Abstract
Although fluoroquinolone antibacterials have a broad therapeutic use, with a relatively low incidence of severe side effects, they have been reported to induce lesions in the cartilage of growing animals by a mechanism that remains unclear. This study was undertaken to determine the potentially deleterious effect of a high dose of pefloxacin (400 mg/kg of body weight) on two main constituents of cartilage in mice, i.e., proteoglycans and collagen. Variations in levels of proteoglycan anabolism measured by in vivo [35S]sulfate incorporation into cartilage and oxidative modifications of collagen assessed by detection of carbonyl derivatives were monitored after administration of pefloxacin. Treatment of mice with 1 day of pefloxacin treatment significantly decreased the rate of biosynthesis of proteoglycan for the first 24 h. However, no difference was observed after 48 h. The decrease in proteoglycan synthesis was accompanied by a marked drop in serum sulfate concentration and a concomitant increase in urinary sulfate excretion. The decrease in proteoglycan synthesis, also observed ex vivo, may suggest a direct effect of pefloxacin on this process, rather than it being a consequence of a low concentration of sulfate. On the other hand, treatment with pefloxacin for 10 days induced oxidative damage to collagen. In conclusion, this study demonstrates, for the first time, that pefloxacin administration to mice leads to modifications in the metabolism and integrity of extracellular proteins, such as collagen and proteoglycans, which may account for the side effects observed. These results offer new insights to explain quinolone-induced disorders in growing articular cartilage.
Fluoroquinolones are potent antimicrobial agents that are widely used to treat infections of the respiratory or urinary tract, skin, and soft tissues. Some infrequent adverse reactions occur during their use, and one of the most important concerns is their potential chondrotoxicity in young patients. Indeed, because of their chondrotoxicity in growing animals (4, 8, 16, 43), quinolones are contraindicated for children and adolescents. Chondrotoxicity has been observed experimentally in growing animals after treatment with first-generation quinolones (21) and with fluoroquinolones (3, 23, 31, 44). Histologic changes were similar in all animals, with lesions occurring, as a rule, during growth (15), except with some fluoroquinolones (8, 5), which also produced characteristic lesions in skeletally mature dogs.
The specific mechanism responsible for the quinolone-induced arthropathy remains unclear, although several explanations have been postulated since its first description approximately 20 years ago (21). Some authors have suggested that chondrocytes are the primary site of quinolone toxicity (24, 45). Others postulated that quinolones interfere directly with the extracellular matrix of joint cartilage (1). More recently, chelation of magnesium ions by quinolones, resulting in changes in the functions of integrin receptors on the chondrocyte surface, was suggested (14, 43). In vitro studies of cartilage from various species have shown an inhibition of the synthesis of either collagen or glycosaminoglycans (3, 23, 24). Moreover, experimental data suggested compromised mitochondrial integrity (20, 24) and an early stimulation by fluoroquinolones of the oxidative metabolism within immature articular chondrocytes (19, 47), suggesting the generation of reactive oxygen species. The hypothesis that an oxidative stress occurs and participates in the pathophysiological effect seems attractive, as cartilage undergoes a chronic hypoxia resulting from the absence of vascularization.
In the present work, the effect of a single dose of pefloxacin (400 mg/kg of body weight) on the biosynthesis of proteoglycans, as revealed by in vivo 35S incorporation, in articular cartilage in mice was investigated. Indeed, 35S incorporation allows the study of the sulfatation of glycosaminoglycan chains covalently bound to the core protein. This approach allowed us to evaluate the time course of the pefloxacin-induced changes on proteoglycan synthesis. Moreover, we determined the oxidative modifications of collagen by monitoring administration of pefloxacin for 1 and 10 days and by measuring carbonyl derivatives. The long half-life of collagen (7) may allow the detection of damage induced by reactive oxygen species over several days.
MATERIALS AND METHODS
Animals.
Three- to 4-week-old male Swiss mice weighing 15 to 20 g (Charles River, Saint-Aubin-les-Elbeuf, France) were housed in solid-bottomed plastic cages designed to allow easy access to standard laboratory food and water ad libitum. The animals were kept on a cycle of 12 h of light and 12 h of dark in a temperature-controlled chamber and were cared for in accordance with the guidelines of the Institutional Animal Care and Use Committee and those of the National Institutes of Health for laboratory animal welfare.
For the first phase of the experiment, two groups of five mice received by gavage either a single dose of pefloxacin dihydrate mesylate (400 mg/kg, 10 μl/g in saline solution; kindly donated by Rhône-Poulenc Rorer Laboratories, Neuilly/Seine, France) or saline only and a concomitant intraperitoneal injection of Na235SO4 (2 μCi/g of body weight; Isotopchim; Amersham, Les Ulis, France). They were decapitated 2, 8, 16, 24, or 48 h later. In the second phase of the experiment (24 to 48 h), mice were given a single oral dose of pefloxacin (400 mg/kg) and 24 h later they received an intraperitoneal injection of radioactive sulfate (2 μCi of Na235SO4/g). Mice were sacrificed 48 h after pefloxacin administration (Fig. 1). Blood samples were collected, and the femoral head caps (FHCs) and patellae were dissected out. Cartilage samples were fixed overnight in 1 ml of cetylpyridinium chloride (Sigma) in phosphate-buffered formalin. Patellae were decalcified in 5% formic acid for 6 h, and then the central area of each patella was sampled with a 1-mm-diameter biopsy punch. The punched-out portions of the patellae (articular cartilage), the remaining peripheral parts of the patellae (fibrocartilage), and FHCs were dissolved overnight in Soluene-350 (Packard, Rungis, France). Blood samples (10 μl) were decolorized by adding 100 μl of propan-2-ol and 100 μl of H2O2 (30%). The amount of [35S]sulfate incorporated in each sample was counted by liquid scintillation spectrometry (Hionic Fluor and Packard). In both treated and untreated mice, the 35S contents in blood paralleled those in the corresponding sera. Therefore, blood samples were used as equivalents of serum samples in measurements of inorganic radiosulfate content. Results are expressed as the differences between the mean amounts of 35S incorporated in FHCs and patellae from animals treated with pefloxacin and values for control animals treated with saline solution.
FIG. 1.
Effects of pefloxacin administration on the kinetics of 35S incorporation in the blood and cartilage of mice in vivo.
Measurement of inorganic sulfate.
The content of inorganic sulfate was measured by turbidimetry, as described by Krijgsheld et al. (26, 27). To 500 μl of serum containing 0.1 to 1.3 mM inorganic sulfate, 2 ml of trichloroacetic acid solution (5% wt/vol) was added, and the mixture was allowed to stand at room temperature for 10 min. After centrifugation, 1 ml of the clear supernatant was mixed into 250 μl of BaCl2 reagent (20 g of BaCl2-2H2O and 100 g of dextran in 1 liter of distilled water) in a disposable semimicrocuvette, which was stirred. After 35 min, the absorbance at 360 nm was recorded against a blank consisting of 1 ml of supernatant and 250 μl of reagent containing 100 g of dextran/liter of distilled water. The amount of inorganic 35S in the serum was measured as described by De Vries et al. (12). After determination of serum sulfate concentration by turbidimetry, the contents of the cuvettes were centrifuged and the radioactivities associated with the pellet (inorganic sulfate fraction in the form of precipitable Ba35SO4) and the supernatant were counted. In normal and treated mice, 97% of 35S was in the inorganic form 35SO42−.
Ex vivo measurement of proteoglycan synthesis.
Proteoglycan synthesis was assayed as described by Van den Berg et al. (50). Briefly, pefloxacin (400 mg/kg daily, 10 μl/g) was administered orally to mice, which were killed by cervical dislocation 24 or 48 h after drug administration. The FHCs and patella samples were carefully dissected and were incubated in RPMI 1640 culture medium (200 μl/patella or FHC) containing gentamicin (50 μg/ml), l-glutamine (2 mM), and Na235SO4 (10 μCi/ml). After incubation for 2 h at 37°C in a 5% CO2 atmosphere, FHCs and patellae were washed with isotonic saline solution and fixed overnight in 0.5% cetylpyridinium chloride in phosphate-buffered formalin. Patellae were decalcified and sampled with a 1-mm-diameter biopsy punch as described above. Samples were then dissolved overnight in Soluene-350. Results are expressed as the differences between the mean amounts of 35S incorporated in FHCs and patellae from animals treated with pefloxacin and those for the control animals treated with the vehicle alone.
Collagen extraction.
Articular cartilage was harvested from the FHCs of mice treated with pefloxacin (400 mg/kg/day) for 1 or 10 days. Collagen was extracted from FHCs, which were washed with water and neutral salt solution (0.05 M Tris-HCl [pH 7.4], 0.9% [wt/vol] NaCl) to remove the salt-soluble material. The tissue was crushed and continuously stirred in a solution of 4 M guanidine-HCl in 0.05 M sodium acetate (pH 5.8) at 4°C for 24 h to remove proteoglycans (48). After centrifugation for 30 min at 30,000 × g, the residue was collected and washed three times with 0.05 M acetic acid. The collagen residue was added to a solution of 1 mg of pepsin per ml of 0.5 M acetic acid in a weight ratio of 1/10 (sample to pepsin) and stirred for 2 days at 4°C (32). Undigested solid material was removed by centrifugation at 30,000 × g, for 30 min. This method of pepsin digestion was chosen experimentally to extract more than 90% of the collagen, as determined by measurement of the hydroxyproline content in the supernatant after pepsin digestion and in FHCs after acid hydrolysis (52). Protein concentration was determined by the Lowry assay, with bovine serum albumin as the standard (30).
Protein derivatization with DNPH and by SDS-PAGE.
Collagen (50 μg) was treated with an equal volume of 0.5 mM dinitrophenylhydrazine (DNPH) (in 0.1 M sodium phosphate buffer, pH 6.3) for 1 h at room temperature. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) was performed according to the method of Laemmli (28) with 1-mm-thick 6% slab gels (16- by 18-cm format). The same amount of collagen (15 μg) was loaded into all lanes. After SDS-PAGE, the gels were transferred onto Immobilon-P membranes with a Trans-Blot electrophoretic transfer apparatus according to the method of Towbin et al. (49). Immunochemical detection of protein carbonyls was performed as described by Keller et al. (25) and Shacter et al. (40). Briefly, the blots were incubated with 0.1% Ponceau S (wt/vol) in 5% acetic acid (vol/vol) and destained in methanol until the bands appeared. Then blots were incubated with bovine serum albumin (3%) for at least 90 min, followed by incubation at room temperature with Sigma rabbit anti-dinitrophenyl antibodies (diluted 1:2,000 in 9 mM Tris-HCl [pH 9.0], 154 mM NaCl, 0.05% Tween 20 [vol/vol] [TBST]). The primary antibody was removed, and the blots were washed three times (10 min each time) with TBST. The blots were incubated with alkaline phosphatase-conjugated goat anti-rabbit immunoglobulin G (Sigma) (diluted 1:5,000 in TBST) for 90 min at room temperature. After washing of the blots with TBST three times (10 min each time), oxidized proteins were revealed by the addition of 5-bromo-4-chloro-3-indolylphosphate (BCIP)–nitroblue tetrazolium. The intensities of the bands were quantified with densitometry analysis software (NIH Image), and results are expressed in arbitrary units.
Statistical analysis.
Biochemical data are presented as means ± standard errors of the means (SEM). Groups were compared by two-way analysis of variance, with a P of <0.05 taken as the significance level.
RESULTS
In vivo effects of a single dose of pefloxacin on the contents of 35S in blood and cartilage in mice. (i) First-phase experiment (early effects).
The effect on proteoglycan synthesis of a single oral dose (400 mg/kg) of pefloxacin depended on the tissue studied. In both treated and control mice, the amount of radioactivity in blood decreased with time until 16 h whereas that in FHCs and patellae increased with a maximum reached at 16 to 24 h (Fig. 2 and 3). This increase was followed by a decrease until 48 h after the injection. Eight hours after injection, pefloxacin-treated animals displayed less radioactivity than control animals in blood (37% less), in cartilaginous structures and FHCs (36% less), in patellar central cartilage (23% less), and in patellar peripheral fibrocartilage (26% less). Similar differences were observed at 16 and 24 h and persisted after 48 h in the central cartilage and peripheral fibrocartilage of patellae but not in blood or FHCs. The amount of radioactivity in blood was 34% lower in the treated mice than in the control mice. Throughout these tests, urinary excretion of 35S was twofold higher in the treated mice than in the controls (data not shown).
FIG. 2.
Effects in mice of treatment with a single oral dose (400 mg/kg) of pefloxacin on the incorporation of simultaneously administered 35S in vivo in blood (log plot) (A) and FHCs (B). Controls received the same volume of saline solution instead of pefloxacin. Values for 35S incorporation are means ± SEM of results from four experiments. ∗, P < 0.05; ∗∗, P < 0.01 versus controls, by Student’s t test.
FIG. 3.
Effect in mice of the same treatment described for Fig. 1 on the incorporation of 35S in patellar central cartilage and peripheral fibrocartilage (log plot). Controls received the same volume of saline solution instead of pefloxacin. Values for 35S incorporation are means ± SEM of results from four experiments with five mice each for levels of radioactivity incorporated. ∗, P < 0.05; ∗∗, P < 0.01 versus controls, by Student’s t test.
(ii) Second-phase experiment (late effects).
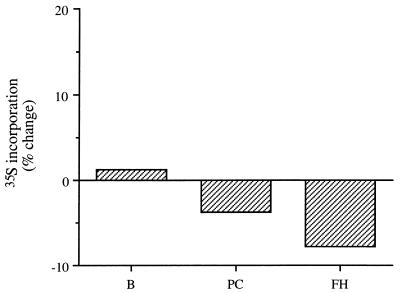
In these second-phase experimental group, 48 h after a single dose of pefloxacin (400 mg/kg) and 24 h after injection of 35S, proteoglycan synthesis was not significantly different from that in controls in patellar cartilage or FHCs (Fig. 4). The amounts of radioactivity in blood were similar in the treated mice and in the controls.
FIG. 4.
Proteoglycan synthesis measured by the in vivo changes in levels of incorporation of 35S 48 h after a single oral dose (400 mg/kg) of pefloxacin and 24 h after intraperitoneal administration of 35S (2 μCi/g). Values are percentages reflecting the difference in 35S incorporation in treated mice from that in controls receiving the same volume of saline only (means of results from two experiments with five mice each). B, blood; PC, central patellar cartilage; FH, FHCs.
Effect of pefloxacin on the endogenous inorganic-sulfate concentration in mice sera 24 h after drug administration.
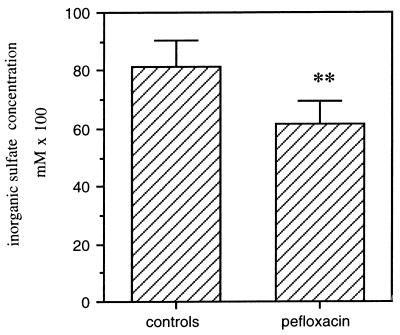
An acute pefloxacin administration (400 mg/kg) caused a marked decrease (30%) in the endogenous sulfate concentration in serum (Fig. 5). The concentration of inorganic sulfate decreased from the physiologic level in serum (0.95 mM) to approximately 0.66 mM and returned to the control level after 48 h.
FIG. 5.
Effect of pefloxacin (400 mg/kg) administered orally to mice on the concentration of inorganic sulfate 24 h after drug administration. Values for inorganic sulfate are means ± SEM of sulfate concentrations from three experiments. ∗∗, P < 0.01 versus controls, by Student’s t test.
Ex vivo effects of a single dose (400 mg/kg) of pefloxacin on the 35S contents in the cartilage of mice. (i) Twenty-four hours after a single oral dose.
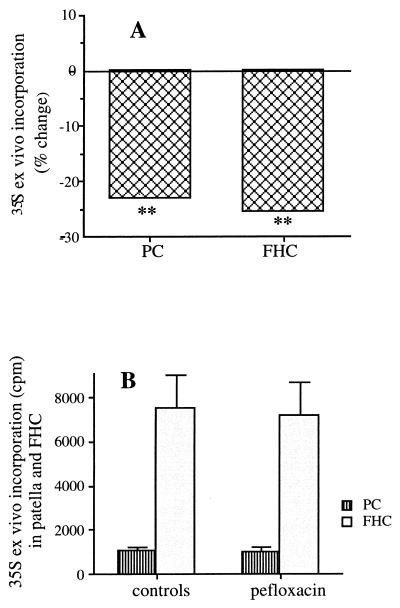
The ex vivo levels of incorporation of 35S into FHCs and patellar central cartilage decreased by 22 and 25%, respectively, 24 h after pefloxacin administration (Fig. 6A).
FIG. 6.
Effects of pefloxacin on proteoglycan synthesis measured by the changes in the levels of incorporation of 35S ex vivo 24 h after a single oral dose (400 mg/kg), relative to the levels of incorporation in controls receiving the same volume of saline solution (A), and 48 h after a single oral dose (400 mg/kg), relative to the levels of incorporation in controls receiving the same volume of saline only (B). Duplicate experiments were performed with eight mice each. PC, central patellar cartilage.
(ii) Forty-eight hours after a single oral dose.
Proteoglycan synthesis in FHCs and patellar central cartilage returned to control values 48 h after pefloxacin administration (Fig. 6B).
Effect of a pefloxacin treatment (400 mg/kg/day) on carbonyl derivative formation in type II collagen.
Oxidized proteins as revealed by Western blot analysis were used to determine the susceptibility of collagen to oxidative modification due to pefloxacin treatment. Tissues from control animals and those treated with pefloxacin for 1 or 10 days were assayed. Collagen was extracted from FHCs and analyzed by SDS-PAGE, after incubation with DNPH. We observed a characteristic band corresponding to the α1(II) chains (Fig. 7). No difference was observed 24 h after a single dose of pefloxacin. A higher carbonyl derivative content, measured by densitometry, was observed in collagen obtained from the FHCs of mice treated daily with pefloxacin for 10 days (400 mg/kg/day) (Fig. 7 and 8).
FIG. 7.
Effect of pefloxacin administration on carbonyl derivative formation in the collagen of articular cartilage revealed by immunochemical detection. Fifteen micrograms of DNPH-derivatized protein was loaded into each lane. (A) Lanes 1 to 3, articular cartilage collagen of mice treated with a single dose of pefloxacin (400 mg/kg); lanes 4 to 6, articular cartilage collagen of mice given saline solution. (B) Lanes 1 to 4, articular cartilage collagen of mice which received pefloxacin (400 mg/kg/day) for 10 days (duplicate loads); lanes 5 to 8, articular cartilage collagen of mice receiving saline solution.
FIG. 8.
Effect of pefloxacin administration on carbonyl derivative formation in articular cartilage collagen. Shown is a densitometric analysis of the blots in Fig. 7. Values are means ± standard deviations of results from two experiments. ∗, P ≤ 0.05 versus controls, by Student’s t test.
DISCUSSION
Although fluoroquinolones have a broad therapeutic potential and a relatively low incidence of serious side effects, they are known to induce lesions of cartilage in growing animals. Pefloxacin has also been reported to induce side effects on articular cartilage in adult dogs, resulting in arthropathy (5, 8, 16). Numerous in vitro studies of cartilage from various species have shown an inhibition of the synthesis of either collagen or glycosaminoglycans under these conditions (3, 23, 24). The damage to articular cartilage promoted by pefloxacin may result from an oxidative stress (17, 47). As free radicals may alter the extracellular matrix, we determined levels of collagen oxidation through the formation of carbonyl derivatives and modifications of cellular activity by proteoglycan anabolism by measuring 35S incorporation in an in vivo mouse model. The doses of pefloxacin administered to mice in this study were much higher than those usually delivered to humans but are common in such toxicological studies. Nevertheless, half-lives of this drug ranged from 1.9 h in mice to 8.6 h in humans (36). Thus, pefloxacin is metabolized more rapidly in mice, and this dose may allow a sufficient tissular diffusion.
The time course study of 35S incorporation showed that in control mice, very little radioactivity remained in the blood 24 h after the 35S injection and that the highest values of radioactivity in the other tissues studied were obtained between 16 and 24 h. When administered simultaneously with 35S, pefloxacin induced a decrease in 35S incorporation in all tissues studied. By contrast, 48 h after a single (400-mg/kg) pefloxacin administration and 24 h after the 35S injection, proteoglycan synthesis in patellar cartilage or FHCs did not significantly differ from that in controls. This reversible change in proteoglycan synthesis in cartilage may reflect a repair process, suggesting that cells recovered their normal functions. This apparent recovery differs from the results of Ingham et al. (21), who observed long-lasting cartilage lesions still present at autopsy. Förster et al. (13) also described irreversible lesions induced by ofloxacin in rats. On the other hand, Kato and Onodera (23) briefly described repair-like processes in the joint cartilage of immature rats that had been treated with ofloxacin. The changes in sulfate incorporation reversibility that we observed 48 h after a single administration of pefloxacin are in agreement with the results of a study of the uptake of 35SO4 by cultured rabbit chondrocytes incubated with levofloxacin (24).
The present data demonstrate a marked fall in the endogenous serum sulfate level 24 h after an acute administration of pefloxacin and a concomitant increase in sulfate urinary excretion. More precisely, the total decrease in serum SO42− concentration at 24 h paralleled the decrease in serum 35S content. Consequently, the specific activities of circulating [35S]sulfate remained identical in both pefloxacin-treated and control mice. A similar situation was also reported in another study of salicylate effects on cartilage, which used a different duration between drug and radiolabel administrations (12). These results suggest that pefloxacin may inhibit the renal absorption of sulfate or may require sulfation for its elimination. Other possible mechanisms for the reduced sulfate incorporation are a direct action of pefloxacin on chondrocyte metabolism and a drug-induced decrease in sulfate availability.
In order to determine if the decrease in 35S incorporation results from a direct effect of pefloxacin on cartilage or from a low concentration of sulfate, we evaluated the pefloxacin effects in an ex vivo system. Using the measured sulfate concentration, we confirmed that pefloxacin inhibits proteoglycan synthesis 24 h after its administration and that this response is reversed after 48 h, as demonstrated in vivo. These results confirm previous work dealing with the in vitro effects of fluoroquinolones on articular chondrocytes (3, 24). A decrease in the ex vivo level of 35S incorporation into the articular cartilage 12 and 24 h after a single administration of ofloxacin (900 and 2,700 mg/kg) has also been described (23). Therefore, the decrease in 35S incorporation we observed during in vivo experiments seemed to be the result of a direct effect of pefloxacin on tissue metabolism rather than the result of a low concentration in sulfate. Thus, these results demonstrate that a single administration of pefloxacin induces early cellular damage which leads to a transitory decrease in proteoglycan anabolism. This matrix component can be replaced relatively quickly, as opposed to collagen, which is much less readily released, but when degradation of collagen does occur, the structural integrity of the tissue is lost (7). Therefore, we investigated the possible oxidative influence of pefloxacin on collagen, which can participate in the damage produced during several days of treatment.
Several reports showed an early stimulation of oxidative metabolism due to fluoroquinolone in immature articular chondrocytes (18, 19, 47), possibly resulting in the generation of reactive oxygen species. Accordingly, compromised mitochondrial integrity has also been described (20, 35). In an attempt to demonstrate in vivo oxidative damage due to pefloxacin, we measured the content of protein carbonyl in purified collagen. It is well known that oxidative stress increases the protein carbonyl content by a direct oxidative attack on amino acid side chains (11). Another mechanism is the modification of side chains by lipid peroxidation products. Therefore, the measurement of protein carbonyls is usually considered a marker of oxidative injury (38, 42). In the present study, we report for the first time that pefloxacin induces oxidative damage to cartilage collagen. An increase of collagen carbonyl derivative content was observed in mice treated for 10 days, whereas no changes appeared after an acute administration of pefloxacin. This oxidative damage to collagen was observed after 5 days of daily treatment. Identical results have been obtained with rats in the same experimental condition (data not shown). These results suggest that repeated administrations of pefloxacin induce the production of oxidizing species in the extracellular matrix of articular cartilage and that this production promotes some oxidative damage to collagen. These oxidizing species may be oxygen-derived reactive species that are formed as a result of modifications in chondrocyte mitochondrial and respiratory activities, as suggested by previous work (18, 19). The formation of oxidative species is sufficient to promote alterations to connective tissue macromolecules, as was observed in collagen. Oxygen-reactive species formation may also explain the inhibition observed on the synthesis of proteoglycan in vivo and ex vivo in mice (46). In this way, previous data demonstrated that reactive oxygen species alter cartilage metabolism and structure when they are overproduced (37). It has been established that oxygen-derived reactive species may promote extracellular matrix modifications, by intensive cross-linking and polymerization of the protein (34, 39, 51) or by activation of latent metalloproteinases (2, 29). Moreover, in vitro localization of radical formation within a collagen molecule was observed at particular sites (17). It was also established that oxidants can directly degrade soluble collagen and at low levels can modify collagen, making it susceptible to proteolytic degradation (10, 33).
These results clearly demonstrated that pefloxacin induced two effects on cartilage, one at the cellular and one at the matrix level. Both effects may contribute to cartilage damage. Cartilage homeostasis is regulated, in part, by the interaction of chondrocytes with their extracellular matrix and depends on interplay between integrins and matrix components related to “outside-in” signaling (9). The oxidative stress induced by pefloxacin may modify cartilage organization by altering mitochondrial function (6), the intracellular redox state, and some cellular signaling pathways (22). For instance, the expression of intercellular adhesion molecule 1 has been shown to be modulated by oxidative stress (53). Moreover, it has been observed that chondrocytes adjacent to fissures in articular cartilage in rats treated with fluoroquinolone have a reduced integrin expression (14), an observation which was confirmed with mice (41). Moreover, cartilage of growing animals may be more susceptible to quinolones than that of adults, as chondrocytes must produce a greater quantity of matrix macromolecules leading to cartilage structural integrity. Also, pefloxacin-induced oxidative stress may modify growing cartilage organization in a faster and more deleterious way than in mature cartilage.
In conclusion, our results indicate that the oral administration of a high dose of pefloxacin to mice induces modifications of the extracellular matrix, as was observed with collagen, suggesting the production of oxygen-derived reactive species. This study also revealed early cellular changes, as evidenced by modifications of proteoglycan anabolism. This double impact, one at the cellular level and one at the matrix level, offers new insights to explain quinolone-induced disorders in articular cartilage. Owing to metabolic needs, a fluoroquinolone-induced oxidative stress may alter immature cartilage organization more seriously than that of mature cartilage. Nevertheless, these results should be considered with care in relation to the adverse effects of pefloxacin reported for humans. On the other hand, further study would be necessary to evaluate the potentially protective effect of antioxidants.
ACKNOWLEDGMENT
This study was supported by a grant from Rhône-Poulenc Rorer.
REFERENCES
- 1.Bendele A M, Hulman J F, Harvey A K, Hrubey P S, Chandrasekhar S. Passive role of articular chondrocytes in quinolone-induced arthropathy in guinea pigs. Toxicol Pathol. 1990;18:304–312. doi: 10.1177/019262339001800209. [DOI] [PubMed] [Google Scholar]
- 2.Brenneisen P, Briviba K, Wlaschek M, Wenk J, Scharffetter- Kochanek K. Hydrogen peroxide (H2O2) increases the steady-state mRNA levels of collagenase/MMP-1 in human dermal fibroblasts. Free Radic Biol Med. 1997;22:515–524. doi: 10.1016/s0891-5849(96)00404-2. [DOI] [PubMed] [Google Scholar]
- 3.Burkhardt J E, Walterspiel J N, Schaad U B. Quinolone arthropathy in animals versus children. Clin Infect Dis. 1997;25:1196–1204. doi: 10.1086/516119. [DOI] [PubMed] [Google Scholar]
- 4.Burkhardt J E, Hill M A, Lamar C H, Smith G N, Carlton W W. Effects of difloxacin on the metabolism of glycosaminoglycans and collagen in organ cultures of articular cartilage. Fundam Appl Toxicol. 1993;20:257–263. doi: 10.1006/faat.1993.1034. [DOI] [PubMed] [Google Scholar]
- 5.Burkhardt J E, Hill M A, Carlton W W, Kesterson J W. Histologic and histochemical changes in articular cartilages of immature Beagle dogs dosed with difloxacin, a fluoroquinolone. Vet Pathol. 1990;27:162–170. doi: 10.1177/030098589002700303. [DOI] [PubMed] [Google Scholar]
- 6.Cardoso S M, Pereira C, Oliveira C R. Mitochondrial function is differentially affected upon oxidative stress. Free Radic Biol Med. 1999;26:3–13. doi: 10.1016/s0891-5849(98)00205-6. [DOI] [PubMed] [Google Scholar]
- 7.Cawston T E, Curry V A, Summers C A, Clark I M, Riley G P, Life P F, Spaull J R, Goldring M B, Koshy P J, Rowan A D, Shingleton W D. The role of oncostatin M in animal and human connective tissue collagen turnover and its localization within the rheumatoid joint. Arthritis Rheum. 1998;41:1760–1771. doi: 10.1002/1529-0131(199810)41:10<1760::AID-ART8>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 8.Christ W, Lehnert T, Ulbrich B. Specific toxicologic aspects of the quinolones. Rev Infect Dis. 1988;10(Suppl. 1):S141–S146. doi: 10.1093/clinids/10.supplement_1.s141. [DOI] [PubMed] [Google Scholar]
- 9.Clancy R M, Rediske J, Tang X, Nijher N, Frenkel S, Philips M, Abramson S B. Outside-in signaling in the chondrocyte. J Clin Investig. 1997;100:1789–1796. doi: 10.1172/JCI119706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Curran S F, Amoruso M A, Goldstein B D, Berg R A. Degradation of soluble collagen by ozone or hydroxyl radicals. FEBS Lett. 1984;176:155–160. doi: 10.1016/0014-5793(84)80931-x. [DOI] [PubMed] [Google Scholar]
- 11.Dean R T, Fu S, Stocker R, Davies M J. Biochemistry and pathology of radical-mediated protein oxidation. Biochem J. 1997;324:1–18. doi: 10.1042/bj3240001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.De Vries B J, van den Berg W B, van de Putte L B. Salicylate-induced depletion of endogenous inorganic sulfate. Arthritis Rheum. 1985;28:922–929. doi: 10.1002/art.1780280812. [DOI] [PubMed] [Google Scholar]
- 13.Förster C, Kociok K, Shakibaei M, Merker H J, Vormann J, Günther T, Stahlmann R. Integrins on joint cartilage chondrocytes and alterations by ofloxacin or magnesium deficiency in immature rats. Arch Toxicol. 1996;70:261–270. doi: 10.1007/s002040050272. [DOI] [PubMed] [Google Scholar]
- 14.Förster C, Kociok K, Shakibaei M, Merker H J, Stahlmann R. Quinolone-induced cartilage lesions are not reversible in rats. Arch Toxicol. 1996;70:474–481. doi: 10.1007/s002040050301. [DOI] [PubMed] [Google Scholar]
- 15.Gough A W, Barsoum N J, Renlund R C, Sturgess J M, de la Iglesia F A. Fine structural changes during reparative phase of canine drug-induced arthropathy. Vet Pathol. 1985;22:82–84. doi: 10.1177/030098588502200113. [DOI] [PubMed] [Google Scholar]
- 16.Gough A W, Kasali O B, Sigler R E, Baragi V. Quinolone arthropathy-acute toxicity to immature articular cartilage. Toxicol Pathol. 1992;20:436–450. doi: 10.1177/019262339202000313. [DOI] [PubMed] [Google Scholar]
- 17.Hawkins C L, Davies M J. Oxidative damage to collagen and related substrates by letal ion/hydrogen peroxide systems: random attack or site-specific damage? Biochim Biophys Acta. 1997;1360:84–96. doi: 10.1016/s0925-4439(96)00069-5. [DOI] [PubMed] [Google Scholar]
- 18.Hayem G, Domarle O, Fay M, Thuong-Guyot M, Pocidalo J J, Carbon C. Lack of correlation between hydrogen peroxide production and nitric oxide production by cultured rabbit articular chondrocytes treated with fluoroquinolone antimicrobial agents. Toxicol In Vitro. 1996;10:551–555. doi: 10.1016/s0887-2333(96)00046-x. [DOI] [PubMed] [Google Scholar]
- 19.Hayem G, Petit P X, Levacher M, Gaudin C, Kahn M F, Pocidalo J J. Cytofluorometric analysis of chondrotoxicity of fluoroquinolone antimicrobial agents. Antimicrob Agents Chemother. 1994;38:243–247. doi: 10.1128/aac.38.2.243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hildebrand H, Kempka G, Schlüten G, Schmidt M. Chondrotoxicity of quinolones in vivo and in vitro. Arch Toxicol. 1993;67:411–415. doi: 10.1007/BF01977402. [DOI] [PubMed] [Google Scholar]
- 21.Ingham B, Brentnall D W, Dale E A, MacFadzean J A. Arthropathy induced by antibacterial fused N-alkyl-3-pyridone-carboxylic acids. Toxicol Lett. 1977;1:21–26. [Google Scholar]
- 22.Kamata H, Hirata H. Redox regulation of cellular signaling. Cell Signal. 1999;11:1–14. doi: 10.1016/s0898-6568(98)00037-0. [DOI] [PubMed] [Google Scholar]
- 23.Kato M, Onodera T. Effects of ofloxacin on the uptake of (3H)thymidine by articular cartilage cells in the rat. Toxicol Lett. 1988;44:131–142. doi: 10.1016/0378-4274(88)90139-7. [DOI] [PubMed] [Google Scholar]
- 24.Kato M, Takada S, Ogawara S, Takayama S. Effect of levofloxacin on glycosaminoglycan and DNA synthesis of cultured rabbit chondrocytes at concentrations inducing cartilage lesions in vivo. Antimicrob Agents Chemother. 1995;39:1979–1983. doi: 10.1128/aac.39.9.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Keller R J, Halmes N C, Hinson J A, Pumford N R. Immunochemical detection of oxidized proteins. Chem Res Toxicol. 1993;6:430–433. doi: 10.1021/tx00034a007. [DOI] [PubMed] [Google Scholar]
- 26.Krijgsheld K R, Glazenburg E J, Scholtens E, Mulder G J. The oxidation of l-and d-cysteine to inorganic sulfate and taurine in the rat. Biochim Biophys Acta. 1981;677:7–12. doi: 10.1016/0304-4165(81)90139-2. [DOI] [PubMed] [Google Scholar]
- 27.Krijgsheld K R, Scholtens E, Mulder G J. An evaluation of methods to decrease the availability of inorganic sulphate for sulphate conjugation in the rat in vivo. Biochem Pharmacol. 1981;30:1973–1979. doi: 10.1016/0006-2952(81)90208-2. [DOI] [PubMed] [Google Scholar]
- 28.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 29.Lo Y Y, Conquer J A, Grinstein S, Cruz T F. Interleukin-1 beta induction of C-fos and collagenase expression in articular chondrocytes: involvement of reactive oxygen species. J Cell Biochem. 1998;69:19–29. doi: 10.1002/(sici)1097-4644(19980401)69:1<19::aid-jcb3>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 30.Lowry O H, Rosebrough N J, Farr A L, Randall R J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- 31.Machida M, Kusajima H, Aijima H, Maeda A, Ishida R, Uchida H. Toxicokinetic study of norfloxacin-induced arthropathy in juvenile animals. Toxicol Appl Pharmacol. 1990;105:403–412. doi: 10.1016/0041-008x(90)90144-j. [DOI] [PubMed] [Google Scholar]
- 32.Miller E J. Structural studies on cartilage collagen employing limited cleavage and solubilization with pepsin. Biochemistry. 1972;11:4903–4909. doi: 10.1021/bi00776a005. [DOI] [PubMed] [Google Scholar]
- 33.Monboisse J C, Poulin G, Braquet P, Randoux A, Ferradini C, Borel J P. Effect of oxygen radicals on several types of collagen. Int J Tissue React. 1984;5:385–390. [PubMed] [Google Scholar]
- 34.Monnier V M, Glomb M, Elgawish A, Sell D R. The mechanism of collagen cross-linking in diabetes: a puzzle nearing resolution. Diabetes. 1996;45:S67–S72. doi: 10.2337/diab.45.3.s67. [DOI] [PubMed] [Google Scholar]
- 35.Mont M A, Mathur S K, Frondoza C G, Hungerford D S. The effects of ciprofloxacin on human chondrocytes in cell culture. Infection. 1996;24:151–155. doi: 10.1007/BF01713325. [DOI] [PubMed] [Google Scholar]
- 36.Montay G, Goueffon Y, Roquet F. Absorption, distribution, metabolic fate, and elimination of pefloxacin mesylate in mice, rats, dogs, monkeys, and humans. Antimicrob Agents Chemother. 1984;25:463–472. doi: 10.1128/aac.25.4.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Panasyuk A, Frati E, Ribault D, Mitrovic D. Effect of reactive oxygen species on the biosynthesis and structure of newly synthesized proteoglycans. Free Radic Biol Med. 1994;16:157–167. doi: 10.1016/0891-5849(94)90139-2. [DOI] [PubMed] [Google Scholar]
- 38.Robinson C E, Keshavarzian A, Pasco D S, Frommel T O, Winship D H, Holmes E W. Determination of protein carbonyl groups by immunoblotting. Anal Biochem. 1999;266:48–57. doi: 10.1006/abio.1998.2932. [DOI] [PubMed] [Google Scholar]
- 39.Sajithlal G B, Chithra P, Chandrakasan G. Effect of curcumin on the advanced glycation and cross-linking of collagen in diabetic rats. Biochem Pharmacol. 1998;56:1607–1614. doi: 10.1016/s0006-2952(98)00237-8. [DOI] [PubMed] [Google Scholar]
- 40.Shacter E, Williams J A, Lim M, Levine R L. Differential susceptibility of plasma proteins to oxidative modification: examination by Western blot immunoassay. Free Radic Biol Med. 1994;17:429–437. doi: 10.1016/0891-5849(94)90169-4. [DOI] [PubMed] [Google Scholar]
- 41.Shakibaei M, Förster C, Merker H J, Stahlmann R. Effects of ofloxacin on integrin expression on epiphyseal mouse chondrocytes in vitro. Toxicol In Vitro. 1995;9:107–116. doi: 10.1016/0887-2333(94)00198-4. [DOI] [PubMed] [Google Scholar]
- 42.Stadtman E R, Oliver C N. Metal-catalysed oxidation of proteins. J Biol Chem. 1991;266:2005–2008. [PubMed] [Google Scholar]
- 43.Stahlmann R, Förster C, Shakibaei M, Vormann J, Günther T, Merker H J. Magnesium deficiency induces joint cartilage lesions in juvenile rats which are identical to quinolone-induced arthropathy. Antimicrob Agents Chemother. 1995;39:2013–2018. doi: 10.1128/aac.39.9.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Stahlmann R, Merker H J, Hinz N, Chahoud I, Webb J, Heger W, Neudert D. Ofloxacin in juvenile non-human primates and rats. Arthropathia and drug plasma concentrations. Arch Toxicol. 1990;64:193–204. doi: 10.1007/BF02010725. [DOI] [PubMed] [Google Scholar]
- 45.Takada S, Kato M, Takayama S. Comparison of lesions induced by intra-articular injections of quinolones and compounds damaging cartilage components in rat femoral condyles. J Toxicol Environ Health. 1994;42:73–88. doi: 10.1080/15287399409531864. [DOI] [PubMed] [Google Scholar]
- 46.Tanaka H, Okada T, Konoshi H, Tsuji T. The effect of reactive oxygen species on the biosynthesis of collagen and glycosaminoglycans in cultured human dermal fibroblasts. Arch Dermatol Res. 1993;285:352–355. doi: 10.1007/BF00371836. [DOI] [PubMed] [Google Scholar]
- 47.Thuong-Guyot M, Domarle O, Pocidalo J J, Hayem G. Effects of fluoroquinolones on cultured articular chondrocytes. Flow cytometric analysis of free radical production. J Pharm Exp Ther. 1994;271:1544–1549. [PubMed] [Google Scholar]
- 48.Todhunter R J, Wootton J A, Altman N, Lust G, Minor R R. Cross-validation of cyanogen bromide-peptide ratios to measure the proportion of type II collagen in pepsin digests of equine articular cartilage, meniscus, and cartilage repair tissue. Anal Biochem. 1994;216:195–204. doi: 10.1006/abio.1994.1025. [DOI] [PubMed] [Google Scholar]
- 49.Towbin H, Stahelin T, Gordon J. Electrophoretic transfer of proteins from acrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1985;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van den Berg W B, Kruijsen M W M, van de Putte L B A. The mouse patella assay: an easy method of quantitating articular cartilage chondrocyte function in vivo and in vitro. Rheumatol Int. 1982;1:165–169. [Google Scholar]
- 51.Wells-Knecht M C, Lyons T J, McCance D R, Thorpe S R, Baynes J W. Age-dependent increase in ortho-tyrosine and methionine sulfoxide in human skin collagen is not accelerated in diabetes. J Clin Investig. 1997;100:839–846. doi: 10.1172/JCI119599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Woessner J F. The determination of hydroxyproline in tissue and protein samples containing small proportions of the amino acid. Biochim Biophys Acta. 1961;93:440–447. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 53.Zwart L L, Meerman J H N, Commandeur J N M, Vermeulen N P E. Biomarkers of free radical damage applications in experimental animals and in humans. Free Radic Biol Med. 1999;26:202–226. doi: 10.1016/s0891-5849(98)00196-8. [DOI] [PubMed] [Google Scholar]