Abstract
The first-line treatment of advanced and metastatic human epidermal growth factor receptor type 2 (HER2+) breast cancer requires two HER2-targeting antibodies (trastuzumab and pertuzumab) and a taxane (docetaxel or paclitaxel). The three-drug regimen costs over $320,000 per treatment course, requires a 4-hour infusion time, and has many adverse side effects, while achieving only 18 months of progression-free survival. To replace this regimen, reduce infusion time, and enhance efficacy, a single therapeutic was developed based on trastuzumab-conjugated nanoparticles for co-delivering docetaxel and siRNA against HER2 (siHER2). The optimal nanoconstruct has a hydrodynamic size of 100 nm and specifically treats HER2+ breast cancer cells over organ-derived normal cells. In a drug-resistant orthotopic HER2+ HCC1954 tumor mouse model, the nanoconstruct inhibited tumor growth more effectively than the docetaxel and trastuzumab combination. When coupled with microbubble-assisted focused ultrasound that transiently disrupted the blood brain barrier, the nanoconstruct inhibited the growth of trastuzumab-resistant HER2+ BT474 tumors residing in brains of mice. The nanoconstruct has a favorable safety profile in cells and in mice. Combination therapies have become the cornerstone of cancer treatment and this versatile nanoparticle platform can co-deliver multiple therapeutic types to ensure that they reach the target cells at the same time to realize their synergy.
Keywords: nanoparticle, siRNA, taxane, HER2+, breast cancer
1. Introduction
Increased understanding of cancer biological pathways has resulted in many new cancer therapies. However, monotherapy generally leads to only incremental, short-lived clinical benefits. To overcome this challenge, combination therapies – in which complementary biological pathways are concurrently targeted by different therapies – have become the cornerstone of cancer therapy including for HER2+ breast cancer.
About 2.3 million new cases of breast cancer were diagnosed worldwide,[1] and HER2+ subtype accounts for about 15–25% of invasive breast cancer.[2, 3] Trastuzumab (anti-HER2 monoclonal antibody) given with a taxane has substantially improved the prognosis of this subtype,[4] but resistance to the treatment is still common. To overcome the resistance, newer HER2-targeted therapies (e.g., pertuzumab, lapatinib, neratinib) have been evaluated clinically,[5–7] but the best outcomes were achieved with a combination of pertuzumab, trastuzumab, and taxane.[8] Thus, this combination serves as the first-line treatment for metastatic and advanced HER2+ breast cancer. Nevertheless, this regimen has improved progression-free survival to only 18.5 months (vs. 12.4 months on trastuzumab + docetaxel) in patients with HER2+ metastatic breast cancer.[8]
Various classes of therapeutics have different pharmacokinetics and often do not reach and uptake into target cells at the same time. This includes the aforementioned drug combination for treating HER2+ breast cancer.[9, 10] To improve combination therapies, herein we report a versatile targeted nanoparticle platform capable of co-delivering multiple types of cargos in order to realize their full synergy.
This nanoparticle platform is also capable of delivering small interfering RNA (siRNA) in our previous report.[11] siRNA holds great promise in cancer therapeutics because over 85% genes important in cancer are not druggable by traditional drugs.[12] siRNA can be designed to silence any gene responsible for different cancer hallmarks,[13] such as angiogenesis, invasion, immune evasion, drug resistance, and metastasis.[14, 15] Thus, a successful siRNA-based therapy has potential to revolutionize cancer therapy. The nanoparticle platform is based on mesoporous silica nanoparticles (MSNP) coated layer-by-layer with (1) bioreducibly cross-linked polyethylenimine (PEI) and (2) polyethylene glycol (PEG). Trastuzumab (anti-HER2 antibody) is conjugated to the PEG chain of the nanoparticle and serves as the homing/targeted delivery to cancer over-expressing HER2. It also has some therapeutic activity on the NP.[11, 16] siRNA against HER2 (siHER2) is loaded last onto the nanoparticle via electrostatic interaction with PEI (and protected under the PEG layer). We found that siHER2 delivered by our particle exhibited superior in vitro and in vivo therapeutic activity than HER2-targeted therapies used in clinics, such as trastuzumab and lapatinib.[11, 16] This is due to more complete inhibition of the targeted protein with RNA interference mechanism. siRNA inhibits the synthesis of the targeted protein, while monoclonal antibodies and small molecule inhibitors merely block its activity without halting its production. Further, we also reported that cells treated long-term with our siHER2-nanoparticles were less prone to develop therapeutic resistance than cells treated long-term with trastuzumab and lapatinib.[17] This result is encouraging, and supports the development of siRNA therapeutics as a superior alternative to current HER2-targeted therapies.
To overcome limitations of the three-drug-regimen in HER2+ breast cancer, we develop a single agent based on the nanoparticle co-delivering trastuzumab (as targeting and therapeutic agent), siHER2, and a taxane (docetaxel, DTX). We hypothesized that co-delivering these therapeutics to the same cells would enhance the on-target efficacy, while also limiting the systemic toxicity of the taxane. The single-agent approach will also reduce patient burden (via shorter infusion time) and cost. We evaluate the efficacy and safety of the newly developed nanoconstruct in cells and in a drug-resistant HER2+ breast tumor model.
HER2+ breast cancer also demonstrates a high propensity for metastatic spread to brain,[18] leading to devastating symptoms (e.g., headaches, neurological deterioration, seizures) and short median survival. Whole brain radiation therapy (WBRT) is a standard treatment regimen,[19] but this approach mediates minimal effect on survival, while leading to neurocognitive toxicity and a decline in quality of life.[20] To address this unmet need, we exploited the microbubble-assisted focused ultrasound (MB-FUS) technique to facilitate crossing the blood-brain barrier (BBB)/blood-tumor barrier (BTB) by the newly developed nanoconstructs. MB-FUS has proven effective to transiently and safely disrupt the BBB and improve the delivery of therapeutic agents into intracranial tumors.[21] When evaluated in clinical trials, this approach demonstrates a favorable safety profile.[22, 23] This is the first time it has been used for brain tumor delivery of nanoparticles.
2. Results and Discussion
Design and optimization of the mesoporous silica based nanoconstruct for targeted delivery of siRNA was previously reported.[11] In this work, we further optimized the nanoconstruct to co-deliver a taxane and siHER2 for HER2+ breast cancer treatment. Docetaxel (DTX) and paclitaxel (PTX) are the two most commonly used taxanes. For breast cancer cells, docetaxel was previously reported to be more potent than paclitaxel.[24] We confirmed that docetaxel was more efficacious than paclitaxel per mass basis in two HER2+ breast cancer cell lines (Figure S1, Supporting Information). Thus, we chose docetaxel as the taxane on our nanoparticle. In addition to targeted delivery, nanoparticles eliminate the need for polysorbate 80, an excipient to improve solubility of docetaxel in clinics, which is associated with side effects, such as hypersensitivity reactions, peripheral neuropathy, vascular toxicity, and fluid retention.[25]
2.1. Material Optimization
2.1.1. Docetaxel loading strategy
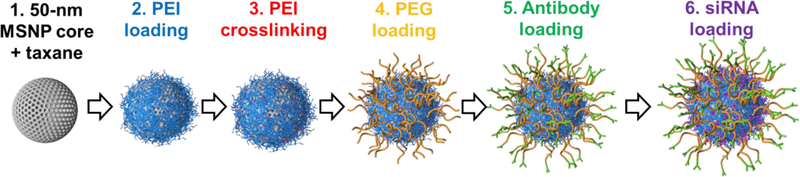
We evaluated strategies to load docetaxel (DTX) on the nanoparticle. The high surface area (666.4 m2/g, measured by BET) and porosity (pore volume of 0.94 cm3/g, measured by BJH) of our MSNP promotes loading of therapeutic cargos, which is particularly advantageous for water-insoluble compounds such as DTX. DTX was loaded via hydrogen bonding between DTX and silanol groups on the MSNP in ethanol, considered a safe solvent. Once the drug loading was completed, we followed with coating the MSNP with PEI, cross-linking PEI, conjugating PEG and antibody, and loading siRNA as shown in Figure 1, following our previously reported procedures.[11] The resulted construct is termed ‘T-siHER2-NP(DTX)’.
Figure 1.
Schematic of our nanoconstruct synthesis. (1) Mesoporous silica nanoparticles were resuspended and loaded with docetaxel (DTX) in ethanol. (2) DTX-loaded MSNPs (MSNP(DTX)) were coated with PEI, which was subsequently (3) cross-linked to enhance the buffering capacity and siRNA delivery efficacy.[11] (4) PEI-coated MSNP(DTX) were conjugated with maleimide-PEG-NHS ester using NHS-amine reaction for the stealth effect. (5) Thiolated antibody was then conjugated to the PEG via the maleimide group. (6) siRNA was loaded last by electrostatic interaction with the cationic PEI layer on the nanoparticles, protected under the PEG layer.
Next, we assessed T-siHER2-NP(DTX)’s hydrodynamic size in serum. Loading DTX on PEI-MSNP (Step 2) resulted in larger particles in 70% serum than loading DTX on the MSNP before PEI binding (Step 1) (Z-average of 128 ± 1.8 nm vs. 113 ± 3.1 nm). The slightly increased size in serum is likely due to lower PEG loading because some amine groups of the PEI were occupied with DTX (when loaded in Step 2) and not available for PEG conjugation. We thus selected loading DTX on the MSNP before PEI binding as our preferred condition.
We varied the initial mass ratios of DTX per MSNP from 0–0.8 in the loading suspension. MSNP and DTX were mixed in ethanol at room temperature overnight. Then, we proceeded to our standard PEI coating, PEI cross-linking, PEG conjugation, antibody conjugation, and siRNA loading as illustrated in Figure 1.[11] The siRNA loading was found best at 2.0 wt.% from our previous work and was not impaired by the drug loading. The nanoconstruct was then characterized for size, composition (polymer, antibody, and DTX loading), gene knockdown efficacy, and cancer killing efficacy.
2.1.2. Particle sizes of nanoconstructs with various DTX loadings
The starting DTX:MSNP mass ratio of 0.2 – 0.6 resulted in the same particle size of the final nanoconstruct (T-siHER2-NP(DTX)) as with no drug loading, while at the mass ratio of 0.8, the material had larger size (Z-average of 142 nm) and size distribution, indicating some agglomeration of nanoparticles (Figure 2 and Table 1). DTX content in each T-siHER2-NP(DTX) was also measured by HPLC-UV (Table 1).
Figure 2.
Hydrodynamic size in PBS of T-siHER2-NP(DTX) made by varying the starting DTX per MSNP mass ratio of 0–0.8.
Table 1.
Docetaxel (DTX) contents and hydrodynamic sizes in PBS of T-siHER2-NP(DTX) made by varying the starting DTX per MSNP mass ratio from 0–0.8. DTX loading was quantified by HPLC-UV.
| DTX:MSNP loading ratio | DTX loading (wt.% of MSNP) | Hydrodynamic size by DLS | |
|---|---|---|---|
| Z-average (nm) | PDI | ||
| 0.0 | 0.00 | 98.5 ± 0.46 | 0.24 ± 0.03 |
| 0.2 | <0.04* | 96.4 ± 0.90 | 0.24 ± 0.01 |
| 0.4 | 0.50 | 98.9 ± 1.93 | 0.27 ± 0.04 |
| 0.6 | 1.03 | 106 ± 1.06 | 0.25 ± 0.03 |
| 0.8 | 1.71 | 142 ± 1.47 | 0.32 ± 0.03 |
Samples are below detection limit, but small content of drug was present as evidenced by cancer cell killing (see Figure 3)
2.1.3. Gene knockdown efficacy of T-siRNA-NP(DTX) with various DTX loading extents
We evaluated these materials for gene knockdown efficacy when delivering siRNA against luciferase (siLUC) to luciferase-expressing HER2+ cells (LM2–4luc+/H2N).[26, 27] Figure 3 shows that the presence of DTX on the nanoparticles did not impair the luciferase knockdown efficacy compared to the material without DTX (DTX/MSNP=0); achieving >70% knockdown for all materials. With DTX on the nanoparticle, cell killing (observed as lower luciferase signal in the scrambled siRNA groups) increased with DTX loading extents (e.g., from DTX/MSNP mass ratio of 0.2 to 0.4, but leveled out at higher DTX/MSNP ratios (from 0.6 to 0.8) under the condition tested.
Figure 3.
Knockdown efficiency of siRNA delivered by T-NP(DTX) with varying DTX loading extents. Luciferase activity of LM2–4luc+/H2N cells after treatment with nanoconstructs with varying starting DTX:MSNP mass ratios, and 2 wt.% siRNA against luciferase (siLUC) or scrambled siRNA (siSCR). Cells were treated with 30 nM siRNA and assayed at 2 days. ** p<0.01, ***p < 0.001, ****p<0.0001. Lower signals from siSCR control groups at high DTX:MSNP ratios were due to cell death caused by the DTX on the nanoparticle.
2.1.4. Characteristics and composition of the optimal T-siHER2-NP(DTX)
We selected the material synthesized with the initial loading ratio of DTX:MSNP of 0.6 as the optimal material since it has the largest amount of drug (1.0% by weight) without increasing the hydrodynamic size (Figure 2 and Table 1) nor reducing siRNA knock down efficacy (Figure 3). Following our prior work[11], the amount of polymer in each layer was determined by thermogravimetric analysis (TGA); and the amount of antibody, by BCA assay. This optimal nanoconstruct has 1.0% DTX, 15% PEI, 11% PEG, 3.5% antibody, and 2.0% siRNA (all by weight of the NP (MSNP-PEI-PEG)). The size of the final nanoconstruct in PBS is 106 nm and the zeta potential in 10 mM NaCl is 12.73 ± 0.47 mV. This material was used for all subsequent studies and is referred to as “T-siHER2-NP(DTX)”.
2.1.5. siRNA protection
Our nanoparticle was designed to have a dense PEG layer, which prevents nanoparticle aggregation in serum, e.g., the hydrodynamic size was maintained at 113 ± 3.1 nm in 70% human serum. The dense PEG layer allows small siRNA to enter and bind to the PEI layer, but prevents large enzymes from entering and degrading siRNA. When incubated in 50% human serum, both T-siHER2-NP and T-siHER2-NP(DTX) greatly protected siRNA from serum enzyme degradation (Figure S2, Supporting Information). Free siHER2 and siHER2-loaded Dharmafect (lipid-based commercial transfection agent) appeared to degrade as soon as 0.5 hours, but the nanoparticle (with or without DTX) kept siHER2 intact for up to 48 hours.
2.2. In vitro efficacy of the optimal T-siHER2-NP(DTX) in HER2+ breast cancer
2.2.1. Specific uptake of T-siRNA-NP(DTX) to HER2+ vs. low-HER2 cancer cells
We measured the preferential uptake of T-NP(DTX) loaded with Alexa-488 dye-tagged siSCR using a panel of two HER2+ cell lines (BT474 and BT474-TRgf[16]) and one low-HER2 cell line (MCF-7). HER2 expression levels were analyzed via flow cytometry after the cells were stained with anti-HER2 antibody and Alexa-488-conjugated secondary antibody (Figure 4a). We incubated T-siSCR(Alexa-488)-NP(DTX) in these cells for one hour. The duration was selected to avoid excessive cell death (e.g., by DTX). We found that longer exposure time would increase the nanoparticle uptake to the HER2+ cells (e.g., 40% uptake at 0.5 hour and 90% at 2 hours as previously reported for T-NP without DTX).[11] The cells were washed to remove any unbound nanoparticles, and quenched with Trypan Blue to exclude signal of nanoparticles bound to the external cell surface. Flow cytometry thus detected only internalized T-siSCR(Alexa-488)-NP(DTX). Figure 4b indicates that nanoparticles were preferentially taken up by HER2+ cells over low-HER2 cells, in a similar manner to our previous report of T-NP with no DTX.[11]
Figure 4.
Preferential uptake of T-siRNA-NP(DTX) into HER2+ over low-HER2 cells. (a) Surface expression of HER2 in BT474, BT474-TRgf, and MCF-7. (b) Percentage of HER2+ (BT474 and BT474-TRgf) and low-HER2 (MCF7) breast cancer cells that were internalized with T-NP(DTX) loaded with Alexa Fluor 488-conjugated scrambled siRNA (siSCR). 10,000 cells were analyzed per event.
2.2.2. In vitro efficacy of T-siHER2-NP(DTX)
Next, we evaluated the efficacy of T-siSCR-NP(DTX) versus free DTX (of the same dose on the nanoparticles) in a panel of HER2+ and low HER2 (HER2-) cells. Figure 5 shows that targeted delivery of DTX by T-NP to HER2+ cancer cells enhanced docetaxel’s therapeutic index by about 2-fold. In particular, T-NP(DTX) was two-fold more toxic than free DTX in HER2+ breast cancer cells, but was not more toxic in the low-HER2 cell lines (MCF12a and HDFa). At this low concentration range, we show in another independent experiment that trastuzumab-conjugated NP without DTX (T-NP) had little effect on cell viability, and free trastuzumab+free DTX did not kill HER2+ cells more than free DTX (Figure S3, Supporting Information).
Figure 5.
Efficacy of free DTX vs. DTX delivered by T-NP. Cell viability of HER2+ breast cancer (HCC1954, BT474, JIMT1) and low-HER2 (HER2-) normal cells (MCF12a and HDFa) after treatment with T-siSCR-NP(DTX) or free DTX. Both treatments contain 90 ng/ml DTX, and cell viability was analyzed at 3 days post-treatment for fast growing cells (HCC1954, JIMT1, MCF12a, HDFa) and 5 days for slow-growing cells (BT474) (media changed at 3 hours after treatment for all). ****p<0.0001. HER2 levels of these cells were reported in our prior work.[11]
We confirmed the ability of T-NP(DTX) to deliver siHER2 and elicit HER2 mRNA knockdown. T-NP with or without DTX could deliver siHER2 and elicit HER2 knockdown in a similar manner (Figure 6).
Figure 6.
HER2 knockdown by T-siHER2-NP(DTX). HER2 mRNA levels of HCC1954 cells at 48 hours after treatment with T-NP or T-NP(DTX) delivering 30 nM siHER2 or siSCR (scrambled control). Media changed overnight. ***p<0.001; ****p<0.0001.
2.2.3. Justification for using trastuzumab (HER2 antibody) as the targeting agent
There was a concern that knocking down HER2 protein with siHER2 may potentially impair the T-siHER2-NP delivery relying on trastuzumab-HER2 mediated endocytosis. To address this potential self-defeating concern, we hypothesized that (1) not all cells within the tumors received T-siHER2-NP(DTX) at once and hence some cells still overexpress HER2 for subsequent delivery; and (2) cells that received sufficient T-siHER2-NP(DTX) underwent apoptosis (hence no longer needing T-siHER2-NP(DTX) delivery), while the surviving cells replenished HER2 protein for subsequent delivery (because the siRNA effect is transient). This hypothesis was supported in our prior study[16, 17] in which a HER2+ cell line (BT474) was continuously treated with siHER2 for 15 weeks. We observed that the majority of cells underwent apoptotic death; however, the remaining cells (either receiving none or insufficient siHER2 dose) replenished their HER2 level to that of naïve cells (see Figure S4c of ref [16]). Thus, they can still be the target for siHER2 delivery by T-NP. In addition to being a targeting agent, we found trastuzumab displayed therapeutic effects on NP.[11] Therefore, this justifies the use of trastuzumab as the targeting agent on our NP.
2.3. In vivo efficacy of T-siHER2-NP(DTX) in mice bearing HER2+ breast tumors
2.3.1. Efficacy in treating drug-resistant orthotopic HER2+ breast tumors
We evaluated the efficacy of systemic T-siHER2-NP(DTX) in an orthotopic HER2+ breast tumor mouse model. The model was established by inoculating HCC1954 cells in the mammary fat pad of mice, and tumor growth was monitored twice weekly (see saline group in Figure 7a). The HCC1954 tumor was previously established by us to be resistant to trastuzumab and taxane in mice.[11] Figure 7a shows that T-siHER2-NP(DTX) is more efficacious at inhibiting tumor growth than the T-NP delivering siHER2 or DTX alone. T-siHER2-NP(DTX) also outperformed its free drug counterpart (DTX+Trastuzumab) delivered at the same dose.
Figure 7.
In vivo efficacy of T-siHER2-NP(DTX) in mice bearing orthotopic HCC1954 tumors. (a) Growth of HCC1954 tumors in mice (n=7–8/group) receiving treatments as specified. Dosing: 50 mg/kg of T-siHER2-NP(DTX) containing 2 wt.% siHER2 and/or 1 wt.% DTX, given i.v. (tail vein) twice a week, 7 doses total (see arrows). In one group, DTX+trastuzumab at the same dose of what on the nanoparticles was given i.v. at the same schedule with nanoparticle treatment. Error bars denote S.E.M. *denotes the significance between T-siHER2-NP(DTX) and saline; $denotes the significance between T-siHER2-NP(DTX) and DTX+trastuzumab; **,$ $ p<0.01; ***,$ $ $ p < 0.005; **** p < 0.0001 [two-way repeated measures ANOVA]. (b) Immunofluorescent images and (c) quantification of the HER2 expression levels of tumor tissues collected from mice (n = 4/group) at 3 days after the third dose of treatments as specified. green = HER2 protein; blue = DAPI staining cell nuclei. Images were taken at 20X and stitched to cover the entire piece of each cut tumor (~70–150 images per tissue). Signals were analyzed by CellProfiler and reported as mean ± SD. *p<0.05; **p<0.01. Graphic in (a) created with Biorender.com.
To confirm the activity of HER2 knockdown, another set of mice with HCC1954 tumors was treated in the same manner for three doses. Three days after the third dose, mice were euthanized, and tumors were collected and stained for HER2 protein. Tumors from mice treated with T-siHER2-NP(DTX) showed ~55% HER2 knockdown vs. saline control, and 40% vs. scrambled control (Figure 7b,c). Some HER2 reduction in the scrambled group is anticipated because trastuzumab on the NP can enhance HER2 receptor internalization and degradation upon NP uptake. Further, it should be noted that this knockdown activity may be underestimated, as some cells successfully treated with T-siHER2-NP(DTX) could have been killed off already from early doses, and their HER2 reduction was not captured in the analysis.
2.3.2. Efficacy of T-siHER2-NP(DTX) in a mouse model of brain metastatic HER2+ breast cancer with focused ultrasound
Mice were intracranially implanted with a BT474 variant (BTGFL1), a gift from Drs. Robert Kerbel and Giulio Francia, which was serially passaged orthotopically in mice in a similar manner as BT474-TRgf, but for over 4 years.[28] The microbubble-facilitated focused ultrasound system (MB-FUS) was designed to allow effective BBB disruption, which can be conducted in the absence of MRI targeting (Figures S4a,b, Supporting Information). The site of initial tumor implantation was used as the reference point for targeting (Figure S4c, Supporting Information). Microbubbles were administered via tail vein catheter 10 seconds after the start of FUS. Effective BBB disruption is noted by Gadovist contrast enhancement in coronal and axial T1-w MRI sections (Figure S4d,e, Supporting Information). This disruption extended approximately 5 mm into the brain and produced a total volume of BBB disruption of approximately 25 mm3, which was sufficient to cover the entirety of the implanted tumor during the period of treatment.
Mice treated with MB-FUS plus T-siHER2-NP(DTX) (Figure 8a) showed inhibition of intracranial BTGFL1 tumor growth during the period of treatment (as determined by T2w MRI). The inhibition achieved throughout this period reached statistical significance by day 53 after the start of treatment relative to control and T-siHER2-NP(DTX) alone (Figure 8b and Figure 8d; Two-way ANOVA; Bonferroni post-test p<0.001 and p<0.01 respectively). Mice subjected to therapy with T-siHER2-NP(DTX) alone showed minimal inhibition and did not reach statistical significance relative to the MB-FUS alone control (Figure 8b). The efficacy associated with MB-FUS plus T-siHER2-NP(DTX) translated into improved survival relative to T-siHER2-NP(DTX) or MB-FUS alone (median survival 80, 54 and 64.5 days respectively; Log-rank p<0.05, Figure 8c).
Figure 8.
In vivo efficacy of T-siHER2-NP(DTX) + MB-FUS in mice bearing BTGFL1 tumors in brain. (a) Schematic representation of treatment protocol using T-siHER2-NP(DTX) (shortened as NP in the figure) alone (50 mg NP/kg per dose via tail vein) or in combination with MB-FUS. (b) Treatment with MB-FUS plus NP mediated a significant inhibitory effect in intracranial BTGFL1 tumors (**p<0.01 vs. NP alone; ***p<0.001 vs. control [MB-FUS alone]). (c) Improved median survival is associated with MB-FUS plus T-siHER2-NP(DTX) treatment (**p < 0.01 vs T-siHER2-NP(DTX) alone,*p < 0.05 vs control). (d) Representative T2w images showing extent of intracranial tumor growth during treatment period (up to day 26) and in follow-up period (day 53). Area of intracranial tumors within brain sections is highlighted by red contours.
2.4. Safety profile of T-siHER2-NP(DTX)
2.4.1. Blood compatibility of T-siHER2-NP(DTX)
Since T-siHER2-NP(DTX) is aimed for systemic administration, its blood compatibility is essential. We thus assessed the blood compatibility profile of T-siHER2-NP(DTX), following published protocols by the National Characterization Lab of the NCI.[29] Abraxane (paclitaxel-albumin nanoparticles) was used as a benchmark herein. Similar to Abraxane, T-siHER2-NP(DTX) did not cause hemolysis when incubated with red blood cells (Figure S5a,b, Supporting Information). Also similar to Abraxane, T-siHER2-NP(DTX) did not cause platelet aggregation when incubated in platelet-rich plasma (PRP), observed as unchanged light transmittance over the incubation period, while collagen-related peptide (CRP, the positive control) caused immediate platelet aggregation (Figure S5c, Supporting Information).
2.4.2. Safety profile of T-siHER2-NP(DTX) in mice after multiple injections
Mice treated with multiple doses of T-siHER2-NP(DTX) (Figure 7a) had overall good general health. Body weight of the treated mice was not significantly different from that of the untreated throughout the entire study (Figure S6, Supporting Information). At sacrifice, mouse serum was collected and analyzed for the levels of BUN and creatinine to evaluate kidney health; AST, ALT, and TBIL to evaluate liver health; and ALT and CK to evaluate general muscle health (e.g., heart). We found that there were no significant differences of these biomarker levels in the serum of treated and untreated groups (Table 2). This is in agreement with histology grading by an independent histopathologist, indicating that there was no obvious toxicity of T-siHER2-NP(DTX) to the key organs (brain, heart, kidney, lung, spleen, and liver) of mice treated with the nanoparticles. (Table S1, Supporting Information).
Table 2:
Serum biomarkers of mice treated twice weekly with T-siHER2-NP(DTX) (7 doses of 50 mg NP/kg, see Figure 7a). Mice were sacrificed one week after the last dose.
| ALT (U/L) | AST (U/L) | TBIL (mg/dL) | BUN (mg/dL) | CRE (mg/dL) | CK (U/L) | |
|---|---|---|---|---|---|---|
| Saline | 25.3 ± 7.0 | 78.5 ± 25.8 | 0.2 ± 0.1 | 25 ± 5.0 | 0.18 ± 0.04 | 320 ± 225 |
| T-siHER2-NP(DTX) | 30.0 ± 3.8 | 83.8 ± 7.8 | 0.1 ± 0.0 | 28 ± 2.9 | 0.21 ± 0.02 | 369 ± 139 |
ALT = Alanine Aminotransferase; AST = Aspartate Aminotransferase; TBIL = Total Bilirubin; BUN = Blood Urea Nitrogen; CRE= Creatinine; CK = Creatine Kinase
2.4.3. Acute toxicity of T-siHER2-NP(DTX) in mice
We evaluated the acute toxicity profile of T-siHER2-NP(DTX) by performing a single injection of T-siHER2-NP(DTX) at 4 different dose levels (50, 100, 150, and 200 mg/kg NP) in tumor-free immunocompetent mice. The highest dose used (200 mg/kg NP) is four times the efficacious dose of 50 mg/kg NP and is not too viscous for bolus tail vain injection. Even at the maximum dose tested, we found no acute toxicity in terms of death or changes in mouse body weight (Figure 9a). Mice also demonstrated behavior that was indicative of good clinical health (i.e., no scruffy fur, appetite loss, or decrease in movement), suggesting that no dose limiting toxicity (DLT) was reached.[30, 31] No significant differences in organ weights were found for the heart, lung, liver, kidney, or spleen from mice in all dose groups (Figure 9b). This agrees with no change in liver and kidney biomarkers (e.g., values were not different than saline or fell within historical range for this mouse strain) (Table S2, Supporting Information). Likewise, histopathology grading of livers from the highest dose group was performed and found to be normal compared to the control group (Table S3, Supporting Information). Altogether, we concluded that we did not reach DLT or MTD at 4-fold the efficacious dose, indicating a favorable safety profile of T-siHER2-NP(DTX).
Figure 9.
(a) Body weights of mice after a single injection of T-siHER2-NP(DTX). Mice were administered with a single dose of T-siHER2-NP(DTX) via tail vein at 50, 100, 150, or 200 mg/kg NP. Error bars denote S.E.M. (n = 3–5). (b) Organ weight of the same tumor-free mice as shown in (a). Organs were collected 12 days post-treatment and weighed. No statistical significance is found in one-way ANOVA analysis with multiple comparisons among groups (p>0.05). Error bars denote S.E.M. (n = 3–5).
2.4.4. Immune response to T-siHER2-NP(DTX) in mice
To complement our acute toxicity and safety profile following a single administration of T-siHER2-NP(DTX), we investigated whether T-siHER2-NP(DTX) delivered at the efficacious dose caused immune-related adverse effects in mice. We measured cytokine levels in the blood of tumor-free immunocompetent mice, considered a sensitive mouse strain for producing an inflammatory response upon stimulation.[32] Serum cytokines (IFN-γ, IL-1β, IL-6, TNF-α, and IFN-α) were measured at 2, 6, and 24 hours after a single injection of T-siHER2-NP(DTX) at the efficacious dose of 50 mg/kg NP. Compared to saline control mice, no induction of IFN-γ, IL-1β, or IFN-α was observed (Figure S7, Supporting Information). TNF-α and IL-6 were induced at 2 hours post-treatment, but subsided by 24 hours, indicating low risk of cytokine storm syndrome. These findings (i.e., IL-6 release profile) are consistent with the mild, transient cytokine induction reported in mice during preclinical evaluation of Patisiran (ALN-TTR02), an siRNA-lipid nanoparticle formulation that has since become the first RNA interference-based drug to be approved by the US Food and Drug Administration.[33]
3. Conclusion
We have reported a novel nanotherapeutic for HER2+ breast cancer. Mesoporous silica nanoparticles were designed and optimized to co-deliver docetaxel and siRNA against HER2 (siHER2), mirroring the current standard treatments for HER2+ breast cancer, which are a combination of a taxane and HER2-targeted antibodies.
We found that the optimized nanoconstruct (T-siHER2-NP(DTX)) performed better than its free-drug counterpart (trastuzumab + docetaxel) when used at the same dose. Furthermore, when combined with microbubble-assisted focused ultrasound, T-siHER2-NP(DTX) successfully elicited treatment effect in breast tumors residing in the brains of mice. T-siHER2-NP(DTX) also showed no apparent toxicity in vitro (cell lines and blood) and in mice treated with multiple doses or in dose-escalation study up to 4-fold the efficacious dose. Therefore, T-siHER2-NP(DTX) holds great promise to replace the standard treatments of HER2+ breast cancer.
Towards clinical translation, next steps include further studies in HER2+ breast cancer patient-derived xenograft (PDX) tumor mouse models and in immunocompetent mouse models, along with comprehensive toxicology studies to enable the Investigational New Drug application. The construct can also be lyophilized for long-term storage with its properties and efficacy remaining intact.[17] We have also demonstrated a favorable safety profile of this MSNP-based platform in Cynomolgus monkeys in our prior reports.[34, 35] MSNPs are biodegradable to non-toxic silicic acid, which can be cleared by the kidneys.[36] Si is also the third most abundant trace metal (after iron and zinc) in the human body.[37] Silica-based delivery of PET tracers has also been evaluated in a clinical trial with a favorable safety profile (e.g., Cornell dots).[38]
Importantly, we have demonstrated that our NP platform is capable of co-delivering multiple classes of cargos simultaneously and effectively (e.g., chemotherapy drug, siRNA, and tumor-targeting antibody herein) while still maintaining a favorable hydrodynamic size of 100 nm. The MSNP platform has many favorable attributes, including tailorable mesoporous structures, high surface areas, large pore volumes, ease of controlling size, and high scalability. We envision that our versatile delivery platform can be utilized to co-deliver other multiple payloads specifically to tumors in the future, serving as an important therapeutic tool to precisely combat cancers.
4. Experimental Section/Methods
Materials, siRNA, and Cell Lines
TEOS, CTAC, NaH2PO4•H2O, Na2HPO4 and TEA were obtained from Sigma Aldrich (St. Louis, MO). Branched-PEI (10 kDa) was obtained from Alfa Aesar (Haverhill, MA). Maleimide-PEG(5 kDa)-NHS was obtained from JenKem Technology USA (Plano, TX). Injectable docetaxel (Accord, Durham, NC), Trastuzumab (Genentech, South San Francisco, CA) and Abraxane (Celgene, Summit, NJ) were obtained from the OHSU pharmacy (Portland, OR). Docetaxel and paclitaxel (powder with no additive) were obtained from LC Labs (Woburn, MA). PBS (pH 7.2) was obtained from Life Technologies (Carlsbad, CA). Desalting columns (MW 40 kDa), RNase free water, Traut’s reagent, DSP, ethanol, concentrated HCl, Dharmafect, and sodium hydroxide were obtained from Thermo Fisher Scientific (Waltham, MA). All reagents are of the highest purity grade available.
siLUC, siHER2, and siSCR (without fluorescent dye tags) were custom synthesized (in vivo HPLC grade) by Dharmacon (Horizon Discovery, Lafayette, CO). siSCR with Alexa Fluor 488 dye was synthesized by Qiagen (Valencia, CA). The sequences are as follows.
| siHER2 | Sense: 5’ CACGUUUGAGUCCAUGCCCAAUU 3’ |
| Antisense: 5’ UUGGGCAUGGACUCAAACGUGUU 3’ | |
| siLUC | Sense: 5’ CGGAUUACCAGGGAUUUCAtt 3’ |
| Antisense: 5’ UGAAAUCCCUGGUAAUCCGtt 3’ | |
| siSCR | Sense: 5’ UGGUUUACAUGUCGACUAA 3’ |
| Antisense: 5’ UUAGUCGACAUGUAAACCA 3’ |
Human breast cancer cell lines (BT474, HCC1954, MCF7) were obtained from American Type Culture Collection. LM2–4luc+/H2N[26, 27] and BTGFL1[28] were gifted by Prof. Robert Kerbel (University of Toronto, Canada) and Giulio Francia (The University of Texas at El Paso). MCF7 was maintained in DMEM media supplemented with 10% FBS. LM2–4luc+/H2N was maintained in RPMI media supplemented with 5% FBS. All other cells were maintained in RPMI media supplemented with 10% FBS. MCF12a and HDFa were obtained from American Type Culture Collection and maintained following manufacturer’s protocols.
Material Synthesis and Characterization
Mesoporous silica nanoparticles (MSNPs) were synthesized by sol-gel method with cetyltrimethylammonium chloride (CTAC) and triethanolamine (TEA) as surfactants. A detailed synthesis method can be found in our prior work.[11] Surface area of MSNPs was determined via 11-point BET analysis; and pore volume, and pore radius of MSNPs were determined via BJH analysis (Micrometrics TriStar II 3020, Particle Technology Labs, Downers Grove, IL) with nitrogen gas as the adsorbate. MSNPs were coated layer-by-layer with PEI (where the PEI was further bio-reducibly crosslinked in situ on the particle surface), PEG, and antibody, following our established protocol.[11, 17] DTX was mixed with MSNPs in ethanol (with a starting DTX:MSNP mass ratio of 0–0.8) for 15 h prior to PEI binding step.
Final nanoconstructs were characterized for size and charge by dynamic light scattering (DLS, Zetasizer Nano ZS-90, Malvern, Westborough, MA); polymer composition by thermogravimetric analysis (TGA Q50, TA Instruments, New Castle, DE), and antibody composition by BCA assay kit (Thermo Fisher Scientific, Waltham, MA) as described in our prior work.[11] To quantify the amount of docetaxel loaded on the nanoparticle, nanoparticles were diluted in PBS/DMSO (1:3 by volume) and shaken for 1 day (400 rpm) to allow for a complete drug release. The mixture was then centrifuged at 21,000xg for 30 min, and the supernatant was collected for docetaxel analysis by reverse-phase high-performance liquid chromatography (HPLC) with a UV detector (Agilent Technologies 1260 Infinity, Santa Clara, CA). The mobile phase consists of solvent A (water with 5% methanol) and solvent B (acetonitrile) mixed on a gradient (0 min; 50/50 A/B, 5 min; 30/70 A/B, 5.5–9 min; 10/90 A/B) at a flow rate of 1.0 mL/min using a 4.6 × 50 mm, 2.7 μm Poroshell 120 C18 column (Agilent Technologies, Santa Clara, CA). UV detection was set at 204 nm, and docetaxel retention time was observed at 2.2 min.
Luciferase Knockdown Efficacy
LM2–4luc+/H2N[26, 27] (over-expressing luciferase and HER2) was used to assess the gene silencing efficacy of nanoparticles loaded with siRNA against luciferase (siLUC). Cells were plated at 3000 cells/well in a 96-well plate and maintained in RPMI + 5% FBS. One day after seeding, cells were treated with siRNA-NPs. The nanoparticles were loaded with siRNA at a NP/siRNA mass ratio of 50. After overnight incubation (~20 hours), cells were washed once and replenished with complete media. At 48 hours post-treatment, cells were lysed and analyzed for luciferase activity by Luciferase Glow Assay Kit (Thermo Fisher Scientific, Waltham, MA), following the manufacturer’s protocols. Luciferase activity of the lysate was reported as a percentage of the untreated control. All treatments were performed in triplicate or quadruplicate.
In Vitro Efficacy: HER2 Protein Knockdown and Cell Viability
Cells were seeded in a 6-well plate and maintained in RPMI + 10% FBS for 24 hours prior to treatment. Nanoparticles were loaded with siHER2 or siSCR at NP/siRNA 50. siRNA dose were 30–60 nM. Media were switched to complete media after overnight siRNA-NP treatment. Two days after treatment with siRNA-NP, RNA was isolated and purified from cells with GeneJet RNA purification kit (Thermo Fisher Scientific, Waltham, MA) according to manufacturer’s instructions. One-Step qRT-PCR was performed using EXPRESS One-Step Superscript™ qRT-PCR Kit (Invitrogen, Carlsbad, CA). 20 ng RNA per reaction was used. Cycling conditions were 50 oC for 2 min, 95 oC for 10 min, 40 cycles of 95 oC for 15 s, and 60 oC for 1 min. TaqMan gene expression primer Human HPRT1 mRNA (Hs99999909_m1) was used as housekeeping gene and Human ERBB2 (HER2) mRNA (Hs01001580_m1) was used to assess HER2 gene knockdown (HER2 relative to HPRT). Data were analyzed using 2–ΔΔC(t) method, and reported as the percentage of the untreated control. For viability, cells (5,000/well for all cells) were plated in a 96-well plate for 24 hours prior to treatment. Cell viability was analyzed 3 days post-treatment for all cells (except BT474 was analyzed 5 days post-treatment because of slower growth rate) using CellTiter-Glo® Luminescent Assay (Promega, Madison, WI) following manufacturer’s protocol. Data were reported as a percentage of the untreated control.
Cellular Uptake Analysis by Flow Cytometry
Cells were harvested and resuspended in 1 million cells per 800 μL per tube. Each tube was mixed with 200 μL of Alexa Fluor 488-conjugated siSCR-nanoparticles in PBS (containing 100 μg nanoparticles). Upon siSCR-nanoparticle addition, cells were placed on a rocker in a cell incubator (37 oC, 5% CO2) for 1.0 hour. After 1.0 hours, cells were washed (centrifuged at 115g, 5 min) with FACS buffer (1 mL, 1X Phosphate Buffered Saline (Ca/Mg++ free) + 1mM EDTA + 25mM HEPES pH 7.0 + 1% Fetal Bovine Serum (Heat-Inactivated)) two times. Cells were then resuspended in FACS buffer (500 μL). Cells were kept on ice until analysis. For cells stained with free antibody (for gating purpose), antibody labeling was performed on ice and under rocking conditions. Cells were stained with primary antibody (trastuzumab (Roche): 2 μg per tube) for 1.0 hour, washed (centrifuged at 115g, 5 min) with PBS one time, stained with secondary antibody (Anti-human Alexa 488 (Life Technologies): 2 μg per tube) for 45 minutes, then washed 2 times and resuspended in FACS buffer (500 μL) before analysis. Then, all tubes (except antibody-labeled cells for gating purpose) were incubated with Trypan Blue (500 μL, 0.4% in PBS) to quench the fluorescence outside of the cells, and subjected to flow cytometry analysis. 10,000 events (cells) were analyzed for each sample. The intensity was processed with FlowJo software (FlowJo LLC, Ashland, OR).
Animal Studies--Mouse Tumor Models and in vivo Efficacy Studies
All animals were recruited and used under approved protocols of the Institutional Animal Care and Use Committee (IACUC) of Oregon Health and Science University and Sunnybrook Research Institute. All animal experiments were carried out under the auspices of the OHSU Department of Comparative Medicine and Sunnybrook Research Institute animal facility.
Orthotopic HER2+ Breast Tumor Mouse Model:
HCC1954 cells in Matrigel (3 × 106 in a total volume of 50 μl) were implanted into the mammary fat pads of 6-week-old female NCI Athymic NCR-nu/nu mice (Charles River, Wilmington, MA) and allowed to grow to an average size of ~150 mm3. Tumor size was measured using a Vernier caliper, and tumor volume was calculated as 0.5 x length x width2. Mice were then grouped and proceeded to receive different test compounds twice a week over a period of 3–4 weeks.
Brain Metastatic HER2+ Breast Cancer Mouse Model
BTGFL1 (1 × 106 in total volume of 4 μl) were implanted intracranially by means of standard stereostatic techniques in 8-week-old SCID female mice. The coordinates ftfor implantation were DV 3, AP 2 and LM −1.1 relative to bregma. Tumor growth was monitored using a 7Tesla MRI scanner (Biospin 7030, Bruker, Billerica) using T2-weighted (T2-w) MR images. Treatment started 2 weeks post-tumor implantation, once tumors were established at a volume of approximately 1 mm3. Mice were divided into 3 groups: control (saline), T-siHER2-NP(DTX), and focused ultrasound plus T-siHER2-NP(DTX). Groups of 4–5 mice were used for experiments. Tumor volume was assessed using T2-w MR coronal and axial scans (slice thickness of 0.5mm, number of slices=12). These scans were visualized and analyzed with the aid of MIPAV software. For survival studies, mice were sacrificed in accordance with institutional guidelines once mice started to show signs of distress (e.g., body weight loss, lack of grooming, changes in behaviour or alertness).
Focused Ultrasound Setup
Experiments were conducted using a custom-made focused ultrasound prototype system. The system consists of a spherically focused transducer matched to 1.1 MHz (F number =0.8, external diameter 25 mm; focal length= 20, focal zone width≈1.4 mm, focal zone length≈10.5 mm). The system uses a 2.5 mm PZT hydrophone. Signal was generated by a Syscomp WGM201 generator and amplified by a 15 Watt (43 dB gain) power amplifier (NP technology NP950 mounted on heat sink NP100/NP101). During sonication, acoustic emissions were monitored with an in-house manufactured 2.5 mm PZT hydrophone mounted at the centre of the transducer with signal capture using Alazar ATS460 signal card. Mice were secured onto an MRI compatible-sled under anesthesia throughout experiment (2% isoflurane). Depilatory cream was used to remove hair from the top of the skull. The transducer was placed within a degassed water chamber and its movements were controlled by a motorized positioning system. The water chamber was lowered to make contact with the top of the skull. Original site of perforation made on skull at the time of tumor implantation was designated as the target (designated as point zero LR=0.0, AP=0.0) for sonication. Three targets spots were chosen around target (point zero; AP+0.5, AP 0.0 and AP-0.5; all with DV =1.0 and LR=0.0). Ten seconds after start of FUS, microbubbles (MB; Definity, Lantheus Medical Imaging, MA) were administered (0.2 ml/kg in saline) via tail vein catheter. Once the ratio of signal above baseline at the sub or first ultra-harmonic frequency passed 3.5, the sonicating pressure was dropped by 50% and maintained at this level for the remainder of sonication.[39] Mice subjected to focused ultrasound for BBB disruption were treated with appropriate dose of nanoparticle immediately following FUS treatment. When performed, BBB disruption was confirmed by injecting gadolinium-based contrast agent (Gadovist, Schering, AG; Berlin) immediately after sonication. Contrast enhancement (as a measure of BBB opening) at the site of treatment was determined by MR T1 weighted scans.
Immunofluorescent Imaging (IF)
For tumor tissue immunofluorescence study, tumors were harvested, formalin-fixed, and paraffin-embedded until the time of analysis. At the time of analysis, the tumor sections were deparaffinized with xylene (X3S-4, Thermo Fisher Scientific), 100% Ethanol (Decon’s Pure Ethanol 200 Proof), 95% Ethanol, 90% Ethanol, 70% Ethanol, 50% Ethanol and 1X PBS, respectively. Following a heat-induced antigen retrieval step before immunohistochemical staining (Thermo Fisher Scientific, Waltham, MA) with high pressure/temperature for 10 minutes (Cuisinart® 6 qt. Electric Pressure Cooker, Stamford, CT), the slides were then transferred to 1X PBS at room temperature, and were washed three times with 1X PBST (Phosphate Buffered Saline with 0.1% Tween 20, Cell Signaling), two times with blocking buffer (1X PBS + 5% Goat Serum + 0.3% Triton-X), and incubated for one hour in the blocking buffer at room temperature. Blocking buffer was then removed, and 50 μL of primary antibodies (anti-HER2 (1:50, 2242 Abcam)) in blocking buffer solution was added and incubated overnight at 4 °C. Slides were washed 3 times with 1X PBST washes (5 minutes each), and incubated in a blocking buffer for 15 minutes. Blocking buffer was removed, and 50 μL of secondary antibodies (AlexaFluor®488 goat anti rabbit IgG (1:200, A11034 Life Technologies, Carlsbad, CA)) were incubated at room temperature for one hour. Tissues were then washed three times with blocking buffer for 5 minutes each. Blocking buffer was then removed, and 50 μL of Hoechst 33342 (1:10,000, H3570 Life Technologies, Carlsbad, CA) in 1X PBS was added and incubated for 15 minutes at room temperature. Following a 1X PBS wash and mounted with antifade mountant (P36965, Invitrogen, Carlsbad, CA), cell nuclei were visualized by DAPI following manufacturer’s protocol. Fluorescence images were obtained with the EVOS FL fluorescence microscope (Life Technologies, Carlsbad, CA) using DAPI and GFP channels. All images were processed for signal intensity by CellProfiler image analysis software (Cambridge, MA). The quantification was done with 20X whole section scan images and 4 animals per treatment.
Acute Toxicity Evaluation
6-week-old female NCI Cr:NIH(S) mice were obtained from Charles River NCI colony (Wilmington, MA). Each mouse was weighed, then injected via tail vein at 50, 100, 150, and 200 mg NP/kg T-siHER2-NP(DTX). The injection volume for all dose groups was 300 uL. Mice were observed for 30 minutes post-injection, then weighed twice weekly and observed daily for the remainder of the study. At 12 days post-injection, mice were euthanized for organ and blood collection. Organs were weighed and blood was processed for analysis of serum biochemistry. Liver samples from saline control and 200 mg/kg mice were fixed, sectioned, and stained with H&E stain (OHSU, OR). Slides were graded by an independent pathologist at Horus Scientific (Worcester, MA).
Cytokine Induction
6-week-old female NCI Cr:NIH(S) mice (Charles River NCI colony, Wilmington, MA) were injected via tail vein at the efficacious dose of 50 mg NP/kg T-siHER2-NP(DTX). The injection volume was 250 uL. Mice were euthanized at 2, 6, and 24 hours post-injection and blood was collected via cardiac puncture, then processed into serum. IFN-γ, IL-1β, IL-6, TNF-α, and IFN-α levels in the serum were analyzed by Eve Technologies (Calgary, AB).
Serum Biochemistry
Upon euthanasia, blood samples were collected via cardiac puncture and processed into serum. Serum biomarkers were measured with Beckman AU680 by IDEXX BioAnalytics (West Sacramento, CA).
Statistical Analysis
Pairwise statistical comparisons were performed using unpaired, two-tailed Student’s t test. One-way ANOVA analysis was used for comparison of more than 2 groups. Tumor growth curve was analyzed with two-way repeated measures ANOVA with post-hoc multiple comparison tests against the saline group, unless otherwise specified. Median survival was analyzed using Log-rank (Mantel-Cox) test (p < 0.05 for statistical significance). Statistical significance was established at p < 0.05. Graphpad Prism 6.0 software (GraphPad Software Inc., San Diego, CA) was utilized for statistical analyses. Unless otherwise specified, all data are presented as mean ± SD.
Supplementary Material
Acknowledgements
Research was supported by the National Cancer Institute of the National Institutes of Health under Award Number R44CA217534, R44CA265751, R44CA265752, the National Institute of Biomedical Imaging and Bioengineering of the National Institutes of Health under Award Number R01EB003268, the Kuni Foundation, the Canadian Institutes for Health Research under Award Number FRN 119312, and the Canada Research Chair Program (awarded to KH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. The authors thank Anh Ngo and Dr. Cristina Puy Garcia for their technical assistance in blood compatibility studies. The authors would like to acknowledge Dr. Wilaiwan Chouyyok, Dr. Soracha Dechaumphai, Shawna Rideout-Gros, Shakthi S. Seerala, and Viva Chen for their valuable assistance with a number of technical procedures. The authors are grateful to Dr. Oleh Taratula for his independent review of the data in this paper as required by OHSU conflict of interest guidelines. OHSU, J.W.G., and W.Y. have a significant financial interest in PDX Pharmaceuticals, Inc, a company that may have a commercial interest in the results of this research and technology. This potential personal and institutional conflict of interest has been reviewed and managed by OHSU.
Received: ((will be filled in by the editorial staff))
Revised: ((will be filled in by the editorial staff))
Published online: ((will be filled in by the editorial staff))
This research paper describes the development of the nanoparticle system that co-delivers siHER2 and docetaxel to mirror the current first-line treatment of HER2-positive breast cancer. The nanotherapeutic has enhanced therapeutic index when compared with free drug counterparts. It shows efficacy in drug-resistant HER2+ breast tumor model. When coupled with microbubble-assisted focused ultrasound, the nanoconstruct can also inhibit HER2+ breast tumors residing in the brain.
Footnotes
Supporting Information
Supporting Information is available from the Wiley Online Library or from the author.
Contributor Information
Dr. Worapol Ngamcherdtrakul, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA
Daniel S. Bejan, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA
William Cruz-Muñoz, Sunnybrook Research Institute, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada.
Dr. Moataz Reda, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA
Husam Y. Zaidan, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA
Dr. Natnaree Siriwon, Department of Biomedical Engineering, Oregon Health & Science University, 3303 S Bond Ave, Portland, OR 97239, USA
Suphalak Marshall, Department of Radiology and Institute of Biomedical Engineering, Faculty of Medicine, Prince of Songkla University, 15 Karnjanavanich Road, Hat Yai Songkhla 90110, Thailand.
Ruijie Wang, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA.
Molly A. Nelson, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA
Justin P.C. Rehwaldt, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA
Prof. Joe W. Gray, Department of Biomedical Engineering, Oregon Health & Science University, 3303 S Bond Ave, Portland, OR 97239, USA
Prof. Kullervo Hynynen, Sunnybrook Research Institute, 2075 Bayview Ave, Toronto, ON M4N 3M5, Canada
Prof. Wassana Yantasee, PDX Pharmaceuticals, Inc, 3303 S Bond Ave, CH13B, Portland, OR 97239, USA Department of Biomedical Engineering, Oregon Health & Science University, 3303 S Bond Ave, Portland, OR 97239, USA.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, CA: A Cancer Journal for Clinicians 2021, 71 (3), 209–249. DOI 10.3322/caac.21660. [DOI] [PubMed] [Google Scholar]
- [2].Burstein HJ, New England Journal of Medicine 2005, 353 (16), 1652–1654. [DOI] [PubMed] [Google Scholar]
- [3].Rexer BN, Arteaga CL, Critical Reviews in Oncogenesis 2012, 17 (1), 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Seidman AD, Fornier MN, Esteva FJ, Tan L, Kaptain S, Bach A, Panageas KS, Arroyo C, Valero V, Currie V, Gilewski T, Theodoulou M, Moynahan ME, Moasser M, Sklarin N, Dickler M, D’Andrea G, Cristofanilli M, Rivera E, Hortobagyi GN, Norton L, Hudis CA, Journal of Clinical Oncology 2001, 19 (10), 2587–2595. [DOI] [PubMed] [Google Scholar]
- [5].Blackwell KL, Burstein HJ, Storniolo AM, Rugo HS, Sledge G, Aktan G, Ellis C, Florance A, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J, Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2012, 30 (21), 2585–92. [DOI] [PubMed] [Google Scholar]
- [6].Blackwell KL, Burstein HJ, Storniolo AM, Rugo H, Sledge G, Koehler M, Ellis C, Casey M, Vukelja S, Bischoff J, Baselga J, O’Shaughnessy J, Journal of Clinical Oncology 2010, 28 (7), 1124–1130. [DOI] [PubMed] [Google Scholar]
- [7].Saura C, Garcia-Saenz JA, Xu B, Harb W, Moroose R, Pluard T, Cortés J, Kiger C, Germa C, Wang K, Martin M, Baselga J, Kim S-B, Journal of Clinical Oncology 2014. [DOI] [PubMed] [Google Scholar]
- [8].Baselga J, Cortes J, Kim SB, Im SA, Hegg R, Im YH, Roman L, Pedrini JL, Pienkowski T, Knott A, Clark E, Benyunes MC, Ross G, Swain SM, The New England Journal of Medicine 2012, 366 (2), 109–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Lim YW, Goh BC, Wang LZ, Tan SH, Chuah BYS, Lim SE, Iau P, Buhari SA, Chan CW, Sukri NB, Cordero MT, Soo R, Lee SC, Annals of Oncology : Official Journal of the European Society for Medical Oncology 2010, 21 (11), 2175–2182. DOI 10.1093/annonc/mdq230. [DOI] [PubMed] [Google Scholar]
- [10].Luo Y, Li W, Jiang Z, Zhang Q, Wang L, Mao Y, Tjan-Heijnen VCG, Im SA, McConnell R, Bejarano S, Fumagalli D, Bines J, Wang B, Garg A, Kirschbrown WP, Xu B, Anti-Cancer Drugs 2019, 30 (8), 866–872. DOI 10.1097/cad.0000000000000808. [DOI] [PubMed] [Google Scholar]
- [11].Ngamcherdtrakul W, Morry J, Gu S, Castro DJ, Goodyear SM, Sangvanich T, Reda MM, Lee R, Mihelic SA, Beckman BL, Hu Z, Gray JW, Yantasee W, Advanced Functional Materials 2015, 25 (18), 2646–2659. DOI: 10.1002/adfm.201404629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Hopkins AL, Groom CR, Nature Reviews Drug Discovery 2002, 1 (9), 727–730. DOI 10.1038/nrd892. [DOI] [PubMed] [Google Scholar]
- [13].Hanahan D, Weinberg RA, Cell 2011, 144 (5), 646–674. [DOI] [PubMed] [Google Scholar]
- [14].Ngamcherdtrakul W, Castro DJ, Gu S, Morry J, Reda M, Gray JW, Yantasee W, Cancer Treatment Reviews 2016, 45, 19–29. DOI 10.1016/j.ctrv.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Ngamcherdtrakul W, Yantasee W, Translational Research 2019, 214, 105–120. DOI 10.1016/j.trsl.2019.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Gu S, Hu Z, Ngamcherdtrakul W, Castro DJ, Morry J, Reda MM, Gray JW, Yantasee W, Oncotarget 2016, 7 (12), 14727–14741. DOI 10.18632/oncotarget.7409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Gu S, Ngamcherdtrakul W, Reda M, Hu Z, Gray JW, Yantasee W, PLOS ONE 2018, 13 (6), e0198141. DOI 10.1371/journal.pone.0198141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Leyland-Jones B, J Clin Oncol. 2009, 27 (31), 5278–5286. [DOI] [PubMed] [Google Scholar]
- [19].Ramakrishna N, Temin S, Chandarlapaty S, Crews J, Davidson N, Esteva F, Giordano S, Gonzalez-Angulo A, Kirshner J, Krop I, Levinson J, Modi S, Patt D, Perez E, Perlmutter J, Winer E, Lin N, Journal of Clinical Oncology : Official Journal of the American Society of Clinical Oncology 2014, 32 (19), 2100–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Yuan P, Gao S, Chronic Dis Transl Med. 2017, 3 (1), 21–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].Burgess A, Hynynen K, in Therapeutic Ultrasound, (Eds: Escoffre J-M; Bouakaz A, Springer International Publishing: Cham, 2016; pp 293–308. [Google Scholar]
- [22].Abrahao A, Meng Y, Llinas M, Huang Y, Hamani C, Mainprize T, Aubert I, Heyn C, Black SE, Hynynen K, Lipsman N, Zinman L, Nat Commun 2019, 10 (1), 4373–4373. DOI 10.1038/s41467-019-12426-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Lipsman N, Meng Y, Bethune AJ, Huang Y, Lam B, Masellis M, Herrmann N, Heyn C, Aubert I, Boutet A, Smith GS, Hynynen K, Black SE, Nat Commun 2018, 9 (1), 2336–2336. DOI 10.1038/s41467-018-04529-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Heiser LM, Sadanandam A, Kuo W-L, Benz SC, Goldstein TC, Ng S, Gibb WJ, Wang NJ, Ziyad S, Tong F, Bayani N, Hu Z, Billig JI, Dueregger A, Lewis S, Jakkula L, Korkola JE, Durinck S, Pepin F, Guan Y, Purdom E, Neuvial P, Bengtsson H, Wood KW, Smith PG, Vassilev LT, Hennessy BT, Greshock J, Bachman KE, Hardwicke MA, Park JW, Marton LJ, Wolf DM, Collisson EA, Neve RM, Mills GB, Speed TP, Feiler HS, Wooster RF, Haussler D, Stuart JM, Gray JW, Spellman PT, Proceedings of the National Academy of Sciences 2012, 109 (8), 2724–2729. DOI 10.1073/pnas.1018854108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Elm’hadi C, Tanz R, Khmamouche MR, Toreis M, Mahfoud T, Slimani KA, Errihani H, Ichou M, Springerplus 2016, 5 (1), 732–732. DOI 10.1186/s40064-016-2351-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Francia G, Man S, Lee C-J, Lee CR, Xu P, Mossoba ME, Emmenegger U, Medin JA, Kerbel RS, Clinical Cancer Research 2009, 15 (20), 6358–6366. DOI 10.1158/1078-0432.ccr-09-0931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Morry J, Ngamcherdtrakul W, Gu S, Reda M, Castro DJ, Sangvanich T, Gray JW, Yantasee W, Molecular Cancer Therapeutics 2017, DOI: 10.1158/1535–7163.mct-16–0644. DOI 10.1158/1535-7163.mct-16-0644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Valenzuela PA, Jallad SN, Parra K, Lerma N, Miramontes I, Gallegos A, Xu P, Cruz-Munoz W, Man S, Kerbel RS, Francia G, Cancer Research 2014, 74 (19 Supplement), 3142–3142. DOI 10.1158/1538-7445.Am2014-3142. [DOI] [Google Scholar]
- [29].Neun BW;, Ilinskaya AN;, Dobrovolskaia MA NCL Method ITA-2.2: Analysis of Platelet Aggregation by Light Transmission Aggregometry; Frederick National Laboratory for Cancer Research: Frederick, MD, 2015. [Google Scholar]
- [30].Aston WJ, Hope DE, Nowak AK, Robinson BW, Lake RA, Lesterhuis WJ, BMC Cancer 2017, 17 (1), 684. DOI 10.1186/s12885-017-3677-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Muqbil I, Philip PA, Mohammad RM, in Animal Models in Cancer Drug Discovery, (Eds: Azmi A; Mohammad RM, Academic Press: 2019; pp 233–248. [Google Scholar]
- [32].Barone M, Chain F, Sokol H, Brigidi P, Bermúdez-Humarán LG, Langella P, Martín R, Frontiers in Microbiology 2018, 9 (565). DOI 10.3389/fmicb.2018.00565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Center for Drug Evaluation and Research, NDA 210922 - Patisiran - Cross-Discipline Team Leader Review, https://www.accessdata.fda.gov/drugsatfda_docs/nda/2018/210922Orig1s000MultiR.pdf, accessed: December, 2021.
- [34].Ngamcherdtrakul W, Reda M, Nelson MA, Wang R, Zaidan HY, Bejan DS, Hoang NH, Lane RS, Luoh S-W, Leachman SA, Mills GB, Gray JW, Lund AW, Yantasee W, Advanced Materials 2021, 33 (31), 2100628. DOI 10.1002/adma.202100628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Moataz R, Worapol N, Natnaree S, Daniel B, Sherif R, Ruijie W, Molly N, Husam Z, Ngoc H, Akash B, Gordon M, Joe G, Wassana Y, Nature Portfolio 2021. DOI 10.21203/rs.3.rs-142908/v1. [DOI] [Google Scholar]
- [36].Tarn D, Ashley CE, Xue M, Carnes EC, Zink JI, Brinker CJ, Accounts of Chemical Research 2013, 46 (3), 792–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Jugdaohsingh R, The Journal of Nutrition, Health & Aging 2007, 11 (2), 99–110. [PMC free article] [PubMed] [Google Scholar]
- [38].Phillips E, Penate-Medina O, Zanzonico PB, Carvajal RD, Mohan P, Ye Y, Humm J, Gönen M, Kalaigian H, Schöder H, Strauss HW, Larson SM, Wiesner U, Bradbury MS, Science Translational Medicine 2014, 6 (260), 260ra149–260ra149. DOI 10.1126/scitranslmed.3009524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].O’Reilly M, Hynynen K, Radiology 2012, 263 (1), 96–106. DOI 10.1148/radiol.11111417. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.