Abstract
We enrolled 98 infants (gestational age <33 weeks) in a pilot randomized trial of antibiotics vs no antibiotics; 55 were randomized (lower maternal infectious risk; symptoms expected for gestation). Adverse events did not differ significantly between the randomization arms. This trial establishes a framework for a larger multicentered trial.
Trial registration
Keywords: microbiome, infants, sepsis, dysbiosis
Antibiotics are routinely used in symptomatic preterm infants after birth for presumed early onset sepsis without clear evidence to guide this practice. Observational studies support an association between routine early antibiotic use in preterm infants and increased risk for morbidities.(1–4) Despite concerns for these morbidities and data showing low rates of culture-confirmed early onset sepsis, most preterm infants are treated with antibiotics in the first days after birth as a standard of care.(5, 6, 7) This practice is based on the hypothesis that preterm deliveries may be precipitated by an infection and that it may be difficult to distinguish between symptoms such as respiratory distress related to prematurity and early onset sepsis. Emerging evidence from our group (8–11) and others (12, 13) has shown that necrotizing enterocolitis (NEC) and late onset sepsis (14) are preceded by intestinal dysbiosis characterized by shifts in bacterial taxa associated with antibiotic use.(15) Furthermore, recent literature has described a connection between intestinal microbial and fungal dysbiosis and antibiotics and lung inflammation.(16–18) Thus most infants born at <33 weeks of gestation are exposed soon after birth to a therapy whose risks may outweigh benefits. Systematic studies that challenge the current therapeutic approach and provide evidence in support or contradict this practice are needed. Here, we present the results of our pilot study randomizing low risk preterm infants with symptoms of prematurity to antibiotics vs no antibiotics after birth.
Methods:
Following multiple discussions with the Neonatology group at the University of Florida (UF), consensus was achieved for the safety of the study protocol, group allocation, and laboratory evaluation. Routine Early Antibiotic use in SymptOmatic preterm Neonates (REASON) was an un-blinded, randomized, pragmatic, pilot clinical trial at a single academic center. REASON was designed to evaluate the feasibility and safety of a larger multicenter trial evaluating the risks and benefits of current practices around the prescribing of antibiotics for preterm infants. The trial was registered at ClinicalTrials.gov: NCT02784821.
Participants.
The planned enrollment was 300 infant-mother dyads (150 infants) during a one-year study period. Per the institutional review board (IRB) policy mothers are enrolled separately if any information from their medical records is to be collected. Written consents for infants and mothers were obtained prenatally or immediately after birth in eligible infants. All infants born at <33 weeks of gestation and admitted to the UF NICU without major congenital anomalies affecting viability were eligible.
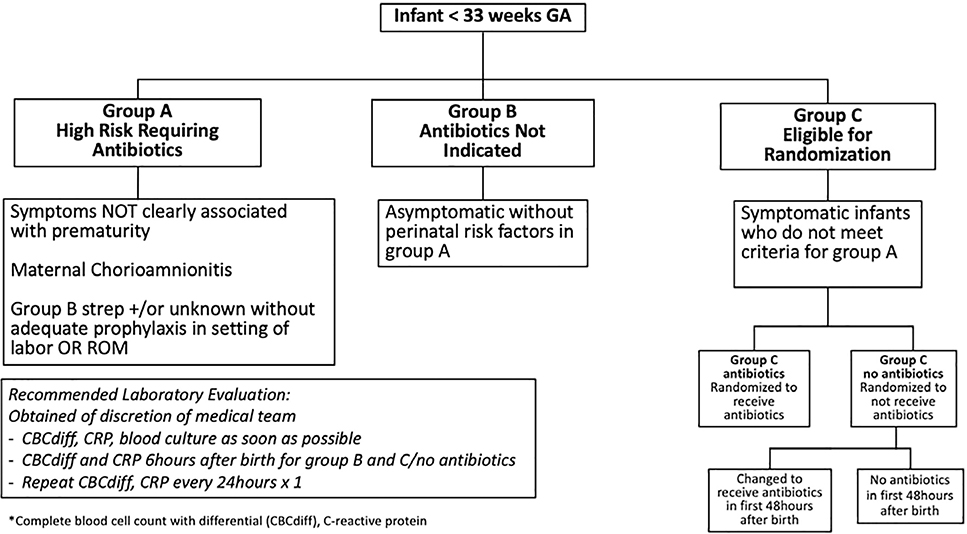
Enrolled infants were assigned to one of three groups based on the infant’s risk of infection and neonatal symptoms: Group A, B, or C. Group allocation was established by the research team after reviewing the maternal and infant charts and discussing the infant’s status with the bedside clinician (Figure 1; available at www.jpesd.com).
Figure 1.
Study Algorithm
Group A consisted of newborn infants with symptoms not clearly associated with prematurity or who were at high risk for infection (maternal chorioamnionitis or colonization with group B streptococcus without prophylaxis). Infants in this group were treated with antibiotics and they were not randomized. Group B consisted of asymptomatic infants at low risk for infection. Antibiotics were not indicated in this group and infants were not randomized.
Group C consisted of infants eligible for randomization. These infants had symptoms associated with prematurity (respiratory distress requiring respiratory support, apnea or bradycardia, ineffective thermoregulation, or hypoglycemia) that historically had been considered indications for treatment with antibiotics after birth. These infants did not meet criteria for Group A and were randomized to either Group C/antibiotics (antibiotics prescribed at birth) or Group C/no antibiotics (no antibiotics prescribed at birth).
Participants were randomized within one hour of birth by block randomization using random block sizes of two and four created in SAS and uploaded in REDCap: Research Electronic Data Capture for the use of the study team. The randomization group was disclosed to the bedside clinician who would order the antibiotics for infants assigned to Group C/antibiotics. The intent was that infants in Group C/antibiotics would receive antibiotics for 48 hours assuming negative cultures.
Risks of intervention and safety mechanisms.
Infants assigned to receive antibiotics immediately after birth if symptomatic (Group A or C/antibiotics) or not receiving antibiotics when asymptomatic (Group B) were exposed to no additional risk due to study enrollment. Symptomatic infants randomized to Group C/no antibiotics could have potentially been exposed to higher risk of infection, morbidity, and mortality. To minimize the risks, the study team recommended obtaining our standard NICU laboratory tests, including blood culture, complete blood cell count with differential, and C-reactive protein in all infants. The medical team was allowed to prescribe antibiotics for infants in Group C/no antibiotics or Group B, based on their clinical judgment.
Outcomes.
The primary outcome was defined prospectively as a composite outcome of late onset sepsis (defined as culture positive infection >48 hours after birth), bronchopulmonary dysplasia (BPD) (defined as oxygen requirement at 36 weeks corrected gestation), NEC (defined as Bell’s stage II or greater), and death. Secondary outcomes were defined as early onset sepsis (defined as culture positive infection in the first 48 hours after birth), intraventricular hemorrhage, periventricular leukomalacia, retinopathy of prematurity and spontaneous intestinal perforation. These outcomes were assessed by review of the medical record at time of discharge from the NICU.
Data from both mothers and infants were collected from electronic medical record system and transferred into REDCap.
A sample size of 50 per group was planned to obtain sufficiently precise rates of anticipated admission to the NICU, consent, and estimates of clinical outcomes to plan a larger trial. Our enrollment goal of 300 infant-mother dyads was not achievable during the study period due to a lower than expected admission rate of infants born at <33 weeks of gestation to the UF neonatal intensive care unit (NICU). We reduced our goal to 200 infant-mother dyads (100 infants). Descriptive statistics; means and rates, were calculated, with corresponding 95% confidence intervals (CIs) to inform plausible ranges of the feasibility and safety outcomes of interest. Statistical comparisons among groups were made via t-tests, ANOVA and chi-square tests for continuous and categorical data, respectively. Our primary focus was on the comparison of the two randomized groups (Groups C/antibiotics and C/no antibiotics). Odds ratios (and 95% CIs) were calculated to compare adverse event rates between the randomized groups. Data management and descriptive analyses were conducted using SAS 9.4 (Cary, NC). Group C infants were analyzed as intention to treat. A secondary post-hoc analysis of group C, compared outcomes in infants who received vs did not receive antibiotics in the first 48 hours after birth.
The study protocol was approved by the IRB at UF on 9/2016, enrollment occurred from 01/2017–01/2019. The study was paused for a full IRB board review due to high number of adverse outcomes and resumed given that the incidence of adverse events was similar to the national standard as shown by Vermont Oxford Network data. There were 46 reportable events during the course of the study.
Results:
Enrollment and Outcome Data.
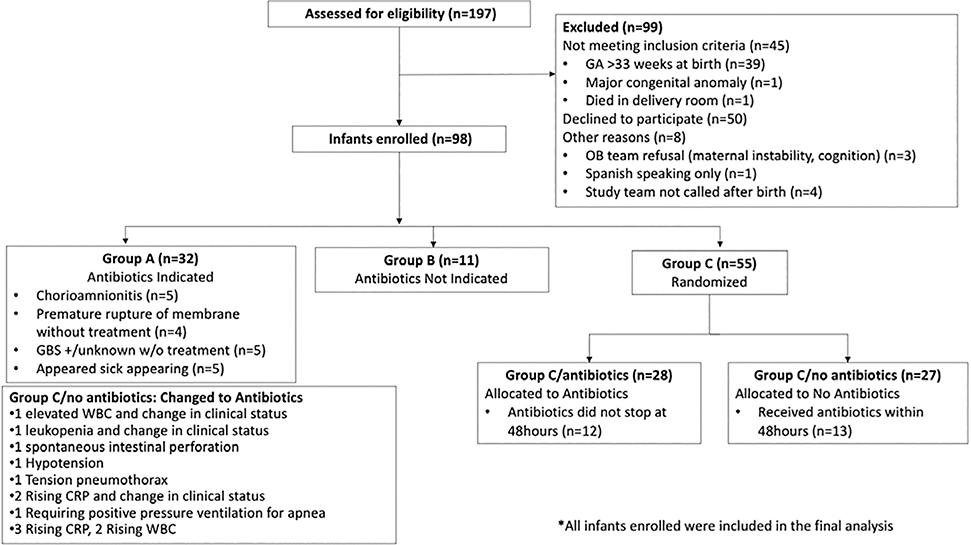
Ninety-eight infants and 88 mothers were enrolled (Figure 2). The baseline characteristics in the randomized groups (Group C/antibiotics and Group C/no antibiotics) were similar (Table I; available at www.jpeds.com). Twenty-seven infants were assigned to Group C/no antibiotics and 13 of these infants (48%) received antibiotics within the first postnatal 48h. Initiation of antibiotics was triggered by a change in clinical status or abnormal labs (Figure 2).
Figure 2.
Consort Diagram
Table 1.
Baseline Characteristics (online only)
| All Study Groups | Randomized Infants | |||||||
|---|---|---|---|---|---|---|---|---|
|
| ||||||||
| Overall | Group A (Antibiotics) | Group B (No antibiotics) | Group C Randomized | p-value† | Group C/Antibiotics | Group C/No Antibiotics | p-value† | |
| (n) | 98 | 32 | 11 | 55 | 28 | 27 | ||
| Male | 50 (51.0) | 16 (50.0) | 4 (36.4) | 30 (54.6) | 0.54 | 13 (46.4) | 17 (63.0) | 0.21 |
| Cesarean delivery | 58 (59.8)1 | 19 (59.4) | 3 (27.3) | 36 (66.7)1 | 0.05 | 17 (63.0)1 | 19 (70.4) | 0.56 |
| Singleton | 75 (76.5) | 24 (75.0) | 10 (90.9) | 41 (74.6) | 0.48 | 20 (71.4) | 21 (77.8) | 0.59 |
| Gestational Age | 29.1 ± 2.8 [23.1, 32.9] | 28.2 ± 2.9 [23.1, 32.9] | 32.1 ± 1.0 [29.3, 32.9] | 29.0 ± 2.6 [23.7, 32.7] | 0.0002 | 29.2 ± 2.5 [23.7, 32.7] | 28.8 ± 2.8 [23.7, 32.7] | 0.59 |
| Weight (g) at Birth | 1240 ± 479 [490, 2770] | 1138 ± 446 [490, 2132] | 1900 ± 417 [1110, 2770] | 1167±407 [525, 2425] | <0.0001 | 1234 ± 424 [525, 2425] | 1098 ± 384 [605, 2116] | 0.22 |
| Length of stay (days) | 56.4 ± 37.1 [0.0, 184.0] | 64.5 ± 40.5 [0.0, 184.0] | 26.5 ± 19.7 [9.0, 81.0] | 57.7 ± 35.2 [1.0, 147.0] | 0.01 | 53.9 ± 30.0 [13.0, 134.0] | 61.6 ± 40.1 [1.0, 147.0] | 0.42 |
Presented as n(%) or the mean ± standard deviation [min, max]
Chi-Square Test or ANOVA
n missing = 1; did not obtain consent to collect maternal data
Safety and Adverse Events.
Individual adverse events were not significantly different between groups (Table 2). Rates of the composite outcome were significantly different between Groups A, B and C. Group B had significantly fewer adverse events than groups A and C. The composite outcome was non-significantly increased in randomized Group C/no antibiotics (51%) compared with Group C/antibiotics (32.1%, P = .14) (Table 2). When group C was analyzed by treatment received, regardless of randomization assignment, there were no significant differences in any individual or composite adverse outcomes. The composite outcome was non-significantly increased in the infants receiving antibiotics in the first 48 hours after birth, compared with those who did not (composite outcome: 46.3% vs. 28.6%, p=0.24) (Table 3; available at www.jpeds.com)
Table 2.
Adverse Events Outcomes Across Study Groups
| All Study Groups | Randomized Infants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Overall | Group A (Antibiotics) | Group B (No Antibiotics) | Group C Randomized | p-value† | Group C antibiotics | Group C no antibiotics | p-value† | OR [95% CI] Unadjusted | OR [95% CI] Adjusted for Gestational Age | |
| (n) | 98 | 32 | 11 | 55 | 28 | 27 | ||||
| Necrotizing enterocolitis | 4(4.1) | 1 (3.1) | -- | 3(5.5) | 0.67 | 2(7.1) | 1 (3.7) | 0.57 | 0.50 [0.04, 5.86] | 0.44 [0.04, 5.41] |
| Late Onset Sepsis | 15 (15.3) | 6 (18.8) | -- | 9 (16.4) | 0.31 | 5 (17.9) | 4 (14.8) | 0.76 | 0.80 [0.19, 3.36] | 0.64 [0.14, 3.01] |
| Bronchopulmonary Dysplasia | 24 (24.5) | 12 (37.5) | 1 (9.1) | 11 (20.0) | 0.08 | 3 (10.7) | 8 (29.6) | 0.08 | 3.51 [0.82, 15.03] | 3.53 [0.73, 17.16] |
| Death | 14 (14.3) | 5 (15.6) | -- | 9 (16.4) | 0.35 | 4 (14.3) | 5 (18.5) | 0.67 | 1.36 [0.32, 5.74] | 1.14 [0.24, 5.43] |
| Adverse Events Composite Outcome†† | 43 (43.9) | 19 (59.4) | 1 (9.1) | 23 (41.8) | 0.01 | 9 (32.1) | 14 (51.9) | 0.14 | 2.27 [0.76, 6.80] | 2.43 [0.68, 8.71] |
Presented as n(%) for categorical data
Chi-Square Test. Odds Ratios referent group: C/antibiotics
Odds Ratios referent group: C/antibiotics
Adverse Event Composite Outcome: Any of the following: Necrotizing enterocolitis, Late-Onset Sepsis, Bronchopulmonary Dysplasia, Death
Table 3.
Adverse Events Outcomes Within Randomized Infants (online only)
| Group C (n=55) | |||
|---|---|---|---|
|
|
|||
| Received Antibiotics† | Did not receive Antibiotics | p-value†† | |
|
|
|||
| (n) | 41 (74.6) | 14 (25.5) | |
| Necrotizing enterocolitis | 3 (7.3) | -- | 0.3 |
| Late Onset Sepsis | 7(17.1) | 2 (14.3) | 0.8 |
| Bronchopulmonary dysplasia | 9 (22.0) | 2 (14.3) | 0.53 |
| Death | 8 (19.5) | 1 (7.1) | 0.28 |
| Adverse events outcome composite | 19 (46.3) | 4 (28.6) | 0.24 |
Infants in group C receiving antibiotics in the first postnatal 48 hours regardless of assigned treatment group Presented as n(%)
Chi-Square Test.
One infant developed early onset sepsis and died in the first 24 hours after birth. This infant was in group C/no antibiotics and switched to receive antibiotics at approximately one hour after birth at the clinician’s discretion for clinical change and laboratory abnormality. Deaths were not significantly different between groups A, C/antibiotics and C/no antibiotics (Table 2). Group B had no mortality and a shorter length of stay in comparison to groups A and C, concordant with an older gestation.
Discussion:
We have shown that a study that challenges the current practice of routinely prescribing antibiotics in newborn infants with symptoms expected for prematurity is feasible. Our study risk stratified and randomized premature infants to receive vs not receive antibiotics in the first 48 hours after birth. Limitations from our study demonstrate the need and provide guidance for how a multicenter randomized and ideally blinded trial can be done to evaluate and expand on our findings.
Enrollment was slower than expected in part due to IRB reviews for adverse events – most in the 23–24 weeks gestation or in group A. Future trials evaluating antibiotic use after birth should consider excluding infants with high likelihood of early morbidity and mortality such as periviable infants or infants with high illness severity scores. Newly developed scoring systems such as the neonatal sequential organ failure assessment score (nSOFA) may assist in the risk stratifying process.(19) Although the goal enrollment was not met, the number studied in this pilot study was determined to be sufficient to determine feasibility for a larger trial.
An increase in the composite outcome that did not meet statistical significance was seen in Group C/no antibiotics vs Group C/antibiotics when analyzed as intent to treat, mostly related to different rates of BPD between the randomized groups. As treatment switching was common in Group C/no antibiotics, we performed a secondary analysis and analyzed Group C by the use of antibiotics in the first 48 postnatal hours. In this analysis, more infants who received antibiotics had composite adverse events and BPD. (p=0.24, Table 3, online only) The increase in adverse events in infants treated with antibiotics could be due to greater illness severity, but this was not specifically studied.
The most common reason for switching treatment in the infants in Group C/no antibiotics was clinical and laboratory abnormalities. The notion of “treatment switching” is a known phenomenon in clinical trials, and statistical methods to account for treatment switching have been developed.(20) Although we did not have the sample size to utilize these methods here, our preliminary data on the rates of switching will be useful in planning larger trials that can use these methods while ensuring sufficient statistical power.
Antibiotics have been proposed to decrease inflammation and subsequently decrease ventilation requirements, which could decrease BPD. However, new studies demonstrate a connection between airway and intestinal dysbiosis with abnormal inflammation that alters lung development, subsequently increasing the risk of BPD.(16, 17) In our study, 8 infants in Group C/no antibiotics developed BPD but 6 of the infants were switched to received antibiotics in the 48 hours after birth. Based on the small numbers it does not appear that withholding antibiotics in the first 48 hours led to BPD, but this needs to be studied further.
Limitations of the study include the randomization of periviable infants at high risk of adverse events, use of an unblinded approach, and a lack of definition of symptoms associated with prematurity as entry criteria for the randomization group. Future trials should better define the symptoms of prematurity for randomization eligibility.
The safety of randomizing premature infants to antibiotics vs no antibiotics has been a major concern in neonatology. Although we do not claim safety, our pilot study’s pragmatic design showed no evidence of harm. Allowing the primary physicians to provide antibiotics based on their clinical judgment was a necessary compromise as more objective validated criteria are lacking in this population. The antibiotics were initiated for abnormal laboratory findings or acute events such as tension pneumothorax or spontaneous intestinal perforation. One infant in the study had culture-positive early onset sepsis. This infant was randomized to Group C/no antibiotics, but ampicillin and gentamicin were administered within one hour after birth due to a clinical status change and laboratory evaluation as outlined in study algorithm. In this infant, the bacterial strain was resistant to the prescribed antibiotics. This infant’s case was reviewed by the data safety monitor board, the neonatology division, and IRB at the UF. It was determined that the death was not study related as serial examinations and early laboratory evaluation accurately detected the infant’s severity of illness and treatment was initiated in a timely manner consistent with the NICU practice. The low rate of early onset sepsis observed in this cohort is similar to other previously published studies and reinforces the goal of challenging the practice of routine antibiotic use in preterm infants.(7, 21)
Future randomized trials should consider methods for blinding the treatment group to improve consistency and safely decrease the rate of treatment switching in infants randomized to no antibiotics. There is a trend of decreasing antibiotic utilization in premature infant care which suggests a growing concern with unnecessary use.(6) However, the overall use remains high demonstrating that a multicenter trial evaluating the use of antibiotics in neonatal care is needed. This study demonstrated the feasibility of a randomized trial of early antibiotics vs no antibiotics in risk-stratified premature infants and provides guidance for larger randomized trials, which in turn may improve clinical care of preterm infants.
Supplementary Material
Acknowledgements
<< >> for the Data Safety Monitoring Board: Susmita Datta, PhD - Biostatistician and Epidemiologist at University of Florida, Michael Cotten, M.D. - Neonatologist at Duke University School of Medicine, William Benitz, M.D. - Neonatologist at Lucile Packard Children’s Hospital, Stanford, Robert Lawrence, M.D. - Pediatric Infectious Disease specialist at University of Florida
Supported by National Institutes of Health (R21HD088005 [to J.N.]) and National Center for Advancing Translational Sciences (UL1 TR000064 [to M.G.]) J.N. is the PI of a study with Infant Bacterial Therapeutics and serves on the Scientific Advisory Boards of Medela and Astarte.
Abbreviations:
- BPD
(bronchopulmonary dysplasia)
- IRB
(Institutional Review Board)
- NEC
(necrotizing enterocolitis)
- NICU
(Neonatal Intensive Care Unit)
- UF
(University of Florida)
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References:
- 1.Alexander VN, Northrup V, Bizzarro MJ. Antibiotic exposure in the newborn intensive care unit and the risk of necrotizing enterocolitis. J Pediatr. 2011;159(3):392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cotten CM, Taylor S, Stoll B, Goldberg RN, Hansen NI, Sanchez PJ, et al. Prolonged duration of initial empirical antibiotic treatment is associated with increased rates of necrotizing enterocolitis and death for extremely low birth weight infants. Pediatrics. 2009;123(1):58–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Abdel Ghany EA, Ali AA. Empirical antibiotic treatment and the risk of necrotizing enterocolitis and death in very low birth weight neonates. Ann Saudi Med. 2012;32(5):521–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Greenwood C, Morrow AL, Lagomarcino AJ, Altaye M, Taft DH, Yu Z, et al. Early empiric antibiotic use in preterm infants is associated with lower bacterial diversity and higher relative abundance of Enterobacter. J Pediatr. 2014;165(1):23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schulman J, Dimand RJ, Lee HC, Duenas GV, Bennett MV, Gould JB. Neonatal intensive care unit antibiotic use. Pediatrics. 2015;135(5):826–33. [DOI] [PubMed] [Google Scholar]
- 6.Greenberg RG, Chowdhury D, Hansen NI, Smith PB, Stoll BJ, Sanchez PJ, et al. Prolonged duration of early antibiotic therapy in extremely premature infants. Pediatr Res. 2019;85(7):994–1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Stoll BJ, Gordon T, Korones SB, Shankaran S, Tyson JE, Bauer CR, et al. Early-onset sepsis in very low birth weight neonates: a report from the National Institute of Child Health and Human Development Neonatal Research Network. J Pediatr. 1996;129(1):72–80. [DOI] [PubMed] [Google Scholar]
- 8.Torrazza RM, Ukhanova M, Wang X, Sharma R, Hudak ML, Neu J, et al. Intestinal microbial ecology and environmental factors affecting necrotizing enterocolitis. PLoS One. 2013;8(12):e83304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mai V, Torrazza RM, Ukhanova M, Wang X, Sun Y, Li N, et al. Distortions in development of intestinal microbiota associated with late onset sepsis in preterm infants. PLoS One. 2013;8(1):e52876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mai V, Young CM, Ukhanova M, Wang X, Sun Y, Casella G, et al. Fecal microbiota in premature infants prior to necrotizing enterocolitis. PLoS One. 2011;6(6):e20647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pammi M, Cope J, Tarr PI, Warner BB, Morrow AL, Mai V, et al. Intestinal dysbiosis in preterm infants preceding necrotizing enterocolitis: a systematic review and meta-analysis. Microbiome. 2017;5(1):31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Hoenig JD, Malin KJ, Qamar S, Petrof EO, Sun J, et al. 16S rRNA gene-based analysis of fecal microbiota from preterm infants with and without necrotizing enterocolitis. ISME J. 2009;3(8):944–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carl MA, Ndao IM, Springman AC, Manning SD, Johnson JR, Johnston BD, et al. Sepsis from the gut: the enteric habitat of bacteria that cause late-onset neonatal bloodstream infections. Clin Infect Dis. 2014;58(9):1211–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Madan JC, Farzan SF, Hibberd PL, Karagas MR. Normal neonatal microbiome variation in relation to environmental factors, infection and allergy. Curr Opin Pediatr. 2012;24(6):753–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Neu J, Pammi M. Pathogenesis of NEC: Impact of an altered intestinal microbiome. Semin Perinatol. 2017;41(1):29–35. [DOI] [PubMed] [Google Scholar]
- 16.Casado F, Morty RE. The emergence of preclinical studies on the role of the microbiome in lung development and experimental animal models of bronchopulmonary dysplasia. Am J Physiol Lung Cell Mol Physiol. 2020. [DOI] [PubMed] [Google Scholar]
- 17.Tirone C, Pezza L, Paladini A, Tana M, Aurilia C, Lio A, et al. Gut and Lung Microbiota in Preterm Infants: Immunological Modulation and Implication in Neonatal Outcomes. Front Immunol. 2019;10:2910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stewart CJ, Nelson A, Scribbins D, Marrs EC, Lanyon C, Perry JD, et al. Bacterial and fungal viability in the preterm gut: NEC and sepsis. Arch Dis Child Fetal Neonatal Ed. 2013;98(4):F298–303. [DOI] [PubMed] [Google Scholar]
- 19.Wynn JL, Polin RA. A neonatal sequential organ failure assessment score predicts mortality to late-onset sepsis in preterm very low birth weight infants. Pediatr Res. 2019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Latimer NR, Abrams KR, Lambert PC, Morden JP, Crowther MJ. Assessing methods for dealing with treatment switching in clinical trials: A follow-up simulation study. Stat Methods Med Res. 2018;27(3):765–84. [DOI] [PubMed] [Google Scholar]
- 21.Kuzniewicz MW, Walsh EM, Li S, Fischer A, Escobar GJ. Development and Implementation of an Early-Onset Sepsis Calculator to Guide Antibiotic Management in Late Preterm and Term Neonates. Jt Comm J Qual Patient Saf. 2016;42(5):232–9. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.