Abstract
Alterations in the TAOK1 gene have recently emerged as the cause of developmental delay with or without intellectual impairment or behavioral abnormalities (MIM # 619575). The 32 cases currently described in the literature have predominantly de novo alterations in TAOK1 and a wide spectrum of neurodevelopmental abnormalities. Here, we report four patients with novel pathogenic TAOK1 variants identified by research genome sequencing, clinical exome sequencing, and international matchmaking. The overlapping clinical features of our patients are consistent with the emerging core phenotype of TAOK1-associated syndrome: facial dysmorphism, feeding difficulties, global developmental delay, joint laxity, and hypotonia. However, behavioral abnormalities and gastrointestinal issues are more common in our cohort than previously reported. Two patients have de novo TAOK1 variants (one missense, one splice site) consistent with most known alterations in this gene. However, we also report the first sibling pair who both inherited a TAOK1 frameshift variant from a mildly affected mother. Our findings suggest that incomplete penetrance and variable expressivity are relatively common in TAOK1-associated syndrome, which holds important implications for clinical genetic testing.
Keywords: autism, failure to thrive in infancy, generalized joint laxity, generalized neonatal hypotonia, moderate global developmental delay, relative macrocephaly
INTRODUCTION
The TAOK1 gene gets its name from the number of amino acids for which it encodes; a “thousand and one” amino acids—thus the name “thousand and one” kinase. TAOK1 is located at cytogenetic band 17p11.2 and is a MAP3K serine/threonine kinase, with the kinase domain located at the amino terminus of the gene. It contains a serine-rich domain as well as two coiled-coil domains. TAOK1 is expressed ubiquitously but has highest expression in testes and brain (Fang et al. 2020; van Woerden et al. 2021). This gene is constrained for both missense variants (234 observed, 553 expected, Z = 4.82) and for loss of function variants (11 observed, 67 expected, pLI = 1) according to the gnomAD database (Karczewski et al. 2020) v2.1.1, which lists only two nonsynonymous variants with minor allele frequency >0.0004 in human population cohorts. TAOK1 has been reported to have a variety of functions including regulation of p38 MAPK, JNK, and Hippo signaling pathways (Plouffe et al. 2016; Fang et al. 2020). It is also thought to play a role in cytoskeleton regulation, neuron development, inflammation, and other pathways (Li et al. 2019; Zhu et al. 2020; van Woerden et al. 2021). Loss of TAOK1 has been shown to result in mitotic abnormalities, suggesting a role for the gene in proper chromosome transmission during cell division (Liskovykh et al. 2019; Fang et al. 2020; Kouprina et al. 2020; Shi et al. 2021).
Alterations in TAOK1 are associated with developmental delay with or without intellectual impairment or behavioral abnormalities (MIM # 619575). A microdeletion of TAOK1 was reported in a patient with neurodevelopmental abnormalities in 2016 (Xie et al. 2016). Dulovic-Mahlow et al. (2019) reported eight individuals with de novo alterations in TAOK1. Five of these patients were among 6504 patients referred to Centogene AG for diagnostic exome sequencing, whereas the other three were identified by international matchmaking (Sobreira et al. 2015). All eight patients presented with nonspecific developmental delay, with some individuals sharing additional clinical features (muscular hypotonia and facial dysmorphism). In 2021, van Woerden et al. reported 23 additional patients with TAOK1 variants (van Woerden et al. 2021) identified by matchmaking efforts. Like the previous study, most of the variants identified were de novo alterations (16/23) with predicted loss-of-function effects (18/23) supporting haploinsufficiency as the mechanism underlying disease (Dulovic-Mahlow et al. 2019; van Woerden et al. 2021). Functional assessment of missense variants, however, revealed that some reduced TAOK1 protein levels consistent with a loss-of-function effect, but others (notably p.Leu548Pro) exhibited increased protein expression suggestive of a dominant negative effect. Even overexpression of wild-type TAOK1 disrupted neuronal morphology and migration, suggesting that tight regulation of this gene during development is required for normal neuronal function (van Woerden et al. 2021).
The clinical presentation of TAOK1-associated neurodevelopmental disorder from the two cohort studies remains variable. The most common features include developmental delay and intellectual disability, macrocephaly, neonatal feeding difficulties, behavioral problems, hypotonia, joint laxity, and some mild facial dysmorphisms (frontal bossing, downslanting palpebral fissures, long philtrum, and a bulbus nasal tip). Given this broad range of reported clinical features, more studies of TAOK1 patients are needed to understand the genotypic and phenotypic spectrum of this disorder. Here, we describe four patients from three families with TAOK1-associated neurodevelopmental disorders, including the first report of an affected sibling pair. Our results extend the clinical manifestations observed in TAOK1 patients and emphasize that both inherited and de novo variants can cause disease.
RESULTS
Clinical Presentation and Family History
Patient 1 (Family 1)
Patient 1 prenatal and perinatal complications included polyhydramnios, maternal uterine rupture at delivery following a failed vaginal birth after caesarean attempt, and intermittent hypoglycemia for ∼2 d. At 6 mo of age this male infant was being treated for feeding issues and gastroesophageal reflux (GERD), but at his last evaluation, his eating behavior had improved and he was noted to be eating a normal diet. At 18 mo of age he was first seen by genetics for macrocephaly, delay, and failure to thrive. He was 3 yr 4 mo at last evaluation at which time he was noted to have global developmental delay including speech delay with only a few words. He had decreased strength, decreased central tone, and joint laxity and was reported to be clumsy. Gross motor skills were delayed (crawling and sitting 2 yr, walked ∼2.5 yr). He was noted to have a tremor when stressed. He was referred to the Child Development Center at our hospital because of disruptive behavior, but was not quantitatively evaluated for autism. He was macrocephalic (53.5 cm; >>99%ile) with frontal prominence of the skull but was overall reported to be nondysmorphic. His linear growth was reported to be normal but his height was 91.4 cm (5%ile; Z = −1.68) and his body mass index (BMI) was 97.75%ile (weight: 61%ile). Brain magnetic resonance imaging (MRI) on two occasions revealed ventriculomegaly and mild bilateral parietal white matter volume loss and cerebellar tonsillar ectopia not meeting Chiari I malformation criteria. Skin findings included livedo reticularis (Table 1).
Table 1.
Demographics and phenotype characteristics of the four patients reported in this study
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | ||
| Demographics and variant information | |||||
| Family | Family 1 | Family 1 | Family 2 | Family 3 | |
| Gender | Male | Female | Male | Female | |
| Age at last evaluation | 3 yr 4 mo | 4 yr 10 mo | 2 yr 10 mo | 5 yr | |
| TAOK1 variant NM_020791.4 (GRCh38): | c.2203delA p.Arg735fs |
c.2203delA p.Arg735fs |
c.132 + 3_132 + 6 delAAGT p.? |
c.1324C > T p.Arg442Trp |
|
| Inheritance | Het/maternal | Het/maternal | Het/de novo | Het/de novo | |
| HPO ID | Term Description | Patient 1 | Patient 2 | Patient 3 | Patient 4 |
| Growth and development | |||||
| HP:0001263 | Global developmental delay | + | + | + | + |
| HP:0002194 | Delayed gross motor development | + | + | + | + |
| HP:0007015 | Poor gross motor coordination | + | + | ||
| HP:0000750 | Delayed speech and language development | + | + | + | + |
| Brain imaging | |||||
| HP:0002119 | Ventriculomegaly | + | + | + | |
| Behavior | |||||
| HP:0000708 | Behavioral abnormality | + | + | + | + |
| HP:0000729 | Autistic behavior | + | + | + | |
| HP:0100852; HP:0000739 | Abnormal fear/anxiety-related behavior (Stress induced tremor) ; Anxiety | + | + | + | |
| Neuromuscular | |||||
| HP:0001252 | Hypotonia | + | + | + | + |
| HP:0001382 | Joint hypermobility | + | + | + | +/– |
| Feeding/gastrointestinal | |||||
| HP:0008872 | Feeding difficulties in infancy | + | + | + | |
| HP:0002020 | Gastroesophageal reflux | + | + | + | + |
| HP:0004324 | Increased BMI | + | + | + | + |
| HP:0002019 | Constipation | + | + | ||
| Genitourinary | |||||
| HP:0000077 | Abnormality of the kidney | + | + | ||
| Craniofacial dysmorphisms | |||||
| HP:0000256 | Macrocephaly | + | + | + | |
| HP:0011220 | Prominent forehead | + | + | + | |
| HP:0001999 | Abnormal facial shape | + | + | ||
| Hands and feet | |||||
| HP:0011297 | Abnormal digit morphology | + | + | + | |
| Prenatal | |||||
| HP:0001561 | Polyhydramnios | + | + | + | |
(BM) Body mass index, (HPO) Human Phenotype Ontology, (+) phenotype present/consistent, (–) mild phenotype.
Clinical microarray testing revealed a 1.27 Mb gain at 8p22 that was classified as a VUS, but considered noncontributory to the patient's phenotype. Prader–Willi/Angelman syndrome methylation studies and myotonic dystrophy testing were normal.
Patient 2 (Family 1)
Patient 2 is the older sibling of Patient 1. Polyhydramnios was noted prenatally. At her latest evaluation at age 4 yr 10 mo of age, this female patient was noted to have global developmental delay and gross motor delay (rolled 13 mo, sat 14 mo, crawled 18 mo, walked at 2 yr). Her speech was significantly delayed and although she has many words, her language is difficult to understand. She communicates by motions and signals in combination with speaking. She was noted to have hypotonia and hyperflexibility. She had a history of GERD and pica, but was eating normal foods at her last evaluation with a BMI of 80.86%ile (weight: 46%ile) and a height of 102.5 cm (19%ile; Z = −0.87). She had stereotypic repetitive behaviors and was referred to the Child Development Center but not quantitatively evaluated for autism. Her growth was slow but steady. She is macrocephalic (fronto-occipital circumference = 52 cm; 90.38% %ile) but was not otherwise noted for dysmorphic features. Brain MRI on two occasions revealed mild dilation of the lateral and third ventricles as well as a small arachnoid cyst. In addition, she had congenital heart defects including tetralogy of Fallot, ventriculoseptal defect, and a bicuspid aortic valve, which are phenotype characteristics not previously reported in other TAOK1 patients (Table 1).
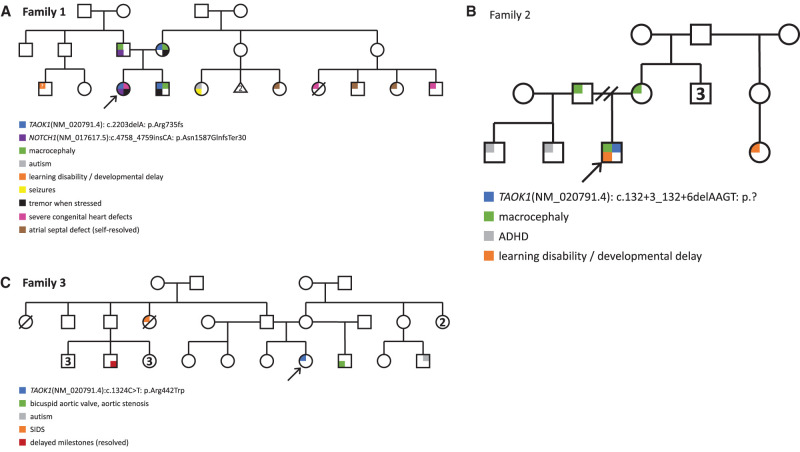
Clinical microarray revealed the same 1.27 Mb gain at 8p22 originally identified in her brother. Clinical exome sequencing (WES) performed at 23 mo of age uncovered a heterozygous likely pathogenic frameshift alteration in NOTCH1 (NM_017617.5: c.4758_4759insCA: p.Asn1587GlnfsTer30) that was reported as the probable cause of her cardiac abnormalities (Garg et al. 2005; McBride et al. 2008). This variant was inherited from an asymptomatic father (Fig. 1A) consistent with reduced penetrance reported for NOTCH1-associated congenital heart defects (Zahavich et al. 2017). Her neurodevelopmental features, however, remained unexplained.
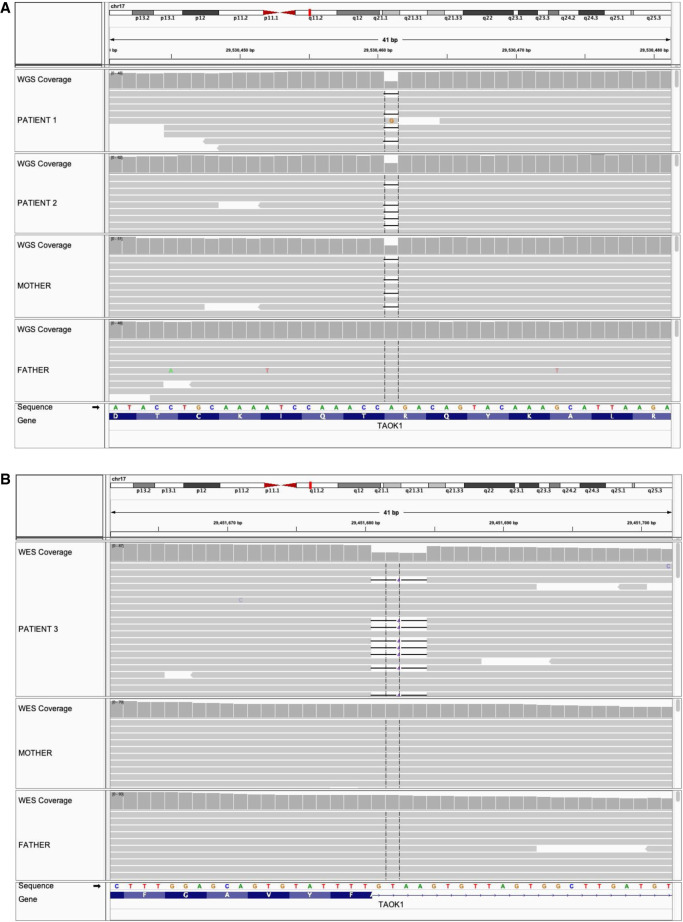
Patients 1 and 2 were referred along with their parents for research whole-genome sequencing (WGS) under an Institutional Review Board (IRB)-approved translational research study at the Institute for Genomic Medicine at Nationwide Children's Hospital. The most plausible candidate variant shared by both siblings was a heterozygous frameshift deletion in TAOK1, NM_020791.4:c.2203delA, p.(Arg735fs) (Fig. 2A). The variant is absent from gnomAD and other public databases and predicted to result in nonsense-mediated decay of the transcript. Retrospective review of the clinical WES data for Patient 2 indicated that this variant was observed but not assessed because of the lack of an OMIM disease association for TAOK1 at the time (late 2018; OMIM did not add the gene–disease association until September 2021). This demonstrates the necessity of reevaluating exome data periodically for negative patients.
Interestingly, the TAOK1 frameshift variant (p.Arg735fs) in patients 1 and 2 was maternally inherited (Fig. 1A). The mother was considered unaffected, was macrocephalic (FOC = 62.6 cm (>>99%ile)), and anecdotally has tremor with stress. She also reported minor learning difficulties and lax ankle joints as a child. The father was noted to be macrocephalic but was otherwise unaffected. The parents report European ancestry. No other family members have reported phenotypes consistent with TAOK1-related disease, though some maternal relatives were noted to have severe congenital heart defects (Fig. 1A).
Figure 1.
Pedigrees for families 1–3. Arrows indicate the index proband in each family; colored shading indicates variant carrier status and reported clinical features. (A) Pedigree for Family 1. (B) Pedigree for Family 2. (C) Pedigree for Family 3.
Several other rare nonsynonymous variants in disease-associated genes were shared by the proband and sibling; these are detailed in Supplemental Table 2. Two rare, maternally inherited missense variants were identified in genes associated with autosomal dominant conditions that did not match the proband or sibling: FN1, associated with glomerulopathy with fibronectin deposits 2 (MIM # 601894) and spondylometaphyseal dysplasia (MIM # 184255), and NODAL, associated with autosomal dominant visceral heterotaxy 5 (MIM # 270100; not evident in either proband or sibling). The siblings also shared a paternally inherited missense variant in MYH9 (MIM # 160775), which is associated with two types of autosomal dominant hearing loss; both they and their father had normal hearing. The proband only was heterozygous for a missense variant in MAGEL2, associated with autosomal dominant Schaaf–Yang syndrome (MIM # 615547), but in which only loss-of-function changes have been reported to cause disease.
Finally, we evaluated a rare missense variant in NF1 (NM_000267.3: c.4363C > T, p.Arg1455Cys) that was heterozygous in both children. This variant has been observed in human populations, albeit at extremely low allele frequency (four heterozygotes in gnomAD; population minor allele frequency [MAF] = 0.0000174). It affects a highly conserved residue and is predicted to be damaging by most in silico tools (24/26, REVEL score = 0.7239). Of more than 2000 pathogenic/likely pathogenic variants in NF1 reported by clinical laboratories (ClinVar), the majority (90%) have loss-of-function effects. The p.Arg1455Cys variant has been reported by two clinical laboratories as a variant of uncertain significance (VUS). Although the children and their father (who also carries this variant) have macrocephaly, a feature sometimes reported in neurofibromatosis syndrome (Clementi et al. 1999), none of them were reported to have café au lait spots or other skin findings characteristic of the disease (for review, see Williams et al. 2009).
Patient 3 (Family 2)
Patient 3 is a 3-yr-old male of European ancestry. Evaluation at 2.5 yr of age revealed intellectual disability and global developmental delay. He was ambulatory but had frequent falls. Brain imaging by MRI revealed ventriculomegaly but no other abnormalities. He was reported to have autism, poor eye contact, staring spells, and restless sleep. He was macrocephalic (frontal occipital circumference [FOC] = 54 cm; >99%ile; Z = 2.99) with some relatively minor facial dysmorphisms including prominent cheeks, slightly prominent forehead, and tenting of the upper lip. Prenatal polyhydramnios was reported and feeding difficulties were noted at birth. Gastrointestinal issues included nausea, reflux, diarrhea, and constipation, and he was eventually found to have tracheoesophageal fistula, annular pancreas, duodenal atresia, mild right caliectasis, and penile adhesions. Despite feeding problems, his BMI was 85.50%ile (weight: 90%ile) and his height was 96.6 cm (76%ile) and was thought to possibly have overgrowth issues and relatively large size. He was noted to have hypotonia, including facial hypotonia, and increased joint mobility along with slightly short appearing digits. Strabismus and ptosis were noted (Table 1). At 2 yr of age he was referred to the Child Development Center at our hospital and diagnosed with autism after a clinical interview and psychological evaluations (ADI-R, AVABO, DP-3). The family history was not particularly suggestive of other similarly affected individuals in the family. Both parents were noted to have some degree of macrocephaly, a relative with learning disabilities was reported, and two paternal half-siblings were noted to have attention deficit disorder (ADHD) (Fig. 1B).
Clinical WES was performed on the proband and both biological parents. A heterozygous 4-bp intronic deletion near the 3′ splice donor site of exon 2 of the TAOK1 gene was detected (NM_020791.4: c.132 + 3_132 + 6delAAGT) in the proband but not present in either parent (Fig. 2B). This alteration was confirmed de novo by Sanger sequencing of the proband and parents, and parentage was confirmed. The variant is absent from gnomAD and other public databases and is predicted to result in complete loss of splicing by multiple algorithms (ESEfinder [Cartegni et al. 2003] and SpliceAI [Smith et al. 2006]; see Table 2). We classify the variant as likely pathogenic (Table 2). Two additional de novo alterations were identified in this patient (Supplemental Table 2). Both are missense changes classified as VUSs; their contributions to Patient 3′s clinical features are unclear. In addition to exome sequencing, fragile X testing and microarray were performed for Patient 3 and reported as normal.
Table 2.
Genomic findings and variant interpretation for the TAOK1 variants identified in our patients
| Patient | Genomic location | HGVS cDNA | HGVS protein | Zygosity/origin | Interpretation |
|---|---|---|---|---|---|
| 1 | Chr 17: 29530460 | NM_020791.4: c.2203delA | TAOK1: p.(Arg735AspfsTer6) | Het/maternal | LP (PVS1, PM2, PP1) |
| 2 | Chr 17: 29530460 | NM_020791.4: c.2203delA | TAOK1: p.(Arg735AspfsTer6) | Het/maternal | LP (PVS1, PM2, PP1) |
| 3 | Chr 17: 29451682 | NM_020791.4:c.132 + 3_132 + 6del | TAOK1: p.(Phe44 + 3) splice | Het/de novo | LP (PS2, PM2, PP3a) |
| 4 | Chr 17: 29502709 | NM_020791.4:c.1324C > T | TAOK1: p.(Arg442Trp) | Het/de novo | LP (PS2, PM2 PP3b) |
Genomic coordinates reflect build GRCh38. Only phenotypes observed in at least two individuals are shown (see Supplemental Table 3 for full details).
aPP3 was applied because of complete loss of donor splicing predicted by BDGP: Splice Site Prediction by Neural Network (fruitfly.org) (native score = 0.95, altered score = not found, threshold 0.1) and ESEFinder (Cartegni et al. 2003) 3.0 (cshl.edu) (native score = 5.41210, altered score = not found, threshold 1.0).
bFor Patient 4, PP3 was applied because the majority of computational algorithms (18 of 25, including SIFT, MutationTaster, PROVEAN, PolyPhen-2, CADD, and others) reported by VarSome (Kopanos et al. 2019) predict pathogenicity.
Patient 4 (Family 3)
Patient 4 is of Dominican and Puerto Rican descent and originally presented with hypotonia and developmental delay (first words 18 mo, pulled to stand 13–14 mo, walking 2.5 yr, not toilet trained at 5 yr). Pregnancy was normal but delivery was induced because of maternal hypertension. Patient 4 was 6 lbs 14 oz. at birth. She has a history of GERD as an infant and is reported to have chronic constipation. Her last evaluation at 5 yr of age determined that her height was 40th%ile, BMI was 98%ile (weight: 96%ile), and head circumference was 20%ile. Her language was delayed, but she is currently talking more and speaking in full sentences. Adaptive skills evaluation revealed an full-scale intelligence quotient (FSIQ) of 58 (subscores 50–60 range except for processing speed score of 77) and a Vineland three adaptive behavioral assessment score of 65 (communication 58, daily living skills 60, socialization 76, motor skills 70). Behavioral concerns included selective mutism, inattention at school, and anxiety. She does not have autism. Medical records also indicate a complex febrile seizure lasting >10 min in the setting of a urinary tract infection. Brain MRI showed multifocal subcortical gliosis thought to be consistent with chronic manifestation of perinatal infection or ischemia. Facial features reported were a prominent forehead, flat nasal bridge with short columella, prominent lips, and a short philtrum. She was noted to have tapered fingers with squared fingertips and persistent fetal finger pads. She also had 2/3 toe syndactyly, sandal gap, and pes planus. She is noted to have astigmatism and wears glasses. Skin findings included a strawberry hemangioma on her chest and a café au lait spot on her neck. Imaging by ultrasound revealed a small right kidney with a simple cortical cyst. Also of note is a history of thrombocytopenia, bruising without trauma, and poor response to vaccinations. She was seen by endocrinology for premature adrenarche (Table 1). Family history did not reveal any relatives with particularly striking phenotype correlation (Fig. 1C).
Patient 4's diagnostic evaluation prior to exome sequencing included normal microarray testing (Affimetrix Cytoscan HD Array), normal Prader–Willi methylation analysis (SNRPN Southern blot), and metabolic screening that was nondiagnostic, showing a mildly elevated ammonia level of 59 and mild nonspecific elevation of serum amino acids. Clinical WES through GeneDx identified a de novo NM_020791.4(TAOK1): c.1324C > T: p.Arg442Trp missense variant that was classified by GeneDX as a VUS but classified as likely pathogenic herein (Table 2). A paternally inherited missense variant in TNFRSF13B (NM_012452.3:c.310T > C:, p.(Cys104Arg)) was classified as pathogenic but not thought to play a major role in the phenotype of Patient 4 (Supplemental Table 2). No other variants of interest were included in the clinical exome report.
Genomic Analyses
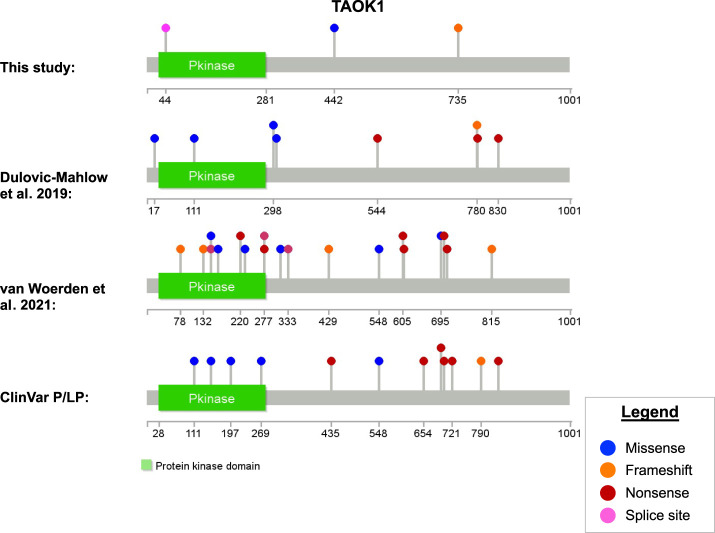
The three TAOK1 variants in this study have not been previously reported in databases or the literature (Fig. 3A). The variant in Family 1 is a frameshift change located in a carboxy-terminal region of the gene in which many loss-of-function variants have been reported by Dulovic-Mahlow et al. (2019) (Fig. 3B), van Woerden et al. (2021) (Fig. 3C), or to the ClinVar database (Fig. 3D) including several that are more carboxy-terminal than the variant reported here. This variant is in exon 18 of 20 (NM_020791.4) and is expected to result in nonsense mediated decay. The c.132 + 3_132 + 6delAAGT variant reported in Patient 3 appears to be the most amino-terminal splice variant reported to date and likely results in nonsense mediated decay. The region in which the missense alteration in Patient 4 (p.Arg442Trp) is found does not belong to a known domain or recognition motif, but UniProt identifies nearby residue 445 as a phosphorylation site (Dephoure et al. 2008; Zhou et al. 2013). Similar numbers of missense and loss of function variants have been reported. There do not appear to be any regions of the gene with greater prevalence of reported disease-causing variants, but rather disease-causing variants of all types are distributed somewhat uniformly across the gene (Fig. 3). As discussed above, loss of function appears to be a genetic mechanism of disease, but the mechanism of disease associated with missense variants is not yet clear.
Figure 3.
Protein structure of TAOK1 and location of reported variants (amino acid position) by source. The first plot shows the variants in this study; the second and third show variants reported in two previous studies. The bottom plot shows ClinVar pathogenic/likely pathogenic variants retrieved 27 September 2021. Variant diagrams were generated using Lollipops v1.5.3 using information from UniProt (ID #Q7L7X3).
Figure 2.
Genomic analysis uncovers rare deletions in TAOK1 in two families. Both panels show sequencing data aligned to the GRCh38 reference sequence as visualized in the Integrative Genomics Viewer (IGV) v2.8.0. (A) Quad whole-genome sequencing data for Family 1, in which Patients 1 and 2 are heterozygous for a single-base deletion inherited from their mother. (B) Trio exome sequencing data for Family 2, in which the proband is heterozygous for a de novo four-base splice site deletion.
Phenotypic Analyses
Overall, the four patients exhibited the most common features of developmental delay with or without intellectual impairment or behavioral abnormalities listed in OMIM (MIM # 619575) including frontal bossing, developmental delay, hypotonia, and neonatal feeding difficulties (Table 1). In addition, consistent features across our patients would add to or provide more definition to the phenotype described in OMIM including joint hypermobility, gross motor delay, speech and language delay, increased BMI, autistic features, and GERD. Patients presented herein were not reported to have ADHD, but Patient 4 was reported to have inattentiveness. Patients all had behavioral abnormalities overlapping with autism spectrum disorder. Despite feeding issues, all patients had elevated BMI, a feature also not listed in OMIM. Although our patients were not reported to be particularly dysmorphic, Patient 3 had some similarities to the mild facial gestalt reported in other TAOK1 patients, whereas Patient 4 had some facial features that differed from previous reports (Dulovic-Mahlow et al. 2019; van Woerden et al. 2021). In addition to the overlapping phenotypes listed in Table 1, detailed evaluation of each patient revealed additional unique phenotype characteristics, some of which could be an expansion of the TAOK1 disease phenotype or phenotypes associated with additional independent or modifier variants (Supplemental Tables 2 and 3).
DISCUSSION
Although a patient with a microdeletion of TAOK1 was reported in 2016 (Xie et al. 2016), most patients with TAOK1 alterations described in the literature (31 of 32) were part of two recent studies (Dulovic-Mahlow et al. 2019; van Woerden et al. 2021). The neurodevelopmental syndrome associated with variants in TAOK1 was recognized by OMIM in September 2021 (MIM # 619575), but our understanding of the genotypic and phenotypic spectrum of disease remains incomplete. Here, we describe four new patients whose overlapping clinical features further solidify the central phenotype TAOK1-associated syndrome: neonatal feeding difficulties, global developmental delay, intellectual disability, behavioral abnormalities, hypotonia, joint hypermobility, and craniofacial dysmorphism (van Woerden et al. 2021). Some variable phenotypes from previous patient cohorts are more consistent in ours, notably gastrointestinal reflux (4/4), increased BMI (4/4), macrocephaly (3/4), and ventriculomegaly (4/4). Also, kidney abnormalities—present in Patients 3 and 4 in this cohort—have only been reported in one other patient to our knowledge.
The TAOK1 variants identified in our patients are novel and have not been previously reported to our knowledge. The variants identified in Patients 3 and 4, like the majority of reported TAOK1 alterations, were demonstrated to be de novo and were absent from confirmed parental samples (Dulovic-Mahlow et al. 2019; van Woerden et al. 2021). The variant identified in Patients 1 and 2, however, was inherited from a mother who was initially considered asymptomatic. Although multiple affected siblings with TAOK1-associated syndrome have never been described (to our knowledge), vertical transmission of TAOK1 variants has been reported in at least three other families (van Woerden et al. 2021). Two of those families had a family history of neurodevelopmental disease, but one did not. Taken together, these observations suggest that inheritance of TAOK1 disease–causing variants from a mildly affected parent may be a relatively common phenomenon. If true, this has important implications for clinical testing; inheritance of a TAOK1 variant from a reportedly asymptomatic parent should no longer be considered evidence against pathogenicity.
Patient 2 in our study had congenital heart defects—including tetralogy of Fallot, ventriculoseptal defect, and a bicuspid aortic valve—which have not been reported in any TAOK1 patients to our knowledge. Instead, we attribute these features to her likely pathogenic variant in NOTCH1, a gene associated with a wide spectrum of developmental heart defects (Garg et al. 2005; McBride et al. 2008; Zahavich et al. 2017). These findings are consistent with the increasingly recognized phenomenon of patients with blended clinical phenotypes caused by multiple independent variants (Smith et al. 2019). These cases highlight the value of comprehensive exome or genome sequencing as a first-line test for diagnosis of genetic disorders.
The emerging picture of TAOK1-associated syndrome, with a wide range of neurodevelopmental and behavioral manifestations as well as de novo and transmitted genetic etiologies, is more reminiscent of autism spectrum disorder than a severe monogenic neurodevelopmental disease. Indeed, TAOK1 first surfaced as a potential disease-associated gene in a single patient in an autism cohort paper (De Rubeis et al. 2014). With the identification and phenotype information of additional patients (Dulovic-Mahlow et al. 2019; Satterstrom et al. 2020; van Woerden et al. 2021), TAOK1 is currently recognized as a high-confidence autism susceptibility gene in the SFARI database (SFARI score = 1.0, EAGLE score = 8.25). As additional TAOK1 variants are identified, the phenotype spectrum will become further solidified.
METHODS
Family 1
Genomic DNA was extracted from blood for the proband, his affected sister, and both parents. Paired-end sequencing (2 × 151 bp) libraries were created with NEBNext Ultra II (New England Biolabs) and sequencing was performed using the NovaSeq6000 instrument (Illumina Inc.) according to the manufacturer protocols. Reads were mapped to the GRCh38/UCSC hg38 reference sequence. Secondary data analysis was performed using Churchill (Kelly et al. 2015), which implements the Genome Analysis Toolkit (GATK) “best practices” workflow to allow for a computationally efficient analysis of WGS data. We generated 179–238 Gbp of uniquely mapped reads per individual, achieving ∼45.8× haploid coverage on average. Sequencing metrics are provided in Supplemental Table 1. VarHouse, an in-house annotation framework, was used to annotate variants with predicted gene/transcript effect (snpEff/ANNOVAR), the damaging scores of various in silico tools, and population allele frequencies from gnomAD and other databases. Our general approach to genomic analysis for rare diseases has been described (Koboldt et al. 2018a,b; Mihalic Mosher et al. 2019). Briefly, we prioritized rare, likely damaging variants that segregate with disease under a plausible inheritance model. Because of the family history, multiple inheritance modes—including recessive, X-linked, and dominant inheritance with incomplete penetrance—were considered.
Family 2
Proband and parental peripheral blood samples were submitted for clinical exome sequencing to The Steve and Cindy Rasmussen Institute for Genomic Medicine at Nationwide Children's Hospital. Genomic DNA extraction from peripheral blood and genotyping assay using a custom Agena MassArray panel (Agena) to ensure sample provenance and verify familial relationships was performed as described by Miller et al. (2020). Libraries underwent target capture using SureSelect Human All Exon V6 (Agilent) followed by paired-end 151-bp sequencing to at least 100× mean depth on a HiSeq 4000 (Illumina), with 95.7% of targeted bases at 20× or greater. Similar to the approach described for Family 1, secondary analysis was performed using Churchill 3.0 and variant annotation was performed by VarHouse. Human Phenotype Ontology (HPO) terms (Köhler et al. 2017) inferred from clinician-provided phenotypes by Mr. Phene (an in-house tool) were used to prioritize variants in phenotypically relevant genes for clinical variant interpretation. Reported variants were confirmed by polymerase chain reaction (PCR) and Sanger sequencing in the proband and both parents as previously described (Miller et al. 2020).
Family 3
Proband and parental peripheral blood samples were submitted for clinical exome sequencing to GeneDx. Genomic DNA was enriched for the complete coding regions and splice site junctions for most genes of the human genome using a proprietary capture system developed by GeneDx for next-generation sequencing with copy number variation calling (NGS–CNV). The enriched targets were simultaneously sequenced with paired-end reads on an Illumina platform. Bidirectional sequence reads were assembled and aligned to the reference sequences based on National Center for Biotechnology Information (NCBI) RefSeq transcripts and human genome build GRCh37/UCSC hg19. Using a custom-developed analysis tool (XomeAnalyzer), data were filtered and analyzed to identify sequence variants and most deletions and duplications involving three or more coding exons (Retterer et al. 2015). The reported variants were converted to GRCh38 coordinates using VarSome (Kopanos et al. 2019).
ADDITIONAL INFORMATION
Data Deposition and Access
Data deposition is not permitted under the current consents, but these variants and our interpretations have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers VCV001333717.1, VCV001328968.2, and VCV001307834.1.
Ethics Statement
Written informed consent was obtained for all patients reported in this study. Families 1 and 2 consented for clinical exome sequencing as well as research studies under a research protocol approved by the Institutional Review Board at Nationwide Children's Hospital (IRB18-00662, Gene Discovery in Clinical Genomic Patients).
Acknowledgments
We thank G.M. van Woerden for discussions and for early access to the accepted version of the 2021 study. We also thank the sequencing services group, laboratory genetic counselors, and variant scientists at the Institute for Genomic Medicine at Nationwide Children's Hospital. We are grateful to all patients and families for their participation in genetic research.
Author Contributions
J.M.H. and D.C.K. performed the analysis and manuscript preparation. K.M., D.B., A.S., and E.d.l.R. performed the clinical evaluations of Families 1 and 2. L.J.M. and J.L.S. performed the clinical evaluation and analysis of Family 3. Ra.K.W. was the study coordinator for Family 1. M.M. performed variant analysis. S.C.R. and C.E.C. performed the clinical laboratory protocols for Families 1 and 2. Ri.K.W. provided research study funding and leadership.
Funding
This study was supported by the Abigail Wexner Research Institute at Nationwide Children's Hospital.
Competing Interest Statement
The authors have declared no competing interest.
Supplementary Material
Footnotes
[Supplemental material is available for this article.]
REFERENCES
- Cartegni L, Wang J, Zhu Z, Zhang MQ, Krainer AR. 2003. ESEfinder: a web resource to identify exonic splicing enhancers. Nucl Acids Res 31: 3568–3571. 10.1093/nar/gkg616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clementi M, Milani S, Mammi I, Boni S, Monciotti C, Tenconi R. 1999. Neurofibromatosis type 1 growth charts. Am J Med Genet 87: 317–323. [DOI] [PubMed] [Google Scholar]
- Dephoure N, Zhou C, Villén J, Beausoleil SA, Bakalarski CE, Elledge SJ, Gygi SP. 2008. A quantitative atlas of mitotic phosphorylation. Proc Natl Acad Sci 105: 10762–10767. 10.1073/pnas.0805139105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Rubeis S, He X, Goldberg AP, Poultney CS, Samocha K, Cicek AE, Kou Y, Liu L, Fromer M, Walker S, et al. 2014. Synaptic, transcriptional and chromatin genes disrupted in autism. Nature 515: 209–215. 10.1038/nature13772 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dulovic-Mahlow M, Trinh J, Kandaswamy KK, Braathen GJ, Di Donato N, Rahikkala E, Beblo S, Werber M, Krajka V, Busk ØL, et al. 2019. De novo variants in TAOK1 cause neurodevelopmental disorders. Am J Hum Genet 105: 213–220. 10.1016/j.ajhg.2019.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang CY, Lai TC, Hsiao M, Chang YC. 2020. The diverse roles of TAO kinases in health and diseases. Int J Mol Sci 21: 7463. 10.3390/ijms21207463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg V, Muth AN, Ransom JF, Schluterman MK, Barnes R, King IN, Grossfeld PD, Srivastava D. 2005. Mutations in NOTCH1 cause aortic valve disease. Nature 437: 270–274. 10.1038/nature03940 [DOI] [PubMed] [Google Scholar]
- Karczewski KJ, Francioli LC, Tiao G, Cummings BB, Alföldi J, Wang Q, Collins RL, Laricchia KM, Ganna A, Birnbaum DP, et al. 2020. The mutational constraint spectrum quantified from variation in 141,456 humans. Nature 581: 434–443. 10.1038/s41586-020-2308-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly BJ, Fitch JR, Hu Y, Corsmeier DJ, Zhong H, Wetzel AN, Nordquist RD, Newsom DL, White P. 2015. Churchill: an ultra-fast, deterministic, highly scalable and balanced parallelization strategy for the discovery of human genetic variation in clinical and population-scale genomics. Genome Biol 16: 6. 10.1186/s13059-014-0577-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt DC, Kastury RD, Waldrop MA, Kelly BJ, Mosher TM, McLaughlin H, Corsmeier D, Slaughter JL, Flanigan KM, McBride KL, et al. 2018a. In-frame de novo mutation in BICD2 in two patients with muscular atrophy and arthrogryposis. Cold Spring Harb Mol Case Stud 4: a003160. 10.1101/mcs.a003160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koboldt DC, Mihalic Mosher T, Kelly BJ, Sites E, Bartholomew D, Hickey SE, McBride K, Wilson RK, White P. 2018b. A de novo nonsense mutation in ASXL3 shared by siblings with Bainbridge–Ropers syndrome. Cold Spring Harb Mol Case Stud 4: a002410. 10.1101/mcs.a002410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S, Vasilevsky NA, Engelstad M, Foster E, McMurry J, Aymé S, Baynam G, Bello SM, Boerkoel CF, Boycott KM, et al. 2017. The Human Phenotype Ontology in 2017. Nucl Acids Res 45: D865–D876. 10.1093/nar/gkw1039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopanos C, Tsiolkas V, Kouris A, Chapple CE, Albarca Aguilera M, Meyer R, Massouras A. 2019. VarSome: the human genomic variant search engine. Bioinformatics 35: 1978–1980. 10.1093/bioinformatics/bty897 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kouprina N, Liskovykh M, Petrov N, Larionov V. 2020. Human artificial chromosome (HAC) for measuring chromosome instability (CIN) and identification of genes required for proper chromosome transmission. Exp Cell Res 387: 111805. 10.1016/j.yexcr.2019.111805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, Liu Z, Wang L, Xu H, Wang Y. 2019. Thousand and one kinase 1 protects MCAO-induced cerebral ischemic stroke in rats by decreasing apoptosis and pro-inflammatory factors. Biosci Rep 39: BSR20190749. 10.1042/BSR20190749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liskovykh M, Goncharov NV, Petrov N, Aksenova V, Pegoraro G, Ozbun LL, Reinhold WC, Varma S, Dasso M, Kumeiko V, et al. 2019. A novel assay to screen siRNA libraries identifies protein kinases required for chromosome transmission. Genome Res 29: 1719–1732. 10.1101/gr.254276.119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McBride KL, Riley MF, Zender GA, Fitzgerald-Butt SM, Towbin JA, Belmont JW, Cole SE. 2008. NOTCH1 mutations in individuals with left ventricular outflow tract malformations reduce ligand-induced signaling. Hum Mol Genet 17: 2886–2893. 10.1093/hmg/ddn187 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mihalic Mosher T, Zygmunt DA, Koboldt DC, Kelly BJ, Johnson LR, McKenna DS, Hood BC, Hickey SE, White P, Wilson RK, et al. 2019. Expansion of B4GALT7 linkeropathy phenotype to include perinatal lethal skeletal dysplasia. Eur J Hum Genet 27: 1569–1577. 10.1038/s41431-019-0464-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller CR, Lee K, Pfau RB, Reshmi SC, Corsmeier DJ, Hashimoto S, Dave-Wala A, Jayaraman V, Koboldt D, Matthews T, et al. 2020. Disease-associated mosaic variation in clinical exome sequencing: a two-year pediatric tertiary care experience. Cold Spring Harb Mol Case Stud 6: a005231. 10.1101/mcs.a005231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plouffe SW, Meng Z, Lin KC, Lin B, Hong AW, Chun JV, Guan KL. 2016. Characterization of Hippo pathway components by gene inactivation. Mol Cell 64: 993–1008. 10.1016/j.molcel.2016.10.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Retterer K, Scuffins J, Schmidt D, Lewis R, Pineda-Alvarez D, Stafford A, Schmidt L, Warren S, Gibellini F, Kondakova A, et al. 2015. Assessing copy number from exome sequencing and exome array CGH based on CNV spectrum in a large clinical cohort. Genet Med 17: 623–629. 10.1038/gim.2014.160 [DOI] [PubMed] [Google Scholar]
- Satterstrom FK, Kosmicki JA, Wang J, Breen MS, De Rubeis S, An JY, Peng M, Collins R, Grove J, Klei L, et al. 2020. Large-scale exome sequencing study implicates both developmental and functional changes in the neurobiology of autism. Cell 180: 568–584.e523. 10.1016/j.cell.2019.12.036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Zhang DD, Liu JB, Yang XL, Xin R, Jia CY, Wang HM, Lu GX, Wang PY, Liu Y, et al. 2021. Comprehensive analysis to identify DLEU2L/TAOK1 axis as a prognostic biomarker in hepatocellular carcinoma. Mol Ther Nucl Acids 23: 702–718. 10.1016/j.omtn.2020.12.016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith PJ, Zhang C, Wang J, Chew SL, Zhang MQ, Krainer AR. 2006. An increased specificity score matrix for the prediction of SF2/ASF-specific exonic splicing enhancers. Hum Mol Genet 15: 2490–2508. 10.1093/hmg/ddl171 [DOI] [PubMed] [Google Scholar]
- Smith ED, Blanco K, Sajan SA, Hunter JM, Shinde DN, Wayburn B, Rossi M, Huang J, Stevens CA, Muss C, et al. 2019. A retrospective review of multiple findings in diagnostic exome sequencing: half are distinct and half are overlapping diagnoses. Genet Med 21: 2199–2207. 10.1038/s41436-019-0477-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobreira N, Schiettecatte F, Valle D, Hamosh A. 2015. GeneMatcher: a matching tool for connecting investigators with an interest in the same gene. Hum Mutat 36: 928–930. 10.1002/humu.22844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Woerden GM, Bos M, de Konink C, Distel B, Avagliano Trezza R, Shur NE, Barañano K, Mahida S, Chassevent A, Schreiber A, et al. 2021. TAOK1 is associated with neurodevelopmental disorder and essential for neuronal maturation and cortical development. Hum Mutat 42: 445–459. 10.1002/humu.24176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams VC, Lucas J, Babcock MA, Gutmann DH, Korf B, Maria BL. 2009. Neurofibromatosis type 1 revisited. Pediatrics 123: 124–133. 10.1542/peds.2007-3204 [DOI] [PubMed] [Google Scholar]
- Xie B, Fan X, Lei Y, Chen R, Wang J, Fu C, Yi S, Luo J, Zhang S, Yang Q, et al. 2016. A novel de novo microdeletion at 17q11.2 adjacent to NF1 gene associated with developmental delay, short stature, microcephaly and dysmorphic features. Mol Cytogenet 9: 41. 10.1186/s13039-016-0251-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahavich L, Bowdin S, Mital S. 2017. Use of clinical exome sequencing in isolated congenital heart disease. Circ Cardiovasc Genet 10: e001581. 10.1161/CIRCGENETICS.116.001581 [DOI] [PubMed] [Google Scholar]
- Zhou H, Di Palma S, Preisinger C, Peng M, Polat AN, Heck AJ, Mohammed S. 2013. Toward a comprehensive characterization of a human cancer cell phosphoproteome. J Proteome Res 12: 260–271. 10.1021/pr300630k [DOI] [PubMed] [Google Scholar]
- Zhu L, Yu Q, Gao P, Liu Q, Luo X, Jiang G, Ji R, Yang R, Ma X, Xu J, et al. 2020. TAOK1 positively regulates TLR4-induced inflammatory responses by promoting ERK1/2 activation in macrophages. Mol Immunol 122: 124–131. 10.1016/j.molimm.2020.04.006 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data deposition is not permitted under the current consents, but these variants and our interpretations have been submitted to ClinVar (https://www.ncbi.nlm.nih.gov/clinvar/) and can be found under accession numbers VCV001333717.1, VCV001328968.2, and VCV001307834.1.