Abstract
Accumulating evidence proves that endothelial dysfunction is involved in coronavirus disease 2019 (COVID-19) progression. We previously demonstrated that the endothelial surface glycocalyx has a critical role in maintenance of vascular integrity. Here, we hypothesised that serum factors of severe COVID-19 patients affect the glycocalyx and result in endothelial dysfunction.
We included blood samples of 32 COVID-19 hospitalised patients at the Leiden University Medical Center, of which 26 were hospitalised in an intensive care unit (ICU) and six on a non-ICU hospital floor; 18 of the samples were obtained from convalescent patients 6 weeks after hospital discharge, and 12 from age-matched healthy donors (control) during the first period of the outbreak. First, we determined endothelial (angiopoietin 2 (ANG2)) and glycocalyx degradation (soluble thrombomodulin (sTM) and syndecan-1 (sSDC1)) markers in plasma.
In the plasma of COVID-19 patients, circulating ANG2 and sTM were elevated in patients in the ICU. Primary lung microvascular endothelial cell (HPMEC) and human glomerular microvascular endothelial cell (GEnC) cultured in the presence of these sera led to endothelial cell glycocalyx degradation, barrier disruption, inflammation and increased coagulation on the endothelial surface, significantly different compared to healthy control and non-ICU patient sera. These changes could all be restored in the presence of fucoidan.
In conclusion, our data highlight the link between endothelial glycocalyx degradation, barrier failure and induction of a procoagulant surface in COVID-19 patients in ICU which could be targeted earlier in disease by the presence of heparan sulfate mimetics.
Short abstract
The heparan sulfate mimetic fucoidan reduces endothelial activation induced by severe #COVID19 serum, restores the endothelial glycocalyx and the cell–cell barrier, and reverses the endothelial pro-coagulable state https://bit.ly/3tsG2mJ
Introduction
The coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus-2 (SARS-CoV-2) has rapidly spread worldwide and has led to an unprecedented global pandemic since late 2019 [1]. Most of the COVID-19 cases are asymptomatic or cause only mild illness [2]. However, in a considerable proportion of patients, respiratory illness that requires hospitalisation occurred, which could then develop into progressive disease and lead to hypoxaemic respiratory failure, requiring long-term ventilatory support [3, 4]. Besides severe respiratory symptoms during hospitalisation at the intensive care unit (ICU), a high incidence of thromboembolism events was observed [5]. Acute respiratory distress syndrome and coagulopathy are leading causes of COVID-19 patient mortality [6], while endothelial dysfunction is reported to be involved in both acute respiratory distress syndrome (ARDS) and coagulopathy [7, 8]. Consistent with autopsy findings that severe lung endothelial injury was observed in patients who succumbed to COVID-19 [9], COVID-19 could be considered as a vascular disease, and vascular dysfunction might play a critical role in the pathogenesis of ARDS and coagulopathy.
The glycocalyx is a key regulator of endothelial cell integrity and homeostasis. It regulates vascular barrier permeability, prevents inflammation and ensures vessel patency [10–13]. It is a gel-like layer composed of the glycosaminoglycans heparan sulfate (HS), hyaluronan (HA), chondroitin sulfate and associated proteins that covers the luminal surface of vascular endothelial cells [14]. Inflammation-induced glycocalyx degradation can lead to hyperpermeability of blood vessels, vasodilation disorders, microvascular thrombosis and increased leukocyte adhesion [15]. In previous studies, glycocalyx destruction and dysfunction was observed in sepsis, acute kidney injury and ARDS, and was associated with worse patient outcome [15, 16]. Recently, it was reported that the endothelial glycocalyx-degrading enzyme heparanase contributed to vascular leakage and inflammation, and its activity was associated with disease severity in COVID-19 patients [11, 17]. Meanwhile, the MYSTIC study also provided evidence that glycocalyx health, as measured by changes in the perfused boundary region, was a predictive prognostic marker for COVID-19 septic patients [18].
Here we test whether preservation of the endothelial glycocalyx might be an effective intervention to improve vascular health. For this, we examined the possible beneficial effects of the HS mimetic fucoidan [19] in restoring endothelial functionality.
Material and methods
For detailed information see the supplementary material.
BEAT-COVID cohort study population and study design
A prospective observational cohort study was set up, in which patients with polymerase chain reaction (PCR)-confirmed SARS-CoV-2 infection after hospital admission were recruited from April 2020 until August 2020, before the use of medication such as dexamethasone and vaccines were implemented (supplementary figure S1). Plasma and serum samples were collected from 32 patients, of which 26 were hospitalised in the ICU and six on a non-ICU medical floor. In 18 of the 32 included patients, follow-up data were obtained 6 weeks after discharge from the hospital. In addition, samples of 12 age-matched controls were obtained. Patient characteristics are shown in supplementary table S1. Owing to the limited amount of material per sample that could be obtained, pooled sera were used in some experiments as indicated. The trial was registered in the Dutch Trial Registry (NL8589). 12 age-matched healthy donors with a male:female ratio of 2:1 were included after confirmed negative SARS-CoV-2 IgG. Ethical approval was obtained from the Medical Ethical Committee Leiden-Den Haag-Delft (NL73740.058.20).
Circulating markers of endothelial dysfunction and glycocalyx shedding
Plasma samples were used to measure angiopoietin 2 (Ang-2, DANG20; R&D Systems, Abingdon, UK), soluble thrombomodulin (sTM, M850720096; Diaclone, Besançon, France) and soluble syndecan-1 (sSDC1, DY2780, R&D Systems) according to the manufacturer's respective protocols.
Endothelial barrier function assay
Endothelial barrier function analysis was performed with impedance-based cell monitoring using the electric cell-substrate impedance sensing system (ECIS Zθ; Applied Biophysics, New York, NY, USA). ECIS plates (96W20idf PET; Applied Biophysics) were pre-treated with 10 mM L-cysteine and coated with 1% gelatine for primary human glomerular microvascular endothelial cells (GEnCs; ACBRI-128; Cell Systems, Kirkland, WA, USA) or without 1% gelatine for primary pulmonary microvascular endothelial cells (HPMECs; PromoCell, Heidelberg, Germany). HPMECs (passage 5) were seeded at a concentration of 4.5×105 cells well−1 and for GEnCs (p5), the concentration was 3×105 cells well−1 in EGM medium (basal medium MV, C-22220; PromoCell) supplemented with C-39220 (PromoCell) and 1% antibiotics (penicillin/streptomycin, 15070063; Gibco, Paisley, UK) at 37°C and 5% CO2.
Initial baseline resistance was measured for 2 h before endothelial cells were seeded into the plate. Multiple frequency/time mode was used for the real-time assessment of the barrier function. Once the stable monolayer was formed, endothelial cells were incubated with 10% serum (healthy (n=12), COVID-19 non-ICU (n=8) and ICU (n=26)) and measured for 20 h. Afterwards, modelling data Rb, which represents barrier function, could be generated.
In additional experiments, HPMECs were exposed to 10% healthy serum (n=12) and COVID-19 ICU serum (n=26) with or without fucoidan (10 µg mL−1, gift from MicroVascular Health Solutions LLC, Alpine, UT, USA). The natural fucoidan provided (Iso 9000 and GMP certified from Omnipharm, S.A.S, Chambéry, France) was extracted from Laminaria japonica as a powder of 91.20% purity and further tested, for instance, on the presence of heavy metals (arsenic, lead, cadmium, mercury) or microbiology parameters (European Pharmacopoeia VIII, Ed 2,6,12: total plate count, yeast, mould, Escherichia coli, Salmonella spp.). A 50× times stock solution was prepared by dissolving the appropriate amount of powder in milliQ water and passed through a 0.22 µm filter before use.
RNA isolation and reverse transcriptase (RT)-PCR
RNA of cultured cells was isolated using RNeasy Mini Kit (74106; Qiagen, Venlo, The Netherlands) according to the manufacturer's protocol. RT-PCR analysis was conducted using SYBR Select Master Mix (4472908; Applied Biosystems, Landsmeer, The Netherlands) and specific primers as indicated in supplementary table S2. Gene expression was normalised to GAPDH of five separate experiments.
Immunoblotting analysis
Western blots were performed from protein extracts of HPMECs [12]. 10% Mini-PROTEAN® TGX™ Protein Gels (4561031; Bio-Rad Laboratories, Veenendaal, The Netherlands) were used for protein size separation, and proteins were transferred to PVDF membranes (1704156; Bio-Rad). Membranes were blocked in 5% milk in phosphate-buffered saline (PBS) with 0.01% tween20 (PBST) at room temperature for 1 h and further incubated with primary antibodies rabbit anti-human intercellular adhesion molecule (ICAM)1 (4915; Cell Signaling Technology Europe B.V., Leiden, the Netherlands), rabbit anti-human total NF-κB p65 (8242; Cell Signalling Technology), rabbit anti-human phosphor-NF-κB p65 (Ser536) (3033; Cell Signalling Technology) or mouse anti-human GAPDH (MA5-15738; Thermo Fisher, Landsdmeer, the Netherlands) overnight at 4°C. Protein was detected using HRP-conjugated antibody (P0447 and P0448; Dako, Amstelveen, The Netherlands) and Western Lightning Plus-ECL, Enhanced Chemiluminescence Substrate (NEL103001EA; PerkinElmer, Groningen, The Netherlands). Intensity of the bands were analysed using ImageJ software. Relative intensity was determined by levels of GAPDH.
Immunofluorescence of cultured cells
HPMECs (p5) were cultured in eight-well chamber slides (80826, ibiTreat, µ-Slide 8 Well). Confluent monolayers were incubated with 10% pooled serum (healthy control (pooled n=12), non-ICU (pooled n=8) and ICU (pooled n=26)) in no fetal calf serum (FCS) EGM medium for 24 h. Next, cells were fixed with 4% PFA and 0.2% Triton-X100 in Hanks’ buffered salt solution (HBSS) or 4% PFA in HBSS (for HS and LEA staining) for 10 minutes at room temperature, blocked with 3% goat serum in HBSS and incubated with fluorescein isothiocyanate (FITC)-labelled Lycopersicon esculentum (LEA-FITC, L0401; Sigma, Houten, The Netherlands), Mouse Anti-Human VE-cadherin (55-7H1; BD Biosciences, Heidelberg, Germany), Mouse Anti-heparan sulfate (10E4, 370255-1; AMSBIO, Abingdon, UK) or Mouse Anti-TM (MA5-11454; Thermo Fisher) at 4°C overnight, followed by an appropriate secondary antibody and phalloidin-TRITC (VE-cadherin samples) for 1 h. Finally, Hoechst 33528 (1/1000) was added and cells were viewed using a LEICA SP8 WLL confocal microscope (Leica, Rijswijk, The Netherlands). Fluorescent images were analysed using Image J software. Quantification is described in the supplementary material.
FX activation by extrinsic tenase complex (TF-FVIIa)
Confluent HPMECs were incubated with 10% control (n=12), COVID-19 non-ICU (n=8) and ICU serum (n=26, with or without presence of fucoidan) in no FCS medium for 24 h. Cell culture supernatants were collected and centrifuged for ELISA assays. Factor VIIa (80 µL, 10 nM, HCVIIA-0031; Haematologic Technologies, Huissen, The Netherlands) was added and incubated for 15 minutes at 37°C and 5% CO2. The reaction was initiated by adding factor X (80 µL, 400 nM, HCX-0050; Haematologic Technologies) to detect the production of active factor X. Aliquots were quenched in HBSS supplemented with 50 mM EDTA to stop the reaction and determined by SpecXa conversion (250 μM) at 405 nm.
Thrombin generation assay
Confluent HPMECs were incubated with 10% healthy (n=12), COVID-19 non-ICU (n=8) and ICU serum (n=26, with or without presence of fucoidan) in no FCS medium for 24 h. Thrombin generation curves were obtained by supplementing normal pooled plasma. Thrombin formation was initiated by adding substrate buffer (FluCa-kit, 86197; Thrombinoscope BV, Leiden, The Netherlands) to the plasma. Thrombin formation was determined using Thrombinoscope software.
Markers of endothelial dysfunction and glycocalyx shedding in cell cultured supernatant
Supernatants collected were used to measure ANG2, sTM, interleukin (IL)-6 (M9316, Sanquin, Amsterdam, The Netherlands) and von Willebrand factor (VWF) (A0082, Dako).
Statistics
Data are presented as mean±sd, unless indicated otherwise. For all experiments, three to five independent experiments were performed. Shapiro-Wilk test and Levene test were performed to evaluate the normality and variances first. Then nonpaired/paired 2-tailed t-tests were used to assess the differences between two groups. One-way ANOVA followed by Tukey's multiple comparisons test and Kruskal–Wallis test followed by Dunn's multiple comparisons test were assessed for multiple groups. Statistical analysis was performed using SPSS statistical software version 25 (SPSS Inc., Chicago, IL, USA) and GraphPad Prism version 8 (Graphpad Inc., La Jolla, CA, USA). A significance level of 0.05 was considered statistically significant.
Results
COVID-19 leads to endothelial dysfunction and glycocalyx degradation
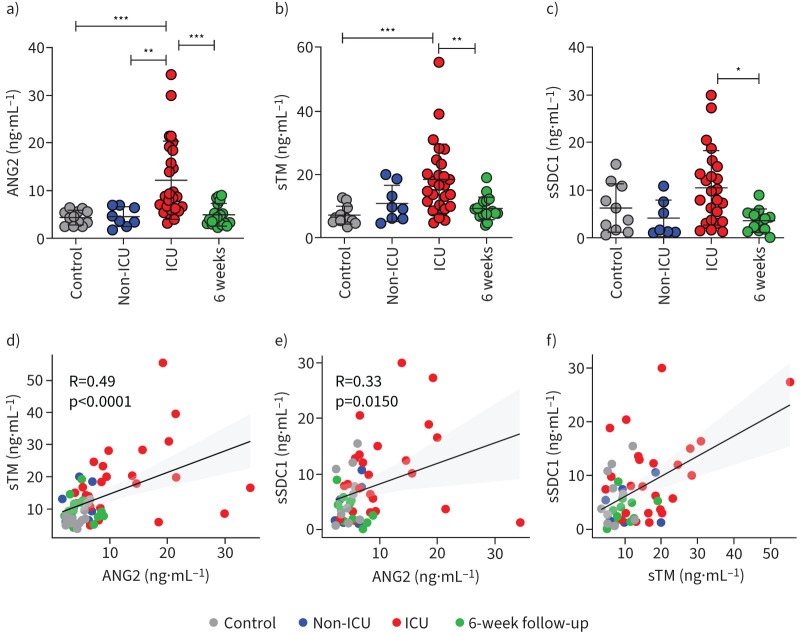
Overall endothelial health in COVID-19 patients was assessed by measuring plasma ANG2 concentrations, which were found to be significantly increased in COVID-19 patients on the ICU (figure 1a). ANG2 levels of non-ICU patients were not distinguishable from control levels or recovered patients 6 weeks after discharge. Circulating fragments of sTM, one of the glycocalyx components, were also markedly increased in COVID-19 ICU patients compared to healthy controls, non-ICU patients and after recovery (figure 1b). Shedding of the glycocalyx core protein, sSDC1, was found not to be significantly raised in COVID-19 ICU patients in comparison to healthy controls or non-ICU patients (figure 1c). Pearson's correlation between each of two markers was analysed and showed that ANG2 moderately correlated with sTM and sSDC1 (R=0.49, p<0.0001 and R=0.33, p=0.015, respectively), and sTM positively correlated with sSDC1 (R=0.50, p=0.0002), indicating that endothelial dysfunction could lead to glycocalyx shedding (figure 1d–f).
FIGURE 1.
Comparison and association of endothelial dysfunction and glycocalyx shedding related markers in coronavirus disease 2019 (COVID-19) patients and healthy controls. Levels of a) angiopoietin 2 (ANG2), b) soluble thrombomodulin (sTM) and c) soluble syndecan-1 (sSDC1) between healthy controls (n=12), COVID-19 non-intensive care unit (ICU) patients (n=8), COVID-19 ICU patients (n=26) and recovered patients (n=18). Pearson's correlation between d) ANG2 and sTM, e) ANG2 and sSDC1, f) sTM and sSDC1. Graphs represent mean±sd. One-way ANOVA followed by Tukey's multiple comparisons test and Pearson's correlation analysis were performed. *: p<0.05; **: p<0.01; ***: p<0.001.
Endothelial glycocalyx degradation in presence of COVID-19 ICU serum
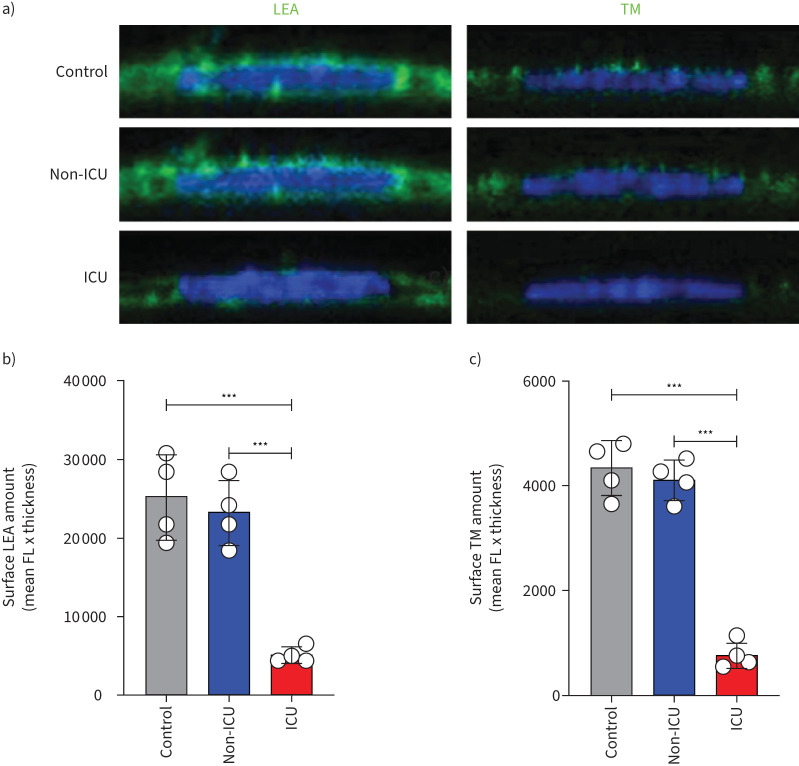
To determine the effect of systemic blood factors during COVID-19 disease on endothelial surface glycocalyx expression, HPMECs were cultured in the presence of 10% pooled serum of either healthy controls, COVID-19 non-ICU and COVID-19 ICU patients for 24 h and stained for overall glycocalyx presence (LEA-FITC), for heparan sulfates (HS) and thrombomodulin (TM). The COVID-19 ICU group showed a marked reduced surface expression of LEA-FITC (figure 2a and b), TM (figure 2a and c) and HS (supplementary figure S2a,b), compared to healthy control and COVID-19 non-ICU groups.
FIGURE 2.
Loss of glycocalyx in primary human pulmonary microvascular endothelial cells in the presence of serum of coronavirus disease 2019 (COVID-19) patients in the intensive care unit (ICU). a) Representative fluorescence (FL) confocal images of Lycopersicon esculentum (LEA)-fluorescein isothiocyanate (FITC) or anti-thrombomodulin (TM) staining on the surface of primary human pulmonary microvascular endothelial cells (HPMECs) in the presence of 10% serum of pooled healthy controls (n=12), COVID-19 non-ICU (n=8) and COVID-19 ICU (n=26) samples for 24 h. HPMEC surface expression quantification of b) LEA-FITC and c) TM in the presence of 10% pooled healthy control, COVID-19 non-ICU and COVID-19 ICU serum for 24 h. All values are given as mean±sd of four independent experiments. One-way ANOVA followed by Tukey's multiple comparisons test was performed. ***: p<0.001.
COVID-19 ICU serum induced loss of endothelial cell barrier function
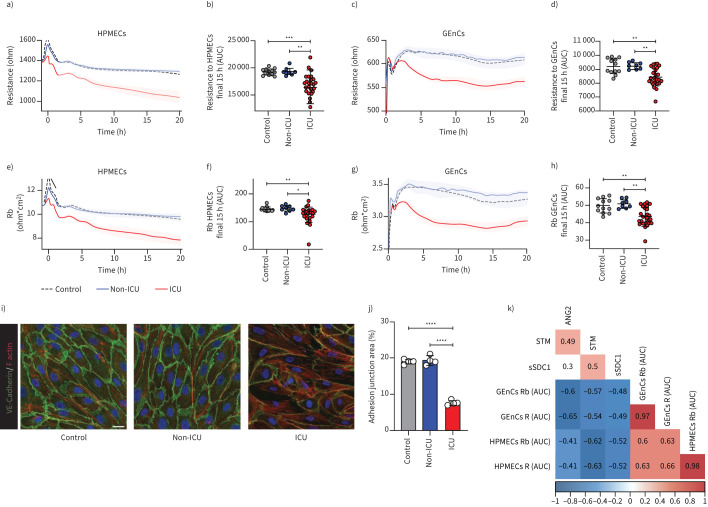
Next, the ability of COVID-19 serum to disrupt the barrier integrity of primary human microvascular endothelial cells was tested. In contrast to healthy controls and COVID-19 non-ICU patients, serum from COVID-19 ICU patients induced a reduction in resistance of both HPMECs and GEnCs (figure 3a and c). The calculated areas under the curve for the final 15 h showed that the COVID-19 ICU samples were significantly below healthy control samples, especially in HPMECs (figure 3b and d). The modelling value for barrier function, Rb, representing cell–cell contacts, resulted in a similar reduction upon exposure to serum of COVID-19 ICU patients (figure 3e–h). Interestingly, we could find differences in vascular bed susceptibility in response to COVID-19 ICU patient sera, showing that the lung-originating HPMECs were more sensitive than the kidney-isolated GEnCs. While resistance and barrier function decreased in HPMECs during the entire incubation time, in GEnCs both factors started to recover after 15 h (figure 3a, c, e and g). Reduction of VE-cadherin, together with reduced cellular junction area, and increasing stress fibre formation in response to COVID-19 ICU patient serum in comparison to COVID-19 non-ICU and healthy control sera confirmed this endothelial cell barrier loss (figure 3i and j). This observed barrier function reduction correlated with the increased plasma levels of ANG2, sTM and sSDC1, linking endothelial cell activation and glycocalyx shedding to possible endothelial loss of barrier function in patients (figure 3k).
FIGURE 3.
Serum mediators of coronavirus disease 2019 (COVID-19) patients in the intensive care unit (ICU) induce microvascular barrier disruption. Barrier integrity parameter, resistance of a and b) primary human pulmonary microvascular endothelial cells (HPMECs) and c and d) primary human glomerular microvascular endothelial cells (GEnCs) assessed by electric cell-substrate impedance sensing system (ECIS Zθ) in response to stimulation with 10% serum (at t=0 h) of healthy controls (n=12), COVID-19 non-ICU (n=8) and COVID-19 ICU (n=26). Cell–cell contact parameter, Rb of e and f) HPMECs and g and h) GEnCs assessed by ECIS Zθ in response to stimulation with 10% serum (at t=0 h) of healthy controls (n=12), COVID-19 non-ICU (n=8) and COVID-19 ICU (n=26). In some cases, multiple samples (in ICU and out of ICU) from the same patient at different time points were obtained. Data of raw resistance and Rb values were shown and presented as mean±sem. Quantification of barrier integrity was based on the measurements of area under the curve (AUC) over the final 15 h of b) HPMECs and d) GEnCs. Quantification of cell–cell contact was based on the measurements of AUC over the final 15 h of f) HPMECs and h) GEnCs. i) Representative confocal images of VE-cadherin (green) and F-actin (red) staining on HPMECs in the presence of 10% pooled healthy control (n=12), COVID-19 non-ICU (n=8) and COVID-19 ICU (n=26) serum for 24 h (scale bar=20 µm). j) Quantification of adhesion junction percentage of HPMECs in the presence of 10% pooled healthy control, COVID-19 non-ICU and COVID-19 ICU serum for 24 h of four independent experiments. Graphs represent the mean±sd. k) Pearson's correlation heatmap between endothelial dysfunction and glycocalyx shedding-related markers (angiopoietin 2 (ANG2), soluble thrombomodulin (sTM) and soluble syndecan-1 (sSDC1)) and barrier function-related parameters (last 15 h AUC of R and Rb in HPMECs and GEnCs). Colours represent correlation, blue means negative correlation and red means positive correlation. Blank means no significance. One-way ANOVA followed by Tukey's multiple comparisons test and Kruskal–Wallis test followed by Dunn's multiple comparisons test were performed. *: p<0.05; **: p<0.01; ***: p<0.001; ****: p<0.0001.
Fucoidan restores glycocalyx and ameliorates endothelial cell activation induced by COVID-19 serum
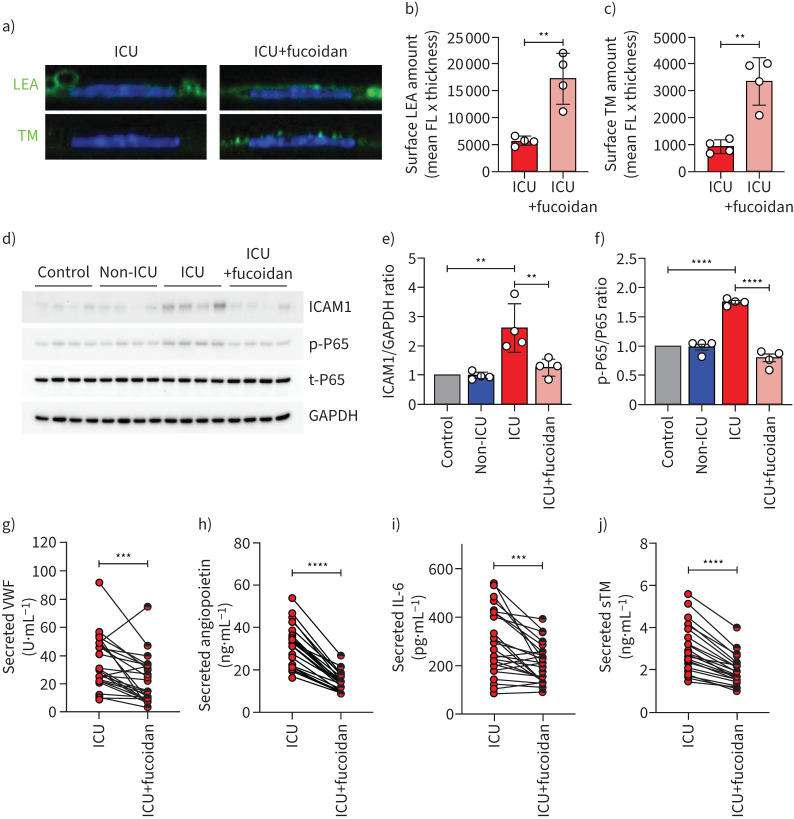
To test whether loss of surface glycocalyx could be restored, we cultured HPMECs in the presence of both ICU patient sera and the HS mimetic fucoidan (10 µg mL−1). Supplementation of fucoidan during culture in the presence of ICU serum dramatically restored the glycocalyx as was shown by LEA-FITC staining (figure 4a and b) and TM expression (figure 4a and c).
FIGURE 4.
Fucoidan restores glycocalyx thickness on human pulmonary microvascular endothelial cells (HPMECs) and reduces endothelial activation. a) Representative confocal images of Lycopersicon esculentum (LEA)-fluorescein isothiocyanate (FITC) or anti-thrombomodulin (TM) staining on the surface of primary HPMECs in the presence of 10% pooled coronavirus disease 2019 (COVID-19) intensive care unit (ICU) (n=26) serum with and without fucoidan (10 µg mL−1) for 24 h. Quantification of HPMECs surface b) LEA and c) TM expression in the presence of 10% pooled COVID-19 ICU serum with and without fucoidan (10 µg mL−1) for 24 h of four independent experiments. d) Western blot images of intercellular adhesion molecule (ICAM)1, p-P65, t-P65 protein expression. Quantification of e) ICAM1/GAPDH ratio and f) p-P65/t-P65 ratio in HPMECs in response to 10% pooled healthy control (n=12), COVID-19 non-ICU (n=8) and COVID-19 ICU (n=26) serum with and without fucoidan (10 µg mL−1) for 24 h of four independent experiments, presented as fold change expression normalised to healthy control. Secreted g) von Willebrand factor (VWF), h) angiopoietin 2, i) interleukin-6 (IL-6) and (j) soluble thrombomodulin (sTM) of HPMECs stimulated with 10% individual COVID-19 ICU sera (n=26) with and without fucoidan (10 µg·mL−1) for 24 h. FL: fluorescence. One-way ANOVA followed by Tukey's multiple comparisons test, Kruskal–Wallis test followed by Dunn's multiple comparisons test and paired two-tailed t-test were performed. **: p<0.01; ***: p<0.001; ****: p<0.0001.
One of the mechanisms of glycocalyx loss is through enzymatic HS degradation by heparanase (HPSE-1), which is elevated in the plasma of COVID-19 patients [17]. In our in vitro cultured HPMECs in the presence of 10% pooled patient or healthy control serum, we observed significantly induced HPSE-1 gene expression in cells from the ICU patient group without a change in the non-ICU group (supplementary figure S3a). Mechanistically, we observed that addition of fucoidan showed a trend of decreasing HPSE-1 gene expression (supplementary figure S3a) in the ICU patient group, which coincided with reduced syndecan-1 gene expression (SDC1) and was significantly restored after fucoidan supplementation (supplementary figure S3b).
Further evaluation of endothelial cell dysfunction in the presence of patient sera showed that compared to healthy controls, gene expression of ANGPT2, ICAM-1 and IL-6 were significantly upregulated in the presence of COVID-19 ICU sera (figure S3c-e), while no effect was found with COVID-19 non-ICU samples. At protein level, ICAM-1 expression was also increased in the presence of COVID-19 ICU serum (figure 4d and e). Since endothelial activation could induce NFκB activation, we then detected the NFκB phosphorylation levels and found a marked activation of these factors in endothelial cells in the presence of COVID-19 ICU sera, without significant changes between healthy control, COVID-19 non-ICU and fucoidan supplementation groups (figure 4d and f). This increased endothelial activation was further corroborated by the induced amount of released VWF and ANG2, from the Weibel–Palade bodies upon activation and secretion of IL-6 and shedding of sTM by these cells (supplementary figure S4). When comparing the response on individual patient serum level, fucoidan reduced these markers significantly (figure 4g–j), in line with the observed normalisation of ICAM-1 expression (figure 4d and e).
Glycocalyx restoration could protect endothelial cell barrier function
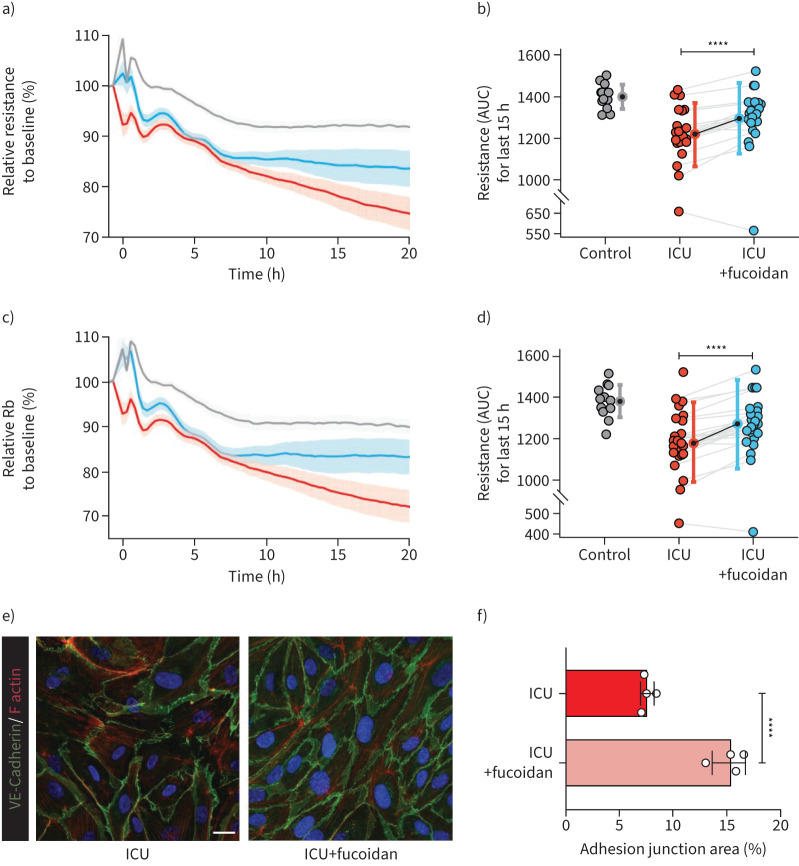
As fucoidan supplementation could restore the glycocalyx and reduce inflammation, we used ECIS to further investigate the effects of fucoidan on protection of the endothelial barrier function. After 24 h of culturing the HPMECs with the various serum samples, addition of fucoidan had no effect during the first 7–8 h. However, in the following time period, endothelial barrier integrity of HPMECs exposed to serum from COVID-19 ICU patients recovered significantly (figure 5a–d). Several samples even reached similar levels to control samples (figure 5b and d). Additionally, VE-cadherin and F-actin staining also showed a similar trend that fucoidan could strengthen the cell–cell contacts against COVID-19 ICU serum exposure (figure 5e and f).
FIGURE 5.
Fucoidan could ameliorate endothelial cell barrier function in presence of serum of coronavirus disease 2019 (COVID-19) intensive care unit (ICU) patients. a) Barrier integrity parameter, resistance of primary human pulmonary microvascular endothelial cells (HPMECs) assessed by electric cell-substrate impedance sensing system (ECIS) in response to stimulation with 10% serum (at t=0 h) of healthy controls (n=12, grey line), COVID-19 ICU (n=26, red line) and COVID-19 ICU with fucoidan (n=26, blue line). Cell–cell contact parameter, Rb of c) HPMECs assessed by ECIS in response to stimulation with 10% serum (at t=0 h) of healthy controls (n=12, grey line), COVID-19 ICU (n=26, red line) and COVID-19 ICU with fucoidan (n=26, blue line). Data were normalised to the baseline resistance or Rb to calculate the relative resistance or Rb to baseline (%) and presented as mean±sem. b) Quantification of barrier integrity was based on the measurements of area under the curve (AUC) of final 15 h. d) Quantification of cell–cell contact was based on the measurements of AUC of final 15 h. e) Representative confocal images of VE-cadherin (green) and F-actin (red) staining on HPMECs in the presence of 10% pooled COVID-19 ICU serum with and without fucoidan (10 µg mL−1) for 24 h (scale bar=20 µm). f) Quantification of adhesion junction percentage of HPMECs in the presence of 10% pooled COVID-19 ICU serum with and without fucoidan (10 µg mL−1) for 24 h of four independent experiments. Graphs represent the mean±sd. Nonpaired and paired two-tailed t-test were performed. ****: p<0.0001.
Fucoidan inhibits the formation of a procoagulant cell surface upon COVID-19 serum treatment
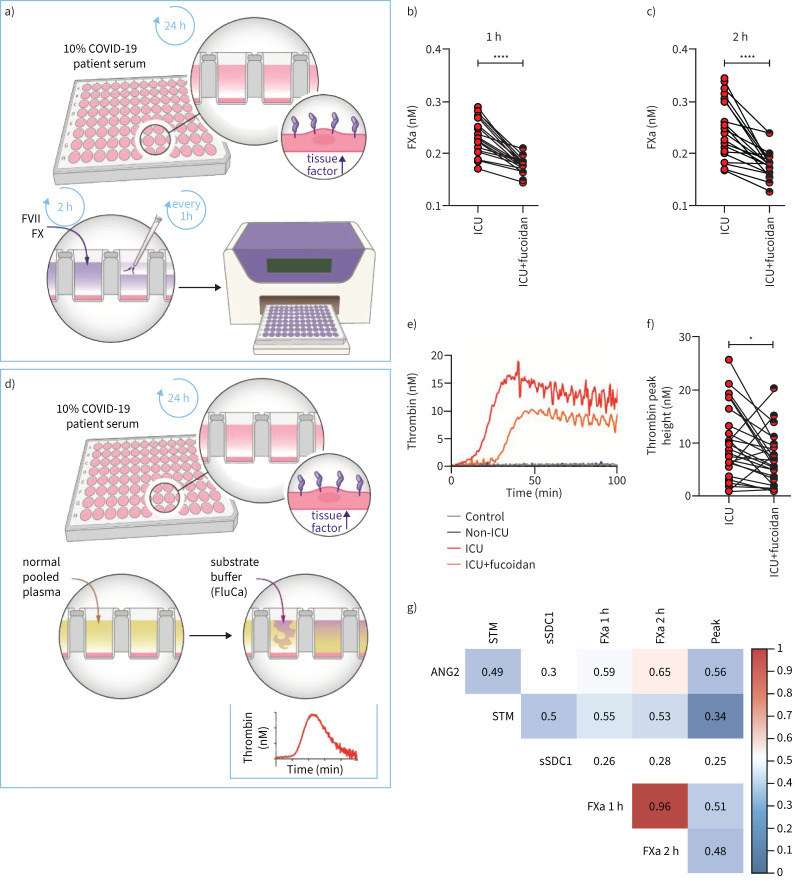
To test whether serum from COVID-19 ICU patients is capable of inducing a procoagulant cell surface, explaining the reported high incidence of (micro)thrombosis in COVID-19 ICU patients [20], we first examined tissue factor gene expression (F3) changes. Indeed, F3 was significantly upregulated in COVID-19 ICU patient samples (supplementary figure S5a), and the presence of fucoidan normalised this expression again to control levels. Next, the production of factor Xa on endothelial cell surface was induced by 10% serum from healthy controls and COVID-19 patients (figure 6a) which resulted in a significant increase in factor Xa production in COVID-19 ICU samples compared to samples from healthy controls and COVID-19 non-ICU, indicating the formation of procoagulant cell surface (supplementary figure S5b and c). Consistent with the observed reduction in F3 mRNA expression with fucoidan present, FX activation was considerably reduced within 1 h and 2 h (figure 6b and c).
FIGURE 6.
Fucoidan inhibits the formation of procoagulant cell surface in response to serum of coronavirus disease 2019 (COVID-19) intensive care unit (ICU) patients. a) Schematic overview showing how to detect FX activation on primary human pulmonary microvascular endothelial cell (HPMEC) surface in response to 10% healthy control or COVID-19 serum. FXa production (nM) in b) first hour and c) second hour on HPMECs surface in the presence of 10% individual COVID-19 ICU sera (n=26) with and without fucoidan (10 µg mL−1) for 24 h. d) Schematic overview showing how to detect thrombin generation on HPMECs cell surface in response to 10% healthy control or COVID-19 serum. e) Representative graph of thrombin generation assay in the presence of 10% healthy control, COVID-19 non-ICU, and COVID-19 ICU with and without fucoidan (same serum induced cell surface). f) Thrombin generation peak height (nM) measured on HPMECs surface in the presence of 10% individual COVID-19 ICU serum (n=26) with and without fucoidan (10 µg mL−1) for 24 h. g) Pearson's correlation heatmap between endothelial dysfunction and glycocalyx shedding-related markers (angiopoietin-2 (ANG2), soluble thrombomodulin (sTM) and soluble syndecan-1 (sSDC1)) and coagulation assay parameters (FXa 1 h, FXa 2 h and thrombin peak height). Colours represent correlation; blue means negative correlated and red means positive correlated. Blank means no significance. Graphs represent the mean±sd and paired two-tailed t-tests were performed. *: p<0.05; ****: p<0.0001.
Thrombin release (figure 6d), another product of the common coagulation pathway, was significantly increased in the COVID-19 ICU group, indicating serum from ICU patients could lead to endothelial cell hypercoagulable state (supplementary figure S5d). When comparing the COVID-19 ICU groups with and without fucoidan added, thrombin peak height, similar to the phenotype in Xa generation assay, showed a significant decrease in fucoidan-treated COVID-19 ICU samples (figure 6f). Correlating FXa and thrombin generation with the plasma levels of ANG2, sTM and sSDC1, we found a link between endothelial activation, glycocalyx degradation and endothelial cell surface coagulable state (figure 6g).
Discussion
Our current study underpins the critical role for loss of the endothelial glycocalyx in severe COVID-19 infections. At a patient level, the endothelial activation marker ANG2 and glycocalyx shedding related markers sTM and sSDC1 were all increased in COVID-19 patients in ICU. At a cellular level, the loss of the endothelial glycocalyx induced by sera of these patients resulted in cellular activation, increased permeability and a hypercoagulable surface. Intervention with the HS mimetic fucoidan restored not only the endothelial glycocalyx but subsequently reduced the endothelial activation state and restored the permeability barrier and anticoagulant cell surface.
Increased circulating ANG2 levels have been associated with diminished respiratory function, increased coagulation activity, acute kidney injury and higher mortality in COVID-19 patients [18, 21–23]. Inhibition of the protective ANG1/TIE2 signalling cascade by ANG2 is a central regulator in protecting the vasculature against thrombus formation and vascular stabilisation [24, 25]. Consistent with our findings, a decrease in plasma ANG2 levels upon recovery argues for ANG2 as a sensitive marker for acute endothelial dysfunction. The observed lower ANG2 levels in our study, together with the relative low levels of sTM and sSDC1, in comparison to non-COVID-19 septic ICU patients, could argue that COVID-19 is a more locally presenting disease [26]. sTM has been reported to be associated with COVID-19 adverse outcome [27], and loss of endothelial surface TM was observed in COVID-19 lung autopsies [28]. We observed that once the microvascular lung endothelial cells were activated, the relatively highly expressed TM (supplementary figure S6) was shed, resulting in a procoagulant cell surface that indicates possible local vulnerability of lung microvessels.
We previously revealed that increased breakdown of HS by heparanase could lead to diminished glycocalyx coverage [29]. Further studies suggest that heparanase activity, the only mammalian enzyme which could degrade HS, was elevated in COVID-19 patients and associated with disease severity [17, 30]. Our observation of increased HPSE-1 mRNA expression upon ICU COVID-19 serum-treated endothelial cells corresponded with reduced LEA-FITC, HS and TM surface expression [31], the induced upregulation of ANG2 and heparanase and loss of endothelial cell barrier function. These findings provide increasing evidence linking endothelial glycocalyx as an additional component of vascular barrier with the ANG/TIE2 signalling pathway to maintain endothelial cell homeostasis. Additionally, lung microvascular endothelial cells were more sensitive to COVID-19 ICU serum than glomerular microvascular endothelial cells, emphasising that organs are differently affected by COVID-19 infection. Notably, the indirect effect by serum mediators, as was also suggested in recent papers [32, 33] in addition to possible direct viral infection, might contribute to patients’ longer hospitalisation or driving the disease progression to severe ARDS.
Coagulation disorder is often seen in COVID-19, especially in severe cases [34]. The endothelial glycocalyx is a crucial compartment for binding and regulating enzymes involved in the coagulation cascade [35]. Tissue factor is a primary trigger of extrinsic coagulation and plays an essential role in haemostasis, which is increased as a result of glycocalyx damage [36, 37]. Combining with our data, the increased endothelial F3 expression corresponded with loss of the endothelial anticoagulant factors, such as TM, in response to incubation with COVID-19 ICU patient serum. This may be a driver of COVID-19 related coagulopathy, which is corroborated by two different ex vivo assays (thrombin generation and Xa generation on endothelial cell surface).
Here we observed that restoration of glycocalyx components by fucoidan leads to reduction of endothelial activation through inactivation of NFκB signalling pathway and downstream ICAM1 expression. Additionally, supplementary fucoidan induced endothelial barrier function recovery, which might open a new therapeutic strategy to treat or prevent respiratory dysfunction. Moreover, fucoidan supplementation has a beneficial effect for anticoagulation. First of all, fucoidan leads to endothelial cell surface tissue factor reduction. Two preprint studies suggested that ANG2 has additional and direct effects on coagulation in COVID-19 [28, 38], and our data demonstrate that fucoidan has a strong effect on decreasing ANG2 expression, preventing TIE2 inactivation. These findings reveal that glycocalyx preservation has promising effects on controlling COVID-19 injury, and our in vitro experiments show that fucoidan as a novel HS mimetic could be a potential therapeutic substance.
Our study had some limitations due to the time of hospitalisation of COVID-19 patients and official ethical approval. The present study only presents a limited group of patients who presented at the Leiden University Medical Center (LUMC) during the first wave before major changes in therapeutic interventions occurred, such as the inclusion of dexamethasone treatment. In addition, no non-COVID-19 ARDS ICU patients were included, limiting the attribution of our data as COVID-19-specific effects. However, similar lung mechanics and increased extravascular lung oedema in COVID-19-related ARDS in comparison to non-COVID-19 ARDS have been found, indicating a similar contribution found in our study [39]. In addition, the fucoidan we used in the present study by itself was not intended for clinical use, but to exemplify possible glycocalyx-restoring mechanisms and endothelial cell functionality.
In conclusion, our present study supports the concept that endothelial cell dysfunction and loss of endothelial glycocalyx might drive worse outcomes like ARDS or coagulopathy in severe COVID-19 at a later disease stage. Fucoidan restored the endothelial glycocalyx and ameliorated endothelial activation, which led to protection of endothelial barrier function and induced antithrombotic effects. Our findings support further validation that glycocalyx preservation or restoration could prevent progression to severe COVID-19 and encourage future clinical trials to evaluate the efficacy of glycocalyx preservation for treatment of more severe forms of COVID-19.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00652-2021.SUPPLEMENT (1.3MB, pdf)
Graphical abstract 00652-2021.GRAPHICAL_ABSTRACT (206.8KB, pdf)
Acknowledgements
We thank all patients and healthy volunteers for taking part in this study. The fucoidan, a main ingredient of the Endocalyx supplement (US patent number 9943572), was exclusively produced for MicroVascular Health Solutions LLC and was provided as a gift.
Proveance: Submitted article, peer reviewed.
BEAT-COVID study group: M.S. Arbous (Dept of Intensive Care, Leiden University Medical Center, Leiden, The Netherlands), B.M. van den Berg (Dept of Internal Medicine, Nephrology, Leiden University Medical Center), S. Cannegieter (Dept of Clinical Epidemiology, Leiden University Medical Center), C.M. Cobbaert (Dept of Clinical Chemistry, Leiden University Medical Center), A. van der Does (Dept of Pulmonology, Leiden University Medical Center), J.J.M. van Dongen (Dept of Immunology, Leiden University Medical Center), J. Eikenboom (Dept of Internal Medicine, Thrombosis and Homeostasis, Leiden University Medical Center), M.C.M. Feltkamp (Dept of Medical Microbiology, Leiden University Medical Centre), A. Geluk (Dept of Infectious Diseases, Leiden University Medical Center), J.J. Goeman (Dept of Biomedical Data Sciences, Leiden University Medical Center), M. Giera (Center for Proteomics and Metabolomics, Leiden University Medical Center), T. Hankemeier (Dept of Analytical Biosciences, Leiden Academic Center for Drug Research, Leiden University Medical Center), M.H.M. Heemskerk (Dept of Haematology, Leiden University Medical Center), P.S. Hiemstra (Dept of Pulmonology, Leiden University Medical Center), C.H. Hokke, J.J. Janse, S.P. Jochems (all Dept of Parasitology, Leiden University Medical Center), S.A. Joosten (Dept of Infectious Diseases), M. Kikkert (Dept of Medical Microbiology), L. Lamont (Dept of Analytical Biosciences), J. Manniën (Dept of Biomedical Data Sciences), T.H.M. Ottenhoff (Dept of Infectious Diseases), M.R. del Prado (Dept of Intensive Care), N. Queralt Rosinach (Dept of Human Genetics, Leiden University Medical Center), M. Roestenberg (Dept of Parasitology), M. Roos (Dept of Human Genetics), A.H.E. Roukens (Dept of Infectious Diseases), H.H. Smits (Dept of Parasitology), E.J. Snijder (Dept of Medical Microbiology), F.J.T. Staal, L.A. Trouw (both Dept of Immunology), R. Tsonaka (Dept of Biomedical Sciences), A. Verhoeven (Center for Proteomics and Metabolomics), L.G. Visser (Dept of Infectious Diseases), J.J.C. de Vries (Dept of Medical Microbiology), D.J. van Westerloo, J. Wigbers (both Dept of Intensive Care), H.J. van der Wijk (Dept of Biomedical Data Sciences), R.C. van Wissen (Dept of Clinical Chemistry), M. Wuhrer (Center for Proteomics and Metabolomics), M. Yazdanbakhsh (Dept of Parasitology) and M. Zlei (Dept of Immunology).
Author contributions: L. Yuan, T.J. Rabelink and B.M. van den Berg conceived and designed the study; L. Yuan, S. Chen, W.M.P.J. Sol and B.M. van den Berg performed experiments and original data collection; L. Yuan, T.J. Rabelink and B.M. van den Berg analysed and interpreted the data; L. Yuan, A.I.M. van der Velden, H. Vink, T.J. Rabelink and B.M. van den Berg edited and discussed the manuscript.
Conflict of interest: L. Yuan reports support for the present manuscript received from the China Scholarship Council grant CSC number 201806270262. H. Vink is Chief Science Officer of MicroVascular Health Solutions LLC (Alpine, UT, USA) and reports the Endocalyx patent (number 9943572) granted to MicroVascular Health Solutions in 2018. The remaining authors have nothing to disclose.
Support statement: This work was supported by the China Scholarship Council grant to L. Yuan (CSC number 201806270262) and BEAT-COVID funding by Leiden University Medical Center.
References
- 1.Cascella M, Rajnik M, Aleem A, et al. Features, Evaluation, and Treatment of Coronavirus (COVID-19). Treasure Island, StatPearls, 2021. [PubMed] [Google Scholar]
- 2.Huang C, Wang Y, Li X, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020; 395: 497–506. doi: 10.1016/S0140-6736(20)30183-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrando C, Suarez-Sipmann F, Mellado-Artigas R, et al. Clinical features, ventilatory management, and outcome of ARDS caused by COVID-19 are similar to other causes of ARDS. Intensive Care Med 2020; 46: 2200–2211. doi: 10.1007/s00134-020-06192-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grasselli G, Zangrillo A, Zanella A, et al. Baseline characteristics and outcomes of 1591 patients infected with SARS-CoV-2 admitted to ICUs of the Lombardy region, Italy. JAMA 2020; 323: 1574–1581. doi: 10.1001/jama.2020.5394 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cui S, Chen S, Li X, et al. Prevalence of venous thromboembolism in patients with severe novel coronavirus pneumonia. J Thromb Haemost 2020; 18: 1421–1424. doi: 10.1111/jth.14830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zhou F, Yu T, Du R, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: a retrospective cohort study. Lancet 2020; 395: 1054–1062. doi: 10.1016/S0140-6736(20)30566-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vassiliou AG, Kotanidou A, Dimopoulou I, et al. Endothelial damage in acute respiratory distress syndrome. Int J Mol Sci 2020; 21: 8793. doi: 10.3390/ijms21228793 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Vallet B, Wiel E. Endothelial cell dysfunction and coagulation. Crit Care Med 2001; 29: Suppl 7, S36–S41. doi: 10.1097/00003246-200107001-00015 [DOI] [PubMed] [Google Scholar]
- 9.Ackermann M, Verleden SE, Kuehnel M, et al. Pulmonary vascular endothelialitis, thrombosis, and angiogenesis in Covid-19. N Engl J Med 2020; 383: 120–128. doi: 10.1056/NEJMoa2015432 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.van den Berg BM, Wang G, Boels MGS, et al. Glomerular function and structural integrity depend on hyaluronan synthesis by glomerular endothelium. J Am Soc Nephrol 2019; 30: 1886–1897. doi: 10.1681/ASN.2019020192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rabelink TJ, van den Berg BM, Garsen M, et al. Heparanase: roles in cell survival, extracellular matrix remodelling and the development of kidney disease. Nat Rev Nephrol 2017; 13: 201–212. doi: 10.1038/nrneph.2017.6 [DOI] [PubMed] [Google Scholar]
- 12.Wang G, Kostidis S, Tiemeier GL, et al. Shear stress regulation of endothelial glycocalyx structure is determined by glucobiosynthesis. Arterioscler Thromb Vasc Biol 2020; 40: 350–364. doi: 10.1161/ATVBAHA.119.313399 [DOI] [PubMed] [Google Scholar]
- 13.Wang G, de Vries MR, Sol W, et al. Loss of endothelial glycocalyx hyaluronan impairs endothelial stability and adaptive vascular remodeling after arterial ischemia. Cells 2020; 9: 824. doi: 10.3390/cells9040824 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Boels MG, Lee DH, van den Berg BM, et al. The endothelial glycocalyx as a potential modifier of the hemolytic uremic syndrome. Eur J Intern Med 2013; 24: 503–509. doi: 10.1016/j.ejim.2012.12.016 [DOI] [PubMed] [Google Scholar]
- 15.Uchimido R, Schmidt EP, Shapiro NI. The glycocalyx: a novel diagnostic and therapeutic target in sepsis. Crit Care 2019; 23: 16. doi: 10.1186/s13054-018-2292-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rovas A, Sackarnd J, Rossaint J, et al. Identification of novel sublingual parameters to analyze and diagnose microvascular dysfunction in sepsis: the NOSTRADAMUS study. Crit Care 2021; 25: 112. doi: 10.1186/s13054-021-03520-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Buijsers B, Yanginlar C, de Nooijer A, et al. Increased plasma heparanase activity in COVID-19 patients. Front Immunol 2020; 11: 575047. doi: 10.3389/fimmu.2020.575047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rovas A, Osiaevi I, Buscher K, et al. Microvascular dysfunction in COVID-19: the MYSTIC study. Angiogenesis 2021; 24: 145–157. doi: 10.1007/s10456-020-09753-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wang J, Zhang Q, Zhang Z, et al. Structural studies on a novel fucogalactan sulfate extracted from the brown seaweed Laminaria japonica. Int J Biol Macromol 2010; 47: 126–131. doi: 10.1016/j.ijbiomac.2010.05.010 [DOI] [PubMed] [Google Scholar]
- 20.McFadyen JD, Stevens H, Peter K. The Emerging threat of (micro)thrombosis in COVID-19 and its therapeutic implications. Circ Res 2020; 127: 571–587. doi: 10.1161/CIRCRESAHA.120.317447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vassiliou AG, Keskinidou C, Jahaj E, et al. ICU admission levels of endothelial biomarkers as predictors of mortality in critically ill COVID-19 patients. Cells 2021; 10: 186. doi: 10.3390/cells10010186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Henry BM, de Oliveira MHS, Cheruiyot I, et al. Circulating level of angiopoietin-2 is associated with acute kidney injury in coronavirus disease 2019 (COVID-19). Angiogenesis 2021; 24: 403–406. doi: 10.1007/s10456-021-09782-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Smadja DM, Guerin CL, Chocron R, et al. Angiopoietin-2 as a marker of endothelial activation is a good predictor factor for intensive care unit admission of COVID-19 patients. Angiogenesis 2020; 23: 611–620. doi: 10.1007/s10456-020-09730-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lovric S, Lukasz A, Hafer C, et al. Removal of elevated circulating angiopoietin-2 by plasma exchange: a pilot study in critically ill patients with thrombotic microangiopathy and anti-glomerular basement membrane disease. Thromb Haemost 2010; 104: 1038–1043. doi: 10.1160/TH10-02-0138 [DOI] [PubMed] [Google Scholar]
- 25.Higgins SJ, De Ceunynck K, Kellum JA, et al. Tie2 protects the vasculature against thrombus formation in systemic inflammation. J Clin Invest 2018; 128: 1471–1484. doi: 10.1172/JCI97488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhatraju PK, Morrell ED, Zelnick L, et al. Comparison of host endothelial, epithelial and inflammatory response in ICU patients with and without COVID-19: a prospective observational cohort study. Crit Care 2021; 25: 148. doi: 10.1186/s13054-021-03547-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goshua G, Pine AB, Meizlish ML, et al. Endotheliopathy in COVID-19-associated coagulopathy: evidence from a single-centre, cross-sectional study. Lancet Haematol 2020; 7: e575–e582. doi: 10.1016/S2352-3026(20)30216-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schmaier AA, Hurtado GP, Manickas-Hill ZJ, et al. Tie2 activation protects against prothrombotic endothelial dysfunction in COVID-19. JCI Insight 2021; 6: e151527. doi: 10.1172/jci.insight.151527 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Boels MG, Avramut MC, Koudijs A, et al. Atrasentan reduces albuminuria by restoring the glomerular endothelial glycocalyx barrier in diabetic nephropathy. Diabetes 2016; 65: 2429–2439. doi: 10.2337/db15-1413 [DOI] [PubMed] [Google Scholar]
- 30.Potje SR, Costa TJ, Fraga-Silva TFC, et al. Heparin prevents in vitro glycocalyx shedding induced by plasma from COVID-19 patients. Life Sci 2021; 276: 119376. doi: 10.1016/j.lfs.2021.119376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stahl K, Gronski PA, Kiyan Y, et al. Injury to the endothelial glycocalyx in critically ill patients with COVID-19. Am J Respir Crit Care Med 2020; 202: 1178–1181. doi: 10.1164/rccm.202007-2676LE [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Birnhuber A, Fliesser E, Gorkiewicz G, et al. Between inflammation and thrombosis: endothelial cells in COVID-19. Eur Respir J 2021; 58: 2100377. doi: 10.1183/13993003.00377-2021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Michalick L, Weidenfeld S, Grimmer B, et al. Plasma mediators in patients with severe COVID-19 cause lung endothelial barrier failure. Eur Respir J 2021; 57: 2002384. doi: 10.1183/13993003.02384-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Iba T, Levy JH, Levi M, et al. Coagulopathy in COVID-19. J Thromb Haemost 2020; 18: 2103–2109. doi: 10.1111/jth.14975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nieuwdorp M, van Haeften TW, Gouverneur MC, et al. Loss of endothelial glycocalyx during acute hyperglycemia coincides with endothelial dysfunction and coagulation activation in vivo. Diabetes 2006; 55: 480–486. doi: 10.2337/diabetes.55.02.06.db05-1103 [DOI] [PubMed] [Google Scholar]
- 36.Grover SP, Mackman N. Tissue factor: an essential mediator of hemostasis and trigger of thrombosis. Arterioscler Thromb Vasc Biol 2018; 38: 709–725. doi: 10.1161/ATVBAHA.117.309846 [DOI] [PubMed] [Google Scholar]
- 37.Ramnath R, Foster RR, Qiu Y, et al. Matrix metalloproteinase 9-mediated shedding of syndecan 4 in response to tumor necrosis factor alpha: a contributor to endothelial cell glycocalyx dysfunction. FASEB J 2014; 28: 4686–4699. doi: 10.1096/fj.14-252221 [DOI] [PubMed] [Google Scholar]
- 38.Hultström M, Fromell K, Larsson A, et al. Elevated angiopoietin-2 inhibits thrombomodulin-mediated anticoagulation in critically ill COVID-19 patients. medRxiv 2021; preprint [ 10.1101/2021.01.13.21249429]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Shi R, Lai C, Teboul JL, et al. COVID-19 ARDS is characterized by higher extravascular lung water than non-COVID-19 ARDS: the PiCCOVID study. Crit Care 2021; 25: 186. doi: 10.1186/s13054-021-03594-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00652-2021.SUPPLEMENT (1.3MB, pdf)
Graphical abstract 00652-2021.GRAPHICAL_ABSTRACT (206.8KB, pdf)