Abstract
Chest Pain Committee of the Chinese Medical Doctor Association, Chinese College of Cardiovascular Physicians, China, Chest Pain Centers Alliance, Executive Committee of China Chest Pain Centers, China Cardiovascular Health Alliance, Headquarter of Chest Pain Centers
In December 2019, an outbreak of novel coronavirus infectious disease (COVID-19)[1] occurred in the city of Wuhan, Hubei Province, China, sparking a grim epidemic. On January 20, 2020, the National Health Commission (NHC) of the People's Republic of China issued a decree (2020 Decree No. 1) that the disease be categorized as Class B infectious diseases as specified in the Law of the People's Republic of China on Prevention and Treatment of Infectious Diseases and be managed as for Class A infectious diseases. Consequently, many regions in China have activated Level I major public health emergency response, and some areas have implemented local traffic control. More than 4000 Chest Pain Centers across China, with regional collaborative rescue as its core mission, are facing new challenges and confusion in actual work. To further standardize and enhance the effectiveness of treatment for patients with acute chest pain and minimize the risk of spread of the COVID-19 epidemic, and fully protect medical staff and patients and their family members, the Chest Pain Committee of the Chinese Medical Doctor Association, Chinese College of Cardiovascular Physicians, China Chest Pain Centers Alliance, Executive Committee of China Chest Pain Centers, China Cardiovascular Health Alliance, and Headquarter of Chest Pain Centers have enlisted the help of Chest Pain Center construction experts across China to timely develop the current consensus. The aim is to provide working guidance during the COVID-19 epidemic for frontline healthcare professionals at Chest Pain Centers across China. The recommendations in the current consensus are only applicable to the unique period of COVID-19 epidemic and are mainly aimed at the patients with both of acute chest pain and suspected/confirmed COVID-19, including patients who do not meet the NHC diagnostic criteria for suspected COVID-19[2],[3] but in whom COVID-19 currently cannot be completely ruled out. Acute chest pain patients in whom COVID-19 has been explicitly excluded are managed according to the Chest Pain Center routine protocols. Once the COVID-19 epidemic has come to an end, Chest Pain Centers shall return to their routine protocols.[4],[5]
The current consensus puts forward the general operating principles of Chest Pain Centers in accordance with the country's COVID-19 prevention and control requirements. Due to regional variations in the severity of the COVID-19 epidemic and control measures and regional disparities in medical resources, the adjustment should be made by each region or hospital on the basis of the current consensus according to local conditions so that region and hospital-compatible workflow procedures and exquisite rules for the implementation may be developed.
Algorithm for Diagnosis and Treatment of Patients
Triage
Each hospital should rearrange its prescreening triage station and fever clinic during the COVID-19 epidemic, according to the NHC requirements for COVID-19 prevention and control.[2],[3] The prescreening triage station should be separate from the Chest Pain Center triage station. The former should be set up at the entrance of the emergency department (ED), and persons who have not undergone prescreening triage are prohibited from entering the outpatient department and ED. All ED patients and outpatients are required to undergo temperature check and complete an epidemiological survey. The prescreening triage personnel should take Level II or above infectious disease protection measures. An epidemiological survey can be waived in severely infected areas.[2] Chest pain patients with suspected COVID-19 should befirst seen at the fever clinic. The fever clinic should be equipped with an electrocardiogram (ECG) machine, particular for the suspected COVID-19 patients if capable. In hospitals without such capabilities, ECG should be conducted by relevant personnel using Level II or above infectious disease protection measures. Chest pain patients in whom COVID-19 has been ruled out by prescreening transfer to the Chest Pain Center triage station to undergo routine procedures. Suspected cases of COVID-19 should be managed according to the Diagnostic and Therapeutic Protocols for Novel Coronavirus Pneumonia (NCP) (Trial Version 5) issued by the NHC of the People' Republic of China.[2]
Chest pain patients with suspected coronavirus infectious disease-19
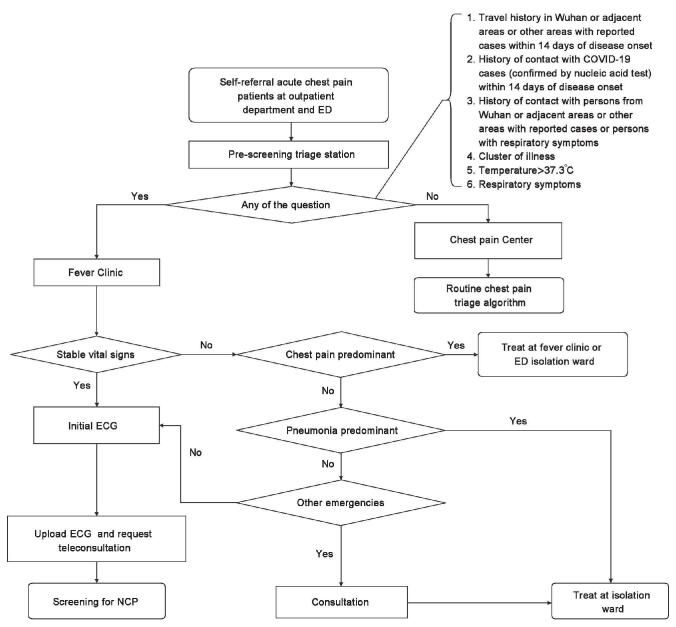
Patients should befirst evaluated for the stability of vital signs. When the vital signs are unstable, the cause of instability should be investigated whether it may be mainly caused by NCP or by chest pain diseases. If the unstable vital signs are due to NCP, the patients are immediately triaged to an isolation ward in the fever clinic for treatment and reported to the public health authorities. When the unstable vital signs are caused by chest pain diseases, the patients should receive immediate rescues in the isolation zone in ED to maintain the stability of vital signs. Hospital staff should take Level II or above infectious disease protection measures and should use Level III infectious disease protection measures during invasive procedures [Figure 1].
Figure 1:
The triage algorithm during the coronavirus infectious disease-19 epidemic
The mean latency period of COVID-19 is 3 days (range: 0–24 days). The general population is susceptible to the disease. Some patients show atypical manifestations, and a sizable proportion of patients may present without fever. Moreover, infected persons may remain asymptomatic and become a source of transmission. Therefore, Level II or above infectious disease protection standards [Appendix 1][6] should be applied for physicians and nurses delivering medical care at ED.[3] Currently, given the lengthy wait for and a relatively high false-negative rate of COVID-19 nucleic acid test results, it is recommended to perform routine chest computed tomography (CT) scans and routine blood tests for patients with chest pain accompanied with fever and/or respiratory symptoms. If time and conditions permit, patients may undergo tests for high-sensitivity C-reactive protein, erythrocyte sedimentation rate, procalcitonin, and respiratory viruses detection.[2],[3] In areas where COVID-19 infection remains prevalent, all hemodynamically stable chest pain patients should undergo a chest CT scan to investigate NCP. Patients in Hubei province should receive a clinical diagnosis of NCP if abnormalities, especially imaging features suggestive of COVID-19 infectious, are demonstrated on a chest CT scan.[2] Such patients in other areas should be placed under the isolation ward for the observation or be transferred to a local COVID-19-designated hospital and undergo nucleic acid testing as soon as possible. Currently, our understanding of COVID-19 is somewhat limited. A proportion of critically ill patients may have concurrent viral pneumonia and fulminant myocarditis with clinical manifestations mimicking acute coronary syndrome (ACS). Such patients, once the diagnosis is established, should be treated according to “the 2017 Chinese Society of Cardiology Expert Consensus Statement on the diagnosis and treatment of adult fulminant myocarditis.”[7] Therefore, great attention should be paid to suspected cases of COVID-19 with chest pain. To provide essential support for the diagnosis and treatment of these patients, each Chest Pain Center should establish a diagnosis and treatment coordination and collaboration system involving ED, fever clinic, and departments of infection control, cardiovascular department, respiratory medicine, the office of medical administration, and other related departments.[INLINE:1]
Algorithm for ST-segment elevation acute myocardial infarction therapy
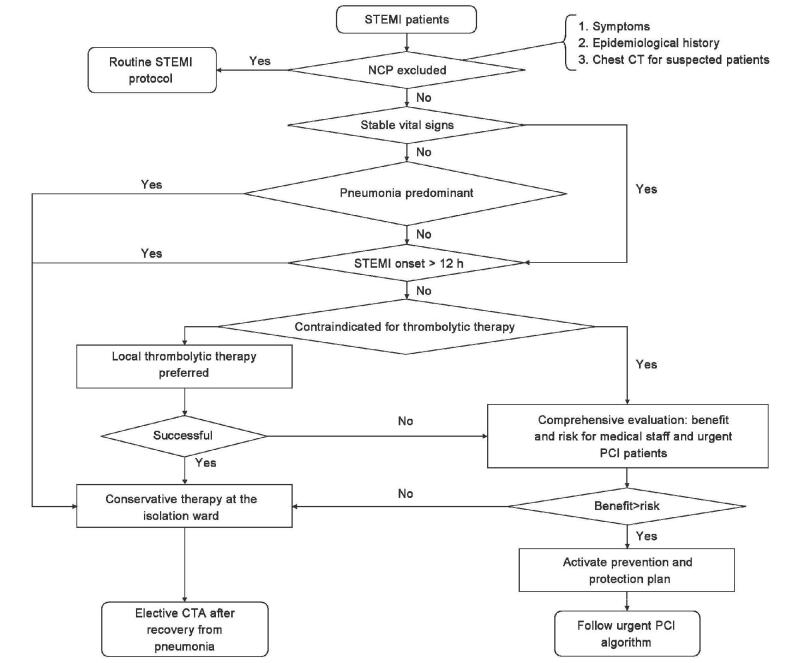
If COVID-19 is explicitly excluded, patients with confirmed ST-segment elevation acute myocardial infarction (STEMI) should be treated routinely according to the Chest Pain Center protocol;[4],[5] however, if patients are initially seen at a non-percutaneous coronary intervention (PCI)-capable hospital, intravenous thrombolytic therapy (third-generation fibrinolytic agents as thefirst choice) should be the therapeutic choice,[8] and patient transfer should be avoided. Regardless whether at standard or grassroots Chest Pain Centers, once STEMI is diagnosed in suspected/confirmed COVID-19 cases, in principle, the patients should receive thrombolytic therapy, if the onset of chest pain is within 12 h. Thrombolytic therapy can be undertaken either in ED or in fever clinic depending on the condition of the hospital but should be in the isolated ward with Level II and preferably Level III infectious disease protection. Patients with successful thrombolysis continue to be observed in the isolation ward and are transferred to the cardiovascular department for elective coronary angiography if COVID-19 is subsequently ruled out and to a COVID-19-designated hospital for further therapy if COVID-19 is confirmed. Patients who have failed to or are contraindicated for thrombolytic therapy should undergo further evaluation for the balance of urgent PCI benefit to the risk shared by both medical staff and patients (risk of infection for physicians and nurses and interventional procedure risk for patients). If the risk outweighs the benefit, or the patient or his family does not agree with the surgery, although the benefit of the PCI results is significant, or the patient's onset time exceeds 12 hours, and the hemodynamic stability is stable, the patient should be transferred to an isolation ward for conservative treatment and investigation of COVID-19. If the benefit is significantly higher than the risk and the patient and family agree to the procedure, then emergency PCI is performed [Figure 2]. For patients who have more than 12 hours of onset but still have symptoms of chest pain or hemodynamic instability, emergency PCI may also be considered after balancing the benefits and risks.
Figure 2:
Reperfusion strategy for ST-segment elevation acute myocardial infarction patients with confirmed/suspected coronavirus infectious disease-19
Algorithm for therapy of confirmed/suspected coronavirus infectious disease patients with non-ST-segment elevation acute coronary syndrome
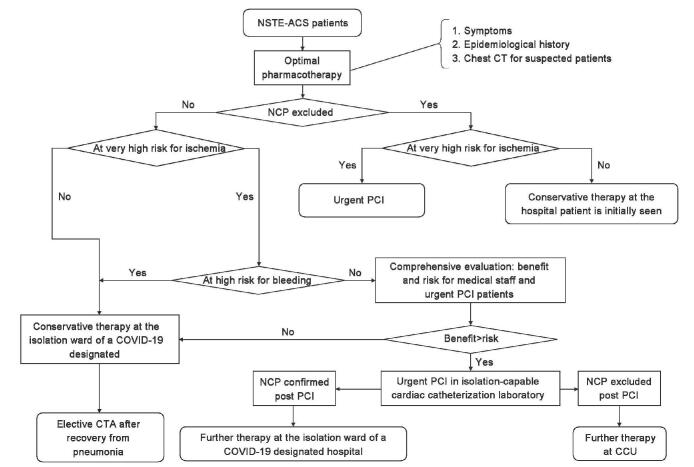
In principle, during the epidemic of COVID-19, all confirmed non-ST-segment elevation ACS (NSTE-ACS) patients are recommended to receive mainly pharmacotherapy at hospitals they are initially seen and undergo investigation for COVID-19. Patients who have failed pharmacotherapy should be transferred to a local urgent PCI-capable and COVID-19-designated hospital for the treatment. Patients receiving adequate pharmacotherapy are stratified for ischemia and bleeding risks, according to the NSTE-ACS diagnosis and treatment guidelines.[9] Patients who are not at very high risk for ischemia or who are at very high risk for both ischemia and bleeding are recommended to undergo conservative therapy in the isolation ward, with the treatment advice by a cardiologist. If the benefit of urgent PCI is expected to outweigh the overall risk for medical staff and patients who are at very high risk for ischemia but not at high risk for bleeding, urgent PCI is recommended and should be conducted in isolation-capable cardiac catheterization laboratory that meets infection control requirements. Patients, if confirmed with COVID-19 post-PCI, receive further treatment in the isolation ward in a COVID-19-designated hospital or are transferred to the coronary care unit (CCU) if COVID-19 is ruled out [Figure 3].
Figure 3:
Algorithm for diagnosis and treatment of confirmed/suspected coronavirus infectious disease-19 patients with non-ST-segment elevation acute coronary syndrome
Algorithm for urgent percutaneous coronary intervention of acute coronary syndrome patients suspected of coronavirus infectious disease-19
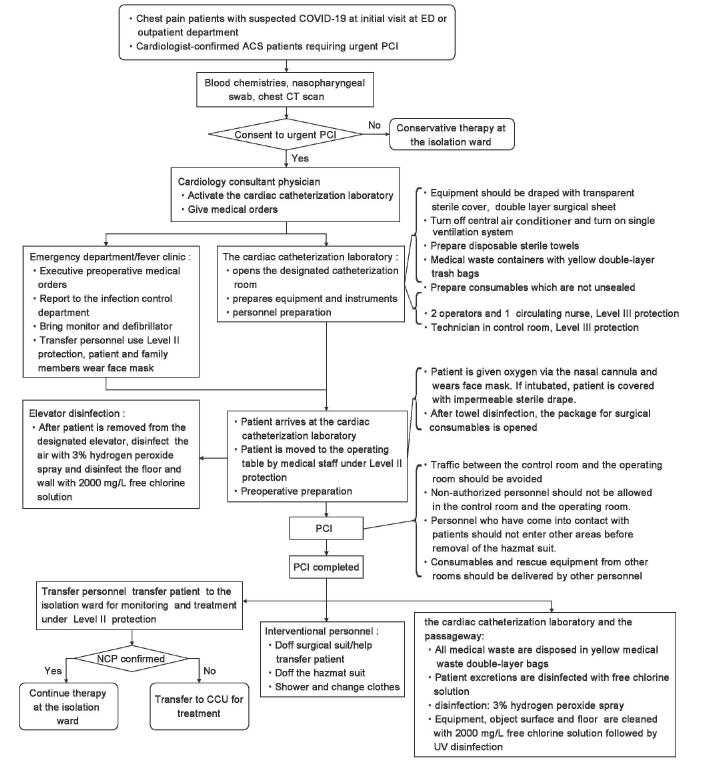
It is rather challenging to prevent and control COVID-19 due to its high infectivity and our current lack of complete understanding of its intermediate host (s) and modes of transmission. Therefore, great caution should be exercised when deciding to proceed with urgent PCI for ACS patients suspected of COVID-19, with full consideration of the benefit of urgent PCI and risk for medical staff and patients. Once a decision to proceed with urgent PCI is made, the key is, apart from adherence to a routine of urgent PCI protocols, effective implementation of infection control measures [Figure 4].
Figure 4:
The flowchart for urgent percutaneous coronary intervention for suspected coronavirus infectious disease-19 cases
Preoperative preparations
Preparations at emergency department/fever clinic
Staff at ED/fever clinic should make sure that patients and family members wear face masks and cap (in principle, only one family member is allowed as a caregiver). Patients with mild hypoxemia should wear a face mask based on nasal cannula oxygen therapy. The faces of intubated mechanically ventilated patients should be covered with disposable impermeable sterile drapes to prevent contamination by nasal or oral secretions. To avoid nonessential on-site consultation, cardiologists are invited for teleconsultation, and on-site consultation, if necessary, should be conducted with Level II infectious disease protection measures. The cardiac catheterization laboratory is activated upon the receipt of informed consent, and preoperative instructions are provided. The physician overseeing the initial visit by a patient is responsible for reporting the case to the appropriate authorities according to infection control requirements,[3] and the Infection Control Department is asked to provide guidance on infection control. Staff at the ED/fever clinic assist the execution of preoperative instructions and preparation of patient transfer. Physicians at ED/fever clinic complete blood chemistries related to the diagnosis of COVID-19 and ACS (blood routine test, liver and kidney function, rapid C-reactive protein, and myocardial biomarkers). All suspected COVID-19 patients should undergo an emergency chest CT scan, and medical staff should take Level II or above infectious disease protection measures. A nasopharyngeal swab is performed if the condition of the patient allows, and the staff performing the task should take Level III infectious disease protection measures. The patient is transferred directly to the cardiac catheterization laboratory upon completion of a chest CT scan.
Preparations at cardiac catheterization laboratory
Urgent PCI for COVID-19 patients should be conducted at a specially designated cardiac catheterization laboratory to avoid cross-infection. On receiving activation instruction, the staff on-duty at the cardiac catheterization laboratory: (1) open the specially designated cardiac catheterization laboratory and close all doors and windows on both sides of the passageway for transporting patients and (2) turn off central air conditioning in the cardiac catheterization laboratory and leave on the independent ventilation system, if any, and the air purifier. (3) Disposable sterile transparent sleeves are used to cover the C arm tube, detector, and control panel on the digital subtraction angiography equipment, and other equipment in the operating room is draped with the disposable sterile transparent cover. (4) Medical waste containers come with yellow double-layer trash bags. (5) Disposable sterile towels are prepared, and (6) consumables of various types to be used for the interventional procedures are prepared, which are not unsealed before arrival, sterilization, and draping of the patient.
Operating room staffing
The number of staff involved in the procedure should be minimized, and the operating room staff include one interventional cardiologist, one assistant, one circulating nurse, and one technician in the control room. The circulating nurse should strictly practice Level III infectious disease protection measures, and in principle, the interventional cardiologist and assistant should also practice Level III infectious disease protection measures. However, Level III infectious disease protection measures may hinder delicate operational maneuvers. Hospitals are recommended to adopt measures based on their conditions that take into consideration infection control, irradiation protection, and the need for delicate operational maneuver. For example, the infection control level may be lowered with the full use of the lead shield to relatively isolating the patient from the operators, or by wearing the isolation suit instead of the hazmat suit or wearing the double-layer surgical gown. In addition, an ultrathin lead apron may be used to minimize the effect of protective measures on the maneuverability of the interventionist. The hazmat suit, lead apron, or surgical gown is donned in the following sequence: perform hand hygienefirst, and then wear a scrub suit, face masks, head cover, and hazmat suit (or isolation suit); put on overshoes, and wear goggles or protective face mask and lead apron; perform hand hygiene, wear a disposable surgical gown (wear the double-layer surgical gown if hazmat suit is not worn), and wear double-layer gloves.
Patient transfer
The followings should be noted during the patient transfer from the ED/fever clinic to the cardiac catheterization laboratory: (1) the patient should wear a face mask and a head cover during transfer. (2) Transfer personnel should use Level II or above infectious disease protection measures and should not stop during the transfer. Family members are not allowed to enter the cardiac catheterization laboratory and should stay in the waiting hall. During the procedure, a second informed consent request should be avoided unless absolutely necessary and contact with patient family members should be minimized. (3) The transfer should be undertaken in a dedicated passageway and, if possible, a dedicated elevator. The elevator should be disinfected immediately by the disinfection team after the patient is pushed out of the elevator; the air (3% hydrogen peroxide spray), floor, wall, and elevator push buttons (chlorine disinfectant) should be disinfected immediately.[10] (4) The patient is transferred to the operating table by medical staff taking Level II or above infectious disease protection measures on arrival at the cardiac catheterization laboratory, and oxygen is continued via the nasal cannula. The face mask is not removed from the patient and the face, if the patient is intubated, is covered with an impermeable sterile drape. After towel disinfection, the package for procedure consumables is opened.
Percutaneous coronary intervention process
Apart from adherence to the basic and procedural requirements for urgent PCI, the following should be noted: (1) Operative procedures should be undertaken gently and cautiously to avoid contamination by blood and body fluid splash. (2) Drugs or equipment from other rooms should be delivered by other personnel for intraoperative use and should not be directly removed from the operating room. (3) Personnel who have come into contact with patients should not enter other areas before the removal of the hazmat suit. (4) Nonauthorized personnel should not be allowed in the control room and the operating room. (5) Traffic between the control room and the operating room should be avoided, and if necessary, personal protective equipment (PPE) should be donned before entry into the operating room; if a person has come into contact with contaminants from the patient, direct return to the control room is prohibited. The person should doff contaminated PPE and don new PPE before return to the control room.
Postpercutaneous coronary intervention management and precautions
In addition to routine management, the following should be noted: (1) Patients: The interventional personnel should doff the outermost surgical gown and transfer the patient by taking Level II or above infectious disease protection measures to a separate isolation CCU ward for further treatment and investigation of COVID-19. The patient is transferred to the CCU ward for further treatment if COVID-19 is ruled out and to a COVID-19-designated hospital for subsequent treatment if COVID-19 is confirmed. (2) Procedure personnel: The surgical gown and the outer gloves are doffedfirst in the operating room, followed by rapid hand hygiene. Then, the lead apron is doffed, followed by hand hygiene. The goggles or protective face shield is removed, followed by hand hygiene and removal of overshoes and doffing of the hazmat suit and the inner gloves, which are disposed of in the medical waste can. After hand hygiene, the interventional personnel enter the buffer zone and doff the head cover and face mask. After leaving the cardiac catheterization laboratory, the interventional personnel take a shower and change clothes.[11] (3) Disinfection of the cardiac catheterization laboratory and the passageway:[9] The operating room, the control room, and the transfer passageway arefirst sprayed with 3% hydrogen peroxide for disinfection. All windows should be closed, if possible, before spraying. Then, the floor, walls, surfaces of objects, and blood stains or contaminated areas are sprayed with 2000 mg/L free chlorine solution and wiped after 30 min. The rest of the floor and walls are wiped with a 2000 mg/L free chlorine solution. (4) Drainage fluid: 2000 mg/L free chlorine solution is added into the suction drain, and after 1 h, the suction drain is decanted into the sewage in the dirty washroom. The disposable drainage container is discarded directly in the medical waste can. Vomitus and feces and excretions are collected in special containers and disinfected with 2000 mg/L free chlorine solution in a ratio of 1:2 for excretions versus disinfectant, and after 2 h, the container is decanted in the sewage. (5) Yellow medical waste double-layer bags are sealed and transported with clear labels. (6) Finally, disinfection by ultraviolet (UV) is carried out. It should be noted that residual chlorine in the air after disinfection with high concentration chlorine disinfectants may cause chlorine poisoning. Doors and windows should be opened to get rid of residual chlorine before UV disinfection.
Recording and reporting
COVID-19 cases should be reported to the public health authorities through a hierarchical system. The procedure record should be entered with “COVID-19” or “suspected COVID-19” by the interventionalist, and the time and the place should be filled in according to the regulation for case tracking.
The urgent percutaneous coronary intervention team and the cardiac catheterization laboratory
A designated urgent PCI team should be established for the COVID-19-infected cases at hospitals with such capabilities. Urgent PCI should be undertaken for all suspected/confirmed COVID-19 patients by taking Level III infectious disease protection measures. Medical staff who are exposed to infection risk during the perioperative period should be placed under isolation, according to the epidemic prevention regulations. Once COVID-19 is confirmed postoperatively in the patient, medical staff in the urgent PCI team are recommended to undergo “medical observation” for 2 weeks; if abnormalities develop during the observation, they should be reported to the public health authorities and treated. PCI-capable COVID-19-designated hospitals are recommended to set up a COVID-19-designated cardiac catheterization laboratory, if the condition allows, for centralized management and special protection.
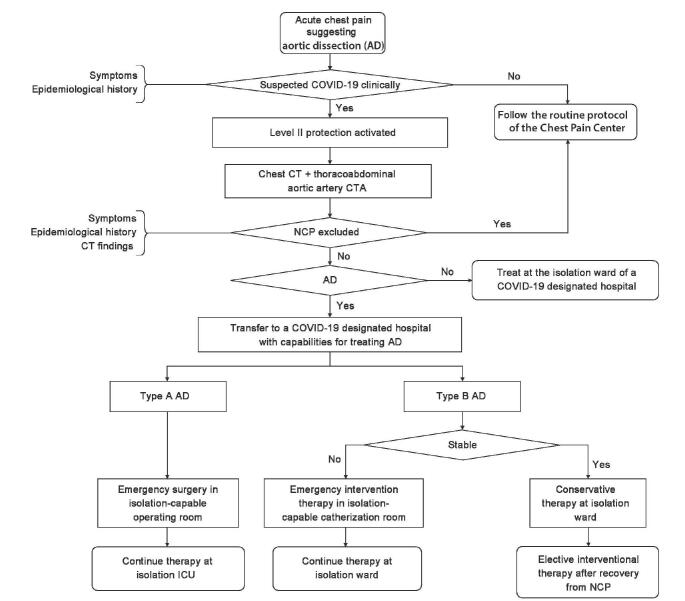
Algorithm for the management of acute aortic dissection patients
The basic workflow for the management of aortic dissection remains identical to that of ACS, as described above. However, aortic dissection should be confirmed by enhancement CT angiography (CTA). CTA and transfer of aortic dissection patients with suspected/confirmed COVID-19 should be undertaken under Level II or above infectious disease prevention. After aortic dissection is confirmed, the decision is made after consideration of aortic dissection types, the stability of vital signs and clinical manifestations, surgery/intervention capabilities of the hospitals, availability of isolation operating room, and whether the hospital is a COVID-19-designated hospital [Figure 5]. If community hospitals encounter difficulties in reading CT and CTA scans, teleconsultation can be provided via the Chest Pain Center regional collaborative rescue network.
Figure 5:
Algorithm for diagnosis and treatment of aortic dissection patients during the coronavirus infectious disease-19 epidemic
Algorithm for the management of acute pulmonary embolism patients
For acute pulmonary embolism management, the basic principle is that patients should complete diagnosis and treatment at CTA-capable hospital where they are initially seen. Thrombolytic therapy can be undertaken, even for high-risk pulmonary embolism, under the guidance by higher-level hospitals. Therefore, the transfer should not be made in principle. High-risk pulmonary embolism patients who are contraindicated for thrombolytic therapy can be managed by referring to the algorithm for urgent PCI for ACS, and interventional therapy can be conducted under close prevention and protection.
Treatment of Chest Pain Patients Admitted Via The Emergency Medical Service
As the latency period of COVID-19 is up to more than 14 days, it is challenging to determine whether a caller with chest pain has COVID-19 or not through simple inquiry by a dispatcher of Emergency Medical Service Center answering the emergency assistance hotline “120.” Therefore, during the COVID-19 epidemic, the protection of the staff on ambulances with the task of providing prehospital emergency care should be strengthened.
In the COVID-19 heavily infected areas, all ambulance personnel are recommended to take Level II or above infectious disease protection measures for personal protection.[12] They should wear face masks upon initial contact with patients and family members. A negative pressure isolation cabin should be installed on the ambulance if the condition allows and disposable instruments and articles for diagnosis and treatment should be used during patient transport, if possible. The outer head cover, face masks, gloves, overshoes, and hazmat suits that have come into contact with the patient during transport are regarded as being contaminated and should be treated according to the infection control requirements. The ambulance should be thoroughly washed and disinfected after each transport mission. Excretions, secretions, vomitus, and other contaminants from the patient during the transport may be carefully removed, if the volume is small, with disposable water-absorbent materials (such as gauze and dishcloth) soaked in 5000–10,000 mg/L chlorine solution, and should be covered, if the volume is large, with hydrous disinfection power or bleach, or with disposable water-absorbent materials. In the latter, absorbent materials (such as a paper towel) arefirst used to remove any visible contaminants, and then, 5000–10,000 mg/L chlorine solution is poured onto the water-absorbent materials. After at least 30 min, careful, thorough cleaning is done.[10]
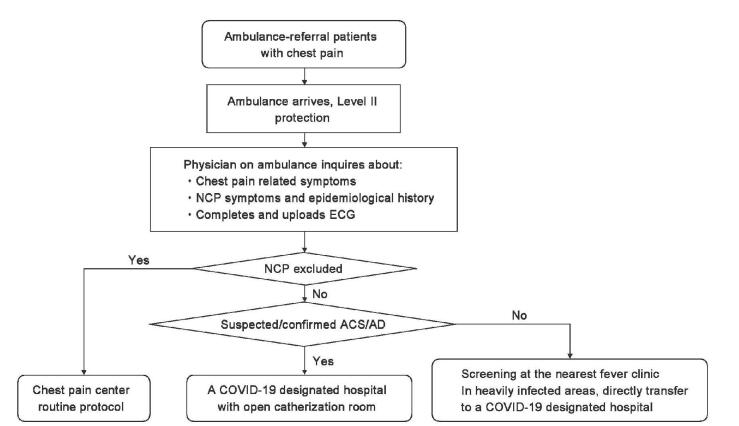
After a patient with acute onset of chest pain makes a phone call to the emergency assistance hotline “120,” the physician in the ambulance, apart from eliciting the history of chest pain, should inquire the COVID-19 infection-associated symptoms and epidemiological history of the patient. Initial ECG is obtained on-site or on the ambulance, if possible, and then immediately transmitted to the nearest Chest Pain Center. The routine protocol of Chest Pain Center should be followed if COVID-19 is completely ruled out clinically. Patients with suspected COVID-19 are screened at the fever clinic. If the diagnosis of COVID-19 remains indeterminate solely based on clinical manifestations, patients are screened at the ED [Figure 6]. In the COVID-19 heavily infected areas, patients are transported directly to a COVID-19-designated hospital with a Chest Pain Center for further investigation without bypass ED.
Figure 6:
The work flowchart for prehospital emergency care during the coronavirus infectious disease-19 epidemic
If the patient is within the reperfusion time window as well as the physician is well experienced in providing prehospital thrombolytic therapy and the prevention and protection condition is excellent, prehospital venous thrombolytic therapy can be conducted in patients with unambiguous STEMI examined by initial ECG on the ambulance and confirmed by teleconsultation. The patient is transported to the ED/fever clinic for further treatment after thrombolytic therapy based on whether COVID-19 can be ruled out according to the above-mentioned algorithm.
Transfer the Chest Pain Patients between Hospitals
Chest Pain Centers emphasize regional coordination and require that patients with acute chest pain are transported in the shortest time to a treatment-capable site for optimal therapy. However, during the COVID-19 epidemic, the infectious disease prevention and control principle is given priority, and the telemedicine network via the regional collaborative rescue network of Chest Pain Centers should be fully utilized so that the patients with acute chest pain are diagnosed early and receive optimized therapy based on the risk/benefit ratio. In principle, local treatment is recommended, and transfer is discouraged to reduce COVID-19 transmission risk. Patients are only transported to other medical institutions when the hospitals' patients have initially seen a lack of necessary diagnostic and treatment capabilities. The rules for referring to other hospitals are detailed below.
Grassroot Chest Pain Centers, after completing diagnosis and treatment of chest pain patients according to the aforementioned triage algorithm, may communicate with higher-level hospitals for transferring patients who have demonstrated need for urgent transfer to higher-level hospitals, including STEMI patients who have failed or are contraindicated for thrombolytic therapy, very high-risk NSTE-ACS patients who remain unstable despite conservative therapy, and patients requiring urgent interventional therapy or surgical aortic dissection patients. Chest Pain Centers' routine procedures for referral are followed after COVID-19 are explicitly ruled out.
Medical staff responsible for transferring chest pain patients with suspected/confirmed COVID-19 should take Level II or above infectious disease protection measures during transfer, and Level III protection measures should be undertaken if available.
If the diagnosis of COVID-19 remains indeterminate or cannot be ruled out, patients with acute chest pain whose diagnosis remains indeterminate and who require transfer to higher-level hospitals for diagnosis should be transferred under infection control measures as for suspected COVID-19 cases to the ED/fever clinic of an urgent interventional therapy-capable COVID-19-designated hospital for further investigation, without the need of bypassing the ED. Modes and methods of transfer differ among different regions, and referral protocols and flowcharts should be refined at various institutions based on their actual conditions and transfer routes.
Ancillary Departments
Given the importance of CT examination in screening COVID-19 infected patients, Chest Pain Centers with such capabilities are recommended to set up a separate CT room for suspected COVID-19 cases. Examiners and transport personnel who are in contact with patients should work under Level II or above infectious disease protection. Immediate disinfection should be conducted after each CT examination. The priority principle should be followed in chest pain patients with suspected COVID-19, especially patients highly suspected of aortic dissection and acute pulmonary embolism. However, infection control requirements should not be less stringent because of emphasis on activating the CT room within 30 min.
The basic requirements for echocardiography of chest pain patients with suspected COVID-19 are identical to those for CT. However, in principle, the transfer of suspected COVID-19 patients to the ultrasound room should be avoided, and bedside ultrasound examination is preferred. Ultrasonographers should conduct bedside ultrasound examination by taking Level II or above infectious disease protection measures.
Time Management
During COVID-19 epidemic, saving time and expediting treatment are recommended under the premise that the infection control requirements and COVID-19 infection screening protocol are honored. However, time management regulations should be adhered to, and data should be reported faithfully. Delays due to infection control or screening for COVID-19 infections should be objectively, accurately, and promptly annotated in the database notes and recorded in the Patient Historical Recordings.
Education and Training and Quality Improvement
During the COVID-19 epidemic, team building and training, joint meetings, quality analysis meetings, and symposium on typical cases should be suspended or conducted via the Internet. It is recommended that each institution should take full use of teleconferences and self-learning via WeChat groups for regular training and discussion. Any work plan that is delayed or canceled due to the epidemic should be recorded and annotated in Chest Pain Center work log, and regular training should be conducted as soon as possible after the epidemic comes to an end and work on improving quality should be continued.
Drafters: Dingcheng Xiang, Shaodong Yi
Participating experts (arranged alphabetically): Aiwu Luo, Bei Shi, Ben He, Bo Yu, Bo Zheng, Cheng Zhang, Chengxing Shen, Chun Liang, Daowen Wang, Difei Shen, Dingcheng Xiang, Dongsheng Li, Feng Liu, Guogang Zhang, Hesong Zeng, Hongwei Yu, Jian'an Wang, Jing Chen, Jingfeng Wang, Jiyan Chen, Jun Bu, Jun Jiang, Junbo Ge, Juying Qian, Kai Huang, Lan Huang, Li Li, Li Shen, Mao Chen, Ning Zhou, Qiutang Zeng, Ruiyan Zhang, Ruizheng Shi, Shaobin Jia, Shaodong Yi, Shuning Zhang, Tongda Xu, Weiyi Fang, Xi Su, Xiang Ma, Xiaoshu Cheng, Xinkai Qu, Xuebo Liu, Yan Wang, Yanjun Gong, Yawei Xu, Yida Tang, Yifei Dong, Yingxian Sun, Yitong Ma, Yong Huo, Yong Zhang, Youqing Tang, Yue Li, Yue Wu, Yuejin Yang, Yuguo Chen, Yun Zhang, Yundai Chen, Zhao Li, Zheng Zhang, Zhenyu Liu.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Appendix
Appendix 1: Infectious disease protection standards
Level I protection measures: Wear work clothes or isolation suit, wear work hat and surgical face masks, and wear latex gloves if necessary.
Level II protection measures: Wear protective face masks (N-95 face masks), wear work clothes, isolation suit or hazmat suit, use overshoes, and wear gloves and work hats.
Level III protection measures: In addition to Level II protection measures, wear a face shield or full-face respirator and wear an impermeable apron if necessary.
Footnotes
For reprints contact: wkhlrpmedknow_reprints@wolterskluwer.com
References
- 1.National Health Commission of People's Republic of China. Notice on Provisional Naming of Novel Coronavirus Pneumonia [EB/OL]. (16-02-2020)[10-03-2020]. http://www.nhc.gov.cn/mohwsbwstjxxzx/s2908/ 202002/f15dda000f6a46b2a1ea1377cd80434d.shtml.
- 2.National Health Commission of People's Republic of China. Diagnostic and Therapeutic Protocols for Novel Coronavirus Pneumonia (Trial Version 5). [EB/OL] (05-02-2020)[10-03-2020]. http://www.nhc.gov.cn/yzygj/s7653p/202002/3b09b894ac9b4204a79db5b8912d4440.shtml.
- 3.National Health Commission of People's Republic of China. Notice on Strengthening Management of Fever Clinics in Key Hospitals in Key Areas and Prevention and Control of Infections in Medical Institutions [EB/OL]. (04-02-2020)[10-03-2020]. http://www.nhc.gov.cn/yzygj/s7659/202002/485aac6af5d54788a05b3bcea5a22e34.shtml.
- 4.Xiang D, Huo Y, Fan W. China Chest Pain Center Accreditation criteria. Chin J Int Cardiol 2016;24:121–30. [Google Scholar]
- 5.Xiang D, Huo Y, Fan W. China grassroots Chest Pain Center accreditation criteria. Chinese J Int Cardiol 2016;24:131–3. [Google Scholar]
- 6.National Health and Family Planning Commission (NHFPC) of the people's Republic of China. Specifications on prevention and control of transmission of airborne diseases at hospitals. WS/ T511-2016[EB/OL]. (17-01-2017) [10-05-2017]. http://www.nhfpc.gov.cn/zhuz/s9496/201701/7e0e8fc6725843aab ba8f841f2f585d2.shtm1.
- 7.Section of Precision Medicine Group of Chinese Medical Association Chinese Society of Cardiology, Editorial Board of Chinese Journal of Cardiology, Working Group of Adult Fulminant Myocarditis. Chinese Society of Cardiology expert consensus statement on the diagnosis and treatment of adult fulminant myocarditis. J Int Intensive Med 2017;23:443–53. [Google Scholar]
- 8.Chinese Medical Association Chinese Society of Cardiology, Editorial Board of Chinese Journal of Cardiology. Guideline on diagnosis and treatment of patients with acute ST-segment elevation myocardial infarction. Chin J Cardiol 2019;47:766–83. [DOI] [PubMed] [Google Scholar]
- 9.Chinese Medical Association Chinese Society of Cardiology. Guideline on diagnosis and treatment of patients with non-ST-segment elevation acute coronary syndrome (2016). Chin J Cardiol 2017;45:359–73. [Google Scholar]
- 10.National Health Commission of People's Republic of China. Technical Specifications for Disinfection at Medical Institutions [EB/OL]. (01-08-2012)[11-02-2020]. http://www.nhc.gov.cn/wjw/s9496/201204/54510.shtml.
- 11.National Health Commission of People's Republic of China. Technical guideline for Prevention and Control of Novel Coronavirus Infection at Medical Institutions (First Edition [EB/OL]). (23-01-2020)[06-02-2020]. http://www.nhc.gov.cn/yzygj/s7659/202001/b91fdab7c304431eb082d67847d27e14.shtml.
- 12.National Health Commission of People's Republic of China. Working Plan on Transfer of Novel Coronavirus Pneumonia Cases (Provisional) [EB/OL]. (28-01-2020)[06-02-2020]. http://www.nhc.gov.cn/xcs/zhengcwj/202001/ccee6ec0942a42a18df8e5ce6329b6f5.shtml.