Abstract
Objectives
In this study, we aimed to determine the correlation between procalcitonin (PCT) levels and clinical outcomes including in-hospital mortality, intensive care unit (ICU) length of stay, and hospital length of stay in patients hospitalized with COVID-19.
Methods
Clinical, laboratory, and demographic data of 223 patients who met inclusion criteria were analyzed. PCT measurements of 0.25 ng/mL and 0.50 ng/mL were used to stratify patients into 2 mutually exclusive groups.
Results
Patients with PCT above 0.25 ng/mL on admission had significantly elevated Acute Physiology and Chronic Health Evaluation II scores (9 vs 8; P = 0.042) and C-reactive proteins levels (111 μg/mL vs 79 μg/mL; P = 0.007). A multivariable binary logistic regression model demonstrated no relationship between PCT and mortality (OR = 1.00; 95% Cl: 0.97 to 1.02; P = 0.713). Kaplan-Meier analysis revealed no statistical evidence of a difference between PCT groups and hospital length of stay (P = 0.144 for 0.25 ng/mL, P = 0.368 for 0.50 ng/mL) or intensive care unit length of stay (P = 0.986 for 0.25 ng/mL, P = 0.771 for 0.50 ng/mL).
Conclusions
Elevated PCT levels were associated with severity of illness but did not correlate with in-hospital mortality, hospital length of stay, or ICU length of stay.
Keywords: Procalcitonin, COVID-19, Clinical outcomes, Prognosis
Introduction
The COVID-19 pandemic has led to more than 249,743,428 confirmed cases, including 5,047,652 deaths worldwide as of November 8, 2021. Early identification of patients at risk for severe outcomes is crucial in the management of this disease. Previous studies have demonstrated that specific inflammatory, biochemical, and immunological biomarkers are of prognostic value in patients infected with SARS-CoV-2 (Liu et al., 2020, Ponti et al., 2020, Hu et al., 2020, Zhang et al., 2020, Ji et al., 2020). A meta-analysis by Tjendra et al. (2020) concluded elevated white blood cell count; lymphopenia; reduced CD3, CD4, or CD8 T-lymphocyte counts; high neutrophil count; and thrombocytopenia were associated with severe disease and the risk of developing sepsis with rapid progression. Another meta-analysis by Figliozzi et al. (2020) studied the association of 18 factors with a composite outcome of death, severe presentation, hospitalization in the intensive care unit, and/or mechanical ventilation. Elevated procalcitonin (PCT) was 1 of these factors with an odds ratio of 4.8.
PCT is a peptide typically produced by the parafollicular cells of the thyroid in healthy individuals. In the setting of bacterial infection, an inflammatory cascade triggers extra-thyroidal production of PCT from adipocytes and the neuroendocrine cells of the lungs and intestine. In contrast, viral infection leads to an increase in interferon gamma, which inhibits the formation of PCT. This mechanism makes PCT a useful biomarker to differentiate systemic inflammation resulting from bacterial origin compared with viral origin in cases of community-acquired pneumonia and sepsis (Lee, 2013). However, PCT levels have been found to be elevated in patients with COVID-19 and have been widely used to monitor disease severity in critically ill patients.
The purpose of this study was to retrospectively determine the correlation of PCT in patients hospitalized with COVID-19 with clinical outcome including in-hospital mortality. Additionally, a secondary aim was to determine if elevated PCT affects intensive care unit or hospital length of stay.
Materials and Methods
A total of 402 consecutive adult patients aged 19 years and older with positive COVID-19 PCR testing were admitted to hospitals within our health system from March 1, 2020, until July 10, 2020. Patients were excluded if they were younger than 19 years, did not have positive COVID-19 PCR testing, or were admitted outside of the dates above. PCT levels were measured in 223 of these patients during hospitalization, and this subset of patients was used for our analysis. There was no formal policy within our institution regarding PCT measurement in patients with COVID-19. In general, PCT was measured at least once in these patients upon admission, and repeat measurement was obtained if there was concern for new infection. All patients included in the study received antimicrobial therapy. Length of therapy was subject to whether the clinician believed there was concurrent bacterial infection.
Baseline patient characteristics including age, biological sex, race, and comorbid conditions such as coronary artery disease, hypertension, malignancy, obesity, asthma, chronic obstructive pulmonary disease, and diabetes were recorded. Data from hospitalization including PCT level, CRP level, lactate dehydrogenase (LDH), ferritin, and Acute Physiology and Chronic Health Evaluation II (APACHE II) scores on admission were collected. Finally, clinical outcomes including in-hospital mortality, length of hospitalization, and intensive care unit (ICU) length of stay were collected.
A PCT measurement of 0.25 ng/mL was used to stratify patients into 2 mutually exclusive groups. Comparisons of patients in the 2 groups were made with the Mann-Whitney test for continuous variables and the chi-square or Fisher exact test on the basis of expected frequencies for discrete variables. Subsequently, a PCT of 0.50 ng/mL was used and similar comparisons were conducted. Continuous variables are presented as median and interquartile range (IQR), whereas categorical variables are presented as count and percent.
Multivariable binary logistic regression models were used to investigate in-hospital mortality. The Pearson correlation coefficient was examined for each pairwise comparison of continuous variables to verify multicollinearity. Variables were selected for inclusion into the multivariable model on the basis of the Gini index calculated using random forests with 1000 classification trees. Furthermore, the investigation of higher-order nonlinear terms for each continuous variable was examined with LOESS methods. Fit of the final multivariable model was evaluated using the c-statistic (ie, area under the curve generated by receiver operating characteristics [ROC] curve). SAS v. 9.4 was used for all analyses; P <0.05 indicated statistical significance. Kaplan-Meier analyses were used to examine hospital length of stay and intensive care unit length of stay used death as the censoring event.
Results
A total of 223 patients met the inclusion criteria (shown in Table 1 ). Median age (95% confidence interval [CI]) was 63 years (52–75 years) with majority (52.5%) of patients as female. In-hospital mortality was 14% in our area of the country. Clinical and demographic factors were stratified by PCT measurements of 0.25 ng/mL (shown in Table 2) and 0.50 ng/mL (shown in Table 3). Patients with PCT above 0.25 ng/mL were significantly more likely to have chronic kidney disease at baseline (21.3% vs 8.8%; P = 0.009) and significantly elevated APACHE II scores (9 vs 8; P = 0.042) and CRP levels (111 μg/mL vs 79 μg/mL; P = 0.007) at hospital admission. Upon stratification via the 0.50 ng/mL point, only CRP levels (131 μg/mL vs 82 μg/mL; P = 0.010) retained statistical significance between groups.
Table 1.
Clinical and demographic characteristics of overall study group and stratified by procalcitonin of 0.25 ng/mL and 0.50 ng/ml.
| Procalcitonin |
|||||||||
|---|---|---|---|---|---|---|---|---|---|
| Overall (N = 223) |
< 0.25 ng/mL (n = 148) |
≥ 0.25 ng/mL (n = 75) |
p | < 0.50 ng/mL (n = 187) |
≥ 0.50 ng/mL (n = 36) |
p | |||
| Age (Years) | 63 [52-75] | 63 [52-75] | 62 [47-75] | 0.651 | 63 [52-75] | 62 [46-76] | 0.647 | ||
| Biological Sex | |||||||||
| Female | 116 (52.5) | 77 (52.7) | 39 (52.0) | 0.917 | 96 (51.9) | 20 (55.6) | 0.687 | ||
| Male | 105 (47.5) | 69 (47.3) | 36 (48.0) | 89 (48.1) | 16 (44.4) | ||||
| Race/Ethnicity | |||||||||
| White | 107 (48.9) | 80 (54.8) | 27 (37.0) | 0.053 | 93 (50.5) | 14 (40.0) | 0.100 | ||
| Black | 28 (12.8) | 15 (10.3) | 13 (17.8) | 24 (13.1) | 4 (11.4) | ||||
| Hispanic | 68 (31.1) | 43 (29.5) | 25 (34.3) | 57 (31.0) | 11 (31.4) | ||||
| Asian | 16 (7.3) | 8 (5.5) | 8 (11.0) | 10 (5.4) | 6 (17.2) | ||||
| Obesity | 153 (69.6) | 101 (69.2) | 52 (70.3) | 0.868 | 132 (71.4) | 21 (60.0) | 0.181 | ||
| CAD | 49 (22.2) | 34 (23.0) | 15 (20.6) | 0.683 | 43 (23.0) | 6 (17.7) | 0.490 | ||
| CKD | 29 (13.0) | 13 (8.8) | 16 (21.3) | 0.009 | 24 (12.8) | 5 (13.9) | 0.863 | ||
| Cancer | 17 (7.6) | 10 (6.8) | 7 (9.3) | 0.493 | 13 (7.0) | 4 (11.1) | 0.389 | ||
| COPD | 24 (11.0) | 16 (11.0) | 8 (11.0) | 1.000 | 17 (9.2) | 7 (20.0) | 0.076 | ||
| Asthma | 17 (7.8) | 12 (8.3) | 5 (6.9) | 0.711 | 14 (7.7) | 3 (8.6) | 0.741 | ||
| Diabetes | 110 (50.2) | 78 (53.4) | 32 (43.8) | 0.181 | 94 (51.1) | 16 (45.7) | 0.560 | ||
| APACHE II | 8 [6-12] | 8 [6-12] | 9 [6-14] | 0.042 | 8 (6-12) | 11 (6-15) | 0.088 | ||
| CRP (μg/mL) | 86 [44-453] | 79 [41-133] | 111 [61-186] | 0.007 | 82 [41-142] | 131 [68-223] | 0.010 | ||
| Maximum Ferritin (μg/L) | 606 [344-1216] | 595 [344-1111] | 628 [316-1328] | 0.741 | 595 [344-1216] | 628 [279-1328] | 0.629 | ||
| LDH (U/L) | 337 [276-460] | 327 [278-440] | 369 [276-517] | 0.077 | 331 [276-454] | 369 [264-461] | 0.386 | ||
| In-hospital death | 31 (14.6) | 18 (12.9) | 13 (18.1) | 0.310 | 25 (14.1) | 6 (17.1) | 0.644 | ||
| Hospital Stay (Days) | 10 [8-13] | 10 [8-14] | 11 [7-14] | 0.144 | 10 [8-13] | 13 [7-16] | 0.368 | ||
| ICU Stay | |||||||||
| No | 114 (53.8) | 82 (58.6) | 32 (44.4) | 0.051 | 100 (56.5) | 14 (40.0) | 0.074 | ||
| Yes | 98 (46.2) | 58 (41.4) | 40 (55.6) | 77 (43.5) | 21 (60.0) | ||||
| ICU Days | 10 [7-13] | 10 [7-14] | 12 [5-15] | 0.986 | 10 [7-14] | 9 [3-13] | 0.218 | ||
| Intubation | |||||||||
| No | 136 (63.9) | 92 (65.3) | 44 (61.1) | 0.552 | 116 (65.2) | 20 (57.1) | 0.366 | ||
| Yes | 77 (36.2) | 49 (34.8) | 28 (38.9) | 62 (34.8) | 15 (42.9) | ||||
| Intubation Days | 13 [10-16] | 13 [8-16] | 13 [10-25] | 0.552 | 14 [8-17] | 13 [7-20] | 0.768 | ||
Note. Data presented as median [IQR] or n (%). For length of stay and length of intubation, data are presented as median [95% CI] based on Kaplan-Meier estimates that accounted for censoring due to in-hospital death.
Results of a multivariable binary logistic regression model for in-hospital mortality demonstrated no relationship between PCT and mortality (OR = 1.00; 95% Cl: 0.97 to 1.02; P = 0.713). However, the model demonstrated that every additional 10 years of age was associated with significantly increased odds of mortality (OR = 2.42; 95% CI: 1.59 to 4.09; P <0.001) after adjusting for all other variables. The ROC curve provided statistical evidence that the model could predict mortality events (AUC = 0.88; 95% CI: 0.80 to 0.96).
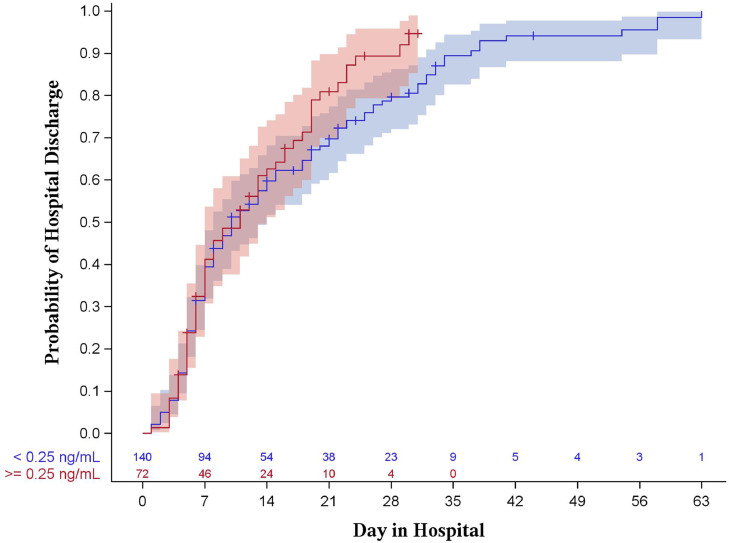
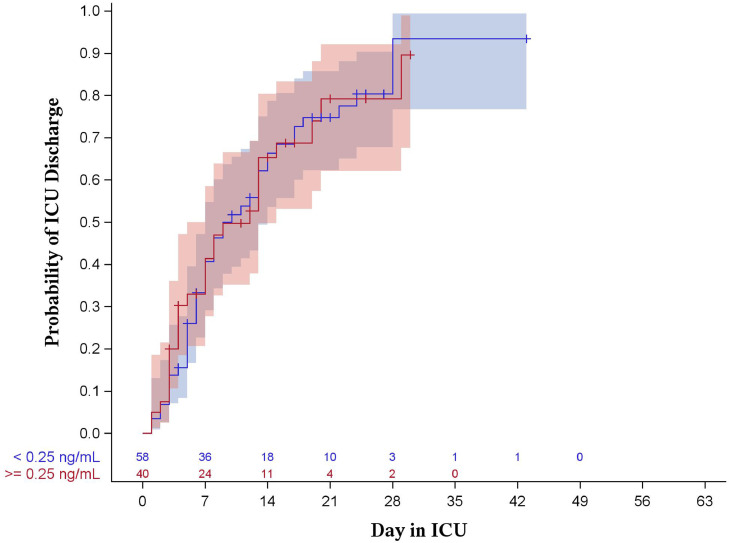
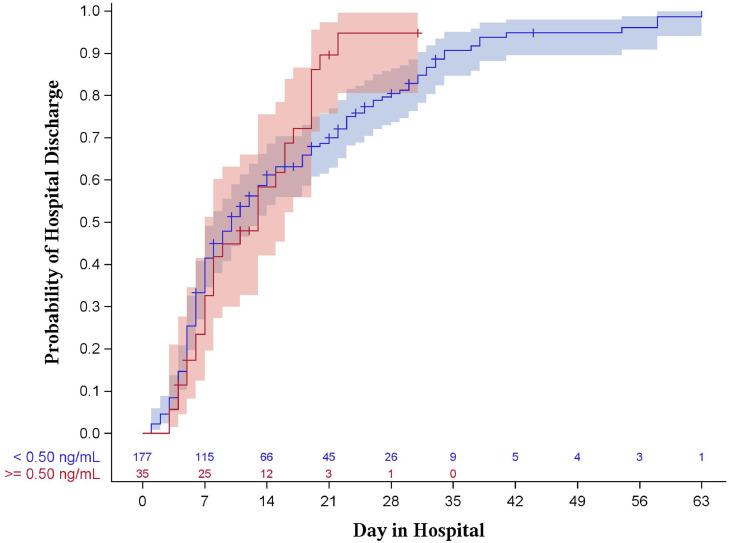
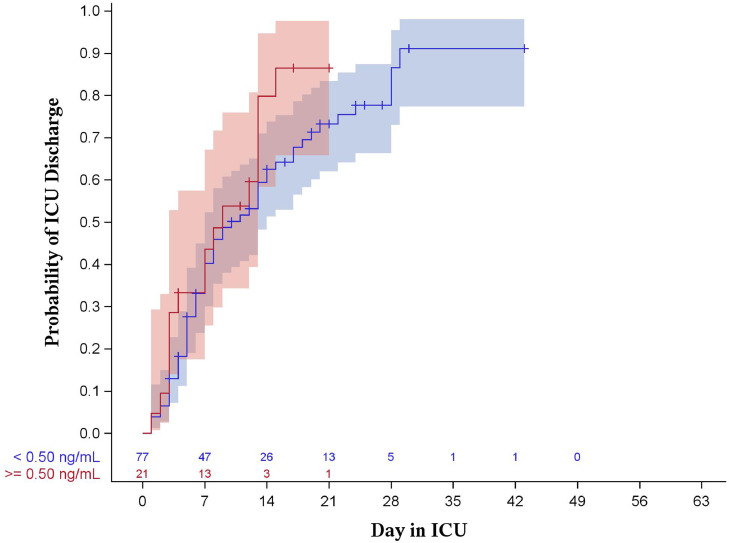
Results of the Kaplan-Meier analysis showed no statistical evidence of a difference between PCT groups with regard to hospital length of stay (P = 0.144 for 0.25 ng/mL, P = 0.368 for 0.50 ng/mL, shown in Figs. 1 , 2 ) or intensive care unit length of stay (P = 0.986 for 0.25 ng/mL, P = 0.771 for 0.50 ng/mL, shown in Figs. 3 , 4 ).
Fig. 1.
Kaplan-Meier curve for hospital length of stay stratified by procalcitonin of 0.25 ng/mL (log-rank p = 0.144). Shaded areas represent 95% confidence intervals.
Fig. 2.
Kaplan-Meier curve for ICU length of stay stratified by procalcitonin of 0.25 ng/mL (log-rank p = 0.986). Shaded areas represent 95% confidence intervals.
Fig. 3.
Kaplan-Meier curve for hospital length of stay stratified by procalcitonin of 0.50 ng/mL (log-rank p = 0.368). Shaded areas represent 95% confidence intervals.
Fig. 4.
Kaplan-Meier curve for ICU length of stay stratified by procalcitonin of 0.50 ng/mL (log-rank p = 0.218). Shaded areas represent 95% confidence intervals.
Discussion/Conclusion
On the basis of this retrospective analysis of patients within our health system who were hospitalized early in the COVID-19 pandemic, elevated PCT level on hospital admission was associated with chronic kidney disease, elevated APACHE II score, and CRP level when PCT was ≥ 0.25 ng/mL. Previous studies have shown that renal dysfunction may reduce the clearance rate of PCT from plasma by 30%–50%, which may explain the observed association with chronic kidney disease (Meisner et al. 2001).
Our study demonstrates that an elevated PCT level on admission may indicate more severe COVID-19 infection, as evidenced by the association with elevated APACHE II score. These findings are consistent with previous studies that demonstrated a relationship between elevated PCT and COVID-19 disease severity. Shen et al. (2021) performed a meta-analysis demonstrating patients with elevated PCT levels on admission were at higher risk of severe and critical COVID-19. Another meta-analysis including 52 studies and 15,296 patients by Ahmed et al found that 85% of the studies reported statistically significant association between PCT and COVID-19 disease severity (Ahmed et al., 2021). The APACHE II score is a severity-of-disease classification system used for adult patients admitted to the ICU. Although the association between PCT and APACHE II score seen in our study is a finding not previously described in the literature, other studies have assessed the relationship between APACHE II score and various other cytokines. A study by Guo et al. (2021) analyzed the correlation between 33 cytokines and APACHE II score in patients in the first week of COVID-19 disease onset. The study found a significant positive correlation between IL-1β and HGF with APACHE II score.
When study groups were instead stratified using ≥0.50 ng/mL PCT value, the association with chronic kidney disease and APACHE II score was no longer observed. A potential explanation for our findings is that decreased plasma clearance of PCT due to renal dysfunction causes only slight elevation of serum PCT, and thus the effect is less significant when using higher PCT threshold. The difference in median APACHE II score increased when stratifying patients using a PCT measurement of 0.50 ng/mL (APACHE II score 8 for PCT <0.25 ng/mL, 9 for PCT >0.25 ng/mL, 8 for PCT <0.50 ng/mL, and 11 for PCT >0.50 ng/mL). However, due to the small sample size of patients with PCT >0.50 ng/mL (N = 36), the difference was not statistically significant (P = 0.042 for PCT stratified by 0.25 ng/mL, P = 0.088 for PCT stratified by 0.50 ng/mL).
Upon stratification using a PCT ≥ 0.50 ng/mL, only CRP levels retained statistical significance. Although PCT is thought to be more specific for bacterial infection than CRP, both PCT and CRP act as acute phase reactants in the setting of inflammation. Previous studies have described the significant inflammatory cascade and systemic response caused by severe COVID-19 infection, which may cause elevation of both markers, potentially explaining the association observed in this study (Melo et al., 2021).
Despite the observed relationship between elevated PCT and APACHE II score, our study found no association between PCT and clinical outcomes, including hospital length of stay, intensive care unit length of stay, and in-hospital mortality. This result contradicts several earlier studies which found that PCT levels correlate with duration of mechanical ventilation and mortality (Liu et al., 2021, Krause et al., 2021). Additionally, a meta-analysis of 13,387 patients by Zare et al. (2020) found that PCT had a pooled diagnostic odds ratio of 13.21 for mortality (95% CI 3.95–44.19).
However, the majority of these studies are from early in the pandemic when the diagnosis of COVID-19 often had to rely on imaging studies alone, and a high proportion of patients may have presented later in their disease course. Several later studies did not replicate these findings. For example, a study by Drewett et al (2019) of 243 patients in Florida found PCT levels in patients with COVID-19 were persistently negative despite a broad spectrum of disease severity. Based on their results, a patient presenting with lower respiratory symptoms and a PCT level ≥0.25 ng/mL is 3.4 times less likely to have COVID-19 (P <0.001). Dolci et al. (2020) concluded that PCT has limited value in predicting poor outcomes in COVID-19, describing publication bias, small-study effects, and a lack of standardization of PCT measurements in the earlier studies. Whether the contrasting results are due to the studies being performed in different timeframes of the pandemic, geography, or study design is unclear and warrants further investigation. Additionally, our results use PCT on admission to the hospital, and other studies may have used PCT from other timeframes during hospitalization.
Although evidence for using PCT as a prognostic marker is mixed, other studies, such as the one by Peters et al. (2021), have found that PCT is still useful in ruling out bacterial co-infection in the setting of COVID-19. The study collected PCT levels on 118 patients and found that PCT testing, when used in combination with clinical assessment, is a safe and effective way of reducing inappropriate antibiotic usage in COVID-19. The limitations of our study include its retrospective nature and comparatively small sample size, as well as other potential factors.
We were unable to characterize the duration of symptoms or the patient timeframe from contraction of COVID-19 to hospitalization in our population, which could affect the results of this study.
In conclusion, these study results demonstrate statistically significant correlation between PCT ≤0.25 ng/ml and admission APACHE II score and CRP levels. Procalcitonin levels did not predict total hospitalization, ICU hospitalization, or mortality. Further studies are needed to fully assess the prognostic utility of PCT in patients with COVID-19.
Statement of Ethics
This study was carried out in accordance with the World Medical Association Declaration of Helsinki. The study protocol was reviewed and approved by the Creighton University Institutional Review Board; approval number 2001057. This project has been determined to be exempt from Federal Policy for Protection of Human Subjects as per 45CFR46.101 (b) 4 by the Creighton University Institutional Review Board and thus did not require written informed consent from participants because of the retrospective nature of the study design.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This study and manuscript did not require any funding or financial backing.
Author Contributions
Conception and design of study: Ian Jackson, Hadi Jaradeh, Shraddha Narechania, Christopher Destache, and Manasa Velagapudi.
Acquisition of data: Ian Jackson, Hadi Jaradeh, Shraddha Narechania, Manasa Velagapudi, and Ali Aldamen.
Analysis and/or interpretation of data: Sarah Aurit, Ian Jackson, Shraddha Narechania, Christopher Destache, and Manasa Velagapudi.
Drafting the manuscript: Ian Jackson, Hadi Jaradeh, Sarah Aurit, Shraddha Narechania, Christopher Destache, and Manasa Velagapudi.
Revising the manuscript critically for important intellectual content: Ian Jackson, Hadi Jaradeh, Shraddha Narechania, Sarah Aurit, Christopher Destache, and Manasa Velagapudi.
Approval of the version of the manuscript to be published: Ian Jackson, Hadi Jaradeh, Shraddha Narechania, Christopher Destache, Manasa Velagapudi, Sarah Aurit, and Ali Aldamen.
Data Availability Statement
The data that support the findings of this study are not publicly available as they contain information that could compromise the privacy of research participants but are available from the corresponding author, Ian Jackson, upon reasonable request.
References
- Ahmed S, Jafri L, Hoodbhoy Z, Siddiqui I. Prognostic Value of Serum Procalcitonin in COVID-19 Patients: A Systematic Review. Indian J Crit Care Med. 2021;25(1):77–84. doi: 10.5005/jp-journals-10071-23706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolci A, Robbiano C, Aloisio E, Chibireva M, Serafini L, Falvella FS, et al. Searching for a role of procalcitonin determination in COVID-19: a study on a selected cohort of hospitalized patients. Clin Chem Lab Med. 2020;59(2):433–440. doi: 10.1515/cclm-2020-1361. [DOI] [PubMed] [Google Scholar]
- Guo J, Wang S, Xia H, Shi D, Chen Y, Zheng S, et al. Cytokine Signature Associated With Disease Severity in COVID-19. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.681516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figliozzi S, Masci PG, Ahmadi N, Tondi L, Koutli E, Aimo A, et al. Predictors of adverse prognosis in COVID-19: A systematic review and meta-analysis. Eur J Clin Invest. 2020;50(10):e13362. doi: 10.1111/eci.13362. [DOI] [PubMed] [Google Scholar]
- Hu R, Han C, Pei S, Yin M, Chen X. Procalcitonin levels in COVID-19 patients. Int J Antimicrob Agents. 2020;56(2) doi: 10.1016/j.ijantimicag.2020.106051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med. 2013;28(3):285–291. doi: 10.3904/kjim.2013.28.3.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu F, Li L, Xu M, Wu J, Luo D, Zhu Y, et al. Prognostic value of interleukin-6, C-reactive protein, and procalcitonin in patients with COVID-19. J Clin Virol. 2020;127 doi: 10.1016/j.jcv.2020.104370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisner M, Lohs T, Huettemann E, Schmidt J, Hueller M, Reinhart K. The plasma elimination rate and urinary secretion of procalcitonin in patients with normal and impaired renal function. Eur J Anaesthesiol. 2001;18(2):79–87. doi: 10.1046/j.0265-0215.2000.00783.x. [DOI] [PubMed] [Google Scholar]
- Melo AKG, Milby KM, Caparroz A, Pinto A, Santos RRP, Rocha AP, et al. Biomarkers of cytokine storm as red flags for severe and fatal COVID-19 cases: A living systematic review and meta-analysis. PLoS One. 2021;16(6) doi: 10.1371/journal.pone.0253894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peters C, Williams K, Un EA, Little L, Saad A, Lendrum K, et al. Use of procalcitonin for antibiotic stewardship in patients with COVID-19: A quality improvement project in a district general hospital. Clin Med (Lond) 2021;21(1):e71–ee6. doi: 10.7861/clinmed.2020-0614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ponti G, Maccaferri M, Ruini C, Tomasi A, Ozben T. Biomarkers associated with COVID-19 disease progression. Crit Rev Clin Lab Sci. 2020;57(6):389–399. doi: 10.1080/10408363.2020.1770685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shen Y, Cheng C, Zheng X, Jin Y, Duan G, Chen M, et al. Elevated Procalcitonin Is Positively Associated with the Severity of COVID-19: A Meta-Analysis Based on 10 Cohort Studies. Medicina (Kaunas) 2021;57(6) doi: 10.3390/medicina57060594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tjendra Y, Al Mana AF, Espejo AP, Akgun Y, Millan NC, Gomez-Fernandez C, et al. Predicting Disease Severity and Outcome in COVID-19 Patients: A Review of Multiple Biomarkers. Arch Pathol Lab Med. 2020;144(12):1465–1474. doi: 10.5858/arpa.2020-0471-SA. [DOI] [PubMed] [Google Scholar]
- Zare ME, Wang Y, Nasir Kansestani A, Almasi A, Zhang J. Procalcitonin Has Good Accuracy for Prognosis of Critical Condition and Mortality in COVID-19: A Diagnostic Test Accuracy Systematic Review and Meta-analysis. Iran J Allergy Asthma Immunol. 2020;19(6):557–569. doi: 10.18502/ijaai.v19i6.4926. [DOI] [PubMed] [Google Scholar]
- Zhang JJY, Lee KS, Ang LW, Leo YS, Young BE. Risk Factors for Severe Disease and Efficacy of Treatment in Patients Infected With COVID-19: A Systematic Review, Meta-Analysis, and Meta-Regression Analysis. Clin Infect Dis. 2020;71(16):2199–2206. doi: 10.1093/cid/ciaa576. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are not publicly available as they contain information that could compromise the privacy of research participants but are available from the corresponding author, Ian Jackson, upon reasonable request.