Abstract
Objectives:
Although the risk of diabetes mellitus has been recognised in rheumatoid arthritis, undiagnosed dysglycaemia remained under-reported. The study aimed to determine the prevalence and associated factors of dysglycaemia among patients with rheumatoid arthritis, utilising the oral glucose tolerance test.
Methods:
This cross-sectional study involved patients with rheumatoid arthritis, aged ⩾30 years. Following an oral glucose tolerance test, they were divided into two: dysglycaemia and normoglycaemia. Demographic and laboratory parameters were compared using logistic regression analyses.
Results:
There were 35.5% (55/155) patients with dysglycaemia (including 25.8% impaired glucose tolerance, 7.1% diabetes mellitus and 1.9% with both impaired fasting glucose and impaired glucose tolerance). Patients with dysglycaemia were heavier (65.5 ± 12.3 versus 60.7 ± 10.6 kg, p = 0.01), had wider waist (89.0 ± 12.5 versus 83.1 ± 9.6 cm, p < 0.01), lower high-density lipoprotein cholesterol (1.4 ± 0.3 versus 1.5 ± 0.4 mmol/L, p = 0.02), higher triglyceride (1.3 (0.9–1.8) versus 0.9 (0.8–1.2) mmol/L, p < 0.01) and intercellular adhesion molecule-1 (361.79 (290.38–481.84) versus 315.92 (251.45–407.93) ng/mL, p = 0.01). History of smoking (odds ratio: 5.70, confidence interval: 1.27–25.7), elevated triglyceride (odds ratio: 2.87, confidence interval: 1.33–6.22) and intercellular adhesion molecule-1 (odds ratio: 1.003, confidence interval: 1.001–1.006) were significantly associated with dysglycaemia.
Conclusions:
Prevalence of undiagnosed dysglycaemia, particularly impaired glucose tolerance, was high in these patients with rheumatoid arthritis, using a 75-g oral glucose tolerance test, which was not associated with disease activity or corticosteroid use. Those with high triglyceride, history of smoking and elevated intercellular adhesion molecule-1 were the two significant predictors for dysglycaemia in our patients with rheumatoid arthritis. Oral glucose tolerance test could be an important laboratory investigation for dysglycaemia in these high-risk patients.
Keywords: Rheumatoid arthritis, type 2 diabetes mellitus, oral glucose tolerance test, dysglycaemia, impaired glucose tolerance
Introduction
Rheumatoid arthritis (RA) is a chronic progressive inflammatory autoimmune disease, mainly affecting the joints. The clinical symptoms of the disease vary from mild self-limited disease to severe joint destruction and physical disability. 1 The global prevalence of RA is estimated to be around 0.24%–1%. 2 , 3 In 2010, the Malaysian National Inflammatory Arthritis Registry (NIAR) reported the prevalence of RA in Malaysia to be approximately 1% 4 and the number increases to six-fold in 2019. 5 Patients with RA have more than twice the mortality rate compared to the general population, with cardiovascular disease as a significant cause. 6 , 7 Studies have also shown that patients with RA have higher risk of type 2 diabetes mellitus (T2DM) and insulin resistance (IR). 8 , 9 Solomon et al., in a study of 48,478 patients, showed that the incidence rate for T2DM among RA patients was 8.6 per 1000 person-years (95% confidence interval (CI): 8.5–8.7) and among controls was 5.8 per 1000 (95% CI: 5.8–5.8), with hazard ratio of 1.5 (95% CI: 1.4–1.5), after adjustment on age, gender and corticosteroid therapy.9
The prevalence of T2DM in patients with RA in Malaysia was estimated at 16.1%. 4 This figure could have easily doubled due to the alarming rise in the prevalence of T2DM, globally, regionally and nationally. 10 The prevalence of IR is higher in RA patients, both in recent-onset and long-standing RA, compared to the general population, (51%, 58% versus 19%, respectively). 11 The increased risk of T2DM and IR in patients with RA was initially thought to be due to the chronic use of corticosteroid. 12 However, this was subsequently disputed, with more recent conflicting discussions that the development of T2DM in RA is independent of corticosteroid use. 9 Interestingly, Tejera-Segura et al. demonstrated that the traditional factors associated with IR or cardiovascular risk factors have less effect on the IR in patients with RA compared to that in healthy individuals. The possible explanation for this was the ongoing inflammation or the pro-inflammatory state in patients with RA plays an important role in development of IR in patients with RA. 13 Inflammatory cytokines such as C-reactive protein (CRP), interleukin-6 (IL-6) and tumour necrosis factor-α (TNF-α) have been shown to be elevated in patients with RA. 14 These inflammatory cytokines play an important role in the pathogenesis of RA. The inflammatory process leads to activation of endothelial cells resulting in endothelial dysfunction, which is an early preclinical marker of atherosclerosis. It induces atherosclerosis via increased expression of leukocyte adhesion molecules such as the intercellular adhesion molecule-1 (ICAM-1), and vascular cell adhesion molecule-1 (VCAM-1). 15 The high levels of these inflammatory markers (CRP, IL-6, TNF-α, ICAM-1 and VCAM-1) were observed in patients with RA several years before the clinical manifestation of RA 16 and they have been demonstrated to be predictors of T2DM and its complications. 14 IR correlates with RA disease activity and inflammatory markers such as TNF-α. 17 TNF-α inhibitors, such as etanercept and adalimumab, have been shown to reduce the risk of future cardiovascular events, through improvement of endothelial function and reduction of IR. 18 , 19
The potential benefit of early screening for individuals at high risk for T2DM in reducing cardiovascular mortality is indisputable. 20 The UK Prospective Diabetes Study (UKPDS) showed that early detection and optimal control of blood glucose reduced morbidity and mortality, and slowed down the progression of microvascular and macrovascular complications in populations with T2DM. 21 Recommendations outlined by the European League Against Rheumatism (EULAR) in management of RA include annual evaluation of cardiovascular risk factors to detect and prevent cardiovascular disease, in line with the national guidelines. 22 Malaysian clinical practice guideline for T2DM recommends screening for diabetes in all adults aged 30 years and above, or earlier in higher risk groups. Venous fasting plasma glucose (FPG) should be done as the initial screening test and to proceed with oral glucose tolerance test (OGTT) if FPG is non-diagnostically elevated. 23 However, in patients on corticosteroid, FPG may not be reliable to diagnose T2DM. Burt et al. reported an FPG of ⩾5.6 mmol/L had 83% (95% CI: 36,100%) sensitivity in glucocorticoid-naive patients but only 33% (95% CI: 8,70%) sensitivity in patients on long-term corticosteroid to diagnose diabetes. 24 This study aimed to determine the prevalence of undiagnosed dysglycaemia among RA patients, with the administration of a 75-g OGTT and to investigate the factors associated with dysglycaemia.
Methods
This was a cross-sectional study, conducted in a rheumatology centre in Malaysia. Recruitment was done over 6 months, from 1 August 2016 until 31 January 2017. Patients aged 30 years and above, with the diagnosis of RA as determined by the 1987 American College of Rheumatology (ACR) or 2010 ACR/EULAR classification criteria, 25 were invited to participate in this study. Patients with prior diagnosis of overlap syndrome, T2DM and pre-diabetes, as well those already receiving any glucose-lowering drugs were excluded. Other exclusion criteria were pregnancy and within 6 months post-partum.
Patients were enrolled based on convenient consecutive sampling method. Demographic and clinical data were obtained from patients’ interviews and electronic medical records. Clinical disease activity index (CDAI)-22 was used to assess RA disease activity, which was performed by one investigator to minimise reporting bias. CDAI indicates RA disease activities, with higher number indicating more severe disease activity. 26
Patients’ weight, height and blood pressure were measured. Two measurements were performed by the primary investigator using the same automated machine (GE ProCare 200 model), which was available at the clinic. A mean of the two measurements was used for analysis. Body mass index (BMI) was calculated by dividing weight in kilograms by the square of heights in metres. All measurements were recorded in standard international (SI) units. After an overnight fast, FPG, lipid profile (low-density lipoprotein cholesterol (LDL-c), high-density lipoprotein cholesterol (HDL-c) and triglyceride), erythrocyte sedimentation rate (ESR), CRP and ICAM-1 were obtained. An OGTT was performed in all study subjects by administration of 75 g of glucose mixed with water, consumed over 15 min. Two-hour post-prandial (2HPP) glucose was obtained. Analysis for ICAM-1 was performed using the enzyme-linked immunosorbent assay (ELISA) method, while other biochemical measurements were performed according to standard laboratory procedures.
Dysglycaemia is defined as the composition of T2DM, impaired fasting glucose (IFG) and impaired glucose tolerance (IGT), according to glucose levels outlined by the Malaysian CPG for Management of T2DM 2019. T2DM was defined as FPG ⩾7.0 mmol/L or 2HPP glucose ⩾11.1 mmol/L, IFG was defined as FPG between 6.1 and 6.9 mmol/L and IGT was defined as 2HPP glucose between 7.8 and 11.0 mmol/L. 21 , 23 Patients who were actively smoking or had previously smoked were labelled as ‘ever smoked’.
This study was approved by Universiti Teknologi MARA Research Ethics Committee on 29 July 2016 (REF: 600-IRMI (5/1/6)) and National Medical Research Register (NMRR) Ethics Committee (NMRR-ID: NMRR-16-1179-31250).
Statistical analysis
The sample size was calculated to achieve precision of 5%, and confidence level of 90%, after considering the normal attrition of 20%. With estimated prevalence of T2DM among RA patients in Malaysia of 16.1% and RA population set at 900 individuals, which corresponded to the number of RA patients attending the Rheumatology Clinic of the institution over 6 months, the calculated sample size was 151.
Statistical Package for the Social Sciences (SPSS for Windows Version 24.0, SPSS Inc., Chicago, IL, USA) was used for all statistical analyses. Continuous data were presented as mean and standard deviations or median followed by standard deviation, while categorical data were presented as numbers of patients and percentages. Based on OGTT results, patients were divided into two groups: dysglycaemia and normoglycaemia. Differences between these groups were analysed using independent t test for the parametric data, Mann–Whitney test for non-parametric data and chi-square test for categorical variables.
All independent variables were analysed for the univariable analysis. Multivariable analysis was performed using binary logistic regression to ascertain the true effects of significant factors found on univariable analysis. Multicollinearity and interactions were checked for between the variables and there were no multicollinearity or interactions. Significance level was set at p < 0.05. The odds ratio (OR) was determined for the degree of association for each significant variable, and adjusted OR was calculated for the significant variables after multivariable analysis.
Only the independent variables that remain significant after multivariable analysis were included in the predictability models. Two predictability models were created: Model 1 with the three significant variables (triglycerides, history of smoking and ICAM-1 level), and Model 2, we excluded ICAM-1 from the model, acknowledging the high cost of ICAM-1 measurements in clinical practise. We would like to know, if without the ICAM-1 in the model, whether triglycerides and smoking would remain significant predictors for dysglycaemia in our cohort. We tested these two models using the receiver operating characteristic (ROC) curve analysis.
Results
Nine hundred ninety-eight patients (998) who attended the Rheumatology Clinic with the diagnosis of RA for at least 6 months were screened. Two hundred six (206) patients with known T2DM prior to the study were excluded. Out of the 792 patients that were eligible for the study, we recruited 156 patients who fulfilled both the inclusion and exclusion criteria. One patient failed the OGTT. A total of 155 patients were included in the statistical analysis, with a mean age of 57 years, out of which 136 (87.7%) were females. The other demographic and clinical data are presented in Table 1.
Table 1.
Demographic and clinical data on 155 patients with RA who underwent OGTT.
| Demographic and clinical data | No. of patients (%) |
|---|---|
| Age (mean ± SD) | 57.18 ± 8.09 |
| Female | 136 (87.7%) |
| Ethnicity | |
| Malay | 60 (38.5%) |
| Chinese | 61 (39.1%) |
| Indian | 34 (21.8%) |
| Others | 1 (0.6%) |
| Employment status | |
| Employed | 41 (26.5%) |
| Unemployed | 59 (38.1%) |
| Retired | 50 (32.2%) |
| Unknown | 5 (3.2%) |
| Educational status | |
| Primary and secondary education | 113 (72.9%) |
| Beyond secondary education | 27 (17.4%) |
| Unknown | 15 (9.7%) |
| Monthly income, n (%) | |
| <RM 3000 | 117 (75.5%) |
| ⩾RM 3000 | 33 (21.3%) |
| Unknown | 5 (3.2%) |
| RA disease duration (mean ± SD) | 11.49 ± 7.99 |
| DMARDs | |
| No DMARDs | 14 (9.0%) |
| Monotherapy | 67 (43.2%) |
| 2 or more DMARDs (including biologics) | 74 (47.7%) |
| Use of corticosteroid within 6 months | 23 (14.74%) |
RA: rheumatoid arthritis; OGTT: oral glucose tolerance test; DMARDs: disease-modifying antirheumatic drugs.
Abnormal OGTT was identified in 55 of 155 patients, revealing a prevalence of dysglycaemia as 35.5%. Within the dysglycaemia group, 40 (25.8%) had IGT, 11 (7.1%) had T2DM, 3 had IFG and IGT (1.9%) and 1 had IFG (0.6%). Among these patients, 35 (60%) were obese, 9 (18%) were overweight, 10 (20%) were of normal weight and 1 (2%) was underweight. Patients with dysglycaemia compared to normoglycaemia were found to be significantly heavier (65.5 ± 12.3 versus 60.7 ± 10.6 kg, p = 0.01), with significantly wider waist circumference (89.0 ± 12.5 versus 83.1 ± 9.6 cm, p < 0.01), higher systolic (134.5 ± 17.5 versus 128.2 ± 18.1 mmHg, p = 0.04) and diastolic blood pressure (79.7 ± 8.7 versus 76.3 ± 10.5 mmHg, p = 0.04), lower HDL-c (1.4 ± 0.3 versus 1.5 ± 0.4 mmol/L, p = 0.02), higher triglycerides (1.3 (0.9–1.8) versus 0.9 (0.8–1.2) mmol/L, p < 0.01) and higher ICAM-1 levels (361.79 (290.38–481.84) versus 315.92 (251.45–407.93) ng/mL, p = 0.02). Refer Table 2.
Table 2.
Comparison of various factors between RA patients with dysglycaemia and normoglycaemia.
| Factors | Dysglycaemia, n = 55 (n (%), mean ± SD or median (IQR)) a | Normoglycaemia, n = 100 (n (%), mean ± SD or median (IQR)) a | p value |
|---|---|---|---|
| Age (years), mean ± SD | 57.15 ± 8.66 | 57.20 ± 7.83 | 0.99 |
| Gender, n (%) | |||
| Female | 45 (81.8) | 91 (91) | 0.1 |
| Ethnicity, n (%) | |||
| Malay | 22 (40.0) | 37 (37.0) | 0.56 |
| Chinese | 21 (38.2) | 40 (40.0) | |
| Indian | 11 (20.0) | 23 (23.0) | |
| Others | 1 (1.8) | 0 | |
| Employment, n (%) | |||
| Employed | 13 (24.5) | 28 (28.9) | 0.29 |
| Unemployed | 18 (34.0) | 41 (42.3) | |
| Retired | 22 (41.5) | 28 (28.9) | |
| Educational status | |||
| Primary school and below | 9 (18.0) | 22 (24.4) | 0.36 |
| Secondary school | 32 (64.0) | 50 (55.6) | |
| Diploma and degree | 9 (18.0) | 18 (20.0) | |
| Monthly income, n (%) | |||
| <RM 3000 | 43 (78.2) | 74 (74.0) | 0.56 |
| ⩾RM 3000 | 12 (36.8) | 26 (26.0) | |
| Family history of T2DM | 25 (47.2) | 47 (48.5) | 0.88 |
| Previous or current smoker | 7 (13.0) | 3 (3.0) | 0.02 |
| Waist circumference, cm | 89.0 ± 12.5 | 83.1 ± 9.6 | <0.01 |
| Weight, kg | 65.5 ± 12.3 | 60.7 ± 10.6 | 0.01 |
| BMI, kg/m2 | 26.85 ± 4.98 | 25.64 ± 4.41 | 0.14 |
| Systolic BP, mmHg | 134.5 ± 17.5 | 128.2 ± 18.1 | 0.04 |
| Diastolic BP, mmHg | 79.7 ± 8.7 | 76.3 ± 10.5 | 0.04 |
| LDL-c, mmol/L | 3.3 ± 0.9 | 3.1 ± 0.8 | 0.15 |
| HDL-c, mmol/L | 1.4 ± 0.3 | 1.5 ± 0.4 | 0.02 |
| Triglycerides, mmol/L | 1.3 (0.9–1.8) | 0.9 (0.8–1.2) | <0.01 |
| RA disease duration, years | 11.0 (6.0–16.0) | 10.0 (5.0–14.7) | 0.52 |
| Steroid use within 6 months | 7 (12.73) | 16 (16) | 0.57 |
| Use of biologics | 4 (7.27) | 3 (3) | 0.23 |
| CDAI | 10.9 ± 6.9 | 9.9 ± 5.6 | 0.33 |
| ICAM-I, ng/mL | 361.79 (290.38–481.84) | 315.92 (251.45–407.93) | 0.01 |
| ESR, mm/h | 30.0 (20.0–40.0) | 34.0 (26.0–40.0) | 0.44 |
| CRP, mg/dL | 0.8 (0.5–1.8) | 0.6 (0.5–1.4) | 0.47 |
BP: blood pressure; RA: rheumatoid arthritis; IQR: interquartile range; T2DM: type 2 diabetes mellitus; BMI: body mass index; LDL-c: low-density lipoprotein cholesterol; HDL-c: high-density lipoprotein cholesterol; CDAI: clinical disease activity index; ICAM-1: intercellular adhesion molecule-1; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein.
n (%) for categorical data, median (IQR) for non-parametric data and mean ± SD for parametric data.
Univariable analysis showed that previous history or current smoker, weight, waist circumference, systolic and diastolic blood pressure, LDL-c, HDL-c, triglyceride and ICAM-1 were the significant associated factors of dysglycaemia in patients with RA. However, when these factors were subsequently included within a multivariable logistic regression analysis, only previous history or current smoker, triglyceride and ICAM-1 levels remained as the significant factors with adjusted OR of 5.70, 2.87 and 1.003, respectively (Table 3).
Table 3.
Factors associated with dysglycaemia in patients with RA, after multivariate logistic regression.
| Factors | Crude OR | 95% CI | p value | Adjusted OR a | 95% CI | p value |
|---|---|---|---|---|---|---|
| Previous or current smoker | 4.77 | 1.18–19.27 | 0.03 | 5.70 | 1.27–25.7 | 0.02 |
| Weight, kg | 1.04 | 1.007–1.07 | 0.02 | |||
| Waist, cm | 1.05 | 1.02–1.09 | <0.01 | |||
| Systolic BP, mmHg | 1.02 | 1.00–1.04 | 0.04 | |||
| Diastolic BP, mmHg | 1.04 | 1.00–1.07 | <0.05 | |||
| LDL-C, mmol/L | 1.33 | 0.90–1.97 | 0.15 | |||
| HDL-C, mmol/L | 0.30 | 0.11–0.82 | 0.02 | |||
| Triglyceride, mmol/L | 2.60 | 1.26–7.50 | <0.01 | 2.87 | 1.33–6.22 | <0.01 |
| ICAM-I | 1.002 | 1.001–1.006 | 0.04 | 1.003 | 1.000–1.006 | 0.03 |
BP: blood pressure; RA: rheumatoid arthritis; OR: odds ratio; CI: confidence interval; LDL-C: low-density lipoprotein cholesterol; HDL-C: high-density lipoprotein cholesterol; ICAM-1: intercellular adhesion molecule-1.
Adjusted OR after adjustment for previous history or current smoker, weight, waist circumference, systolic and diastolic blood pressure, LDL-C, HDL-C, triglyceride and ICAM-1.
A predictability model which consisted of these factors was found to be significant with area under the curve (AUC) of 0.728 (95% CI: 0.65–0.81, p < 0.001). However, considering that ICAM-1 is expensive in clinical practice, a second predictability model excluding it was created. This model demonstrated that interaction between previous history or current smoker and elevated triglyceride level were significant predictors of dysglycaemia in the study population of patients with RA with AUC of 0.712 (95% CI: 0.63–0.80, p < 0.001). Refer Table 4.
Table 4.
Comparing the predictability model using ROC curve.
| Predictability model | AUC | Standard error | 95% CI | p value |
|---|---|---|---|---|
| Model 1 Previous or current smoker High TG (⩾1.7 mmol/L) High ICAM-1 (⩾347.48 ng/mL) |
0.648 | 0.049 | 0.552–0.743 | 0.003 |
| Model 2 Previous or current smoker High TG (⩾1.7 mmol/L) |
0.610 | 0.050 | 0.512–0.707 | 0.041 |
ROC: receiver operating characteristic; AUC: area under the curve; CI: confidence interval; TG: triglyceride; ICAM-1: intercellular adhesion molecule-1.
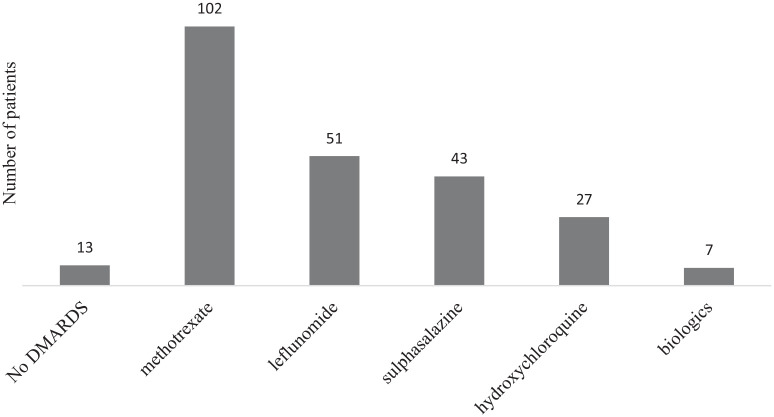
We also analysed the cohort of our patients who were on disease-modifying antirheumatic drugs (DMARDs). There were 142 patients on DMARDs, with the highest DMARD usage with methotrexate. However, only a small number of patients used biologics (n = 7/155, 4.5%). We provided the details of DMARDs used in our patients (Figure 1) and the cardiovascular risk profiles according to DMARDs (Table 5).
Figure 1.
Number of patients on different types of DMARDs.
Table 5.
Cardiovascular risk profile in patients with RA according to DMARDs used.
| Factors | No DMARDs, n = 13 | Conventional DMARDs, n = 135 | Biologic and JAK inhibitor, n = 7 |
|---|---|---|---|
| Waist circumference, cm | 81.5 (72.5–88.5) | 84.0 (77.0–92.0) | 90.2 (74.9–99.0) |
| Weight, kg | 65.4 (53.6–75.3) | 60.0 (54.7–67.8) | 63.1 (52.3–69.5) |
| BMI, kg/m | 25.88 (21.50–28.96) | 25.18 (22.74–28.76) | 25.92 (22.34–30.07) |
| Systolic BP, mmHg | 127 (111–137) | 130 (120–140) | 139 (120–148) |
| Diastolic BP, mmHg | 73 (66–84) | 78 (70–85) | 77 (75–84) |
| LDL-c, mmol/L | 3.10 (2.65–3.85) | 3.15 (2.60–3.77) | 3.30 (2.50–3.80) |
| HDL-c, mmol/L | 1.60 (1.25–1.85) | 1.50 (1.20–1.70) | 1.60 (1.20–1.90) |
| Triglycerides, mmol/L | 0.8 (0.7–1.3) | 1.0 (0.8–1.3) | 1.0 (0.8–1.4) |
| ICAM-I, ng/mL | 347.23 (288.62–445.65) | 323.47 (257.44–435.93) | 270.63 (200.88–378.52) |
| ESR, mm/hr | 34 (26–39) | 33 (22–40) | 36 (29–48) |
| CRP, mg/dL | 0.5 (0.5–0.58) | 0.75 (0.50–1.78) | 0.87 (0.50–3.72) |
RA: rheumatoid arthritis; DMARDs: disease-modifying antirheumatic drugs; JAK: Janus Kinase; BP: blood pressure; BMI: body mass index; LDL-C: low-density lipoprotein cholesterol; HDL-c: high-density lipoprotein cholesterol; ICAM-1: intercellular adhesion molecule-1; ESR: erythrocyte sedimentation rate; CRP: C-reactive protein; IQR: interquartile range.
All data were presented as median (IQR).
Discussion
The prevalence of patients with undiagnosed dysglycaemia following OGTT in this present study was 35.5% (n = 55/155) which was predominantly IGT (25.8%) followed by T2DM (7.1%). Ursini et al. reported a higher percentage of undiagnosed dysglycaemia of 43% and T2DM of 10%, 8 while Hoes et al. reported 11% undiagnosed T2DM and 35% IGT. 27 A plausible explanation for the lower prevalence of undiagnosed T2DM in our study cohort could be due to the regular annual screening with FPG in all patients with RA, which could have resulted in early detection of T2DM. However, this would have neglected patients with elevated post-prandial blood glucose and IGT. In addition, fasting blood glucose has been reported to frequently be in the normal range, while post-prandial levels are elevated in patients with endogenous 28 and acute exogenous 29 glucocorticoid excess. Our finding was somewhat similar with a recent report by Rajput et al. who demonstrated a prevalence of IGT of 14.67% following an OGTT. 30 Comparatively, both studies demonstrated high number of subjects with IGT, that is, Rajput et al. reported a ratio of 27/44 patients, while this study demonstrated 40/55 patients. The rate of pre-diabetes which included IFG was, however, not statistically different from the control group. 30 This observation would clearly underscore the need to perform glucose tolerance test to identify the post-prandial abnormality in this group of patients.
We demonstrated that there were significantly more patients with metabolic risks in the dysglycaemia group compared to the normoglycaemia group, as they were heavier, had wider waist circumference, higher systolic and diastolic BP, higher TG and lower HDL-C, which concur with previous studies. 31 , 32 The link between lipid profile and RA has been shown to be non-linear, and some studies suggested that there is a complex genetic mechanism behind this. 33 Univariate analysis revealed HDL-C and triglyceride to be significantly associated with dysglycaemia. However, only triglyceride remained as a significant risk factor following the multivariable analysis. This further highlights the importance of detecting dysglycaemia, particularly hypertriglyceridaemia, in this group of a seemingly low-risk population. Previous history or current smoking was identified as a strong risk factor associated with dysglycaemia, with an adjusted OR of 5.70, which concurred with previous reports demonstrating increased risk of metabolic syndrome in this population. 34
Based on the findings by Burt et al., the use of oral corticosteroid is an expected risk factor of dysglycaemia. 24 However, our study revealed that oral corticosteroid use was not significantly associated with dysglycaemia. A plausible explanation could have been due to the restriction in the use of oral corticosteroid within 6 months of study period, while Burt et al. included long-term corticosteroid use. In clinical practice, long-term use of corticosteroid is usually avoided, while favouring short courses as bridging therapy towards more definitive treatment for newly diagnosed disease or during acute flares. In this cohort, most patients had a low to lower limit of moderate RA disease activity; thus, corticosteroid may not have been indicated in them. The other plausible explanation is the limitation of the small number of patients on oral corticosteroid and the lack of further details on preparation and dosages of the corticosteroid, which could affect the glycaemic status.
Disease activity and inflammatory markers had also been recognised to be associated with development of T2DM, possibly due to ongoing inflammation in patients with RA. 14 , 35 In this study, we demonstrated that the ICAM-1 level was a significant risk factor for dysglycaemia in this cohort. This finding would be consistent with the pathophysiology of RA, in which the chronic inflammation had been shown to lead to endothelial dysfunction and increased expression of leukocyte adhesion molecules (ICAM-1 and VCAM-1) which are the early preclinical markers of atherosclerosis. 15 However, we did not find significant differences in CDAI, ESR or CRP between the dysglycaemia and normoglycaemia groups. The plausible explanation could be due to the low to lower limit of moderate RA disease activity. However, our study showed that dysglycaemia in long-standing RA with a mean disease duration of 11.7 years was associated with significantly higher surrogate marker for endothelial dysfunction, as represented by ICAM-I. Further studies to investigate the different types of leukocyte adhesion molecules in RA and their relationship with inflammatory markers such as TNF, IL6, rheumatoid factor and anti-cyclic citrullinated peptide would be able to confirm these findings. Also, it would be interesting to further study the associations between these molecules and RA clinical presentation such as number of swollen and tender joints, presence of deformities as well as measurement of carotid intima–medial thickness to assess for atherosclerosis.
We acknowledge that different types of DMARDs including conventional, biologics or JAK inhibitors deserve special interest as different biologics may have different impact on cardiovascular risk in RA patients. Unfortunately, this study was conducted in a government-funded hospital, whereby the use of biologics and targeted synthetic DMARDs were very limited. According to the latest national registry on inflammatory arthritis, only 288 out of 6542 (2.9%) patients attending 11 government hospital over 10 years were treated with biologic and targeted synthetic DMARDs.5
We acknowledge the limitations in this study which included the single-centre data collection, a cross-sectional design and the relatively small number of subjects. Furthermore, the effect of corticosteroid would require more information on the methods of administration, duration of therapy and the exact dose throughout disease duration.
Conclusion
In conclusion, our study found a high prevalence of undiagnosed dyslgycaemia, especially IGT, in patients with RA using a 75-g OGTT. Dysglycaemia is significantly associated with common cardiovascular risk factors, including weight, blood pressure, dyslipidaemia and smoking history. However, it was not associated with disease activity or use of oral corticosteroid. High triglycerides and previous or current smoker were the two significant predictors for dysglycaemia in our patients with RA. OGTT could be an important laboratory investigation for dysglycaemia in these high-risk patients; however, more studies are needed to support this notion.
Acknowledgments
We wish to thank all the doctors and nurses of the Rheumatology Unit Hospital Selayang for their assistance during this study. We are grateful to the Director General of Ministry of Health and the Dean of the Faculty of Medicine UiTM for allowing us to conduct our study in their facilities.
Footnotes
Author contributions: First and second authors wrote the first draft of the article. All authors reviewed and edited the article and approved the final version of the article.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship and/or publication of this article.
Ethical approval: This study was approved by Universiti Teknologi MARA Research Ethics Committee on 29 July 2016 (REF: 600-IRMI (5/1/6)) and National Medical Research Register (NMRR) Ethics Committee (NMRR-ID: NMRR-16-1179-31250).
Informed consent: Written informed consent was obtained from all subjects prior to the initiation of the study.
ORCID iD: Nur ‘Aini Eddy Warman  https://orcid.org/0000-0002-8204-102X
https://orcid.org/0000-0002-8204-102X
References
- 1. Plenge RM. Rheumatoid arthritis genetics: 2009 update. Curr Rheumatol Rep 2009; 11(5): 351–356. [DOI] [PubMed] [Google Scholar]
- 2. Silman AJ, Pearson JE. Epidemiology and genetics of rheumatoid arthritis. Arthritis Res Ther 2002; 4(3): S265–S272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Cross M, Smith E, Hoy D, et al. The global burden of rheumatoid arthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 2014; 73(7): 1316–1322. [DOI] [PubMed] [Google Scholar]
- 4. Rosman A, Hussein H, Chyn GS, et al. National Inflammatory Arthritis Registry (NIAR). National Inflammatory Arthritis Registry (NIAR) Preliminary Report (2009-2010), 2012, https://www.crc.gov.my/wp-content/uploads/documents/report/NIAR_Report.pdf
- 5. Rosman M. Zain M, Ch’ng SS, et al. NIAR and MARBLE rheumatoid arthritis. Malaysian National Inflammatory Arthritis Registry, Ministry of Health Malaysia, 2019, https://www.moh.gov.my/moh/resources/Penerbitan/CPG/2)_CPG_Management_of_Rheumatoid_Arthritis.pdf
- 6. Gonzalez A, Maradit Kremers H, Crowson CS, et al. The widening mortality gap between rheumatoid arthritis patients and the general population. Arthritis Rheum 2007; 56(11): 3583–3587. [DOI] [PubMed] [Google Scholar]
- 7. Wolfe F, Mitchell DM, Sibley JT, et al. The mortality of rheumatoid arthritis. Arthritis Rheum 1994; 37(4): 481–494. [DOI] [PubMed] [Google Scholar]
- 8. Ursini F, Russo E, D’Angelo S, et al. Prevalence of undiagnosed diabetes in rheumatoid arthritis: an OGTT study. Medicine 2016; 95(7): e2552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Solomon DH, Love TJ, Canning C, et al. Risk of diabetes among patients with rheumatoid arthritis, psoriatic arthritis and psoriasis. Ann Rheum Dis 2010; 69(12): 2114–2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. National Institutes of Health (NIH), Ministry of Health Malaysia. National health and morbidity survey 2019, 2019, https://iptk.moh.gov.my/images/technical_report/2020/4_Infographic_Booklet_NHMS_2019_-_English.pdf
- 11. Giles JT, Danielides S, Szklo M, et al. Insulin resistance in rheumatoid arthritis. Arthritis Rheumatol 2015; 67: 626–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Caldwell JR, Furst DE. The efficacy and safety of low-dose corticosteroids for rheumatoid arthritis. Semin Arthritis Rheum 1991; 21(1): 1–11. [DOI] [PubMed] [Google Scholar]
- 13. Tejera-Segura B, López-Mejías R, De Vera-González AM, et al. Relationship between insulin sensitivity and β-cell secretion in nondiabetic subjects with rheumatoid arthritis. J Rheumatol 2019; 46(3): 229–236. [DOI] [PubMed] [Google Scholar]
- 14. Goldberg RB. Cytokine and cytokine-like inflammation markers, endothelial dysfunction, and imbalanced coagulation in development of diabetes and its complications. J Clin Endocrinol Metab 2009; 94(9): 3171–3182. [DOI] [PubMed] [Google Scholar]
- 15. Yang X, Chang Y, Wei W. Endothelial dysfunction and inflammation: immunity in rheumatoid arthritis. Mediators Inflamm 2016; 2016: 6813016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Van Steenbergen HW, Huizinga TW, Van Der Helm-Van Mil AH. The preclinical phase of rheumatoid arthritis: what is acknowledged and what needs to be assessed? Arthritis Rheum 2013; 65(9): 2219–2232. [DOI] [PubMed] [Google Scholar]
- 17. Wasko MC, Kay J, Hsia EC, et al. Diabetes mellitus and insulin resistance in patients with rheumatoid arthritis: risk reduction in a chronic inflammatory disease. Arthritis Care Res 2011; 63(4): 512–521. [DOI] [PubMed] [Google Scholar]
- 18. Deodhar A, Bitman B, Yang Y, et al. The effect of etanercept on traditional metabolic risk factors for cardiovascular disease in patients with rheumatoid arthritis. Clin Rheumatol 2016; 35(12): 3045–3052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Gonzalez-Juanatey C, Llorca J, Sanchez-Andrade A, et al. Short-term adalimumab therapy improves endo-thelial function in patients with rheumatoid arthritis refractory to infliximab. Clin Exp Rheumatol 2006; 24(3): 309–312. [PubMed] [Google Scholar]
- 20. Simmons R, Rahman M, Jakes R, et al. Effect of population screening for type 2 diabetes on mortality: long-term follow-up of the Ely cohort. Diabetologia 2011; 54(2): 312–319. [DOI] [PubMed] [Google Scholar]
- 21. UK Prospective Diabetes Study Group. Tight blood pressure control and risk of macrovascular and microvascular complications in type 2 diabetes: UKPDS 38. Br Med J 1998; 317(7160): 703–713. [PMC free article] [PubMed] [Google Scholar]
- 22. Peters M, Symmons D, McCarey D, et al. EULAR evidence-based recommendations for cardiovascular risk management in patients with rheumatoid arthritis and other forms of inflammatory arthritis. Ann Rheum Dis 2010; 69(2): 325–331. [DOI] [PubMed] [Google Scholar]
- 23. Ministry of Health Malaysia. Clinical practice guideline on management of type2 diabetes mellitus (6th edn), 2020, https://www.moh.gov.my/moh/resources/Penerbitan/CPG/Endocrine/QR_T2DM_6th_Edition_QR_Guide_Digital.pdf
- 24. Burt MG, Willenberg VM, Petersons CJ, et al. Screening for diabetes in patients with inflammatory rheumatological disease administered long-term prednisolone: a cross-sectional study. Rheumatology 2012; 51(6): 1112–1119. [DOI] [PubMed] [Google Scholar]
- 25. Kay J, Upchurch KS. 2010. ACR/EULAR rheumatoid arthritis classification criteria. Rheumatology 2012; 51(Suppl. 6): vi5–vi9. [DOI] [PubMed] [Google Scholar]
- 26. Aletaha D, Nell VP, Stamm T, et al. Acute phase reactants add little to composite disease activity indices for rheumatoid arthritis: validation of a clinical activity score. Arthritis Res Ther 2005; 7(4): R796–R806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Hoes J, Van Der Goes M, Van Raalte D, et al. Glucose tolerance, insulin sensitivity and β-cell function in patients with rheumatoid arthritis treated with or without low-to-medium dose glucocorticoids. Ann Rheum Dis 2011; 70(11): 1887–1894. [DOI] [PubMed] [Google Scholar]
- 28. Arnaldi G, Angeli A, Atkinson A, et al. Diagnosis and complications of Cushing’s syndrome: a consensus statement. J Clin Endocrinol Metab 2003; 88(12): 5593–5602. [DOI] [PubMed] [Google Scholar]
- 29. Burt MG, Roberts GW, Aguilar-Loza NR, et al. Continuous monitoring of circadian glycemic patterns in patients receiving prednisolone for COPD. J Clin Endocrinol Metab 2011; 96(6): 1789–1796. [DOI] [PubMed] [Google Scholar]
- 30. Rajput R, Dangi A, Singh H. Prevalence of glucose intolerance in rheumatoid arthritis patients at a tertiary care centre in Haryana. Diabetes Metab Syndr 2017; 11(Suppl. 2): S1013–S1016. [DOI] [PubMed] [Google Scholar]
- 31. Hallajzadeh J, Safiri S, Mansournia MA, et al. Metabolic syndrome and its components among rheumatoid arthritis patients: a comprehensive updated systematic review and meta-analysis. PLoS One 2017; 12(3): e0170361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Zhang J, Fu L, Shi J, et al. The risk of metabolic syndrome in patients with rheumatoid arthritis: a meta-analysis of observational studies. PLoS One 2013; 8(10): e78151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Andreassen OA, Desikan RS, Wang Y, et al. Correction: abundant genetic overlap between blood lipids and immune-mediated diseases indicates shared molecular genetic mechanisms. PLoS One 2015; 10(5): e0128048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Zonana-Nacach A, Santana-Sahagún E, Jiménez-Balderas FJ, et al. Prevalence and factors associated with metabolic syndrome in patients with rheumatoid arthritis and systemic lupus erythematosus. J Clin Rheumatol 2008; 14(2): 74–77. [DOI] [PubMed] [Google Scholar]
- 35. Wang X, Bao W, Liu J, et al. Inflammatory markers and risk of type 2 diabetes: a systematic review and meta-analysis. Diabetes Care 2013; 36(1): 166–175. [DOI] [PMC free article] [PubMed] [Google Scholar]