Abstract
Shedding light on the mechanisms that underlie durable response to immunotherapy, a new study evaluating memory T cell responses in long-term melanoma survivors treated with immunotherapy finds that tumor-associated clonotypes are sustained over years and persist as expanded, IFNγ-expressing TRM cells in the skin with TEM counterparts in the blood.
Cancer immunosurveillance mediated by CD8+ cytotoxic T cells (CTLs) leads to T cell receptor (TCR) recognition of tumor-associated antigens followed by killing of tumor cells, enabling favourable patient outcomes. More recently, CD8+ tissue-resident memory cells (TRM), a distinct subset of non-recirculating memory CD8+ T cells, have been recognised as critical players in anti-tumor immune responses, suggesting that the quality rather than just the quantity of tumor-infiltrating CD8+ T cells is important for clinical outcomes1–3. Despite the protective effect of CD8+ CTLs, most tumors fail to regress as a result of the immunosuppressive milieu fostered by the tumor. In addition to suppressive populations such as CD4+ regulatory T cells (Tregs), tumor-associated fibroblasts and macrophages, tumor immune escape is facilitated by T cell “exhaustion”. Chronic antigen stimulation within tumors leads to a state of T cell dysfunction and increased expression of multiple inhibitory receptors or immune checkpoints4. Immunotherapy serves to counteract these inhibitory pathways, and can reinvigorate the T cells and enhance T cell infiltration, expansion and effector functions within tumors5.
Immunotherapy achieves remarkable and often durable clinical response in a variety of advanced cancers, however such responses occur only in a minority of patients6. The factors that promote therapeutic response versus treatment resistance has been the subject of much investigation. A comprehensive understanding of the multifaceted mechanisms, globally as well as at the level of the individual cancer and patient, would guide choice of immunotherapy to promote robust anti-tumor immune responses required for tumor regression and improved patient outcomes. Tumor-intrinsic features such as tumor neoantigen load on the one hand, PD-L1 expression, disruption of antigen presentation and co-optation of mesenchymal transition or angiogenesis on the other, have been reported to impact response7. Recently, response to immunotherapy has been related to host-intrinsic factors such as HLA genotype8. The immune correlates of response to immunotherapy have also been studied in different compartments during treatment. Pre-existing tumor immune infiltration and an increase in the numbers of CD8+ TILs early during immunotherapy has been reported in treatment responders9. In peripheral blood, the ratio of reinvigorated PD-1+CTLA-4+ “exhausted” CD8+ T cells to tumor burden has been proposed as a predictive biomarker of response10. In addition, several studies have found a positive correlation with signatures of T cell states, including activation, exhaustion, cytotoxicity, or the presence of a TCF1+ stem-like CTL subset to clinical response11,12. Whilst these elegant studies provide insights into potential mechanisms activated by treatment, the features of durable anti-tumor immune responses in those patients that demonstrate long-term clinical remission remain unknown.
In this issue of Nature Cancer, Han et al. report the molecular features of CD8+ memory T cell responses across tissues and over years in patients with malignant melanoma (MM) who are long-term survivors following immunotherapy (Figure 1). The authors previously showed that vitiligo, resulting from autoimmune destruction of normal host melanocytes, is required to sustain melanocyte antigen-specific T cells in mouse models, consistent with its recognition as a positive prognostic factor in melanoma patients13. In the current study, they evaluated matched melanoma tissue, distant vitiligo-affected skin and blood collected at least one year after initiation of a variety of immunotherapy regimens, and one month after therapy completion, from four long-term MM survivors. By application of paired single-cell RNA sequencing (scRNA-seq) and T-cell receptor (TCR) sequencing (scTCR-seq) to flow-sorted CD8+ T cells, they generated an integrated map of transcriptional profiles and TCR specificities and tracked them over time.
Figure 1:
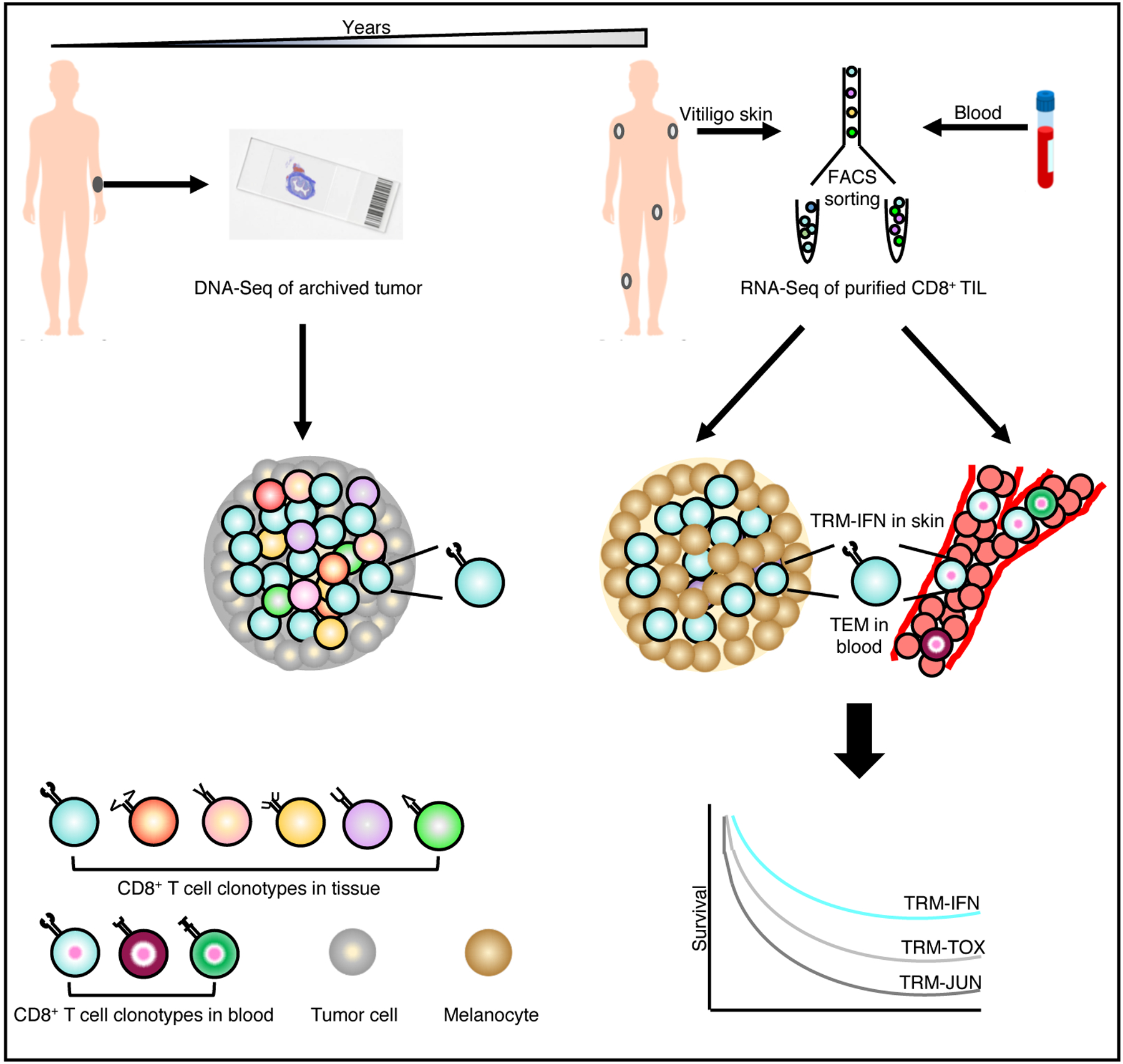
In long-term melanoma survivors, tracking of tumor-infiltrating CD8+ T cell clonotypes over years reveals their persistence as tissue-resident memory cells with effector capabilities (TRM-IFN) in vitiligo-affected skin and as effector memory T cells (TEM) circulating in the blood. TRM-IFN subset was the most significant positive prognostic indicator relative to the other TRM subsets, TRM-TOX and TRM-JUN, with features of exhaustion, and TCR activation, respectively.
Based on gene expression program, the authors report enrichment of TRM cells in tumor and vitiligo-affected skin, which was confirmed by flow cytometry and immunohistochemistry. Unsupervised clustering analysis demonstrated five clusters (C1–5) comprising predominantly of CD8+ T cells from blood and five clusters (C6–10) consisting of those from tumor and/or skin. Blood-derived clusters (C1–5) were each enriched for CD8+ T cells with different circulating phenotypes such as effector memory (TEM), effector (TEFF), central memory (TCM), naïve (TNAV) and mucosal-associated invariant T cells (MAIT). Among the tissue-derived clusters, C6 was enriched for skin CD8+ T cells whilst C7 was composed of CD8+ T cells with a TCF7+ stem cell memory phenotype (TSCM) from the tumor. Three clusters (C8, C9, C10) were composed of CD8+ T cells from both skin and tumor, and strikingly, each of these three clusters were enriched for TRM cells with distinct functional and phenotypic features. TRM cells in C8 (TRM-FOS) showed features linked to TCR signaling whilst those in C10 (TRM-TOX) were enriched for transcripts encoding immune checkpoints (TOX, LAG3, PDCD1 and CTLA4), cytotoxicity mediators (PRF1, GZMB), transcription factor BLIMP1 and the classical TRM marker, CD103. Cluster C9 (TRM-IFNG) was composed of TRM cells marked by high expression of effector molecules such as cytokines (IFNG, TNF) and chemokines (CCL3, CCL4). By overlaying TRM subset signatures onto data from the TCGA dataset, the authors report that TRM-IFNG cells were the most significant predictor of patient survival, even after adjusting for confounders such as TIL density and other clinical variables. Interestingly, the TRM-TOX+ cells also had positive predictive value despite displaying an “exhausted” phenotype. We envision that this subset may be important particularly in the setting of large tumors early in treatment given that the “exhausted” phenotype imposed by TOX following chronic antigenic stimulation is critical for preventing T cell overstimulation, activation-induced cell death and limiting collateral tissue damage14. Although the authors did not correlate the non-TRM clusters with patient survival, it will be interesting and pertinent to elucidate if the tumor TCF7-expressing TSCM subset is linked to long-term outcome, given that expansion of these cells has been favourably associated with response to immunotherapy in prior studies11.
Next, Han et al. leveraged paired scTCR-seq data from the same four MM patients to correlate transcriptomic profiles with T cell clonality across tissue compartments. Of all the tumor-associated clonotypes that were expanded within tumors, they found that thirty-three clonotypes contained counterparts in vitiligo-affected skin. Among the thirty-three clonotypes, fifteen were also identified in the blood (“Resident/Circulating” clonotypes) whilst eighteen were confined to tumor and skin (“Resident-Only” clonotypes). Notably, the “Resident/Circulating” clonotypes were enriched in TRM-IFNG cells in tumor and skin, and existed as TEM cells in the blood, suggesting a common clonal precursor for these cells. The “Resident/Circulating” clonotypes also had the highest frequencies indicating robust expansion among the TRM-IFNG cells. Furthermore, the authors identified melanoma differentiation antigen (MDA)-specific responses in both skin and blood in a separate cohort of MM survivors, although the precise specificities of the “Resident/Circulating” clonotypes were not evaluated and warrants future investigation.
A unique aspect of this study is the demonstration of durable CD8+ T cell memory. In seven long-term MM survivors, the authors performed bulk TCRβ DNA sequencing on skin, blood, and archival primary and metastatic tumors and showed long-term persistence of tumor-associated clonotypes in vitiligo-affected skin and blood, even up to nine years following tumor resection. The authors report that skin, relative to blood, showed greater enrichment for tumor-associated TCR repertoire and those clonotypes that were expanded in the skin were also more expanded in the blood. These findings suggest that the skin not only can act as a ‘museum’ of how immunological challenges were overcome in the past, but that the skin may also play a crucial role in the maintenance of anti-melanoma immunity. To shed light on the gene expression profile of these long-lived clonotypes, Han et al. analyzed one patient who had optimal archival tumor TCR DNA sequencing data and scTCR-seq data and found that the persistent tumor-associated clonotypes were indeed TRM-IFNG cells in the skin and TEM cells in the blood.
In summary, systematic analysis by Han et al. links long-term response and patient survival following immunotherapy to the persistence of a distinct TRM subset with effector function and shared tumor-associated TCRs in vitiligo-affected skin. Given that the skin-resident tumor-associated clonotypes had clonally expanded counterparts in the blood as TEM cells, their findings suggest that skin may serve as a niche of host-wide memory response. TRM cells have been associated with prognosis in a wide variety of cancers even in treatment-naïve settings. Whether and how the phenotypic and functional properties of TRM cells change in a tissue-specific and context-dependent manner, and which subsets are most critical for tumor elimination at specific stages are not known. Future studies that will construct a trajectory of CD8+ T cell profiles integrated with multi-omics data in a longitudinal fashion will provide comprehensive insights into T cell responses and enable rational choice of immunotherapy at every stage of disease to promote long-term remission.
References
- 1.Ganesan AP, et al. Tissue-resident memory features are linked to the magnitude of cytotoxic T cell responses in human lung cancer. Nature immunology 18, 940–950 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Savas P, et al. Single-cell profiling of breast cancer T cells reveals a tissue-resident memory subset associated with improved prognosis. Nature medicine 24, 986–993 (2018). [DOI] [PubMed] [Google Scholar]
- 3.Edwards J, et al. CD103(+) Tumor-Resident CD8(+) T Cells Are Associated with Improved Survival in Immunotherapy-Naive Melanoma Patients and Expand Significantly During Anti-PD-1 Treatment. Clinical cancer research: an official journal of the American Association for Cancer Research 24, 3036–3045 (2018). [DOI] [PubMed] [Google Scholar]
- 4.Wherry EJ T cell exhaustion. Nature immunology 12, 492–499 (2011). [DOI] [PubMed] [Google Scholar]
- 5.Sharma P & Allison JP Immune checkpoint targeting in cancer therapy: toward combination strategies with curative potential. Cell 161, 205–214 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Topalian SL, et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. The New England journal of medicine 366, 2443–2454 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rizvi NA, et al. Cancer immunology. Mutational landscape determines sensitivity to PD-1 blockade in non-small cell lung cancer. Science 348, 124–128 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chowell D, et al. Patient HLA class I genotype influences cancer response to checkpoint blockade immunotherapy. Science 359, 582–587 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen PL, et al. Analysis of Immune Signatures in Longitudinal Tumor Samples Yields Insight into Biomarkers of Response and Mechanisms of Resistance to Immune Checkpoint Blockade. Cancer Discov 6, 827–837 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang AC, et al. T-cell invigoration to tumour burden ratio associated with anti-PD-1 response. Nature 545, 60–65 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sade-Feldman M, et al. Defining T Cell States Associated with Response to Checkpoint Immunotherapy in Melanoma. Cell 175, 998–1013 e1020 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Riaz N, et al. Tumor and Microenvironment Evolution during Immunotherapy with Nivolumab. Cell 171, 934–949 e916 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Malik BT, et al. Resident memory T cells in the skin mediate durable immunity to melanoma. Science immunology 2(2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scott AC, et al. TOX is a critical regulator of tumour-specific T cell differentiation. Nature 571, 270–274 (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]


