Abstract
Background:
Tyrosine kinase inhibitors (TKIs) are effective for treating human epidermal growth factor receptor 2 (HER2)-positive metastatic breast cancer. However, therapies subsequent to TKI progression remain controversial, and effective treatments for TKI resistance are urgently needed. We evaluate the practice of exchange of TKIs, which involves treatment with a different TKI following prior TKI failure. Specifically, this study investigated the efficacy of pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer (NCT04899128).
Methods:
This real-world study included 76 patients diagnosed with HER2-positive metastatic breast cancer who received pyrotinib-based therapy after lapatinib progression at four Chinese institutions between August 2018 and March 2020. Progression-free survival (PFS), overall survival (OS), objective response rate (ORR), clinical benefit rate (CBR), and toxicity profiles were reported.
Results:
All patients received pyrotinib-based therapy in two or later line therapy. The median PFS was 8.0 months (95% CI 5.1–10.9). OS has not reached. The ORR and CBR were 17.1% and 60.5%, respectively. The median PFS was 7.1 months (95% CI 5.633–8.567) and intracranial ORR was 42.9% in patients who had brain metastasis (n = 14). Patients who benefited from lapatinib ⩾ 6.0 months prior exhibited a longer PFS (10.6 versus 6.0 months, p = 0.034, stratified hazard ratio (HR) 0.534, 95% CI 0.293–0.975). The most common adverse effects were diarrhea (n = 34, 44.7%) and hand-foot syndrome (n = 10, 13.2%).
Conclusion:
Pyrotinib-based therapy has the potential to improve survival in patients with lapatinib-resistant HER2-positive metastatic breast cancer, including those with brain metastases. Pyrotinib could provide a clinically significant increase in PFS for patients who benefited from prior lapatinib.
Keywords: breast cancer, human epidermal growth factor receptor 2, lapatinib, metastasis, pyrotinib
Introduction
Breast cancers with amplified or overexpression of human epidermal growth factor receptor 2 (HER2, also referred to as ERBB2) account for approximately 15–20% of all breast cancers and are historically aggressive, commonly resulting in poor prognosis. 1 The anti-HER2 therapies, including trastuzumab, pertuzumab, lapatinib, and ado-trastuzumab emtansine (T-DM1), have improved the prognosis of patients with HER2-positive breast cancer.2–4 Despite the improvement in survival with anti-HER2 therapies, therapeutic resistance remains a challenge, highlighting the clinical need for alternative therapies. 5
Tyrosine kinase inhibitors (TKIs) are considered advanced third line and later treatments by the National Comprehensive Cancer Network (NCCN) guidelines for their encouraging anti-HER2 effects. Data from three large randomized trials showed that lapatinib, neratinib, and tucatinib significantly improved survival in patients with HER2-positive metastatic breast cancer. These therapies inhibit HER2 autophosphorylation, effectively blocking downstream signaling.6–8 Pyrotinib, which irreversibly inhibits epidermal growth factor receptor (EGFR, HER1), HER2, and human epidermal growth factor receptor 4 (HER4), was approved in China in 2018 for patients with advanced or metastatic HER2-positive breast cancer when combined with capecitabine. Both PHOEBE and PHENIX demonstrate substantial clinical benefits of pyrotinib combined with capecitabine in patients with HER2-positive relapsed or metastatic breast cancer after trastuzumab and taxane.9,10 However, the choice of treatment after TKI treatment remains controversial. The benefits and drawbacks of switching to trastuzumab, TKIs, or antibody-drug conjugates (ADC) after therapeutic resistance remain elusive.
Our study aimed to evaluate the practice of exchange of TKIs in the context of HER2 positive breast cancer with prior TKI resistance. This multicenter real-world study evaluated the efficacy and safety of pyrotinib subsequent to lapatinib resistance.
Patients and methods
Study design
This real-world study was conducted in four medical institutions, including the Jiangsu Province Hospital, Fudan University Shanghai Cancer Center, First Affiliated Hospital of Soochow University, and the Affiliated Hospital of Jiangnan University. The ethics committee and institutional review board of Jiangsu Province Hospital approved this study (Approval No. 2021-SR-357). Informed consent was waived due to the retrospective design, according to institutional requirements and national legislation. This study was conducted in accordance with Good Clinical Practice and the Declaration of Helsinki. The study was registered at clinicaltrials.gov (NCT04899128).
Patients’ eligibility
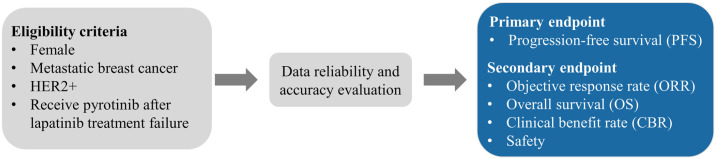
Eligibility criteria included (1) females aged 18–70 years; (2) HER2-positive breast cancer diagnosed by histopathology (immunochemistry 3+, or immunochemistry 1/2+ together with HER2 gene amplification by fluorescence in situ hybridization); (3) metastatic or locally recurrent breast cancer with at least one measurable lesion of metastasis according to the Response Evaluation Criteria in Solid Tumors guidelines version 1.1 (RECIST 1.1); (4) patients who received lapatinib therapy for relapsed or metastatic disease and received pyrotinib therapy after lapatinib failure; and (5) patients with complete and accurate medical data. Trastuzumab resistance was identified as recurrence detected during or within 12 months after adjuvant trastuzumab, or disease progression diagnosed during the first radiological assessment (8–12 weeks) or within 3 months after first-line trastuzumab. Patients with recurrence detected 12 months after completing adjuvant trastuzumab, or disease progression diagnosed after two or more lines of trastuzumab that achieved response or stabilization at the first radiological assessment, were classified as being refractory to trastuzumab. 11 Patients with incomplete or inaccurate medical data were excluded.
Treatment administration
All patients were treated with lapatinib for metastasis or local recurrence. After lapatinib progression, patients received pyrotinib; the starting dose, dose modification, discontinuation, and combination therapy were determined as per the physician’s opinion based on clinical guidelines, previous clinical trials, general health status, and patient willingness. The minimum time between lapatinib and pyrotinib treatments was not restricted.
Outcomes
The primary endpoint was progression-free survival (PFS), defined as the time from the date of drug administration to the first occurrence of any event, including local relapse, distant metastasis, or death by any cause. Secondary endpoints included the objective response rate (ORR), which was the proportion of participants whose best outcome was complete response (CR) or partial response (PR); clinical benefit rate (CBR), the proportion of participants who achieved CR, PR, or stable disease (SD) for more than 24 weeks; overall survival (OS), the time from drug administration to death by any cause; and safety.
Tumor response was evaluated using computed tomography (CT) and magnetic resonance imaging (MRI), according to the RECIST 1.1 system. Adverse events (AEs) were assessed according to the National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE, 4.03).
Statistical analyses
Quantitative data are presented as the mean, median, and interquartile range. Qualitative and ranked data are summarized by rate and proportion. PFS and OS with 95% confidence intervals (CIs) were analyzed using the Kaplan–Meier method. Hazard ratios (HRs) and corresponding 95% CIs were assessed using the Cox proportional hazards regression model. Multivariate analyses were performed based on the results of univariate analyses. All tests were two-sided, and p-values less than 0.05 were considered significant. SPSS 26.0, and GraphPad Prism 8.0, were employed in all analyses.
Results
Patients
The patient selection process is illustrated in Figure 1. Baseline characteristics are presented in Table 1. The median age of the patients was 55 (range, 46–60) years. In total, 35 (46.1%) patients were hormone receptor (HR)-positive. However, 23 (30.3%) patients were exhibited trastuzumab resistance, and 48 (63.2%) were refractory to trastuzumab. Visceral and brain metastases were observed in 49 (64.5%) and 14 (18.4%) patients, respectively.
Figure 1.
Diagram of the treatment schema.
Table 1.
Baseline characteristics of patients who received pyrotinib after lapatinib failure.
| Characteristic | N (%) (n = 76) |
|---|---|
| Age | |
| Median (interquartile range) | 55 (46–60) |
| HR status | |
| HR positive | 35 (46.1) |
| HR negative | 33 (43.4) |
| Unknown | 8 (10.5) |
| Trastuzumab resistance | |
| Resistance | 23(30.3) |
| Refractoriness | 48(63.2) |
| Unknown | 5(6.6) |
| Visceral metastases | |
| Yes | 49 (64.5) |
| No | 27 (35.5) |
| Metastatic sites | |
| Lymph nodes | 16 (21.1) |
| Lung | 35 (46.1) |
| Liver | 17 (22.4) |
| Bone | 14 (18.4) |
| Brain | 14 (18.4) |
| Chest wall | 4 (5.3) |
| PFS of lapatinib therapy (months) | |
| <6.0 | 29 (38.2) |
| ⩾6.0 | 43 (56.6) |
| Unknown | 4 (5.3) |
| Lines of pyrotinib therapy | |
| 2 | 13 (17.1) |
| 3 | 23 (30.3) |
| ⩾4 | 40 (52.6) |
| Pyrotinib regimens | |
| Pyrotinib | 7 (9.2) |
| Pyrotinib + capecitabine | 38 (50.0) |
| Pyrotinib + vinorelbine | 12 (15.8) |
| Pyrotinib + trastuzumab | 6 (7.9) |
| Other | 13 (17.1) |
HR, hormone receptor; PFS, progression-free survival.
Lapatinib was administered to 43 (56.6%) patients ⩾ 6 months prior and 29 (38.2%) patients < 6 months prior. However, 13 (17.1%), 23 (30.3%), and 40 (52.6%) patients received pyrotinib-based therapies in two, three, and four or later lines, respectively. Combination therapy, including pyrotinib + capecitabine, vinorelbine, or trastuzumab, was administered to 69 (90.8%) patients, whereas 7 (9.2%) received pyrotinib alone.
Efficacy outcomes
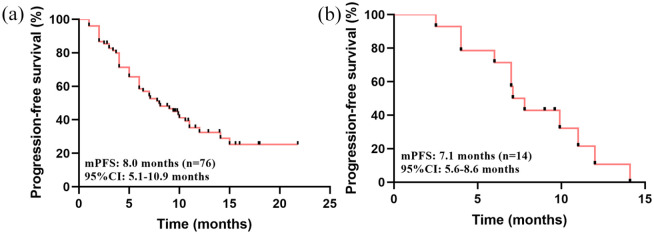
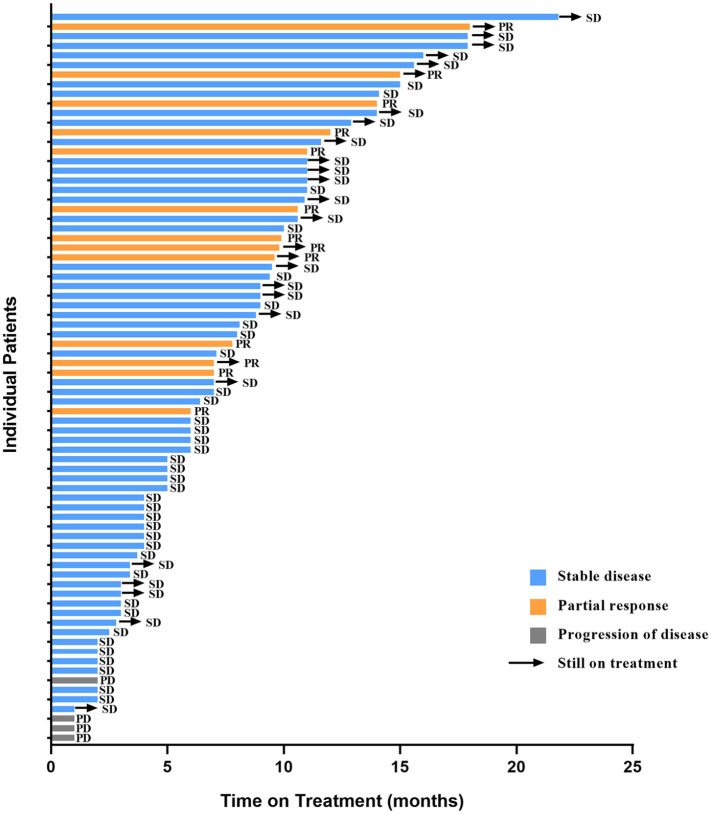
The median PFS was 8.0 months (95% CI 5.1–10.9) (Figure 2(a)). OS has not reached. The ORR and CBR were 17.1% and 60.5%, respectively. Though, no patients achieved a CR, 13 (17.1%) and 59 (77.6%) patients achieved a PR and SD, respectively (Figure 3). The survival data of the pyrotinib treatment combinations are summarized in the supplementary materials (Supplementary Figures S1–S5).
Figure 2.
Kaplan–Meier analysis of patients who received pyrotinib-based therapy after lapatinib resistance. (a) PFS of all patients who received pyrotinib-based therapy. (b) PFS of patients with brain metastases who received pyrotinib-based therapy.
Figure 3.
Summary of pyrotinib-based therapy response in lapatinib-resistant HER2-positive metastatic breast cancer patients.
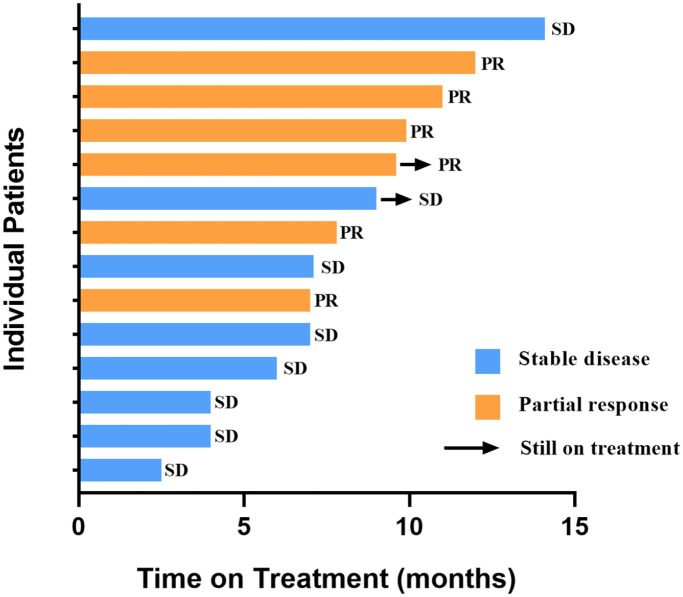
The median PFS in patients with brain metastases was 7.1 months (95% CI 5.6–8.6) (Figure 2(b)). The CBR was 78.6%, and the intracranial ORR was 42.9%. No patients achieved CR, but six (42.9%) achieved a PR and eight (57.1%) achieved SD (Figure 4).
Figure 4.
Anti-tumor activity of pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer patients with brain metastases.
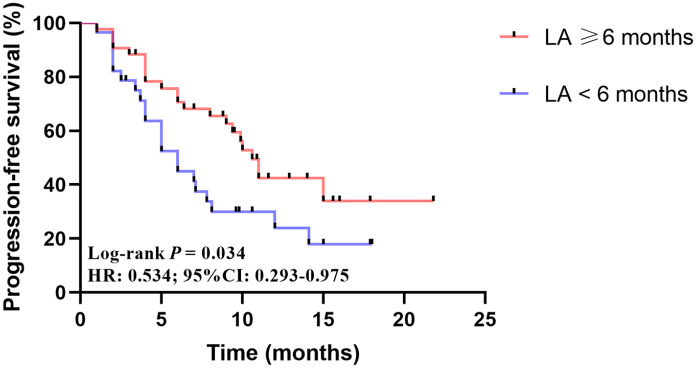
Univariate analysis indicated that age, HR status, trastuzumab resistance, metastasis type, and pyrotinib lines were not related to the efficacy of pyrotinib in lapatinib-resistant HER2-positive breast cancer (Table 2). However, patients who benefited from lapatinib ⩾ 6 months prior had a longer PFS after pyrotinib treatment (10.6 versus 6.0 months, p = 0.034) (Figure 5). The Cox multivariate analysis also suggested that prior lapatinib PFS could be an independent predictor for the efficacy of subsequent pyrotinib treatments (HR 0.534, 95% CI 0.293–0.975) (Table 2).
Table 2.
Log-rank and Cox analysis of factors associated with pyrotinib PFS.
| Characteristic | Log-rank analysis | Cox multivariate analysis | |
|---|---|---|---|
| p | p | HR (95% CI) | |
| Age (< 60 versus ⩾60) | 0.247 | ||
| HR status (negative versus positive) | 0.76 | ||
| Trastuzumab resistance (resistance versus refractoriness) | 0.585 | ||
| Metastasis type (non-visceral versus visceral) | 0.298 | ||
| Line of pyrotinib (⩽3 versus > 3) | 0.347 | ||
| Lapatinib PFS (<6 versus ⩾6 months) | 0.034 | 0.041 | 0.534 (0.293–0.975) |
CI, confidence interval; HR, hazard ratio; PFS, progression-free survival.
Figure 5.
Kaplan–Meier analysis of pyrotinib in patients who benefited from lapatinib ⩾ 6.0 and < 6.0 months. LA: lapatinib PFS.
Safety outcomes
The safety profile of pyrotinib-based therapy is presented in Table 3. The most common adverse event was diarrhea (n = 34, 44.7%). Other adverse events included hand-foot syndrome (n = 10, 13.2%), nausea (n = 4, 5.2%), and neutropenia (n = 2, 2.6%). Diarrhea was also the most common Grade 3–4 adverse event (n = 11, 14.5%). Adverse events related to pyrotinib combination therapies are summarized in the supplementary materials (Supplementary Table S1). Overall, pyrotinib treatment after lapatinib resistance did not increase the risk of overlapping toxicities, which supports the clinical potential of TKI exchange.
Table 3.
Adverse events of patients who received pyrotinib after lapatinib failure.
| Adverse events | All grades | Grade 3–4 |
|---|---|---|
| Diarrhea | 34 (44.7%) | 11 (14.5%) |
| Hand-foot syndrome | 10 (13.2%) | 0 |
| Nausea | 4 (5.2%) | 0 |
| Anemia | 3 (3.9%) | 1 (1.3%) |
| Neutropenia | 2 (2.6%) | 0 |
| Vomiting | 2 (2.6%) | 1 (1.3%) |
| Increased alanine or aspartate aminotransferase | 2 (2.6%) | 0 |
| Dizziness | 2 (2.6%) | 0 |
| Rash | 1 (1.3%) | 0 |
| Cardiac dysfunction | 1 (1.3%) | 1 (1.3%) |
| Nipple ulceration | 1 (1.3%) | 0 |
Discussion
Recently, TKIs, including lapatinib, neratinib, pyrotinib, and tucatinib, have greatly improved the survival of patients with HER2-positive breast cancer. Pyrotinib, a novel TKI synthesized in China, has been highly effective in metastatic breast cancer treatments. For example, the PHENIX study indicated that pyrotinib improved the prognosis of HER2-positive metastatic breast cancer after prior trastuzumab and taxane treatment. 10 The PHOEBE study also showed that pyrotinib provided a better PFS and ORR in metastatic HER2-positive breast cancer than lapatinib. 9
We evaluated the practice of exchange of TKIs and observed that pyrotinib was effective against lapatinib-resistant HER2-positive metastatic breast cancer. Patients with brain metastases also benefited from pyrotinib therapy after lapatinib failure. Patients who benefited from prior lapatinib treatment also had a significantly longer PFS, but no relationship was detected between pyrotinib effectiveness and age, HR status, trastuzumab resistance, metastasis type, or number of pyrotinib lines.
The use of pyrotinib in lapatinib-resistant HER2-positive breast cancer is controversial. A recent real-world study demonstrated that pyrotinib improved the survival of lapatinib-naïve patients more than those who received lapatinib. 12 Similarly, lapatinib-naïve patients reportedly had a longer PFS than lapatinib-treated patients who received subsequent pyrotinib + vinorelbine therapy. 13 However, another real-world study suggested that pyrotinib provided a significant longer PFS than T-DM1 among patients who initially responded to lapatinib. 14 These results imply that the efficacy of exchanging TKIs might depend on the response to prior TKI treatment. Patients who gained clinical benefits from prior TKI treatment could be recommended for another TKI to circumvent the drug resistance.
Lapatinib is a small molecule inhibitor that reversibly blocks EGFR (HER1) and HER2. 15 Various mechanisms are implicated in lapatinib resistance, including the activation of compensatory signaling pathways, mutation of HER2 or other key genes, changes in cell metabolism, and dysregulation of apoptosis or autophagy.16–18 The HER2 L755 S mutation reportedly induces lapatinib resistance in HER2-positive breast cancer, but could be overcome by a neratinib, a pan-HER TKI that targets HER1, HER2, and HER4.19,20 Pyrotinib, as an irreversible inhibitor of HER1, HER2, and HER4, 21 may also overcome lapatinib resistance by more broadly inhibiting receptor tyrosine kinases. Additional studies are required to further explore the mechanism by which pyrotinib overcomes lapatinib resistance and to identify key biomarkers for retreatment with TKIs and establish the benefits of exchange of TKI therapy.
In addition, we observed that pyrotinib improved the survival of patients with brain metastases after lapatinib failure. Similarly, in the PHENIX study, pyrotinib + capecitabine led to a longer PFS for patients with baseline brain metastases. 10 Yan et al. also reported that pyrotinib improved patient survival in patients with radiotherapy-naïve and radiotherapy-treated brain metastases. 22 These results support the promising role of pyrotinib in brain metastasis treatment.
Moreover, our data demonstrate that pyrotinib was well tolerated in patients with lapatinib-resistant HER2-positive breast cancer. Diarrhea is the most common adverse event associated with TKI therapy, 18 which was consistent with our findings. Therefore, the potential for overlapping toxicity must be considered in the practice of exchange of TKIs. While our data showed a relatively mild safety profile of pyrotinib, we believe that more studies are required to comprehensively evaluate the safety and quality of life with subsequent TKI therapy.
During the study period, neratinib, tucatinib, and T-DM1 were not accessible to most Chinese patients with breast cancer. For a long time, treatment of breast cancer with trastuzumab and lapatinib resistance has been a challenge for Chinese oncologists. Therefore, evaluating the exchange of TKI therapy has the potential to provide new rationale for and enhance the efficiency of anti-HER2 treatments and support optimal use of medical resources.
This study had several limitations. First, the retrospective design may have caused selection bias. Second, the sample size was relatively small, and the study design was not as rigorous as prior randomized trials. Third, this study lacked information regarding long-term survival. Further studies are required to verify the safety and efficacy of exchange of TKI therapy.
Conclusion
This real-world study suggests that pyrotinib could prolong the survival of patients with lapatinib-resistant HER2-positive breast cancer patients with moderate toxicity. Patients with brain metastases may also benefit from the subsequent use of pyrotinib, as can patients who benefited from prior lapatinib treatment.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221085232 for Treatment with pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer: a multicenter real-world study by Yijia Hua, Wei Li, Nan Jin, Dongyan Cai, Jie Sun, Chunxiao Sun, Fan Yang, Xinyu Wu, Xiang Huang, Biyun Wang and Yongmei Yin in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank Editage (www.editage.cn) for English language editing.
Footnotes
Author contributions: Yijia Hua: Data curation; Formal analysis; Writing – original draft.
Wei Li: Conceptualization; Methodology; Supervision; Writing – review & editing.
Nan Jin: Data curation; Formal analysis; Writing – original draft.
Dongyan Cai: Investigation; Resources; Writing – review & editing.
Jie Sun: Investigation; Resources; Writing – review & editing.
Chunxiao Sun: Methodology; Resources; Writing – review & editing.
Fan Yang: Methodology; Writing – review & editing.
Xinyu Wu: Methodology; Writing – review & editing.
Xiang Huang: Investigation; Writing – review & editing.
Biyun Wang: Conceptualization; Investigation; Project administration; Writing – review & editing.
Yongmei Yin: Conceptualization; Investigation; Project administration; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was financially supported by the National Key Research and Development Program of China (ZDZX2017ZL-01), High-level Innovation Team of Nanjing Medical University (JX102GSP201727), Wu Jieping Foundation (320.6750.17006), Key Medical Talents (ZDRCA2016023), 333 Project of Jiangsu Province (BRA2017534 and BRA2015470), the Collaborative Innovation Center for Tumor Individualization Focuses on Open Topics (JX21817902/008), and Project of China Key Research and Development Program Precision Medicine Research (2016YFC0905901).
ORCID iDs: Biyun Wang  https://orcid.org/0000-0002-7829-1544
https://orcid.org/0000-0002-7829-1544
Yongmei Yin  https://orcid.org/0000-0003-3335-369X
https://orcid.org/0000-0003-3335-369X
Data availability: All data analyzed in the study are included in the article. Further inquiries can be directed to the corresponding authors.
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Yijia Hua, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China; The First Clinical College of Nanjing Medical University, Nanjing, China.
Wei Li, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Nan Jin, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China; The First Clinical College of Nanjing Medical University, Nanjing, China.
Dongyan Cai, Department of Medical Oncology, The Affiliated Hospital of Jiangnan University Wuxi, China.
Jie Sun, Department of General Surgery, The First Affiliated Hospital of Soochow University, Suzhou, China.
Chunxiao Sun, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Fan Yang, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Xinyu Wu, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China; The First Clinical College of Nanjing Medical University, Nanjing, China.
Xiang Huang, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, Nanjing, China.
Biyun Wang, Department of Breast Cancer and Urological Medical Oncology, Fudan University Shanghai Cancer Center; Department of Oncology, Shanghai Medical College, Fudan University, No.270, Dong’an Road, Xuhui District, Shanghai 200032, China.
Yongmei Yin, Department of Oncology, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing 210029, China; Jiangsu Key Lab of Cancer Biomarkers, Prevention and Treatment, Collaborative Innovation Center for Personalized Cancer Medicine, Nanjing Medical University, Nanjing, China.
References
- 1. Waks AG, Winer EP. Breast cancer treatment: a review. JAMA 2019; 321: 288–300. [DOI] [PubMed] [Google Scholar]
- 2. Eroglu Z, Tagawa T, Somlo G. Human epidermal growth factor receptor family-targeted therapies in the treatment of HER2-overexpressing breast cancer. Oncologist 2014; 19: 135–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ross JS, Slodkowska EA, Symmans WF, et al. The HER-2 receptor and breast cancer: ten years of targeted anti-HER-2 therapy and personalized medicine. Oncologist 2009; 14: 320–368. [DOI] [PubMed] [Google Scholar]
- 4. Slamon DJ, Clark GM, Wong SG, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science 1987; 235: 177–182. [DOI] [PubMed] [Google Scholar]
- 5. Loibl S, Gianni L. HER2-positive breast cancer. Lancet 2017; 389: 2415–2429. [DOI] [PubMed] [Google Scholar]
- 6. Geyer CE, Forster J, Lindquist D, et al. Lapatinib plus capecitabine for HER2-positive advanced breast cancer. N Engl J Med 2006; 355: 2733–2743. [DOI] [PubMed] [Google Scholar]
- 7. Murthy RK, Loi S, Okines A, et al. Tucatinib, trastuzumab, and capecitabine for HER2-positive metastatic breast cancer. N Engl J Med 2020; 382: 597–609. [DOI] [PubMed] [Google Scholar]
- 8. Saura C, Oliveira M, Feng YH, et al. Neratinib plus capecitabine versus lapatinib plus capecitabine in HER2-positive metastatic breast cancer previously treated with >/= 2 HER2-directed regimens: phase III NALA trial. J Clin Oncol 2020; 38: 3138–3149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Xu B, Yan M, Ma F, et al. Pyrotinib plus capecitabine versus lapatinib plus capecitabine for the treatment of HER2-positive metastatic breast cancer (PHOEBE): a multicentre, open-label, randomised, controlled, phase 3 trial. Lancet Oncol 2021; 22: 351–360. [DOI] [PubMed] [Google Scholar]
- 10. Yan M, Bian L, Hu X, et al. Pyrotinib plus capecitabine for human epidermal growth factor receptor 2-positive metastatic breast cancer after trastuzumab and taxanes (PHENIX): a randomized, double-blind, placebo-controlled phase 3 study. Transl Breast Cancer Res 2020; 1: 13. [Google Scholar]
- 11. Wong H, Leung R, Kwong A, et al. Integrating molecular mechanisms and clinical evidence in the management of trastuzumab resistant or refractory HER-2(+) metastatic breast cancer. Oncologist 2011; 16: 1535–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lin Y, Lin M, Zhang J, et al. Real-world data of pyrotinib-based therapy in metastatic HER2-positive breast cancer: promising efficacy in lapatinib-treated patients and in brain metastasis. Cancer Res Treat 2020; 52: 1059–1066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Li Y, Qiu Y, Li H, et al. Pyrotinib combined with vinorelbine in HER2-positive metastatic breast cancer: a multicenter retrospective study. Front Oncol 2021; 11: 664429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li F, Xu F, Li J, et al. Pyrotinib versus trastuzumab emtansine for HER2-positive metastatic breast cancer after previous trastuzumab and lapatinib treatment: a real-world study. Ann Transl Med 2021; 9: 103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Xia W, Mullin RJ, Keith BR, et al. Anti-tumor activity of GW572016: a dual tyrosine kinase inhibitor blocks EGF activation of EGFR/erbB2 and downstream Erk1/2 and AKT pathways. Oncogene 2002; 21: 6255–6263. [DOI] [PubMed] [Google Scholar]
- 16. D’Amato V, Raimondo L, Formisano L, et al. Mechanisms of lapatinib resistance in HER2-driven breast cancer. Cancer Treat Rev 2015; 41: 877–883. [DOI] [PubMed] [Google Scholar]
- 17. Shi H, Zhang W, Zhi Q, et al. Lapatinib resistance in HER2+ cancers: latest findings and new concepts on molecular mechanisms. Tumour Biol 2016; 37: 15411–15431. [DOI] [PubMed] [Google Scholar]
- 18. Schlam I, Swain SM. HER2-positive breast cancer and tyrosine kinase inhibitors: the time is now. NPJ Breast Cancer 2021; 7: 56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Xu X, De Angelis C, Burke KA, et al. HER2 Reactivation through Acquisition of the HER2 L755S mutation as a mechanism of acquired resistance to HER2-targeted therapy in HER2(+) breast cancer. Clin Cancer Res 2017; 23: 5123–5134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Veeraraghavan J, Mistry R, Nanda S, et al. Abstract1911: HER2 L755S mutation is associated with acquired resistance to lapatinib and neratinib, and confers cross-resistance to tucatinib in HER2-positive breast cancer models. J Cancer Res 2020; 80: 1911. [Google Scholar]
- 21. Li X, Yang C, Wan H, et al. Discovery and development of pyrotinib: a novel irreversible EGFR/HER2 dual tyrosine kinase inhibitor with favorable safety profiles for the treatment of breast cancer. Eur J Pharm Sci 2017; 110: 51–61. [DOI] [PubMed] [Google Scholar]
- 22. Yan M, Ouyang Q, Sun T, et al. Pyrotinib plus capecitabine for HER2-positive metastatic breast cancer patients with brain metastases (PERMEATE): a multicenter, single-arm phase II study. J Clin Oncol 2021; 39: 1037–1037. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221085232 for Treatment with pyrotinib-based therapy in lapatinib-resistant HER2-positive metastatic breast cancer: a multicenter real-world study by Yijia Hua, Wei Li, Nan Jin, Dongyan Cai, Jie Sun, Chunxiao Sun, Fan Yang, Xinyu Wu, Xiang Huang, Biyun Wang and Yongmei Yin in Therapeutic Advances in Medical Oncology