Abstract
Juvenile dermatomyositis is a rare autoimmune myopathy of childhood, associated with systemic vasculopathy, primarily affecting the capillaries. Panniculitis is seen histologically in about 10% of patients with dermatomyositis; however, its clinical presentation is rare, with only 30 cases presented in the literature to date. The histopathology overlaps with other inflammatory disease states, and is almost identical to the panniculitis seen in lupus erythematous panniculitis. In the cases with both panniculitis and dermatomyositis, skin and muscle inflammation is usually the first clinical manifestation. We present a case of a 16-year-old female with panniculitis as the initial presenting feature of juvenile dermatomyositis in the context of a prior diagnosis of indeterminate colitis.
Keywords: Juvenile dermatomyositis, lupus erythematosus panniculitis, inflammatory bowel disease, lupus panniculitis, panniculitis
Introduction
Juvenile dermatomyositis (JDM) is a rare autoimmune myopathy of childhood, associated with a systemic vasculopathy, primarily affecting the capillaries. 1 The disease is characterized by cutaneous findings, such as heliotrope rash and Gottron’s papules, proximal muscle weakness, elevated creatinine kinase, and endomysial infiltration of mononuclear cells surrounding myofibers. 2 JDM has an annual incidence rate of two to three cases per one million children, with females affected two to five times more than males. 1 Current standard treatment for JDM includes high-dose systemic steroids, methotrexate (MTX), and hydroxychloroquine for initial treatment, and intravenous immunoglobulin (IVIG) and/or cyclophosphamide or biologic therapies for refractory cases. 3 Panniculitis has rarely been described in the setting of dermatomyositis (DM) in adult and pediatric patients despite 10% of patients having subclinical evidence of panniculitis on muscle biopsy. 4 In the cases reported, myositis almost always occurs before the panniculitis manifests.5–7 The histopathological findings of the panniculitis in JDM overlap with other inflammatory diseases, notably lupus erythematous panniculitis (LEP). We present the case of a 16-year-old female who developed panniculitis as the first manifestation of JDM, thought initially to be LEP, in the context of a prior diagnosis of inflammatory bowel disease (IBD).
Case report
The patient initially presented with fatigue, bloody stools, and low-grade fevers at the age of 14.5 years, and was diagnosed with atypical indeterminate colitis based on rectal biopsy findings. She was trialed on multiple formulations of mesalazine, oral budesonide, and oral prednisone, before symptom remission with a colon-specific oral mesalazine and rectal mesalazine. She was referred to pediatric rheumatology and dermatology clinics at age 16 years for new concerns of bruising and leg pain without any obvious injury, associated with underlying painful, firm palpable lesions on her thighs and upper arms (Figure 1). These lesions were suspected to be erythema nodosum in the context of her IBD diagnosis, but the distribution affecting the proximal limbs was atypical. Her rheumatologic review of systems was negative at that time, and her investigations demonstrated positive antinuclear antibody (ANA) ⩾1:640, anti-neutrophil cytoplasmic antibody with perinuclear pattern (P-ANCA), myeloperoxidase antibody (MPO) 1.8 Antibody Index Units (AI) (0.0–0.9 AI), and centromere B 1.4 AI (0.0–0.9 AI). Anti-ds DNA antibody and rheumatoid factor were negative; C3, C4, and immunoglobulins (Igs) were normal. A deep skin biopsy including the fascia was performed, and the pathology was consistent with LEP (Figure 2). She was started on hydroxychloroquine 300 mg PO daily with improvement.
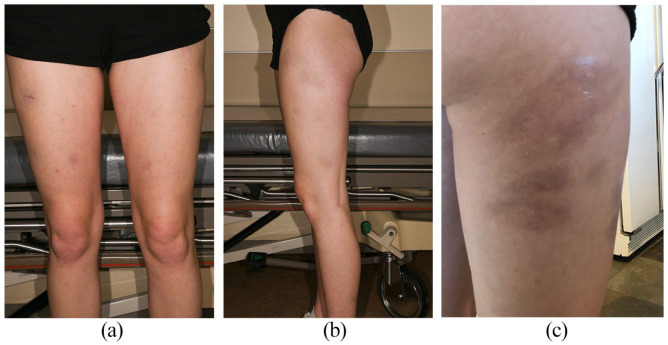
Figure 1.
Multiple painful gray-brown subtly indurated and depressed round nodules most prominent on the anterior (a) and lateral thighs bilaterally (b and c), initially diagnosed as lupus erythematosus panniculitis on skin biopsy before other clinical manifestations of juvenile dermatomyositis presented.
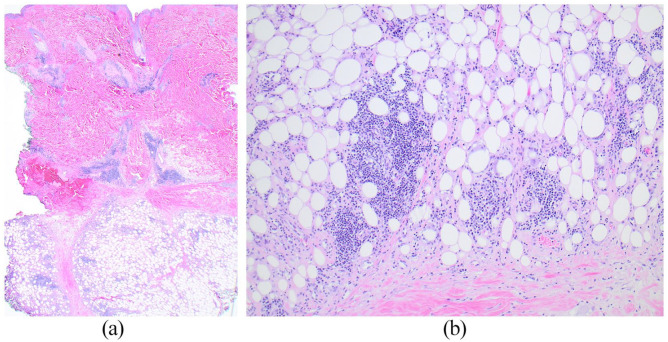
Figure 2.
Deep skin and subcutaneous biopsy of the lateral thigh: scanning magnification (a) shows a lobular panniculitis and a patchy superficial and deep dermal perivascular, perifollicular, and periadnexal lymphoplasmacytic inflammatory infiltrate without accompanying interface or epidermal changes (Hematoxylin & Eosin ×20). Higher magnification (b) shows the lymphoplasmacytic inflammation within lobules of subcutaneous fat and small foci of fat necrosis (Hematoxylin & Eosin ×100).
After 3 months of treatment, she developed periorbital edema with suborbital ecchymosis, facial rash in the malar distribution, myalgia, arthralgia, worsening fatigue, and 30 min of morning stiffness in her fingers with marked dilated nailfold capillaries. She was trialed on a 5-day course of 5 mg oral prednisone with no improvement so increased to 50 mg daily dosing with some improvement. Repeat investigations showed positive RNP-A at 1.3 AI (0.0–0.9 AI), medium positive anti-histone antibody, creatine kinase (CK) 312 (20–300 U/L), and Epstein–Barr virus (EBV) IgM positive, IgG negative. It was thought she had an intercurrent EBV infection with the Hoagland sign 8 as an explanation for the periorbital edema, and thus, her steroids were tapered. At lower doses of oral prednisone, she had increased myalgias, muscle weakness particularly with lifting arms overhead, and worsening periorbital edema with more prominent malar rash. Her myositis antibody panel was negative. As her weakness began to progress, her prednisone dose was increased to 60 mg PO daily when she became severely unwell with fever, hypotension, and profound weakness, resulting in an admission to the intensive care unit. A magnetic resonance imaging (MRI) of her muscles demonstrated widespread myositis (Figure 3). A muscle biopsy showed classic features of immune myopathy with perimysial pathology that was most consistent with the clinical entity of JDM.
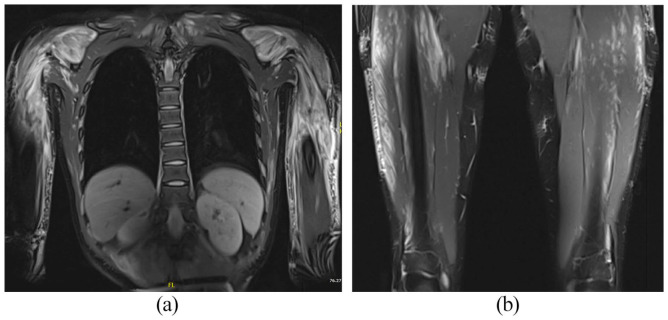
Figure 3.
Magnetic resonance imaging demonstrates diffuse extensive patchy hyperintense T2 signal intensity seen in multiple muscles of the shoulders, upper arms, chest wall, abdominal wall, bilateral paraspinal regions (a), bilateral psoas, and bilateral thighs (b), keeping with myositis.
She was treated with IVIG 2 g/kg, IV methylprednisolone 1 g daily for 5 days, MTX 25 mg subcutaneous weekly, and remained on hydroxychloroquine 300 mg daily. Her symptoms improved significantly throughout her stay and she was discharged after 11 days in hospital.
Discussion
Our patient demonstrates a rare occurrence of lobular panniculitis as a first dermatologic feature of JDM. Our initial diagnosis of LEP was reasonable, as the histopathologic findings of DM panniculitis are largely identical to those of lupus panniculitis,9,10 demonstrating the challenge of distinguishing between these entities histopathologically. LEP is a rare form of chronic cutaneous lupus erythematous (LE), characterized by inflammation of the subcutaneous fat, presenting in 1%–3% of patients with cutaneous LE. 11 The disease manifests as indurated plaques or nodules usually on the proximal extremities and trunk, which are tender or painful, may progress to calcifications or ulcerations, and frequently result in lipoatrophy upon resolution. 12 As with DM panniculitis, histopathology, demonstrates dermal perivascular infiltrates of mononuclear cells with lobular panniculitis, hyaline fat necrosis, paraseptal lymphoid aggregates, and lymphocytic vasculitis. 13 Usually, LEP and DM panniculitis can be differentiated based on the broader clinical picture. When the patient started demonstrating significant myositis, the diagnosis of JDM was considered more likely. Since her weakness worsened with the initial steroid wean, it was unlikely that her presentation was due to steroid-induced myopathy. 14 The patient’s periorbital edema was another early cutaneous finding, which has been reported as part of the clinical presentation of JDM in case reports only, but is not one of the classic cutaneous manifestations of JDM. 15
Our patient had another unique feature, with her prior diagnosis of indeterminate colitis and treatment with mesalazine. Mesalazine has been associated with drug-induced lupus. 16 Anti-histone antibodies are present in more than 95% of cases of drug-induced lupus, but can also occur in up to 80% of patients with idiopathic lupus. 17 Interestingly, anti-histone antibodies have also been reported in up to 17% of patients with DM. 18 It is known that IBD and DM can occur together. 19 In adult studies, the incidence of DM is higher in patients with ulcerative colitis (UC) compared to control groups, and the presence of UC may actually be a risk factor for DM. 20 Given that the entire clinical picture is compatible with JDM, and that there are no reports of mesalazine inducing JDM, her disease was likely not drug-induced. As a precaution, our patient discontinued mesalazine and continued MTX, high-dose prednisone, IVIG, and hydroxychloroquine to treat her colitis, skin manifestations, and myositis. At time of publication—3 months after her acute presentation—her panniculitis has resolved with lipoatrophy, she has normal functional muscle strength and her IBD symptoms are well controlled. This case is an example of overlapping inflammatory diseases occurring in a single patient, and the complex diagnostic dilemma of panniculitis as a presenting feature of evolving JDM.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Informed consent: Informed consent for information and images to be published was provided by the patient.
ORCID iD: Dylan C Ginter  https://orcid.org/0000-0002-6949-1085
https://orcid.org/0000-0002-6949-1085
References
- 1. Feldman BM, Rider LG, Reed AM, et al. Juvenile dermatomyositis and other idiopathic inflammatory myopathies of childhood. Lancet 2008; 371(9631): 2201–2212. [DOI] [PubMed] [Google Scholar]
- 2. Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of Rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups. Annals of the Rheumatic Diseases 2017; 76(12): 1955–1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Stringer E, Bohnsack J, Bowyer SL, et al. Treatment approaches to juvenile dermatomyositis (JDM) across North America: the childhood arthritis and rheumatology research alliance (CARRA) JDM treatment survey. J Rheumatol 2010; 37(9): 1953–1961. [DOI] [PubMed] [Google Scholar]
- 4. Castillo RL, Femia AN. Covert clues: the non-hallmark cutaneous manifestations of dermatomyositis. Ann Transl Med 2021; 9(5): 346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Polcari IC, Stein SL. Panniculitis in childhood. Dermatol Ther 2010; 23(4): 356–367. [DOI] [PubMed] [Google Scholar]
- 6. Salman A, Kasapcopur O, Ergun T, et al. Panniculitis in juvenile dermatomyositis: report of a case and review of the published work. J Dermatol 2016; 43(8): 951–953. [DOI] [PubMed] [Google Scholar]
- 7. Yoo JY, Jo SJ, Cho KH. Lupus panniculitis with combined features of dermatomyositis resulting in severe lipoatrophy. J Dermatol 2004; 31(7): 552–555. [DOI] [PubMed] [Google Scholar]
- 8. Inokuchi R, Iida H, Ohta F, et al. Hoagland sign. Emerg Med J 2014; 31(7): 561. [DOI] [PubMed] [Google Scholar]
- 9. Braunstein I, Werth VP. Update on management of connective tissue panniculitides. Dermatol Ther 2012; 25(2): 173–182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Santos-Briz A, Calle A, Linos K, et al. Dermatomyositis panniculitis: a clinicopathological and immunohistochemical study of 18 cases. J Eur Acad Dermatol Venereol 2018; 32(8): 1352–1359. [DOI] [PubMed] [Google Scholar]
- 11. Park HS, Choi JW, Kim B, et al. Lupus erythematosus panniculitis: clinicopathological, immunophenotypic, and molecular studies. Am J Dermatopathol 2010; 32(1): 24–30. [DOI] [PubMed] [Google Scholar]
- 12. Peters MS, Daniel Su WP. Lupus erythematosus panniculitis. Medical Clinics of North America 1989; 73(5): 1113–1126. [DOI] [PubMed] [Google Scholar]
- 13. Crowson AN, Magro C. The cutaneous pathology of lupus erythematosus: a review. J Cutan Pathol 2001; 28(1): 1–23. [DOI] [PubMed] [Google Scholar]
- 14. Gupta Y, Gupta A. Glucocorticoid-induced myopathy: pathophysiology, diagnosis, and treatment. Indian J Endocrinol Metab 2013; 17(5): 913–916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sevigny GM, Mathes BM. Periorbital edema as the presenting sign of juvenile dermatomyositis. Pediatr Dermatol 1999; 16(1): 43–45. [DOI] [PubMed] [Google Scholar]
- 16. Katsanos KH, Voulgari PV, Tsianos EV. Inflammatory bowel disease and lupus: a systematic review of the literature. J Crohn Colitis 2012; 6(7): 735–742. [DOI] [PubMed] [Google Scholar]
- 17. Yung RL, Johnson KJ, Richardson BC. New concepts in the pathogenesis of drug-induced lupus. Lab Invest 1995; 73(6): 746–759. [PubMed] [Google Scholar]
- 18. Kubo M, Ihn H, Yazawa N, et al. Prevalence and antigen specificity of anti-histone antibodies in patients with polymyositis/dermatomyositis. J Invest Dermatol 1999; 112(5): 711–715. [DOI] [PubMed] [Google Scholar]
- 19. Meneghel A, Zulian F, Martini G, et al. Ischemic ulcerative colitis in juvenile dermatomyositis. J Pediatr Gastroenterol Nutr 2009; 49(5): 549. [DOI] [PubMed] [Google Scholar]
- 20. Tseng C-C, Chang S-J, Liao W-T, et al. Increased cumulative incidence of dermatomyositis in ulcerative colitis: a nationwide cohort study. Sci Rep 2016; 6(1): 28175. [DOI] [PMC free article] [PubMed] [Google Scholar]