Abstract
Background
The association between childhood diarrheal disease and linear growth faltering in developing countries is well described. However, the impact attributed to specific pathogens has not been elucidated, nor has the impact of recommended antibiotic treatment.
Methods
The Global Enteric Multicenter Study enrolled children with moderate to severe diarrhea (MSD) seeking healthcare at 7 sites in sub-Saharan Africa and South Asia. At enrollment, we collected stool samples to identify enteropathogens. Length/height was measured at enrollment and follow-up, approximately 60 days later, to calculate change in height-for-age z scores (ΔHAZ). The association of pathogens with ΔHAZ was tested using linear mixed effects regression models.
Results
Among 8077 MSD cases analyzed, the proportion with stunting (HAZ below −1) increased from 59% at enrollment to 65% at follow-up (P < .0001). Pathogens significantly associated with linear growth decline included Cryptosporidium (P < .001), typical enteropathogenic Escherichia coli (P = .01), and untreated Shigella (P = .009) among infants (aged 0–11 months) and enterotoxigenic E. coli encoding heat-stable toxin (P < .001) and Cryptosporidium (P = .03) among toddlers (aged 12–23 months). Shigella-infected toddlers given antibiotics had improved linear growth (P = .02).
Conclusions
Linear growth faltering among children aged 0–23 months with MSD is associated with specific pathogens and can be mitigated with targeted treatment strategies, as demonstrated for Shigella.
Keywords: Diarrhea, pathogens, growth faltering, stunting, children, antibiotics
Diarrheal disease is associated with linear growth faltering among young children [1]. Community-based studies in low-resource settings demonstrate an increasing risk of stunting at age 24 months with each diarrheal episode and each day of diarrhea before that age [2]. In turn, stunting is a risk factor for poor health and development [3]. Even mild stunting predicts an increased risk of death during the first 2 years of life [4].
Although most acute diarrhea is caused by infection, few studies have elucidated the impact of specific pathogens on growth, and a limited array of pathogens has been examined [5–8]. Moreover, trials designed to evaluate the efficacy of pathogen-specific treatment have used short-term clinical and bacteriologic cure as end points [9, 10], while the impact on growth has not been characterized.
The Global Enteric Multicenter Study (GEMS) is a prospective, matched case-control study of the burden, etiology, and adverse clinical outcomes of moderate to severe diarrhea (MSD) in children aged 0–59 months in sub-Saharan Africa and South Asia. GEMS found an association between MSD and linear growth faltering [11, 12]. In this study, we assessed pathogen-specific associations and whether antibiotic treatment of Shigella dysentery, according to World Health Organization (WHO) recommendations, improved growth outcomes.
MATERIALS AND METHODS
Study Design and Participants
For 36 months at each site, children aged 0–59 months were enrolled into GEMS at sites in Basse (The Gambia), Bamako (Mali), Manhiça (Mozambique), Siaya County (Kenya), Kolkata (India), Mirzapur (Bangladesh), and Karachi, Bin Qasim town (Pakistan), according to published methods [13–15]. To be eligible for enrollment, a child had to reside in the site’s demographic surveillance area, seek care at a study health center with diarrhea (defined as ≥3 abnormally loose stools in the previous 24 hours) that began in the previous 7 days, and meet ≥1 of the following criteria for MSD: sunken eyes, decreased skin turgor, visible blood in stool, or a clinician recommendation for intravenous rehydration or hospitalization. We aimed to enroll approximately 220 eligible children per site per year into each of 3 age groups: infants (0–11 months), toddlers (12–23 months), and young children (24–59 months). Children were eligible for reenrollment if a new episode of MSD developed after the 60-day follow-up visit [13]. For this report, only children with MSD were included. Although GEMS included a case-control component described elsewhere [12, 13], for this paper, only cases (children with MSD) were analyzed.
Data Collection
During the enrollment visit at the health center, all children underwent a standardized clinical assessment and anthropometric measurements and provided a stool sample to identify enteropathogens [13, 15]. Stool collection, transport, and pathogen identification methods have been described elsewhere [16]. Management of diarrhea at the health center, including antibiotic use, was documented. Approximately 60 days after enrollment (49–91 days), participants were visited at home for assessment of vital status and repeated anthropometric measurements.
Anthropometric Measurements
We measured standing height for children ≥2 years old, and recumbent length for younger children and those unable to stand unassisted, thrice to the nearest 0.1 cm, using a ShorrBoard measuring board and following a 2-person standardized measurement procedure [17]. The median of the 3 measurements was used to calculate the height-for-age z score (HAZ) [18, 19].
Before study initiation, staff at each site underwent anthropometry training followed by a standardization exercise to calculate intrarater and interrater (trainee vs instructor) variability [13]. Trainees who exceeded the acceptable measurement error (0.5 cm) at least half the time did not undertake field activities until retrained and deemed competent. A proficient member of each local team provided training and standardization exercises for newly hired staff and refresher training for existing staff every 4–6 months. The core team visited each site approximately twice annually to train, observe field activities, and review standardization results.
The study was approved by ethics committees at the University of Maryland, Baltimore, and each field site. Informed consent was obtained from the parent or guardian of each child before study procedures were performed.
Statistical Methods
Variable Definitions
Age was measured on a continuous scale, and analyses were stratified by age group. The HAZ was calculated at enrollment and follow-up, using the median of the 3 length/height measurements and age, according to WHO standards [19]. We defined stunting as HAZ below −1 and the degree of stunting as mild (HAZ <−1 and >/=−2), moderate (HAZ <−2 and >/=−3), or severe (HAZ<−3), at enrollment and follow-up.
Data Analysis
This analysis included children with MSD with both enrollment and follow-up measurements; implausible or inconsistent measurements between enrollment and follow-up were excluded (Supplementary Figure 1).
The primary outcome is change in linear growth (change in HAZ [ΔHAZ]), which was calculated for each child as the difference in HAZ from enrollment to follow-up. A negative change in linear growth is deemed growth faltering. We initially compared enrollment and follow-up HAZ within each age group (and study site), using a 1-sample t test for ΔHAZ and adjusted for individuals with multiple episodes of MSD during the study period (SAS proc surveymeans t test with CLUSTER statement). we compared the presence and degree of stunting at enrollment and follow-up, using a Wilcoxon signed-rank test.
Pathogens were tested for association with change in linear growth within each age group. We limited the analysis to pathogens that were (1) significantly associated with MSD in ≥4 sites [12] and/or (2) associated with increased risk of dying in a pooled analysis of children with MSD at all sites [12]. For the first criterion, we included, for all age groups, rotavirus, Cryptosporidium, Shigella, and enterotoxigenic E. coli (ETEC) encoding heat-stable toxin (ST-ETEC) with or without targets for heat-labile enterotoxin, and for the 2 youngest groups, adenovirus serotypes 40 and 41. For the second criterion, we also included typical enteropathogenic E. coli (tEPEC) in the analyses involving infants [12]. We used a linear mixed effects regression model (LMM) to examine the association of each of the above-named pathogens individually with change in linear growth among children with MSD by age group. In addition to the pathogen, the model also included HAZ at enrollment, age at enrollment in months, duration to follow-up in days, and study site.
Treatment of Shigella with a WHO-recommended antibiotic (ciprofloxacin, third-generation cephalosporins, azithromycin, or pivmecillinam) [20] could modify any potential association of Shigella with linear growth. We therefore examined the need for an interaction term between the presence and absence of Shigella with or without antibiotic treatment. The interaction term was retained if the associated P value was <.10. Pathogens associated (P < .10) with ΔHAZ in the individual models were combined into a single age-stratified model, which included the same confounding variables described previously for the individual models. Unless otherwise stated, associations were considered statistically significant at P < .05. SAS software, version 9.4 (SAS Institute), was used for all summary statistics and associated tests; STATA/SE software, version 17, was used to fit the LMMs.
RESULTS
Study Participants
Between 1 December 2007 and 3 March 2011, a total of 8077 MSD episodes in 7545 children were analyzed: 3408 were aged 0–11 months, 2741 aged 12–23 months, and 1928 aged 24–59 months. There were 1362 episodes excluded from analysis owing to missing measurements (63.2%), implausible values (17.8%), death before follow-up (14.0%), and follow-up visit outside the acceptable time window (5.0%) (Supplementary Figure 1). Children with excluded and included episodes had similar demographic features (Supplementary Table 1), except those excluded because of death had significantly lower enrollment HAZ than included children in 14 of 21 comparisons (3 age groups at 7 sites).
Mean enrollment HAZ in each age group, at every site, was <0 and became more negative with increasing age (Supplementary Table 2), while the proportion with stunting at enrollment increased with age (Table 1). A total of 58.7% of children were stunted at enrollment (mild stunting in 32.2%, moderate in 18.0%, and severe in 8.5%).
Table 1.
Degree of Stunting in Children With Moderate to Severe Diarrhea at Enrollment and at Follow-up, by Age Group
| Degree of Stunting at Follow-up, No. (%)a | |||||
|---|---|---|---|---|---|
| Degree of Stunting at Enrollment by Age Group | Total | None | Mild | Moderate | Severe |
| Age 0–11 mo | |||||
| Total | 3408 | 1414 (41.5) | 1093 (32.1) | 616 (18.1) | 285 (8.3) |
| None | 1727 (50.7) | 1302 (75.4) | 391 (22.6) | 32 (1.9) | 2 (0.1) |
| Mild | 1049 (30.8) | 104 (9.9) | 640 (61.0) | 291 (27.7) | 14 (1.3) |
| Moderate | 435 (12.7) | 8 (1.8) | 57 (13.1) | 257 (59.1) | 113 (26.0) |
| Severe | 197 (5.8) | 0 | 5 (2.5) | 36 (18.3) | 156 (79.2) |
| Age 12–23 mo | |||||
| Total | 2741 | 838 (30.6) | 951 (34.7) | 591 (21.6) | 361 (13.2) |
| None | 998 (36.4) | 774 (77.6) | 222 (22.2) | 2 (0.2) | 0 |
| Mild | 911 (33.2) | 63 (6.9) | 654 (71.8) | 191 (21.0) | 3 (0.3) |
| Moderate | 556 (20.3) | 1 (0.2) | 74 (13.3) | 374 (67.3) | 107 (19.2) |
| Severe | 276 (10.1) | 0 | 1 (0.4) | 24 (8.7) | 251 (90.9) |
| Age 24–59 mo | |||||
| Total | 1928 | 582 (30.2) | 647 (33.6) | 457 (23.7) | 242 (12.6) |
| None | 612 (31.7) | 528 (86.3) | 84 (13.7) | 0 | 0 |
| Mild | 642 (33.3) | 54 (8.4) | 509 (79.3) | 79 (12.3) | 0 |
| Moderate | 462 (24.0) | 0 | 54 (11.7) | 358 (77.5) | 50 (10.8) |
| Severe | 212 (11.0) | 0 | 0 | 20 (9.4) | 192 (90.6) |
aFollow-up visits were approximately 60 days after enrollment (range, 49–91 days). Stunting was significantly more severe at follow-up in all 3 age groups (P < .001; Wilcoxon signed-rank test). The degree of stunting was defined according to height-for-age z scores (HAZ), No stunting defined as HAZ ≥ −1, with mild stunting defined as HAZ between <−1 and >/+−2, moderate stunting as HAZ between <−2 and ≥−3, and severe stunting as HAZ below <−3.
The proportion of children with stunting was significantly higher at follow-up than at enrollment in every age group (Table 1 and Supplementary Table 2), with a difference of 9.2% in infants, 5.8% in toddlers, and 1.5% in young children. A total of 64.9% of children were stunted at follow-up (mild stunting in 33.3%, moderate in 20.6%, and severe in 11.0%).
Pathogens Associated With Linear Growth Faltering
In the individual-pathogen analyses for infants, the presence of Cryptosporidium or tEPEC was significantly associated with a greater decline in linear growth than in those without either pathogen (difference in ΔHAZ, −0.09 for Cryptosporidium [95% confidence interval (CI), −.14 to −.04; P < .001] and −0.08 for tEPEC [−.15 to −.02; P = .01]) (Table 2). In addition, the interaction between Shigella and WHO-recommended antibiotics was statistically significant (P = .01). Shigella episodes not treated with antibiotics resulted in a greater decline in linear growth compared with treated episodes (difference in ΔHAZ, −0.16 [95% CI, −.29 to −.03]; P = .02), while Shigella episodes treated with antibiotics were not associated with positive or negative linear growth. These 3 pathogens, including the interaction term, were included in a final fully adjusted model. Rotavirus and ST-ETEC were not associated with linear growth faltering among infants.
Table 2.
Change in Linear Growth Between Enrollment and Follow-up in Children From 7 Global Enteric Multicenter Study Sites, by Age Group, Using Individual- and Multiple-Pathogen Models
| Individual-Pathogen Model | Multiple-Pathogen Model | ||||
|---|---|---|---|---|---|
| Pathogen by Age Group | Episodes With pathogen, No. (%) | Difference in ΔHAZ (95% CI) | P Value | Difference in ΔHAZ (95% CI) | P Value |
| Age 0–11 mo (n = 3408) | |||||
| Cryptosporidium | 525 (15.4) | −0.09 (−.14 to −.04) | <.001 | −0.09 (−.14 to −.04) | <.001 |
| Shigella, not treated with antibiotic | 72 (2.1) | −0.16 (−.29 to −.03) | .02 | −0.17 (−.31 to −.04) | .009 |
| Shigella, treated with antibiotic | 93 (2.7) | 0.05 (−.07 to .17) | .38 | 0.05 (−.07 to .17) | .41 |
| Typical EPEC | 304 (8.9) | −0.08 (−.15 to −.02) | .01 | −0.08 (−.15 to −.02) | .01 |
| Rotavirus | 859 (25.2) | 0.02 (−.02 to .06) | .40 | … | … |
| ST-ETEC | 203 (6.0) | 0.006 (−.07 to .08) | .89 | … | … |
| Adenovirus 40/41 | 104 (3.0) | −0.06 (−.17 to .05) | .27 | … | … |
| Age 12–23 mo (n = 2741) | |||||
| Cryptosporidium | 312 (11.4) | −0.05 (−.09 to −.003) | .04 | −0.05 (−.09 to −.005) | .03 |
| Shigella, not treated with antibiotic | 159 (5.8) | −0.06 (−.12 to .006) | .08 | −0.06 (−.12 to .001) | .054 |
| Shigella, treated with antibiotic | 282 (10.3) | 0.07 (0.01 to 0.13) | .02 | 0.06 (.009 to .13) | .02 |
| Rotavirus | 492 (18.0) | −0.04 (−.07 to .002) | .06 | … | … |
| ST-ETEC | 199 (7.3) | −0.12 (−.17 to −.06) | <.001 | −0.12 (−.17 to −.06) | <.001 |
| Adenovirus 40/41 | 76 (2.8) | 0.008 (−.08 to .09) | .86 | … | … |
Abbreviations: ΔHAZ, change in height-for-age z scores; CI, confidence interval; ST-ETEC, enterotoxigenic Escherichia coli encoding heat-stable toxin.
Among toddlers, Cryptosporidium was associated with a greater decline in linear growth compared with those without Cryptosporidium (difference in ΔHAZ, −0.05 [95% CI, −.09 to −.003]; P = .04) (Table 2), with the difference in ΔHAZ very similar to that in infants. The presence of rotavirus or ST-ETEC also resulted in a greater decline in linear growth (difference in ΔHAZ, −0.04 for rotavirus [95% CI, −.07 to −.002; P = .06] and −0.12 for ST-ETEC [95% CI, −.17 to −.06; P < .001]). The result for rotavirus was not statistically significant but met our criteria for inclusion in the final adjusted model. The interaction term between Shigella and antibiotic treatment was significant (P = .003), and antibiotic treatment improved linear growth in toddlers with shigellosis (difference in ΔHAZ 0.07 [95% CI, .01 to .13]; P = .02). Shigella not treated with antibiotics resulted in further declines in linear growth (difference in ΔHAZ, −0.06 [95% CI, −.12 to .006]; P = .08). Adenovirus 40/41 was not associated with linear growth among children with MSD aged 12–23 months.
Each of Cryptosporidium, rotavirus, ST-ETEC, and the interaction between Shigella and antibiotic treatment were included in a final model; however, rotavirus was subsequently excluded from the final model because the association was not statistically significant. For those pathogens remaining in the final model, the results of this pathogen-adjusted analysis were very similar to those of the individual-pathogen analyses (Table 2). None of the individual pathogens were associated with linear growth faltering in the oldest age group (24−59 months).
Antibiotic Prescribing Practices and Susceptibility Patterns
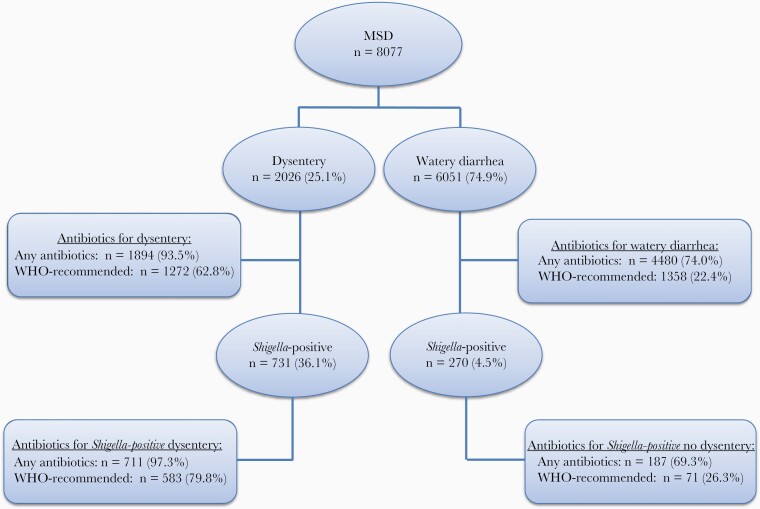
In an ad hoc analysis, we further explored the observed association between linear growth faltering and failure to treat Shigella with antibiotics recommended by WHO for dysentery. For context, we first examined antibiotic prescribing practices for Shigella dysentery (Figure 1 and Supplementary Table 3) and the susceptibility patterns of offending strains.
Figure 1.
Distribution of diarrhea episodes included in the analysis according to the presence of dysentery, Shigella isolation, and antibiotic treatment. Abbreviation: MSD, moderate to severe diarrhea; WHO, World Health Organization.
Of all MSD episodes in children, 2026 (25.1%) had dysentery; 1894 (93.5%, range 79.9% [Pakistan] to 99.6% [Bangladesh]) were prescribed antibiotics. The most common antibiotics were ciprofloxacin, trimethoprim-sulfamethoxazole, and metronidazole (59.9%, 22.6%, and 11.6%, respectively). A WHO-recommended antibiotic was prescribed for 1272 dysentery episodes (62.8%) and for 583 Shigella-positive dysentery episodes (79.8%) (Figure 1 and Supplementary Table 3).
There was considerable regional diversity in antibiotic prescribing practices. A WHO-recommended antibiotic was prescribed for 614 of the 621 Shigella dysentery episodes in Asia (98.9%), but for only 20 of the 110 episodes at the 4 African sites (18.1%). Ciprofloxacin was the most prescribed antibiotic at the Asian sites, including India (77.8%), Bangladesh (86.5%), and Pakistan (78.9%), but it was a rare choice in Africa. At the African sites, the most commonly prescribed antibiotic was trimethoprim-sulfamethoxazole in The Gambia (60.0%), Mali (60.0%), and Kenya (40.5%), and nalidixic acid in Mozambique (87.5%).
Virtually all (≥99%) Shigella strains were susceptible to ciprofloxacin in The Gambia, Mali, Mozambique, Kenya, and Pakistan, compared with 87.0% in Bangladesh and only 35.4% in India. At the African sites, >80% of Shigella isolates were resistant to trimethoprim-sulfamethoxazole, while 85% were susceptible to nalidixic acid in Mozambique.
Impact of Antibacterial Treatment of Shigellosis on Linear Growth
Shigella was cultured in 731 of the dysentery episodes (36.1%) and 270 watery diarrhea episodes (4.5%) (Figure 1). A WHO-recommended antibiotic was prescribed for 583 Shigella-positive dysentery episodes (79.8%) and 71 Shigella-positive watery diarrhea episodes (26.3%) (Figure 1). Analysis of the impact of antibiotic treatment of shigellosis on linear growth was performed separately for Shigella-positive watery diarrhea and dysentery (Table 3).
Table 3.
Linear Growth After Episodes of Shigella-Positive Dysentery or Watery Diarrhea, According to Whether World Health Organization–Recommended Antibiotic Treatment Was Prescribed
| Prescription Status by Age Groupa | ||||||
|---|---|---|---|---|---|---|
| 0–11 mo | 12–23 mo | 24–59 mo | ||||
| Shigella-Positive Diarrhea or Dysentery | Prescribed | Not Prescribed | Prescribed | Not Prescribed | Prescribed | Not Prescribed |
| Watery diarrhea | n = 11 | n = 45 | n = 29 | n = 92 | n = 31 | n = 62 |
| ΔHAZ (95% CI)b | −0.40 (−.63 to −.17) | −0.44 (−.57 to −.31) | −0.16 (−.36 to .04) | −0.25 (−.34 to −.16) | −0.15 (−.27 to −.02) | −0.13 (−.21 to −.05) |
| Dysentery | n = 82 | n = 26 | n = 253 | n = 67 | n = 248 | n = 55 |
| ΔHAZ (95% CI)b | −0.21 (−.28 to −.13) | −0.47 (−.72 to −.23) | −0.10 (−.18 to .03) | −0.37 (−.48 to −.26)c | −0.12 (−.15 to −.08) | −0.14 (−.28 to −.009) |
Abbreviations: ΔHAZ, change in height-for-age z scores; CI, confidence interval.
aPrescription of a World Health Organization–recommended antibiotic for dysentery (ciprofloxacin, third-generation cephalosporin, azithromycin, or pivmecillinam).
bAssessed using linear regression, controlling for age, site, enrollment height-for-age z score (HAZ), and days to follow-up visit.
c P = .03.
In toddlers, whose incidence of Shigella-positive MSD was almost double that of the other 2 age groups [12], an approximately 4-fold reduction in linear growth faltering was observed among Shigella-positive dysentery treated with WHO-recommended antibiotics, compared with untreated children (ΔHAZ, −0.10 [95% CI, −.18 to .03] vs −0.37 [−.48 to −.26]; P = .03). A similar trend was observed among infants with Shigella-positive dysentery and toddlers with Shigella-positive watery diarrhea, but the differences did not reach statistical significance with the small sample sizes (Table 3).
To address whether antibiotics exert a nonspecific growth-promoting effect on children with diarrhea, we compared ΔHAZ among children with MSD associated with rotavirus (n = 1493) or Cryptosporidium (n = 945) who were offered ciprofloxacin, third-generation cephalosporins, azithromycin, or pivmecillinam with those who were not offered these antibiotics. Overall, 33.0% of rotavirus-positive and 32.0% of Cryptosporidium-positive children received 1 of these antibiotics, but no significant associations with linear growth were observed.
DISCUSSION
We previously demonstrated that among children 0–59 months of age living in low-resource settings in South Asia and sub-Saharan Africa, an episode of MSD was associated with an increased risk of stunting over the ensuing 2–3 months. Now we present findings involving a broad array of pathogens indicating that 4 pathogens (Cryptosporidium, tEPEC, Shigella, and ST-ETEC) exert the largest negative effect on linear growth among infants and toddlers. We found that the risk associated with Shigella was largely limited to episodes not treated with WHO-recommended antibiotics.
To our knowledge, this is the first study to demonstrate that antibiotic treatment of dysentery according to WHO guidelines significantly ameliorates the linear growth impairment associated with Shigella infection, and in toddlers also augments growth. Previous clinical trials of children with shigellosis examined the short-term benefits of antibiotics (resolution of diarrheal disease symptoms and fecal shedding) but did not evaluate the impact on linear growth faltering, widely believed to be a proxy for mortality rate and poor health outcomes in the longer term. We observed a statistically significant improvement in linear growth among children with Shigella-positive dysentery with WHO-recommended antibiotic in the 12–23-month age group, and a similar trend among infants with Shigella-positive dysentery and toddlers with Shigella-positive watery diarrhea that did not reach statistical significance.
Regional differences in executing WHO guidelines were apparent. Although most Asian sites administered recommended first-line therapy with ciprofloxacin, 20% of episodes were missed, and the escalating prevalence of ciprofloxacin resistance threatens the efficacy of recommended treatment, particularly in India. The African sites provided treatment to only 20% of Shigella-positive dysentery episodes, but in most cases Shigella was resistant to the antibiotic chosen (trimethoprim-sulfamethoxazole). The prevalence of resistance to trimethoprim-sulfamethoxazole precludes its routine use unless supported by local susceptibility patterns.
Our findings corroborate and expand results of studies in Peru [6] and Guinea Bissau [7], which suggested that Cryptosporidium infection in infancy imparted a lasting adverse effect on linear growth. Our group previously reported that this risk period extends to include the second year of life, when Cryptosporidium was strongly associated with MSD at all GEMS sites, regardless of HIV prevalence [12], and was associated with death during the approximately 60 days after an MSD episode in toddlers. Our current findings suggest that the impact of Cryptosporidium on mortality rates may be linked to its considerable nutritional insult, as measured by a negative ΔHAZ. Strategies for point-of-care diagnosis and identification of appropriate therapeutic agents for case management of cryptosporidiosis in low-resource settings are needed and should be evaluated for their impact on growth, and if possible, survival.
Our findings also demonstrate an association between ST-ETEC diarrhea and linear growth faltering during the second year of life. Previous studies of the relationship between ST-ETEC and stunting have conflicting results. One study of children aged 3–48 months in rural Bangladesh demonstrated a significant association between the percentage of days with ETEC diarrhea during 60-day intervals and failure to gain weight, but not an association with impeded linear growth [5]. Another study involving a birth cohort followed up for 2 years in urban Bangladesh found that stunted or undernourished children aged 12–24 months were significantly more likely to have experienced ETEC diarrhea than those who were not stunted [21].
Our analysis differs from previous studies in that it distinguishes ST-ETEC from the less virulent ETEC pathotype encoding only LT, and we limited enrollment to more clinically severe forms of diarrheal illness. In addition, we examined linear growth over a relevant time frame (2–3 months after MSD onset) and demonstrated the impact of ST-ETEC relative to other pathogens that were significantly associated with MSD. Nonetheless, the observed effect of ST-ETEC infection on linear growth was somewhat unexpected, since ETEC is often considered a self-limited secretory diarrhea, which, in animal models [22] and human challenge studies, caused little or no inflammation or alteration of intestinal integrity that might interfere with growth [23, 24]. Other data, however, suggest that ST-ETEC can elicit an inflammatory response involving interleukin 8 expression [25, 26]. Peruvian children younger than 2 years with ETEC infection had fecal leukocytes in their diarrheal stools [27].
Several limitations of this study are worthy to mention. First, unmeasured events between enrollment and follow-up may have influenced growth, so it is notable that children with MSD grew significantly less during follow-up than their matched controls, despite having comparable HAZ at enrollment [12]. Second, it is possible that the detrimental effects of growth faltering after MSD can be overcome by catch-up growth. Even if that were the case, the 2–3 months after MSD onset was a particularly vulnerable period when mortality rates among children with MSD were 8.5-fold higher than among matched controls [12]. We recognize that observational studies are suboptimal for evaluating the impact of antibiotics against Shigella-associated linear growth faltering; however, the inclusion of objective end points (height/length, predefined diarrhea and dysentery, and findings of fecal microbiology) mitigate these concerns. Finally, we used directly observed inpatient administration of antibiotics and/or prescription of antibiotics as a proxy for antibiotic use and were unable to measure compliance.
In conclusion, our findings suggest that prevention or treatment of infection with 4 pathogens (Cryptosporidium, ST-ETEC, tEPEC, and Shigella) may reduce the burden of linear growth faltering in children. The adverse effects of Shigella were mitigated by administration of WHO-recommended antibiotics; however, these antibiotics were prescribed suboptimally in only 63% of dysentery episodes, and in several sites antibiotic practices did not match local susceptibility patterns. We recognize that antibiotic use has been associated with increasing resistance of Shigella to antibiotics, which globally could leave few options for effective therapy [28]. Accordingly, WHO has declared antibiotic-resistant Shigella to be a serious threat [29]. It seems prudent that for benefit to exceed risk, treatment of shigellosis should be judicious, guided by susceptibility data when possible, and directed toward individuals at risk for severe disease or complications. Because stunting is prevalent worldwide and directly associated with poor outcomes, interventions with a relatively small but significant effect have the potential to benefit many children’s lives.
Supplementary Material
Notes
Acknowledgments. We thank the families who participated in these studies, the project field staff for their hard work and dedication, and the physicians and administration at every site who generously provided facilities for the conduct of the study. We also thank Irwin J. Shorr for anthropometry training and development of anthropometry training materials, Annemieke Van ejik and Lynette Berkeley for their role in clinical and microbiologic data collection, Leslie Jamka for reviewing the manuscript, and Rebecca J. Stoltzfus for her advice on analysis and review of the manuscript.
Author contributions. M. M. L. conceived the project and acquired the grant funds. J. P. N., M. M. L., and K. L. K. designed the protocol. D. N., with W. C. B., H. S., Y. W., A. R., U. R., H. P., Y. L., and K. L. K., did the statistical analysis. D. N., T. H. F., S. P., D. Saha, M. J. H., S. O. S., R. F. B., D. Sur, A. S. G. F., A. K. M. Z., R. A. A., C. E. O. R., E. D. M., J. P. N., and K. L. K. planned and supervised the study. D. Sanogo, S. K., R. O., S. K. D., S. A., F. Q., T. N., and Q. B. coordinated clinical data collection; B. T., T. R., J. B. O., J. O. O., S. Q., M. A., I. M., did the laboratory assays; and K. B., M. J. H., U. O., and B. M. participated in data management. D. N., W. C. B., Y. W., T. H. F., J. P. N., M. M. L., and K. L. K. had full access to all the data in the study and did data analysis. D. N. and K. L. K. wrote the manuscript with input from all authors and had final responsibility for the decision to submit for publication. All authors reviewed the draft and approved the decision to submit for publication.
Financial support. This work was supported by the Bill & Melinda Gates Foundation.
Potential conflicts of interest. All authors: No reported conflicts. All authors have submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Conflicts that the editors consider relevant to the content of the manuscript have been disclosed.
References
- 1. Richard SA, Black RE, Gilman RH, et al. ; Childhood Malnutrition and Infection Network. Diarrhea in early childhood: short-term association with weight and long-term association with length. Am J Epidemiol 2013; 178:1129–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Checkley W, Buckley G, Gilman RH, et al. ; Childhood Malnutrition and Infection Network. Multi-country analysis of the effects of diarrhoea on childhood stunting. Int J Epidemiol 2008; 37:816–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Hoddinott J, Behrman JR, Maluccio JA, et al. Adult consequences of growth failure in early childhood. Am J Clin Nutr 2013; 98:1170–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Olofin I, McDonald CM, Ezzati M, et al. ; Nutrition Impact Model Study (anthropometry cohort pooling). Associations of suboptimal growth with all-cause and cause-specific mortality in children under five years: a pooled analysis of ten prospective studies. PloS One 2013; 8:e64636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Black RE, Brown KH, Becker S. Effects of diarrhea associated with specific enteropathogens on the growth of children in rural Bangladesh. Pediatrics 1984; 73:799–805. [PubMed] [Google Scholar]
- 6. Checkley W, Epstein LD, Gilman RH, Black RE, Cabrera L, Sterling CR. Effects of Cryptosporidium parvum infection in Peruvian children: growth faltering and subsequent catch-up growth. Am J Epidemiol 1998; 148:497–506. [DOI] [PubMed] [Google Scholar]
- 7. Mølbak K, Andersen M, Aaby P, et al. Cryptosporidium infection in infancy as a cause of malnutrition: a community study from Guinea-Bissau, west Africa. Am J Clin Nutr 1997; 65:149–52. [DOI] [PubMed] [Google Scholar]
- 8. Qadri F, Ahmed T, Ahmed F, et al. Mucosal and systemic immune responses in patients with diarrhea due to CS6-expressing enterotoxigenic Escherichia coli. Infect Immun 2007; 75:2269–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Traa BS, Walker CL, Munos M, Black RE. Antibiotics for the treatment of dysentery in children. Int J Epidemiol 2010; 39(suppl 1):i70–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kabir I, Butler T, Khanam A. Comparative efficacies of single intravenous doses of ceftriaxone and ampicillin for shigellosis in a placebo-controlled trial. Antimicrob Agents Chemother 1986; 29:645–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Levine MM, Kotloff KL, Nataro JP, Muhsen K. The Global Enteric Multicenter Study (GEMS): impetus, rationale, and genesis. Clin Infect Dis 2012; 55(suppl 4):S215–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kotloff KL, Nataro JP, Blackwelder WC, et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): a prospective, case-control study. Lancet 2013; 382:209–22. [DOI] [PubMed] [Google Scholar]
- 13. Kotloff KL, Blackwelder WC, Nasrin D, et al. The Global Enteric Multicenter Study (GEMS) of diarrheal disease in infants and young children in developing countries: epidemiologic and clinical methods of the case/control study. Clin Infect Dis 2012; 55(suppl 4):S232–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Blackwelder WC, Biswas K, Wu Y, et al. Statistical methods in the Global Enteric Multicenter Study (GEMS). Clin Infect Dis 2012; 55(suppl 4):S246–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Panchalingam S, Antonio M, Hossain A, et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis 2012; 55(suppl 4):S294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Panchalingam S, Antonio M, Hossain A, et al. Diagnostic microbiologic methods in the GEMS-1 case/control study. Clin Infect Dis 2012; 55(suppl 4):S294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. United Nations. How to weigh and measure children: assessing the nutritional status of young children in household surveys: preliminary version. New York, NY: United Nations, Department of Technical Co-operation for Development and Statistical Office, 1986. Available at: https://digitallibrary.un.org/record/145378?ln=en. Accessed 15 January 2021. [Google Scholar]
- 18. World Health Organization. WHO child growth standards. Geneva, Switzerland: World Health Organization; 2006. city of publication: Geneva, Switzerland publisher: World Health Organization. Available at: https://apps.who.int/iris/handle/10665/43413. Accessed 10 January 2021.
- 19. World Health Organization. WHO Anthro for personal computer. Version 3.2.2. Geneva, Switzerland: World Health Organization; 2011. Available at: https://www.who.int/tools/child-growth-standards/software. Accessed 10 January 2021. [Google Scholar]
- 20. World Health Organization. Guidelines for the control of shigellosis, including epidemics due to Shigella dysenteriae type 1. Geneva, Switzerland: World Health Organization, 2005. Available at: https://apps.who.int/iris/handle/10665/43252. Accessed 20 February 2021. [Google Scholar]
- 21. Qadri F, Saha A, Ahmed T, Al Tarique A, Begum YA, Svennerholm AM. Disease burden due to enterotoxigenic Escherichia coli in the first 2 years of life in an urban community in Bangladesh. Infect Immun 2007; 75:3961–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. DuPont HL, Formal SB, Hornick RB, et al. Pathogenesis of Escherichia coli diarrhea. N Engl J Med 1971; 285:1–9. [DOI] [PubMed] [Google Scholar]
- 23. Harris JC, Dupont HL, Hornick RB. Fecal leukocytes in diarrheal illness. Ann Intern Med 1972; 76:697–703. [DOI] [PubMed] [Google Scholar]
- 24. Levine MM, Caplan ES, Waterman D, Cash RA, Hornick RB, Snyder MJ. Diarrhea caused by Escherichia coli that produce only heat-stable enterotoxin. Infect Immun 1977; 17:78–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Huang DB, DuPont HL, Jiang ZD, Carlin L, Okhuysen PC. Interleukin-8 response in an intestinal HCT-8 cell line infected with enteroaggregative and enterotoxigenic Escherichia coli. Clin Diagn Lab Immunol 2004; 11:548–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Roselli M, Finamore A, Britti MS, et al. The novel porcine Lactobacillus sobrius strain protects intestinal cells from enterotoxigenic Escherichia coli K88 infection and prevents membrane barrier damage. J Nutr 2007; 137:2709–16. [DOI] [PubMed] [Google Scholar]
- 27. Mercado EH, Ochoa TJ, Ecker L, et al. Fecal leukocytes in children infected with diarrheagenic Escherichia coli. J Clin Microbiol 2011; 49:1376–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Kotloff KL, Riddle MS, Platts-Mills JA, Pavlinac P, Zaidi AKM. Shigellosis. Lancet 2018; 391:801–12. [DOI] [PubMed] [Google Scholar]
- 29. World Health Organization. Global priority list of antibiotic-resistant bacteria to guide research, discovery, and development of new antibiotics. Geneva, Switzerland: World Health Organization. Available at: https://www.who.int/medicines/publications/WHO-PPL-Short_Summary_25Feb-ET_NM_WHO.pdf. Accessed 20 March 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.