Abstract
Background
The aim of this research was to investigate the analgesic effects of intravenous lidocaine on postoperative pain management in orthopedic patients after total joint arthroplasty and fractures of the limbs and to compare lidocaine efficacy between these orthopedic surgical procedures.
Material/Methods
Ninety patients scheduled for elective orthopedic surgery were recruited: 46 patients with total knee arthroplasty, and 35 patients with femoral fractures. Patients in the lidocaine group received lidocaine during the induction phase of anesthesia as a bolus injection of 1.5·kg−1·mg over 10 min, followed by intravenous infusion of 1.5 mg·kg−1·h−1 for 24 postoperative hours. Patients in the control group received an equal volume of saline as placebo administered at the same rate. Pain scores were assesed at intervals of 0, 15, 30, 60 min, and 6, 12, and 24 h postoperatively. The reduction rate of additional analgesics, total analgesic use, incidence of nausea and vomiting, mobilization, length of hospital stay, adverse effects, and hemodynamic parameters were secondary outcomes.
Results
Pain scores at rest and during movement were significantly lower in the lidocaine group compared to those in controls starting at 30 min (P=0.03), the first postoperative hour, and also at 6, 12, and 24 h (P<0.001). Additional analgesics were administered at a significantly lower rate in the lidocaine group (P<0.05). Total analgesic use in the postoperative period was significantly higher in the control group (P<0.001).
Conclusions
This study showed that intravenous lidocaine provided adequate postoperative analgesia for orthopedic patients undergoing elective total joint arthroplasty and limb fracture repair.
Keywords: Arthroplasty, Replacement, Ankle; Orthopedic Procedures; Analgesia; Pain, Postoperative; Lidocaine
Background
Postoperative pain treatment remains one of the biggest challenges in surgical patient care, despite the efforts of anesthesiologists and other healthcare providers. Most patients experience high-intensity postoperative pain even after receiving multimodal analgesia [1]. Furthermore, many studies suggest that postoperative pain is not routinely well-managed, and the incidence of postoperative pain is overlooked [2]. Proper management of postoperative pain shortens rehabilitation time and hospital stays and reduces the incidence of morbidity and chronic pain [3].
Opioids, the most commonly used medications for the treatment of postoperative pain, have many adverse effects, whereas invasive methods, such as an epidural catheter, involve higher costs and difficulties during application. Adverse effects of opioids, such as nausea, vomiting, pruritis, urinary retention, constipation, hyperalgesia, and respiratory depression [4], significantly reduce their utility and affect the length of the rehabilitation period and discharge time [5]. An epidural catheter is an expensive procedure that reduces the patient’s mobility and delays hospital discharge [6]. In addition, its use is associated with complications such as urinary infections [7], abscesses, and epidural hematoma [8]. Moreover, as an analgesic technique, epidural catheters are contraindicated in patients with coagulopathies and those receiving long-term anticoagulant therapies [9]. Therefore, other analgesic approaches are warranted, including various analgesic techniques or non-opioid medications, with the goal that postoperative pain not remain untreated and, more importantly, not evolve into chronic pain.
Lidocaine is a local anesthetic frequently used in daily medical practice. Although lidocaine is mainly used for peripheral nerve and neuroaxial blocks, it has an analgesic effect when injected intravenously [10]. Intravenous lidocaine has multiple mechanisms of actions [11]: blockade of sodium gated channels [12,13], blockade of potassium current [13], blockade of presynaptic dopamine and muscarinic receptors [14,15], reduced conduction and excitability of unmyelinated C fibers [16], reduced excitability of the spinal dorsal horn [16], inhibition of N-methyl-D-aspartate receptors (NMDA) [17], and inhibiting release of endogenous opiods [18].
Numerous randomized clinical trials have analyzed the analgesic effect of intravenous lidocaine in the postoperative period. Most studies have concluded that intravenous lidocaine lowers pain scores [19,20], decreases postoperative opioid requirements [19,20], offers early improvement of bowel function [19,21–23], and reduces the length of hospital stays [19,22,23].
Recommendations from the updated procedure-specific postoperative pain management (PROSPECT) guideline for total hip arthroplasty [24] includes administration of paracetamol, a non-steroidal antinflammatory drug, or a cyclo-oxygenase-2-selective inhibitor as a first-line choice analgesics, spinal or general anesthesia, and intravenous dexamethasone 8–10 mg given intraoperatively. This systematic review also recommends that opiods should be kept as last-resort postoperatively analgesics [24].
The present study was designed to test the primary hypothesis that intravenous lidocaine reduces postoperative pain, measured at rest and during movement, using a visual analog scale (VAS) in elective orthopedic surgery patients. Secondary outcome measures were the rate of reduction of additional analgesics, total analgesic use in the postoperative period, initiation of intestinal motility, mobilization, incidence of nausea and vomiting, adverse effects (eg, headache, hypotension, arrhythmias, dizziness, tinnitus, perioral numbness, metallic taste, seizures), and length of hospital stay. Orthopedic procedures have an increased risk for long-term opioid use [25]; therefore, the use of an alternative, non-opioid analgesic approach would have considerable clinical importance.
Only a limited number of relevant studies have been reported, with controversial results on the analgesic efficacy of lidocaine administered intravenously in patients undergoing elective orthopedic surgery, including total joint arthroplasty and extremity fractures. Therefore, we conducted this study to evaluate this novel analgesic strategy, which could be suitable, in particular, for patients in whom the use of epidural anesthesia is contraindicated.
The present study investigated the use of intravenous lidocaine in the management of postoperative analgesia in 90 patients with total joint arthroplasty and limb fracture.
Material and Methods
After receiving approval from the Institutional Ethical Review Board (Ref No 2017-12073), a single-center, study was conducted between May 2019 and June 2021 at the American Hospital of Kosova. The study was registered at www.clinicaltrials.gov (NCT03921567) by the principal investigator (R.N.) on 18 April 2019, prior to the enrollment of patients.
Participants
After obtaining written informed consent, we prospectively enrolled 90 patients undergoing elective orthopedic surgery: 46 patients with total knee arthroplasty (TKA), and 35 patients with limb fractures (LF) under general anesthesia. The inclusion criteria were: participants ages 18–75 years, undergoing elective orthopedic surgery (TKA, LF), and American Society of Anesthesiologists classification (ASA) I–III. The exclusion criteria were: patient rejection, hypersensitivity to lidocaine, long-term opioid intake, renal or hepatic dysfunction, heart failure, cardiac arrhythmias, organ transplant history, history of seizures, pregnancy, and family history of malignant hyperthermia.
The orthopedic patients scheduled for elective orthopedic surgery – total knee arthroplasty (TKA) and limb fractures (LF) – were randomly assigned to 1 of 2 groups: (1) in the lidocaine group, 40 patients received lidocaine (Lidokaine, solution containing 1000 mg of lidocaine hydrochloride in 50 mL of water Profarma, Tirana, Albania), 22 patients with total knee arthroplasty and18 patients with femoral fractures, as bolus injection of 1.5·mg·kg−1 over 10 min during the induction of anesthesia followed by an i.v. infusion of 1.5 mg·kg−1·h−1 for 24 h postoperatively; and (2) in the control group (41 patients), 24 patients with total knee arthroplasty and 17 patients with femoral fractures, received saline, as a bolus dose and continuous infusion at the same volume and rate.
Patients were randomly assigned to 1 of 2 groups according to a computer-generated table of random numbers. An instructor in charge of randomization kept the opaque envelopes in a box and delivered them according to the randomization codes after obtaining consent from patients. Sealed envelopes contained the group allocation, and the opaque envelopes were opened only after the patient had arrived in the operating theater [26]. An independent anesthesia assistant prepared the syringes with study drugs according to the randomization numbers. Those syringes contained the same volume of colored liquid, and the lidocaine and saline were indistinguishable. Another anesthesia assistant blind to the study administered those syringes to patients. The anesthesiologist, patients, anesthesia assistants in the operation room, the anesthesia resident, and nurses assessing the outcomes in the post-anesthesia care unit (PACU) and surgical ward were blinded to the study.
Anesthesia Procedure
Standard anesthesia procedure and standard monitoring, according to the American Society of Anesthesiologists, was performed. Patients were preoxygenated with a facemask with 100% O2. In the induction of general anesthesia, we used midazolam (0.04 mg·kg−1), fentanyl (1.5–2 μg·kg−1), rocuronium (0.6–1.2 mg·kg−1), and propofol (2 mg·kg−1). Continuous intravenous drips of propofol and fentanyl were used for the maintenance of anesthesia. The bispectral index (BIS), as a part of standard monitoring, was used to follow the depth of anesthesia, propofol, and fentanyl requirements to maintain values at 45–60. During surgery, noninvasive blood pressure, hemoglobin oxygen saturation (SpO2), heart and respiratory rate, electrocardiogram, and capnography were continuously monitored.
After the completion of the surgical procedure and extubation, patients were transferred to the post-anesthesia care unit (PACU) for postoperative monitoring. Analgesia in the postoperative period was maintained with the standard analgesic multimodal regimen of our hospital, which included: non-steroid anti-inflamatory drugs (NSAIDS): 30 mg ketorolac (Eumat, ampoule containing 30 mg/1 ml, Epifarma Srl, Episcopia, Italy), every 8 h with the first dose administered at the end of surgical procedure, and 1 g acetaminophen (Paracetamol B. Braun, solution for infusion containing 1000 mg/100 ml, B. Braun Melsungen AG 34209 Melsungen, Germany) every 6 h. When postoperative pain according to VAS was ≥4, 5 mg morphine (Morfina cloridrato monico, ampoule containing 10 mg/1 ml, Monico spa, Venezia, Italy) or 100 mg tramadol (Tramadol Alkaloid, ampoule containing 100 mg/2 ml, Alkaloid, Skopje, Republic of North Macedonia) was administered. The total amount of analgesics administered in the postoperative period converted into equivalent doses of morphine was recorded in the electronic medical records.
Surgical Techniques
Total knee arthroplastys were performed using a anterior approach. This procedure was performed with the patient in supine position. After the skin preparation, skin, subcutaneous fat, capsula articularis, and quadriceps lateralis muscle tendon were incised. For knee implants, a Smith+Nephew cemented prosthesis were used.
Limb fractures included in this study were all diaphyseal femoral fractures. Intramedullary nailing was the surgical procedure used for the surgical management of diaphyseal femoral fractures. This technique consists on a permanent nail or rod inserted into the center of the fractured bone.
Assessment of Outcomes
The primary outcome was postoperative pain at rest and during movement. The severity of postoperative pain was assessed with a visual analog scale (VAS): 0=no pain, 10=most severe pain). Participants were trained during the preoperative visit to score their pain at rest and during movement. VAS pain scores were assessed at intervals of 0, 15, 30, and 60 min, and at 6, 12, and 24 h in the postoperative period. During movement, pain management efficacy for total knee arthroplasty was evaluated during 60–90 degree knee flexion, whereas pain management efficacy during movement for limb fractures was assessed during 40–60 degree leg flexion. All these assessments were performed under the supervision of the responsible physiatrist.
Patients were monitored in the post-anesthesia care unit, and according to the protocol of discharge, when they were free from pain and met the criteria of Aldrete score ≥9, they were transferred to the surgical ward. In the PACU, mean arterial pressure, heart rate, and saturation of hemoglobin were observed at intervals of 0 and 30 min and 1, 2, 6, 12, and 24 h during the postoperative period. All outcomes were assessed by an independent anesthesiology resident in the post-anesthesia care unit (PACU) and nurses in the surgical ward, who were all blinded to the study intervention.
Secondary outcome measures were: the rate of reduction of additional analgesics, total analgesic use in the postoperative period, beginning of intestinal motility, mobilization, incidence of nausea and vomiting in the postoperative period, adverse effects (headache, hypotension, arrhythmias, dizziness, tinnitus, perioral numbness, metallic taste, seizures), and length of hospital stay. Additional outcomes included mean arterial pressure (MAP), heart rate, and saturation of hemoglobin (SpO2). These data were recorded for each participant in their personal worksheet.
Total analgesic use in the postoperative period was recorded as morphine equivalent doses. This conversion is done based on the conversion factors presented in the literature (intravenous morphine 10 mg=fentanyl, 100 μg=tramadol, 100 mg=ketorolac 30 mg) [27,28]. Adverse effects were registered during the entire postoperative period. The beginning of intestinal motility was recorded as the time to first flatus. Mobilization time was also recorded in hours starting from the hour when the patient arrived in the PACU until the first active movement. Surgical complications were also recorded. Length of hospital stay was registered as number of nights in hospital.
Statistical Analysis
Demographic data and clinical characteristics are presented as means and standard deviation for continuous variables and as frequencies for categorical variables. Testing of data with normal distribution was done with the t test, while those with abnormal distribution were assessed with the Mann-Whitney test. Categorical data were tested with the Fisher exact test or the chi-square test. P<0.05 was set as the level of statistical significance. We performed a logistic regression analysis for comparison of the VAS between groups using age, sex, body mass index (BMI), time of surgery, and time of anesthesia as confounders. Data processing was performed with the SPSS software version 26 (SPSS, Inc., Chicago, IL, USA).
Sample Size Calculation
A minimum sample size of 40 patients for each group was prospectively estimated according to the data given from previous studies to detect a significant difference of 2 in the mean VAS scale [29], a standard deviation of 3 [30], a 2-sided α=0.05, and β=0.2 [31]. The total required number of patients was 80. To avoid errors from possible dropouts, 90 patients were included in this study.
Results
Ninety patients were enrolled between May 2019 and June 2021 in this single-center study. Nine patients were excluded because 4 did not meet the inclusion criteria (n=4) and 5 declined to participate (n=5). The remaining 81 patients were randomized to 1 of the 2 groups. Therefore, the final analysis included 81 patients: 40 patients were analyzed in the lidocaine group and 41 patients in the control group (Figure 1).
Figure 1.
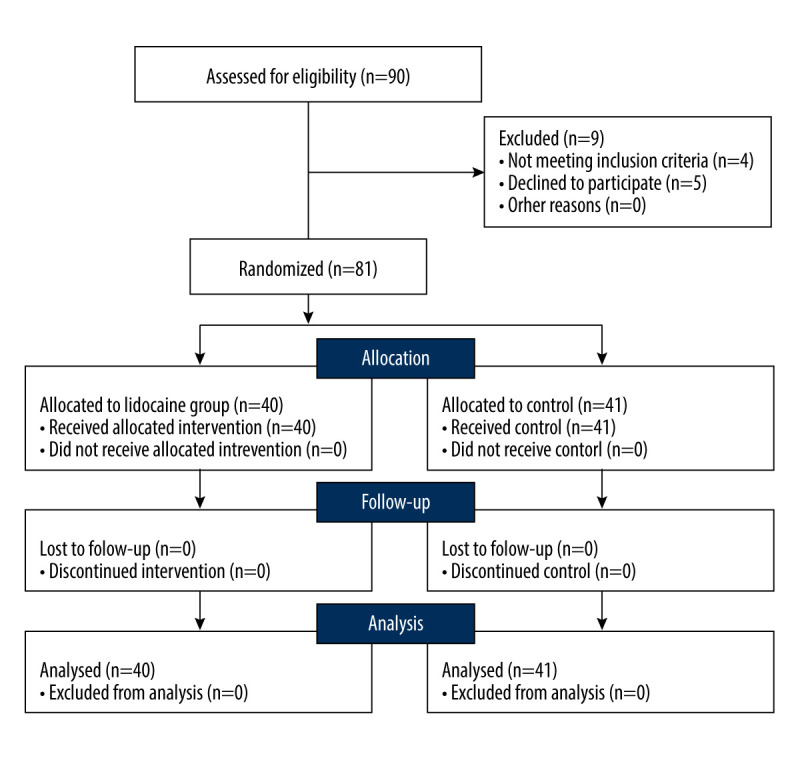
Flow diagram of patients included in randomization, allocation, follow-up and analysis (CONSORT).
Characteristics of Participants
The demographic characteristics were similar in both groups of patients according to age, weight, height, and ASA status (Table 1). There were more males than females in our cohort, but without a significant difference between groups. There were no significant differences between groups in anesthesia and surgery time, mobilization, length of hospital stay, and the frequency of nausea and vomiting.
Table 1.
Demographic and clinical data of the patients included in the study.
| Lidocaine n=40 |
Control n=41 |
P-value | |
|---|---|---|---|
| Gender F, n (%) | 17 (42.5) | 18 (45) | P=0.82 |
| Age (years) | 54.4±8.3 | 56.6±9.9 | P=0.28 |
| Wheight (kg) | 78.8±7.2 | 78.7±6.3 | P=0.94 |
| Height (cm) | 166.9±6.1 | 169.9±7.5 | P=0.34 |
| BMI, mean±SD | 23.6±2.2 | 23.2±1.9 | P=0.35 |
| Hypertension, n (%) | 22 (55) | 14 (35) | P=0.07 |
| Diabetes, n (%) | 1 (2.5) | 1 (2.5) | P=1.0 |
| Smoking, n (%) | 3 (7.5) | 4 (10) | P=0.69 |
| Alcohol consumption, n (%) | 0 (0) | 2 (5) | P=0.15 |
| Anesthesia time (min), mean±SD | 163.7±38.5 | 162.7±39.4 | P=0.90 |
| Surgical time (min), mean±SD | 141.5±41.7 | 138.1±39.2 | P=0.70 |
| Mobilization, mean±SD | 10.2±0.6 | 10.4±0.8 | P=0.21 |
| Length of hospital stay, mean±SD | 7.2±0.4 | 7.2±0.4 | P=0.79 |
| ASA, n (%) | |||
| 1 | 6 (15) | 8 (20) | P=0.14 |
| 2 | 32 (80) | 25 (62.5) | |
| 3 | 2 (5) | 7 (17.5) | |
| Nausea, vomiting, n (%) | 20 (50) | 15 (37.5) | P=0.26 |
| Additional analgesia, n (%) | 24 (42.1) | 33 (57.9) | P=0.03 |
| Morphine miliecuivalent, mean±SD | 37.2±19.2 | 54±4.5 | P<0.001 |
| Type of surgery, n (%) | |||
| Total knee arthroplasthy | 22 (55) | 24 (58.5) | P=0.74 |
| Femoral fractures | 18 (45) | 17 (41.5) |
Data are expressed as mean±standard deviation or numbers (proportion%). ASA – American Association of Anesthesiologists; BMI – body mass index.
Primary Outcomes
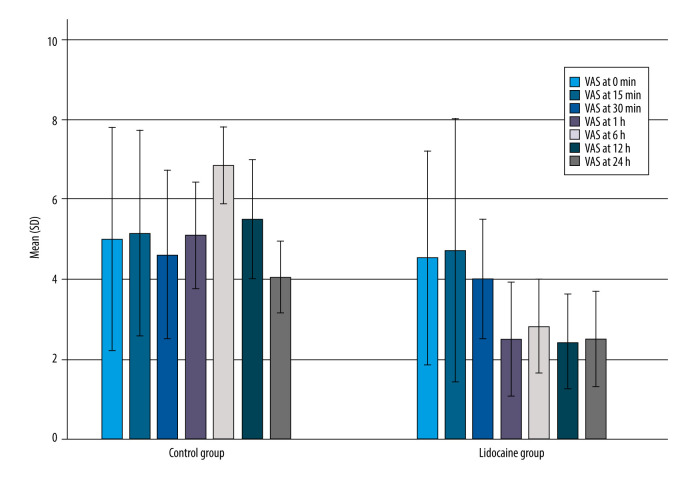
Postoperative pain scores as primary outcomes at rest according to the VAS scale (0–10) at intervals 0 min and 15 min were similar, with no significant difference between groups. Pain scores at rest were higher in the control group than the lidocaine group at 30 min,1, 6, 12 h, and 24 h, with a significant difference between groups (P<0.001). The highest level of postoperative pain was observed at 6 h (2.8±0.5 vs 6.8±0.4, P<0.001), and 12 h (6.8±0.4 vs 5.5±0.7, respectively). The results are shown in Table 2 and Figure 2.
Table 2.
Comparison of mean VAS at rest and during movement in patients treated with lidocaine and controls.
| VAS at rest | Lidocaine | Control | P-value | VAS during movement | Lidocaine | Control | P-value |
|---|---|---|---|---|---|---|---|
| n | 40 | 41 | 40 | 41 | |||
| 0 min | 4.5±1.3 | 5.0±1.3 | P=0.125 | 0 min | 4.6±0.7 | 5.1±0.7 | P=0.01 |
| 15 min | 4.7±1.6 | 5.1±1.2 | P=0.203 | 15 min | 4.5±0.8 | 5.2±1.0 | P=0.003 |
| 30 min | 4.0±0.7 | 4.6±1.0 | P=0.003 | 30 min | 4.7±0.8 | 5.5±0.9 | P<0.001 |
| 1 h | 2.5±0.7 | 5.1±0.6 | P<0.001 | 1 h | 4.5±0.5 | 5.8±0.7 | P<0.001 |
| 6 h | 2.8±0.5 | 6.8±0.4 | P<0.001 | 6 h | 3.6±0.7 | 7.9±0.8 | P<0.001 |
| 12 h | 2.4±0.5 | 5.5±0.7 | P<0.001 | 12 h | 3.1±0.5 | 7.0±1.0 | P<0.001 |
| 24 h | 2.5±0.5 | 4.0±0.4 | P<0.001 | 24 h | 2.6±0.5 | 5.5±1.0 | P<0.001 |
Data are expressed as mean±standard deviation or number (%). VAS – visual analog scale.
Figure 2.
Comparison of the VAS at rest between lidocaine and controls at different times VAS – visual analog scale. Lower VAS scores were observed in the lidocaine group compared to the control group.
VAS pain scores during movement were not significantly different between groups at time intervals 0 and 15 min (all P>0.05). Significant differences between groups were observed starting at 30 min in the postoperative period, 1 h (P<0.001), 6 h (3.6±0.7 vs 7.9±0.8, P<0.001), 12 h (3.1±0.5 vs 7.0±1.0, P<0.001) and 24 h (P<0.001). Patients in the control group had higher VAS than patients in the lidocaine group, with a significant difference between groups (P<0.001) (Table 2).
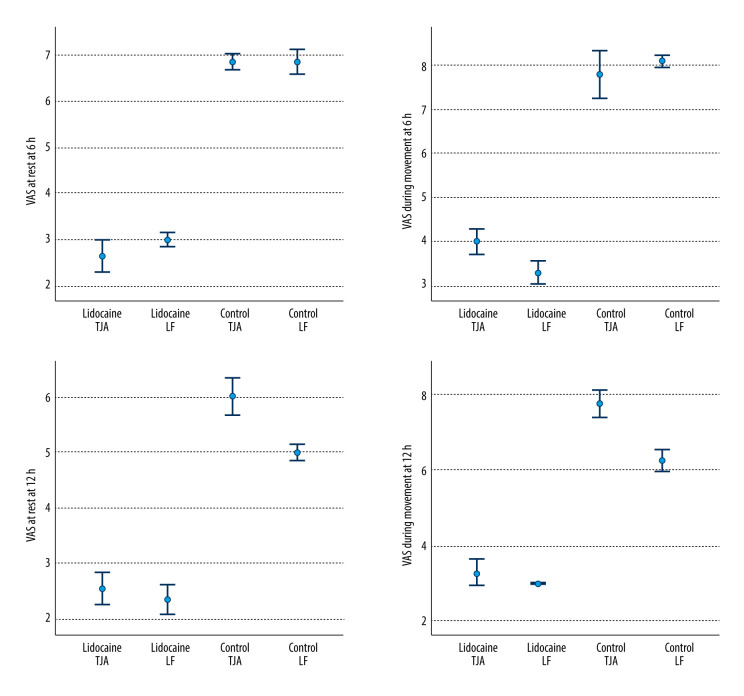
Even after adjusting for several risk factors and confounders, VAS at rest and VAS during movement remained significantly different between groups, starting at 1 h (odds ratio, 9.54; 95% confidence interval, 6.59–12.50; P<0.001), 6 h (odds ratio, 3.66; 95% confidence interval, 1.69–5.64; P<0.001), 12 h (odds ratio, 4.36; 95% confidence interval, 1.53–4.36; P<0.001), and 24 h (odds ratio, 3.76; 95% confidence interval, 1.70–5.72; P<0.001). The results are shown in Table 3 and Figure 3.
Table 3.
Logistic regression analysis of VAS in patients treated with lidocaine and controls. Controls had significantly higher VAS scores after 30 min, even after adjusting for age, sex, BMI, time of surgery, anesthesia, and risk factors (hypertension, diabetes, smoking).
| VAS at rest | OR | (95% CI) | P-value | VAS during movement | OR | (95% CI) | P-value |
|---|---|---|---|---|---|---|---|
| 0 min | 0.179 | (−0.39 to 0.78) | P=0.51 | 0 min | 2.25 | (0.95 to 3.54) | P=0.07 |
| 15 min | 0.39 | (−0.22 to 0.96) | P=0.203 | 15 min | 1.64 | (1.1 to 2.35) | P=0.006 |
| 30 min | 2.62 | (1.75 to 3.51) | P=0.007 | 30 min | 2.4 | (1.5 to 3.2) | P<0.001 |
| 1 h | 9.54 | (6.59 to 12.50) | P<0.001 | 1 h | 3.7 | (1.8 to 5.6) | P<0.001 |
| 6 h | 3.66 | (1.69 to 5.64) | P<0.001 | 6 h | 9.8 | (6.9 to 12.8) | P<0.001 |
| 12 h | 4.36 | (1.53 to 4.36) | P<0.001 | 12 h | 2.5 | (1.3 to 3.6) | P<0.001 |
| 24 h | 3.71 | (1.70 to 5.72) | P<0.001 | 24 h | 3.6 | (1.6 to 5.6) | P<0.001 |
Data are expressed as odds ratios and 95% confidence intervals. VAS – visual analog scale; BMI – body mass index.
Figure 3.
Error bars of the VAS at rest and during movement at 6 h and 12 h. VAS was lower in lidocaine group in both TJA and LF groups at both time intervals. VAS – visual analog scale; TJA – total joint arthroplasty; LF – limb fractures.
A subgroup analysis was performed to assess whether intravenous lidocaine was more effective in the management of postoperative pain after total knee arthroplasty versus limb fractures. According to the pain scores level at rest and movement, we did not find any significant differences at time intervals 0, 15, and 30 min, but we recorded significant differences at time intervals 1, 6, 12, and 24 hours between the lidocaine group and control group with total knee arthroplasty (P<0.001). We observed that the pain scores at rest and movement were significantly different between the patients in the lidocaine group and controls with limb fractures. Pain score levels were significantly different at all time pointsmeasured in the postoperative period (P<0.05).
Secondary Outcomes
In 33 patients (57.9%) from the control group and in 24 patients (42.1%) from the lidocaine group, additional analgesics were administered, with significant differences between groups (P<0.05). The rate of additional analgesics administered in the control group was significantly higher than that in the lidocaine group. Total analgesic use in the postoperative period converted in milliequivalents of morphine was significantly higher in the control group than in the lidocaine group (37.2±19.2 vs 54±4.5) (P<0.001).
Nausea and vomiting in the postoperative period were observed in 13 patients (37.5%) in the lidocaine group and 20 patients (50%) in the control group, but without significant difference between groups (P>0.05). No signs of toxicity related to lidocaine (eg, headache, arrhythmias, dizziness, tinnitus, perioral numbness, metallic taste, seizures) were observed. Three patients were treated with intravenous ephedrine 5 mg and atropine 0.5 mg due to hypotension and bradycardia. No significant differences were observed between groups according to the beginning of the intestinal motility, mobilization, and length of hospital stay.
We noticed lower values of mean arterial pressure and heart rate in the lidocaine group than in the control group. The hemoglobin saturation was similar in the 2 groups. No surgical complications were noticed.
Discussion
The findings of our study show that intravenous lidocaine significantly lowers pain scores at rest and during movement in elective orthopedic patients compared with the control group. Postoperative pain scores at rest and movement intervals at 0 and 15 min were similar, and no significant difference was observed between groups. However, starting from 30 min in the postoperative period and continuing for 1, 6, 12, and 24 h, significant differences between groups were observed. No significant differences between groups were observed according to the beginning of intestinal motility, mobilization, and length of hospital stay. A significant difference between groups was observed in the rate of reduction of additional analgesics and total analgesic use in the postoperative period. Additional analgesics were administered at a lower rate in the lidocaine group than in controls. Total analgesic use in the postoperative period was significantly higher in the control group. We did not observe significant differences in adverse effects (eg, headache, arrhythmias, dizziness, tinnitus, perioral numbness, metallic taste, seizures) between groups. Lower values of mean arterial pressure and heart rate in the lidocaine group compared to the control group were observed, but without a significant difference.
We found a very limited number of studies in the literature that evaluated the analgesic effect of intravenous lidocaine in elective orthopedic patients. Forouzaan et al [32] concluded that intravenous lidocaine compared to intravenous morphine was a significantly more effective technique for the management of pain in patients with extremity fractures. In a retrospective analysis, Choi et al [33] concluded that intravenous lidocaine significantly reduces opioid requirements in patients with traumatic rib fractures. Another retrospective cohort study has shown that intravenous lidocaine has a similar analgesic effect as epidural analgesia in traumatic rib fractures [34]. Intravenous lidocaine was shown to be a more beneficial and safer analgesic technique when compared with regional analgesia in terms of pain management in young patients with forearm fractures [35]. Alebouyeh et al [36] demonstrated a significant reduction in pain scores and opioid requirements in patients after orthopedic surgery when lidocaine was added to morphine used in patient-controlled intravenous analgesia. We did not use PCA (patient-controlled analgesia) in our study because opioids are the most common drugs used in PCA [36], whereas the main goal of our study was to investigate opioid-free analgesia to avoid the adverse effects of opioids. The basic analgesics used in our study were paracetamol and ketorolac, and we used tramadol or morphine only when the VAS score was ≥4.
In our study, we observed significant differences in pain scores at rest and movement in patients after total knee arthroplasty. In the literature, is hard to find similar studies that investigated the analgesic efficacy of intravenous lidocaine for total joint arthroplasty, especially for total knee arthroplasty. A randomized, double-blind, placebo-controlled trial by Yu Wong et al [37] analyzed the intravenous administration of lidocaine in patients after total knee arthroplasty, finding that patients in the lidocaine group had lower VAS scores at rest and during movement, and also had shorter length of hospitalization, compared to the control group [37]. In another randomized clinical trial, which compared intravenous lidocaine with saline in patients after total hip arthroplasty, no significant differences in pain scores between the lidocaine group and control group were observed [38]. In this RCT, lidocaine was administered 1.5 mg·kg−1·h−1 as a bolus dose followed by intravenous infusion of 1.5 mg·kg−1·h−1, but the infusion of lidocaine was stopped 1 h after skin closure. Therefore, this short time of intravenous lidocaine application may be the reason why no difference in pain scores was observed. A meta-analysis for total joint arthroplasty found no evidence for analgesic efficacy, opioid-sparing effect, or the impact of intravenous lidocaine on the length of hospital stay [39].
Lidocaine, as the most extensively used local anesthetic, has an analgesic effect, antihyperalgesic and anti-inflammatory properties, and is associated with faster recovery of bowel function after surgery [40]. The mechanism by which lidocaine exhibits its analgesic effect includes blocking of muscarinic receptors (M1, M3) and N-methyl-D-aspartate receptors (NMDA), whereas at higher doses, lidocaine blocks nicotinic receptors and 5-hydroxytryptamine-3, voltage-gated calcium channels [41]. Numerous randomized controlled trials and meta-analyses have shown the analgesic effects of intravenous lidocaine on reduction of pain scores in the postoperative period, nausea and vomiting, and an early return of bowel function, principally in abdominal surgeries [42–45]. We did not observe any significant differences between groups according to the beginning of intestinal motility, mobilization, and length of hospital stay.
A significant difference between groups was observed in the rate of reduction of additional analgesics and total analgesic use in the postoperative period. Additional analgesics were administered at a lower rate in the lidocaine group than in controls. Total analgesic use in the postoperative period converted to milliequivalents of morphine was significantly higher in the control group. Similarly, Choi et al [33] and Alebouyeh et al [36] concluded that intravenous lidocaine reduces the opioid requirements in orthopedic patients. We did not observe significant differences in adverse effects (eg, headache, arrhythmias, dizziness, tinnitus, perioral numbness, metallic taste, seizures) between groups. Three patients were treated with intravenous ephedrine and atropine due to hypotension and bradycardia. In addition, we noticed lower values of mean arterial pressure and heart rate in the lidocaine group compared to the control group, but without a significant difference. Those lower values of mean arterial pressure and heart rate were constant throughout the intraoperative and postoperative period, without significant fluctuations of these hemodynamic parameters affecting hemodynamic stability.
The standard analgesic multimodal regimen of our institution included: non-steroid anti-inflamatory drugs (ketorolac 30 mg every 8 h with the first dose administered at the end of surgical procedure), and acetaminophen 1 g every 6 h. A similar analgesic regimen is also recommended in an updated procedure-specific postoperative pain management (PROSPECT) guideline for total hip arthroplasty [24]. This systematic review also recommends that opiods should be kept as analgesics for moderate and severe pain [24], and in our study we used tramadol or morphine only when the VAS score was ≥4.
Several studies suggest that intravenous lidocaine has an analgesic effect similar to that of epidural analgesia and can be a safe analgesic technique when epidural analgesia is contraindicated [34], and intravenous lidocaine is even more effective than epidural analgesia in pain management [38]. Further investigations are needed in various fields of surgery to demonstrate the analgesic efficacy of intravenous lidocaine compared to that of epidural analgesia. Currently, an increasing number of patients are being treated with anticoagulant therapy, or who have coagulopathies or other contraindications to epidural analgesia. Given that epidural analgesia is a much more costly technique and is sometimes contraindicated, utilizing a low-cost and easily administered analgesic procedure with intravenous lidocaine would be of considerable clinical importance.
One of the most interesting findings in the present study, which we noted during the analysis of subgroups, was the higher efficacy of lidocaine in lowering postoperative pain scores at most time points in patients with limb fractures, where we observed a significant difference between groups. However, this finding does not have a high statistical value due to the small number of patients included in this subgroup. Further multicenter trials are required, especially in patients with surgery repair fractures, to prove the analgesic efficacy of intravenous lidocaine. Also, further multicenter studies are needed to compare the analgesic efficacy of intravenous lidocaine with epidural analgesia. If future research proves the superiority of lidocaine versus epidural anesthesia, this would be a great achievement for the growing population of people in whom regional analgesia is contraindicated.
In the world’s current epidemiological circumstances, especially in patients with SARS-CoV-2, in whom regional analgesia has limited feasibility due to concurrent anticoagulant therapy and respiratory failure, this novel analgesic technique would be of tremendous clinical importance.
Limitations
One limitation of this research was that we conducted a single-center clinical study; therefore, the data are limited. Another limitation was that we did not determine the concentration of lidocaine in the serum during the intraoperative and postoperative periods. In the present study, the anti-inflamatory properties of lidocaine were not examined, as they were not the focus of our research, although these effects of lidocaine have been reported by numerous studies.
Conclusions
This study shows that intravenous lidocaine provided adequate postoperative analgesia for orthopedic patients undergoing elective total joint arthroplasty and limb fracture repair. A significant difference was observed between groups in the reduction of additional analgesics and total analgesic use. No significant differences were observed in mobilization and length of hospital stay. Large-scale clinical studies and controlled clinical trials are required to investigate the role of postoperative intravenous lidocaine analgesia in specific types of orthopedic surgical procedures.
Acknowledgements
We thank Fisnik Jashari for his contribution to statisical analysis and Arjan Ademi and Flamur Krasniqi for their contribution in data collection.
Footnotes
Conflict of interest: None declared
Department and Institution Where Work Was Done
Department of Anesthesiology and Reanimation, American Hospital of Kosova, Pristina, Republic of Kosova.
Declaration of Figures’ Authenticity
All figures submitted have been created by the authors, who confirm that the images are original with no duplication and have not been previously published in whole or in part.
Financial support: None declared
References
- 1.Dale GJ, Phillips S, Falk GL. The analgesic efficacy of intravenous lidocaine infusion after laparoscopic fundoplication: A prospective, randomized, double-blind, placebo-controlled trial. Local Reg Anesth. 2016;9:87–93. doi: 10.2147/LRA.S119483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Apfelbaum JL, Chen C, Mehta SS, Gan TJ. Postoperative pain experience: Results from a national survey suggest postoperative pain continues to be undermanaged. Anesth Analg. 2003;97:534–40. doi: 10.1213/01.ANE.0000068822.10113.9E. [DOI] [PubMed] [Google Scholar]
- 3.Barreveld A, Witte J, Chahal H, et al. Preventive analgesia by local anesthetics: The reduction of postoperative pain by peripheral nerve blocks and intravenous drugs. Anesth Analg. 2013;116(5):1141–61. doi: 10.1213/ANE.0b013e318277a270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marret E, Kurdi O, Zufferey P, Bonnet F. Effects of nonsteroidal antiinflammatory drugs on patient-controlled analgesia morphine side effects: Meta-analysis of randomized controlled trials. Anesthesiology. 2005;102:1249–60. doi: 10.1097/00000542-200506000-00027. [DOI] [PubMed] [Google Scholar]
- 5.Holbek BL, Horsleben Petersen R, Kehlet H, et al. Fast-track video-assisted thoracoscopic surgery: Future challenges. Scand Cardiovasc J. 2016;50(2):78–82. doi: 10.3109/14017431.2015.1114665. [DOI] [PubMed] [Google Scholar]
- 6.Joshi GP, Bonnet F, Kehlet H PROSPECT collaboration. Evidence-based postoperative pain management after laparoscopic colorectal surgery. Colorectal Dis. 2013;15:146–55. doi: 10.1111/j.1463-1318.2012.03062.x. [DOI] [PubMed] [Google Scholar]
- 7.Halabi WJ, Kang CY, Nguyen VQ, et al. Epidural analgesia in laparoscopic colorectal surgery: A nationwide analysis of use and outcomes. JAMA Surg. 2014;149:130–36. doi: 10.1001/jamasurg.2013.3186. [DOI] [PubMed] [Google Scholar]
- 8.Cook TM, Counsell D, Wildsmith JAW Royal College of Anaesthetists Third National Audit Project. Major complications of central neuraxial block: Report on the Third National Audit Project of the Royal College of Anaesthetists. Br J Anaesth. 2009;102:179–90. doi: 10.1093/bja/aen360. [DOI] [PubMed] [Google Scholar]
- 9.Dewinter G, Van de Velde M, Fieuws S, et al. Transversus abdominis plane block versus perioperative intravenous lidocaine versus patient-controlled intravenous morphine for postoperative pain control after laparoscopic colorectal surgery: Study protocol for a prospective, randomized, double-blind controlled clinical trial. Trials. 2014;15(1):1–7. doi: 10.1186/1745-6215-15-476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kumar K, Kirksey MA, Duong S, Wu CL. A review of opioid-sparing modalities in perioperative pain management: Methods to decrease opioid use postoperatively. Anesth Analg. 2017;125(5):1749–60. doi: 10.1213/ANE.0000000000002497. [DOI] [PubMed] [Google Scholar]
- 11.Chu R, Umukoro N, Greer T, et al. Intravenous lidocaine infusion for the management of earlypostoperative pain: A comprehensive review of controlled trials. Psychopharmacol Bull. 2020;50:216–59. [PMC free article] [PubMed] [Google Scholar]
- 12.Butterworth JF, Strichartz GR. Molecular mechanisms of local anesthesia: A review. Anesthesiology. 1990;72(4):711–34. doi: 10.1097/00000542-199004000-00022. [DOI] [PubMed] [Google Scholar]
- 13.Olschewski A, Hempelmann G, Vogel W, Safronov BV. Blockade of Na+ and K+ currents by local anesthetics in the dorsal horn neurons of the spinal cord. Anesthesiology. 1998;88(1):172–79. doi: 10.1097/00000542-199801000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Aguilar JS, Criado M, De Robertis E. Inhibition by local anesthetics, phentolamine and propranolol of (3H)quinuclydinyl benzylate binding to central muscarinic receptors. Eur J Pharmacol. 1980;68(3):317–26. doi: 10.1016/0014-2999(80)90529-4. [DOI] [PubMed] [Google Scholar]
- 15.Bittencourt AL, Takahashi RN. Mazindol and lidocaine are antinociceptives in the mouse formalin model: Involvement of dopamine receptor. Eur J Pharmacol. 1997;330(2–3):109–13. doi: 10.1016/s0014-2999(97)00182-9. [DOI] [PubMed] [Google Scholar]
- 16.Woolf CJ, Wiesenfeld-Hallin Z. The systemic administration of local anaesthetics produces a selective depression of C-afferent fibre evoked activity in the spinal cord. Pain. 1985;23(4):361–74. doi: 10.1016/0304-3959(85)90006-5. [DOI] [PubMed] [Google Scholar]
- 17.Muth-Selbach U, Hermanns H, Stegmann JU, et al. Antinociceptive effects of systemic lidocaine: Involvement of the spinal glycinergic system. Eur J Pharmacol. 2009;613(1–3):68–73. doi: 10.1016/j.ejphar.2009.04.043. [DOI] [PubMed] [Google Scholar]
- 18.Cohen SP, Mao J. Is the analgesic effect of systemic lidocaine mediated through opioid receptors? Acta Anaesthesiol Scand. 2003;47(7):910–41. doi: 10.1034/j.1399-6576.2003.00163.x. [DOI] [PubMed] [Google Scholar]
- 19.Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology. 2007;106:11–18. doi: 10.1097/00000542-200701000-00007. [DOI] [PubMed] [Google Scholar]
- 20.Koppert W, Weigand M, Neumann F, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg. 2004;98:1050–55. doi: 10.1213/01.ANE.0000104582.71710.EE. [DOI] [PubMed] [Google Scholar]
- 21.Herroeder S, Pecher S, Schonherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: A double-blinded, randomized, placebo-controlled trial. Ann Surg. 2007;246:192–200. doi: 10.1097/SLA.0b013e31805dac11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bryson GL, Charapov I, Krolczyk G, et al. Intravenous lidocaine does not reduce length of hospital stay following abdominal hysterectomy. Can J Anaesth. 2010;57:759–66. doi: 10.1007/s12630-010-9332-2. [DOI] [PubMed] [Google Scholar]
- 23.Lauwick S, Kim DJ, Mistraletti G, Carli F. Functional walking capacity as an outcome measure of laparoscopic prostatectomy: The effect of lidocaine infusion. Br J Anaesth. 2009;103:213–19. doi: 10.1093/bja/aep103. [DOI] [PubMed] [Google Scholar]
- 24.Anger M, Valovska T, Beloeil H, et al. PROSPECT Working Group and the European Society of Regional Anaesthesia and Pain Therapy. PROSPECT guideline for total hip arthroplasty: A systematic review and procedure-specific postoperative pain management recommendations. Anaesthesia. 2021;76(8):1082–97. doi: 10.1111/anae.15498. [DOI] [PubMed] [Google Scholar]
- 25.Sun EC, Darnall BD, Baker LC, Mackey S. Incidence of and risk factors for chronic opioid use among opioid-naive patients in the postoperative period. JAMA Intern Med. 2016;176:1286–93. doi: 10.1001/jamainternmed.2016.3298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Viera AJ, Bangdiwala SI. Eliminating bias in randomized controlled trials: Importance of allocation concealment and masking. Fam Med. 2007;39:132–37. [PubMed] [Google Scholar]
- 27.Cepeda MS, Carr DB, Miranda N, et al. Comparison of morphine, ketorolac, and their combination for postoperative pain: Results from a large, randomized, double-blind trial. Anesthesiology. 2005;103:1225–32. doi: 10.1097/00000542-200512000-00018. [DOI] [PubMed] [Google Scholar]
- 28.McPherson ML. Demystifying opioid conversion calculations: A guide for effective dosing Bethesda. Maryland, MD: American Society of Health-System Pharmacists; 2009. [Google Scholar]
- 29.Farrar JT, Polomano RC, Berlin JA, Strom BL. A comparison of change in the 0–10 numeric rating scale to a pain relief scale and global medication performance scale in a short-term clinical trial of breakthrough pain intensity. Anesthesiology. 2010;112:14641472. doi: 10.1097/ALN.0b013e3181de0e6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carli F, Clemente A, Asenjo JF, et al. Analgesia and functional outcome after total knee arthroplasty: Periarticular infiltration vs continuous femoral nerve block. Br J Anaesth. 2010;105:185195. doi: 10.1093/bja/aeq112. [DOI] [PubMed] [Google Scholar]
- 31.Choi S, O’Hare T, Gollish J, et al. Optimizing pain and rehabilitation after knee arthroplasty: A two-center, randomized trial. Anesth Analg. 2016;123(5):1316–24. doi: 10.1213/ANE.0000000000001469. [DOI] [PubMed] [Google Scholar]
- 32.Forouzan A, Barzegari H, Motamed H, et al. Intravenous lidocaine versus morphine sulfate in pain management for extremity fractures; A clinical trial. Emergency. 2017;5(1):e68. [PMC free article] [PubMed] [Google Scholar]
- 33.Choi J, Zamary K, Barreto NB, et al. Intravenous lidocaine as a non-opioid adjunct analgesic for traumatic rib fractures. PLoS One. 2020;15:e0239896. doi: 10.1371/journal.pone.0239896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lii TR, Aggarwal AK. Comparison of intravenous lidocaine versus epidural anesthesia for traumatic rib fracture pain: A retrospective cohort study. Reg Anesth Pain Med. 2020;45:628–33. doi: 10.1136/rapm-2019-101120. [DOI] [PubMed] [Google Scholar]
- 35.Juliano PJ, Mazur JM, Cummings RJ, McCluskey WP. Low-dose lidocaine intravenous regional anesthesia for forearm fractures in children. J Pediatr Orthop. 1992;12(5):633–35. [PubMed] [Google Scholar]
- 36.Alebouyeh MR, Imani F, Rahimzadeh P, et al. Analgesic effects of adding lidocaine to morphine pumps after orthopedic surgeries. J Res Med Sci. 2014;19(2):122–27. [PMC free article] [PubMed] [Google Scholar]
- 37.Wong SY, Lam DMH, Mak HCY, et al. Effects of intravenous lidocaine in total knee joint arthroplasty: A randomized, double-blind, placebo-controlled trial. International Journal of Clinical Studies and Medical Case Reports. 2021;11(4):1–8. [Google Scholar]
- 38.Martin F, Cherif K, Gentili ME, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology. 2008;109:118–23. doi: 10.1097/ALN.0b013e31817b5a9b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Soffin EM, Gibbons MM, Ko CY, et al. Evidence review conducted for the agency for healthcare research and quality safety program for improving surgical care and recovery: Focus on anesthesiology for total knee arthroplasty. Anesth Analg. 2019;128(3):441–53. doi: 10.1213/ANE.0000000000003564. [DOI] [PubMed] [Google Scholar]
- 40.Beaussier M, Delbos A, Maurice-Szamburski A, et al. Perioperative use of intravenous lidocaine. Drugs. 2018;78:1229–46. doi: 10.1007/s40265-018-0955-x. [DOI] [PubMed] [Google Scholar]
- 41.Hermanns H, Hollmann MW, Stevens MF, et al. Molecular mechanisms of action of systemic lidocaine in acute and chronic pain: a narrative review. Br J Anaesth. 2019;123:335–49. doi: 10.1016/j.bja.2019.06.014. [DOI] [PubMed] [Google Scholar]
- 42.Kaszynski M, Lewandowska D, Sawicki P, et al. Efficacy of intravenous lidocaine infusions for pain relief in children undergoing laparoscopic appendectomy: A randomized controlled trial. BMC Anesth. 2021;21:2. doi: 10.1186/s12871-020-01218-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ghimire A, Subedi A, Bhattarai B, Sah BP. The effect of intraoperative lidocaine infusion on opioid consumption and pain after totally extraperitoneal laparoscopic inguinal hernioplasty: A randomized controlled trial. BMC Anesth. 2020;20:137. doi: 10.1186/s12871-020-01054-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sun Y, Li T, Wang N, et al. Perioperative systemic lidocaine for postoperative analgesia and recovery after abdominal surgery: A meta-analysis of randomized controlled trials. Dis Colon Rectum. 2012;55:1183–94. doi: 10.1097/DCR.0b013e318259bcd8. [DOI] [PubMed] [Google Scholar]
- 45.McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: A systematic review of randomized controlled trials. Drugs. 2010;70:1149–63. doi: 10.2165/10898560-000000000-00000. [DOI] [PubMed] [Google Scholar]