Abstract
A quantitative, simple, and rapid assay has been developed to assess Giardia lamblia trophozoite sensitivity to metronidazole [1-(2-hydroxyetyl)-2-methyl-5-nitroimidazole] (MTZ). This new assay utilizes the ability of live (surviving) trophozoites to take up oxygen after have been exposed to MTZ. The effect of MTZ on oxygen uptake was compared with its effect on viability as evaluated by a culture method and morphological assays. Oxygen uptake rates decreased in trophozoites treated with MTZ, and this effect was drug concentration dependent: O2 uptake rates went from 3.04 μM O2 min−1 per 106 cells to 0.72 μM O2 min−1 per 106 cells with increasing drug concentration (0.15 to 0.6 mM) in the preincubation. Concentrations of the drug which inhibited oxygen uptake by 28 to 76% in trophozoites killed from 39 to 82% of trophozoites, as evaluated by the culture method, and altered the morphology of 21 to 86% of the trophozoites. Thus, the trophozoites killed by MTZ are nonmotile cells and do not take up oxygen. A good correlation was found between the inhibitory effects of MTZ, as evaluated by oxygen uptake, and cellular viability. Similar 50% inhibitory concentrations were obtained: 0.33 mM by oxygen uptake, 0.26 mM by the culture method, and 0.35 mM by morphological criteria. Oxygen uptake appears to be a good indicator of parasite viability. Therefore, this new method can provide a convenient means to assess MTZ susceptibility in G. lamblia and can be applied for screening potential antigiardial agents.
Giardia lamblia is a binucleated flagellar protozoan that causes intestinal infection in humans. This parasite is endemic throughout the world, and it is the most common isolated enteric pathogenic in Portugal. A striking feature of giardiasis is the wide variability of the clinical symptoms. Giardiasis may be entirely asymptomatic, may produce a mild self-limiting illness, or may produce chronic diarrhea with or without malabsorption. Although various drugs have been available for several decades to treat this infection, none of them is entirely satisfactory due to a high incidence of undesirable side effects and a significant failure rate in clearing parasites from the gastrointestinal tract (15, 19). Some evidence suggests that drug resistance may be responsible for these failures (1, 15, 16).
Previously published methods for the assessment of antigiardial activity in vitro rely on the viability of G. lamblia trophozoites. However, in vitro drug studies have been hampered by the lack of a good method for determining G. lamblia viability. The morphological assay (13), inhibition of clonal growth (8), culture (10), inhibition of radiolabelled substrate incorporation (11), and adherence studies (2, 6, 9) offer some problems. Problems with morphological assays may include the underestimation of parasite death if cells remain morphologically intact, although they are actually unable to replicate. The clonal growth method has low efficiency, regrowth and adherence studies are time-consuming, and other methods require the use of radiolabelled materials. Thus, considering the problems with in vitro susceptibility tests and the clinical and epidemiological relevance of drug resistance, the aim of this work was to evaluate a new method for assessing drug susceptibility in G. lamblia.
G. lamblia is an aerotolerant organism. When exposed to oxygen, it takes up oxygen at rates comparable to those of aerobic protozoa (12). The ability of G. lamblia NADH oxidase to use O2 as an electron acceptor under aerobic conditions explains the apparent respiration of the amitochondrial fermentative metabolism of Giardia (3). Paget et al. have also described that metronidazole (MTZ) inhibits the O2 uptake of G. lamblia trophozoites and that oxygen uptake can be correlated with metabolic function (17).
These findings prompted us to compare the in vitro effects of MTZ on oxygen uptake and viability of G. lamblia trophozoites to evaluate the eventual use of O2 uptake as an index of viability. Oxygen uptake was measured with a Clark-type oxygen electrode and was compared with the trophozoites’ viability as determined by the culture method and morphological criteria. The G. lamblia strain WB (ATCC 30957) was used as a cellular model.
MATERIALS AND METHODS
Antimicrobial agent.
MTZ [1-(2-hydroxyetyl)-2-methyl-5-nitroimidazole] was obtained from Sigma Chemical Corporation. Fresh stock solutions of 4.8 mM were prepared by dissolving pure MTZ in phosphate-buffered saline (PBS; 8 mM; pH 7.1).
Parasites and cultures.
G. lamblia (WB strain [ATCC 30957] originally from a patient with chronic diarrhea) was obtained from the American Type Culture Collection, Rockville, Md. Trophozoites were maintained in axenic culture at 37°C in 10 ml of Diamond’s TYI-S-33 medium, as modified by Keister (14), in screw-cap cell culture vials. Penicillin G (250 μg/ml), streptomycin sulfate (250 μg/ml), gentamicin sulfate (50 μg/ml), and amphotericin B (0.25 μg/ml) were added during routine culture. After 2 days, the cultures were harvested by cooling of the culture vials at 4°C for 15 min and centrifugation at 400 × g for 10 min. Trophozoites were washed three times in PBS (8 mM; pH 7.1), and cells were counted in a hemocytometer (Neubauer cell-counter chamber). These cells were used as the inoculum with which to study the effects of MTZ on G. lamblia trophozoites.
Test procedure.
An inoculum of 4.5 × 106 cells was exposed to 0.15 to 0.6 mM of MTZ (around 25 to 100 μg/ml) in fresh medium (without serum and antibiotics) for 3 h at 37°C, by using 10-ml polystyrene screw-cap vials. Control experiments were performed under similar experimental conditions, without the drug, in the presence of only the drug solvent (PBS; 8 mM; pH 7.1). After incubation, the vials were cooled at 4°C and the suspension was centrifuged at 400 × g for 10 min. Trophozoites were washed three times in cold PBS, and cell pellets were resuspended in 100 μl of PBS (pH 7.1) and then processed to measure the oxygen uptake and cellular viability.
Oxygen uptake.
Measurements of oxygen uptake were made in a closed glass vessel (1 ml), thermostated at 37°C and provided with a stirrer, by using a Clark-type oxygen electrode (YSI model 5331; Yellow Springs Instrument Co.). Oxygen uptake was calculated assuming an oxygen concentration of 227 nmol/ml in the initial incubation at 37°C. To determine O2 uptake, 30 μl of each sample of trophozoites was applied to the equilibrated system through a small pot in the top of the vessel. With this technique, oxygen uptake rates were calculated within 10 min of introducing the sample into the electrode vessel. The effect of MTZ was determined by comparing respiratory rates in control cells and in cells preexposed to the drug. The results were expressed as O2 uptake (micromolar O2 per minute per 106 cells) and as inhibition of O2 uptake (percentage of control).
Viability study.
Trophozoite viability was directly determined by phase-contrast microscopy, with the live and dead cells counted in a hemocytometer. Parasites were considered viable if they had a characteristic pear-shaped structure, flagellar motility, normal architecture of the ventral disc and refractory quality (10).
The regrowth assay, based on the ability of viable G. lamblia trophozoites to multiply and grow in fresh culture medium after being exposed to a lethal agent, has been extensively used for the determination of parasite viability (4, 6, 9, 10). Thus, for the regrowth assay, 30 μl of each incubation was subcultivated for 48 h at 37°C in 10 ml of fresh TYI-S-33 medium supplemented with antibiotics. Subsequently, the vials were cooled at 4°C, and the total number of parasites was determined microscopically with a Neubauer cell-counter chamber. The initial number of viable parasites in each sample was deduced by extrapolation from the standard curve of G. lamblia growth according to the total number of parasites at 48 h as previously described (4). The experiments were performed three times, and the results were expressed as the number of viable cells and as a percentage of the control.
RESULTS
Oxygen consumption.
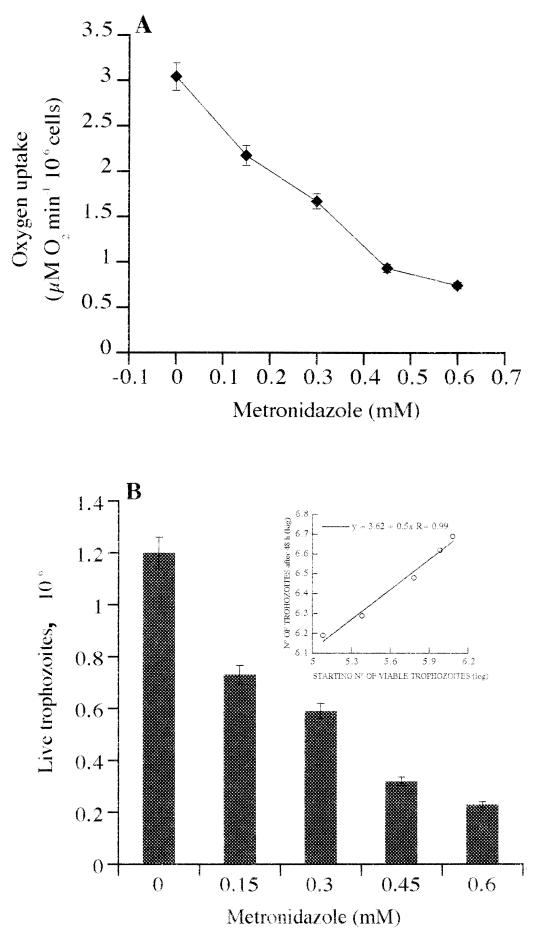
Oxygen uptake rates of G. lamblia trophozoites are shown in Fig. 1A. There was a positive correlation between increasing drug concentration and decreasing O2 uptake rates of trophozoites. Oxygen uptake rates went from 3.04 μM O2 min−1 per 106 cells in untreated cells to 0.72 μM O2 min−1 per 106 cells with the highest concentration of MTZ (0.6 mM) in the preincubation.
FIG. 1.
(A) Changes in oxygen uptake, after pretreatment for 3 h at 37°C with MTZ, of G. lamblia trophozoites. Oxygen uptake (⧫) was determined with an open oxygen electrode set at 227 μM oxygen. All assays were performed at 37°C. Standard errors of the mean (vertical bars) were calculated from data in four experiments. (B) Killing of G. lamblia trophozoites by MTZ evaluated by the regrowth assay. The starting number of viable parasites of each sample was obtained by extrapolation from the standard curve of G. lamblia growth, represented by the inset, by using the total number of cells at 48 h. Standard errors of the mean (vertical bars) were calculated from data of four experiments.
Viability studies.
MTZ induced loss of motility of G. lamblia trophozoites. The nonmotile trophozoites had, by phase-contrast microscopy, a typical pear-shaped structure and refractory quality (not shown). MTZ induced loss of cellular viability as evaluated by regrowth assay. The initial number of viable trophozoites in each sample (Fig. 1B) was obtained by extrapolation from the standard curve of G. lamblia growth (inset of Fig. 1B) according to the total number of cells at 48 h.
Comparison of methods.
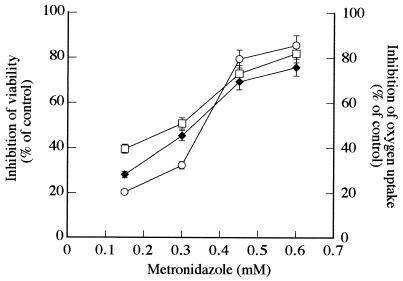
The dose-response curves obtained for MTZ determined by oxygen uptake and those obtained by viability tests (morphological and regrowth criteria) are shown in Fig. 2. These results are expressed as the percentage of inhibition of the control. The MTZ concentrations which inhibited oxygen uptake by 28 to 76% of trophozoites killed from 39 to 82% of trophozoites, as evaluated by the culture method, and altered the morphology of 21 to 86% of the cells.
FIG. 2.
Percentage of inhibition of G. lamblia trophozoites by MTZ as determined by oxygen uptake (⧫), viability determined by culture method (□), and viability determined by morphological assay (○). The IC50 determined by O2 uptake was 0.33 mM (r = 0.98), that determined by the culture method was 0.26 mM (r = 0.98), and that determined by the morphological assay was 0.35 mM (r = 0.95). Standard errors of the mean (vertical bars) were calculated from data of four experiments.
The morphological changes in trophozoites after exposure to MTZ were correlated with the loss of viability as evaluated by the culture method; in both cases, similar 50% inhibitory concentrations (IC50s [0.35 and 0.26 mM, respectively]) were obtained. Thus, the MTZ concentrations which induce loss of motility also induce loss of the ability of the trophozoites to multiply in fresh culture medium.
The alterations in O2 uptake rates correlate very well with death as determined by the culture method and morphologic assay; a similar IC50 (0.33 mM) was obtained.
DISCUSSION
The optimal method for performing susceptibility studies of G. lamblia has not been established. A reliable viability assay is required for in vitro susceptibility studies and to screen drugs for antigiardial activity. Several drugs are currently used for treatment of giardiasis in humans. Of these, MTZ, a nitroimidazole antibiotic, is recommended for chemotherapy of Giardia infections and is the drug of choice in many countries.
MTZ and other 5-nitroimidazoles are selectively toxic to Giardia. However, the killing mechanisms of these drugs have not been studied with this parasite. By analogy with antimicrobial activity of MTZ against trichomonads and bacteria, it has been proposed that effectors of cytotoxicity are the free nitro radicals, which are formed during the metabolic reduction of the drug, which oxidizes DNA, causing strand breaks and subsequent cell death (5). It has been postulated that electrons generated by energy-yielding pathways are transferred to the drug’s nitro group by ferredoxin (18). However, neither the donors of the electrons nor the cytotoxic intermediates and their target molecules have been identified.
The antibiotics with anaerobic activity should be compared with MTZ, which is considered a “gold standard” antibiotic (7). Considering this finding and the clinical and epidemiological relevance of drug resistance (1, 15, 16), we developed a new method to study the susceptibility of G. lamblia trophozoites to MTZ. The assay exploits the fact that G. lamblia trophozoites take up oxygen at rates comparable to those of aerobic protozoa, which is abolished with death by MTZ. This alteration in metabolic activity, induced by MTZ, was compared with cellular viability measured by morphological criteria and the culture method.
Considering our results, showing the high correlation between oxygen uptake rates by trophozoites and cellular viability, we can conclude that the oxygen uptake of G. lamblia trophozoites could be used as an index of cellular viability. This conclusion may have additional implications with regard to characterizing the killing mechanisms of MTZ. Recent studies have shown that NADH oxidase is responsible for the oxygen uptake of Giardia trophozoites and may be involved in the maintenance of an optimum intracellular redox ratio (3). Oxygen uptake alteration, induced by MTZ, could be correlated with cytotoxicity mechanisms. Drug concentrations that altered O2 uptake rates induced death of trophozoites, suggesting a link between metabolic activity and MTZ action.
Considerable differences in vitro sensitivity have been found according to the assay employed, which may reflect different modes of action of antigiardial drugs. Based on our data and the fact that MTZ is cidal in its activity, it is reasonable to accept that O2 uptake rates can be used as a convenient method to study the lethal (irreversible drug effects) drug’s activity. Our preliminary studies showed that furazolidone was two to three times more active than MTZ and that quinacrine was less active than MTZ (not shown); these results were consistent with previously published values (15).
The methodology described in this study, based on the oxygen uptake of trophozoites, is a quantitative, rapid, and simple method for the assessment of the lethal effects of MTZ in G. lamblia trophozoites. The method has some advantages over other methods because it does not involve radioisotopes or complex instrumentation and the results are available within a few hours. This assay can be applied to assessing MTZ susceptibility in G. lamblia and can be used to screen new antigiardial agents.
ACKNOWLEDGMENT
This work was partially supported by PRODEP II.
REFERENCES
- 1.Barat L M, Bloland P B. Drug resistance among malaria and other parasites. Infect Dis Clin N Am. 1997;11:969–987. doi: 10.1016/s0891-5520(05)70400-1. [DOI] [PubMed] [Google Scholar]
- 2.Baveja U K, Bhatia V N, Warhurst D C. Giardia lamblia: in-vitro sensitivity to some chemotherapeutic agents. J Commun Dis. 1998;30:79–84. [PubMed] [Google Scholar]
- 3.Brown D M, Upcroft J A, Upcroft P. A H2O-producing NADH oxidase from the protozoan parasite Giardia duodenalis. Eur J Biochem. 1996;241:155–161. doi: 10.1111/j.1432-1033.1996.0155t.x. [DOI] [PubMed] [Google Scholar]
- 4.Cedilo-Reviera R, Muñoz O. In-vitro susceptibility of Giardia lamblia to albendazole, mebendazole and other chemotherapeutic agents. J Med Microbiol. 1992;37:221–224. doi: 10.1099/00222615-37-3-221. [DOI] [PubMed] [Google Scholar]
- 5.Edwards D I. Nitroimidazole drugs: action and resistance mechanism. I. Mechanism of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 6.Farbey M D, Reynoldson J A, Thompson R C A. In vitro drug susceptibility of 29 isolates of Giardia duodenalis from humans as assessed by an adhesion assay. Int J Parasitol. 1995;25:593–599. doi: 10.1016/0020-7519(94)00174-m. [DOI] [PubMed] [Google Scholar]
- 7.Freeman C D, Klutman N E, Lamp K C. Metronidazole. A therapeutic review and update. Drugs. 1997;54:679–708. doi: 10.2165/00003495-199754050-00003. [DOI] [PubMed] [Google Scholar]
- 8.Gillin F D, Diamond L S. Clonal growth of Giardia lamblia trophozoites in a semisolid agarose medium. J Parasitol. 1980;66:350–352. [PubMed] [Google Scholar]
- 9.Hemphill S, Gottstein B, Muller N. Electron microscopical investigation of surface alterations on Giardia lamblia trophozoites after exposure to a cytotoxic monoclonal antibody. Parasitol Res. 1996;82:206–210. doi: 10.1007/s004360050096. [DOI] [PubMed] [Google Scholar]
- 10.Hill D R, Pohl R, Pearson R D. Giardia lamblia: a culture method for determining parasite viability. Am J Trop Med Hyg. 1986;35:1129–1133. doi: 10.4269/ajtmh.1986.35.1129. [DOI] [PubMed] [Google Scholar]
- 11.Inge M P G, Farthing M J G. A radiometric assay for antigiardial drugs. Trans R Soc Trop Med Hyg. 1987;77:487–488. doi: 10.1016/0035-9203(87)90260-4. [DOI] [PubMed] [Google Scholar]
- 12.Jarrol E L, Manning P, Berrade A, Hare D, Lindmark D G. Biochemistry and metabolism of Giardia. J Protozool. 1989;36:190–197. doi: 10.1111/j.1550-7408.1989.tb01073.x. [DOI] [PubMed] [Google Scholar]
- 13.Jokipii L, Jokipii A M M. In vitro susceptibility of Giardia lamblia trophozoites to metronidazole and tinidazole. J Infect Dis. 1980;141:317–325. doi: 10.1093/infdis/141.3.317. [DOI] [PubMed] [Google Scholar]
- 14.Keister D B. Axenic culture of Giardia lamblia in TYI-S-33 medium supplemented with bile. Trans R Soc Trop Med Hyg. 1983;77:487–488. doi: 10.1016/0035-9203(83)90120-7. [DOI] [PubMed] [Google Scholar]
- 15.Kulda J, Nohyhová E. Therapy of giardiasis. In: Kreier J P, editor. Parasitic protozoa. New York, N.Y: Academic Press; 1995. pp. 369–381. [Google Scholar]
- 16.MacIntyre P, Boreham P F L, Phillips R E, Shepherd R W. Chemotherapy in giardiasis: clinical responses and in vitro drug sensitivity of human isolates in axenic culture. J Pediatr. 1986;108:1005–1010. doi: 10.1016/s0022-3476(86)80950-7. [DOI] [PubMed] [Google Scholar]
- 17.Paget T A, Macechho P T, Jarrol E L. Metabolic changes in Giardia intestinalis during differentiation. J Parasitol. 1998;84:222–226. [PubMed] [Google Scholar]
- 18.Townson S M, Boreham P F L, Upcroft P, Upcroft J A. Resistance to the nitroheterocyclic drugs. Acta Trop. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 19.Zaat J O, Mank T G, Assendelf W J. A systematic review on the treatment of giardiasis. Trop Med Int Health. 1997;2:63–82. doi: 10.1046/j.1365-3156.1997.d01-132.x. [DOI] [PubMed] [Google Scholar]