Abstract
Background:
Real-world data on treatment and outcomes in patients with synchronous metastatic disease compared with patients with metachronous metastatic disease in esophagogastric cancer have not been published before. The aim of our study was to explore treatment, overall survival (OS), and time to treatment fialure (TTF) in patients with synchronous and metachronous metastatic esophagogastric adenocarcinoma.
Methods:
Patients with synchronous metastatic disease (2015–2017) and patients with metachronous metastatic disease initially treated with curative intent for nonmetastatic disease (2015–2016) were selected from the Netherlands Cancer Registry. OS and TTF were assessed from metastatic diagnosis for patients with synchronous, early metachronous (⩽6 months) or late metachronous (>6 months) metastatic disease using Kaplan–Meier curves with two-sided log-rank test.
Results:
Median OS was 4.2, 2.1, and 4.4 months in patients with synchronous, early metachronous, and late metachronous metastatic disease, respectively (p < 0.001). The proportion of patients receiving systemic treatment was 41.3%, 21.5%, and 32.5% for synchronous, early metachronous, and late metachronous metastatic disease, respectively (p = 0.001). Among patients receiving systemic treatment, median OS was 8.8, 4.5, and 9.1 months (p < 0.001) and median TTF was 6.1, 3.8, and 5.7 months (p < 0.001) in synchronous, early metachronous, and late metachronous metastatic disease, respectively.
Conclusion:
Patients with early metachronous metastatic disease have a worse survival compared with patients with synchronous or late metachronous metastatic disease. These patients less often receive systemic treatment, and even when treated, survival is worse compared with patients with synchronous or late metachronous metastatic disease, suggesting a more aggressive tumor behavior.
Keywords: esophageal cancer, gastric cancer, metachronous metastatic disease, palliative treatment, synchronous metastatic disease
Introduction
Esophageal and gastric cancer are the tenth and sixth most frequently occurring cancers worldwide, respectively. 1 Prognosis of both esophageal and gastric cancer is poor, particularly when metastases are present. 2 Up to 40% of patients with esophagogastric cancer present with metastatic disease at primary diagnosis,3–5 defined as synchronous metastatic disease. In addition, patients might develop metastases after primary nonmetastatic disease, which is referred to as metachronous metastatic disease.
Palliative systemic therapy is available for patients with metastatic disease who are in good physical condition and is intended to extend survival and maintain or improve quality of life.6,7 For both metastatic esophageal and gastric adenocarcinoma, the recommended first-line treatment is fluoropyrimidine and platinum with the addition of trastuzumab in patients with HER2 overexpression, paclitaxel plus ramucirumab at second-line treatment.6,8–11
No distinction in treatment recommendations is provided for patients with synchronous or metachronous metastatic disease. In fact, comparison of survival in patients with synchronous or metachronous metastatic disease in a real-world setting has not been published previously and survival of patients with metachronous metastatic disease is currently unknown.
Therefore, we described characteristics, treatment, overall survival (OS), and time-to-treatment failure (TTF) in patients with synchronous or metachronous metastatic esophagogastric adenocarcinoma in a real-world setting.
Methods
Study population
Patients with an adenocarcinoma of the esophagus, gastroesophageal junction, or stomach diagnosed with synchronous metastases (cTallcNallcM1, 2015–2017) or metachronous metastases after primary nonmetastatic diagnosis (cTallcNallcM0, 2015–2016) were selected from the Netherlands Cancer Registry (NCR) (Figure 1).12,13 The NCR is a nationwide population-based cancer registry that covers the total Dutch population and is based on notification of all newly diagnosed malignancies by the national automated pathology archive. Specially trained data managers routinely extract information on diagnosis and treatment from medical records. Data on progression (including metachronous metastases) were collected in the second half of 2019. Information on vital status was available through linkage with the Dutch Personal Records Database and follow-up was complete until 1 February 2021. According to the Central Committee on Research involving Human Subjects, this type of study does not require approval from an ethics committee in the Netherlands. Based on current Dutch legislation, it is not necessary to retrieve informed consent from patients for registration into the NCR. The privacy review board of the NCR reviews all data requests for studies with data of the NCR regarding privacy issues. This study was approved by the Privacy Review Board of the NCR and the scientific committee of the Dutch Upper GI Cancer Group (K19.045).
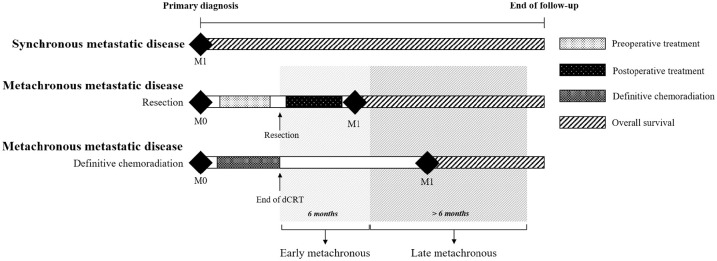
Figure 1.
Schematic illustration of the definition synchronous or metachronous metastatic disease. Patients with primary nonmetastatic disease are at risk for metachronous metastatic disease after resection (with or without preoperative and/or postoperative treatment) or definitive chemoradiation. Early metachronous metastatic disease is defined as metastatic disease within 6 months of resection or end date of definitive chemoradiation and late metachronous metastatic disease after 6 months of resection or end date of definitive chemoradiation.
Synchronous metastases were defined as diagnosis of metastases before or within the first 5 days after start of treatment. 14 Except in patients who had surgical resection without preoperative treatment, metastases needed to be diagnosed before resection. In patients receiving best supportive care, metastases needed to be diagnosed within 6 weeks after primary diagnosis as treatment usually starts within the first few weeks after diagnosis.
Metachronous metastases were defined as diagnosis of metastases after end of treatment with curative intent for primary nonmetastatic disease. Treatment with curative intent included either resection [endoscopic resection, esophagectomy, or (sub)total gastrectomy] or definitive chemoradiotherapy (chemotherapy with concurrent radiotherapy consisting of ⩾28 fractions or total radiation dose of ⩾50 Gy). Metastases needed to be diagnosed at least 5 days after resection to account for delay in pathological confirmation of metastases. Patients who had a diagnosis of metastases during treatment with curative intent were defined as having interval metastases. The aim of our study was to compare patients with metachronous versus synchronous metastatic disease and we consider patients with interval metastases a different population. Analysis of patients with interval metastases were therefore beyond the scope of this study and information of these patients are presented elsewhere.15,16
There is currently no consensus on the definition of metachronous metastatic disease. In colorectal cancer, a period of 12 months has been used to distinguish between early or late metachronous metastatic disease.17,18 Two studies investigating metachronous liver metastases in gastric cancer defined an interval of at least 6 months before metastases were considered metachronous.19,20 We defined early and late metachronous metastatic disease as metastases diagnosed within and after 6 months of resection or definitive chemoradiation, respectively (Figure 1).
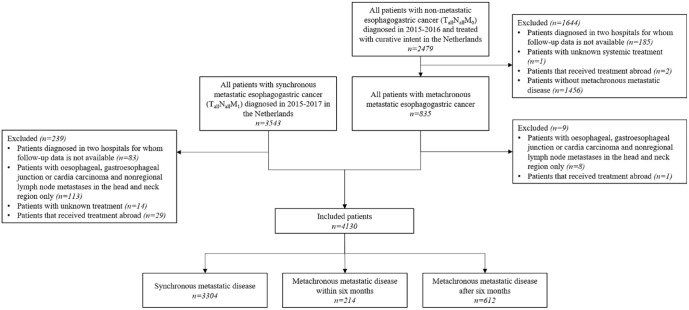
Patients diagnosed in two hospitals were excluded as data on progression (including metachronous metastases) were not available (n = 268) (Figure 2). Patients without metachronous metastatic disease were excluded (n = 1456). Patients with nonregional lymph node metastases limited to the head and neck region were excluded to avoid inclusion of patients possibly still eligible for treatment with curative intent (n = 121).14,21 Patients with unknown (systemic) treatment (n = 15) or receiving treatment abroad (n = 32) were also excluded.
Figure 2.
Flowchart of patient selection.
Treatment definitions
Treatment after metastatic disease was classified as radical treatment of primary tumor or locoregional recurrence, radical treatment of distant metastases, systemic therapy (as previously defined by Dijksterhuis et al.14,22), or best supportive care only. Radical treatment of primary tumor or locoregional recurrence included surgical resection of the primary tumor or chemoradiotherapy (chemotherapy with concurrent radiotherapy with a maximum dose per fraction of 2.2 Gy). Radical treatment of distant metastases included metastasectomy, chemoradiotherapy directed at metastases or stereotactic body radiotherapy for metastases (⩾10 Gy per fraction if ⩾1 fraction, ⩾7 Gy per fraction if ⩾5 fractions, or ⩾5 Gy per fraction if ⩾12 fractions). If patients did not receive any of the above-mentioned treatment, patients were considered receiving best supportive care. Treatment groups were not mutually exclusive (with exception of best supportive care) and based on the treatment after diagnosis of synchronous or metachronous metastatic disease prior to (potential) progression.
Overall survival and time-to-treatment failure
In patients with synchronous metastatic disease, OS and TTF were assessed from primary diagnosis and in patients with metachronous metastatic disease from diagnosis of metastases. OS was assessed until death or end of follow-up for vital status. TTF was assessed until the first progression that resulted in end of the regimen. If no progression was registered, patients were censored at the end of follow-up (the date of the last hospital visit). If no subsequent systemic regimen was administered and the patient died within 90 days after the last hospital visit, the date of death was considered as an event for TTF analyses. 14 For the OS and TTF analyses in the subgroup of patients who received systemic therapy, patients who also received radical treatment of the primary tumor, locoregional recurrence or distant metastases were excluded due to potential effect of these treatments on survival.
Statistical analyses
Characteristics between patients were analyzed with chi-square test, Fisher’s exact test, or ANOVA. To evaluate the OS and TTF, Kaplan–Meier analyses and log-rank tests were used, as well as univariable and multivariable Cox proportional hazard models. The multivariable models were adjusted for clinically relevant patient demographics and tumor characteristics. The proportional hazards assumptions for these variables were tested by creating time-dependent covariates as a function of survival time. If these interaction terms were significant, the Schoenfeld residual plots were graphically inspected and if the residuals of the covariates changed over time the variables were deemed nonproportional and the model was stratified for these variables instead of adjusted. Two-sided p-values of <0.05 were considered statistically significant. All analyses were conducted using SAS® version 9.4 (SAS Institute, Cary, North Carolina, USA).
Results
We included 4130 patients (Figure 2), 3304 with synchronous, 214 with early metachronous, and 612 with late metachronous metastatic disease. Treatment after primary diagnosis prior to metachronous metastatic disease consisted of endoscopic resection (0.8%), surgical resection (92.3%), or definitive chemoradiotherapy (6.9%). In patients who had surgical resection, 14.6% had surgery only, 68.6% preoperative treatment, 15.5% preoperative and postoperative treatment, and 1.3% postoperative treatment (Supplementary Figure 2). Twenty-five patients had treatment for nonmetastatic locoregional recurrence prior to metachronous metastatic disease (Supplementary Table 1).
Patient characteristics
The number of distant metastatic sites of ⩾2 was highest in the early metachronous population (52.8%) compared with synchronous (41.6%) or late metachronous (46.2%) population (p = 0.001; Table 1). In the synchronous population, distant lymph nodes and liver metastases were more common (38.8% and 48.3%, respectively) than the early (lymph nodes: 29.9% and liver: 33.6%) or late metachronous population (lymph nodes: 33.3% and liver: 24.5%) (lymph nodes: p = 0.002 and liver: p < 0.001). Whereas lung, bone, and peritoneal metastases were less common in the synchronous compared with the early or late metachronous population (Table 1). Among patients with esophageal cancer, peritoneal metastases were present in 5.9%, 24.2%, and 22.2% of patients with synchronous, early metachronous, and late metachronous metastatic disease (p < 0.001; further details in Supplementary Table 2).
Table 1.
Characteristics for patients with synchronous or metachronous metastatic disease at metastatic diagnosis.
| Synchronous (n = 3304) | Metachronous (n = 826) | Early metachronous (n = 214) | Late metachronous (n = 612) | p value a | |
|---|---|---|---|---|---|
| Male | 2459 (74.4%) | 643 (77.8%) | 169 (79.0%) | 474 (77.5%) | 0.11 |
| Age (years)–median (IQR) | 69 (61–76) | 66 (59–73) | 66 (60–73) | <0.001 | |
| Comorbidities | 0.03 | ||||
| 0 | 1561 (47.2%) | 406 (49.2%) | 108 (50.5%) | 298 (48.7%) | |
| 1 | 1003 (30.4%) | 273 (33.1%) | 65 (30.4%) | 208 (34%) | |
| ⩾2 | 594 (18.0%) | 129 (15.6%) | 35 (16.4%) | 94 (15.4%) | |
| Unknown | 146 (4.4%) | 18 (2.2%) | 6 (2.8%) | 12 (2.0%) | |
| Performance status | <0.001 | ||||
| 0–1 | 1509 (45.7%) | 295 (35.7%) | 60 (28.0%) | 235 (38.4%) | |
| ⩾2 | 544 (16.5%) | 159 (19.2%) | 47 (22.0%) | 112 (18.3%) | |
| Unknown | 1251 (37.9%) | 372 (45.0%) | 107 (50.0%) | 265 (43.3%) | |
| Primary tumor location | <0.001 | ||||
| Esophagus | 1552 (47.0%) | 502 (60.8%) | 132 (61.7%) | 370 (60.5%) | |
| Gastroesophageal junction – Cardia | 521 (15.8%) | 94 (11.4%) | 29 (13.6%) | 65 (10.6%) | |
| Noncardia stomach | 1231 (37.3%) | 230 (27.8%) | 53 (24.8%) | 177 (28.9%) | |
| cT stage at primary diagnosis | <0.001 | ||||
| cT1 | 16 (0.5%) | 13 (1.6%) | 3 (1.4%) | 10 (1.6%) | |
| cT2 | 1127 (34.1%) | 264 (32.0%) | 51 (23.8%) | 213 (34.8%) | |
| cT3 | 786 (23.8%) | 455 (55.1%) | 132 (61.7%) | 323 (52.8%) | |
| cT4 | 322 (9.7%) | 34 (4.1%) | 11 (5.1%) | 23 (3.8%) | |
| cTX | 1053 (31.9%) | 60 (7.3%) | 17 (7.9%) | 43 (7.0%) | |
| cN stage at primary diagnosis | <0.001 | ||||
| cN0 | 593 (17.9%) | 339 (41%) | 68 (31.8%) | 271 (44.3%) | |
| cN1 | 955 (28.9%) | 268 (32.4%) | 69 (32.2%) | 199 (32.5%) | |
| cN2 | 1113 (33.7%) | 170 (20.6%) | 66 (30.8%) | 104 (17%) | |
| cN3 | 292 (8.8%) | 28 (3.4%) | 7 (3.3%) | 21 (3.4%) | |
| cNX | 351 (10.6%) | 21 (2.5%) | 4 (1.9%) | 17 (2.8%) | |
| Lauren’s classification at primary diagnosis | 0.03 | ||||
| Intestinal | 1319 (39.9%) | 340 (41.2%) | 78 (36.4%) | 262 (42.8%) | |
| Diffuse | 869 (26.0%) | 205 (24.8%) | 57 (26.6%) | 148 (24.2%) | |
| Mixed | 79 (2.4%) | 5 (0.6%) | 1 (0.5%) | 4 (0.7%) | |
| Indeterminate | 120 (3.6%) | 40 (4.8%) | 9 (4.2%) | 31 (5.1%) | |
| Adenocarcinoma NOS | 917 (27.8%) | 236 (28.6%) | 69 (32.2%) | 167 (27.3%) | |
| Tumor differentiation at primary diagnosis | <0.001 | ||||
| Well/moderate | 688 (20.8%) | 276 (33.4%) | 56 (26.2%) | 220 (35.9%) | |
| Poorly/undifferentiated | 1292 (39.1%) | 407 (49.3%) | 125 (58.4%) | 282 (46.1%) | |
| Unknown | 1324 (40.1%) | 143 (17.3%) | 33 (15.4%) | 110 (18.0%) | |
| HER2 status | 0.01 | ||||
| Positive | 411 (12.4%) | 70 (8.5%) | 12 (5.6%) | 58 (9.5%) | |
| Negative | 1488 (45.0%) | 388 (47.0%) | 98 (45.8%) | 290 (47.4%) | |
| Unknown | 1405 (42.5%) | 368 (44.6%) | 104 (48.6%) | 264 (43.1%) | |
| Metastatic sites | 0.001 | ||||
| 1 | 1930 (58.4%) | 430 (52.1%) | 101 (47.2%) | 329 (53.8%) | |
| ⩾2 | 1374 (41.6%) | 396 (47.9%) | 113 (52.8%) | 283 (46.2%) | |
| Distant lymph node metastases | 1281 (38.8%) | 268 (32.4%) | 64 (29.9%) | 204 (33.3%) | 0.002 |
| Liver metastases | 1597 (48.3%) | 222 (26.9%) | 72 (33.6%) | 150 (24.5%) | <0.001 |
| Lung metastases | 581 (17.6%) | 189 (22.9%) | 45 (21.0%) | 144 (23.5%) | 0.001 |
| Bone metastases | 479 (14.5%) | 155 (18.8%) | 49 (22.9%) | 106 (17.3%) | 0.002 |
| Peritoneal metastases | 889 (26.9%) | 292 (35.4%) | 78 (36.4%) | 214 (35.0%) | <0.001 |
| Other metastases | 469 (14.2%) | 293 (35.5%) | 88 (41.1%) | 205 (33.5%) | <0.001 |
HER2, human epidermal growth factor receptor 2; IQR, interquartile range; NOS, not otherwise specified.
Statistical analysis between patients with synchronous, early metachronous, or late metachronous metastatic disease.
Treatment patterns
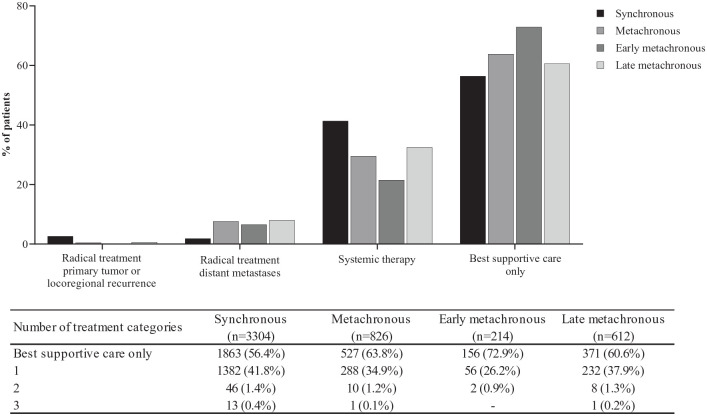
Radical treatment of primary tumor or locoregional recurrence was received by 2.6% and 0.7% of patients with synchronous and late metachronous metastatic disease, respectively (p = 0.002; Figure 3). None of the patients with early metachronous metastatic disease had radical treatment of locoregional recurrence. Radical treatment of distant metastases was received by 1.9%, 6.5%, and 8.0% of patients with synchronous, early metachronous, and late metachronous metastatic disease, respectively (p < 0.001). Systemic therapy was highest in patients with synchronous metastatic disease (41.3%) compared with early (21.5%) or late metachronous metastatic disease (32.5%) (p < 0.001). Best supportive care was received by 56.4%, 72.9%, and 60.6% of patients with synchronous, early, or late metachronous metastatic disease, respectively (p < 0.001). Among patients receiving best supportive care, 14.1% received a stent and 24.6% received radiotherapy for symptom control.
Figure 3.
Type of treatment and number of treatment categories after metastatic diagnosis in patients synchronous, metachronous, early metachronous, or late metachronous metastatic disease.
Among patients receiving systemic therapy, the proportions of patients receiving doublet and triplet chemotherapy differed between synchronous metastatic disease (doublet: 56.2% and triplet: 20.6%), early (doublet: 63.0% and triplet: 13.0%), and late metachronous metastatic disease (doublet: 70.7% and triplet: 7.1%) (doublet: p < 0.001 and triplet: p < 0.001) (Supplementary Table 3). Among patients receiving systemic treatment, the HER2 status did not differ (p = 0.17) and the proportion of patients receiving a trastuzumab-containing systemic regimen was similar (p = 0.27). Among patients receiving a subsequent systemic regimen, the most frequent was paclitaxel and ramucirumab (Supplementary Table 4).
Overall survival and time-to-treatment failure
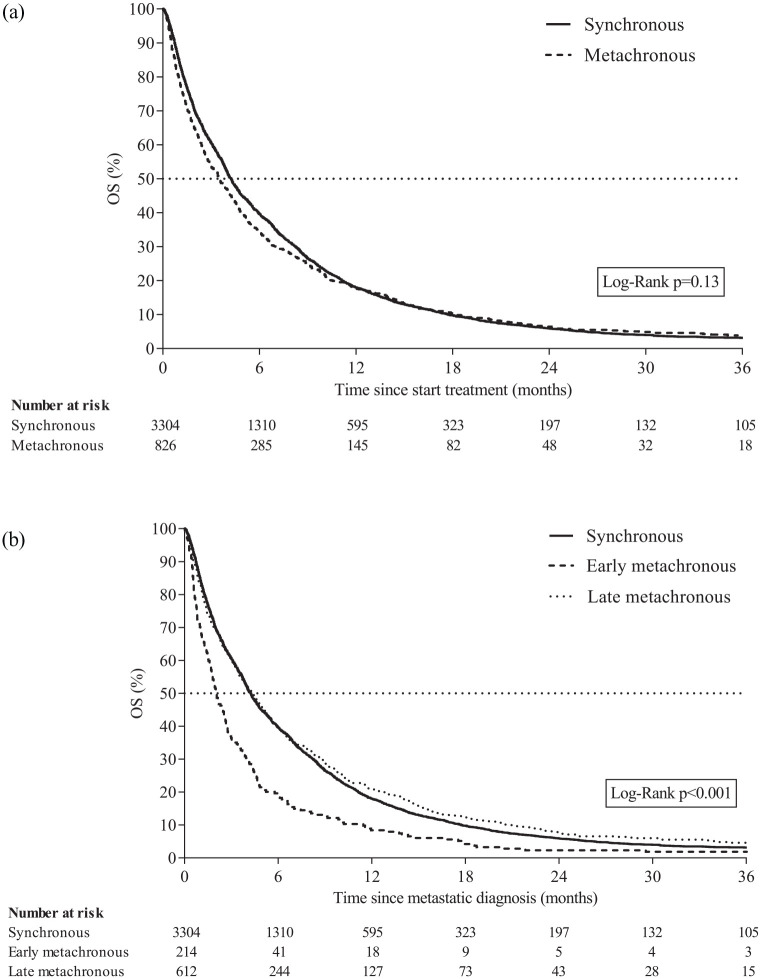
Median OS for all patients was 4.1 months [interquartile range (IQR) 1.5–9.4]. Among all patients with (both early and late) metachronous metastatic disease, median OS was similar (3.5 months, IQR 1.2–8.9) compared with patients with synchronous metastatic disease (4.2 months, IQR 1.6–9.5) (p = 0.13; Figure 4(a)). Median OS was 4.2 (IQR 1.6–9.5), 2.1 (IQR 0.8–4.6), and 4.4 months (IQR 1.4–10.1) for patients with synchronous, early metachronous, and late metachronous metastatic disease, respectively (p < 0.001; Figure 4(b)).
Figure 4.
(a) Overall survival of patients with synchronous metastatic disease or metachronous metastatic disease. (b) Overall survival of patients with synchronous metastatic disease, early metachronous metastatic disease, or late metachronous metastatic disease.
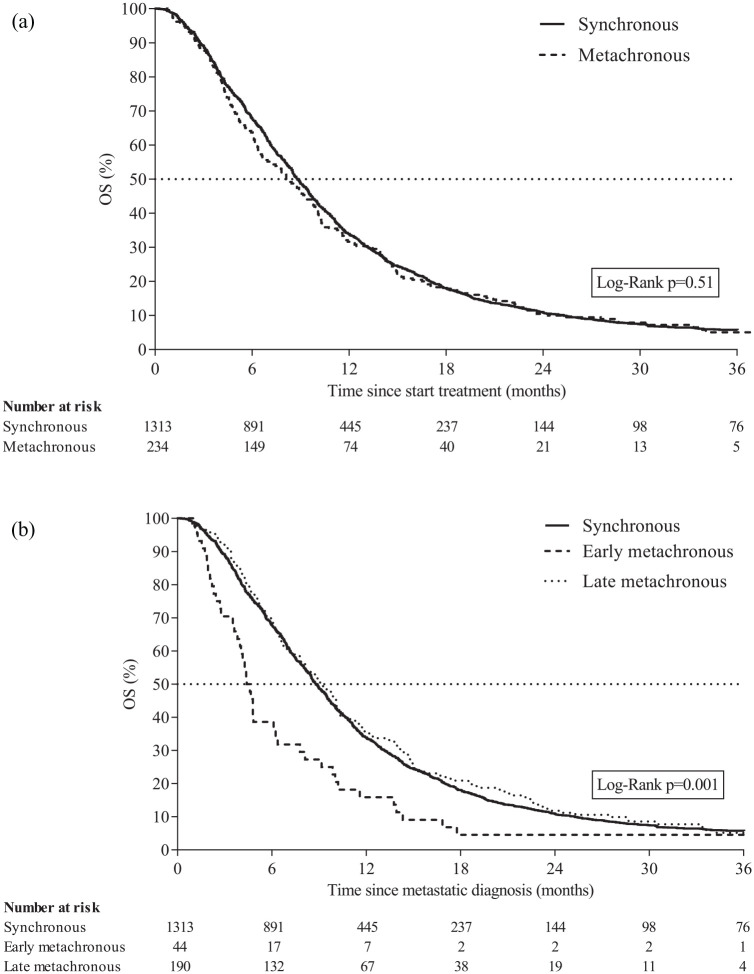
Median OS and median TTF for patients receiving systemic treatment (and not receiving radical treatment) were 8.7 months (IQR 4.8–14.7) and 5.9 months (IQR 3.5–9.5), respectively. Among all patients with metachronous metastatic disease receiving systemic treatment median OS (8.1 months, IQR 4.4–14.7) and TTF (5.4 months, IQR 3.4–5.8) were similar compared with patients with synchronous metastatic disease receiving systemic treatment (OS: 8.8 months, IQR 4.9–14.8 and TTF: 6.1 months, IQR 3.5–9.7) (OS: p = 0.51; Figure 5(a) and TTF: p = 0.29; Supplementary Figure 1A). Among patients receiving systemic therapy, the median OS was 8.8 (IQR 4.9–14.8), 4.5 (IQR 2.6–9.5), and 9.1 months (IQR 5.2–15.0) for patients with synchronous, early metachronous, and late metachronous metastatic disease, respectively (p = 0.001; Figure 5(b)). Median TTF was 6.1 (IQR 3.5–9.7), 3.8 (IQR 2.3–5.3), and 5.7 months (IQR 3.7–9.7) for patients with synchronous, early metachronous, and late metachronous metastatic disease, respectively (p < 0.001; Supplementary Figure 1B). Median OS and TTF were also assessed from start of systemic treatment after metastatic diagnosis and presented in Supplementary Table 5. Among patients receiving doublet or triplet chemotherapy (and not receiving radical treatment), no differences in median OS were observed for patients with synchronous (doublet: 7.7 versus triplet: 6.8 months, p = 0.57) or metachronous (doublet 6.3 versus triplet 9.3 months, p = 0.82) metastatic disease. Separately analyses for patients with early or late metachronous receiving doublet or triplet chemotherapy were not performed due to limited sample size.
Figure 5.
(a) Overall survival of patients with synchronous metastatic disease or metachronous metastatic disease receiving systemic treatment after diagnosis. Patients receiving radical treatment of primary tumor, locoregional recurrence, or distant metastases in addition to systemic therapy were excluded. (b) Overall survival of patients with synchronous metastatic disease, early metachronous metastatic disease, or late metachronous metastatic disease receiving systemic treatment after diagnosis. Patients receiving radical treatment of primary tumor, locoregional recurrence, or distant metastases in addition to systemic therapy were excluded.
Among patients receiving systemic treatment, the multivariable analysis showed that patients with synchronous metastatic disease had a better OS and TTF compared with early metachronous metastatic disease [OS: hazard ratio (HR) 1.70 (1.23–2.36); TTF: HR 1.89 (1.37–2.61)] and a similar OS and TTF compared with late metachronous metastatic disease [OS: HR 1.00 (0.84–1.20); TTF: HR 1.01 (0.84–1.21); Table 2].
Table 2.
Cox regression for overall survival and time to treatment failure in patients receiving systemic treatment after diagnosis of metastatic disease.
| Overall survival | Time-to-treatment failure | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Events | Median OS (months) | Univariable regression | Multivariable regressiona,b | Events | Median TTF (months) | Univariable regression | Multivariable regressiona,b | |||||
| HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | HR (95% CI) | p value | |||||
| Synchronous | 1269 | 8.8 | Ref | Ref | 1222 | 6.1 | Ref | Ref | ||||
| Early metachronous | 42 | 4.5 | 1.75 (1.29–2.38) | <0.001 | 1.70 (1.23–2.36) | 0.001 | 43 | 3.8 | 1.96 (1.44–2.65) | <0.001 | 1.89 (1.37–2.61) | <0.001 |
| Late metachronous | 177 | 9.1 | 0.96 (0.82–1.12) | 0.59 | 1.00 (0.84–1.20) | 0.99 | 161 | 5.7 | 0.97 (0.82–1.14) | 0.70 | 1.01 (0.84–1.21) | 0.93 |
CI, confidence interval; HR, hazard ratio; OS, overall survival; TTF, time to treatment failure.
Patients receiving radical treatment of primary tumor, locoregional recurrence, or distant metastases in addition to systemic therapy were excluded.
Adjusted for gender, age, number of comorbidities, tumor location at primary diagnosis, cT stage at primary diagnosis, cN stage at primary diagnosis, Lauren’s classification at primary diagnosis, tumor differentiation at primary diagnosis, number of metastatic sites, location of metastases (distant lymph nodes, liver metastases, lung, bone, peritoneal, and other), and receiving a trastuzumab-containing regimen.
Performance status did not meet the proportional hazard assumptions and the models were stratified for this covariate.
Median OS for patients receiving best supportive care only was 2.0 months (IQR 1.0–4.4). Multivariable analyses showed that patients with early metachronous [HR 1.41 (1.18–1.68)] and not late metachronous metastatic disease [HR 1.02 (0.89–1.15)] had a worse OS compared with synchronous metastatic disease (Table 3).
Table 3.
Cox regression for overall survival in patients receiving best supportive care only after diagnosis of metastatic disease.
| Events | Median OS (months) | Univariable regression | Multivariable regressiona,b | |||
|---|---|---|---|---|---|---|
| HR (95% CI) | p value | HR (95% CI) | p value | |||
| Synchronous | 1857 | 2.0 | Ref | Ref | ||
| Early metachronous | 156 | 1.5 | 1.33 (1.13–1.56) | <0.001 | 1.41 (1.18–1.68) | <0.001 |
| Late metachronous | 364 | 2.2 | 0.90 (0.81–1.00) | 0.07 | 1.02 (0.89–1.15) | 0.81 |
CI, confidence interval; HR, hazard ratio; OS, overall survival.
Adjusted for gender, age, number of comorbidities, tumor location at primary diagnosis, cT stage at primary diagnosis, cN stage at primary diagnosis, Lauren’s classification at primary diagnosis, tumor differentiation at primary diagnosis, number of metastatic sites and location of metastases (distant lymph nodes, liver metastases, lung, bone, peritoneal, and other).
Performance status did not meet the proportional hazard assumptions and the model was stratified for these covariates.
Discussion
In this nationwide study of patients with synchronous or metachronous metastatic esophagogastric adenocarcinoma, survival was especially poor in patients with early metachronous metastatic disease compared with patients with synchronous metastatic disease or late metachronous metastatic disease. Patients with early metachronous metastatic disease were less often treated with systemic therapy and treatment failure occurred more rapidly compared with patients with synchronous or late metachronous metastatic disease.
Patients with early metachronous metastatic disease had the highest number of distant metastatic sites. This finding may reflect a more aggressive evolution of the disease that has escaped previous treatment with curative intent.23–25 Locations of metastases were different between patients with synchronous, early, or late metachronous metastatic disease. Particularly, peritoneal metastases occurred almost four times more frequently in patients with esophageal cancer in early or late metachronous compared with synchronous metastatic disease. This shows that peritoneal metastases occur in a late stage of disease in esophageal cancer also indicating a higher metastatic potential due to resistance after treatment with curative intent. 25
In the Netherlands, imaging to detect disease recurrence is not routinely performed during follow-up care after treatment with curative intent.26,27 Follow-up after treatment with curative intent is focused on quality of life and symptom control. Guidelines recommend a follow-up visit every 3 months in the first year and (half)yearly after the first year until 5 years after treatment with curative intent.8,9 Radiological examinations or endoscopies to detect recurrent or metastatic disease are only performed when patients experience disease symptoms. The vast majority of patients with early metachronous metastatic disease (78.0%) were diagnosed after experiencing symptoms (results not shown). If these patients would have had routine imaging metastatic disease would have been diagnosed even sooner. This indicates that either metastases developed within a short period of time after treatment with curative intent or metastases were already present during treatment with curative intent but were undetected.
Our study identified a poor prognosis of patients with early metachronous metastatic disease compared with patients with synchronous or late metachronous metastatic disease. Besides differences in metastatic sites, certain characteristics, such as a higher cT and cN stage stage at primary diagnosis, and more often a poorly/undifferentiated tumor at primary diagnosis, appear to be associated with early metachronous metastatic disease and a more aggressive tumor biology. Future research should focus on the identification of patients at primary diagnosis with a high risk for early metachronous metastatic disease as this could have clinical implications for treatment strategies (e.g. intensification or addition adjuvant therapy 28 ) and/or follow-up strategies (e.g. more frequent restaging investigations, including clinical examinations, radiological and/or laboratory investigations).
Systemic treatment was also least common among patients with early metachronous metastatic disease. A potential explanation could be that patients with early metachronous metastatic disease were still recovering from more recent treatment with curative intent and by definition have a more aggressive disease than patients with late metachronous metastatic disease.8–11 Even if patients with early metachronous metastatic disease received systemic treatment, their survival was very poor, suggesting a rapid progression and unresponsive tumor biology.
In the current guidelines, no distinct recommendation is provided for the type of palliative systemic treatment for synchronous or metachronous metastatic disease. Nevertheless, patients with synchronous metastatic disease more often received triplet therapy compared with patients with metachronous metastatic disease. A possible explanation could be the presence of long-term toxicity or adverse events from prior chemotherapy in patients with metachronous metastatic disease. During the study period, triplet therapy was still routinely used, however, recent evidence has shown that doublet therapy leads to similar survival and less toxicity compared with triplet therapy.6,14,29 In our study no difference in survival was observed between patients receiving doublet or triplet chemotherapy. These results are in line with a previous study using data from the NCR, which reported no survival benefit for patients diagnosed with esophagogastric cancer between 2010 and 2016 receiving triplet chemotherapy compared with doublet chemotherapy. 14 In addition, a network meta-analysis of 50 studies reported no difference in survival between triplet chemotherapy compared with fluoropyrimidine doublet therapy. 6
Regardless of the availability of palliative systemic treatment, more than half of all patients received best supportive care only. Information with respect to the decision to refrain from palliative systemic treatment was not available. This high percentage could be due to patients’ preference, performance status, comorbidities, low life expectancy, or for patients with metachronous metastatic disease toxicity of previous chemotherapy. 30 In addition, medical oncologists in the Netherlands might have a more conservative approach on the use of chemotherapy compared with other European countries as a previous study reported lower chemotherapy usage in the Netherlands (39%) compared with Belgium (63%) in patients with metastatic gastric cancer. 31 Furthermore, it is likely that hospital variation plays a role in the probability of receiving first-line palliative systemic therapy as a study including seven Dutch hospitals identified the hospital of diagnosis as an independent factor for treatment decisions in the palliative care of esophageal cancer. 32
The major strength of this study is the use of population-based data, resulting in a representative reflection of patients in clinical practice. However, this study also has some limitations. First, the retrospective design of the study. Second, data for certain variables, for example, performance status and tumor differentiation, were incomplete which might have resulted in suboptimal adjustment in multivariable models. Finally, sample size of certain subgroups was limited and resulted in wide confidence intervals of the point estimates for OS and TTF.
In conclusion, in this nationwide study for patients with synchronous or metachronous metastatic esophagogastric adenocarcinoma, we showed that patients with early metachronous metastatic disease have a worse survival compared with patients with synchronous or late metachronous metastatic disease, suggesting a more aggressive tumor biology. Therefore, patients with early metachronous metastatic disease should be considered as a separate group compared with patients with synchronous metastatic disease and late metachronous metastatic disease.
Supplemental Material
Supplemental material, sj-docx-1-tam-10.1177_17588359221085557 for A population-based study in synchronous versus metachronous metastatic esophagogastric adenocarcinoma by Marieke Pape, Pauline A. J. Vissers, David Bertwistle, Laura McDonald, Marije Slingerland, Nadia Haj Mohammad, Laurens V. Beerepoot, Jelle P. Ruurda, Grard A. P. Nieuwenhuijzen, Paul M. Jeene, Hanneke W. M. van Laarhoven and Rob H. A. Verhoeven in Therapeutic Advances in Medical Oncology
Acknowledgments
The authors thank the registration team of the Netherlands Comprehensive Cancer Organisation (IKNL) for the collection of data for the Netherlands Cancer Registry. The authors thank all participating hospitals in the Netherlands.
Footnotes
Author contributions: Marieke Pape: Conceptualization; Data curation; Formal analysis; Investigation; Methodology; Writing – original draft; Writing – review & editing.
Pauline A. J. Vissers: Conceptualization; Methodology; Supervision; Writing – review & editing.
David Bertwistle: Conceptualization; Methodology; Writing – review & editing.
Laura McDonald: Conceptualization; Methodology; Writing – review & editing.
Marije Slingerland: Conceptualization; Writing – review & editing.
Nadia Haj Mohammad: Methodology; Writing – review & editing.
Laurens V. Beerepoot: Conceptualization; Writing – review & editing.
Jelle P. Ruurda: Conceptualization; Writing – review & editing.
Grard A. P. Nieuwenhuijzen: Conceptualization; Writing – review & editing.
Paul M. Jeene: Methodology; Writing – review & editing.
Hanneke W. M. van Laarhoven: Conceptualization; Methodology; Supervision; Writing – review & editing.
Rob H. A. Verhoeven: Conceptualization; Funding acquisition; Methodology; Supervision; Writing – review & editing.
Conflict of interest statement: The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: D.B. is employee of BMS and holds stock in BMS and GSK. L.M. is employee of BMS and holds stock in BMS. N.H.M. reports personal fees (consultancy) from BMS, Eli Lilly, Astra Zeneca, Servier, and MSD. G.N. reports unrestricted research funding from Medtronic and fees for consultancy from Medtronic and Lilly. H.W.M.v.L. reports grants from Roche, has served as a consultant for BMS, Celgene, Lilly, and Nordic, and has received unrestricted research funding from Bayer, BMS, Celgene, Lilly, Merck Serono, MSD, Nordic, Philips, and Roche. R.H.A.V. reports grants from BMS and Roche. M.P., P.V., M.S., L.B., J.R., and P.J. have no disclosures to declare.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by Bristol Myers Squibb (CA209-77E). The funder has financed part of the data collection and two employees of the funder have contributed as co-authors on the article. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
ORCID iDs: Marieke Pape  https://orcid.org/0000-0001-9054-7541
https://orcid.org/0000-0001-9054-7541
Laurens V. Beerepoot  https://orcid.org/0000-0002-3040-4626
https://orcid.org/0000-0002-3040-4626
Supplemental material: Supplemental material for this article is available online.
Contributor Information
Marieke Pape, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, The Netherlands; Department of Medical Oncology, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
Pauline A. J. Vissers, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Utrecht, The Netherlands Department of Surgery, Radboud University Medical Centre, Nijmegen, The Netherlands.
David Bertwistle, Worldwide Health Economics & Outcomes Research, Bristol-Myers Squibb, Uxbridge, UK.
Laura McDonald, Centre for Observational Research & Data Sciences, Bristol-Myers Squibb, Uxbridge, UK.
Marije Slingerland, Department of Medical Oncology, Leiden University Medical Center, Leiden, The Netherlands.
Nadia Haj Mohammad, Department of Medical Oncology, University Medical Center Utrecht, Utrecht University, Utrecht, The Netherlands.
Laurens V. Beerepoot, Department of Medical Oncology, Elisabeth-TweeSteden Hospital, Tilburg, The Netherlands
Jelle P. Ruurda, Department of Surgery, University Medical Center Utrecht, Utrecht, The Netherlands
Grard A. P. Nieuwenhuijzen, Department of Surgery, Catharina Hospital, Eindhoven, The Netherlands
Paul M. Jeene, Department of Radiation Oncology, Amsterdam University Medical Centers, Amsterdam, The Netherlands Radiotherapiegroep, Deventer, The Netherlands.
Hanneke W. M. van Laarhoven, Department of Medical Oncology, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands
Rob H. A. Verhoeven, Department of Research & Development, Netherlands Comprehensive Cancer Organisation (IKNL), Godebaldkwartier 419, 3511 DT Utrecht, The Netherlands; Department of Medical Oncology, Cancer Center Amsterdam, Amsterdam UMC, University of Amsterdam, Amsterdam, The Netherlands.
References
- 1. Sung H, Ferlay J, Siegel RL, et al. Global cancer statistics 2020: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J Clin 2021; 71: 209–249. [DOI] [PubMed] [Google Scholar]
- 2. van Putten M, de Vos-Geelen J, Nieuwenhuijzen GAP, et al. Long-term survival improvement in oesophageal cancer in the Netherlands. Eur J Cancer 2018; 94: 138–147. [DOI] [PubMed] [Google Scholar]
- 3. Riihimaki M, Hemminki A, Sundquist K, et al. Metastatic spread in patients with gastric cancer. Oncotarget 2016; 7: 52307–52316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Napier KJ, Scheerer M, Misra S. Esophageal cancer: a review of epidemiology, pathogenesis, staging workup and treatment modalities. World J Gastrointest Oncol 2014; 6: 112–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Haj Mohammad N, Bernards N, van Putten M, et al. Volume-outcome relation in palliative systemic treatment of metastatic oesophagogastric cancer. Eur J Cancer 2017; 78: 28–36. [DOI] [PubMed] [Google Scholar]
- 6. Ter Veer E, Haj Mohammad N, van Valkenhoef G, et al. The efficacy and safety of first-line chemotherapy in advanced esophagogastric cancer: a network meta-analysis. J Natl Cancer Inst 2016; 108: djw166. [DOI] [PubMed] [Google Scholar]
- 7. van Kleef JJ, Ter Veer E, van den Boorn HG, et al. Quality of life during palliative systemic therapy for esophagogastric cancer: systematic review and meta-analysis. J Natl Cancer Inst 2020; 112: 12–29. [DOI] [PubMed] [Google Scholar]
- 8. Dutch clinical practice guidelines for esophageal carcinoma version 31, 2015, www.oncoline.nl (accessed October 2020).
- 9. Dutch clinical practice guidelines for gastric carcinoma version 20, 2017, www.oncoline.nl (accessed October 2020).
- 10. Lordick F, Mariette C, Haustermans K, et al. Oesophageal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v50–v57. [DOI] [PubMed] [Google Scholar]
- 11. Smyth EC, Verheij M, Allum W, et al. Gastric cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 2016; 27: v38–v49. [DOI] [PubMed] [Google Scholar]
- 12. International Union against Cancer (UICC). TNM classification of malignant tumours. 7th ed. Chichester: Wiley-Liss, 2009. [Google Scholar]
- 13. International Union against Cancer (UICC). TNM classification of malignant tumours. 8th ed. Chichester: Wiley-Blackwell, 2017. [Google Scholar]
- 14. Dijksterhuis WPM, Verhoeven RHA, Slingerland M, et al. Heterogeneity of first-line palliative systemic treatment in synchronous metastatic esophagogastric cancer patients: a real-world evidence study. Int J Cancer 2020; 146: 1889–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Dijksterhuis WPM, Kroese TE, Verhoeven RHA, et al. Management and outcomes of gastric cancer patients with interval distant metastases in clinical practice [manuscript submitted for publication]. 2022. [Google Scholar]
- 16. Kroese TE, Dijksterhuis WPM, van Rossum PSN, et al. Prognosis of interval distant metastases after neoadjuvant chemoradiotherapy for esophageal cancer. Ann Thorac Surg 2022; 113: 482–490. [DOI] [PubMed] [Google Scholar]
- 17. Adam R, de Gramont A, Figueras J, et al. Managing synchronous liver metastases from colorectal cancer: a multidisciplinary international consensus. Cancer Treat Rev 2015; 41: 729–741. [DOI] [PubMed] [Google Scholar]
- 18. Vayrynen V, Wirta EV, Seppala T, et al. Incidence and management of patients with colorectal cancer and synchronous and metachronous colorectal metastases: a population-based study. BJS Open 2020; 4: 685–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Luo Z, Rong Z, Huang C. Surgery strategies for gastric cancer with liver metastasis. Front Oncol 2019; 9: 1353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Thelen A, Jonas S, Benckert C, et al. Liver resection for metastatic gastric cancer. Eur J Surg Oncol 2008; 34: 1328–1334. [DOI] [PubMed] [Google Scholar]
- 21. Jeene PM, Versteijne E, van Berge Henegouwen MI, et al. Supraclavicular node disease is not an independent prognostic factor for survival of esophageal cancer patients treated with definitive chemoradiation. Acta Oncol 2017; 56: 33–38. [DOI] [PubMed] [Google Scholar]
- 22. Dijksterhuis WPM, Verhoeven RHA, Pape M, et al. Hospital volume and beyond first-line palliative systemic treatment in metastatic oesophagogastric adenocarcinoma: a population-based study. Eur J Cancer 2020; 139: 107–118. [DOI] [PubMed] [Google Scholar]
- 23. Ebbing EA, Steins A, Fessler E, et al. Esophageal adenocarcinoma cells and xenograft tumors exposed to Erb-b2 receptor tyrosine kinase 2 and 3 inhibitors activate transforming growth factor beta signaling, which induces epithelial to mesenchymal transition. Gastroenterology 2017; 153: 63–76. [DOI] [PubMed] [Google Scholar]
- 24. Ebbing EA, van der Zalm AP, Steins A, et al. Stromal-derived interleukin 6 drives epithelial-to-mesenchymal transition and therapy resistance in esophageal adenocarcinoma. Proc Natl Acad Sci USA 2019; 116: 2237–2242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Steins A, Ebbing EA, Creemers A, et al. Chemoradiation induces epithelial-to-mesenchymal transition in esophageal adenocarcinoma. Int J Cancer 2019; 145: 2792–2803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dutch clinical practice guidelines for esophageal carcinoma – follow-up, 2010, www.richtlijnendatabase.nl (accessed March 2021).
- 27. Dutch clinical practice guidelines for gastric carcinoma – follow-up version 2.2, www.richtlijnendatabase.nl (accessed June 2021).
- 28. Kelly RJ, Kuzdzal J, Zander T, et al. Adjuvant nivolumab in resected esophageal or gastroesophageal junction cancer following neoadjuvant chemoradiation therapy: first results of the CheckMate 577 study. ESMO Virtual Congress 2020; 31: S1142–S1215. [Google Scholar]
- 29. Carmona-Bayonas A, Jimenez-Fonseca P, Custodio A, et al. Anthracycline-based triplets do not improve the efficacy of platinum-fluoropyrimidine doublets in first-line treatment of advanced gastric cancer: real-world data from the AGAMEMON National Cancer Registry. Gastric Cancer 2018; 21: 96–105. [DOI] [PubMed] [Google Scholar]
- 30. Wagner AD, Grothe W, Haerting J, et al. Chemotherapy in advanced gastric cancer: a systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006; 24: 2903–2909. [DOI] [PubMed] [Google Scholar]
- 31. Claassen YHM, Bastiaannet E, Hartgrink HH, et al. International comparison of treatment strategy and survival in metastatic gastric cancer. BJS Open 2019; 3: 56–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Opstelten JL, de Wijkerslooth LR, Leenders M, et al. Variation in palliative care of esophageal cancer in clinical practice: factors associated with treatment decisions. Dis Esophagus 2017; 30: 1–7. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-tam-10.1177_17588359221085557 for A population-based study in synchronous versus metachronous metastatic esophagogastric adenocarcinoma by Marieke Pape, Pauline A. J. Vissers, David Bertwistle, Laura McDonald, Marije Slingerland, Nadia Haj Mohammad, Laurens V. Beerepoot, Jelle P. Ruurda, Grard A. P. Nieuwenhuijzen, Paul M. Jeene, Hanneke W. M. van Laarhoven and Rob H. A. Verhoeven in Therapeutic Advances in Medical Oncology