Abstract
Background:
The Surgical Treatment for Ischemic Heart Failure Trial (STICH) demonstrated that coronary artery bypass grafting (CABG) reduced all-cause mortality rates out to 10 years compared with medical therapy alone (MED) in patients with ischemic cardiomyopathy and reduced left ventricular function (ejection fraction ≤35%). We examined the economic implications of these results.
Methods:
We used a decision-analytic patient-level simulation model to estimate the lifetime costs and benefits of CABG and MED using patient-level resource use and clinical data collected in the STICH trial. Patient-level costs were calculated by applying externally derived US cost weights to resource use counts during trial follow-up. A 3% discount rate was applied to both future costs and benefits. The primary outcome was the incremental cost-effectiveness ratio (ICER) assessed from the US healthcare sector perspective.
Results:
For the CABG arm, we estimated 6.53 quality-adjusted life years (QALYs) (95% confidence internal (CI) 5.70 to 7.53) and a lifetime cost of $140,059 (95% CI $106,401 to $180,992). For the MED arm, the corresponding estimates were 5.52 (95% CI 5.06 to 6.09) QALYs and $74,894 lifetime cost (95% CI $58,372 to $93,541). The ICER for CABG compared to MED was $63,989 per QALY gained. At a societal willingness-to-pay threshold of $100,000 per QALY gained, CABG was found to be economically favorable compared to medical therapy in 87% of microsimulations.
Conclusion:
In STICH patients with ischemic cardiomyopathy and reduced left ventricular function, CABG was economically attractive relative to medical therapy at current benchmarks for value in the United States.
Clinical Trial Registration:
URL: https://www.clinicaltrials.gov/ct2/show/NCT00023595; Unique Identifier: NCT00023595
INTRODUCTION
The natural history of ischemic cardiomyopathy is one of progressive left ventricular damage and dysfunction, leading to impaired quality of life, accelerating medical care costs, and eventually premature death.1–3 Medical therapy has a central role to play in favorably modifying prognosis for patients with heart failure (HF) with reduced ejection fraction.4 When significant ischemia due to obstructive coronary artery disease (CAD) is believed to be the primary cause of the HF with reduced ejection fraction, clinicians must decide whether medical therapy alone is sufficient or whether the patient will also benefit from coronary revascularization. The use of such interventions is motivated by pathophysiologic reasoning and by some observational data.5, 6 However, only one major clinical trial has tested the value of a revascularization strategy added to medical therapy. The Surgical Treatment for Ischemic Heart Failure (STICH) trial showed that, relative to MED alone, coronary artery bypass grafting (CABG) improved survival at 10 years of follow up. Quality of life (QOL) was also significantly improved with CABG.7, 8 Substitution of percutaneous coronary intervention (PCI) for CABG, while appealing on the grounds of being both less invasive and less costly, was not tested in STICH and is not supported by any other large trial evidence.9–12
The long-term economic consequences of selecting CABG in STICH-eligible patients with ischemic cardiomyopathy has not been defined. While clinicians rarely regard cost issues as relevant to their treatment decisions, such data are currently included in American College of Cardiology / American Heart Association clinical guideline documents and are often pivotal in policy-level health care funding decisions.13, 14 In this report, we used patient-level resource use and clinical data collected in the STICH trial to assess the cost effectiveness of coronary artery bypass surgery for patients with ischemic cardiomyopathy from the perspective of the United States (US) health care system.
METHODS
The data underlying this analysis can be requested from the National Heart, Lung, and Blood Institute and can be accessed via the Biologic Specimen and Data Repository Information Coordinating Center (https://biolincc.nhlbi.nih.gov/studies/stich/).
Trial Design and Patient Population
In the STICH trial, 1,212 patients with a left ventricular ejection fraction (LVEF) of ≤35% and coronary artery disease amenable to CABG were randomized to either CABG plus medical therapy (CABG) or medical therapy alone (MED).7, 8 Participants ≥18 years of age from 22 countries were enrolled between 2002 and 2007. Follow-up extended to 2015. Approval of the appropriate ethics committees was obtained at all sites, and all patients provided written informed consent (ClinicalTrials.gov Identifier NCT00023595).
As previously reported, the patients enrolled in STICH had a median age of 60 years, 12% were female, and 35% identified as an ethnic or racial minority.7, 8 A history of prior myocardial infarction was present in 77%, 39% had diabetes, 37% had New York Heart Association (NYHA) Class III or IV functional status, 54% had an ejection fraction ≤28%, 36% had 3-vessel CAD, and 69% had significant left main or proximal left anterior descending CAD.
Economic Model Design and Structure
We constructed an individual patient-level state transition simulation model to project the benefits and costs of the CABG group and MED group over a lifetime horizon from the perspective of the US health system (Supplemental Figure I). The model consisted of two health states, in which a HF patient each month may survive or die over monthly transition cycles.15, 16 To account for patient heterogeneity, individual patient estimates of life expectancy were estimated by bootstrapping (i.e., 5,000 first order simulations) baseline characteristic risk profiles from patients randomized to medical therapy alone in the STICH trial (N=610) and applying a multivariable parametric survival model to these patient risk profiles (see section below on Survival Probabilities). Each simulated patient in the model accrued costs, life-years (LYs), and quality-adjusted life years (QALYs) based on parameters derived from the prospectively collected trial data by intention-to-treat groups. Parameter uncertainty was incorporated into the model through a probabilistic sensitivity analysis (i.e., 10,000 second-order simulations per each simulated patient). Model outcomes included costs, LYs, QALYs, and the incremental cost-effectiveness ratio (ICER). All outcomes are reported based on the intention-to-treat population.
Survival Probabilities
All-cause mortality was modelled using patient-level data from the STICH trial and its extended follow up trial (STICHES).7, 8 At the end of the extended median follow up of 9.8 years, 58.9% of patients randomized to CABG and 66.1% of patients randomized to medical therapy alone had died.8 From the individual patient-level data, all-cause mortality for the MED group was fit to a parametric survival model using a Gompertz distribution, which was used to estimate within-trial survival and to extrapolate the tail end of the survival curve beyond trial follow-up.17 The Gompertz distribution was selected according to smallest Akaike or Bayesian Information Criteria goodness-of-fit statistics.18, 19
The multivariable Gompertz model was then used to derive a survival curve for each patient over their lifetime (Supplemental Table I). Mortality rates for each cycle were calculated as a function of baseline patient characteristics including age, sex, smoking status, New York Heart Association symptom class, diabetes, LVEF, creatinine, hemoglobin, number of vessels with >75% stenosis, and severity of mitral regurgitation. To account for the interdependence of patient characteristics in survival modeling and in probabilistic sensitivity analyses based on that modeling, a Cholesky decomposition of the covariance matrix was estimated from the output of the regression models.20
Hazard ratios, comparing CABG to MED, were applied to cycle-specific all-cause death rates in the medical management arm to estimate the survival curves for patients randomized to the CABG group. In the STICH trial, hazards were not proportional over time due to an increased risk of mortality associated with CABG during the early post-operative period. To account for the varying treatment effect over time, the hazard ratios for all-cause death were estimated using a Cox proportional hazard model, stratified by time period (≤60 days, 61 days to 365 days, 366 days to 2 years, and ≥2 years) (Supplemental Table II). After 2 years, the HR for death did not violate proportionality assumption (Schoenfeld Residuals test, p=0.18) and the HR beyond 2 years was not further partitioned.
To assess model agreement with empirical STICH survival results, we compared the model estimates of undiscounted life expectancy to the restricted mean survival times derived directly from the STICH trial data. The modelled and empiric survival estimates were compared over the trial follow up.
Health-Related Quality of Life (QOL)
The STICH trial collected QOL data at 4, 12, 24, and 36 months using the EQ-5D 3-level instrument. EQ-5D responses were converted to a summary preference weighted health index, or utility index, ranging from 0 to 1 with 1 representing perfect health and 0 representing death.21 The STICH utilities, by intention to treat, have been previously reported.22 At baseline assessment, the CABG group reported a lower mean utility score than the MED group (0.693 versus 0.723). The mean utility difference between CABG and MED over trial follow up was +0.045 (95% confidence interval (CI) 0.015 to 0.075, p=0.004). Our economic model incorporated the temporal changes in utility scores (Supplemental Table II).
Since QOL data were not collected in the extended follow up STICHES trial, we adopted the conservative assumption that QOL gains attenuated at the end of trial follow up. That is, at 10 years, the mean utility of the CABG group was assumed to be equal to the mean utility of the MED group (i.e. 0.815). As a sensitivity analysis, we modelled an alternative scenario where the QOL benefits were sustained beyond trial follow up.
Medical Resource Use and Costs
Costs were based on medical resource use data prospectively collected over trial follow up. Resource consumption data included dates of inpatient care for rehospitalization, and hospital-based testing and procedures. External cost weights were developed to value resource use collected in the STICHES trial (Supplemental Tables III and IV). Weights for hospital-based services were estimated using encounter-level cost data for patients with a diagnosis of ischemic cardiomyopathy extracted from the Premier Healthcare Database (1/1/2016–12/31/2016).23 This database contains discharge and cost data for all inpatient and hospital-based outpatient encounters from geographically diverse US hospitals. Two-thirds of these hospitals provide detailed, service-level data from resource-based cost-accounting systems, while the remainder provides itemized charges that are converted to costs using department level cost-to-charge ratios. Patient-level costs were calculated by applying cost weights to collected resource use during trial follow-up.
Index hospitalization and follow up costs were estimated separately. Costs associated with initial CABG hospitalization included inpatient room and board, nursing care, operative room services, medical supplies, pharmacy, laboratory and imaging tests. During the follow up, mean costs for hospitalization and inpatient procedures were estimated in 3-month intervals by treatment group (Supplemental Figure II). For extrapolation of inpatient costs beyond trial-follow, costs per patient appeared stable in the STICHES extended follow up period (Supplemental Figure II). We assumed a constant inpatient cost per patient per period based on the average per patient costs beyond 6 years. For hospital-based outpatient procedures, we estimated a constant 3-month interval cost by treatment group from the cumulative costs of outpatient procedures over trial follow up.
Since physician costs were excluded from the Premier Database, the cost of inpatient physician services, including daily hospital care and procedures, was estimated from professional fee ratios reported for HF patients; specifically, physician fees represented an additional 14% of total inpatient HF costs.24 Costs for outpatient management included hospital-based ambulatory visits, office-based visits, and medications. These costs have been previously reported using data from the 2002–2011 Medical Expenditure Panel Survey Household component, which is the largest nationally representative survey of medical costs in the United States.25 Annual medication costs were estimated at an average of $4,773, and annual medical visits were estimated at $3,407. The CABG and MED groups were assumed to have similar annual medication and outpatient medical care costs. For patients who died, a one-time end-of-life cost was added to account for the increased resource use in the last 6 months of end-stage heart failure,26 approximated by an additional year-worth of outpatient costs. Costs were valued in 2019 US dollars and adjusted using the Personal Health Care Index or the Centers for Medicare & Medicaid Services inpatient market basket indicator, as appropriate.27 A 3% discount rate was applied to all future costs and benefits.28
Statistical Analyses
In order to understand how key model inputs influenced the estimated cost effectiveness, one-way deterministic sensitivity analyses were performed by varying a single input parameter at a time and recording the change in incremental cost per QALY gained. Key assumptions varied in these analyses included the time horizon, discount rate, and extrapolation of clinical effectiveness. For external cost inputs (i.e. outpatient management and medication costs), we varied the parameters over wide range (±50%).
We also conducted a probabilistic sensitivity analysis for each simulated patient using a Monte Carlo simulation of 10,000 iterations to propagate the uncertainty in model parameters in order to generate a distribution of expected costs and QALYs. We applied log-normal distributions for all hazard ratios, β-distributions to all probabilities and utilities, and γ-distributions to all costs. Incremental cost-effectiveness ratios were calculated as the difference in mean lifetime cost divided by the difference in mean quality-adjusted life expectancy. Cost effectiveness was displayed on the incremental cost-effectiveness plane and summarized using cost-effectiveness acceptability curves.29 We assumed a willingness-to-pay threshold of $100,000 per QALY gained,30 but we also considered the value assessment proposed by American College of Cardiology and American Heart Association: high-value represents cost-savings or ICER <$50,000 per QALY gained; intermediate value is represented by ICERs between $50,000 to <$150,000 per QALY gained; and low value is described by ICERs ≥$150,000 per QALY gained.13 The probabilities of cost effectiveness according to these different thresholds were estimated.
Secondary cost-effectiveness analyses were performed for two clinically relevant, pre-specified subgroups based on LVEF (i.e., ≤28% versus >28%) and extent of coronary artery disease (i.e., 0 to 2-vessel disease versus 3-vessel disease) with cut-points selected to be consistent with prior STICH clinical analyses.7, 8
Statistical analyses were performed in Stata/IC 15.1 (College Station, TX) and SAS/STAT 15.1 (Cary, NC). Modelling was performed in TreeAge Pro Healthcare 2021 (Williamstown, MA).
RESULTS
Model Agreement with Empirical STICH Survival
In the MED arm, the modelled life expectancies compared with restricted mean survival times were 0.95 LY versus 0.93 LY (0.92 – 0.95) at 1 year, 3.89 LYs versus 3.88 LY (3.74 – 4.01) at 5 years, and 6.14 LYs versus 6.11 LY (5.82 – 6.40) at 10 years. The modelled versus empirical estimate agreements were similarly consistent in the CABG arm (Supplemental Table V).
Within Trial Results (10-year Time Horizon)
Over the trial follow up period, patients in the MED group accrued an average of 5.41 LYs or 4.31 QALYs and a cumulative cost of $60,634. Patients randomized to the CABG group gained an additional 0.29 LY and 0.45 QALYs at an additional cost of $54,050. Mean cost of initial hospitalization, including physician fees, for the CABG group was estimated at $53,041. The within-trial ICER for CABG compared to MED alone was $120,288 per QALY gained, with an 34% likelihood of meeting a $100,000 per QALY willingness-to-pay threshold and a 69% likelihood of meeting a $150,000 per QALY gained threshold (Table 1). Without quality-of-life adjustment, the within-trial ICER for CABG compared to MED was $187,314 per LY gained.
Table 1.
Base Case and Sensitivity Analyses - Discounted Cost, Life Expectancy, and Cost Effectiveness*
| Scenario | CABG | Medical Therapy | Difference | Incremental Cost-Effectiveness Ratio | Probability of Cost-Effectiveness by Willingness-to-Pay threshold ($/QALY) | ||
|---|---|---|---|---|---|---|---|
| $50,000 | $100,000 | $150,000 | |||||
|
| |||||||
| Base Case | |||||||
| Lifetime Costs, USD | 140,059 (106,401 – 180,992) |
74,896 (58,372 – 93,541) |
65,163 (36,772 – 102,074) |
- | - | - | - |
| QALYs | 6.53 (5.70 – 7.53) |
5.52 (5.06 – 6.09) |
1.02 (0.40 – 1.71) |
$63,989 per QALY | 0.25 | 0.87 | 0.97 |
| LYs | 7.88 (6.90 – 9.07) |
6.90 (6.34 – 7.59) |
0.99 (0.25 – 1.80) |
$66,124 per LY | 0.25 | 0.81 | 0.93 |
|
| |||||||
| Sensitivity Analyses | |||||||
| Sustained QoL Benefit† | |||||||
| QALYs (base costs) | 6.65 (5.84 – 7.78) |
5.51 (5.07 – 6.05) |
1.14 (0.51 – 1.90) |
$55,703 per QALY | 0.36 | 0.91 | 0.98 |
|
| |||||||
| 10-Year Time Horizon | |||||||
| Total Costs, USD | 114,684 (83,400 – 154,075) |
60,634 (47,878 – 74,949) |
54,050 (25,391 – 90,814) |
- | - | - | - |
| QALYs | 4.76 (4.41 – 5.09) |
4.31 (4.11 – 4.50) |
0.45 (0.16 – 0.75) |
$120,288 per QALY | 0.02 | 0.34 | 0.69 |
| LYs | 5.70 (5.32 – 6.06) |
5.41 (5.18 – 5.63) |
0.29 (−0.02 – 0.61) |
$187,314 per LY | 0.003 | 0.13 | 0.35 |
|
| |||||||
| Attenuated Mortality Benefit‡ | |||||||
| Total Costs | 136,399 (104,548 – 180,485) |
75,288 (59,316 – 93,720) |
61,111 (33,418 – 95,711) |
- | - | - | - |
| QALYs | 6.22 (5.56 – 6.98) |
5.53 (5.08 – 6.06) |
0.69 (0.27 – 1.15) |
$88,448 per QALY | 0.07 | 0.63 | 0.89 |
| LYs | 7.49 (6.69 – 8.42) |
6.91 (6.36 – 7.55) |
0.58 (0.09 – 1.08) |
$105,275 per LY | 0.05 | 0.47 | 0.76 |
|
| |||||||
| 0% Discount Rate | |||||||
| Total Costs | 165,955 (125,636 – 216,037) |
91,996 (71,230 – 117,998) |
73,959 (42,248 – 112,674) |
- | - | - | - |
| QALYs | 8.47 (7.14 – 10.45) |
6.94 (6.16 – 8.00) |
1.53 (0.55 – 2.71) |
$48,375 per QALY | 0.52 | 0.95 | 0.99 |
|
| |||||||
| 5% Discount Rate | |||||||
| Total Costs | 128,887 (96,734 – 170,224) |
67,332 (53,782 – 84,162) |
61,554 (33,323 – 99,793) |
- | - | - | - |
| QALYs | 5.71 (5.12 – 6.45) |
4.92 (4.57 – 5.33) |
0.79 (0.31 – 1.32) |
$78,110 per QALY | 0.12 | 0.73 | 0.92 |
Reported costs and benefits are discounted at 3%, except for sensitivity analyses that use a 0% and 5% discount rate. Costs, LYs, and QALYs are represented by means and 95% confidence intervals.
Sustained QOL benefit – In this scenario, the CABG group continues to sustain additional quality of life gains compared to the MED group, beyond trial follow up at 10 years.
Attenuated mortality benefit – In this scenario, CABG does not confer a mortality benefit over MED beyond trial follow up. That is, the hazard ratio of mortality is set to 1.0 starting at 10 years.
Abbreviations: CABG – coronary artery bypass grafting, LY – life years, QALY – quality-adjusted life years, QoL – quality of life, USD – US dollars.
Discounted Base Case Results (Lifetime Horizon)
As per economic best practices, we estimated cost-effectiveness over a lifetime horizon to capture all potential treatment benefits and costs. Patients in the MED group accrued an average of 6.90 LYs or 5.52 QALYs at a lifetime cost of $74,896. Patients randomized to the CABG group experienced an additional 0.99 LY and 1.02 QALYs at an additional cost of $65,163. The ICER for CABG compared to MED alone was $63,989 per QALY gained, with an 87% likelihood of meeting a $100,000 per QALY willingness-to-pay threshold a 97% likelihood of meeting a $150,000 per QALY gained threshold (Table 1, Figure 1). Without quality-of-life adjustment, the ICER for CABG compared to MED was $66,124 per LY gained.
Figure 1.
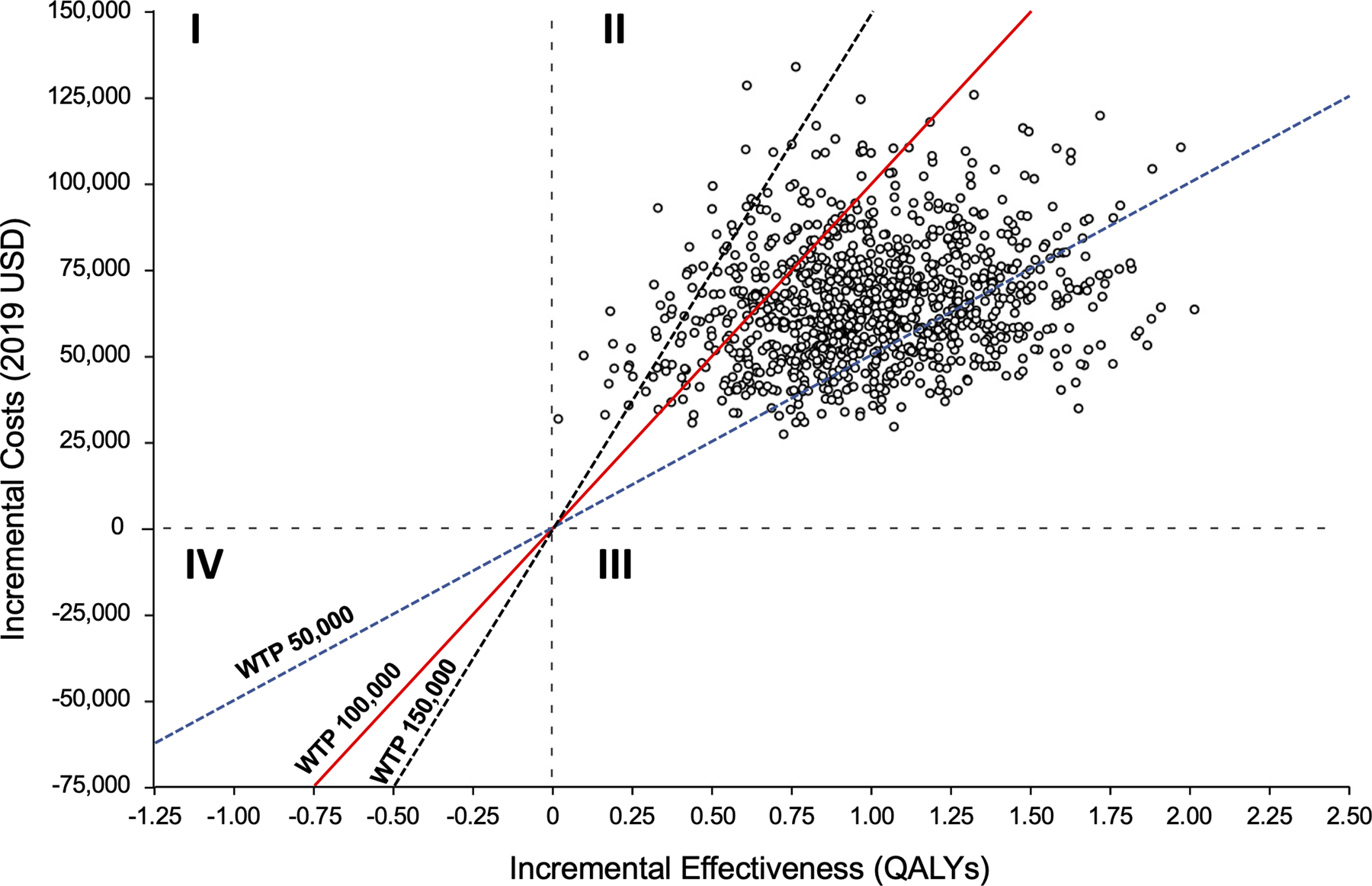
Incremental cost-effectiveness plane comparing CABG to Medical Therapy. Quadrant I represent scenarios where CABG is more costly and less effective, Quadrant II represents scenarios where CABG is more costly and effective, Quadrant III represents scenarios where CABG is less costly but more effective, and Quadrant IV represents scenarios where CABG is less costly and less effective. Abbreviations: QALY – quality-adjusted life year; WTP – willingness-to-pay; USD – United States Dollars
Sensitivity Analyses
The input with the greatest variation effect on the results (Figure 2) was the long-term clinical effectiveness of CABG (i.e., risk reduction in all-cause mortality compared to medical therapy beyond 2 years of follow up). When the hazard ratio was varied from 0.63 to 0.89, the range of the incremental cost per QALY gained was between $45,431 and $116,298.
Figure 2.
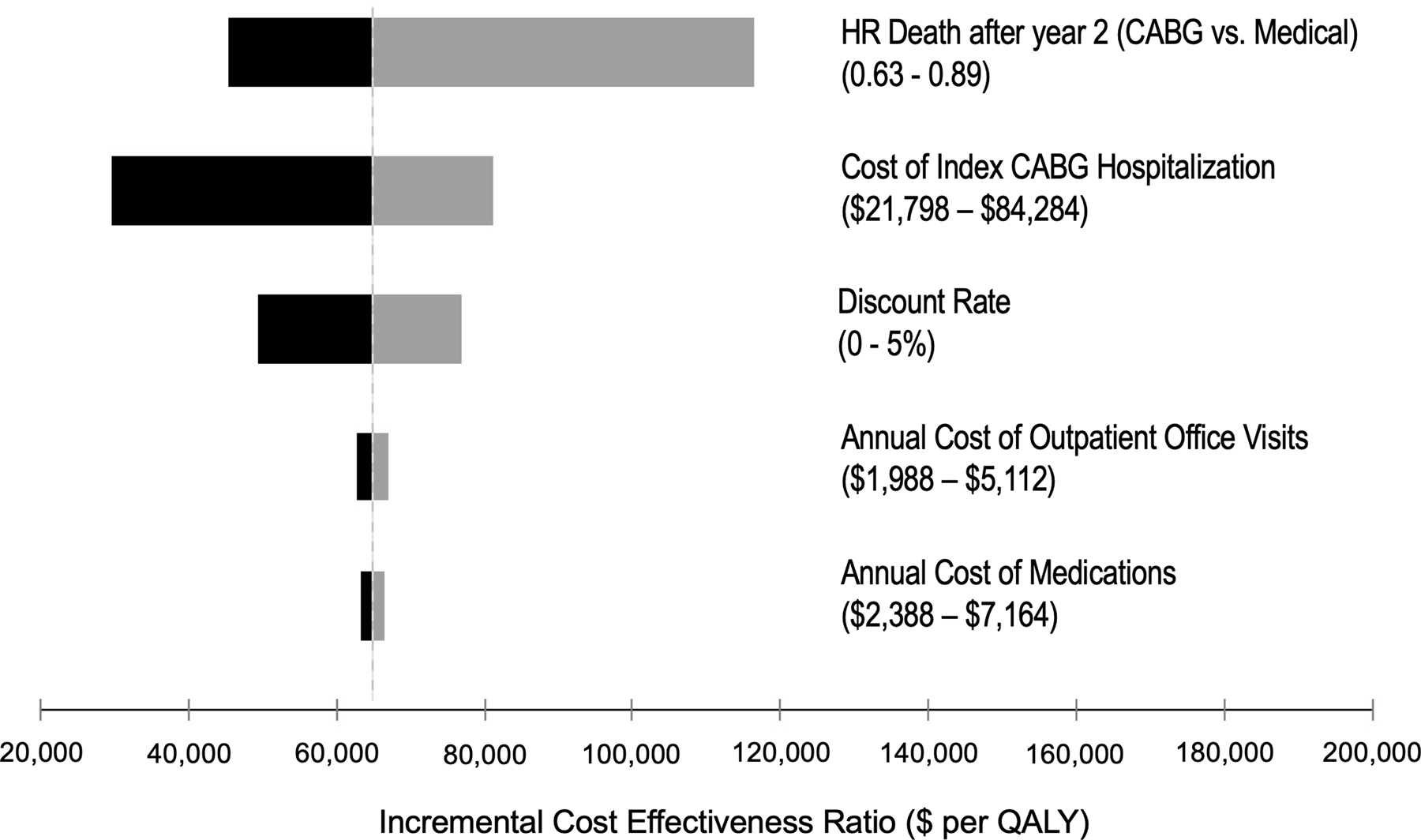
Tornado diagram summarizing one-way sensitivity analyses on incremental cost-effectiveness ratio (cost per quality-adjusted life years gained). Grey and black bars denote the effects of the upper and lower bounds of each variable input, respectively. Abbreviations: HR, hazard ratio; QALY, quality-adjusted life year.
The model was also moderately sensitive to the upfront costs associated with cardiac surgery in the CABG group. For instance, when the cost of initial hospitalization for CABG was varied from $21,798 to $84,284, the incremental cost per QALY gained ranged from $29,753 to $81,187. We conducted a post-hoc threshold analysis to identify the theoretical upper limit of initial CABG hospitalization costs that would yield a cost per QALY gained below a willingness-to-pay threshold of $50,000 per QALY. In this analysis, the initial cost of CABG hospitalization would need to be less than $46,397.
Our base case analysis made the conservative assumption that QOL gains associated with the CABG group were attenuated beyond trial follow up. If we modify this assumption to allow sustained QOL benefit of CABG beyond trial follow up, the CABG and MED groups accrued 6.65 and 5.51 QALYs, respectively, and the estimated ICER was $55,703 per QALY.
Finally, as there are limited data to extrapolate the long-term clinical effectiveness of CABG, we conducted a sensitivity analysis that attenuated the effect of CABG on mortality beyond the 10-year follow up of STICH. Specifically, we set the hazard ratio of mortality (CABG versus MED) to 1.0 at 10 years in the model. In this conservative analysis, CABG was associated with an ICER of $88,448 per QALY gained compared to MED with a 63% likelihood of meeting a $100,000 per QALY willingness-to-pay threshold.
Subgroup Analyses
Two subgroups prespecified in the parent STICH Trial were examined in our analysis (Supplemental Table VI). Among patients with an LVEF ≤28%, CABG led to greater incremental QALYs (1.31) and higher costs ($67,495) compared to patients with an LVEF >28% (i.e., 0.69 incremental QALYs and $62,936 in incremental costs). Accordingly, compared to MED, CABG among patients with an LVEF ≤28% yielded a more favorable ICER of $51,370 per QALY gained compared to patients with an LVEF >28% (ICER of $90,687 per QALY gained) (Table 2, Figure 3A).
Table 2.
Subgroup Analyses - Cost, Life Expectancy, and Cost Effectiveness*
| Subgroup | CABG | Medical Therapy | Difference | Incremental Cost-Effectiveness Ratio | Probability of Cost-Effectiveness by Willingness-to-Pay threshold ($/QALY) | ||
|---|---|---|---|---|---|---|---|
| $50,000 | $100,000 | $150,000 | |||||
|
| |||||||
| 0–2 Vessel Disease | |||||||
| Total Costs, USD | 137,652 (103,640 – 177,631) |
78,226 (61,406 – 97,974) |
59,426 (30,694 – 95,906) |
- | - | - | - |
| QALYs | 6.38 (5.37 – 7.54) |
5.82 (5.25 – 6.49) |
0.56 (−0.25 – 1.42) |
$106,752 per QALY | 0.08 | 0.46 | 0.65 |
|
| |||||||
| 3 Vessel Disease | |||||||
| Total Costs, USD | 143,567 (107,360 – 188,165) |
69,810 (54,622 – 87,386) |
73,756 (42,741 – 113,276) |
- | - | - | - |
| QALYs | 6.80 (5.52 – 8.24) |
5.02 (4.49 – 5.64) |
1.78 (0.72 – 2.98) |
$41,476 per QALY | 0.71 | 0.98 | 1.00 |
|
| |||||||
| LVEF ≤ 28% | |||||||
| Total Costs | 135,441 (101,502 – 176,159) |
67,916 (53,112 – 84,421) |
67,495 (38,187 – 104,831) |
- | - | - | - |
| QALYs | 6.15 (5.20 – 7.28) |
4.84 (4.38 – 5.35) |
1.31 (0.54 – 2.21) |
$51,370 per QALY | 0.47 | 0.95 | 0.99 |
|
| |||||||
| LVEF > 28% | |||||||
| Total Costs | 146,356 (109,993 – 190,063) |
83,420 (64,780 – 104,702) |
62,936 (32,209 – 100,657) |
- | - | - | - |
| QALYs | 7.04 (5.72 – 8.52) |
6.35 (5.69 – 7.15) |
0.69 (−0.38 – 1.80) |
$90,687 per QALY | 0.14 | 0.55 | 0.71 |
Reported costs and benefits are discounted at 3%, except for sensitivity analyses that use a 0% and 5% discount rate. Costs, LYs, and QALYs are represented by means and 95% confidence intervals.
Abbreviations: CABG – coronary artery bypass grafting, LVEF – left ventricular ejection fraction, LY – life years, QALY – quality-adjusted life years, USD – US dollars.
Figure 3.
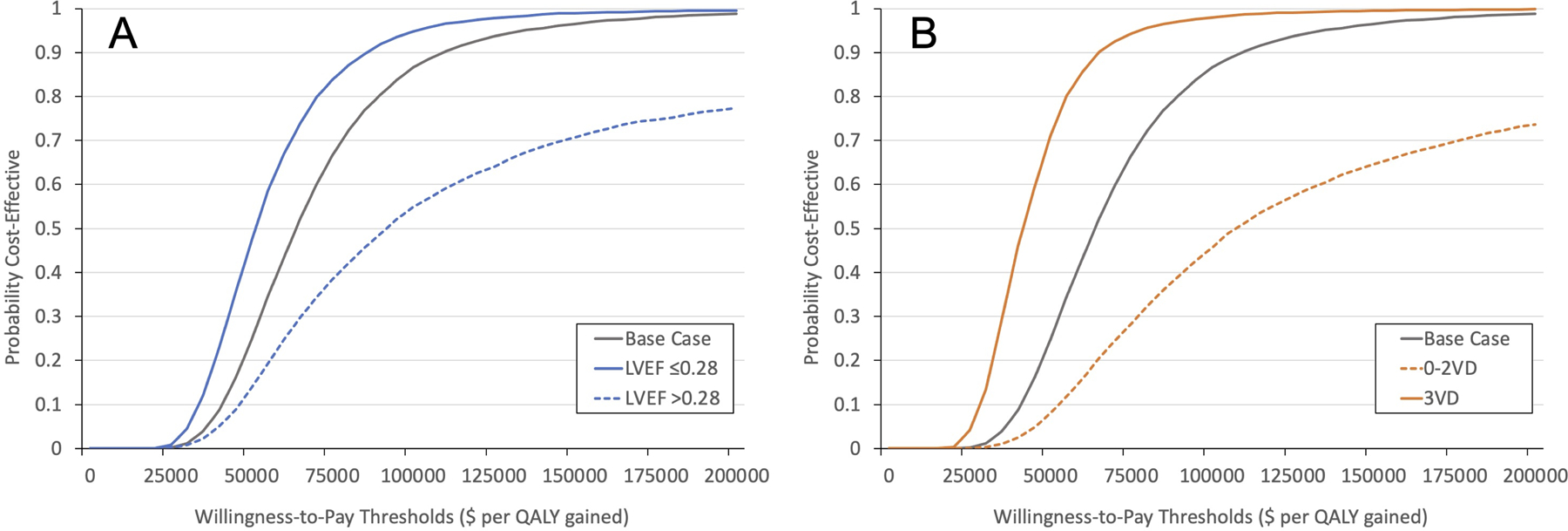
Cost-effectiveness acceptability curves for subgroups versus base case: (A) LVEF (≤28% versus >28%) (B) Number of Vessel Disease (0–2 vessels versus 3 vessel). These figures show the probability that the cost-effectiveness ratio for surgery (versus medicine) falls below willingness-to-pay thresholds <$200,000.
Similarly, CABG was more economically attractive among patients with 3-vessel disease (ICER $41,476 per QALY gained) compared to patients with <3 vessel disease (ICER $106,752 per QALY gained) (Table 2, Figure 3B).
DISCUSSION
The primary result of our analysis indicates that routine use of CABG in patients with ischemic cardiomyopathy eligible for STICH is economically attractive by conventional US health care system benchmarks, or “intermediate-value” as per the American College of Cardiology/American Heart Association Cost and Value framework.13 That is, while the CABG group had higher lifetime costs, the additional QALYs provided by CABG relative to MED provided reasonable value for money. In our base case analysis, the CABG:MED incremental cost-effectiveness ratio was $63,989 per QALY gained, and in the probabilistic sensitivity analysis, 87% of microsimulations yielded a value below $100,000 per QALY. Notable strengths of the current study include use of an individual patient microsimulation model to better represent patient heterogeneity in medical resource use and therapeutic response among patients enrolled in STICH. Additionally, our economic analysis benefits from a relatively long study follow up period (i.e., median 9.8 years), which reduces the amount of extrapolation needed to complete a lifetime cost-effectiveness analysis. Our comparison of model-based with observed survival outcomes out to 10 years showed excellent agreement, increasing confidence that our post-empirical extrapolations are also reasonable.
The current economic analysis has important implications in the United States given the prevalence of ischemic cardiomyopathy, which comprises approximately 50% of all HF with reduced LV function.31 The relevance of ischemic cardiomyopathy as a public health issue is anticipated to become even more urgent in the context of an aging population and rising prevalence of coronary artery disease and HF.32 Thus, it is critical to understand the relative value of high-cost surgical interventions by considering the downstream economic and clinical consequences.
In the current study, our estimates of cost effectiveness were not substantially affected by changes to health resource unit costs, discount rate, quality-of-life weighting or the assumptions related to extrapolation of clinical effectiveness. As economic models require extrapolation beyond trial follow up, our sensitivity analyses assessed a key assumption regarding the long-term clinical benefit of CABG on ischemic cardiomyopathy relative to medical therapy alone. Similar to the base case analysis, the CABG strategy was associated with an ICER less than $100,000 per QALY gained in the conservative scenario where the mortality benefit of CABG was attenuated at 10 years. Nevertheless, this assumption may be considered too conservative. In the base case analysis where we allowed for sustained clinical benefit, our model predicted a 30-year survival among CABG patients of 4%, which is similar to the survival reported in observational cohort studies. For example, Domburg and colleagues reported a 30-year survival of 6% (95% CI 0 to 13%) among a Dutch cohort with impaired LV function undergoing CABG.33
There are few prior economic evaluations that assess the value of CABG relative to medical therapy in the context of a large, randomized trial.34 In a trial-based analysis of the BARI-2D (Bypass Angioplasty Revascularization Investigation 2 Diabetes) study, Hlatky and colleagues found that CABG was economically attractive compared to medical therapy at a cost of $50,000 (2006 USD) per QALY gained over lifetime horizon.35 However, their economic findings cannot be generalized to the STICHES cohort due to differences in baseline characteristics of enrolled participants. Specifically, BARI-2D only enrolled participants with type 2 diabetes (compared to 40% diabetes in STICH), and only 6% of participants enrolled to the CABG stratum had reduced LV function (LVEF <40%).36, 37 Similarly, prior economic analyses from Brazil and the United Kingdom compared CABG and medical therapy in patient populations with primarily preserved LV function.38, 39
Our model found that patients assigned to the CABG group had greater total costs than medical therapy, driven by higher initial costs associated with surgery. However, the upfront costs were offset by sufficient gains in life expectancy and quality of life to represent good value.13 Notably, the incremental cost-effectiveness ratio remained below $100,000 per QALY gained even at the upper range of index CABG costs (approximately $84,000). Finally, CABG was most economically attractive among patients with more severe forms of ischemic cardiomyopathy, such as those with more extensive coronary artery disease and lower LVEF. These results are concordant with the clinical findings of STICH,8, 22, 40 where these higher risk subgroups had greater relative gains in survival and quality of life to offset the expected higher cumulative costs compared to their lower risk counterparts.
Limitations
Several caveats should be considered in the interpretation of our study. First, because most patients were enrolled outside the US, medical resource use was valued using externally derived unit costs rather than using empirical trial cost data collected from the hospitals and clinicians providing care to STICH patients. Unit cost estimates in our analysis were based on the costs of a large cohort of patients with ischemic cardiomyopathy from geographically diverse US hospitals. Second, QOL data were not collected beyond the follow up period of the original STICH trial. In the base case analysis, we adopted a conservative assumption where QOL gains were attenuated beyond trial follow up. This assumption may underestimate the value of CABG since potential quality of life differences due to rehospitalization or progression in HF would not be captured. Accordingly, we conducted an optimistic sensitivity analysis where QOL gains were sustained beyond trial follow up. However, varying the duration of QOL benefit did not substantially affect the value of CABG (i.e., optimistic ICER of $55,703 per QALY versus base case ICER of $63,989 per QALY gained). Finally, while ICERs are reported to the nearest dollar, these estimates are based on extrapolation of several model inputs including cost, effectiveness, and quality-of-life. These estimates therefore necessarily incorporate important uncertainties at multiple levels, many of which are unavoidable in such an analysis. For this reason, interpretation of the cost-effectiveness ratios and component estimates should always be done with an appreciation that they are approximations that make use of the best information available together with plausible assumptions. The associated uncertainty is assessed in our analysis using bootstrap analysis and quantified as the percentage probability of being under the major cost-effectiveness interpretive benchmarks.
CONCLUSION
In STICH patients with ischemic cardiomyopathy and reduced left ventricular function, CABG was economically attractive relative to medical therapy at current benchmarks for societal willingness to pay in the United States.
Supplementary Material
CLINICAL PERSPECTIVE.
What is New?
Although STICH (Surgical Treatment for Ischemic Heart Failure Trial) showed that coronary artery bypass grafting (CABG) improved survival relative to medical therapy (MED) alone at 10-years of follow up, the long-term economic consequences of selecting CABG in this population have not previously been described.
In this patient-level simulation model using resource use and clinical data collected in the STICH trial, CABG was estimated to cost $63,989 per quality-adjusted life-year gained compared to MED.
What are the Clinical Implications?
In STICH-eligible patients with left ventricular ejection fraction (LVEF) of ≤35% and coronary artery disease amenable to CABG, routine use of CABG increases the quality-adjusted life expectancy compared to medical therapy alone for an increased cost within current benchmarks for good value in healthcare within the United States.
Together with the improved clinical outcomes seen in the 10-year extended follow up of STICH, these findings provide additional economic support for the use of CABG in patients with ischemic cardiomyopathy eligible for STICH.
SOURCES OF FUNDING
The STICH trial was supported by cooperative agreements (U01-HL-069009, HL-069010, HL-069011, HL-069012, HL-069013, HL-069015, HL-070011, and HL-072683) with the National Heart, Lung, and Blood Institute. The STICHES trial was supported by a separate grant (R01-HL105853) from the National Institutes of Health.
ABBREVIATIONS
- BARI-2D
Bypass Angioplasty Revascularization Investigation 2 Diabetes
- CABG
coronary artery bypass grafting
- CAD
coronary artery disease
- CI
confidence interval
- HF
heart failure
- ICER
incremental cost effectiveness ratio
- LVEF
left ventricular ejection fraction
- LY
life year
- MED
medical therapy
- NYHA
New York Heart Association
- PCI
percutaneous coronary intervention
- QALY
quality-adjusted life year
- QOL
quality of life
- STICH
Surgical Treatment for Ischemic Heart Failure Trial
- USD
United States dollars
Footnotes
DISCLOSURES
DSC is supported by a Canadian Institutes of Health Research Banting Fellowship and an Arthur JE Child Cardiology Fellowship. DBM reports grant support from Merck & Co., Inc., HeartFlow and Mayo Clinic. JR reports consulting fees for Novartis, AstraZeneca, Abbott, Bayer, Aventis and Myokardia. The remaining authors report no relevant disclosures.
REFERENCES
- 1.Balmforth C, Simpson J, Shen L, Jhund PS, Lefkowitz M, Rizkala AR, Rouleau JL, Shi V, Solomon SD, Swedberg K, Zile MR, Packer M and McMurray JJV. Outcomes and Effect of Treatment According to Etiology in HFrEF: An Analysis of PARADIGM-HF. JACC Heart Fail. 2019;7:457–465. [DOI] [PubMed] [Google Scholar]
- 2.Khatibzadeh S, Farzadfar F, Oliver J, Ezzati M and Moran A. Worldwide risk factors for heart failure: a systematic review and pooled analysis. Int J Cardiol. 2013;168:1186–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Virani SS, Alonso A, Aparicio HJ, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Cheng S, et al. , American Heart Association Council on Epidemiology, Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2021 Update: A Report From the American Heart Association. Circulation. 2021;143:e254–e743. [DOI] [PubMed] [Google Scholar]
- 4.Murphy SP, Ibrahim NE and Januzzi JL. Heart failure with reduced ejection fraction: a review. JAMA. 2020;324:488–504. [DOI] [PubMed] [Google Scholar]
- 5.Braunwald E and Rutherford JD. Reversible ischemic left ventricular dysfunction: evidence for the “hibernating myocardium”. J Am Coll Cardiol. 1986;8:1467–1470. [DOI] [PubMed] [Google Scholar]
- 6.O’Connor CM, Velazquez EJ, Gardner LH, Smith PK, Newman MF, Landolfo KP, Lee KL, Califf RM and Jones RH. Comparison of coronary artery bypass grafting versus medical therapy on long-term outcome in patients with ischemic cardiomyopathy (a 25-year experience from the Duke Cardiovascular Disease Databank). Am J Cardiol. 2002;90:101–107. [DOI] [PubMed] [Google Scholar]
- 7.Velazquez EJ, Lee KL, Deja MA, Jain A, Sopko G, Marchenko A, Ali IS, Pohost G, Gradinac S, Abraham WT, et al. Coronary-artery bypass surgery in patients with left ventricular dysfunction. N Engl J Med. 2011;364:1607–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Velazquez EJ, Lee KL, Jones RH, Al-Khalidi HR, Hill JA, Panza JA, Michler RE, Bonow RO, Doenst T, Petrie MC, et al. Coronary-Artery Bypass Surgery in Patients with Ischemic Cardiomyopathy. N Engl J Med. 2016;374:1511–1520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun LY, Gaudino M, Chen RJ, Bader Eddeen A and Ruel M. Long-term Outcomes in Patients With Severely Reduced Left Ventricular Ejection Fraction Undergoing Percutaneous Coronary Intervention vs Coronary Artery Bypass Grafting. JAMA Cardiol. 2020;5:631–641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Pei J, Wang X, Xing Z, Zheng K and Hu X. Short-term and long-term outcomes of revascularization interventions for patients with severely reduced left ventricular ejection fraction: a meta-analysis. ESC Heart Fail. 2021;8:634–643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Perera D, Clayton T, Petrie MC, Greenwood JP, O’Kane PD, Evans R, Sculpher M, McDonagh T, Gershlick A, de Belder M, et al. Percutaneous Revascularization for Ischemic Ventricular Dysfunction: Rationale and Design of the REVIVED-BCIS2 Trial: Percutaneous Coronary Intervention for Ischemic Cardiomyopathy. JACC Heart Fail. 2018;6:517–526. [DOI] [PubMed] [Google Scholar]
- 12.Velazquez EJ. Percutaneous Coronary Intervention or Coronary Artery Bypass Grafting to Treat Ischemic Cardiomyopathy? JAMA Cardiol. 2020;5:641–642. [DOI] [PubMed] [Google Scholar]
- 13.Anderson JL, Heidenreich PA, Barnett PG, Creager MA, Fonarow GC, Gibbons RJ, Halperin JL, Hlatky MA, Jacobs AK, Mark DB, et al. ACC/AHA statement on cost/value methodology in clinical practice guidelines and performance measures: a report of the American College of Cardiology/American Heart Association Task Force on Performance Measures and Task Force on Practice Guidelines. J Am Coll Cardiol. 2014;63:2304–2322. [DOI] [PubMed] [Google Scholar]
- 14.Chew DS and Mark DB. Dapagliflozin-Does Cost Make 4-Pillar Heart Failure Therapy Too Herculean a Labor for Medicine? JAMA Cardiol. 2021;6:875–876. [DOI] [PubMed] [Google Scholar]
- 15.Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ, Rouleau JL, Zile MR, McMurray JJ and Solomon SD. Cost-effectiveness Analysis of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction. JAMA Cardiol. 2016;1:666–672. [DOI] [PubMed] [Google Scholar]
- 16.Gold MR, Padhiar A, Mealing S, Sidhu MK, Tsintzos SI and Abraham WT. Economic Value and Cost-Effectiveness of Cardiac Resynchronization Therapy Among Patients With Mild Heart Failure: Projections From the REVERSE Long-Term Follow-Up. JACC Heart Fail. 2017;5:204–212. [DOI] [PubMed] [Google Scholar]
- 17.Lee ET and Go OT. Survival analysis in public health research. Annu Rev Public Health. 1997;18:105–134. [DOI] [PubMed] [Google Scholar]
- 18.Royston P and Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002;21:2175–2197. [DOI] [PubMed] [Google Scholar]
- 19.Soikkeli F, Hashim M, Ouwens M, Postma M and Heeg B. Extrapolating Survival Data Using Historical Trial-Based a Priori Distributions. Value Health. 2019;22:1012–1017. [DOI] [PubMed] [Google Scholar]
- 20.Edlin R, McCabe C, Hulme C, Hall P and Wright J. Correlated Parameters and the Cholesky Decomposition Cost Effectiveness Modelling for Health Technology Assessment: Springer; 2015: 119–132. [Google Scholar]
- 21.Shaw JW, Johnson JA and Coons SJ. US valuation of the EQ-5D health states: development and testing of the D1 valuation model. Med Care. 2005;43:203–220. [DOI] [PubMed] [Google Scholar]
- 22.Mark DB, Knight JD, Velazquez EJ, Wasilewski J, Howlett JG, Smith PK, Spertus JA, Rajda M, Yadav R, Hamman BL, et al. Quality-of-life outcomes with coronary artery bypass graft surgery in ischemic left ventricular dysfunction: a randomized trial. Ann Intern Med. 2014;161:392–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Premier Healthcare Database White Paper: Data that informs and performs, March 2, 2020. Premier Applied Sciences®, Premier Inc. https://learn.premierinc.com/white-papers/premier-healthcare-database-whitepaper.
- 24.Peterson C, Xu L, Florence C, Grosse SD and Annest JL. Professional Fee Ratios for US Hospital Discharge Data. Med Care. 2015;53:840–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Echouffo-Tcheugui JB, Bishu KG, Fonarow GC and Egede LE. Trends in health care expenditure among US adults with heart failure: The Medical Expenditure Panel Survey 2002–2011. Am Heart J. 2017;186:63–72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kaul P, McAlister FA, Ezekowitz JA, Bakal JA, Curtis LH, Quan H, Knudtson ML and Armstrong PW. Resource use in the last 6 months of life among patients with heart failure in Canada. Arch Intern Med. 2011;171:211–217. [DOI] [PubMed] [Google Scholar]
- 27.Dunn A, Grosse SD and Zuvekas SH. Adjusting Health Expenditures for Inflation: A Review of Measures for Health Services Research in the United States. Health Serv Res. 2018;53:175–196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sanders GD, Neumann PJ, Basu A, Brock DW, Feeny D, Krahn M, Kuntz KM, Meltzer DO, Owens DK, Prosser LA, et al. Recommendations for Conduct, Methodological Practices, and Reporting of Cost-effectiveness Analyses: Second Panel on Cost-Effectiveness in Health and Medicine. JAMA. 2016;316:1093–103. [DOI] [PubMed] [Google Scholar]
- 29.Willan AR and Briggs AH. Statistical analysis of cost-effectiveness data. Chichester, West Sussex, England: John Wiley & Sons, Ltd.; 2006. [Google Scholar]
- 30.Vanness DJ, Lomas J and Ahn H. A Health Opportunity Cost Threshold for Cost-Effectiveness Analysis in the United States. Ann Intern Med. 2021;174:25–32. [DOI] [PubMed] [Google Scholar]
- 31.Maggioni AP, Dahlstrom U, Filippatos G, Chioncel O, Crespo Leiro M, Drozdz J, Fruhwald F, Gullestad L, Logeart D, Fabbri G, et al. EURObservational Research Programme: regional differences and 1-year follow-up results of the Heart Failure Pilot Survey (ESC-HF Pilot). Eur J Heart Fail. 2013;15:808–817. [DOI] [PubMed] [Google Scholar]
- 32.Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, et al. American Heart Association Council on Epidemiology, Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart Disease and Stroke Statistics-2020 Update: A Report From the American Heart Association. Circulation. 2020;141:e139–e596. [DOI] [PubMed] [Google Scholar]
- 33.van Domburg RT, Kappetein AP and Bogers AJ. The clinical outcome after coronary bypass surgery: a 30-year follow-up study. Eur Heart J. 2009;30:453–458. [DOI] [PubMed] [Google Scholar]
- 34.Gholami SS, Azar FEF, Rezapour A and Tajdini M. Cost-effectiveness of coronary artery bypass graft and percutaneous coronary intervention compared to medical therapy in patients with coronary artery disease: a systematic review. Heart Fail Rev. 2019;24:967–975. [DOI] [PubMed] [Google Scholar]
- 35.Hlatky MA, Boothroyd DB, Melsop KA, Kennedy L, Rihal C, Rogers WJ, Venkitachalam L, Brooks MM and Bypass Angioplasty Revascularization Investigation 2 Diabetes Study Group. Economic outcomes of treatment strategies for type 2 diabetes mellitus and coronary artery disease in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial. Circulation. 2009;120:2550–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Schwartz L, Kip KE, Alderman E, Lu J, Bates ER, Srinivas V, Bach RG, Mighton LD, Feit F, King S, et al. Baseline coronary angiographic findings in the Bypass Angioplasty Revascularization Investigation 2 Diabetes trial (BARI 2D). Am J Cardiol. 2009;103:632–638. [DOI] [PubMed] [Google Scholar]
- 37.Group BDS, Frye RL, August P, Brooks MM, Hardison RM, Kelsey SF, MacGregor JM, Orchard TJ, Chaitman BR, Genuth SM, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. N Engl J Med. 2009;360:2503–2515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Griffin SC, Barber JA, Manca A, Sculpher MJ, Thompson SG, Buxton MJ and Hemingway H. Cost effectiveness of clinically appropriate decisions on alternative treatments for angina pectoris: prospective observational study. BMJ. 2007;334:624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brandao SMG, Rezende PC, Rocca HB, Ju YT, de Lima ACP, Takiuti ME, Hueb W and Bocchi EA. Comparative cost-effectiveness of surgery, angioplasty, or medical therapy in patients with multivessel coronary artery disease: MASS II trial. Cost Eff Resour Alloc. 2018;16:55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Panza JA, Velazquez EJ, She L, Smith PK, Nicolau JC, Favaloro RR, Gradinac S, Chrzanowski L, Prabhakaran D, Howlett JG, et al. Extent of coronary and myocardial disease and benefit from surgical revascularization in ischemic LV dysfunction. J Am Coll Cardiol. 2014;64:553–561. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


