogen efficacy in smokers [232].
Thus, gonadal hormone plays a crucial role in nicotine metabolism which is evident in pregnancy, menstruation as well as hormonal therapy suggesting the sex-different effect on nicotine pharmacokinetics.
8.2. Role of sex on nicotine pharmacodynamics:
8.2.1. Nicotinic receptor:
As previously mentioned, nicotine can upregulate the receptor density of nAChRs and comparative upregulation of nAChRs between male and female has been observed in different animal models. Studies have shown that chronic nicotine treatment upregulates nAChRs in the brains of male rats but not in female. However, the observed upregulation in male rats does not persist after a withdrawal period of 20 days. Sex differences in upregulation of nAChRs following chronic treatment with nicotine has been also observed in male mice compared to female mice using a specific radioligand [125I] IPH [233]. However, no sex difference in the upregulation of nAChRs has been observed when rats self-administer nicotine [234] suggesting the possible role of route of nicotine administration in inducing nicotinic receptor regulation in rodents [235].
As stated before, β2-nAChR is crucial for the reinforcing effects of nicotine. Therefore, sex difference in β2-nAChR availability and action may explain sex differences in tobacco smoking behaviors. Several studies have demonstrated the upregulation of β2-nAChRs level in nicotine treated male rodents compared to nicotine naïve counterparts [219, 236]. On the contrary, β2-nAChR upregulation has not been observed in nicotine exposed female rodents [237]. It has been demonstrated that a higher level of β2-nAChR was present in male smokers in the striatum, cortex, and cerebellum compared to male non-smokers, whereas female smokers have similar β2-nAChR density compared to non-smokers. These results suggest one possible neurochemical explanation for the sex difference in treatment response using nicotine replacement therapy (NRT). Interestingly, higher efficacy of NRT has been observed in male regarding smoking cessation compared to female [238]. As smoking-induced β2-nAChRs upregulation has been observed in men compared to women, it is likely that NRT may help to reduce β2-nAChRs in men down to non-smoker levels over time [239], yielding better smoking cessation efficacy.
8.2.2. DA receptor and 5HT transporters:
Several studies have identified sex differences in the regulatory effects of smoking on the mesolimbic DA system. Lower level of DA D2 receptor availability has been observed in the caudate and putamen of male smokers compared to non-smokers, although female group was not included in that study [240]. Another study reported that male smokers showed downregulation of DA D2/D3 level in the dorsal striatum compared to non-smokers [241]. Interestingly, similar levels of striatal DA D2/D3 availability was observed in both female smokers and female non-smokers [241] suggesting the importance of inclusion of female groups in experimental design. Okita et al reported that female smokers exhibited higher levels of midbrain DA D2/D3 receptor density compared to female non-smokers whereas no difference was observed in midbrain DA D2/D3 receptor availability between male smokers and non-smokers [242]. This study also suggests that upregulation of midbrain DA D2/D3 receptor availability in female smokers may mitigate against the downregulation of striatal DA D2/D3 receptors previously found in male smokers [242]. This helps explain the availability of DA D2/D3 receptor levels but does not describe the function of the DA system. Additionally, changes in DA release during in-vivo smoking was measured by novel PET technique revealing the consistent and rapid upregulation of DA level in ventral striatum of male smoker. On the contrary, no upregulation of DA level was observed in ventral striatum of female smokers [243]. The same study revealed that women respond faster to smoking than men in a discrete subregion of the dorsal putamen [243]. These findings suggest that male tobacco smokers generally exhibit lower D2 availability and higher DA release in the striatum, while female tobacco smokers show higher D2 availability in the midbrain and lower DA release in the striatum [239]. Future, controlled studies are needed to determine the influence of sex on dopamine reward pathways in smokers.
In addition, smoking can increase brain serotonin (5HT) levels and may alter expression and function of 5HT transporters. Sex hormones can modulate 5HT transporters indicating the role of sex difference on transporter function in smokers. Single photon emission computed tomography (SPECT) and [123I] β-CIT were used to label DA and 5HT transporters in brain where smokers showed higher uptake of [123I] β-CIT compared to non-smokers. Higher uptake in the striatum (10%), diencephalon (15%), and brainstem (15%) have been observed in females compared to males, regardless of smoking status. Although brainstem uptake was found to be 20% higher in male smokers and only 5% in female smokers compared to nonsmokers, sex and smoking interaction was not significant. These results demonstrate higher availability of DA and 5-HT transporter in women relative to men and no overall smoking effect with the exception of a moderate elevation in brainstem 5-HT transporters in male smokers. Brainstem 5-HT transporters may be regulated by smoking in a sex-specific manner [244].
8.2.3. Locomotor activity:
Nicotine effects on the DA system are associated with altered locomotor activity. It has been observed long before that nicotine stimulated locomotor activity in females however no effect was observed in males [245]. The impact of nicotine on locomotor activity involves both stimulant and depressant action [246]. Extracellular DA level has been found to be increased in the nucleus accumbens (NAc) following systemically administered nicotine [247]. Moreover, nicotine infusions into the ventral tegmental area resulted in enhanced locomotor activation [248] and this locomotor activity can be lessened by lesioning the ascending mesolimbic DA pathway [249]. These studies confirm the association of nicotine induced locomotor activity through the dopaminergic system and this dopaminergic system is controlled by hormonal environment. Studies have shown that estrogens and progesterone can regulate the function of DA systems in a complex manner. Estrogens can also increase nicotine-induced DA release in striatal slices in the brains of ovariectomized female rats [250]. Several studies have investigated the effects of sex and ovarian hormone on the nicotine effect on locomotor activity. Kanyt et al reported about acute and chronic effect of nicotine on locomotor activity in male, female, and ovariectomized hooded rats in series of experiments. This study showed that female rats experienced higher locomotor activity compared to male rates. Acute administration of nicotine reduced locomotor activity which was found slightly higher in female rats than male. Daily nicotine administration for 21 days resulted in similar and gradual increase in locomotor activity in both male and female [251]. In another experiment of the same study, ovariectomized rats were primed with 17-β-estradiol and progesterone. Acute administration of nicotine showed reduced activity in both groups in similar way. However, chronic nicotine administration for 21 days produced enhanced activity as a function of both chronic nicotine and hormonal priming. This study clearly suggests the role ovarian hormones on the chronic locomotor-activating effect of nicotine [251]. Other studies have reported significant reduction in spontaneous locomotor activity in nicotine treated female group compared to male [252, 253]. Also, an earlier study reported that lower sensitivity to nicotine’s motor depressant effects was observed in females compared to males [254].
Some studies have examined the effect of nicotine on locomotor activity during adolescent periods including both sexes. Higher locomotor activity was observed in female rats compared to male rats at low dose of nicotine injection [255]. However, Faraday et al reported contradictory results demonstrating higher locomotor activity in adolescent males compared to females when single dose nicotine was administered with minipumps [256]. Another study demonstrated that lower dose of nicotine administration caused decreased locomotor activity in female than male, using osmotic mini pumps [257].
Even though the nicotine-exposed adolescent demonstrated contradictory results, it is apparent that females are more sensitive to the nicotine induced locomotor activity compared to male, and most likely, ovarian hormones play a role in this greater responsivity. Future, experimental design should also focus on acute vs. chronic nicotine dosing when interpreting effects on locomotor activity.
8.2.4. Cognitive effects:
The nAChRs are present in different brain region including cortex, striatum, and ventral tegmental area which are critical for cognitive function and addiction [235]. Usually, cognitive function is similar in male and female humans however, task dependent sex differences have been observed [258]. Cognitive task performance depends not only on cognitive ability but also on cognitive style and behavioral strategy. Moreover, cognitive style or strategy can be affected by several factors including age, sex, hormonal influences and pharmacological manipulations [235]. Several studies have demonstrated sex difference in cognitive tasks and problem-solving strategies [259–261]. Klingenberg et al reported that males follow impulsive-global strategy whereas females prefer a reflective and sequential task-solving strategy which made them slower but accurate in problem solving [260]. Several studies have explored the effect of sex and nicotine on cognitive function. Algan et al reported that smoking has a gender-specific effect on cognitive function. Better verbal task performance was observed in males while increased subjective confidence was found in females, thus impacting the preferred cognitive strategies for problem solving [262]. Alteration of ‘no-response’ was observed in female due to smoking. Female nonsmokers showed higher no-response rate than males, while female smokers responded to almost all the stimuli presented in both verbal and spatial cognitive tasks. However, no smoking-effect was observed on the ‘no-response’ rate in males, suggesting the impact of smoking on females, such that their approach to the solution of a problem was modified [262]. Another study on rats showed sex difference effects of nicotine on cognitive function and strategy. This study measured cognitive function using water maze involving both navigational and visual cues. Without nicotine exposure, female rats showed different strategies using visual cues to find the platform than males, however nicotine treated female group exhibited a more male-like strategy to find the platform suggesting the nicotine effect on shifting strategy in female rats in problem solving and cognitive task [263].
In addition to altering cognitive strategy, enhanced performance in cognitive tasks by nicotine exposure was observed in several studies using both sexes. Yilmaz et al demonstrated that cognitive function in rats can be improved by nicotine during the acquisition phase of active avoidance learning trials in a dose-dependent manner. It was observed that male rats benefited from nicotine at all doses tested, whereas female rates experienced deteriorated learning performance at the higher dose, indicating the effect of nicotine on active avoidance in a sex and dose-dependent manner [264]. A recent cohort study involving around 70,000 participant also reported that smoking is associated with higher learning performance in women compared to men suggesting the role of nicotine in sex-difference on verbal learning and memory function [265].
Surprisingly, tobacco abstinence has also showed sex-difference effects on memory and cognitive function. A pilot study comprised of 25 participants (moderate to heavy smokers) examined how 24 hours tobacco abstinence affects cognitive function in male and female. Tobacco abstinence significantly deteriorated memory performance under full attention conditions for males but not for females, suggesting possible sex differences in the cognitive effects of tobacco abstinence [266].
The studies summarized above suggest an apparent sex-difference impact of nicotine on cognitive function. However, other factors including type of test, route of nicotine administration, species, strains, and age of animals should be considered to evaluate sex-difference effect of nicotine on cognitive and memory function.
8.2.5. Smoking cessation and withdrawal:
There are several studies focused on sex differences in smoking cessation and withdrawal involving several factors [267, 268]. Although tobacco smoking prevalence has been declining over the decades, interestingly this decline is less prominent in female compared to male. Some studies reported that women faced more difficulties in smoking cessation compared to men in clinical trials [269, 270]. Women are observed to be less successful and maintain abstinence for a shortened period of time compared to men [235]. The sex-difference regarding smoking cessation becomes even more evident with nicotine replacement therapy. It has been suggested in several studies that nicotine can be less reinforcing relative to nonpharmacological aspects of smoking [271–273]. Poorer pharmacotherapy outcome has been reported in women compared to men, which can be related to nicotine addiction as well as depression in women [274]. However, the reasons behind sex differences are yet to be elucidated. Figure 1 summarizes the impact of sex on pharmacokinetics and pharmacodynamics of nicotine.
Figure 1:
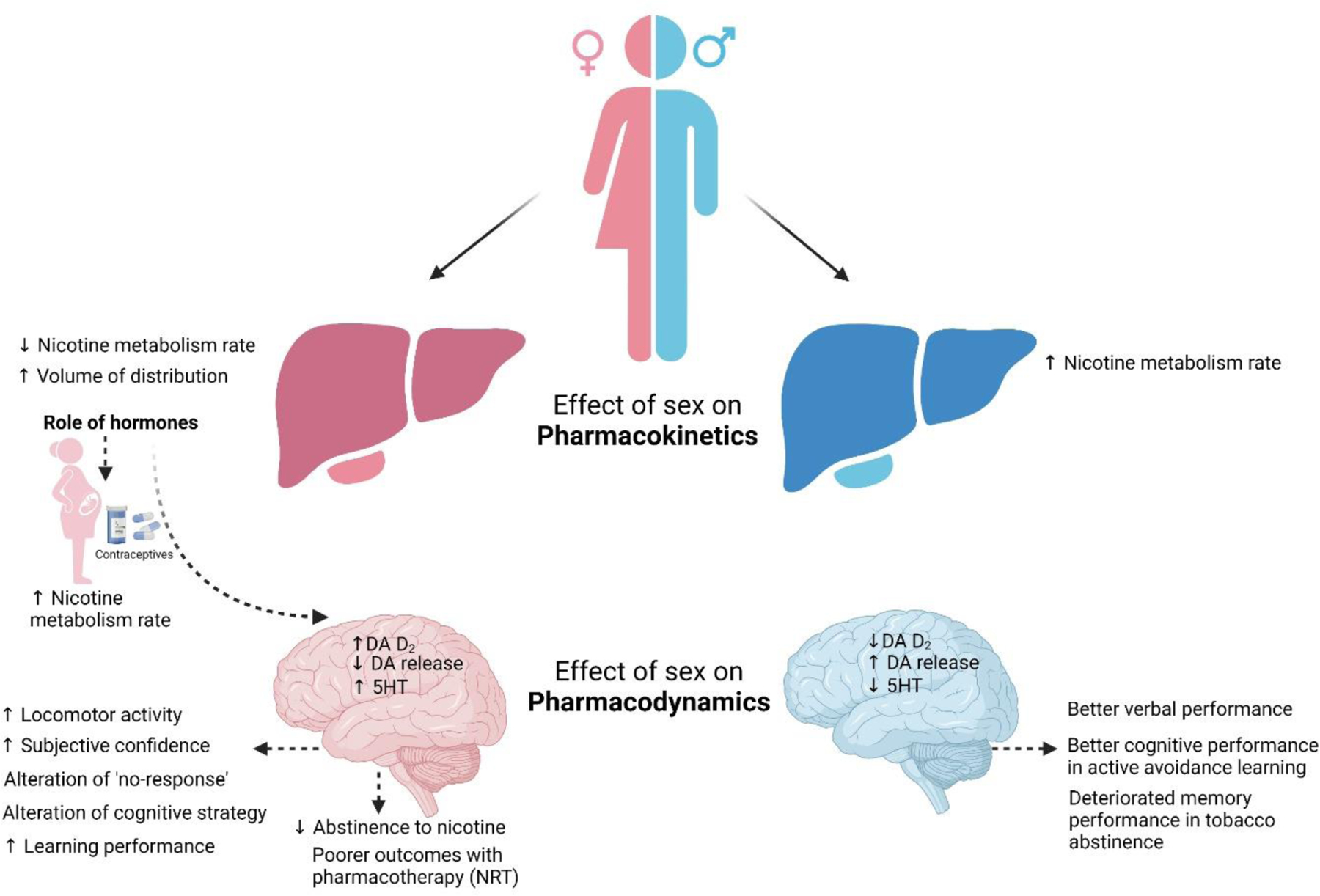
Role of sex on pharmacokinetics and pharmacodynamics of nicotine. (DA: Dopamine, DA D2: Dopamine receptor 2, 5 HT: 5 Hydroxytryptamine or serotonin, NRT: nicotine replacement therapy)
9. Role of age on nicotine pharmacokinetics and pharmacodynamics:
9.1. Role of age on nicotine pharmacokinetics:
Age related physiological changes can alter pharmacokinetic processes in several ways which ultimately affect drug availability and action. Pharmacokinetic parameters including absorption [275], distribution [276, 277], excretion [278], and metabolism [279] can be altered by age. Nicotine is no exception of this. Studies have shown that age has significant effect on nicotine pharmacokinetics. Reduced clearance of nicotine has been observed in elderly people (> 65 age) [277] and neonates [280]. However, no differences in nicotine pharmacokinetics have been observed between human adolescents and adults [281, 282]. On the contrary, lower level of plasma nicotine and cotinine was observed in adolescent rats compared to adult rats [283]. Craig et al., investigated the effects of age (adolescence vs adult) on nicotine pharmacokinetics and subsequently the plasma and brain levels of nicotine and its metabolites [284]. Nicotine was administered by SC or IV route to early adolescent and adult rats. Lower levels of nicotine, metabolites cotinine, and nicotine-19-N-oxide in plasma and brain of early adolescent rats compared to adult rats were found at different time points following SC nicotine administration which resulted in lower area under the plasma concentration-time curve. Similar results were observed following IV nicotine administration, suggesting a substantial age difference in nicotine pharmacokinetics [284]. The changes in pharmacokinetics of nicotine depends on age related changes in absorption, distribution, metabolism, and elimination.
Distribution:
Body mass composition plays an important role in nicotine distribution and this parameter changes with age. Younger animals have a higher ratio of lean to fatty mass than older animals [285]. As nicotine primarily distributes into lean mass, therefore a change in this body mass composition due to age could potentially affect nicotine’s volume of distribution [277]. For instance, as adolescent animals tend to have primarily lean body mass, they would be expected to have a larger volume of distribution, relative to their body weight, for nicotine, resulting in lower plasma nicotine levels [284, 286]. Moreover, decreased volume of distribution at steady state is observed in the elderly subjects and could be due to age-related decrease in lean body mass [287, 288].
Hepatic clearance:
Age is considered as an important factor affecting CYP2A6 activity. Different studies have reported that CYP2A6 activity increases with increased age [289, 290]. Johnstone et al demonstrated that metabolic ration (3OH/COT) was marginally increased with age [291]. Increased CYP2A6 activity with age has been observed in in-vitro study using microsomes [292]. However, some studies have reported contradictory results on effect of age on nicotine metabolism. Surprisingly, it has been observed that nicotine clearance was decreased in elderly (age > 65) compared to adults [293]. In case of newborn, prolonged elimination half-life of nicotine but not cotinine was found compared to adult, suggesting the lower CYP2A6 activity or different specificity in newborn [294]. Studies have also suggested decreased renal clearance, non-renal clearance, and total clearance of nicotine in elderly population compared to adults. Since around 80% of nicotine is cleared by hepatic metabolism [295], lower non-renal clearance suggests a decreased hepatic extraction of nicotine in the elderly population. Reduced hepatic extraction could be due to reduced hepatic blood flow in elderly subjects. Moreover, liver volume and mass also decrease with age [296]. However, nicotine is a high extraction ration drug and hence it is speculated that its clearance is less affected by hepatic enzyme expression and activity [185]. Therefore, the faster clearance of nicotine observed in younger animals could be due to increased blood flow in liver [297].
Renal clearance:
Nicotine is excreted by renal clearance which involves glomerular filtration and tubular secretion with varying levels of absorption, based on urinary pH. Age has been found to associated with reduced renal function. Glomerular filtration rate [298, 299] and tubular function [300] gradually decline with age. Lower nicotine dependance (ND) was observed in aged (> 65) population compared to young and middle-aged population. Decreased total clearance (23%) and renal clearance (49%) in elderly resulted in lower nicotine metabolism contributing to reduction of urge to smoke and decreased nicotine dependance [301]. As renal clearance accounts for only 5% of total nicotine clearance in humans [185] therefore, a change in renal function will only have a limited effect on nicotine disposition [277]. However, decreased glomerular filtration rate and reduced urinary flow could explain reduced renal clearance of nicotine in elderly populations [277]. Furthermore, increased mRNA expression of renal transporter has been observed in early adolescent rats [302]. As protein expression of transporters changes with age, it is expected to observe the age-dependent differences in distribution and elimination of systemically available substrates as well [302]. Increased nicotine clearance indicating the lower nicotine levels in plasma of adolescents compared to adult can explain the age related alteration of nicotine elimination [284].
In summary, the studies presented above highlight the importance of age as a factor contributing to differences in nicotine pharmacokinetics which may result in altered nicotine pharmacodynamics and addiction rates (see Figure 2).
Figure 2:
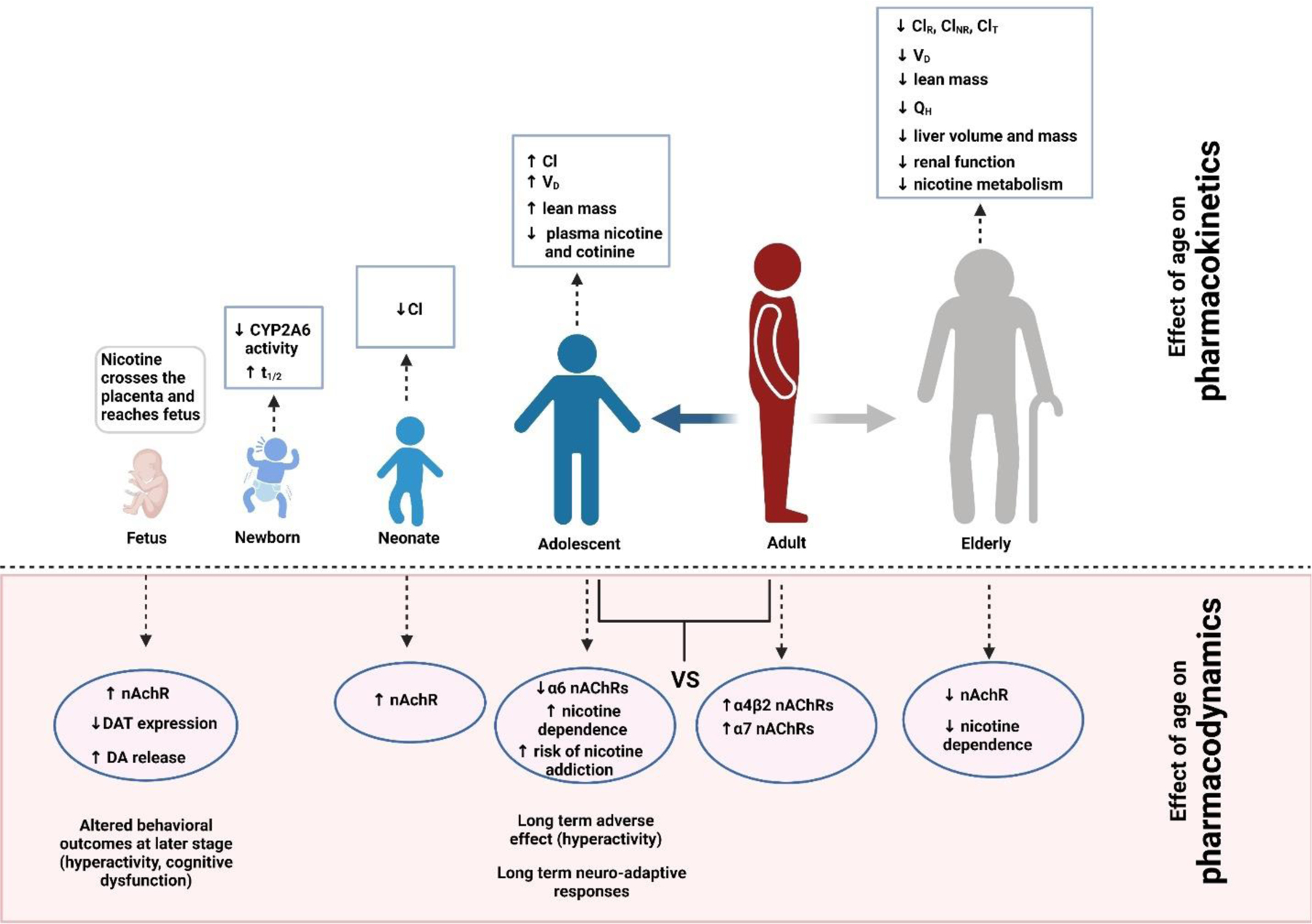
Role of age on pharmacokinetics and pharmacodynamics of nicotine. Pharmacokinetic parameters at different ages (newborn, neonates, adolescent, and elderly) are compared with adult stage. (t1/2: elimination half-life, Cl: Clearance, VD: Volume of distribution, ClR: Renal clearance, ClNR: Non-renal or hepatic clearance, ClT: Total clearance, QH: Hepatic clearance). Pharmacodynamics of nicotine has been compared between adolescent and adult as well as elderly and adult mainly (nAchR: Nicotinic acetyl choline receptor, DA: Dopamine, DAT: Dopamine transporter)
9.2. Role of age on nicotine pharmacodynamics:
9.2.1. Nicotinic receptor:
Neuronal nicotinic acetylcholine receptors (nAChRs) are associated with different neurophysiological process including learning and memory function [303–305]. Several studies have demonstrated the age-related decline of nicotinic receptor expression in the central nervous system resulting in various types of dementia [306]. Postmortem studies on Alzheimer’s patients reported significant reduction (25–70%) in regional nAChR expression at different stages of the disease that correlate with decline in cognitive function [307]. However, some studies have reported increase, decrease or no change in high affinity nicotine binding in brain with age depending on the brain region studied [308–316]. Age related decline in high affinity binding has been observed in frontal cortex, temporal cortex, entorhinal cortex and hippocampus in several studies [316]. However, no age-related difference in nAChR high affinity binding has been observed in thalamus and cerebellum [310, 312–314].
Age dependent nicotine induced alteration in nAChR activity has been observed in different studies. Increased level of high-affinity nicotinic receptor binding in brain has been observed in adult animals following chronic nicotine administration [317]. Likewise, elevated levels of brain nicotinic receptors were found in human smokers compared to same age nonsmokers [204].
Maternal smoking during pregnancy has detrimental effects on neonatal health including but not limited to low birth weight, higher perinatal mortality rate, long term effects on physical, emotional, and intellectual growth of child, learning difficulties, cognitive dysfunction, and hyperactivity [318–323]. A recent study from our lab has also demonstrated that prenatal exposure to e-cig containing 2.4% nicotine decreases brain glucose utilization and worsens outcome in offspring hypoxic–ischemic brain injury [324]. Several studies using rat have demonstrated that chronic exposure of pregnant rats to nicotine resulted in upregulation of nAChRs in fetal and neonatal brain [325–327]. Similar results have been observed in studies using mice [328]. This altered level of nAChRs can result in altered behavioral outcomes at later stage.
Adolescence is the most vulnerable stage of human life and nicotine exposure through TS and e-cig may induce long-term effects. Recent studies have shown that e-cig has been become extremely popular among youth in US. The most popular e-cig device among youth and adolescent are refillable cartridges or pods. It has been reported that, in 2020, about half of e-cig users in high school use prefilled pods or cartridges [329]. Adolescence is a time of significant brain development, and exposure to nicotine during this period is associated with higher subsequent rates of dependence. Nicotine has been demonstrated to cause long-term adverse effects on adolescent rat brain, including altered proliferation, differentiation, synaptic activity, synaptic maturation, and increased cell damage and cell death [330–332]. Adolescent rats differ from adult rats in their nicotine-induced behavioral responses [333, 334] and the effects of adolescent nicotine exposure can persist into adulthood. Pre-exposure to nicotine during adolescence, sensitizes rats to nicotine effects on conditioned place preference and locomotion during adulthood [335]. Also, several recent studies have shown that pretreatment with nicotine during adolescence sensitizes rats to the rewarding effects of other drugs [336–338]. Comparative study involving chronic nicotine exposure by osmotic minipump in adult and periadolescent rats demonstrated the elevated level of α4β2 nAChRs was prominent and widespread in adult animals whereas in periadolescents, up-regulation of α4β2 occurred in fewer regions at lesser extent. Similarly, in case of α7 receptors, adults were found to be more responsive than periadolescents to nicotine-induced upregulation. In adult animals, chronic nicotine exposure did not cause upregulation of α6 nAChRs; binding was downregulated in three regions. Unlike the other subtypes, the response of α6 nAChRs to chronic nicotine was greater in periadolescents, with more regions showing greater down-regulation compared to adults. These differences in receptor expression and regulation between age groups are likely to be important given the unique vulnerability of adolescents to nicotine-induced behavioral changes and susceptibility to drug abuse [339].
Chronic nicotine treatment to adult mice also demonstrates dose-dependent elevation of nicotinic receptors [340–342]. These studies reported that chronic nicotine treatment resulted in dose-dependent increases in [3H] nicotine binding that vary somewhat among brain regions. The increase in binding is reversible in that levels of binding return to control approximately seven days after chronic infusion is stopped [340]. Chronic nicotine administration also results in tolerance to many of nicotine’s behavioral and physiological effects.
9.2.2. DA receptor:
The dopamine (DA) system of the ventral midbrain plays a crucial role as mammals learn adaptive behaviors driven by environmental salience and reward. Nicotine has significant impact on the mesolimbic DA system by activating and desensitizing nicotinic acetylcholine receptors (nAChRs) in a subtype-dependent manner as explained before. Nicotine induced synaptic plasticity at excitatory synapses onto DA neurons increases DA signals and participates during the reinforcement of addictive behaviors. As humans and animals at different developmental ages are vulnerable to the drug-induced effects, several clinical and epidemiological studies suggest a higher risk of addiction among adolescents involving age-dependent sensitivity to nicotine. One of the mechanisms to explain this is drug-induced synaptic plasticity at excitatory synapses onto the dopamine neurons in the ventral midbrain. Nicotine mediated glutamatergic transmission differences are expressed as an increased ratio of AMPA receptors to NMDA receptors at glutamatergic synapses. Also, age-dependent effects in the excitability and the nicotine sensitivity in the DA system of midbrain may contribute to the higher risk of nicotine addiction in adolescent animals and humans [343].
9.2.3. Locomotor activity:
Nicotine induced locomotor activity or hyperactivity has been observed in different age groups in different studies. An association between gestational exposure to nicotine or tobacco smoke and attention deficit hyperactivity disorder (ADHD) in children has been reported in many studies [344–349]. Hyperactive offspring that were prenatally exposed to nicotine also exhibited a significant increase in cortical receptor densities without changes in binding affinity, demonstrating the role of upregulated cortical nicotinic receptor on hyperactivity in offspring [350]. Another study reported that nicotine induced hyperactivity appears to be mediated by central nicotine receptors, possibly located on dopaminergic neurons, and also requires the activation of both D1 and D2 dopamine receptors [351].
Also, a recent study showed paternal nicotine exposure led to hyperactivity in the offspring [352]. This study reported that nicotine exposure induced a rise in the total DNA methylation level of Dat in murine speratozoa, and the hyper-methylation could transmit in the offspring mice brains. These epigenetic modifications also decreased the expression of DAT in the brain of the offspring, resulting in increased level of extracellular dopamine. The D2 receptors activation resulted in dephosphorylation of AKT, which led to increase in activation of GSK3α/β, and ultimately resulted in hyperactivity in the offspring mice [352].
Adolescence is a vulnerable stage of life when there is a high chance of tobacco dependence. Several factors are associated with increased risk of adolescent smoking, including low socioeconomic status, peer smoking, parental smoking, risk taking, and comorbid psychopathology [353]. Psychopathologies associated with adolescent smoking include anxiety disorders, depressive disorders, conduct disorders and attention-deficit hyperactivity disorder (ADHD) [354]. Several studies have demonstrated an age difference effect of nicotine on locomotor activity comparing adolescence and adult. Chronically administered nicotine (via osmotic minipump) had greater activity-stimulating effects in adolescent male rats than in adult male rats, and nicotine exposure resulted in long-term hyperactivity (in the absence of nicotine) in adolescent males but not in adult males [355]. Another study reported that initial nicotine exposure in adolescence (0.50 and 1.0 mg/kg), but not in adulthood, resulted in hyperactivity in adulthood in the absence of nicotine (interim phase) suggesting the age differences in nicotine sensitivity that could predispose individuals initially exposed to nicotine in adolescence to long-term smoking [356].
9.2.4. Cognitive effects:
Association of nicotine with alteration of behavioral outcomes has been confirmed in several studies. Prenatal smoking during pregnancy is reported to be harmful for postnatal cognitive function. Several epidemiological studies have shown that prenatal nicotine administration or exposure is directly associated with cognitive deficits in children [318, 322, 357–362]. Similarly, animal models of in utero exposure to nicotine have demonstrated behavioral, neurochemical, and cognitive abnormalities in offspring [350, 363–373]. The effects of nicotine are most likely mediated by nAChRs that are present in fetal brain by mid-gestation and are believed to play a critical role in neuronal differentiation and development [328, 372, 374–376]. Torres et al reported that exposure of neonatal mice to tobacco smoke disrupts synaptic proteins and spatial learning and memory function from late infancy to early adulthood [377]. Comparative studies between adolescent and adult rats showed different behavioral outcomes [378]. This study demonstrates that adolescent rats did not show signs of nicotine-cue conditioning and exhibited less robust sensitization to the locomotor effects of nicotine compared to adults. In addition, adolescent rats showed increased ambulation, and less sensitivity to the depressant effects of nicotine than adults. Initial exposure to nicotine resulted in increased sensitivity to the motor-activating effects of nicotine but less sensitivity to the depressant effects of nicotine in rearing in adolescents. These results suggest that adolescent animals can display different long-term neuroadaptive responses to nicotine compared to adult animals, possibly related to immature or still-developing plasticity mechanisms in the prefrontal cortex [378]. Persistent and delayed behavioral changes were also observed in adolescent rats after nicotine administration [379]. These studies clearly demonstrated the long-term effect of nicotine exposure in age-dependent manner.
9.2.5. Smoking cessation and withdrawal:
It is evident that success of smoking cessation depends on the characteristics of the smokers. Therefore, it is extremely important to understand the differences among major sub-groups so that effective smoking cessation therapies can be designed [380]. Smoking cessation success has been found to be related to tobacco dependance including the number of cigarettes smoked per day and age of smoking initiation. Initiation of smoking at early age has been found to be related to lower rate of smoking cessation [381–383]. Early initiation is also associated with higher relapse rate after smoking cessation [384]. Therefore, young adolescents have been reported to develop nicotine dependence after shorter smoking duration [385]. It has been also found that smokers with higher nicotine dependence are less likely to quit smoking. Hence, successful smoking cessation has been observed in older age who had lower nicotine dependence, and poor health condition [386]. Figure 2 has demonstrated the effect of age-difference on pharmacodynamics of nicotine.
10. Conclusion
In conclusion, preclinical and clinical studies suggest a strong role of sex and age as biological determinants that alter brain function, behavior, and metabolic systems of our body. Sex-different and age-dependent effects have also been observed in central nervous system pathologies, including alterations in the BBB, nicotinic acetylcholine receptors, and serotonin and dopamine systems leading to differences in several cerebrovascular and neurological dysfunction. Sex and age based biological determinants appear to have a strong influence on nicotine neurotoxicity. Hence, it is expected that the pharmacokinetics and pharmacodynamics of nicotine may vary depending on sex and age. Although several reports have shown the detrimental effects of nicotine exposure in animals and humans, some age and sex dependent discrepancies exist in these studies which need to be followed up by appropriate research design including sex and age as biological determinants. Sexually dimorphic pharmacokinetics can cause variance in nicotine concentration in blood and brain which may ultimately affect nicotine action. Gonadal hormone induced CYP450 activity increases nicotine metabolism in female during pregnancy and oral contraceptive use, suggesting the increased dependency on nicotine and lower abstinence characteristics in female. Differences in nAChRs and DA system between male and female also explain the variances in nicotine action on addiction, reward, hyperactivity as well as cognitive function. Higher renal and non-renal clearance of nicotine in young populations can help explain the urge of smoking and nicotine dependence in this age group. Differences in nAChR and DA expression and regulation in adolescents clarify the susceptibility of adolescents to nicotine-induced long-term behavioral changes (hyperactivity, cognitive dysfunction) and susceptibility to drug abuse. In addition, prenatal nicotine exposure also causes long term effects on postnatal hyperactivity, learning and memory function, suggesting some detrimental effects of nicotine which transmits from generation to generation. Therefore, it is imperative to consider the impact of sex and age for evaluating the neurotoxic effects of nicotine on several neurodegenerative and cerebrovascular diseases. Additionally, due to the noticeable growth in nicotine containing e-cigarette usage among the younger population and lack of data related to its harmful impact on health, it has also become crucial to focus research on e-cigarette product usage considering biological determinants like sex and age.
Highlights:
Nicotine is associated with cerebrovascular and neurological impairments
Sex and age can impact nicotine pharmacokinetics and pharmacodynamics
Sex and age are key biological determinants that can impact nicotine neurotoxicity
Acknowledgments
Funding:
This work was supported by the National Institutes of Health National Institute of Neurologic Disorders and Stroke [R01NS106879] and National Institute on Drug Abuse [R01DA049737, R01DA029121].
List of Abbreviations:
- TS
tobacco smoke
- e-cig
electronic cigarette
- COPD
chronic obstructive pulmonary disease
- CDC
centers for disease control and prevention
- BBB
blood-brain barrier
- NVU
neurovascular unit
- AJ
adherens junction
- TJ
tight junction
- ZO
zona occludin
- nAchRs
nicotinic acetylcholine receptors
- Na+
sodium ion
- Ca2+
calcium ion
- K+
potassium ion
- GABA
gamma-aminobutyric acid
- 5HT
5-hydroxytryptamine or serotonin
- VTA
ventral tegmental area
- DA
dopamine
- CNS
central nervous system
- mHB
medial habenula
- IPN
interpeduncular nucleus
- iPSC
induced pluripotent stem cell
- BMEC
brain microvascular endothelial cells
- TEER
trans-endothelial electrical resistance
- PECAM-1
platelet endothelial cell adhesion molecule
- JAMA
junction adhesion molecule A
- BDNF
brain-derived neurotrophic factor
- CD31
cluster of differentiation 31
- EDH
endothelial derived hyperpolarization
- GPCR
G-protein coupled receptor
- NO
nitric oxide
- GFAP
glial fibrillary acidic protein
- MS
multiple sclerosis
- TBI
traumatic brain injury
- AD
Alzheimer’s disease
- PD
Parkinson’s disease
- AIDS
acquired immunodeficiency syndrome
- HIV
human immunodeficiency virus
- Nrf2
nuclear factor-erythroid factor 2-related factor 2
- TNF-α
tumor necrosis factor α
- ROS
reactive oxygen species
- MMPs
matrix metalloproteinases
- CYP450 (2A6, 2B6)
cytochrome P450 enzyme
- CPP
conditioned place preference
- PET
positron emission tomography
- SPECT
single photon emission computed tomography
- NAc
nucleus accumbens
- D2
dopamine receptor 2
- IV
intravenous route
- SC
subcutaneous route
- NRT
nicotine replacement therapy
- ND
nicotine dependance
- mRNA
messenger RNA
- AMPA
α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid
- NMDA
N-methyl-D-aspartic acid
- ADHD
attention deficit hyperactivity disorder
- DNA
deoxyribonucleic acid
- DAT
dopamine transporter
- AKT
protein kinase B or PKB
- ClR
renal clearance
- ClNR
non-renal or hepatic clearance
- ClT
total clearance
- QH
hepatic blood flow
- t1/2
elimination half life
- VD
volume of distribution
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of Competing Interest:
The authors report no declarations of interest.
Conflicts of Interest:
The authors declare no conflicts of interest.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper
References:
- 1.(CDC), C.f.D.C.a.P. Smoking and Tobacco Use:Fast Facts. 2021. [cited 2021 8th September]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm.
- 2.(CDC), C.f.D.C.a.P. Tips From Former Smokers. 2021. [cited 2021 8th September]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/fast_facts/index.htm.
- 3.(WHO), W.H.O. WHO Report on the Global Tobacco Epidemic. 2017. [cited 2021 8th September]. [Google Scholar]
- 4.(NIDA), N.I.o.D.A. Substance Use in Women Research Report. 2021. [cited 2021 9th September]; Available from: https://www.drugabuse.gov/publications/research-reports/substance-use-in-women/summary.
- 5.National Center for Chronic Disease, P., S. Health Promotion Office on, and Health, Reports of the Surgeon General, in The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General 2014, Centers for Disease Control and Prevention (US): Atlanta (GA). [Google Scholar]
- 6.Bhalerao A, et al. , Public Health Policies on E-Cigarettes. Current Cardiology Reports, 2019. 21(10): p. 111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Melikian AA, et al. , Gender differences relative to smoking behavior and emissions of toxins from mainstream cigarette smoke. Nicotine Tob Res, 2007. 9(3): p. 377–87. [DOI] [PubMed] [Google Scholar]
- 8.Cosgrove KP, et al. , Sex differences in the brain’s dopamine signature of cigarette smoking. The Journal of neuroscience : the official journal of the Society for Neuroscience, 2014. 34(50): p. 16851–16855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Torres OV and O’Dell LE, Stress is a principal factor that promotes tobacco use in females. Progress in neuro-psychopharmacology & biological psychiatry, 2016. 65: p. 260–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Thun MJ, et al. , 50-year trends in smoking-related mortality in the United States. N Engl J Med, 2013. 368(4): p. 351–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Farley TM, et al. , Combined oral contraceptives, smoking, and cardiovascular risk. Journal of epidemiology and community health, 1998. 52(12): p. 775–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cornelius ME WT, Jamal A, Loretan CG, Neff LJ. . Tobacco Product Use Among Adults — United States, 2019 2020. [cited 2021 9th September]; Available from: https://www.cdc.gov/mmwr/volumes/69/wr/mm6946a4.htm?s_cid=mm6946a4_w#References [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.(CDC), C.f.D.C.a.P. Youth and Tobacco Use. 2020. [cited 2021 9th September]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/youth_data/tobacco_use/index.htm. [Google Scholar]
- 14.West R, Tobacco smoking: Health impact, prevalence, correlates and interventions. Psychology & health, 2017. 32(8): p. 1018–1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Whittington JR, et al. , The Use of Electronic Cigarettes in Pregnancy: A Review of the Literature. Obstet Gynecol Surv, 2018. 73(9): p. 544–549. [DOI] [PubMed] [Google Scholar]
- 16.(CDC), C.f.D.C.a.P. Smoking and Tobacco Use: Current Cigarette Smoking Among Adults in the United States 2020. [cited 2021 9th September]; Available from: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm.
- 17.Villarroel MA, Cha AE, and Vahratian A, Electronic Cigarette Use Among U.S. Adults, 2018. NCHS Data Brief, 2020(365): p. 1–8. [PubMed] [Google Scholar]
- 18.Pogun S, et al. , Sex differences in nicotine preference. Journal of Neuroscience Research, 2017. 95(1–2): p. 148–162. [DOI] [PubMed] [Google Scholar]
- 19.Obisesan OH, et al. , E-Cigarette Use Patterns and High-Risk Behaviors in Pregnancy: Behavioral Risk Factor Surveillance System, 2016–2018. American journal of preventive medicine, 2020. 59(2): p. 187–195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kapaya M DAD, Tong VT, et al. Use of Electronic Vapor Products Before, During, and After Pregnancy Among Women with a Recent Live Birth — Oklahoma and Texas, 2015 2019. [cited 2021 9th September]; Available from: https://www.cdc.gov/mmwr/volumes/68/wr/mm6808a1.htm#suggestedcitation. [DOI] [PMC free article] [PubMed]
- 21.Drake P DA, Mathews TJ Cigarette smoking during pregnancy: United States, 2016 2018. [cited 2021 9th September]; Available from: https://www.cdc.gov/nchs/products/databriefs/db305.htm. [PubMed] [Google Scholar]
- 22.(CDC), C.f.D.C.a.P. Smoking and Tobacco Use: About Electronic Cigarettes (E-Cigarettes) 2021. [cited 2021 9th September]; Available from: https://www.cdc.gov/tobacco/basic_information/e-cigarettes/about-e-cigarettes.html.
- 23.Polesskaya OO, et al. , Nicotine causes age-dependent changes in gene expression in the adolescent female rat brain. Neurotoxicology and Teratology, 2007. 29(1): p. 126–140. [DOI] [PubMed] [Google Scholar]
- 24.Chang YP and Seo YS, E-cigarette use and concurrent risk behaviors among adolescents. Nurs Outlook, 2021. 69(3): p. 302–310. [DOI] [PubMed] [Google Scholar]
- 25.Wang TW, et al. , Disposable E-Cigarette Use among U.S. Youth — An Emerging Public Health Challenge. New England Journal of Medicine, 2021. 384(16): p. 1573–1576. [DOI] [PubMed] [Google Scholar]
- 26.MacDonald A and Middlekauff HR, Electronic cigarettes and cardiovascular health: what do we know so far? Vascular health and risk management, 2019. 15: p. 159–174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tjakra M, et al. , Overview of crosstalk between multiple factor of transcytosis in blood brain barrier. Frontiers in neuroscience, 2020. 13: p. 1436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bhalerao A, et al. , In vitro modeling of the neurovascular unit: advances in the field. Fluids and Barriers of the CNS, 2020. 17(1): p. 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wolburg H, et al. , Brain endothelial cells and the glio-vascular complex. Cell and tissue research, 2009. 335(1): p. 75–96. [DOI] [PubMed] [Google Scholar]
- 30.Abbott NJ, et al. , Structure and function of the blood–brain barrier. Neurobiology of disease, 2010. 37(1): p. 13–25. [DOI] [PubMed] [Google Scholar]
- 31.Bell AH, et al. , The neurovascular unit: Effects of brain insults during the perinatal period. Frontiers in neuroscience, 2020. 13: p. 1452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.T Ronaldson P and P Davis T, Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Current pharmaceutical design, 2012. 18(25): p. 3624–3644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Abbott NJ, Rönnbäck L, and Hansson E, Astrocyte–endothelial interactions at the blood–brain barrier. Nature reviews neuroscience, 2006. 7(1): p. 41–53. [DOI] [PubMed] [Google Scholar]
- 34.Sá-Pereira I, Brites D, and Brito MA, Neurovascular unit: a focus on pericytes. Molecular neurobiology, 2012. 45(2): p. 327–347. [DOI] [PubMed] [Google Scholar]
- 35.Engelhardt B, Development of the blood-brain interface. Blood-brain barriers, 2006. 1: p. 11–39. [Google Scholar]
- 36.Butt AM, Jones HC, and Abbott NJ, Electrical resistance across the blood‐brain barrier in anaesthetized rats: a developmental study. The Journal of physiology, 1990. 429(1): p. 47–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Archie SR, Al Shoyaib A, and Cucullo L, Blood-Brain Barrier Dysfunction in CNS Disorders and Putative Therapeutic Targets: An Overview. Pharmaceutics, 2021. 13(11). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu J, Understanding of nicotinic acetylcholine receptors. Acta Pharmacologica Sinica, 2009. 30(6): p. 653–655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Clarke PB, et al. , Nicotinic binding in rat brain: autoradiographic comparison of [3H]acetylcholine, [3H]nicotine, and [125I]-alpha-bungarotoxin. J Neurosci, 1985. 5(5): p. 1307–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Séguéla P, et al. , Molecular cloning, functional properties, and distribution of rat brain alpha 7: a nicotinic cation channel highly permeable to calcium. J Neurosci, 1993. 13(2): p. 596–604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zoli M, et al. , Identification of four classes of brain nicotinic receptors using beta2 mutant mice. J Neurosci, 1998. 18(12): p. 4461–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Grady SR, et al. , Rodent habenulo-interpeduncular pathway expresses a large variety of uncommon nAChR subtypes, but only the alpha3beta4* and alpha3beta3beta4* subtypes mediate acetylcholine release. J Neurosci, 2009. 29(7): p. 2272–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scholze P, et al. , Subunit composition of α5-containing nicotinic receptors in the rodent habenula. J Neurochem, 2012. 121(4): p. 551–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shih PY, et al. , Differential expression and function of nicotinic acetylcholine receptors in subdivisions of medial habenula. J Neurosci, 2014. 34(29): p. 9789–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brunzell DH, McIntosh JM, and Papke RL, Diverse strategies targeting α7 homomeric and α6β2* heteromeric nicotinic acetylcholine receptors for smoking cessation. Ann N Y Acad Sci, 2014. 1327(1): p. 27–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Weber CM and Clyne AM, Sex differences in the blood–brain barrier and neurodegenerative diseases. APL Bioengineering, 2021. 5(1): p. 011509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lippmann ES, et al. , Derivation of blood-brain barrier endothelial cells from human pluripotent stem cells. Nature Biotechnology, 2012. 30(8): p. 783–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wimmer I, et al. , PECAM-1 Stabilizes Blood-Brain Barrier Integrity and Favors Paracellular T-Cell Diapedesis Across the Blood-Brain Barrier During Neuroinflammation. Frontiers in Immunology, 2019. 10: p. 711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kamm DR, The Effects of Diet and Sex Differences on Cortical Tight-Junction Protein Expression in Senescence-Accelerated Mouse-Prone 8 (SAMP8) Mice 2019, Southern Illinois University at Edwardsville: Ann Arbor. p. 49. [Google Scholar]
- 50.Cipolla MJ, The Cerebral Circulation, Second Edition. Colloquium Series on Integrated Systems Physiology: From Molecule to Function, 2016. 8(1): p. 1–80. [Google Scholar]
- 51.Holder SM, et al. , Relationship Between Endothelial Function and the Eliciting Shear Stress Stimulus in Women: Changes Across the Lifespan Differ to Men. Journal of the American Heart Association, 2019. 8(4): p. e010994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Huang A, et al. , Gender difference in flow-induced dilation and regulation of shear stress: role of estrogen and nitric oxide. American Journal of Physiology-Regulatory, Integrative and Comparative Physiology, 1998. 275(5): p. R1571–R1577. [DOI] [PubMed] [Google Scholar]
- 53.Reckelhoff JF, Gender Differences in the Regulation of Blood Pressure. Hypertension, 2001. 37(5): p. 1199–1208. [DOI] [PubMed] [Google Scholar]
- 54.Shore AC, Sandeman DD, and Tooke JE, Capillary pressure, pulse pressure amplitude, and pressure waveform in healthy volunteers. American Journal of Physiology-Heart and Circulatory Physiology, 1995. 268(1): p. H147–H154. [DOI] [PubMed] [Google Scholar]
- 55.Hakim MA, et al. , Aging Alters Cerebrovascular Endothelial GPCR and K+ Channel Function: Divergent Role of Biological Sex. The Journals of Gerontology: Series A, 2020. 75(11): p. 2064–2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Bauersachs J, Popp R, Hecker M, Sauer E, Fleming I, and Busse R. Nitric oxide attenuates the release of endothelium-derived hyperpolarizing factor. Circulation, 1996. 94: p. 3341–3347. [DOI] [PubMed] [Google Scholar]
- 57.Min J, 17β-Estradiol-stimulated eNOS gene transcriptional activation is regulated through the estrogen-responsive element in eNOS promoter. Biotechnology and Bioprocess Engineering, 2007. 12(4): p. 446–449. [Google Scholar]
- 58.Erdő F, Denes L, and de Lange E, Age-associated physiological and pathological changes at the blood-brain barrier: A review. Journal of cerebral blood flow and metabolism : official journal of the International Society of Cerebral Blood Flow and Metabolism, 2017. 37(1): p. 4–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hunziker O, et al. , Quantitative studies in the cerebral cortex of aging humans. Gerontology, 1978. 24(1): p. 27–31. [DOI] [PubMed] [Google Scholar]
- 60.Hicks P, et al. , Age-related changes in rat brain capillaries. Neurobiol Aging, 1983. 4(1): p. 69–75. [DOI] [PubMed] [Google Scholar]
- 61.Burns EM, Kruckeberg TW, and Gaetano PK, Changes with age in cerebral capillary morphology. Neurobiol Aging, 1981. 2(4): p. 283–91. [DOI] [PubMed] [Google Scholar]
- 62.Grammas P, Martinez J, and Miller B, Cerebral microvascular endothelium and the pathogenesis of neurodegenerative diseases. Expert Rev Mol Med, 2011. 13: p. e19. [DOI] [PubMed] [Google Scholar]
- 63.Elahy M, et al. , Blood-brain barrier dysfunction developed during normal aging is associated with inflammation and loss of tight junctions but not with leukocyte recruitment. Immun Ageing, 2015. 12: p. 2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Enciu A-M, Gherghiceanu M, and Popescu BO, Triggers and effectors of oxidative stress at blood-brain barrier level: relevance for brain ageing and neurodegeneration. Oxidative medicine and cellular longevity, 2013. 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Candiello J, Cole GJ, and Halfter W, Age-dependent changes in the structure, composition and biophysical properties of a human basement membrane. Matrix Biol, 2010. 29(5): p. 402–10. [DOI] [PubMed] [Google Scholar]
- 66.Ravens JR, Vascular changes in the human senile brain. Adv Neurol, 1978. 20: p. 487–501. [PubMed] [Google Scholar]
- 67.Rodríguez-Arellano JJ, et al. , Astrocytes in physiological aging and Alzheimer’s disease. Neuroscience, 2016. 323: p. 170–82. [DOI] [PubMed] [Google Scholar]
- 68.Harris JL, Choi IY, and Brooks WM, Probing astrocyte metabolism in vivo: proton magnetic resonance spectroscopy in the injured and aging brain. Front Aging Neurosci, 2015. 7: p. 202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Chisholm NC and Sohrabji F, Astrocytic response to cerebral ischemia is influenced by sex differences and impaired by aging. Neurobiol Dis, 2016. 85: p. 245–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Middeldorp J and Hol EM, GFAP in health and disease. Prog Neurobiol, 2011. 93(3): p. 421–43. [DOI] [PubMed] [Google Scholar]
- 71.Stewart PA, et al. , A quantitative analysis of blood-brain barrier ultrastructure in the aging human. Microvasc Res, 1987. 33(2): p. 270–82. [DOI] [PubMed] [Google Scholar]
- 72.Sturrock RR, A comparative quantitative and morphological study of ageing in the mouse neostriatum, indusium griseum and anterior commissure. Neuropathol Appl Neurobiol, 1980. 6(1): p. 51–68. [DOI] [PubMed] [Google Scholar]
- 73.Blau CW, et al. , The age-related deficit in LTP is associated with changes in perfusion and blood-brain barrier permeability. Neurobiol Aging, 2012. 33(5): p. 1005.e23–35. [DOI] [PubMed] [Google Scholar]
- 74.Lucke-Wold BP, et al. , Aging, the metabolic syndrome, and ischemic stroke: redefining the approach for studying the blood-brain barrier in a complex neurological disease. Adv Pharmacol, 2014. 71: p. 411–49. [DOI] [PubMed] [Google Scholar]
- 75.Cerbai F, et al. , The neuron-astrocyte-microglia triad in normal brain ageing and in a model of neuroinflammation in the rat hippocampus. PLoS One, 2012. 7(9): p. e45250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Buschini E, et al. , Age related macular degeneration and drusen: neuroinflammation in the retina. Prog Neurobiol, 2011. 95(1): p. 14–25. [DOI] [PubMed] [Google Scholar]
- 77.Kettenmann H, et al. , Physiology of microglia. Physiol Rev, 2011. 91(2): p. 461–553. [DOI] [PubMed] [Google Scholar]
- 78.Ronaldson PT and Davis TP, Blood-brain barrier integrity and glial support: mechanisms that can be targeted for novel therapeutic approaches in stroke. Curr Pharm Des, 2012. 18(25): p. 3624–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Health, U.D.o. and H. Services, The health consequences of smoking—50 years of progress: a report of the Surgeon General 2014, Atlanta, GA: US Department of Health and Human Services, Centers for Disease; …. [Google Scholar]
- 80.Morgan JC, et al. , How people think about the chemicals in cigarette smoke: a systematic review. Journal of behavioral medicine, 2017. 40(4): p. 553–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Cataldo JK, Prochaska JJ, and Glantz SA, Cigarette smoking is a risk factor for Alzheimer’s Disease: an analysis controlling for tobacco industry affiliation. Journal of Alzheimer’s disease, 2010. 19(2): p. 465–480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Sharma S, et al. , Repurposing metformin to treat age-related neurodegenerative disorders and ischemic stroke. Life Sciences, 2021: p. 119343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Sivandzade F, et al. , The cerebrovascular and neurological impact of chronic smoking on post-traumatic brain injury outcome and recovery: an in vivo study. Journal of neuroinflammation, 2020. 17(1): p. 1–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Salzer J, et al. , Smoking as a risk factor for multiple sclerosis. Multiple Sclerosis Journal, 2013. 19(8): p. 1022–1027. [DOI] [PubMed] [Google Scholar]
- 85.Bhalerao A and Cucullo L, Impact of tobacco smoke in HIV progression: a major risk factor for the development of NeuroAIDS and associated CNS disorders. Journal of Public Health, 2020. 28(3): p. 259–270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kaisar MA, et al. , Offsetting the impact of smoking and e-cigarette vaping on the cerebrovascular system and stroke injury: Is Metformin a viable countermeasure? Redox biology, 2017. 13: p. 353–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Hawkins BT, et al. , Nicotine increases in vivo blood–brain barrier permeability and alters cerebral microvascular tight junction protein distribution. Brain research, 2004. 1027(1–2): p. 48–58. [DOI] [PubMed] [Google Scholar]
- 88.Choi S, Krishnan J, and Ruckmani K, Cigarette smoke and related risk factors in neurological disorders: an update. Biomedicine & Pharmacotherapy, 2017. 85: p. 79–86. [DOI] [PubMed] [Google Scholar]
- 89.Sifat AE, et al. , Nicotine and electronic cigarette (E‐Cig) exposure decreases brain glucose utilization in ischemic stroke. Journal of neurochemistry, 2018. 147(2): p. 204–221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Kaisar MA, et al. , Conventional and electronic cigarettes dysregulate the expression of iron transporters and detoxifying enzymes at the brain vascular endothelium: In vivo evidence of a gender-specific cellular response to chronic cigarette smoke exposure. Neuroscience letters, 2018. 682: p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Sifat AE, et al. , Neurovascular unit transport responses to ischemia and common coexisting conditions: Smoking and diabetes. American Journal of Physiology-Cell Physiology, 2019. 316(1): p. C2–C15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Alberg AJ, Shopland DR, and Cummings KM, The 2014 Surgeon General’s report: commemorating the 50th Anniversary of the 1964 Report of the Advisory Committee to the US Surgeon General and updating the evidence on the health consequences of cigarette smoking. American journal of epidemiology, 2014. 179(4): p. 403–412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Paulson JR, et al. , Nicotine exacerbates brain edema during in vitro and in vivo focal ischemic conditions. Journal of pharmacology and experimental therapeutics, 2010. 332(2): p. 371–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Abdullahi W, Tripathi D, and Ronaldson PT, Blood-brain barrier dysfunction in ischemic stroke: targeting tight junctions and transporters for vascular protection. American Journal of Physiology-Cell Physiology, 2018. 315(3): p. C343–C356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Paulson JR, et al. , Tobacco smoke chemicals attenuate brain-to-blood potassium transport mediated by the Na, K, 2Cl-cotransporter during hypoxia-reoxygenation. Journal of Pharmacology and Experimental Therapeutics, 2006. 316(1): p. 248–254. [DOI] [PubMed] [Google Scholar]
- 96.Abbruscato TJ, et al. , Regulation of blood-brain barrier Na, K, 2Cl-cotransporter through phosphorylation during in vitro stroke conditions and nicotine exposure. Journal of pharmacology and experimental therapeutics, 2004. 310(2): p. 459–468. [DOI] [PubMed] [Google Scholar]
- 97.Rodu B and Plurphanswat N, E-cigarette use among US adults: population assessment of tobacco and health (PATH) study. Nicotine and Tobacco Research, 2018. 20(8): p. 940–948. [DOI] [PubMed] [Google Scholar]
- 98.Shah KK, Boreddy PR, and Abbruscato TJ, Nicotine pre-exposure reduces stroke-induced glucose transporter-1 activity at the blood–brain barrier in mice. Fluids and Barriers of the CNS, 2015. 12(1): p. 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Abbruscato TJ, et al. , Nicotine and cotinine modulate cerebral microvascular permeability and protein expression of ZO-1 through nicotinic acetylcholine receptors expressed on brain endothelial cells. Journal of pharmaceutical sciences, 2002. 91(12): p. 2525–2538. [DOI] [PubMed] [Google Scholar]
- 100.Herson PS, Palmateer J, and Hurn PD, Biological sex and mechanisms of ischemic brain injury. Translational stroke research, 2013. 4(4): p. 413–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Suzuki S, Brown CM, and Wise PM, Mechanisms of neuroprotection by estrogen. Endocrine, 2006. 29(2): p. 209–215. [DOI] [PubMed] [Google Scholar]
- 102.Banerjee A, et al. , Phenotypic changes in immune cell subsets reflect increased infarct volume in male vs. female mice. Translational stroke research, 2013. 4(5): p. 554–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Carswell H, Dominiczak A, and Macrae I, Estrogen status affects sensitivity to focal cerebral ischemia in stroke-prone spontaneously hypertensive rats. American Journal of Physiology-Heart and Circulatory Physiology, 2000. 278(1): p. H290–H294. [DOI] [PubMed] [Google Scholar]
- 104.Liu F, et al. , Changes in experimental stroke outcome across the life span. Journal of Cerebral Blood Flow & Metabolism, 2009. 29(4): p. 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Gaignard P, et al. , Effect of sex differences on brain mitochondrial function and its suppression by ovariectomy and in aged mice. Endocrinology, 2015. 156(8): p. 2893–2904. [DOI] [PubMed] [Google Scholar]
- 106.Mirza MA, et al. , Sexually dimorphic outcomes and inflammatory responses in hypoxic-ischemic encephalopathy. Journal of neuroinflammation, 2015. 12(1): p. 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Gupta NC, et al. , Soluble epoxide hydrolase: sex differences and role in endothelial cell survival. Arteriosclerosis, thrombosis, and vascular biology, 2012. 32(8): p. 1936–1942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Liu M, et al. , Role of P450 aromatase in sex-specific astrocytic cell death. Journal of Cerebral Blood Flow & Metabolism, 2007. 27(1): p. 135–141. [DOI] [PubMed] [Google Scholar]
- 109.Roy-O’Reilly M and McCullough LD, Age and Sex Are Critical Factors in Ischemic Stroke Pathology. Endocrinology, 2018. 159(8): p. 3120–3131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Appelros P, Nydevik I, and Viitanen M, Poor outcome after first-ever stroke: predictors for death, dependency, and recurrent stroke within the first year. Stroke, 2003. 34(1): p. 122–126. [DOI] [PubMed] [Google Scholar]
- 111.Bots SH, Peters SA, and Woodward M, Sex differences in coronary heart disease and stroke mortality: a global assessment of the effect of ageing between 1980 and 2010. BMJ global health, 2017. 2(2): p. e000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Towfighi A, et al. , A midlife stroke surge among women in the United States. Neurology, 2007. 69(20): p. 1898–1904. [DOI] [PubMed] [Google Scholar]
- 113.Rambaud T, et al. , Acute ischemic stroke in adolescents. Neurology, 2020. 94(2): p. e158. [DOI] [PubMed] [Google Scholar]
- 114.Singhal AB, et al. , Recognition and management of stroke in young adults and adolescents. Neurology, 2013. 81(12): p. 1089–1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Sharma R, et al. , Infections after a traumatic brain injury: The complex interplay between the immune and neurological systems. Brain, behavior, and immunity, 2019. 79: p. 63–74. [DOI] [PubMed] [Google Scholar]
- 116.Dewan MC, et al. , Estimating the global incidence of traumatic brain injury. Journal of neurosurgery, 2018. 130(4): p. 1080–1097. [DOI] [PubMed] [Google Scholar]
- 117.Mendes Arent A, et al. , Perspectives on molecular biomarkers of oxidative stress and antioxidant strategies in traumatic brain injury. BioMed research international, 2014. 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Durazzo TC, et al. , The influence of chronic cigarette smoking on neurocognitive recovery after mild traumatic brain injury. Journal of neurotrauma, 2013. 30(11): p. 1013–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Bayir H, et al. , Marked gender effect on lipid peroxidation after severe traumatic brain injury in adult patients. Journal of neurotrauma, 2004. 21(1): p. 1–8. [DOI] [PubMed] [Google Scholar]
- 120.Ley EJ, et al. , Gender impacts mortality after traumatic brain injury in teenagers. Journal of trauma and acute care surgery, 2013. 75(4): p. 682–686. [DOI] [PubMed] [Google Scholar]
- 121.Späni CB, Braun DJ, and Van Eldik LJ, Sex-related responses after traumatic brain injury: Considerations for preclinical modeling. Frontiers in neuroendocrinology, 2018. 50: p. 52–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Skolnick BE, et al. , A clinical trial of progesterone for severe traumatic brain injury. New England Journal of Medicine, 2014. 371(26): p. 2467–2476. [DOI] [PubMed] [Google Scholar]
- 123.Wright DW, et al. , Very early administration of progesterone for acute traumatic brain injury. New England Journal of Medicine, 2014. 371(26): p. 2457–2466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Biswas RK, Kabir E, and King R, Effect of sex and age on traumatic brain injury: a geographical comparative study. Archives of public health, 2017. 75(1): p. 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Kraus JF, Peek-Asa C, and McArthur D, The independent effect of gender on outcomes following traumatic brain injury: a preliminary investigation. Neurosurgical focus, 2000. 8(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 126.Mychasiuk R, et al. , The direction of the acceleration and rotational forces associated with mild traumatic brain injury in rodents effect behavioural and molecular outcomes. Journal of neuroscience methods, 2016. 257: p. 168–178. [DOI] [PubMed] [Google Scholar]
- 127.Edwards P, et al. , CRASH trial collaborators. Final results of MRC CRASH, a randomised placebo-controlled trial of intravenous corticosteroid in adults with head injury-outcomes at 6 months. Lancet, 2005. 365(9475): p. 1957–1959. [DOI] [PubMed] [Google Scholar]
- 128.Davis DP, et al. , Traumatic brain injury outcomes in pre-and post-menopausal females versus age-matched males. Journal of neurotrauma, 2006. 23(2): p. 140–148. [DOI] [PubMed] [Google Scholar]
- 129.Arambula SE, et al. , Sex differences in pediatric traumatic brain injury. Experimental Neurology, 2019. 317: p. 168–179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Carroll L, et al. , Prognosis for mild traumatic brain injury: results of the WHO Collaborating Centre Task Force on Mild Traumatic Brain Injury. Journal of rehabilitation medicine, 2004. 36(0): p. 84–105. [DOI] [PubMed] [Google Scholar]
- 131.Biswas RK, Kabir E, and King R, Effect of sex and age on traumatic brain injury: a geographical comparative study. Archives of Public Health, 2017. 75(1): p. 43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Ilie G, et al. , Adolescent traumatic brain injuries: Onset, mechanism and links with current academic performance and physical injuries. PloS one, 2020. 15(3): p. e0229489–e0229489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Reeder KP, et al. , Impact of age on functional outcome following traumatic brain injury. The Journal of Head Trauma Rehabilitation, 1996. [Google Scholar]
- 134.Cifu DX, et al. , Functional outcomes of older adults with traumatic brain injury: a prospective, multicenter analysis. Archives of physical medicine and rehabilitation, 1996. 77(9): p. 883–888. [DOI] [PubMed] [Google Scholar]
- 135.Frankel JE, et al. , A follow-up study of older adults with traumatic brain injury: taking into account decreasing length of stay. Archives of physical medicine and rehabilitation, 2006. 87(1): p. 57–62. [DOI] [PubMed] [Google Scholar]
- 136.(CDC), C.f.D.C.a.P. Rates of TBI-related Emergency Department Visits by Sex — United States, 2001–2010 2016. [cited 2022 20th January]; Available from: https://www.cdc.gov/traumaticbraininjury/data/rates_ed_bysex.html. [Google Scholar]
- 137.Almeida OP, et al. , Smoking is associated with reduced cortical regional gray matter density in brain regions associated with incipient Alzheimer disease. The American Journal of Geriatric Psychiatry, 2008. 16(1): p. 92–98. [DOI] [PubMed] [Google Scholar]
- 138.Moreno-Gonzalez I, et al. , Smoking exacerbates amyloid pathology in a mouse model of Alzheimer’s disease. Nature communications, 2013. 4(1): p. 1–10. [DOI] [PubMed] [Google Scholar]
- 139.Li R and Singh M, Sex differences in cognitive impairment and Alzheimer’s disease. Frontiers in neuroendocrinology, 2014. 35(3): p. 385–403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Vina J and Lloret A, Why women have more Alzheimer’s disease than men: gender and mitochondrial toxicity of amyloid-β peptide. Journal of Alzheimer’s disease, 2010. 20(s2): p. S527–S533. [DOI] [PubMed] [Google Scholar]
- 141.Dye RV, et al. , Hormone replacement therapy and risk for neurodegenerative diseases. International journal of Alzheimer’s Disease, 2012. 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Carter CL, et al. , Sex and gender differences in Alzheimer’s disease: recommendations for future research. Journal of women’s health, 2012. 21(10): p. 1018–1023. [DOI] [PubMed] [Google Scholar]
- 143.Cui J, Shen Y, and Li R, Estrogen synthesis and signaling pathways during aging: from periphery to brain. Trends in molecular medicine, 2013. 19(3): p. 197–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Galea LA, et al. , Sex, hormones and neurogenesis in the hippocampus: hormonal modulation of neurogenesis and potential functional implications. Journal of neuroendocrinology, 2013. 25(11): p. 1039–1061. [DOI] [PubMed] [Google Scholar]
- 145.Singh M, et al. , Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. Journal of Neuroscience, 1999. 19(4): p. 1179–1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Anastasio TJ, Exploring the contribution of estrogen to amyloid-Beta regulation: a novel multifactorial computational modeling approach. Frontiers in pharmacology, 2013. 4: p. 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Li R, et al. , Early reproductive experiences in females make differences in cognitive function later in life. Journal of Alzheimer’s Disease, 2013. 34(3): p. 589–594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.National Institute on Aging, N. What Causes Alzheimer’s Disease? 2019. [cited 2022 15th January]; Available from: https://www.nia.nih.gov/health/what-causes-alzheimers-disease.
- 149.Nakamura T, et al. , Effect of cigarette smoking on plasma metalloproteinase-9 concentration. Clinica chimica acta, 1998. 276(2): p. 173–177. [DOI] [PubMed] [Google Scholar]
- 150.Tomassini V and Pozzilli C, Sex hormones: a role in the control of multiple sclerosis? Expert Opinion on Pharmacotherapy, 2006. 7(7): p. 857–868. [DOI] [PubMed] [Google Scholar]
- 151.Nicot A, Gender and sex hormones in multiple sclerosis pathology and therapy. Frontiers in bioscience (Landmark edition), 2009. 14: p. 4477–4515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Keith AB, Effect of pregnancy on experimental allergic encephalomyelitis in guinea pigs and rats. J Neurol Sci, 1978. 38(3): p. 317–26. [DOI] [PubMed] [Google Scholar]
- 153.McClain MA, et al. , Pregnancy suppresses experimental autoimmune encephalomyelitis through immunoregulatory cytokine production. J Immunol, 2007. 179(12): p. 8146–52. [DOI] [PubMed] [Google Scholar]
- 154.Brenner T, Evron S, and Abramsky O, Effect of experimental autoimmune encephalomyelitis on pregnancy: studies in rabbits and rats. Isr J Med Sci, 1991. 27(4): p. 181–5. [PubMed] [Google Scholar]
- 155.Mertin LA and Rumjanek VM, Pregnancy and the susceptibility of Lewis rats to experimental allergic encephalomyelitis. J Neurol Sci, 1985. 68(1): p. 15–24. [DOI] [PubMed] [Google Scholar]
- 156.Elenkov IJ, et al. , IL-12, TNF-α, and hormonal changes during late pregnancy and early postpartum: implications for autoimmune disease activity during these times. The Journal of Clinical Endocrinology & Metabolism, 2001. 86(10): p. 4933–4938. [DOI] [PubMed] [Google Scholar]
- 157.Confavreux C and Vukusic S, Age at disability milestones in multiple sclerosis. Brain, 2006. 129(3): p. 595–605. [DOI] [PubMed] [Google Scholar]
- 158.Cossburn M, et al. , Age at onset as a determinant of presenting phenotype and initial relapse recovery in multiple sclerosis. Multiple Sclerosis Journal, 2012. 18(1): p. 45–54. [DOI] [PubMed] [Google Scholar]
- 159.Sanai SA, et al. , Aging and multiple sclerosis. Multiple Sclerosis Journal, 2016. 22(6): p. 717–725. [DOI] [PubMed] [Google Scholar]
- 160.Roy S, et al. , Differential effects of aging on motor and cognitive functioning in multiple sclerosis. Multiple Sclerosis Journal, 2017. 23(10): p. 1385–1393. [DOI] [PubMed] [Google Scholar]
- 161.Pizza V, et al. , Neuroinflamm-aging and neurodegenerative diseases: an overview. CNS & Neurological Disorders-Drug Targets (Formerly Current Drug Targets-CNS & Neurological Disorders), 2011. 10(5): p. 621–634. [DOI] [PubMed] [Google Scholar]
- 162.Dorszewska J, Cell biology of normal brain aging: synaptic plasticity–cell death. Aging clinical and experimental research, 2013. 25(1): p. 25–34. [DOI] [PubMed] [Google Scholar]
- 163.Dendrou CA, Fugger L, and Friese MA, Immunopathology of multiple sclerosis. Nature Reviews Immunology, 2015. 15(9): p. 545–558. [DOI] [PubMed] [Google Scholar]
- 164.Di Benedetto S, et al. , Contribution of neuroinflammation and immunity to brain aging and the mitigating effects of physical and cognitive interventions. Neuroscience & Biobehavioral Reviews, 2017. 75: p. 114–128. [DOI] [PubMed] [Google Scholar]
- 165.Bolton C and Smith PA, The influence and impact of ageing and immunosenescence (ISC) on adaptive immunity during multiple sclerosis (MS) and the animal counterpart experimental autoimmune encephalomyelitis (EAE). Ageing research reviews, 2018. 41: p. 64–81. [DOI] [PubMed] [Google Scholar]
- 166.Clinic C Pediatric Multiple Sclerosis 2020. [cited 2022 15th January]. [Google Scholar]
- 167.Reinhardt K, et al. , Multiple sclerosis in children and adolescents: incidence and clinical picture - new insights from the nationwide German surveillance (2009–2011). Eur J Neurol, 2014. 21(4): p. 654–9. [DOI] [PubMed] [Google Scholar]
- 168.Shapshak P, et al. , Editorial neuroAIDS review. AIDS (London, England), 2011. 25(2): p. 123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Jin M, et al. , A LC-MS/MS method for concurrent determination of nicotine metabolites and role of CYP2A6 in nicotine metabolism in U937 macrophages: implications in oxidative stress in HIV+ smokers. Journal of Neuroimmune Pharmacology, 2012. 7(1): p. 289–299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.McLaurin KA, et al. , Sex matters: robust sex differences in signal detection in the HIV-1 transgenic rat. Frontiers in behavioral neuroscience, 2017. 11: p. 212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Robertson KR, et al. , No gender differences in the progression of nervous system disease in HIV infection. JAIDS Journal of Acquired Immune Deficiency Syndromes, 2004. 36(3): p. 817–822. [DOI] [PubMed] [Google Scholar]
- 172.Royal III W, et al. , Associations between cognition, gender and monocyte activation among HIV infected individuals in Nigeria. PloS one, 2016. 11(2): p. e0147182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.McLaurin KA, Booze RM, and Mactutus CF, Evolution of the HIV-1 transgenic rat: utility in assessing the progression of HIV-1-associated neurocognitive disorders. Journal of neurovirology, 2018. 24(2): p. 229–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Rowson SA, et al. , Neuroinflammation and behavior in HIV-1 transgenic rats exposed to chronic adolescent stress. Frontiers in psychiatry, 2016. 7: p. 102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Griesbeck M, Scully E, and Altfeld M, Sex and gender differences in HIV-1 infection. Clinical Science, 2016. 130(16): p. 1435–1451. [DOI] [PubMed] [Google Scholar]
- 176.Nasi M, et al. , Aging with HIV infection: a journey to the center of inflammAIDS, immunosenescence and neuroHIV. Immunology letters, 2014. 162(1): p. 329–333. [DOI] [PubMed] [Google Scholar]
- 177.Appay V and Sauce D, Immune activation and inflammation in HIV‐1 infection: causes and consequences. The Journal of Pathology: A Journal of the Pathological Society of Great Britain and Ireland, 2008. 214(2): p. 231–241. [DOI] [PubMed] [Google Scholar]
- 178.Streit W, Microglia and the response to brain injury. Neuroinflammation—from bench to bedside, 2002: p. 11–24. [DOI] [PubMed] [Google Scholar]
- 179.Jahanshad N, et al. , Disrupted brain networks in the aging HIV+ population. Brain connectivity, 2012. 2(6): p. 335–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Ettenhofer ML, et al. , Aging, neurocognition, and medication adherence in HIV infection. The American Journal of Geriatric Psychiatry, 2009. 17(4): p. 281–290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Khanlou N, et al. , Increased frequency of α-synuclein in the substantia nigra in human immunodeficiency virus infection. Journal of neurovirology, 2009. 15(2): p. 131–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Anthony IC, et al. , Accelerated Tau deposition in the brains of individuals infected with human immunodeficiency virus-1 before and after the advent of highly active anti-retroviral therapy. Acta neuropathologica, 2006. 111(6): p. 529–538. [DOI] [PubMed] [Google Scholar]
- 183.Wilmshurst JM, et al. , Chapter 8 - NeuroAIDS in children, in Handbook of Clinical Neurology, Brew BJ, Editor. 2018, Elsevier. p. 99–116. [DOI] [PubMed] [Google Scholar]
- 184.McKinney DL and Vansickel AR, Chapter 9 - Nicotine Chemistry, Pharmacology, and Pharmacokinetics, in Neuropathology of Drug Addictions and Substance Misuse, Preedy VR, Editor. 2016, Academic Press: San Diego. p. 93–103. [Google Scholar]
- 185.Benowitz NL, Hukkanen J, and Jacob P 3rd, Nicotine chemistry, metabolism, kinetics and biomarkers. Handbook of experimental pharmacology, 2009(192): p. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Hukkanen J, Jacob P, and Benowitz NL, Metabolism and Disposition Kinetics of Nicotine. Pharmacological Reviews, 2005. 57(1): p. 79. [DOI] [PubMed] [Google Scholar]
- 187.Benowitz NL, et al. , Nicotine absorption and cardiovascular effects with smokeless tobacco use: Comparison with cigarettes and nicotine gum. Clinical Pharmacology & Therapeutics, 1988. 44(1): p. 23–28. [DOI] [PubMed] [Google Scholar]
- 188.Willems EW, et al. , Significance of ammonium compounds on nicotine exposure to cigarette smokers. Food and Chemical Toxicology, 2006. 44(5): p. 678–688. [DOI] [PubMed] [Google Scholar]
- 189.Talih S, et al. , Effect of free-base and protonated nicotine on nicotine yield from electronic cigarettes with varying power and liquid vehicle. Scientific Reports, 2020. 10(1): p. 16263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.McKinney DL, et al. , Evaluation of the Effect of Ammonia on Nicotine Pharmacokinetics Using Rapid Arterial Sampling. Nicotine & Tobacco Research, 2012. 14(5): p. 586–595. [DOI] [PubMed] [Google Scholar]
- 191.Rose JE, et al. , Kinetics of brain nicotine accumulation in dependent and nondependent smokers assessed with PET and cigarettes containing <sup>11</sup>C-nicotine. Proceedings of the National Academy of Sciences, 2010. 107(11): p. 5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Berridge MS, et al. , Smoking produces rapid rise of [11C]nicotine in human brain. Psychopharmacology (Berl), 2010. 209(4): p. 383–94. [DOI] [PubMed] [Google Scholar]
- 193.Yildiz D, Nicotine, its metabolism and an overview of its biological effects. Toxicon, 2004. 43(6): p. 619–632. [DOI] [PubMed] [Google Scholar]
- 194.Benowitz NL, Hukkanen J, and Jacob P, Nicotine Chemistry, Metabolism, Kinetics and Biomarkers, in Nicotine Psychopharmacology, Henningfield JE, London ED, and Pogun S, Editors. 2009, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 29–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Feyerabend C, Ings RM, and Russel MA, Nicotine pharmacokinetics and its application to intake from smoking. British Journal of Clinical Pharmacology, 1985. 19(2): p. 239–247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Benowitz NL, Nicotine addiction. N Engl J Med, 2010. 362(24): p. 2295–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Mineur YS and Picciotto MR, Genetics of nicotinic acetylcholine receptors: Relevance to nicotine addiction. Biochem Pharmacol, 2008. 75(1): p. 323–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Cosgrove KP, et al. , Imaging Tobacco Smoking with PET and SPECT. Curr Top Behav Neurosci, 2015. 24: p. 1–17. [DOI] [PubMed] [Google Scholar]
- 199.Staley JK, et al. , Human tobacco smokers in early abstinence have higher levels of beta2* nicotinic acetylcholine receptors than nonsmokers. J Neurosci, 2006. 26(34): p. 8707–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Esterlis I, et al. , CHRNA4 and ANKK1 Polymorphisms Influence Smoking-Induced Nicotinic Acetylcholine Receptor Upregulation. Nicotine Tob Res, 2016. 18(9): p. 1845–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Benowitz NL, NICOTINE ADDICTION. Primary Care: Clinics in Office Practice, 1999. 26(3): p. 611–631. [DOI] [PubMed] [Google Scholar]
- 202.Dani JA, Overview of nicotinic receptors and their roles in the central nervous system. Biological Psychiatry, 2001. 49(3): p. 166–174. [DOI] [PubMed] [Google Scholar]
- 203.Wooltorton JR, et al. , Differential desensitization and distribution of nicotinic acetylcholine receptor subtypes in midbrain dopamine areas. J Neurosci, 2003. 23(8): p. 3176–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 204.Benwell MEM, Balfour DJK, and Anderson JM, Evidence that Tobacco Smoking Increases the Density of (−)-[3H]Nicotine Binding Sites in Human Brain. Journal of Neurochemistry, 1988. 50(4): p. 1243–1247. [DOI] [PubMed] [Google Scholar]
- 205.Gray R, et al. , Hippocampal synaptic transmission enhanced by low concentrations of nicotine. Nature, 1996. 383(6602): p. 713–716. [DOI] [PubMed] [Google Scholar]
- 206.Pidoplichko VI, et al. , Nicotine activates and desensitizes midbrain dopamine neurons. Nature, 1997. 390(6658): p. 401–404. [DOI] [PubMed] [Google Scholar]
- 207.Mansvelder HD and McGehee DS, Long-term potentiation of excitatory inputs to brain reward areas by nicotine. Neuron, 2000. 27(2): p. 349–357. [DOI] [PubMed] [Google Scholar]
- 208.Mansvelder HD, Keath JR, and McGehee DS, Synaptic mechanisms underlie nicotine-induced excitability of brain reward areas. Neuron, 2002. 33(6): p. 905–919. [DOI] [PubMed] [Google Scholar]
- 209.Saal D, et al. , Drugs of abuse and stress trigger a common synaptic adaptation in dopamine neurons. Neuron, 2003. 37(4): p. 577–582. [DOI] [PubMed] [Google Scholar]
- 210.Pidoplichko VI, et al. , Nicotinic cholinergic synaptic mechanisms in the ventral tegmental area contribute to nicotine addiction. Learning & memory, 2004. 11(1): p. 60–69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Balfour DJK, The Role of Mesoaccumbens Dopamine in Nicotine Dependence, in The Neuropharmacology of Nicotine Dependence, Balfour DJK and Munafò MR, Editors. 2015, Springer International Publishing: Cham. p. 55–98. [DOI] [PubMed] [Google Scholar]
- 212.Koob GF and Volkow ND, Neurocircuitry of Addiction. Neuropsychopharmacology, 2010. 35(1): p. 217–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Cosgrove KP, et al. , Imaging Tobacco Smoking with PET and SPECT, in The Neuropharmacology of Nicotine Dependence, Balfour DJK and Munafò MR, Editors. 2015, Springer International Publishing: Cham. p. 1–17. [Google Scholar]
- 214.Pogun S and Yararbas G, Sex differences in nicotine action. Handb Exp Pharmacol, 2009(192): p. 261–91. [DOI] [PubMed] [Google Scholar]
- 215.Rosecrans JA, Effects of nicotine on brain area 5-hydroxytryptamine function in male and female rats separated for differences of activity. Eur J Pharmacol, 1971. 16(1): p. 123–7. [DOI] [PubMed] [Google Scholar]
- 216.Rosecrans JA and Schechter MD, Brain area nicotine levels in male and female rats of two strains. Arch Int Pharmacodyn Ther, 1972. 196(1): p. 46–54. [PubMed] [Google Scholar]
- 217.Schepers G, et al. , Metabolism of S-nicotine in noninduced and aroclor-induced rats. Eur J Drug Metab Pharmacokinet, 1993. 18(2): p. 187–97. [DOI] [PubMed] [Google Scholar]
- 218.Kyerematen GA, et al. , Sexual dimorphism of nicotine metabolism and distribution in the rat. Studies in vivo and in vitro. Drug Metab Dispos, 1988. 16(6): p. 823–8. [PubMed] [Google Scholar]
- 219.Koylu E, et al. , Sex difference in up-regulation of nicotinic acetylcholine receptors in rat brain. Life Sciences, 1997. 61(12): p. PL185–PL190. [DOI] [PubMed] [Google Scholar]
- 220.Tyndale RF and Sellers EM, Genetic variation in CYP2A6-mediated nicotine metabolism alters smoking behavior. Ther Drug Monit, 2002. 24(1): p. 163–71. [DOI] [PubMed] [Google Scholar]
- 221.Dempsey D, Jacob P 3rd, and Benowitz NL, Accelerated metabolism of nicotine and cotinine in pregnant smokers. J Pharmacol Exp Ther, 2002. 301(2): p. 594–8. [DOI] [PubMed] [Google Scholar]
- 222.Selby P, et al. , Heavily smoking women who cannot quit in pregnancy: evidence of pharmacokinetic predisposition. Ther Drug Monit, 2001. 23(3): p. 189–91. [DOI] [PubMed] [Google Scholar]
- 223.Berlin I, Gasior MJ, and Moolchan ET, Sex-based and hormonal contraception effects on the metabolism of nicotine among adolescent tobacco-dependent smokers. Nicotine Tob Res, 2007. 9(4): p. 493–8. [DOI] [PubMed] [Google Scholar]
- 224.Chenoweth MJ, et al. , Known and novel sources of variability in the nicotine metabolite ratio in a large sample of treatment-seeking smokers. Cancer Epidemiol Biomarkers Prev, 2014. 23(9): p. 1773–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Benowitz NL, et al. , Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther, 2006. 79(5): p. 480–8. [DOI] [PubMed] [Google Scholar]
- 226.Strasser AA, et al. , Nicotine metabolite ratio predicts smoking topography and carcinogen biomarker level. Cancer Epidemiol Biomarkers Prev, 2011. 20(2): p. 234–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Sofuoglu M, et al. , Rapid nicotine clearance is associated with greater reward and heart rate increases from intravenous nicotine. Neuropsychopharmacology, 2012. 37(6): p. 1509–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Rubinstein ML, et al. , Rate of nicotine metabolism and withdrawal symptoms in adolescent light smokers. Pediatrics, 2008. 122(3): p. e643–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Chenoweth MJ, et al. , The Nicotine Metabolite Ratio is Associated With Early Smoking Abstinence Even After Controlling for Factors That Influence the Nicotine Metabolite Ratio. Nicotine Tob Res, 2016. 18(4): p. 491–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Vaz LR, et al. , The Nicotine Metabolite Ratio in Pregnancy Measured by trans-3’-Hydroxycotinine to Cotinine Ratio: Characteristics and Relationship With Smoking Cessation. Nicotine Tob Res, 2015. 17(11): p. 1318–23. [DOI] [PubMed] [Google Scholar]
- 231.Hukkanen J, et al. , Influence of menstrual cycle on cytochrome P450 2A6 activity and cardiovascular effects of nicotine. Clin Pharmacol Ther, 2005. 77(3): p. 159–69. [DOI] [PubMed] [Google Scholar]
- 232.Mueck AO and Seeger H, Smoking, estradiol metabolism and hormone replacement therapy. Curr Med Chem Cardiovasc Hematol Agents, 2005. 3(1): p. 45–54. [DOI] [PubMed] [Google Scholar]
- 233.Mochizuki T, et al. , Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: gender differences. Synapse, 1998. 30(1): p. 116–8. [DOI] [PubMed] [Google Scholar]
- 234.Donny EC, et al. , Nicotine self-administration in rats: estrous cycle effects, sex differences and nicotinic receptor binding. Psychopharmacology (Berl), 2000. 151(4): p. 392–405. [DOI] [PubMed] [Google Scholar]
- 235.Pogun S and Yararbas G, Sex Differences in Nicotine Action, in Nicotine Psychopharmacology, Henningfield JE, London ED, and Pogun S, Editors. 2009, Springer Berlin Heidelberg: Berlin, Heidelberg. p. 261–291. [Google Scholar]
- 236.Mochizuki T, et al. , Nicotine induced up-regulation of nicotinic receptors in CD-1 mice demonstrated with an in vivo radiotracer: Gender differences. Synapse, 1998. 30(1): p. 116–118. [DOI] [PubMed] [Google Scholar]
- 237.Cosgrove KP, et al. , Sex Differences in Availability of β2*-Nicotinic Acetylcholine Receptors in Recently Abstinent Tobacco Smokers. Archives of General Psychiatry, 2012. 69(4): p. 418–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Smith PH, et al. , Gender differences in the real-world effectiveness of smoking cessation medications: Findings from the 2010–2011 Tobacco Use Supplement to the Current Population Survey. Drug and Alcohol Dependence, 2017. 178: p. 485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Verplaetse TL, et al. , Sex differences in the nicotinic acetylcholine and dopamine receptor systems underlying tobacco smoking addiction. Current Opinion in Behavioral Sciences, 2018. 23: p. 196–202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 240.Fehr C, et al. , Association of Low Striatal Dopamine D 2 Receptor Availability With Nicotine Dependence Similar to That Seen With Other Drugs of Abuse. American Journal of Psychiatry, 2008. 165(4): p. 507–514. [DOI] [PubMed] [Google Scholar]
- 241.Brown AK, et al. , Sex differences in striatal dopamine D2/D3 receptor availability in smokers and non-smokers. International Journal of Neuropsychopharmacology, 2012. 15(7): p. 989–994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 242.Okita K, et al. , Sex Differences in Midbrain Dopamine D2-Type Receptor Availability and Association with Nicotine Dependence. Neuropsychopharmacology, 2016. 41(12): p. 2913–2919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Cosgrove KP, et al. , Sex Differences in the Brain's Dopamine Signature of Cigarette Smoking. The Journal of Neuroscience, 2014. 34(50): p. 16851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Staley JK, et al. , Sex differences in [123I]beta-CIT SPECT measures of dopamine and serotonin transporter availability in healthy smokers and nonsmokers. Synapse, 2001. 41(4): p. 275–84. [DOI] [PubMed] [Google Scholar]
- 245.Rosecrans JA, Brain area nicotine levels in male and female rats with different levels of spontaneous activity. Neuropharmacology, 1972. 11(6): p. 863–70. [DOI] [PubMed] [Google Scholar]
- 246.Schwartz RD and Kellar KJ, In vivo regulation of [3H]acetylcholine recognition sites in brain by nicotinic cholinergic drugs. J Neurochem, 1985. 45(2): p. 427–33. [DOI] [PubMed] [Google Scholar]
- 247.Nisell M, Nomikos GG, and Svensson TH, Systemic nicotine-induced dopamine release in the rat nucleus accumbens is regulated by nicotinic receptors in the ventral tegmental area. Synapse, 1994. 16(1): p. 36–44. [DOI] [PubMed] [Google Scholar]
- 248.Reavill C and Stolerman IP, Locomotor activity in rats after administration of nicotinic agonists intracerebrally. Br J Pharmacol, 1990. 99(2): p. 273–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Clarke PB, et al. , Evidence that mesolimbic dopaminergic activation underlies the locomotor stimulant action of nicotine in rats. J Pharmacol Exp Ther, 1988. 246(2): p. 701–8. [PubMed] [Google Scholar]
- 250.Dluzen DE and Anderson LI, Estrogen differentially modulates nicotine-evoked dopamine release from the striatum of male and female rats. Neurosci Lett, 1997. 230(2): p. 140–2. [DOI] [PubMed] [Google Scholar]
- 251.Kanýt L, et al. , Influence of sex and female hormones on nicotine-induced changes in locomotor activity in rats. Pharmacol Biochem Behav, 1999. 62(1): p. 179–87. [DOI] [PubMed] [Google Scholar]
- 252.Craft RM and Milholland RB, Sex differences in cocaine- and nicotine-induced antinociception in the rat. Brain Res, 1998. 809(1): p. 137–40. [DOI] [PubMed] [Google Scholar]
- 253.Cheeta S, et al. , In adolescence, female rats are more sensitive to the anxiolytic effect of nicotine than are male rats. Neuropsychopharmacology, 2001. 25(4): p. 601–7. [DOI] [PubMed] [Google Scholar]
- 254.Hatchell PC and Collins AC, The influence of genotype and sex on behavioral sensitivity to nicotine in mice. Psychopharmacology (Berl), 1980. 71(1): p. 45–9. [DOI] [PubMed] [Google Scholar]
- 255.Elliott BM, et al. , Effects of nicotine on elevated plus maze and locomotor activity in male and female adolescent and adult rats. Pharmacol Biochem Behav, 2004. 77(1): p. 21–8. [DOI] [PubMed] [Google Scholar]
- 256.Faraday MM, Elliott BM, and Grunberg NE, Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacol Biochem Behav, 2001. 70(4): p. 475–89. [DOI] [PubMed] [Google Scholar]
- 257.Trauth JA, Seidler FJ, and Slotkin TA, Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Res, 2000. 880(1–2): p. 167–72. [DOI] [PubMed] [Google Scholar]
- 258.Kimura D, Sex and cognition 1999. [Google Scholar]
- 259.Fallon JH, et al. , Gender: a major determinant of brain response to nicotine. Int J Neuropsychopharmacol, 2005. 8(1): p. 17–26. [DOI] [PubMed] [Google Scholar]
- 260.Klinteberg BA, Levander SE, and Schalling D, Cognitive sex differences: speed and problem-solving strategies on computerized neuropsychological tasks. Percept Mot Skills, 1987. 65(3): p. 683–97. [DOI] [PubMed] [Google Scholar]
- 261.Pratt MW, et al. , From inquiry to judgment: age and sex differences in patterns of adult moral thinking and information-seeking. Int J Aging Hum Dev, 1988. 27(2): p. 109–24. [DOI] [PubMed] [Google Scholar]
- 262.Algan O, et al. , Effects of tobacco smoking and gender on interhemispheric cognitive function: performance and confidence measures. Behav Pharmacol, 1997. 8(5): p. 416–28. [PubMed] [Google Scholar]
- 263.Kanit L, et al. , Nicotine interacts with sex in affecting rat choice between “look-out” and “navigational” cognitive styles in the Morris water maze place learning task. Brain Res Bull, 1998. 46(5): p. 441–5. [DOI] [PubMed] [Google Scholar]
- 264.Yilmaz O, et al. , Effects of nicotine on active avoidance learning in rats: sex differences. Behav Pharmacol, 1997. 8(2–3): p. 253–60. [PubMed] [Google Scholar]
- 265.Lewis CR, et al. , Smoking is associated with impaired verbal learning and memory performance in women more than men. Scientific Reports, 2021. 11(1): p. 10248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 266.Merritt P, Cobb A, and Cook G, Sex Differences in the Cognitive Effects of Tobacco Abstinence: A Pilot Study. Experimental and clinical psychopharmacology, 2012. 20: p. 258–63. [DOI] [PubMed] [Google Scholar]
- 267.Gritz ER, Nielsen IR, and Brooks LA, Smoking cessation and gender: the influence of physiological, psychological, and behavioral factors. Journal of the American Medical Women’s Association (1972), 1996. 51(1–2): p. 35–42. [PubMed] [Google Scholar]
- 268.Tanoue LT, CIGARETTE SMOKING AND WOMEN’S RESPIRATORY HEALTH. Clinics in Chest Medicine, 2000. 21(1): p. 47–65. [DOI] [PubMed] [Google Scholar]
- 269.Jarvis M, Gender and Smoking: Do Women Really Find it Harder to Give Up? British Journal of Addiction, 1984. 79(4): p. 383–387. [DOI] [PubMed] [Google Scholar]
- 270.Smith PH, et al. , Gender Differences in Medication Use and Cigarette Smoking Cessation: Results From the International Tobacco Control Four Country Survey. Nicotine & Tobacco Research, 2015. 17(4): p. 463–472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 271.Perkins KA, Donny E, and Caggiula AR, Sex differences in nicotine effects and selfadministration: review of human and animal evidence. Nicotine Tob Res, 1999. 1(4): p. 301–15. [DOI] [PubMed] [Google Scholar]
- 272.Perkins KA, Smoking cessation in women. Special considerations. CNS Drugs, 2001. 15(5): p. 391–411. [DOI] [PubMed] [Google Scholar]
- 273.Wetter DW, et al. , Gender differences in smoking cessation. J Consult Clin Psychol, 1999. 67(4): p. 555–62. [DOI] [PubMed] [Google Scholar]
- 274.Frank E, Carpenter LL, and Kupfer DJ, Sex differences in recurrent depression: are there any that are significant? Am J Psychiatry, 1988. 145(1): p. 41–5. [DOI] [PubMed] [Google Scholar]
- 275.Mangoni AA and Jackson SHD, Age-related changes in pharmacokinetics and pharmacodynamics: basic principles and practical applications. British journal of clinical pharmacology, 2004. 57(1): p. 6–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Turnheim K, Drug dosage in the elderly. Is it rational? Drugs Aging, 1998. 13(5): p. 357–79. [DOI] [PubMed] [Google Scholar]
- 277.Molander L, Hansson A, and Lunell E, Pharmacokinetics of nicotine in healthy elderly people. Clin Pharmacol Ther, 2001. 69(1): p. 57–65. [DOI] [PubMed] [Google Scholar]
- 278.McLean AJ and Le Couteur DG, Aging biology and geriatric clinical pharmacology. Pharmacol Rev, 2004. 56(2): p. 163–84. [DOI] [PubMed] [Google Scholar]
- 279.Klinger W, Developmental pharmacology and toxicology: biotransformation of drugs and other xenobiotics during postnatal development. Eur J Drug Metab Pharmacokinet, 2005. 30(1–2): p. 3–17. [DOI] [PubMed] [Google Scholar]
- 280.Dempsey D, Jacob P 3rd, and Benowitz NL, Nicotine metabolism and elimination kinetics in newborns. Clin Pharmacol Ther, 2000. 67(5): p. 458–65. [DOI] [PubMed] [Google Scholar]
- 281.Gourlay SG and Benowitz NL, The benefits of stopping smoking and the role of nicotine replacement therapy in older patients. Drugs Aging, 1996. 9(1): p. 8–23. [DOI] [PubMed] [Google Scholar]
- 282.Al Koudsi N, et al. , Hepatic CYP2A6 levels and nicotine metabolism: impact of genetic, physiological, environmental, and epigenetic factors. Eur J Clin Pharmacol, 2010. 66(3): p. 239–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Shram MJ, et al. , Interactions between age and the aversive effects of nicotine withdrawal under mecamylamine-precipitated and spontaneous conditions in male Wistar rats. Psychopharmacology (Berl), 2008. 198(2): p. 181–90. [DOI] [PubMed] [Google Scholar]
- 284.Craig EL, et al. , Nicotine Pharmacokinetics in Rats Is Altered as a Function of Age, Impacting the Interpretation of Animal Model Data. Drug Metabolism and Disposition, 2014. 42(9): p. 1447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 285.Wolden-Hanson T, Changes in body composition in response to challenges during aging in rats. Interdiscip Top Gerontol, 2010. 37: p. 64–83. [DOI] [PubMed] [Google Scholar]
- 286.Tutka P, Mosiewicz J, and Wielosz M, Pharmacokinetics and metabolism of nicotine. Pharmacol Rep, 2005. 57(2): p. 143–53. [PubMed] [Google Scholar]
- 287.Novak LP, Aging, total body potassium, fat-free mass, and cell mass in males and females between ages 18 and 85 years. J Gerontol, 1972. 27(4): p. 438–43. [DOI] [PubMed] [Google Scholar]
- 288.Forbes GB and Reina JC, Adult lean body mass declines with age: some longitudinal observations. Metabolism, 1970. 19(9): p. 653–63. [DOI] [PubMed] [Google Scholar]
- 289.Nowell S, et al. , CYP2A6 Activity Determined by Caffeine Phenotyping. Cancer Epidemiology Biomarkers & Prevention, 2002. 11(4): p. 377. [PubMed] [Google Scholar]
- 290.Sinues B, et al. , CYP2A6 activity in a healthy Spanish population: effect of age, sex, smoking, and oral contraceptives. Human & Experimental Toxicology, 2008. 27(5): p. 367–372. [DOI] [PubMed] [Google Scholar]
- 291.Johnstone E, et al. , Determinants of the rate of nicotine metabolism and effects on smoking behavior. Clinical pharmacology and therapeutics, 2006. 80: p. 319–30. [DOI] [PubMed] [Google Scholar]
- 292.Parkinson A, et al. , The effects of gender, age, ethnicity, and liver cirrhosis on cytochrome P450 enzyme activity in human liver microsomes and inducibility in cultured human hepatocytes. Toxicol Appl Pharmacol, 2004. 199(3): p. 193–209. [DOI] [PubMed] [Google Scholar]
- 293.Molander L and Lunell E, Pharmacokinetic investigation of a nicotine sublingual tablet. European Journal of Clinical Pharmacology, 2001. 56(11): p. 813–819. [DOI] [PubMed] [Google Scholar]
- 294.Dempsey D, Jacob Iii P, and Benowitz NL, Nicotine metabolism and elimination kinetics in newborns. Clinical Pharmacology & Therapeutics, 2000. 67(5): p. 458–465. [DOI] [PubMed] [Google Scholar]
- 295.Benowitz NL, et al. , Interindividual variability in the metabolism and cardiovascular effects of nicotine in man. J Pharmacol Exp Ther, 1982. 221(2): p. 368–72. [PubMed] [Google Scholar]
- 296.Wynne HA, et al. , The effect of age upon liver volume and apparent liver blood flow in healthy man. Hepatology, 1989. 9(2): p. 297–301. [DOI] [PubMed] [Google Scholar]
- 297.Woodhouse K and Wynne HA, Age-related changes in hepatic function. Implications for drug therapy. Drugs Aging, 1992. 2(3): p. 243–55. [DOI] [PubMed] [Google Scholar]
- 298.Rowe JW, et al. , The effect of age on creatinine clearance in men: a cross-sectional and longitudinal study. J Gerontol, 1976. 31(2): p. 155–63. [DOI] [PubMed] [Google Scholar]
- 299.Fliser D, et al. , Renal handling of drugs in the healthy elderly. Creatinine clearance underestimates renal function and pharmacokinetics remain virtually unchanged. Eur J Clin Pharmacol, 1999. 55(3): p. 205–11. [DOI] [PubMed] [Google Scholar]
- 300.Davies DF and Shock NW, Age changes in glomerular filtration rate, effective renal plasma flow, and tubular excretory capacity in adult males. J Clin Invest, 1950. 29(5): p. 496–507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 301.Li H, et al. , The Relationship between Nicotine Dependence and Age among Current Smokers. Iranian journal of public health, 2015. 44(4): p. 495–500. [PMC free article] [PubMed] [Google Scholar]
- 302.de Zwart L, et al. , The ontogeny of drug metabolizing enzymes and transporters in the rat. Reprod Toxicol, 2008. 26(3–4): p. 220–30. [DOI] [PubMed] [Google Scholar]
- 303.Levin ED, Nicotinic systems and cognitive function. Psychopharmacology, 1992. 108(4): p. 417–431. [DOI] [PubMed] [Google Scholar]
- 304.Levin ED, Nicotinic receptor subtypes and cognitive function. Journal of Neurobiology, 2002. 53(4): p. 633–640. [DOI] [PubMed] [Google Scholar]
- 305.Rezvani AH and Levin ED, Cognitive effects of nicotine. Biological Psychiatry, 2001. 49(3): p. 258–267. [DOI] [PubMed] [Google Scholar]
- 306.Perry EK, et al. , Alteration in nicotine binding sites in Parkinson’s disease, Lewy body dementia and Alzheimer’s disease: Possible index of early neuropathology. Neuroscience, 1995. 64(2): p. 385–395. [DOI] [PubMed] [Google Scholar]
- 307.Court J, et al. , Nicotinic receptor abnormalities in Alzheimer’s disease. Biological Psychiatry, 2001. 49(3): p. 175–184. [DOI] [PubMed] [Google Scholar]
- 308.Court JA, et al. , Nicotinic and muscarinic cholinergic receptor binding in the human hippocampal formation during development and aging. Developmental Brain Research, 1997. 101(1): p. 93–105. [DOI] [PubMed] [Google Scholar]
- 309.Flynn DD and Mash DC, Characterization of l-[3H]Nicotine Binding in Human Cerebral Cortex: Comparison Between Alzheimer’s Disease and the Normal. Journal of Neurochemistry, 1986. 47(6): p. 1948–1954. [DOI] [PubMed] [Google Scholar]
- 310.Hellström-Lindahl E, Winblad B, and Nordberg A, Muscarinic and nicotinic receptor changes in the cortex and thalamus of brains of chronic alcoholics. Brain Research, 1993. 620(1): p. 42–48. [DOI] [PubMed] [Google Scholar]
- 311.Hellström-Lindahl E and Court JA, Nicotinic acetylcholine receptors during prenatal development and brain pathology in human aging. Behavioural Brain Research, 2000. 113(1): p. 159–168. [DOI] [PubMed] [Google Scholar]
- 312.Marutle A, et al. , Regional distribution of subtypes of nicotinic receptors in human brain and effect of aging studied by (±)-[3H]epibatidine. Brain Research, 1998. 801(1): p. 143–149. [DOI] [PubMed] [Google Scholar]
- 313.Nordberg A, Alafuzoff I, and Winblad B, Nicotinic and muscarinic subtypes in the human brain: Changes with aging and dementia. Journal of Neuroscience Research, 1992. 31(1): p. 103–111. [DOI] [PubMed] [Google Scholar]
- 314.Nordberg A and Winblad B, Brain Nicotinic and Muscarinic Receptors in Normal Aging and Dementia, in Alzheimer’s and Parkinson’s Disease: Strategies for Research and Development, Fisher A, Hanin I, and Lachman C, Editors. 1986, Springer US: Boston, MA. p. 95–108. [Google Scholar]
- 315.Perry EK, et al. , Cholinergic Receptors in Cognitive Disorders. Canadian Journal of Neurological Sciences / Journal Canadien des Sciences Neurologiques, 1986. 13(S4): p. 521–527. [DOI] [PubMed] [Google Scholar]
- 316.Mitsis EM, et al. , Age-related decline in nicotinic receptor availability with [123I]5-IA-85380 SPECT. Neurobiology of Aging, 2009. 30(9): p. 1490–1497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 317.Wonnacott S, The paradox of nicotinic acetylcholine receptor upregulation by nicotine. Trends in Pharmacological Sciences, 1990. 11(6): p. 216–219. [DOI] [PubMed] [Google Scholar]
- 318.Butler NR and Goldstein H, Smoking in Pregnancy and Subsequent Child Development. British Medical Journal, 1973. 4(5892): p. 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 319.Douglas JWB, Population Studies, 1973. 27(3): p. 599–601. [Google Scholar]
- 320.Denson R, Nanson JL, and McWatters MA, Hyperkinesis and maternal smoking. Can Psychiatr Assoc J, 1975. 20(3): p. 183–7. [DOI] [PubMed] [Google Scholar]
- 321.Dunn HG, et al. , Maternal Cigarette Smoking during Pregnancy and the Child’s Subsequent Development: II. Neurological and Intellectual Maturation to the Age of 6½ Years. Canadian Journal of Public Health / Revue Canadienne de Sante’e Publique, 1977. 68(1): p. 43–50. [PubMed] [Google Scholar]
- 322.Naeye RL and Peters EC, Mental development of children whose mothers smoked during pregnancy. Obstetrics and Gynecology, 1984. 64(5): p. 601–607. [PubMed] [Google Scholar]
- 323.Nasrat HA, Al-Hachim GM, and Mahmood FA, Perinatal Effects of Nicotine. Neonatology, 1986. 49(1): p. 8–14. [DOI] [PubMed] [Google Scholar]
- 324.Sifat AE, et al. , Prenatal electronic cigarette exposure decreases brain glucose utilization and worsens outcome in offspring hypoxic–ischemic brain injury. Journal of Neurochemistry, 2020. 153(1): p. 63–79. [DOI] [PubMed] [Google Scholar]
- 325.Hagino N and Lee JW, Effect of maternal nicotine on the development of sites for [3H]nicotine binding in the fetal brain. International Journal of Developmental Neuroscience, 1985. 3(5): p. 567–571. [DOI] [PubMed] [Google Scholar]
- 326.Sershen H, et al. , Effects of prenatal administration of nicotine on amino acid pools, protein metabolism, and nicotine binding in the brain. Neurochemical Research, 1982. 7(12): p. 1515–1522. [DOI] [PubMed] [Google Scholar]
- 327.Slotkin TA, Orband-Miller L, and Queen KL, Development of [3H]nicotine binding sites in brain regions of rats exposed to nicotine prenatally via maternal injections or infusions. Journal of Pharmacology and Experimental Therapeutics, 1987. 242(1): p. 232. [PubMed] [Google Scholar]
- 328.Van de Kamp JL and Collins AC, Prenatal nicotine alters nicotinic receptor development in the mouse brain. Pharmacology Biochemistry and Behavior, 1994. 47(4): p. 889–900. [DOI] [PubMed] [Google Scholar]
- 329.Wang TW NL, Park-Lee E, Ren C, Cullen KA, King BA., E-cigarette Use Among Middle and High School Students — United States, 2020. MMWR Morb Mortal Wkly Rep, 2020. 69: p. 1310–1312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 330.Trauth JA, et al. , Modeling adolescent nicotine exposure: effects on cholinergic systems in rat brain regions. Brain Research, 2000. 873(1): p. 18–25. [DOI] [PubMed] [Google Scholar]
- 331.Slotkin TA, Nicotine and the adolescent brain: Insights from an animal model. Neurotoxicology and Teratology, 2002. 24(3): p. 369–384. [DOI] [PubMed] [Google Scholar]
- 332.Abreu-Villaça Y, Seidler FJ, and Slotkin TA, Impact of adolescent nicotine exposure on adenylyl cyclase-mediated cell signaling: enzyme induction, neurotransmitter-specific effects, regional selectivities, and the role of withdrawal. Brain Research, 2003. 988(1): p. 164–172. [DOI] [PubMed] [Google Scholar]
- 333.Belluzzi JD, et al. , Age-dependent effects of nicotine on locomotor activity and conditioned place preference in rats. Psychopharmacology, 2004. 174(3): p. 389–395. [DOI] [PubMed] [Google Scholar]
- 334.Adriani W, et al. , Evidence for Enhanced Neurobehavioral Vulnerability to Nicotine during Periadolescence in Rats. The Journal of Neuroscience, 2003. 23(11): p. 4712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 335.Adriani W, et al. , Preexposure during or following adolescence differently affects nicotine-rewarding properties in adult rats. Psychopharmacology, 2006. 184(3): p. 382–390. [DOI] [PubMed] [Google Scholar]
- 336.Collins SL and Izenwasser S, Chronic nicotine differentially alters cocaine-induced locomotor activity in adolescent vs. adult male and female rats. Neuropharmacology, 2004. 46(3): p. 349362. [DOI] [PubMed] [Google Scholar]
- 337.McMillen BA, et al. , Periadolescent nicotine exposure causes heterologous sensitization to cocaine reinforcement. European Journal of Pharmacology, 2005. 509(2): p. 161–164. [DOI] [PubMed] [Google Scholar]
- 338.McQuown SC, Belluzzi JD, and Leslie FM, Low dose nicotine treatment during early adolescence increases subsequent cocaine reward. Neurotoxicology and Teratology, 2007. 29(1): p. 66–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 339.Doura MB, et al. , Adult and periadolescent rats differ in expression of nicotinic cholinergic receptor subtypes and in the response of these subtypes to chronic nicotine exposure. Brain Research, 2008. 1215: p. 40–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 340.Marks MJ, Stitzel JA, and Collins AC, Time course study of the effects of chronic nicotine infusion on drug response and brain receptors. Journal of Pharmacology and Experimental Therapeutics, 1985. 235(3): p. 619. [PubMed] [Google Scholar]
- 341.Marks MJ, Stitzel JA, and Collins AC, Dose-response analysis of nicotine tolerance and receptor changes in two inbred mouse strains. J Pharmacol Exp Ther, 1986. 239(2): p. 358–64. [PubMed] [Google Scholar]
- 342.Marks MJ, Stitzel JA, and Collins AC, Influence of kinetics of nicotine administration on tolerance development and receptor levels. Pharmacology Biochemistry and Behavior, 1987. 27(3): p. 505–512. [DOI] [PubMed] [Google Scholar]
- 343.Placzek AN, Zhang TA, and Dani JA, Age dependent nicotinic influences over dopamine neuron synaptic plasticity. Biochemical Pharmacology, 2009. 78(7): p. 686–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 344.Philpot RM, Engberg ME, and Wecker L, Effects of nicotine exposure on locomotor activity and pCREB levels in the ventral striatum of adolescent rats. Behavioural brain research, 2012. 230(1): p. 62–68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 345.Abbott LC and Winzer-Serhan UH, Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Critical reviews in toxicology, 2012. 42(4): p. 279–303. [DOI] [PubMed] [Google Scholar]
- 346.Behnke M, Smith VC, and Abuse C.o.S., Prenatal substance abuse: short-and long-term effects on the exposed fetus. Pediatrics, 2013. 131(3): p. e1009–e1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 347.Latimer K, et al. , Disruptive behaviour disorders: a systematic review of environmental antenatal and early years risk factors. Child: care, health and development, 2012. 38(5): p. 611–628. [DOI] [PubMed] [Google Scholar]
- 348.Linnet KM, et al. , Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: review of the current evidence. American Journal of Psychiatry, 2003. 160(6): p. 1028–1040. [DOI] [PubMed] [Google Scholar]
- 349.Rosenthal DG and Weitzman M, Examining the effects of intrauterine and postnatal exposure to tobacco smoke on childhood cognitive and behavioral development. International Journal of Mental Health, 2011. 40(1): p. 39–64. [Google Scholar]
- 350.Tizabi Y, et al. , Hyperactivity Induced by Prenatal Nicotine Exposure Is Associated with an Increase in Cortical Nicotinic Receptors. Pharmacology Biochemistry and Behavior, 1997. 58(1): p. 141–146. [DOI] [PubMed] [Google Scholar]
- 351.O’Neill MF, Dourish CT, and Iversen SD, Evidence for an involvement of D1 and D2 dopamine receptors in mediating nicotine-induced hyperactivity in rats. Psychopharmacology, 1991. 104(3): p. 343–350. [DOI] [PubMed] [Google Scholar]
- 352.Zhang M, et al. , Paternal nicotine exposure induces hyperactivity in next-generation via down-regulating the expression of DAT. Toxicology, 2020. 431: p. 152367. [DOI] [PubMed] [Google Scholar]
- 353.Wittchen HU, et al. , What are the high risk periods for incident substance use and transitions to abuse and dependence? Implications for early intervention and prevention. International Journal of Methods in Psychiatric Research, 2008. 17(S1): p. S16–S29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 354.Lasser K, et al. , Smoking and mental illness: a population-based prevalence study. Jama, 2000. 284(20): p. 2606–2610. [DOI] [PubMed] [Google Scholar]
- 355.Faraday MM, Elliott BM, and Grunberg NE, Adult vs. adolescent rats differ in biobehavioral responses to chronic nicotine administration. Pharmacology Biochemistry and Behavior, 2001. 70(4): p. 475–489. [DOI] [PubMed] [Google Scholar]
- 356.Faraday MM, et al. , Adolescent and adult male rats differ in sensitivity to nicotine’s activity effects. Pharmacology Biochemistry and Behavior, 2003. 74(4): p. 917–931. [DOI] [PubMed] [Google Scholar]
- 357.Denson R, Nanson J, and McWatters M, Hyperkinesis and maternal smoking. Canadian Psychiatric Association Journal, 1975. 20(3): p. 183–187. [DOI] [PubMed] [Google Scholar]
- 358.Fergusson DM, Horwood LJ, and Lynskey MT, Maternal smoking before and after pregnancy: effects on behavioral outcomes in middle childhood. Pediatrics, 1993. 92(6): p. 815–822. [PubMed] [Google Scholar]
- 359.Fergusson DM, Woodward LJ, and Horwood LJ, Maternal smoking during pregnancy and psychiatric adjustment in late adolescence. Archives of general psychiatry, 1998. 55(8): p. 721–727. [DOI] [PubMed] [Google Scholar]
- 360.Kristjansson EA, Fried P, and Watkinson B, Maternal smoking during pregnancy affects children’s vigilance performance. Drug and alcohol dependence, 1989. 24(1): p. 11–19. [DOI] [PubMed] [Google Scholar]
- 361.Milberger S, et al. , Is maternal smoking during pregnancy a risk factor for attention deficit hyperactivity disorder in children? The American journal of psychiatry, 1996. [DOI] [PubMed] [Google Scholar]
- 362.Streissguth AP, et al. , Intrauterine alcohol and nicotine exposure: Attention and reaction time in 4-year-old children. Developmental Psychology, 1984. 20(4): p. 533. [Google Scholar]
- 363.Fung Y, Postnatal behavioural effects of maternal nicotine exposure in rats. Journal of pharmacy and pharmacology, 1988. 40(12): p. 870–872. [DOI] [PubMed] [Google Scholar]
- 364.Levin ED, et al. , Prenatal nicotine effects on memory in rats: pharmacological and behavioral challenges. Developmental Brain Research, 1996. 97(2): p. 207–215. [DOI] [PubMed] [Google Scholar]
- 365.Lichtensteiger W, et al. , Prenatal adverse effects of nicotine on the developing brain. Progress in brain research, 1988. 73: p. 137–157. [DOI] [PubMed] [Google Scholar]
- 366.Martin JC, et al. , Growth, development and activity in rat offspring following maternal drug exposure. Experimental aging research, 1976. 2(3): p. 235–251. [DOI] [PubMed] [Google Scholar]
- 367.Miao H, et al. , Nicotine exposure during a critical period of development leads to persistent changes in nicotinic acetylcholine receptors of adult rat brain. Journal of neurochemistry, 1998. 70(2): p. 752–762. [DOI] [PubMed] [Google Scholar]
- 368.Navarro H, et al. , Effects of prenatal nicotine exposure on development of central and peripheral cholinergic neurotransmitter systems. Evidence for cholinergic trophic influences in developing brain. Journal of Pharmacology and Experimental Therapeutics, 1989. 251(3): p. 894–900. [PubMed] [Google Scholar]
- 369.Popke EJ, et al. , Prenatal exposure to nicotine: effects on prepulse inhibition and central nicotinic receptors. Pharmacology Biochemistry and Behavior, 1997. 58(4): p. 843–849. [DOI] [PubMed] [Google Scholar]
- 370.Richardson SA and Tizabi Y, Hyperactivity in the offspring of nicotine-treated rats: role of the mesolimbic and nigrostriatal dopaminergic pathways. Pharmacology Biochemistry and Behavior, 1994. 47(2): p. 331–337. [DOI] [PubMed] [Google Scholar]
- 371.Schlumpf M, et al. , A new device for monitoring early motor development: prenatal nicotine-induced changes. Pharmacology Biochemistry and Behavior, 1988. 30(1): p. 199–203. [DOI] [PubMed] [Google Scholar]
- 372.Slotkin TA, Fetal nicotine or cocaine exposure: which one is worse? Journal of pharmacology and experimental therapeutics, 1998. 285(3): p. 931–945. [PubMed] [Google Scholar]
- 373.Sorenson CA, Raskin LA, and Suh Y, The effects of prenatal nicotine on radial-arm maze performance in rats. Pharmacology Biochemistry and Behavior, 1991. 40(4): p. 991–993. [DOI] [PubMed] [Google Scholar]
- 374.Agulhon C, et al. , Distribution of mRNA for the α4 subunit of the nicotinic acetylcholine receptor in the human fetal brain. Molecular brain research, 1998. 58(1–2): p. 123–131. [DOI] [PubMed] [Google Scholar]
- 375.Cairns NJ and Wonnacott S, [3H](−) nicotine binding sites in fetal human brain. Brain research, 1988. 475(1): p. 1–7. [DOI] [PubMed] [Google Scholar]
- 376.Hellström-Lindahl E, et al. , Regional distribution of nicotinic receptors during prenatal development of human brain and spinal cord. Developmental brain research, 1998. 108(1–2): p. 147–160. [DOI] [PubMed] [Google Scholar]
- 377.Torres LH, et al. , Exposure of Neonatal Mice to Tobacco Smoke Disturbs Synaptic Proteins and Spatial Learning and Memory from Late Infancy to Early Adulthood. PLOS ONE, 2015. 10(8): p. e0136399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 378.Schochet TL, Kelley AE, and Landry CF, Differential behavioral effects of nicotine exposure in adolescent and adult rats. Psychopharmacology, 2004. 175(3): p. 265–273. [DOI] [PubMed] [Google Scholar]
- 379.Trauth JA, Seidler FJ, and Slotkin TA, Persistent and delayed behavioral changes after nicotine treatment in adolescent rats. Brain Research, 2000. 880(1): p. 167–172. [DOI] [PubMed] [Google Scholar]
- 380.Kviz FJ, et al. , Age and Smoking Cessation Behaviors. Preventive Medicine, 1995. 24(3): p. 297–307. [DOI] [PubMed] [Google Scholar]
- 381.Breslau N and Peterson EL, Smoking cessation in young adults: age at initiation of cigarette smoking and other suspected influences. American journal of public health, 1996. 86(2): p. 214–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 382.Chen J and Millar WJ, Age of smoking initiation: implications for quitting. Health reports-statistics Canada, 1998. 9: p. 39–48. [PubMed] [Google Scholar]
- 383.Pesce G, et al. , Time and age trends in smoking cessation in Europe. PLOS ONE, 2019. 14(2): p. e0211976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 384.Koçak ND, et al. , Relapse rate and factors related to relapse in a 1-year follow-up of subjects participating in a smoking cessation program. Respiratory care, 2015. 60(12): p. 1796–1803. [DOI] [PubMed] [Google Scholar]
- 385.Breslau N, Fenn N, and Peterson EL, Early smoking initiation and nicotine dependence in a cohort of young adults. Drug and alcohol dependence, 1993. 33(2): p. 129–137. [DOI] [PubMed] [Google Scholar]
- 386.Qiu D, et al. , Smoking cessation and related factors in middle-aged and older Chinese adults: Evidence from a longitudinal study. PLOS ONE, 2020. 15(10): p. e0240806. [DOI] [PMC free article] [PubMed] [Google Scholar]


