Abstract
Objective:
To test the impact of a multicomponent behavioral intervention to reduce the use of high-risk anticholinergic medications in primary care older adults.
Design:
Cluster-randomized controlled trial.
Setting and Participants:
Ten primary care clinics within Eskenazi Health in Indianapolis.
Intervention:
The multicomponent intervention included provider- and patient-focused components. The provider-focused component was computerized decision support alerting of the presence of a high-risk anticholinergic and offering dose- and indication-specific alternatives. The patient-focused component was a story-based video providing education and modeling an interaction with a healthcare provider resulting in a medication change. Alerts within the medical record triggered staff to play the video for a patient. Our design intended for parallel, independent priming of both providers and patients immediately before an outpatient face-to-face interaction.
Measurement:
Medication orders were extracted from the electronic medical record system to evaluate the prescribing behavior and population prevalence of anticholinergic users. The intervention was introduced April 1, 2019, through March 31, 2020, and a preintervention observational period of April 1, 2018, through March 31, 2019, facilitated difference in difference comparisons.
Results:
A total of 552 older adults had visits at primary care sites during the study period, with mean age of 72.1 (SD 6.4) years and 45.3% African American. Of the 259 provider-focused alerts, only three (1.2%) led to a medication change. Of the 276 staff alerts, 4.7% were confirmed to activate the patient-focused intervention. The intervention resulted in no significant differences in either the number of discontinue orders for anticholinergics (intervention: two additional orders; control: five fewer orders, p = 0.7334) or proportion of the population using anticholinergics following the intervention (preintervention: 6.2% and postintervention: 5.1%, p = 0.6326).
Conclusion:
This multicomponent intervention did not reduce the use of high-risk anticholinergics in older adults receiving primary care. Improving nudges or a policy-focused component may be necessary to reduce use of high-risk medications.
Keywords: anticholinergic, clinical decision support, deprescribing
Editor’s Notes
Overprescribing of drugs with anticholinergic activity to older adults has been a concern for nearly four decades. Anticholinergic burden continues to plague older adults resulting in adverse effects and contributing to the prescribing cascade. Numerous tools and interventions have been devised to reduce anticholinergic burden with variable success. The study by Campbell and colleagues employed interventions targeting both providers and patients in an effort to increase the rate of anticholinergic deprescribing. Despite the intervention’s low acceptance rate and resultant negligible change in anticholinergic prescribing, the trial is worth publishing despite its “negative” results. The literature is rich with articles documenting the prescribing of potentially inappropriate medications to older adults; intervention trials are less common. This trial has value to other investigators designing interventions to deprescribe potentially inappropriate medications to older adults.
—Todd Semla, MS, PharmD, AGSF
INTRODUCTION
Older adults continue to use high-risk medications,1–3 defined as those with the potential for more risks than benefits.4 Lists of high-risk medications have been available for nearly 30 years with periodic updates,4 and despite recommendations from expert organizations and stakeholders such as the American Geriatrics Society (AGS), Centers for Medicare and Medicaid Services, and the National Committee for Quality Assurance, their use continues with similar frequency.1,2 Data from the Medical Expenditure Panel Survey has shown that 42% of older adults were prescribed at least one potentially inappropriate drug from the 2012 AGS Beers Criteria.3
Anticholinergics represent one type of high-risk medication and have been associated with a decline in cognitive and physical function in multiple studies.5–11 Two studies including more than 2000 community-dwelling adults with mean age over 70 years found that anticholinergic users had lower cognitive performance than those not using these medications.12,13 In multiple large international observational studies, anticholinergic use has been associated with an increased risk of cognitive decline and incident dementia.6–9,14,15 Although these studies focus on the long-term effect of anticholinergics, prior research has shown that these medications can cause delirium and shorter-term cognitive dysfunction.5,16,17 Reducing the use of high-risk anticholinergic medications in older adults may represent an opportunity to reduce unnecessary harm, costs, and resource utilization.18
Attempts to reduce prescribing of anticholinergics through provider-focused computerized decision support have not had success in prior clinical trials. We previously reported results from three randomized clinical trials that failed to significantly reduce exposure to high-risk anticholinergics in the inpatient setting.19–21 These trials employed interventions focused only on provider prescribing behavior, with progressive levels of computerized and human-based decision support. Alternatively, two trials emerged providing support for patient-focused interventions to reduce high-risk medications in older adults.22,23
Our objective in the current study was to deploy a multicomponent approach that included both provider- and patient-focused interventions to reduce exposure to potentially inappropriate anticholinergic medications among older adults receiving care in a 10-site, community-based primary care network operating as a Federally Qualified Health Center (FQHC).
METHODS
Design and development of the multicomponent intervention
To develop and test the intervention, we employed a systems engineering approach to problem solving, comprising problem analysis, design, development, implementation, and evaluation.24 In the problem analysis phase, we conducted stakeholder interviews to understand decisions and demands for the use of anticholinergics from the perspectives of the patient, provider, and pharmacist. Findings included a lack of awareness of the long-term risks of anticholinergics among patients, demand from patients for symptom control in the present, and future risks discounted by providers.25 Poor awareness of recommended alternatives was also identified as a reason for persistent use. Additionally, the majority of older adults believed their provider was the primary decision-maker regarding prescription medication use.
Because the problem of high-risk anticholinergic use in primary care older adults is infrequent (approximately 20–25% of older adults use one such medication annually), the potential for harm is delayed, and the decision to titrate to an alternative treatment is complex, deprescribing these medications can be viewed as a behavior that may be responsive to principles of behavioral economics or “nudge” techniques.26 Therefore, our design of the multicomponent intervention incorporated “nudge” techniques such as priming for both patients and providers, incorporating influential messengers and setting defaults and norms.26
The intervention also addressed the realities identified in the problem analysis, while remaining implementable within the boundaries of routine clinical care, often referred to as pragmatic approaches.27 We designed and developed interventions targeting the primary agents responsible for both supply (providers) and demand (patients) in the process of prescribing anticholinergics. Our premise was that independent parallel priming of both providers and patients immediately before their face-to-face interaction, patient-focused videos focused on risks and norms of anticholinergic use, and provider-focused support in the form of defaults and titration aids would increase the chance that a high-risk medication would be discontinued and changed to a safer alternative.
Based on our local review of anticholinergic dispensing volume and risk attributed to certain classes of anticholinergics,6,7 we focused the intervention on anticholinergics from two classes: tricyclic antidepressants and urinary antispasmodics, which investigators determined to hold the highest potential risk, highest prevalence, and availability of safer pharmacologic alternatives.
The provider-focused intervention component was designed to alert providers to the presence of a target anticholinergic, provide a brief statement of alignment with organizational priorities, and offer easy navigation to titration plans for safer alternatives. We developed a “Best Practice Alert” (BPA) as a standard method for provider alerting in the EPIC® ambulatory electronic medical record system (see File S1 and Figure S1). Alerts were triggered each time a patient record was opened on the day of an outpatient visit among patients aged 65 years and older who were current users of one of the target anticholinergics. New orders for target anticholinergics in older adults also triggered an alert during outpatient visits.
If the provider chose to make a change in the medication, autopopulated fields for cross-tapering to an alternative treatment were generated (see Figure S2), making it as easy as possible to safely discontinue the target, high-risk anticholinergic medications. Although no previously published deprescribing or titration guideline was available, recommendations were based on evidence where available and expert local opinion in an effort to minimize adverse withdrawal reactions or recurrence of symptoms, which are rare in general, though more likely if abrupt changes are made. The autopopulated fields were customizable to personalize the discontinuation or taper schedule as needed. The design and programming of the BPA followed standardized formatting and delivery offered by the EPIC® suite of interventions.
The patient-focused intervention component included animated awareness videos that were written and produced by the investigators working with a digital storyteller and graphic illustrator. Each of three videos tells the story of an older adult (with variation in sex, race, and Hispanic origin) who learns about the risks of a specifically referenced anticholinergic medication, consults with a provider or pharmacist about their personal risk and safer alternatives, and replaces the anticholinergic with an alternative medication. The videos ranged in duration from 3.5 to 4.5 minutes and were displayed on a tablet device attached to a mobile stand. Medical assistants received a noninterruptive BPA during rooming procedures (see Figure 1 and File S1), directing them to play one of three videos (Figure S3), selected to best match each patient’s medication and demographic characteristics. Spanish language videos were available for Spanish-speaking patients.
FIGURE 1.
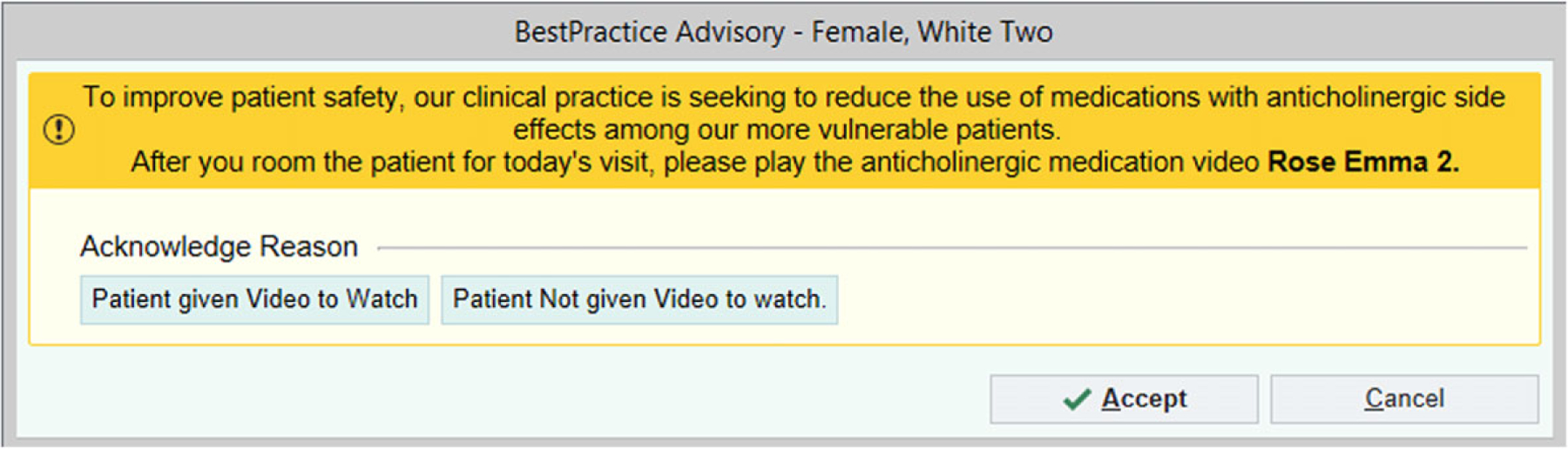
Example best practice advisory intended for medical assistants
Setting and study participants
The design, development, and implementation of the intervention were conducted in collaboration with patients, providers, and administrators of the Eskenazi Health organization in Indianapolis, IN. The intervention was endorsed by the Chief Medical Officer, Chief Nursing Officer, and Director of Pharmacy through written and verbal communication, in alignment with system-level priorities for safe medication use. We presented the goals and rationale for the study in group meetings with the leadership of the primary care sites and sought feedback for pragmatic changes to improve outcomes. Eskenazi Health provides care to the underresourced and underserved population of Greater Indianapolis. Care is provided in 10 community-based health centers strategically located around the Greater Indianapolis area to optimize access to more than 5000 adults aged 65 years and older. The intervention was activated for adults aged 65 years and older with either an existing or new medication order for a target anticholinergic.
Design, measures, and analytic plan
We conducted a cluster-randomized trial nested within 10 primary care clinics at Eskenazi Health Primary Care. Clinic sites were randomized to intervention or control in a 1:1 ratio. The intervention period was 12 months, from April 1, 2019, through March 31, 2020. To evaluate the aim of reducing the number of users of high-risk anticholinergics in primary care older adults, we extracted medication orders from the electronic medical records system, reflecting prescribing behavior as an intended target of the intervention. The proportion of anticholinergic orders prescribed as discontinuation orders in the preintervention and postintervention periods were used as a primary outcome measure. We also compared the population prevalence of anticholinergic use in the 12 months before the intervention and the intervention period. We included any patient and visit data within the pre- or postintervention time period. Descriptive statistics and a difference in difference analysis were conducted to evaluate differences by group and time. Mixed effect logistic regression models were used to comparatively examine the probabilities of an anticholinergic discontinuation in the two groups, as well as before and after the implementation of the treatment. In these analyses, we included in the models random clinic effects to account for the potential correlations among patients seen at the same clinic. As a quality improvement project developed and conducted in partnership with the Eskenazi Health system, patients did not provide consent to the study, but ethical approval for data collection and reporting was obtained from the Indiana University Institutional Review Board in Indianapolis. Statistical analyses were performed using SAS, version 9.4 (SAS Institute, Inc., Cary, NC).
RESULTS
A total of 552 older adults were included in the evaluation (see Figure 2), with 254 unique participants in the intervention group and 298 in the control group contributing data for evaluation. The overall mean age was 72.1 (SD 6.4) years, and 45.3% were African American. Table 1 describes those receiving care at one of the five intervention sites were slightly younger (p = 0.0026), more likely to be African American (p < 0.0001), and less likely to be depressed (p = 0.0019) than those receiving care at one of the five control sites.
FIGURE 2.
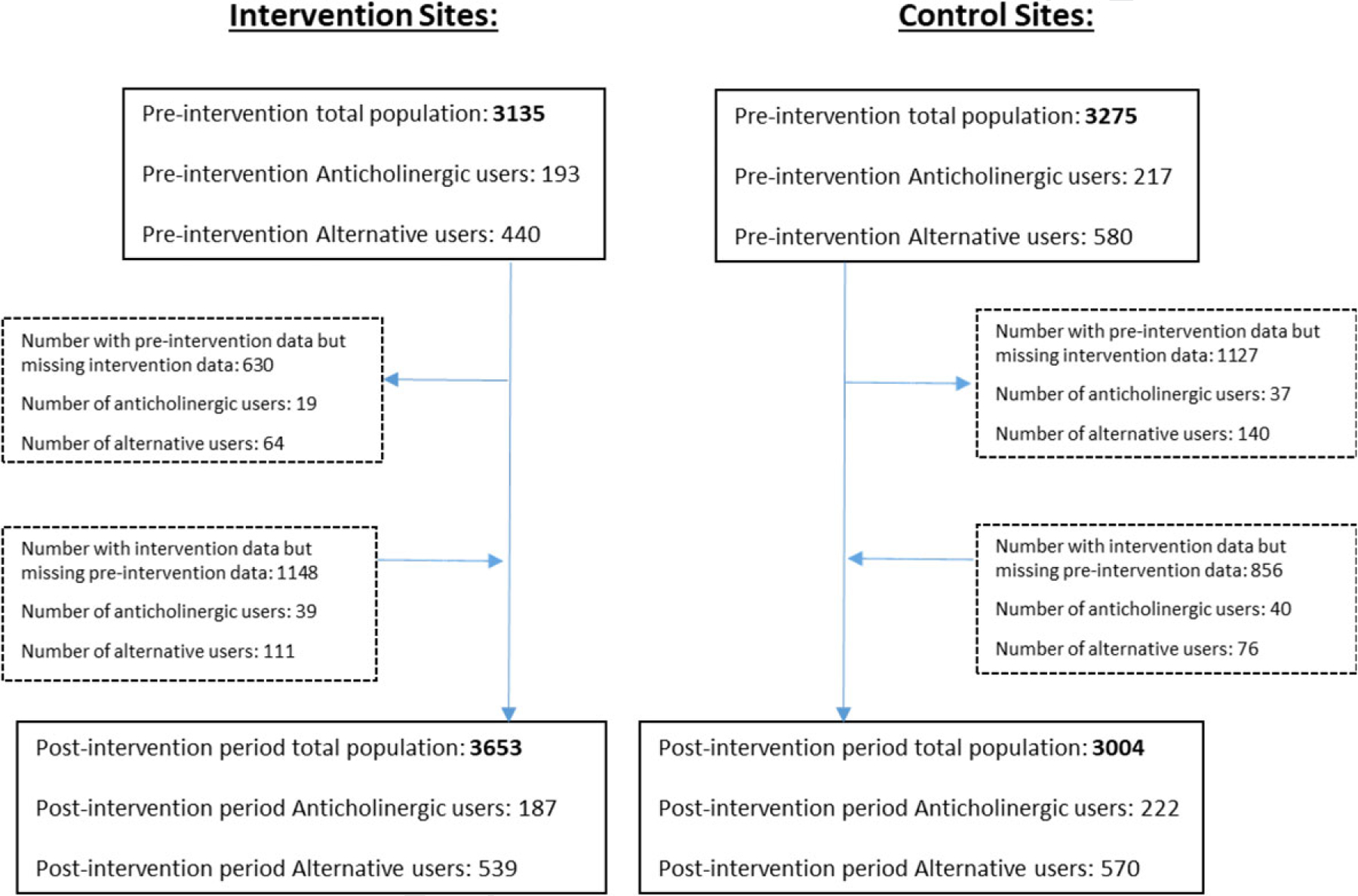
Flow of patients by year and site. Given the change in eligible population over time, the analysis is based on unique users contributing data to preintervention and postintervention years. Participants may have contributed data in the preintervention, postintervention, or both years. Among the 380 anticholinergic users at intervention sites, 254 unique patients were included in the analysis, and among the 439 anticholinergic users at control sites, 298 unique patients were included in the analysis
TABLE 1.
Demographics of anticholinergic users by study group
| Overall N = 552 |
Intervention sites N = 254 | Usual care sites N = 298 | p-Value for between-group comparisons | |
|---|---|---|---|---|
| Age, mean (SD) | 72.1 (± 6.4) | 71.2 (± 5.9) | 72.9 (± 6.8) | 0.0026 |
| Gender, % female | 442 (80.1%) | 204 (80.3%) | 238 (79.9%) | 0.6353 |
| Race | ||||
| % African American | 250 (45.3%) | 132 (52.0%) | 118 (39.6%) | <0.0001 |
| % Caucasian | 232 (42.0%) | 81 (31.9%) | 151 (50.7% | |
| % other | 70 (12.7%) | 41 (16.1%) | 29 (9.7%) | |
| Coronary artery disease, n (%) | 86 (15.6%) | 33 (13.0%) | 53 (17.8%) | 0.1217 |
| Congestive heart failure, n (%) | 56 (10.1%) | 23 (9.0%) | 33 (11.1%) | 0.4337 |
| Hypertension, n (%) | 471 (85.3%) | 214 (84.2%) | 257 (86.2%) | 0.5103 |
| Diabetes mellitus, n (%) | 251 (45.5%) | 118 (46.4%) | 133 (44.6%) | 0.6677 |
| Cancer, n (%) | 57 (10.3%) | 25 (9.8%) | 32 (10.7%) | 0.7303 |
| Depression, n (%) | 210 (38.0%) | 79 (31.1%) | 131 (44.0%) | 0.0019 |
| Stroke, n (%) | 35 (6.3%) | 12 (4.7%) | 23 (7.7%) | 0.1503 |
| Arthritis, n (%) | 172 (31.2%) | 79 (31.1%) | 93 (31.2%) | 0.9787 |
| Liver disease, n (%) | 35 (6.3%) | 19 (7.5%) | 16 (5.4%) | 0.3104 |
| Renal disease, n (%) | 104 (18.8%) | 51 (20.1%) | 53 (17.8%) | 0.4922 |
| Alzheimer’s and/or dementia, n (%) | 90 (16.3%) | 38 (15.0%) | 52 (17.4%) | 0.4301 |
The intervention resulted in no significant change in the number of discontinue orders for target anticholinergics between the preintervention and postintervention period (intervention: increase of two orders and control: reduction of five orders; p = 0.7334 for the probability an anticholinergic is discontinued in the preintervention compared with the postintervention period) (Table 2). The intervention did not result in a change in the proportion of the population using target anticholinergics in the preintervention versus postintervention period (preintervention: 6.2% and postintervention: 5.1%; p = 0.6326 for the probability of a target anticholinergic being discontinued in the preintervention period compared with the postintervention period) (Table 3). Similarly, mixed effect logistic regression models showed no differences in anticholinergic orders between sites (p = 0.6535) or the prevalence of anticholinergic users between sites (p = 0.0610). Given findings that no significant difference in medication use was identified by the intervention, no assessment of safety was pursued as it is unlikely to show differences attributable to the intervention.
TABLE 2.
Prevalence of discontinuation or active orders for study medications among users of those medicationsa
| Order typeb | Intervention | Control | p-Value for difference by timec | p-Value for difference by sited | |
|---|---|---|---|---|---|
| Target anticholinergics | Number of preintervention discontinuation orders divided by all orders (%) | 21/289 (7.3%) | 34/362 (9.4%) | 0.7334 | 0.6535 |
| Number of postintervention discontinuation orders divided by all orders (%) | 23/294 (7.8%) | 29/356 (8.2%) | |||
| Change | 2 | −5 | |||
| Recommended alternatives | Number of preintervention active orders divided by all orders (%) | 672/708 (94.9%) | 1019/1092 (93.3%) | 0.2220 | 0.4444 |
| Number of postintervention active orders divided by all orders (%) | 913/962 (94.9%) | 979/1034 (94.7%) | |||
| Change | 241 | −40 |
Cell denominators include orders for target anticholinergics or recommended alternatives among all patients with a visit to the clinic site within the designated time frame.
Discontinuation orders: number (percent) of all anticholinergic orders that were intended for discontinuation during the 12 months before (preintervention) and 12 months after the onset of the intervention (postintervention). Active orders: number (percent) of all orders that were intended as new or continuation orders for recommended alternative medications during the 12 months before (preintervention) and 12 months after the onset of the intervention (postintervention).
p-Value for estimating the probability of a target anticholinergic order being discontinued or a recommended alternative being active using mixed effect logistic regression model with covariates of time period (pre/post), treatment site, and random effect to account for the correlation within clinics.
p-Value for estimating the probability of a target anticholinergic order being discontinued or a recommended alternative being active using mixed effect logistic regression model with covariates of time period (pre/post), treatment site, and random effect to account for the correlations within clinics.
TABLE 3.
Prevalence of users of target anticholinergics or recommended alternatives among all primary care older adults by group and timea
| Intervention | Control | p-Value for difference by timeb | p-Value for difference by sitec | |
|---|---|---|---|---|
| Target anticholinergics | ||||
| Preintervention | 193/3135 (6.2%) | 217/3275 (6.6%) | 0.6326 | 0.0610 |
| Postintervention | 187/3653 (5.1%) | 222/3004 (7.4%) | ||
| Recommended alternatives | ||||
| Preintervention | 440/3135 (14.0%) | 580/3275 (17.7%) | 0.1076 | 0.5360 |
| Postintervention | 539/3653 (14.8%) | 570/3004 (19.0%) |
Cell denominators include patients with any visit data at the designated clinic site in the period indicated.
p-Value for estimating the probability of a target anticholinergic order being discontinued or a recommended alternative being active using a mixed effect logistic regression model with covariates of time period (pre/post), treatment site, and random effect to account for the correlations within clinics.
p-Value estimating the probability of a target anticholinergic order being discontinued or a recommended alternative being active using a mixed effect logistic regression model with covariates of time period (pre/post), treatment site, and random effect to account for the correlations within clinics.
Alerts directed toward providers were triggered 259 times, with 94% of alerts firing for existing medications and only 6% for new orders of target anticholinergics. Alerts were fired among 113 patients, with a mean of 2.3 alerts per patient, whereas 141 patients did not receive an alert due to the lack of a visit with their primary care provider. Among the alerts triggered, autopopulated order sets were opened by providers 39 times (15% of all alerts), yet only three (1.2% of all alerts) led to a discontinuation. Thus, three discontinuation orders were generated from the alerts and 20 discontinuation orders were generated outside of the alerts (Table 2). Reported differently, the number needed to remind28 to discontinue a high-risk anticholinergic medication was 86.3, meaning 86 alerts need to fire to result in one new discontinue order for a high-risk anticholinergic in a primary care older adult.
Alerts to medical assistants were triggered 276 times among 52 patients for a mean of 5.3 alerts per patient throughout the intervention period. Alerts were not fired in 202 patients due to the lack of a visit or missing information in the EMR required to trigger an alert. Among the 276 alerts, confirmation that a video was shown was recorded by medical assistants in only 13 instances of the alert firing, or 4.7% of all alerts.
DISCUSSION
Our deprescribing nudge targeting both providers and patients was not able to produce a significant reduction in the proportion of older adult patients using anticholinergics. With 85% of alerts to providers and 95% of alerts to medical assistants not reaching the intended target, we cannot conclude that independently priming patients and providers is an ineffective deprescribing approach. Rather, we have identified a need to improve interaction rates or demand for computerized deprescribing alerts (a nudge for a nudge).
A recent review of computerized decision support for deprescribing suggests a reduction in the number of high-risk medications can be achieved; however, a variety of alert designs have been employed, and statistically significant differences were often not achieved.29 Two recent computerized decision support tools intending to deprescribe high-risk medications provided recommendations to providers and showed mixed results, with little overall change in medications.30,31 Neither provider- nor patient-focused alerts employed in our study forced a decision but were noninterruptive or passive in nature, potentially explaining the low rate of acceptance. Interruptive alerts that force an interaction, for example, default to a change in medication or require a justification for refusal carries ethical considerations that limit use but may be more effective in provoking change.32
In addition to considerations of interruptive and non-interruptive alerts, our results may have been attributed to other characteristics of alerts or deprescribing processes in primary care. First, given the low acceptance rate of alert recommendations, the BPA may have been poorly designed. More creative design may be necessary to increase provider and medical staff interest, attention, and interaction with alerts. The low rates of interactions between our alerts and the intended recipients were due to missed or absence of visits and missing information required to trigger the alerts. Second, despite our attempted priming, there may be a low rate of demand for deprescribing support in the clinical environment. We intended to increase demand for deprescribing by priming patients and providers. However, there are alternative approaches for increasing demand, including more effective nudges than those employed in our intervention. Providers may have viewed deprescribing as an unplanned or low-priority activity.32 Until the practice of deprescribing is normalized in routine clinical care, or timing of alerts can be improved with contextual awareness, deprescribing alerts may continue to be unsuccessful.
The development and implementation of our intervention was supported by the chief medical officer and shared with medical leadership during provider business meetings at which opportunities for improvement were discussed. The chief nursing officer also approved the patient-focused intervention that targeted clinic staff and supported implementation activities. Additionally, the director of pharmacy reviewed and approved the alternative suggestions for the target anticholinergics. Our study was conducted in an environment in which a number of services and prior research supporting best practices in the care of older adults have been conducted. First, as noted previously, the study site has conducted research identifying the risks of anticholinergics for several years9,15,33 and previously failed to reduce exposure to anticholinergics and other high-risk medications in clinical trials.19–21 Second, before the trial, the site was a partner for a geriatric workforce enhancement program that provided intermittent education to primary care sites about the risks of certain medications in older adults. This program provided intermittent, face-to-face education on the risks of anticholinergic and other high-risk medications in older adults to primary care staff and providers, as well as pocket cards to aid in recognition during routine clinical care. Taken as a whole, neither the clinical trials nor educational programs were able to demonstrate a significant reduction in anticholinergic use.
Other considerations for our findings include a sample size that may have been too small for a cluster-randomized design. Other clustered trials of interventions targeting prescribing behavior utilized more than 150 sites per arm to identify group differences, suggesting the effect size of computerized decision support to reduce high-risk medications is small.34,35 Second, the duration of the intervention may have been insufficient. One study utilizing computerized decision support suggests differences in clinical outcomes may occur after 1 year of implementation.34 In support of this finding, Alagiakrishnan showed that alerts were triggered on average 5.8 times before a user interaction was achieved, and the alert success was similar to our results at 1.2%, with a similar number needed to remind (82.8).36 By comparison, Einbinder reported number needed to remind of approximately 2.0 for reminders guiding providers to quality metrics for diabetes mellitus and cardiovascular care.28 Although we pilot-tested our intervention before implementation, only the technical aspects of the alerts were evaluated rather than acceptability and fidelity with respect to behavior change. Thus, it is unknown whether improving the rate of interaction with the alerts or the alert content would be needed to improve efficacy to reduce the sample size or duration required to measure an effect. Third, a differential effect of CDS interventions on prevalent compared with incident prescriptions may exist; Tamblyn and colleagues showed no impact of a CDS on existing prescriptions, though new prescriptions of high-risk medications were reduced among the 12,560 patients.37 Lastly, attempts to reduce or discontinue anticholinergics may not have been captured in the medical record order until the result of the attempt was known; therefore, medication orders may have underestimated the impact of the intervention.
Further explanation for our findings should consider that deprescribing anticholinergics possibly requires human-intensive approaches. Conversations about risks and benefits; identification of appropriate alternatives; and monitoring symptoms before, during, and after titration plans are required and lack high-quality evidence directing such activities. Other studies have found human or expert-intensive approaches to deprescribing anticholinergic medications have been successful in reducing use of these medications, often by as much as 73%; however, the clinical impact of such changes has yet to be shown.38–40 Additionally, policy-based restrictions on high-risk medications may be required to raise demand for deprescribing to drive behavior change and prevent adverse outcomes, as in the case of opioid prescribing.41,42 A combination of effective nudges and policy may be required to reduce use of high-risk medications in older adults.
CONCLUSION
This multicomponent intervention targeting both providers and patients did not reduce the use of high-risk anticholinergics in older adults receiving primary care. Although this was a multicomponent intervention providing alternative medication options, a larger sample size or longer duration of alerts over time might have demonstrated clinical effects. Interventions with improved nudge characteristics or stronger policy-focused components may be necessary to reduce use of high-risk medications in vulnerable populations.
Supplementary Material
Figure S1: Provider-focused best practice advisory alert trigger
Figure S2: Provider-focused best practice advisory order set
Figure S3: Thumbnail images for patient-focused videos
Data S1: File S1: Development and description of alert triggers
Key Points
We incorporated principles of behavioral economics into the design of a coordinated, multicomponent deprescribing intervention that was built within the electronic medical record and tested in a pragmatic clinical trial.
The intervention targeted both providers and patients with the goal of increasing the rate of deprescribing high-risk anticholinergics in primary care older adults.
A low rate of acceptance of the alert-based approach did not allow the intervention to penetrate the target audience, and no change in deprescribing high-risk anticholinergics was realized.
Why Does this Paper Matter?
The electronic medical record offers a scalable opportunity to support deprescribing; however, our results contribute to the evidence requiring improvements in the design of interventions and demand for deprescribing before widespread adoption of electronic interventions.
ACKNOWLEDGMENTS
The authors acknowledge the important contributions to this work including the design, production, and animation of the interventions: Thomas Lewis, MFA, Jiaichi Liu, Kunal Bodke, and Preethi Srinivas, PhD. We also thank Epic Systems Corporation for permission to include Best Practice Alert interfaces used in primary care practices at Eskenazi Health during the study period.
Funding information
Agency for Healthcare Research and Quality, Grant/Award Number: P30HS24384
FINANCIAL DISCLOSURE
This study is supported by a grant from the Agency for Healthcare Research and Quality (AHRQ) (P30HS24384). The content is solely the responsibility of the authors and does not necessarily represent the official views of the AHRQ. The views expressed in this manuscript are those of the authors and not necessarily those of the AHRQ, Eskenazi Health, or the Epic Systems Corporation.
Footnotes
CONFLICT OF INTEREST
All authors report no financial or personal conflicts of interest for this article.
SUPPORTING INFORMATION
Additional supporting information may be found online in the Supporting Information section at the end of this article.
REFERENCES
- 1.Felton M, Hanlon JT, Perera S, Thorpe JM, Marcum ZA. Racial differences in anticholinergic use among community-dwelling elders. Consult Pharm 2015;30(4):240–245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sumukadas D, McMurdo MET, Mangoni AA, Guthrie B. Temporal trends in anticholinergic medication prescription in older people: repeated cross-sectional analysis of population prescribing data. Age Ageing 2014;43(4):515–521. [DOI] [PubMed] [Google Scholar]
- 3.Davidoff AJ, Miller GE, Sarpong EM, Yang E, Brandt N, Fick DM. Prevalence of potentially inappropriate medication use in older adults using the 2012 Beers criteria. J Am Geriatr Soc 2015;63(3):486–500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.By the 2019 American Geriatrics Society Beers Criteria Update Expert Panel. American Geriatrics Society 2019 updated AGS beers criteria for potentially inappropriate medication use in older adults. J Am Geriatr Soc 2019;67(4):674–694. [DOI] [PubMed] [Google Scholar]
- 5.Fox C, Smith T, Maidment I, et al. Effect of medications with anti-cholinergic properties on cognitive function, delirium, physical function and mortality: a systematic review. Age Ageing 2014;43(5):604–615. [DOI] [PubMed] [Google Scholar]
- 6.Coupland CA, Hill T, Dening T, Morriss R, Moore M, Hippisley-Cox J. Anticholinergic drug exposure and the risk of dementia: a nested case-control study. JAMA Intern Med 2019; 179:1084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Richardson K, Fox C, Maidment I, et al. Anticholinergic drugs and risk of dementia: case-control study. BMJ 2018;k1315:361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gray SL, Anderson ML, Dublin S, et al. Cumulative use of strong anticholinergics and incident dementia: a prospective cohort study. JAMA Intern Med 2015;175(3):401–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Campbell NL, Lane KA, Gao S, Boustani MA, Unverzagt F. Anticholinergics influence transition from normal cognition to mild cognitive impairment in older adults in primary care. Pharmacotherapy 2018;38(5):511–519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Szabo SM, Gooch K, Schermer C, et al. Association between cumulative anticholinergic burden and falls and fractures in patients with overactive bladder: US-based retrospective cohort study. BMJ Open 2019;9(5):e026391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Rudolph JL, Salow MJ, Angelini MC, McGlinchey RE. The anticholinergic risk scale and anticholinergic adverse effects in older persons. Arch Intern Med 2008;168(5):508–513. [DOI] [PubMed] [Google Scholar]
- 12.Lechevallier-Michel N, Molimard M, Dartigues J-F, Fabrigoule C, Fourrier-Reglat A. Drugs with anticholinergic properties and cognitive performance in the elderly: results from the PAQUID Study. Br J Clin Pharmacol 2005;59(2):143–151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Risacher SL, McDonald BC, Tallman EF, et al. Association between anticholinergic medication use and cognition, brain metabolism, and brain atrophy in cognitively normal older adults. JAMA Neurol 2016;73(6):721–732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carriere I, Fourrier-Reglat A, Dartigues JF, et al. Drugs with anticholinergic properties, cognitive decline, and dementia in an elderly general population: the 3-city study. Arch Intern Med 2009;169(14):1317–1324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Campbell NL, Boustani MA, Lane KA, et al. Use of anticholinergics and the risk of cognitive impairment in an African American population. Neurology 2010;75(2):152–159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Campbell N, Boustani M, Limbil T, et al. The cognitive impact of anticholinergics: a clinical review. Clin Interv Aging 2009;4: 225–233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mondimore FM, Damlouji N, Folstein MF, Tune L. Post-ECT confusional states associated with elevated serum anticholinergic levels. Am J Psychiatry 1983;140(7):930–931. [DOI] [PubMed] [Google Scholar]
- 18.Campbell NL, Holden R, Boustani MA. Preventing Alzheimer disease by deprescribing anticholinergic medications. JAMA Intern Med 2019;179:1093. [DOI] [PubMed] [Google Scholar]
- 19.Boustani MA, Campbell NL, Khan BA, et al. Enhancing care for hospitalized older adults with cognitive impairment: a randomized controlled trial. J Gen Intern Med 2012;27(5):561–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan BA, Perkins AJ, Campbell NL, et al. Pharmacological management of delirium in the intensive care unit: a randomized pragmatic clinical trial. J Am Geriatr Soc 2019;67(5): 1057–1065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Campbell NL, Perkins AJ, Khan BA, et al. Deprescribing in the pharmacologic management of delirium: a randomized trial in the intensive care unit. J Am Geriatr Soc 2019;67(4): 695–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tannenbaum C, Martin P, Tamblyn R, Benedetti A, Ahmed S. Reduction of inappropriate benzodiazepine prescriptions among older adults through direct patient education: the EMPOWER cluster randomized trial. JAMA Intern Med 2014; 174(6):890–898. [DOI] [PubMed] [Google Scholar]
- 23.Martin P, Tamblyn R, Benedetti A, Ahmed S, Tannenbaum C. Effect of a pharmacist-led educational intervention on inappropriate medication prescriptions in older adults: the D-PRESCRIBE randomized clinical trial. JAMA 2018;320(18): 1889–1898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Meister D, Enderwick TP. Human Factors in System Design, Development, and Testing Boca Raton, FL: CRC Press; 2001. [Google Scholar]
- 25.Holden RJ, Srinivas P, Campbell NL, et al. Understanding older adults’ medication decision making and behavior: a study on over-the-counter (OTC) anticholinergic medications. Res Social Adm Pharm 2019;15(1):53–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Dolan P, Hallsworth M, Halpern D, King D, Metcalfe R, Vlaev I. Influencing behavior: the mindspace way. J Econ Psychol 2012;33(1):264–277. [Google Scholar]
- 27.Mitchell SL, Mor V, Harrison J, McCarthy EP. Embedded pragmatic trials in dementia care: realizing the vision of the NIA IMPACT collaboratory. J Am Geriatr Soc 2020;68:S1–S7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Einbinder J, Hebel E, Wright A, Panzenhagen M, Middleton B. The number needed to remind: a measure for assessing CDS effectiveness. AMIA Annu Symp Proc 2014;506–515. [PMC free article] [PubMed] [Google Scholar]
- 29.Monteiro L, Maricoto T, Solha I, Ribeiro-Vaz I, Martins C, Monteiro-Soares M. Reducing potentially inappropriate prescriptions for older patients using computerized decision support tools: systematic review. J Med Internet Res 2019;21(11): e15385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fried TR, Niehoff KM, Street RL, et al. Effect of the tool to reduce inappropriate medications on medication communication and deprescribing. J Am Geriatr Soc 2017;65(10):2265–2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McDonald EG, Wu PE, Rashidi B, et al. The MedSafer study: a controlled trial of an electronic decision support tool for deprescribing in acute care. J Am Geriatr Soc 2019;67(9):1843–1850. [DOI] [PubMed] [Google Scholar]
- 32.Valvona SN, Rayo MF, Abdel-Rasoul M, et al. Comparative effectiveness of best practice alerts with active and passive presentations: a retrospective study. Proc Int Symp Hum Factors Ergon Health Care 2020;9(1):105–109. [Google Scholar]
- 33.Cai X, Campbell N, Khan B, Callahan C, Boustani M. Long-term anticholinergic use and the aging brain. Alzheimers Dement 2013;9(4):377–385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rieckert A, Reeves D, Altiner A, et al. Use of an electronic decision support tool to reduce polypharmacy in elderly people with chronic diseases: cluster randomized controlled trial. BMJ 2020;m1822:369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sonnichsen A, Trampisch U, Rieckert A, et al. Polypharmacy in chronic disease-reduction of inappropriate medication and adverse drug events in older populations by electronic decision support (PRIMA-eDS): study protocol for a randomized controlled trial. Trials 2016;17:57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alagiakrishnan K, Ballermann M, Rolfson D, et al. Utilization of computerized clinical decision support for potentially inappropriate medications. Clin Interv Aging 2019;14:753–762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tamblyn R, Huang A, Perreault R, et al. The medical office of the 21st century (MOXXI): effectiveness of computerized decision-making support in reducing inappropriate prescribing in primary care. CMAJ 2003;169(6):549–556. [PMC free article] [PubMed] [Google Scholar]
- 38.Boustani MA, Sachs GA, Alder CA, et al. Implementing innovative models of dementia care: the healthy aging brain center. Aging Ment Health 2011;15(1):13–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kersten H, Molden E, Tolo IK, Skovlund E, Engedal K, Wyller TB. Cognitive effects of reducing anticholinergic drug burden in a frail elderly population: a randomized controlled trial. J Gerontol A Biol Sci Med Sci 2013;68(3):271–278. [DOI] [PubMed] [Google Scholar]
- 40.Efjestad AS, Molden E, Oksengard AR. Pharmacist-initiated management of antagonistic interactions between anticholinergic drugs and acetyl cholinesterase inhibitors in individuals with dementia. J Am Geriatr Soc 2013;61(9):1624–1625. [DOI] [PubMed] [Google Scholar]
- 41.Beaudoin FL, Banerjee GN, Mello MJ. State-level and system-level opioid prescribing policies: the impact on provider practices and overdose deaths, a systematic review. J Opioid Manag 2016;12(2):109–118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Moyo P, Simoni-Wastila L, Griffin BA, et al. Impact of prescription drug monitoring programs (PDMPs) on opioid utilization among Medicare beneficiaries in 10 US states. Addiction 2017; 112(10):1784–1796. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1: Provider-focused best practice advisory alert trigger
Figure S2: Provider-focused best practice advisory order set
Figure S3: Thumbnail images for patient-focused videos
Data S1: File S1: Development and description of alert triggers


