Abstract
Background
Toxic epidermal necrolysis is a rare condition where a drug reaction induces skin loss, similar to that seen in extensive burns. It is associated with high morbidity and mortality and there is no clear agreement on effective treatment.
Objectives
To assess the effects of all interventions for the treatment of toxic epidermal necrolysis.
Search methods
We searched the Cochrane Skin Group Specialised Register (March 2001), the Cochrane Controlled Trials Register (March 2001), MEDLINE (1966 to December 2001), EMBASE (1980 to December 2001), DARE (4th Quarter 2001) and CINAHL (1982 to October 2001).
Selection criteria
Randomised controlled trials of therapeutic and supportive interventions that included participants clinically diagnosed with toxic epidermal necrolysis were included.
Data collection and analysis
Two independent authors carried out study selection and assessment of methodological quality.
Main results
Only one randomised controlled trial of treatment was identified. This trial compared the effectiveness of thalidomide with placebo and included 22 patients, 12 in the treatment group and 10 in the placebo group. Patients on the treatment arm received thalidomide 200 mg twice daily for 5 days. The main end point was the measurement of the progression of skin detachment after seven days. Other end points were the overall mortality and severity of the disease evaluated with the simplified acute physiology score. The study was terminated as the mortality on the treatment arm was 83% compared to 30% on the control arm (relative risk 2.78, 95% confidence interval 1.04 to 7.40). No randomised controlled trials of the most commonly used current treatments i.e. systemic steroids, cyclosporin A and intravenous immunoglobulins were found.
Authors' conclusions
Treatment with thalidomide was not shown to be effective and was associated with significantly higher mortality than placebo. There is no reliable evidence on which to base treatment for toxic epidermal necrolysis, a disease commonly associated with mortality rates of around 30%. More research is required to understand the mechanisms of toxic epidermal necrolysis. International multi‐centre studies are needed in the form of randomised controlled trials, to evaluate treatments for toxic epidermal necrolysis, especially those using high doses of steroid and intravenous immunoglobulins.
Keywords: Humans, Dermatologic Agents, Dermatologic Agents/therapeutic use, Randomized Controlled Trials as Topic, Stevens‐Johnson Syndrome, Stevens‐Johnson Syndrome/drug therapy, Thalidomide, Thalidomide/therapeutic use
Plain language summary
Interventions for toxic epidermal necrolysis
Toxic epidermal necrolysis (TEN or Lyell's disease) is a rare life‐threatening skin condition. It is probably an immune response triggered by some drugs or infection, which is more likely to happen in people with suppressed immunity. TEN causes extensive blistering and shedding of skin, similar to burns. Drugs used include oral steroids, thalidomide, immunosuppressants and immunoglobulins. This review of trials did not find any reliable evidence for the treatment of TEN. The only trial available used thalidomide, but this trial did not show any benefit from treatment compared against placebo but highlighted increased chances of dying from the treatment. Thalidomide is not safe or effective for the skin condition toxic epidermal necrolysis, but there is not enough evidence to show which treatments are effective.
Background
Description of the condition
Definition
Toxic epidermal necrolysis (TEN) is a rare dermatological condition of sudden onset characterised by extensive blistering and shedding of the skin. It is usually associated with high morbidity and mortality (Dolan 1989). This condition is also known as Lyell's disease after Alan Lyell who first described it in four patients and coined the term TEN (Lyell 1956).
The terminology of severe skin reactions such as TEN, Steven‐Johnson syndrome (SJS) and erythema multiforme majus (EEMM) has been confusing. Some specialists in this field believe that EEMM has to be separated from SJS and TEN not only clinically but also etiologically (Assier 1995; Mockenhaupt 1999). Furthermore, they believe that SJS and TEN represent a continuous spectrum of the same disease, i.e. one disorder of different severity (Revuz 1996). A consensus definition has been developed by an international group of dermatologists, which has been used successfully in several epidemiological studies (Bastuji‐Garin 1993).
Epidemiology
TEN is a rare condition. The annual reported incidence of TEN, SJS and TEN/SJS overlap is 1.89 cases per million population (Mockenhaupt 1996). The incidence of SJS is probably higher than TEN (Roujeau 1995). The incidence of TEN may be greater in the elderly population, which could be due to their relative over‐exposure to medication. There is some predisposition to TEN among patients with systemic lupus erythematosus, HIV/AIDS and recipients of bone marrow transplants. The reported mortality in TEN is around 30% (Kelly 1995) but can be as high as 45% when there is skin detachment of more than 30% (Mockenhaupt 1996).
Causes and mechanisms of action
The cause of TEN is far from clear although hypersensitivity to drugs is by far the commonest reported cause (Roujeau 1995). Drugs that are known to trigger such reactions frequently include antimicrobials (such as sulphonamides), allopurinol (a drug used to prevent gout), anticonvulsants (such as phenytoin), and non‐steroidal anti‐inflammatory agents (Revuz 1996). Certain infections and immunosuppression may act as predisposing factors to TEN. If a drug precipitates TEN, removal of the offending medication does not always clear the skin and mucous membrane lesions immediately, especially if the drug takes a long time to be cleared from the body. Removal of the offending drug may still be a crucial aspect of disease management, especially for short acting drugs. The exact pathological mechanism of TEN is not known. It has been suggested that people who metabolise certain drugs more slowly than normal may be more prone to drug reactions in general, but whether this is true for TEN remains to be seen. The clinical manifestation of TEN is likely to be mediated by an immunological response of the host rather than a direct toxic action of the drug or its metabolites. Some people who have received a bone marrow transplant have developed a form of TEN, and it is still unclear if this is due to administered drugs, a form of graft‐versus‐host disease or both (Villada 1990).
Diagnosis
TEN is clinically diagnosed usually by dermatologists. Skin biopsy helps to confirm the diagnosis and also to differentiate it from staphylococcal scalded skin syndrome, a more superficial form of epidermal loss induced by toxins produced by certain strains of the bacterium Staphylococcus aureus (Revuz 1987).
Clinical features
The onset of TEN can be from a few hours to several weeks depending on the patient's underlying disease and co‐medications as well as the suspected drug itself. In affected patients the skin becomes reddened, and large blisters develop quickly (Revuz 1987). Complete death of the epidermis leads to sloughing similar to that seen in an extensive burn. This loss of the epidermis increases the risk of life‐threatening infections. The skin failure resulting from this loss leads to loss of fluid and electrolytes which can result in shock. Hypothermia can also develop. TEN usually involves the eyes and the mucous membranes such as the mouth, nose, genitalia and anus.
Description of the intervention
There is no clear agreement on the treatment for TEN. People with TEN are often treated at burn centres because of extensive loss of skin (Pruitt 1987). Some authors claim this is the only useful treatment (Halebian 1986). Oral steroids (Stables 1993), immuno suppressants e.g. cyclosporine (Renfro 1989), cyclophosphamide (Heng 1991), azathioprine (Bunger 1968), plasmapheresis (Sakellariou 1991) and intravenous immunoglobulin (IVIG) (Viard 1998) have all been tried in the management of patients with TEN, but it is far from clear whether any of these treatments are effective. A recent study suggests that the death rate among SJS and TEN patients treated with IVIG is even higher than expected from earlier epidemiological studies. In addition, no measurable effect, either on the progression of detachment or on the speed of re‐epithelisation, could be determined (Bachot 2001).
Why it is important to do this review
This systematic review attempts to clarify which of the therapeutic or supportive interventions used in TEN are effective, ineffective or hazardous.
Objectives
To assess the effects of therapeutic agents (steroids, immuno suppressants, cytotoxic drugs) for the treatment of TEN.
To compare the efficacy of burn centre treatment versus conventional supportive care.
Methods
Criteria for considering studies for this review
Types of studies
All randomised controlled trials of interventions for TEN.
Types of participants
Anyone with a clinical diagnosis of TEN.
Types of interventions
Any therapeutic or supportive intervention used to treat patients with TEN.
Types of outcome measures
Primary outcomes
Mortality.
Quality of life after recovery.
Pain during acute episode.
Secondary outcomes
Loss of total body surface area.
Serious infection.
Renal failure.
Length of hospital stay.
Bone marrow toxicity.
Search methods for identification of studies
Electronic searches
a) MEDLINE (from 1966 to December 2001), EMBASE (from 1980 to December 2001), DARE (4th Quarter 2001) and CINAHL (from 1982 to October 2001).
b) The Skin Group Specialised Register and the Cochrane Controlled Trials Register were searched (March 2001) for all trials of therapeutic or supportive interventions for TEN.
Searching other resources
Bibliographic searches
The bibliographies of all the literature identified were searched.
Correspondence
Personal communication with trialists and other knowledgeable persons was made to obtain information otherwise not available.
Handsearching
The conference proceedings and published abstracts of four international meetings on cutaneous adverse drug reactions (ADR) and other similar conference proceedings were hand searched.
Data collection and analysis
Selection of studies
Titles and abstracts identified from the searches were checked by two authors (SM and AT). The full text of all studies identified as of possible relevance was obtained and assessed by two independent authors (SM and AT). These authors decided which trials fitted the criteria for a RCT and these were included.
Data extraction and management
Data was extracted by one author (AT), using the Cochrane Skin Group's data extraction form.
Potential sources of study heterogeneity such as disease severity of study participants, different doses of interventions and different study designs were also recorded.
Assessment of risk of bias in included studies
The identified study was critically assessed by two authors (SM and AT) with particular attention paid to the methods of randomisation. We looked for explanations in the methods section for:
allocation concealment
blinding
follow‐up
The following additional sources of systematic errors in trials of health care interventions were checked:
Selection bias: systematic differences in groups compared in terms of age, co‐morbidity and extent of skin loss
Performance bias: systematic difference in the care package apart from the intervention on trial
Detection bias: systematic differences in the assessment of outcome
Results
Description of studies
Results of the search
The literature search resulted in 88 citations. Only one of these was an RCT and it met the inclusion criteria.
Included studies
The included study was a multi‐centre randomised double blind placebo controlled trial (Characteristics of included studies). The trial included 22 randomised patients in 2 arms. The treatment arm had 12 patients while the control arm had 10 patients. All patients were over 18 years and had 10 to 90% body surface area of skin loss within the first 4 days since the appearance of the first mucocutaneous symptom. Diagnosis was made on clinical and histopathological evidence.
The intervention was thalidomide 2 x 100 mg twice daily for 5 days as an oral capsule or by nasogastric tube feeding. The placebo was identical in appearance. The outcome measurements were skin detachment and simplified acute physiology score (SAPS) score at five and seven days, and mortality. Plasma and blister fluid tumour necrosis factor‐alpha (TNF‐alpha) and interleukin six concentration were measured at day zero and day two.
Nine of the 15 patients at a single centre died (60%). This high mortality rate alerted the local investigators who informed the trial coordinator. A safety board of three experts was gathered who first looked at the data without breaking the code. Across all centres, 13 of the 22 patients enrolled had died (59%). A decision to break the code was taken and it was confirmed that the death rate was significantly higher in the thalidomide group. The safety board thus advised that the trial be stopped
Excluded studies
Please see Characteristics of excluded studies.
Risk of bias in included studies
The general quality of the included study was good.
Allocation
Appropriate methods of allocation generation and concealment were used. The randomisation sequence was generated by a private randomisation service and administered by telephone. The sequence was balanced in blocks of six patients, stratified according to two categories of study centres.
Blinding
Blinding of participant, clinician and outcome assessor was present and appropriately done.
Incomplete outcome data
All losses to follow up were due to death. Three patients (one placebo, two thalidomide) died before day five and so were unavailable for outcome measurements other than mortality at day five. A further three patients in the thalidomide group died before day seven.
Other potential sources of bias
Selection bias
Participants in the two groups were similar in terms of age, body weight, percentage skin detachment and simplified acute physiology score (SAPS).
Effects of interventions
Only one randomised controlled trial of treatment of TEN was available. This study was terminated because of significantly high mortality on the treatment arm.
Mortality
Mortality was 83% (10 out of 12 patients) on the thalidomide arm compared to 30% (3 out of 10 patients) on the placebo arm, relative risk 2.78 (95% confidence interval 1.04 to 7.40; Analysis 1.1). This increased risk persisted after adjustment for other possible prognostic factors such as the simplified acute physiology score.
1.1. Analysis.
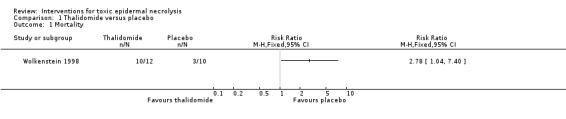
Comparison 1 Thalidomide versus placebo, Outcome 1 Mortality.
Loss of total body surface area
The progression of skin detachment did not show a difference at either day five or day seven between the groups.
Plasma TNF‐alpha concentration
The plasma TNF‐alpha concentration was found to be higher in the thalidomide group on day 2 (median value of 93 ng/L in the thalidomide group versus 36 ng/L in the placebo group), although the difference was not significant (Wilcoxon rank sum test p = 0.07).
Causes of death
The causes of death were as follows: multiple organ failure (treatment arm six, placebo arm two), septic shock (treatment arm five, placebo arm three), acute respiratory distress syndrome (treatment arm three, placebo arm none). Two out of 12 in the thalidomide group completed (survived) the study. In the placebo group seven out of ten completed (survived) the study.
Discussion
The available literature has many contradictory claims of both primary and supportive interventions. There were no controlled trials of supportive therapy and only one controlled trial of primary therapeutic intervention using thalidomide. This study, although well conducted, had to be terminated due to the very high mortality on the treatment arm. The reason for the excess mortality in the thalidomide group of patients in this study is still unclear, although the authors of the study have suggested some possible mechanisms. First, thalidomide might have increased mortality by impairing breathing through a central sedative effect. Second, a chemical messenger called TNF‐alpha, which becomes raised in the bloodstream during TEN, might be paradoxically overproduced by thalidomide ‐ a drug thought to prevent TNF‐alpha release. This hypothesis was partly supported by the study data.
The study emphasises the dangers of using therapeutic agents based on the study of known mechanisms only and underpins the need for high quality randomised controlled trials in this area using clinical markers (i.e. death, quality of life in survivors) rather than surrogate markers (e.g. blister TNF‐alpha levels) as outcome measures. Toxic epidermal necrolysis is a rare disease associated with a mortality as high as 30 to 45%. The rarity of the disease makes it difficult (but not impossible) to enrol adequate numbers of participants for randomised controlled trials to obtain conclusive answers. There are also ethical concerns of allocating patients to a treatment arm with possible benefit in a disease with high mortality.
This review did not find any good evidence to support the use of oral steroids, immunoglobulins or cyclosporin A which are commonly used to treat this condition. There are claims and counterclaims of effectiveness of various therapies and supportive treatments in the literature not substantiated by hard evidence. To date, there is no high quality evidence to help doctors, guide patients and the public on what is the most effective treatment for this potentially fatal disease.
Authors' conclusions
Implications for practice.
Only one RCT looking at effectiveness of treatments for TEN was available. Thalidomide treatment was not shown to be effective and was associated with significantly high mortality. There is no reliable evidence on commonly used interventions, such as systemic steroids, immunoglobulins and cyclosporin A, for treating TEN, a disease associated with high mortality.
Implications for research.
More research is required to determine the exact pathophysiology of TEN. Multi‐centre, carefully designed, randomised controlled trials are needed, especially to evaluate the effects of treatment with high‐dose oral steroids and intravenous immunoglobulins compared against best supportive care. Comparison of standard supportive care in an acute hospital versus care in a special burns unit might also be worthwhile especially if the components of these complex interventions can be adequately defined. Other interventions that might improve prognosis such as early withdrawal of the suspected drug, types of skin care dressing and the role of infection prophylaxis are worthy of further study. Such trials need to take into account the variable severity of the condition as baseline severity may be the largest predictor of outcome. Other research could focus on the management of people who survive the acute phase e.g. by evaluating the impact of different educational strategies for subsequent medication use and modalities to prevent long term complications such as ocular scarring.
What's new
| Date | Event | Description |
|---|---|---|
| 17 February 2015 | Amended | This review is going to be updated. We have written a published note to say that because the scope of the review has substantially expanded, a new protocol and then a new review will be written. |
History
Protocol first published: Issue 1, 1999 Review first published: Issue 4, 2002
| Date | Event | Description |
|---|---|---|
| 20 October 2008 | Amended | Converted to new review format. |
Notes
This review is being updated by way of a new protocol and then a review, as the scope of the review has substantially expanded.
Acknowledgements
The authors would like to thank Professor Hywel Williams and Dr Tina Leonard of the Cochrane Skin group, for their unwavering support.
The editorial base would like to thank the following people who were the external referees for this review: Sima Halevy and Nick Craven (content experts) and Kay Coulson (consumer).
Appendices
Appendix 1. Search strategy for MEDLINE (OVID)
This strategy was adjusted accordingly for other electronic databases.
Search strategy to locate RCTs
Search terms 1‐29, as given in the Cochrane Reviewers' Handbook, appendix 5c.2 (Clarke 2000):
Search strategy to locate toxic epidermal necrolysis
30. Epidermal Necrolysis, toxic/ or toxic epidermal necrolysis.mp. 31. Lyell's disease.mp. or Dermatitis, Exfoliative/ or Stevens‐Johnson Syndrome/ 32. erythema multiforme majus.mp. or Erythema Multiforme/ 33. 30 or 31 or 32
Search strategy to locate treatments
34. STEROIDS/ or steroid.mp. 35. Immunosuppressive Agents/ or immunosuppressant.mp. 36. AZATHIOPRINE/ or azathioprine.mp. 37. CYCLOPHOSPHAMIDE/ or cyclophosphamide.mp. 38. CYCLOSPORINE/ or cyclosporine.mp. 39. THALIDOMIDE/ or thalidomide.mp. 40. Immunoglobulins/ or immunoglobulin.mp. 41. exp THERAPEUTICS/ 42. or/34‐41
Search strategy to locate RCTs of therapeutic interventions for TEN
43. 29 and 33 and 42
Data and analyses
Comparison 1. Thalidomide versus placebo.
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mortality | 1 | Risk Ratio (M‐H, Fixed, 95% CI) | Totals not selected |
Characteristics of studies
Characteristics of included studies [ordered by study ID]
Wolkenstein 1998.
| Methods | parallel group. 3rd party telephone randomisation in blocks of 6, stratified to 2 centres. participant, clinician and outcome assessor blinded. | |
| Participants | 22 adults with TEN and 10 to 90% BSA skin detachment taken from dermatology, burns and intensive care units (total of 9 centres). | |
| Interventions | 2 x 100 mg twice daily thalidomide vs placebo capsule or NG tube feeding for 5 days. | |
| Outcomes | Death Change in % BSA skin detachment from day 0 to days 5 and 7 Change in SAPS from day 0 to days 5 and 7 |
|
| Notes | Trial terminated due to high mortality (13/22). Information in fig 2 conflicts with that given in fig 1 and the text. The authors have confirmed that fig 1 and the text is correct. | |
| Risk of bias | ||
| Bias | Authors' judgement | Support for judgement |
| Allocation concealment (selection bias) | Low risk | A ‐ Adequate |
SAPS ‐ simplified acute physiology score BSA ‐ body surface area
Contributions of authors
Link with editorial base (SM) Design search (SM) Identify relevant titles and abstracts from searches (SM & AT) Obtain copies of trials (SM & AT) Select which trials to include (SM & AT) Extract data from trials (SM & AT) Enter data into RevMan (AT) Draft final review (SM with contributions from AT & MM and the editorial base)
Declarations of interest
None
Edited (no change to conclusions)
References
References to studies included in this review
Wolkenstein 1998 {published and unpublished data}
- Wolkenstein P, Latarjet J, Roujeau JC, Duguet C, Boudeau S, Vaillant L, et al. Randomized comparison of thalidomide versus placebo in toxic epidermal necrolysis. Lancet 1998;352(9140):1586‐9. [DOI] [PubMed] [Google Scholar]
Additional references
Assier 1995
- Assier H. Bastuji‐Garin S, Revuz J, Roujeau JC. Erythema multiforme with mucous membrane involvement and Stevens‐Johnson syndrome are clinically different disorders with distinct causes. Archives of Dermatology 1995;131:540. [PubMed] [Google Scholar]
Bachot 2001
- Bachot N, Revuz J, Roujeau JC. Are intravenous immunoglobulins the treatment of Stevens‐Johnson syndrome or toxic epidermal necrolysis?. Allergologie 2001;24:214. [Google Scholar]
Bastuji‐Garin 1993
- Bastuji‐Garin S, Rzany B, Stern RS, Shear NH, Naldi L, Roujeau JC. Classification of cases of toxic epidermal necrolysis, Stevens‐Johnson syndrome, and erythema multiforme. Archives of Dermatology 1993;129(1):92‐6. [PubMed] [Google Scholar]
Bunger 1968
- Bunger P, Delventhal G. Azathioprinbehandlung bei einem schweren Krankheitsfall von "Lyell Syndrom". Zeitschrift für Haut und Geschlechtskrankheiten 1968;43:853‐60. [PubMed] [Google Scholar]
Clarke 2000
- Clarke M, Oxman AD. Optimal search strategy for RCTs. Cochrane Reviewer's Handbook 4.1 [updated June 2000]. Oxford: The Cochrane Collaboration, 2000:appendix 5c. [Google Scholar]
DerSimonian 1986
- DerSimonian R, Laird N. Meta analysis in clinical trials. Controlled Clinical Trials 1986;7:177‐88. [DOI] [PubMed] [Google Scholar]
Dolan 1989
- Dolan PA, Flowers FP, Sheretz EF. Toxic epidermal necrolysis. Journal of Emergency Medicine 1989;7(1):65‐9. [DOI] [PubMed] [Google Scholar]
Fine 1985
- Fine J. Management of acquired bullous skin diseases. New England Journal of Medicine 1995;133:1475‐84. [DOI] [PubMed] [Google Scholar]
Halebian 1986
- Halebian PH, Corder VJ, Madden MR, Finklestein JL, Shires GT. Improved burn centre survival of patients with toxic epidermal necrolysis managed without corticosteroids. Annals of Surgery 1986;204:503‐12. [DOI] [PMC free article] [PubMed] [Google Scholar]
Hedges 1985
- Hedges LV, Olkin I. Statistical methods for meta‐analysis. New York: Academic Press, 1985. [Google Scholar]
Heimbach 1987
- Heimbach DM, Engrav LH, Marvin JA, Harnar TA, Grube BJ. Toxic epidermal necrolysis: a step forward in treatment. Journal of the American Medical Association 1987;257:2171‐5. [PubMed] [Google Scholar]
Heng 1991
- Heng MYC, Allen SG. Efficacy of cyclophosphamide in toxic epidermal necrolysis: clinical and pathophysiological aspects. Journal of the American Academy of Dermatology 1991;25:778‐86. [DOI] [PubMed] [Google Scholar]
Kelly 1995
- Kelly JP, Auquier A, Rzany B, Naldi L, Bastuji‐Garin S, et al. An international collaborative case control study of severe cutaneous adverse reactions (SCAR). Journal of Clinical Epidemiology 1995;48(9):1099‐8. [DOI] [PubMed] [Google Scholar]
Lyell 1956
- Lyell A. Toxic epidermal necrolysis: an eruption resembling scalding of the skin. British Journal of Dermatology 1956;68:355‐61. [DOI] [PubMed] [Google Scholar]
Mockenhaupt 1996
- Mockenhaupt M, Schopf E. Epidemiology of drug induced severe skin reactions. Seminar in Cutaneous Medicine & Surgery 1996;15(4):236‐43. [DOI] [PubMed] [Google Scholar]
Mockenhaupt 1999
- Mockenhaupt M, Schröder W, Schlingmann J, Schneck B, Hering O, Schöpf E. Clinical re‐evaluation of erythema exsudativum multiforme majus and Stevens‐Johnson syndrome resulting in a different etiology. The Journal of Investigative Dermatology 1999;112:661. [Google Scholar]
Pruitt 1987
- Pruitt BA. Burn treatment for the unburned. Journal of the American Medical Association 1987;257:2207‐8. [PubMed] [Google Scholar]
Renfro 1989
- Renfro L, Grant‐Kels JM, Daman LA. Drug induced toxic epidermal necrolysis treated with cyclosporin. International Journal of Dermatology 1989;28:441‐4. [DOI] [PubMed] [Google Scholar]
Revuz 1987
- Revuz J, Penso D, Roujeau JC, Guillaume JC, Payne CR, Wechsler J, et al. Toxic epidermal necrolysis: clinical finding and prognosis factors in 87 patients. Archives of Dermatology 1987;123:1160‐65. [DOI] [PubMed] [Google Scholar]
Revuz 1996
- Revuz J, Roujeau JC. Advances in toxic epidermal necrolysis. Seminar in Cutaneous Medicine & Surgery 1996;15(4):258‐66. [DOI] [PubMed] [Google Scholar]
Roujeau 1995
- Roujeau JC, Kelly JP, Naldi L, Rzany B, Stern RS, Anderson T, et al. Medication use and the risk of Stevens‐Johnson syndrome or toxic epidermal necrolysis. New England Journal of Medicine 1990;333:1600‐7. [DOI] [PubMed] [Google Scholar]
Sakellariou 1991
- Sakellariou G, Koukoudis P, Karpouzas J, Alexopoulos E, Papadopoulou D, Chrisomalis F, et al. Plasma exchange (PE) treatment in drug‐induced toxic epidermal necrolysis (TEN). International Journal of Artificial Organs 1991;14(10):634‐8. [PubMed] [Google Scholar]
Schulz 1995
- Schulz KF, Chalmers I, Haynes RJ, Altman DG. Empirical evidence of bias: dimensions of methodological quality associated with estimates of treatment effects in controlled trials. Journal of the American Medical Association 1995;273:408‐12. [DOI] [PubMed] [Google Scholar]
Stables 1993
- Stables GI, Lever RS. Toxic epidermal necrolysis and systemic corticosteroids. British Journal of Dermatology 1993;128:357‐60. [DOI] [PubMed] [Google Scholar]
Viard 1998
- Viard I, Wehrli P, Bullani R, Schneider P, Holler N, Salomon D, et al. Inhibition of toxic epidermal necrolysis by blockade of CD95 with human intravenous immunoglobulin. Science 1998;282:490‐3. [DOI] [PubMed] [Google Scholar]
Villada 1990
- Villada G, Roujeau JC, Cordonnier C, Bagot M, Keuntz M, Wechsler J, et al. Toxic epidermal necrolysis after bone marrow transplantation: study of nine cases. Journal of the American Academy of Dermatology 1990;23:870‐5. [DOI] [PubMed] [Google Scholar]
Whitehead 1991
- Whitehead A, Whitehead J. A general parametric approach to the meta‐analysis of randomized clinical trials. Statistics in Medicine 1991;10:1665‐77. [DOI] [PubMed] [Google Scholar]


