Abstract
Background Serosurveillance can be used to investigate the extent and distribution of immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) within a population. Characterisation of humoral immune responses gives insight into whether immunity is infection- or vaccine-derived.
Methods A longitudinal study of health care workers (HCWs) in Dili, Timor-Leste, was conducted during vaccine rollout (ChAdOx1) and a concurrent SARS-CoV-2 outbreak.
Results A total of 324 HCWs were included at baseline (April-May 2021). Out of those, 32 (9.9%) were seropositive for anti-nucleocapsid protein (anti-N) IgG antibodies, indicating a significant sub-clinical infection among HCWs early in the local outbreak. Follow-up was conducted in 157 (48.5%) participants (July-September 2021), by which time there had been high uptake of vaccination (91.7%), and 86.0% were seropositive for anti-spike protein antibodies. Acquisition of anti-N antibodies was observed in partially vaccinated HCWs (30/76, 39.5%), indicating some post-dose-1 infections.
Discussion Serosurveillance of HCWs may provide early warning of SARS-CoV-2 outbreaks and should be considered in non-endemic settings, particularly where there is limited availability/uptake of testing for acute infection. Characterisation of humoral immune responses may be used to assess vaccine impact and coverage. Such studies should be considered in national and international efforts to investigate and mitigate against future emerging pathogens.
Keywords: ChAdOx1, COVID-19, healthcare worker, SARS-CoV-2, serological surveillance, vaccination
Background
Serosurveillance can be used to determine the proportion of a population for whom there is evidence of humoral immunity to severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of the coronavirus disease (COVID-19) pandemic (Bobrovitz et al., 2021, Xinhua et al., 2021; Kayı et al., 2021; Rostami et al., 2021; Vaselli et al., 2021). Detection of specific antibodies against the viral nucleocapsid protein (anti-nucleocapsid protein [anti-N] antibodies) indicates previous infection or vaccination with a whole-virus vaccine, whereas antibodies against the spike protein may indicate previous infection and/or vaccination with any vaccine. Characterisation using a spectrum of humoral immune responses therefore gives insight into whether immunity is derived from natural infection (where anti-N and anti-spike protein [anti-S] antibodies are usually detectable) or spike protein-based vaccines (where anti-S antibodies are detectable, but anti-N antibodies are not) (Duarte et al., 2022; Wei et al., 2021; Whitaker et al., 2021).
Health care workers (HCWs), through occupational exposure, are generally at higher risk of SARS-CoV-2 infection, although infection prevention and control measures can mitigate this. Studies of HCWs in settings with established SARS-CoV-2 transmission have found high seropositivity when compared with general population estimates and have identified significant asymptomatic and/or undiagnosed infection (Galanis et al., 2021; Grant et al., 2021; Meinus et al., 2022; Rudberg et al., 2020). However, only a minority of studies have been conducted in low- and middle-income countries (and none previously in Timor-Leste) (Rostami et al., 2021; SeroTracker, n.d.).
Timor-Leste has a population of 1.3 million and occupies the eastern side of an island located between Australia and Indonesia. Throughout 2020, widespread SARS-CoV-2 community transmission was avoided, largely because of border controls and quarantine measures. By 1st January 2021, 44 COVID-19 cases had been detected using Nucleic Acid Amplification Tests (NAAT), mostly among returned international travellers. However, no onward community transmission was detected despite widespread use of NAAT in the capital city, Dili. In February 2021, the first community cases not linked to an imported case were confirmed. This led to a large outbreak in Dili Municipality between April and June 2021 and subsequently community transmission across the country, which continues presently (Novel Coronavirus (2019-nCoV) situation reports, n.d.).
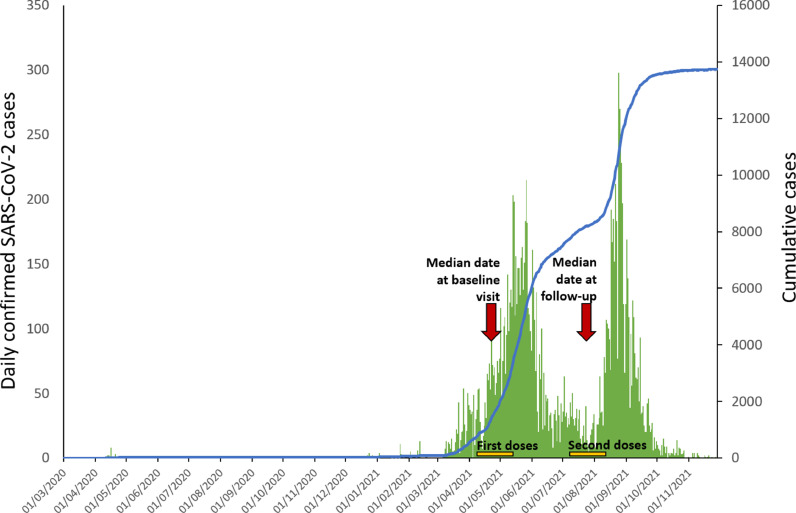
The AstraZeneca (ChAdOx1 nCoV-19, Belgium) viral-vector COVID-19 vaccine became available in April 2021. HCWs were given the highest priority and were offered first doses from April-May 2021, with second doses being administered between July and August 2021. Sinovac (China) inactivated COVID-19 vaccine became available in June 2021 but was not offered to HCWs and made up only 6.3% of all COVID-19 vaccine doses given in Timor-Leste by 29th November 2021 (Novel Coronavirus (2019-nCoV) situation reports, n.d.). Figure 1 shows the epidemic curve for Dili Municipality and the timing of HCW vaccinations.
Figure 1.
SARS-CoV-2 epidemic curve for Dili Municipality showing timing of study visits and periods of HCW vaccination HCW, health care worker; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
A longitudinal serosurveillance study of HCWs in Dili, Timor-Leste, was conducted between April and September 2021 to determine the extent of natural infection- and/or vaccine-derived immunity in this population.
Methods
This study took place at 3 sites: the single large referral hospital in Dili (Hospital Nacional Guido Valadares [HNGV]), the national health laboratory in Dili (Laboratório Nacional de Saúde [LNS]), and the regional ambulance service in Dili (Servico Nacional Ambulancia de Emergencia Medica [SNAEM], in the headquarters building). All individuals working in patient-facing and/or clinical sample-processing roles at any of these facilities were eligible. Individuals attended 1 of 3 research clinics set up at each site, for collection of serum samples and recording epidemiological information. Demographic data (age, gender, nationality), self-reported vaccine status (doses, dates of administration), occupational data (occupation, perceived risk of COVID-19 at work, known personal protective equipment [PPE] breach), and clinical data (history of recent illness, symptoms [if applicable], known COVID-19 diagnosis) were collected during a ‘baseline visit’ (occurring between 7th April and 31st May 2021) using a structured interview-questionnaire. After 3 months, participants attended a ‘follow-up visit’ (occurring between 9th July and 8th September 2021) where collection of data and serum samples were repeated. Figure 1 shows the timing of study visits in relation to confirmed cases of COVID-19 in Dili Municipality and the timing of HCW vaccination.
Samples were stored and analysed at LNS. Anti-S IgG antibodies were detected using the qualitative Ortho Clinical Diagnostics chemiluminescent assay on the VITROS ECiQ platform. Samples positive for anti-S were also tested using the qualitative Epitope Diagnostics COVID-19 enzyme-linked immunosorbent assay to detect anti-N IgG antibodies. These assays were chosen on the basis of their high sensitivity and specificity for detecting previous infection (including among individuals with non-severe illness) (Harritshøj et al., 2021; Yassine et al., 2021), their immediate availability for shipment to Timor-Leste, and local laboratory expertise. Testing was conducted in accordance with manufacturers’ instructions and published cut-offs were used (positive if sample value / cut-off value [automatically calculated during calibration] ≥ 1.0 for the Ortho Clinical Diagnostics assay, and positive if sample optical density [OD] ≥ 1.1 x [OD of negative control + 0.18] for the Epitope Diagnostics assay).
Individuals’ vaccination status was stratified by the number of doses received, and whether their most recent dose had been within the previous 21 days. Anti-S and anti-N seropositivity were determined for each time point and compared across demographic, vaccine-related, occupational, and clinical variables in univariate analyses. To assess bias caused by loss-to-follow-up, baseline characteristics of those who attended follow-up were compared with those who did not, in univariate analyses. Fisher's exact and Mann–Whitney U tests were used to test associations between categorical and ordinal data, respectively. Results were considered significant if p<0.05.
All participants gave written informed consent and were informed of their serology results. This study received ethical approval from Institute National of Health-Research Ethics and Technical Committee, Timor-Leste (Reference: 265/MS-INS/DE/III/2021) and Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, Australia (Reference: 2020-3925).
Results
Description of the cohort: There were 324 HCWs in the study (representing 51.3% of all eligible HCWs registered as working in 1 of the 3 participating institutions); 263 worked at HNGV (50.6% of all patient-facing employees), 30 worked at LNS (45.5% of all laboratory employees), and 31 worked at SNAEM (68.9% of all patient-facing employees). Among these, there were 101 (31.2%) nurses, 64 (19.8%) cleaners, 51 (15.7%) doctors, 37 (11.4%) laboratory scientists, 18 (5.6%) midwives, 13 (4.0%) ambulance staff members, and 40 (12.3%) miscellaneous health care staff members; 191 (59.0%) were female. The median age was 29 years (interquartile range 34-43 years), and 317 (97.8%) described themselves as having Timorese nationality.
Results at baseline: At baseline (between 7th April and 31st May 2021), 156 (48.1%) reported receiving 1 SARS-CoV-2 vaccine dose, with 25 of 156 (16.0%) of these doses received in the previous 21 days. There were 56 (17.3%) anti-S seropositive participants (consistent with vaccination and/or previous infection) and 32 (9.9%) anti-N seropositive participants (consistent with previous infection). Most of the 32 anti-N positive participants (27/32, 84.4%) did not report a previous laboratory-confirmed diagnosis of SARS-CoV-2 infection. Male gender (p=0.013), occupation as an ambulance staff member (p=0.030), and history of any acute illness (p=0.047) were significantly associated with anti-N seropositivity.
Results at follow-up: A follow-up visit was attended by 157 of 324 (48.5%) participants (between 9th July and 8th September 2021). On univariate analysis, a significantly lower proportion of nurses (p=0.022) and higher proportion of laboratory scientists (p=0.002) attended follow-up than participants of other occupations. Those followed up were similar to those lost to follow-up in terms of all other baseline characteristics assessed, including gender (p=0.991), age group (p=0.476), and baseline vaccination status (p=0.244). There were 127 of 157 (80.9%) participants who reported receiving 2 SARS-CoV-2 vaccine doses, with 106 of 157 (67.5%) having had both doses >21 days before their follow-up visit. Seventeen (10.8%) reported having received 1 SARS-CoV-2 vaccine dose, and 13 (8.3%) remained unvaccinated, of whom 10 (10/13, 76.9%) stated they wished to be vaccinated. A total of 148 (94.3%) participants were anti-S seropositive (consistent with vaccination and/or previous infection), including all 127 (100.0%) individuals who had received 2 vaccine doses.
Of those who were anti-S seropositive, 72 were also anti-N seropositive (indicating previous infection), representing 45.9% of those who had a follow-up visit. On univariate analysis, increasing age (p=0.013) and previous laboratory-confirmed SARS-CoV-2 infection (p=0.008) were significantly associated with anti-N seropositivity. More complete vaccination status (p=0.039) and occupation as a laboratory scientist (p=0.033) were inversely associated with anti-N seropositivity.
Table 1 shows seropositivity in different groups at both time points.
Table 1.
SARS-CoV-2 seropositivity in different groups of health care workers at baseline and follow-up
| Baseline visit (Apr-May 2021) | Follow-up visit (Jul-Sep 2021) | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| n=324 |
n=157 |
||||||||||||||
| Anti-S positive | p= | Anti-N positivea | p= | Total | Anti-S positive | p= | Anti-N positivea | p= | Total | ||||||
| Gender | Female | 23 | 12.0% | 12 | 6.3% | 191 | 87 | 94.6% | 41 | 44.6% | 92 | ||||
| Male | 33 | 24.8% | 0.004 | 20 | 15.0% | 0.013 | 133 | 61 | 93.8% | 1.000 | 31 | 47.7% | 0.746 | 65 | |
| Age group (years) | 18-20 | 0 | 0.0% | 0 | 0.0% | 2 | 0 | 0.0% | 0 | 0.0% | 2 | ||||
| 21-30 | 18 | 16.7% | 10 | 9.3% | 108 | 52 | 94.5% | 21 | 38.2% | 55 | |||||
| 31-40 | 20 | 17.1% | 8 | 6.8% | 117 | 57 | 98.3% | 25 | 43.1% | 58 | |||||
| 41-50 | 6 | 10.2% | 5 | 8.5% | 59 | 21 | 87.5% | 14 | 58.3% | 24 | |||||
| 50+ | 12 | 31.6% | 0.407 | 9 | 23.7% | 0.117 | 38 | 18 | 100.0% | 0.249 | 12 | 66.7% | 0.013 | 18 | |
| Occupation | Ambulance staff | 4 | 30.8% | 0.251 | 4 | 30.8% | 0.030 | 13 | 6 | 100.0% | 1.000 | 4 | 66.7% | 0.414 | 6 |
| Cleaner | 19 | 29.7% | 0.005 | 10 | 15.6% | 0.101 | 64 | 23 | 88.5% | 0.171 | 16 | 61.5% | 0.089 | 26 | |
| Doctor | 9 | 17.6% | 1.000 | 2 | 3.9% | 0.197 | 51 | 29 | 96.7% | 1.000 | 12 | 40.0% | 0.544 | 30 | |
| Laboratory scientist | 1 | 2.7% | 0.010 | 1 | 2.7% | 0.150 | 37 | 26 | 96.3% | 1.000 | 7 | 25.9% | 0.033 | 27 | |
| Midwife | 0 | 0.0% | 0.051 | 0 | 0.0% | 0.235 | 18 | 5 | 83.3% | 0.302 | 3 | 50.0% | 1.000 | 6 | |
| Nurse | 17 | 16.8% | 1.000 | 11 | 10.9% | 0.690 | 101 | 36 | 92.3% | 0.395 | 21 | 53.8% | 0.166 | 39 | |
| Other | 6 | 15.0% | 0.825 | 4 | 10.0% | 1.000 | 40 | 23 | 100.0% | 0.358 | 9 | 39.1% | 0.507 | 23 | |
| SARS-CoV-2 vaccine statusa | Unvaccinated | 17 | 10.1% | 16 | 9.5% | 168 | 8 | 61.5% | 8 | 61.5% | 13 | ||||
| Dose 1 < 21 days ago | 19 | 14.5% | 8 | 6.1% | 131 | 2 | 66.7% | 2 | 66.7% | 3 | |||||
| Dose 1 21+ days ago | 20 | 80.0% | 8 | 32.0% | 25 | 11 | 78.6% | 9 | 64.3% | 14 | |||||
| Dose 2 <21 days ago | 0 | - | 0 | - | 0 | 21 | 100.0% | 10 | 47.6% | 21 | |||||
| Dose 2 21+ days ago | 0 | - | <0.001 | 0 | - | 0.242 | 0 | 106 | 100.0% | <0.001 | 43 | 40.6% | 0.039 | 106 | |
| Occupational riska | Perceived 'at risk of COVID-19 at work' | 0 | 0.0% | 0.827 | 0.0% | 1 | 65 | 94.2% | 1.000 | 28 | 40.6% | 0.262 | 69 | ||
| PPE breach with COVID-19 pos. patient/sample | 0 | - | - | 0 | - | - | 0 | 7 | 100.0% | 1.000 | 1 | 14.3% | 0.126 | 7 | |
| Recent illnessa | Any acute illness | 14 | 24.6% | 0.123 | 10 | 17.5% | 0.047 | 57 | 49 | 96.1% | 0.719 | 25 | 49.0% | 0.611 | 51 |
| Fever or chills | 5 | 16.7% | 1.000 | 4 | 13.3% | 0.518 | 30 | 32 | 94.1% | 1.000 | 16 | 47.1% | 1.000 | 34 | |
| Cough | 11 | 24.4% | 0.201 | 8 | 17.8% | 0.063 | 45 | 38 | 95.0% | 1.000 | 21 | 52.5% | 0.362 | 40 | |
| Shortness of breath | 1 | 25.0% | 0.534 | 1 | 25.0% | 0.342 | 4 | 9 | 100.0% | 1.000 | 6 | 66.7% | 0.303 | 9 | |
| Loss of smell or taste | 0 | 0.0% | 0.359 | 0 | 0.0% | 1.000 | 8 | 16 | 100.0% | 0.599 | 9 | 56.3% | 0.432 | 16 | |
| Sore throat | 6 | 20.0% | 0.619 | 4 | 13.3% | 0.518 | 30 | 29 | 96.7% | 1.000 | 15 | 50.0% | 0.686 | 30 | |
| Muscle or joint aches | 9 | 25.7% | 0.162 | 7 | 20.0% | 0.063 | 35 | 32 | 94.1% | 1.000 | 16 | 47.1% | 1.000 | 34 | |
| Nausea or vomiting | 1 | 16.7% | 1.000 | 1 | 16.7% | 0.467 | 6 | 10 | 100.0% | 1.000 | 7 | 70.0% | 0.188 | 10 | |
| Fever or chills / Cough / Loss of smell or taste | 11 | 23.4% | 0.295 | 8 | 17.0% | 0.107 | 47 | 41 | 95.3% | 1.000 | 22 | 51.2% | 0.474 | 43 | |
| Previous lab-confirmed SARS-CoV-2 | Yes | 5 | 83.3% | 5 | 83.3% | 6 | 25 | 100.0% | 18 | 72.0% | 25 | ||||
| No | 51 | 16.0% | 0.001 | 27 | 8.5% | <0.001 | 318 | 123 | 93.2% | 0.356 | 54 | 40.9% | 0.008 | 132 | |
| TOTAL | 56 | 17.3% | 32 | 9.9% | 324 | 148 | 94.3% | 72 | 45.9% | 157 | |||||
Only samples which were positive for anti-S IgG underwent testing for anti-N IgG. Therefore, all anti-N-positive samples were also anti-S-positive.
anti-N, anti-nucleocapsid; anti-S, anti-spike protein; PPE, personal protective equipment; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
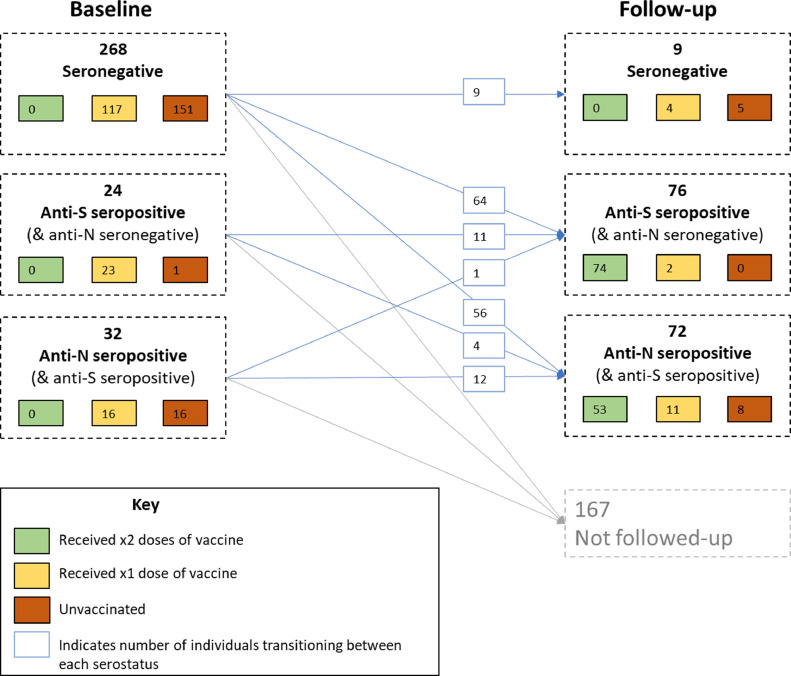
Longitudinal analysis to assess protective effect of vaccination: A longitudinal analysis was performed on the 157 individuals for whom follow-up data were available. Out of 129 (82.2%) who were anti-S seronegative at baseline, 9 (7.0%) had unchanged serology at follow-up, 64 (49.6%) had become anti-S seropositive but were anti-N seronegative, and 56 (43.4%) had become anti-N (and anti-S) seropositive. Out of 15 (9.6%) individuals who were anti-S seropositive but anti-N seronegative at baseline, 11 (73.3%) had unchanged serology at follow-up, and 4 (26.7%) had become anti-N (and anti-S) seropositive. Serological status of participants at baseline and follow-up is shown in Figure 2.
Figure 2.
SARS-CoV-2 serostatus and vaccination status of participants at baseline and follow-up anti-N, anti-nucleocapsid; anti-S, anti-spike protein; SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
During the follow-up period, incidence of laboratory-diagnosed (self-reported) SARS-CoV-2 infection was 18 in 129 (14.0%) in those who were seronegative at baseline, compared with 1 in 15 (6.7%) among those who had an antibody profile consistent with vaccination (anti-S seropositive, anti-N seronegative) at baseline. Development of anti-N seropositivity occurred in 56 of 129 (43.4%) individuals who were seronegative at baseline, compared with 4 of 15 (26.7%) of those who had an antibody profile consistent with vaccination. Thus, anti-S seropositivity at baseline appeared protective against both laboratory-diagnosed (self-reported) SARS-CoV-2 infection and development of anti-N seropositivity, although these associations were not statistically significant (p=0.693 and p=0.274, respectively). Table 2 shows associations between baseline vacation status and serostatus with markers of SARS-CoV-2 infection during the follow-up period.
Table 2.
Associations of baseline vaccination status and serostatus with markers of SARS-CoV-2 infection during the follow-up period
| Laboratory-confirmed SARS-CoV-2 infection occurring during follow-up period |
Serostatus at follow-up |
Total | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Yes | No | p= | Remained anti-N seronegative | Became anti-N seropositive | p= | |||||||
| Vaccination status at baseline | Unvaccinated | 10 | 14.7% | 58 | 85.3% | 38 | 55.9% | 30 | 44.1% | 68 | ||
| Dose 1 < 21 days ago | 9 | 13.0% | 60 | 87.0% | 42 | 60.9% | 27 | 39.1% | 69 | |||
| Dose 1 21+ days ago | 0 | 0.0% | 7 | 100.0% | 0.555 | 4 | 57.1% | 3 | 42.9% | 0.631 | 7 | |
| Serostatus at baseline | Seronegative | 18 | 14.0% | 111 | 86.0% | 73 | 56.6% | 56 | 43.4% | 129 | ||
| Anti-S seropositive (& anti-N seronegative) | 1 | 6.7% | 14 | 93.3% | 0.693 | 11 | 73.3% | 4 | 26.7% | 0.274 | 15 | |
| Total | 19 | 13.2% | 125 | 86.8% | 84 | 58.3% | 60 | 41.7% | 144 | |||
SARS-CoV-2, severe acute respiratory syndrome coronavirus 2.
Those with evidence of previous SARS-CoV-2 infection (anti-N seropositive) at baseline have been excluded. anti-N, anti-nucleocapsid; anti-S, anti-spike protein.
Discussion
This longitudinal serosurveillance study characterised humoral immunity to SARS-CoV-2 among HCWs during the period of vaccine rollout and a concurrent outbreak in Dili Municipality, Timor-Leste. At baseline (between April and May 2021), there was evidence of previous infection in a significant proportion of HCWs (9.9%). This was early in the local outbreak when cases of laboratory-confirmed SARS-CoV-2 infection in Dili Municipality were low (see Figure 1). Most of these individuals did not report having a recent illness, nor a previous laboratory-confirmed diagnosis of COVID-19. This highlights the propensity for SARS-CoV-2 to cause sub-clinical disease and the importance of serosurveillance in detecting transmission, particularly where there is low access/uptake of testing for acute infection. Other studies frequently described high numbers of cases detected through serosurveillance, compared with those diagnosed acutely and reported through systems of passive surveillance (Byambasuren et al., 2021; Rostami et al., 2021). Infections following vaccination are frequently asymptomatic or mild, which may further reduce the likelihood that diagnostic testing is performed (Voysey et al., 2021).
Studies in other settings have shown high SARS-CoV-2 seroprevalence among HCWs relative to other groups (Galanis et al., 2021; Rudberg et al., 2020). This is likely to be driven by occupational and community exposure. Among HCWs included in a systematic review and meta-analysis (conducted before widespread availability of vaccines), those with male gender and those doing patient-related work (in addition to those with self-reported previous infection or a positive PCR test) were more likely to be seropositive (Galanis et al., 2021). These were also identified as risk factors for anti-N seropositivity in our study. From June to December 2020, a SARS-CoV-2 seroprevalence study was conducted in Java, Indonesia (an island near Timor-Leste) and found that laboratory technicians had higher seroprevalence than other HCWs (Megasari et al., 2021). This contrasts with the findings of our study and may reflect differences in occupational role, working practice, infection prevention and control procedures, and/or availability of PPE between the 2 settings. Surveillance of HCWs may be useful in providing early warning of more widespread SARS-CoV-2 outbreaks and should be considered in settings that have not yet reached a state of endemicity.
By July-September 2021, almost half of HCWs enrolled in the present study were anti-N seropositive. In settings where spike-based vaccines are exclusively used, anti-N seroconversion is a specific indicator of naturally occurring SARS-CoV-2 infection (Duarte et al., 2022; Whitaker et al., 2021). This includes detection of breakthrough infections among individuals who are fully vaccinated but not previously infected, although anti-N seroconversion may be less sensitive in this group (Allen et al., 2021; Whitaker et al., 2021a). Therefore, studies which make this determination can provide information about the extent of SARS-CoV-2 transmission that is independent from that of vaccination. Longitudinal surveillance of anti-N antibodies among cohorts of individuals who have received different spike-based vaccines (and/or those who choose to be unvaccinated), correlated with data on clinical outcomes, may be a useful way to estimate real-life vaccine impact in such settings. Furthermore, individuals who are seropositive typically experience a rise in anti-N antibody titre upon re-infection, which could be used to monitor transmission in populations which already have high levels of exposure (Atti et al., 2022).
In the period when increasing numbers of infections occurred, there was rollout of vaccination with high uptake among health care staff, which resulted in 91.7% of HCWs with a history of at least 1 vaccine dose at follow-up. Acquisition of anti-N antibodies was observed in HCWs who had been partially vaccinated at baseline, indicating some post-dose-1 infections. It was not possible to ascertain how many individuals were infected after the second dose because none had received 2 doses at baseline. However, it is most likely that infections occurred just before or after dose 1. Ultimately, high anti-S seropositivity was observed (94.3%), reflecting a combination of natural infection- and vaccine-derived immunity in this important cohort.
This study provided an opportunity for strengthening surveillance and serological testing capacity within LNS, as part of a wider programme that will examine seroprevalence of SARS-CoV-2 and other vaccine-preventable diseases in both HCWs and other population groups across Timor-Leste. Limitations include a small sample size, which precluded multivariate analysis to investigate associations with vaccination and/or SARS-CoV-2 infection. There was a significant dropout rate, and followed-up individuals were significantly different from those who were not followed up in terms of occupation. Association between baseline serostatus and markers of subsequent SARS-CoV-2 infection were non-significant and could therefore have been due to chance of confounding by other factors. Additionally, there was lack of adjustment of serological results using previously determined estimates of test sensitivity and specificity, meaning a small minority of individuals could have been assigned false-negative or false-positive results.
The role of HCW sentinel-site surveillance including the routine collection and storage of serum should be considered as part of national and international efforts to investigate and mitigate against emerging pathogens, including SARS-CoV-2.
Conflicts of interest
Authors do not have any commercial or other associations that might pose conflicts of interest.
Acknowledgments
Ethical approval statement
This study received ethical approval from Institute National of Health-Research Ethics and Technical Committee, Timor-Leste (Reference: 265/MS-INS/DE/III/2021) and Human Research Ethics Committee of the Northern Territory Department of Health and Menzies School of Health Research, Australia (Reference: 2020-3925).
Funding source
This work was supported by the Department for Foreign Affairs and Trade of the Australian Government (Complex Grant Agreement Number 75889).
Contributions
Dr Paul Randell, Consultant Clinical Virologist, Imperial College Healthcare NHS Trust.
Previous presentation of findings
Data in this manuscript have been presented to collaborators at the Ministry of Health, Timor-Leste to assist with local outbreak response. To date, findings have not been presented or published at academic meetings.
Contributor Information
Paul Arkell, Email: paularkell@doctors.org.uk.
Joshua R Francis, Email: josh.francis@menzies.edu.au.
References
- Allen N, Brady M, Martin AIC, Domegan L, Walsh C, Doherty L, et al. Serological markers of SARS-CoV-2 infection; anti-nucleocapsid antibody positivity may not be the ideal marker of natural infection in vaccinated individuals. J Infect. 2021;83:e9–10. doi: 10.1016/J.JINF.2021.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atti A, Ferrari M, Castillo-Olivares J, Monk EJM, Gopal R, Patel M, et al. Serological profile of first SARS-CoV-2 reinfection cases detected within the SIREN study. J Infect. 2022;84:248–288. doi: 10.1016/j.jinf.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobrovitz N, Arora RK, Cao C, Boucher E, Liu M, Donnici C, et al. Global seroprevalence of SARS-CoV-2 antibodies: A systematic review and metaanalysis. PLoS One. 2021;16 doi: 10.1371/journal.pone.0252617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byambasuren O, Dobler CC, Bell K, Rojas DP, Clark J, McLaws ML, et al. Comparison of seroprevalence of SARS-CoV-2 infections with cumulative and imputed COVID-19 cases: Systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xinhua Chen, Chen Z, Azman AS, Deng X, Sun R, Zhao Z, et al. Serological evidence of human infection with SARS-CoV-2: a systematic review and meta-analysis. Lancet Glob Heal. 2021;9:e598–e609. doi: 10.1016/S2214-109X(21)00026-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte N, Yanes-Lane M, Arora RK, Bobrovitz N, Liu M, Bego MG, et al. Adapting Serosurveys for the SARS-CoV-2 Vaccine Era. Open Forum Infect Dis. 2022;9 doi: 10.1093/ofid/ofab632. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galanis P, Vraka I, Fragkou D, Bilali A, Kaitelidou D. Seroprevalence of SARS-CoV-2 antibodies and associated factors in healthcare workers: a systematic review and meta-analysis. J Hosp Infect. 2021;108:120–134. doi: 10.1016/j.jhin.2020.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant JJ, Wilmore SMS, McCann NS, Donnelly O, Lai RWL, Kinsella MJ, et al. Seroprevalence of SARS-CoV-2 antibodies in healthcare workers at a London NHS Trust. Infect Control Hosp Epidemiol. 2021;42:212–214. doi: 10.1017/ice.2020.402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harritshøj LH, Gybel-Brask M, Afzal S, Kamstrup PR, Jørgensen CS, Thomsen MK, et al. Comparison of 16 serological SARS-CoV-2 immunoassays in 16 clinical laboratories. J Clin Microbiol. 2021;59 doi: 10.1128/JCM.02596-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kayı İ, Madran B, Keske Ş, Karanfil Ö, Arribas JR, Psheniсhnaya N, et al. The seroprevalence of SARS-CoV-2 antibodies among health care workers before the era of vaccination: a systematic review and meta-analysis. Clin Microbiol Infect. 2021;27:1242–1249. doi: 10.1016/j.cmi.2021.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megasari NLA, Utsumi T, Yamani LN, Juniastuti Gunawan E, Furukawa K, et al. Seroepidemiological study of SARS-CoV-2 infection in East Java. Indonesia. PLoS One. 2021;16 doi: 10.1371/JOURNAL.PONE.0251234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meinus C, Singer R, Nandi B, Jagot O, Becker-Ziaja B, Karo B, et al. SARS-CoV-2 prevalence and immunity: a hospital-based study from Malawi. Int J Infect Dis. 2022;116:157–165. doi: 10.1016/j.ijid.2021.12.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novel Coronavirus (2019-nCoV) situation reports. n.d. https://www.who.int/timorleste/emergencies/novel-coronavirus-2019/novel-coronavirus-(2019-ncov)-situation-reports (accessed December 13, 2021).
- Rostami A, Sepidarkish M, Fazlzadeh A, Mokdad AH, Sattarnezhad A, Esfandyari S, et al. Update on SARS-CoV-2 seroprevalence: regional and worldwide. Clin Microbiol Infect. 2021;27:1762–1771. doi: 10.1016/j.cmi.2021.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudberg AS, Havervall S, Månberg A, Jernbom Falk A, Aguilera K, Ng H, et al. SARS-CoV-2 exposure, symptoms and seroprevalence in healthcare workers in Sweden. Nat Commun. 2020;11 doi: 10.1038/S41467-020-18848-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sero Tracker. n.d. https://serotracker.com/en/Explore (accessed November 2, 2021).
- Vaselli NM, Hungerford D, Shenton B, Khashkhusha A, Cunliffe NA, French N. The seroprevalence of SARS-CoV-2 during the first wave in Europe 2020: A systematic review. PLoS One. 2021;16 doi: 10.1371/journal.pone.0250541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voysey M, Clemens SAC, Madhi SA, Weckx LY, Folegatti PM, Aley PK, et al. Safety and efficacy of the ChAdOx1 nCoV-19 vaccine (AZD1222) against SARS-CoV-2: an interim analysis of four randomised controlled trials in Brazil, South Africa, and the UK. Lancet. 2021;397:99–111. doi: 10.1016/S0140-6736(20)32661-1/ATTACHMENT/BD910DFE-2C8A-4512-A277-6B1DBA6322EE/MMC2.PDF. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei J, Matthews PC, Stoesser N, Maddox T, Lorenzi L, Studley R, et al. Anti-spike antibody response to natural SARS-CoV-2 infection in the general population. Nat Commun 2021 121. 2021;12:1–12. doi: 10.1038/s41467-021-26479-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker Heather J, Elgohari S, Rowe C, Otter AD, Brooks T, Linley E, et al. Impact of COVID-19 vaccination program on seroprevalence in blood donors in England, 2021. J Infect. 2021;83:237–279. doi: 10.1016/j.jinf.2021.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitaker Heather J, Gower C, Otter AD, Simmons R, Kirsebom F, Letley L, et al. Nucleocapsid antibody positivity as a marker of past SARS-CoV-2 infection in population serosurveillance studies: impact of variant, vaccination, and choice of assay cut-off. MedRxiv. 2021 doi: 10.1101/2021.10.25.21264964. 2021.10.25.21264964. [DOI] [Google Scholar]
- Yassine HM, Al-Jighefee H, Al-Sadeq DW, Dargham SR, Younes SN, Shurrab F, et al. Performance evaluation of five ELISA kits for detecting anti-SARS-COV-2 IgG antibodies. Int J Infect Dis. 2021;102:181–187. doi: 10.1016/j.ijid.2020.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]