Abstract
Vicilin Buried Peptides (VBPs) from edible plants are derived from the N-terminal leader sequences (LS) of seed storage proteins. VBPs are defined by a common α-hairpin fold mediated by conserved CxxxCx(10–14)CxxxC motifs. Here, peanut and walnut VBPs were characterized as potential mediators of both peanut/walnut allergenicity and cross-reactivity despite their low (~17%) sequence identity. The structures of one peanut (AH1.1) and 3 walnut (JR2.1, JR2.2, JR2.3) VBPs were solved using solution-NMR, revealing similar α-hairpin structures stabilized by disulfide bonds with high levels of surface similarity. Peptide microarrays identified several peptide sequences primarily on AH1.1 and JR2.1 which were recognized by peanut, walnut, and dual-allergic patient IgE, establishing these peanut and walnut VBPs as potential mediators of allergenicity and cross-reactivity. JR2.2 and JR2.3 displayed extreme resilience against endosomal digestion, potentially hindering epitope generation and contributing to their reduced allergic potential.
Keywords: α-hairpin scaffold, cross-reactivity, IgE epitopes, Vicilin-buried peptide
Graphical Abstract

Introduction
Peanut allergy is among the most commonly reported food allergies, with a prevalence of 1–9% among Western European cultures.1 Peanut allergy is responsible for over 50% of food-induced anaphylaxis cases and is rarely outgrown, making it a key health concern.2,3 Furthermore, up to 86% of peanut-allergic patients show some level of sensitization to tree-nuts, particularly walnut, with ~30% displaying clinically-relevant levels of IgE cross-reactivity.4,5 While allergens from peanuts and tree-nuts can be grouped into similar protein families, their overall sequence identity falls well below the 70% threshold previously considered to support cross-reactivity, raising questions regarding the basis for this phenomenon.6
Vicilins are a family of ubiquitous seed storage proteins which are translated as preproproteins consisting of three conserved regions: a short, hydrophobic signal peptide that is removed upon location to a storage vacuole; an N-terminal, cysteine-rich leader sequence (LS) which is removed by asparaginyl endopeptidase (AEP)7; and the mature vicilin domain.8–10 Recent studies have identified a new family of peptides, termed Vicilin-Buried Peptides (VBP) within this LS region. These VBPs are defined by a common cysteine motif (CxxxCx(10–14)CxxxC), and are widely dispersed across most angiosperm families including trees, grasses, nightshades and legumes9,10 where they have been implicated in a variety of biological functions including ribosome inactivation, protease inhibition and antimicrobial defense.11–16 Despite this diversity, the structure of all VBPs that have been solved to date reveal a common α-hairpin fold/motif mediated by disulfide bonds between the highly conserved CxxxC motifs, potentially providing a common structural scaffold that can mediate peanut/tree-nut cross-reactivity.17,18
The walnut vicilin Jug r 2 leader sequence (JRLS) contains three VBP motifs (JR2.1, JR2.2, JR2.3), while the peanut 7S globulin Ara h 1 contains just one (AH1.1) (Figure 1). These VBPs have been identified in both peanut and walnut extracts,19,20 and are shown to bind IgE from allergic patients. Curiously, sensitization to the peanut VBP among peanut-allergic patients did not correlate with that of its parent vicilin domain, suggesting that VBP’s could potentially represent an independent family of allergens with distinct physiochemical determinants of allergenicity.18,20,21 However, information on the cross-reactivity and biophysical properties of these allergen-derived VBPs remains sparse relative to the more studied vicilin/globulin domains. In this work, we present full 3D structures of all four peanut and walnut VBPs, revealing the characteristic α-hairpin motif with a high degree of surface similarity both among themselves, and with other previously characterized VBPs from non-allergic sources. Peptide microarrays confirm the presence of numerous peanut and walnut epitopes on the structured regions of AH1.1 and JR2.1 with IgE prevalence of up to 100% among allergic patients, establishing the VBP α-hairpin as a potent mediator of both peanut and walnut allergy. Despite their shared motif, the IgE reactivity of JR2.2 and JR2.3 was significantly diminished. This correlated with subtle structural differences in and amino acid physiochemical properties between the various VBP sequences. In addition, JR2.2 and JR2.3 showed a markedly higher resistance to simulated endosomal degradation, providing potential insight into the biophysical determinants of VBP sensitization. These studies establish VBPs as a potential family of pan-allergens, whose highly conserved α-hairpin structure provides a potent scaffold for cross-reactivity across distantly related species.
Figure 1:

Amino acid sequence of Peanut/Walnut VBPs. A) N-terminal leader sequence (LS) from Ara h 1 and Jug r 2. Individual VBP sequences are highlighted in orange. Residues present in the NMR structures, but not the native sequence are denoted in parentheses. Conserved cysteine forming the characteristic CxxxC motif highlighted in red B) sequence identity matrix comparing the various Peanut/Walnut VBP sequences.
Materials and Methods
Constructs and Purification
A full description of the expression and purification methods is provided in the supplemental material. In brief, VBP sequences from the peanut (Arachis hypogaea) and English walnut (Juglans regia) allergens Ara h 1 and Jug r 2 were identified and cloned into the pDest expression system with an N-terminal Glutathione S-transferase tag separated from the main sequence by a tobacco etch virus (TEV) protease cleavage site. The vectors were expressed in E. coli (BL21 DE3, Millipore Sigma) and the resulting protein purified using an immobilized glutathione column. TEV protease was used to remove the GST tag, and the final product was isolated using a Superdex75 26/600 gel filtration column in phosphate-buffer saline (PBS).
Patient sera
Sera from 40 individuals (male/female ratio: 17/23) with walnut (12 patients), peanut (12 patients), and peanut/walnut allergy (16 patients) were collected in accordance with rules and regulations of the institutional review board of their respective institutions (Tulane University Biomedical IRB 09-00231, REF #: 140613; University of California at Davis IRB protocol No. 200210194-6) and in accordance with U.S. federal policy for the protection of human subjects (Table 1). Patients were over the age of 18 and have experienced recurrent severe, systemic allergic reactions to peanut, walnut or both. These patients were included in this study based on their convincing clinical history of food allergy and did not undergo food challenge due to the potential severity of reactions.
Table 1.
Patient allergies
| Patient # | Age | Sex, race | Symptoms | IgE kU/L | PN Allergy | WN Allergy | Other allergy |
|---|---|---|---|---|---|---|---|
| 1 | 54 | M, W | UA, GI, nLT | ✓ | Sesame, other TN | ||
| 2 | 33 | F, W | OAS, nLT | ✓ | Pumpkin, other TN | ||
| 3 | 22 | F, W | OAS, nLT | ✓ | Coconut, fruits, carrots other TN | ||
| 4 | - | M | MO, UA, LA, nLT | ✓ | Other TN | ||
| 5 | 50 | M, W | MO, UA, LA, LT | ✓ | Coconut, other TN | ||
| 6 | 38 | M | MO, UA, LA, LT | ✓ | Coconut, fish, other TN | ||
| 7 | 35 | F | MO, UA, LA, LT | ✓ | |||
| 8 | 53 | F | MO, UA, LA, LT | ✓ | Tomatoes, other TN | ||
| 9 | 44 | M | MO, UA, LA, LT | ✓ | |||
| 10 | 48 | F | MO, UA, LA, LT | ✓ | Apple, pear, other TN | ||
| 11 | 40 | F | MO, UA, LA, LT | ✓ | Sesame, fruits, other TN | ||
| 12 | 36 | F | MO, UA, LA, LT | ✓ | Fruits, other TN | ||
| 13 | - | F | MO, nLT, OFC | 59 | ✓ | Sunflower | |
| 14 | 24 | M | MO, nLT, OFC | >100 | ✓ | Soy, other TN | |
| 15 | 25 | M, W | MO, nLT, OFC | >100 | ✓ | Cod, egg | |
| 16 | 35 | F, W | MO, nLT, OFC | ✓ | Soy, other TN | ||
| 17 | 12 | F, B | MO, LT | 68 | ✓ | ||
| 18 | 40 | F, W | UA, OAS, nLT | ✓ | |||
| 19 | 25 | F, W | UA, LA, nLT | 40 | ✓ | ||
| 20 | 35 | M, W | MO, UA, LA, LT | 36 | ✓ | ||
| 21 | 45 | M, W | UA, OAS, nLT | ✓ | |||
| 22 | 40 | F, W | MO, nLT, OFC | ✓ | Shrimp | ||
| 23 | 19 | M | MO, LT | 75.4 | ✓ | ||
| 24 | 29 | M, W | MO, nLT | ✓ | Shrimp | ||
| 25 | 63 | M, W | MO, LT | >100 | ✓ | ✓ | Crab, lobster, flax, soy, other TN |
| 26 | 25 | F, W | MO, LT | >100 | ✓ | ✓ | Other TN |
| 27 | 21 | F, W | MO, LT | 52.7 | ✓ | ✓ | |
| 28 | 38 | F, A | MO, LT | ✓ | ✓ | Poppy, sesame, coconut, other TN | |
| 29 | 42 | M, W | OFC, nLT | ✓ | ✓ | Other TN | |
| 30 | 48 | F, W | MO, nLT | ✓ | ✓ | Sesame, other TN | |
| 31 | 62 | M, W | MO, nLT, both | ✓ | ✓ | Crab, lobster, other TN | |
| 32 | 38 | M, W | MO, LT, both | 46 | ✓ | ✓ | Shellfish, other TN |
| 33 | 36 | F, W | MO, LT, both | ✓ | ✓ | Shellfish, other TN | |
| 34 | 25 | F, W | MO, LT, both | ✓ | ✓ | Other TN | |
| 35 | 36 | F, W | MO, LT, both | ✓ | ✓ | ||
| 36 | 33 | F | MO, LT, both | ✓ | ✓ | ||
| 37 | 17 | F | MO, LT, both | ✓ | ✓ | Shellfish, flax, soy, other TN | |
| 38 | 27 | M | MO, LT, both | ✓ | ✓ | Poppy seeds, other TN | |
| 39 | 30 | M | MO, LT to PN, class 2 to WN | PN: 82, W: 2.2 | ✓ | ✓ | |
| 40 | 42 | F | MO, nLT, both | ✓ | ✓ | sunflower |
Acronyms: PN: peanut; WN: Walnut; TN: Tree-nut; MO: Multi-organ involvement; LT: Life Threatening; nLT: non-life threatening; OAS: Oral allergy syndrome; OFC+: Oral food challenge positive; UA: upper airway; LA: lower airway
In sex/race column: M=male; F=female, W=white; B=black; A=Asian. In some cases the race was not specified. Final M/F: 17/23
Peptide microarrays
The entire amino acid sequences of Ara h 1 and Jug r 2 were printed onto microarray slides (JPT Peptide Technologies GmbH, Berlin, Germany) as sequential overlapping 15 amino acid spots, offset by 5 amino acids. Slides were placed into a HS400 Pro (Tecan, San Jose, CA), blocked in filtered SuperBlock TBS (Thermo Fisher Scientific, Waltham, MA) for 30 minutes at room temperature under agitation and then washed with Tris-buffered saline containing 0.5% Tween-20 (TBST) (Bio-Rad, Hercules, California). After centrifugation, 200 μL of each patient’s undiluted sera was injected into individual chambers containing microarray slides and incubated at 4°C for 16 hours with agitation. Microarray slides were then washed and injected with 170 μL of mouse α-human IgE (Life Technologies, Grand Island, NY) diluted into filtered Superblock at a dilution of 1:5000 for 30 minutes at room temperature. The slides were washed and dried before scanning on a GenePix-4000B (Molecular Devices, San Jose, CA). IgE binding was measured by Cy3 green fluorescence at 532 nm. The data was analyzed by GenePix Pro 7.2 software.
Statistics
Statistical analyses were performed using R (version 3.6.3). Modified z values were calculated from the microarray median signal-to-noise ratios (SNRs). IgE reactive peptides were defined as being recognized by 50% or more or patients within an allergy group.
Structural and biochemical characterization of VBPs
Detailed NMR and biophysical characterization methods can be found in supplemental material. Briefly, triple-resonance and NOESY spectra were collected on 0.1–1 mM protein samples in PBS using either a 600 or 800 MHz Agilent DD2 console equipped with cryogenically cooled probe. Backbone and side-chain assignments and T1/T2 relaxation times for the oxidized samples were obtained using standard triple resonance techniques employing either the standard VARIAN Biopack or modified BEST-TROSY pulse sequences as described previously22,23 supplemented by additional 3D or 4D experiments to resolve peak overlap in the reduced samples.24,25 3D structures were calculated using the PONDEROSA web server.26,27 Surface similarity was assessed using the SPADE (Surface comparison-based Prediction of Allergen Discontinuous Epitopes) algorithm accessed via the provided web server.28,29 Amino acid physicochemical property similarities assessed using the Property Distance (PD) tool accessed via the Structural Database of Allergenic Proteins.30
Simulated gastric and endosomal digestion was carried out as described previously using 1.15 μg/mL trypsin (Sigma) an 0.25 U Cathepsin S (Human, recombinant from E-coli – Millipore) respectively in the absence or presence of 2 mM DTT.31 Circular dichroism spectra were collected using a Jasco J-815 CD spectropolarimeter (Jasco, Easton MD) and analyzed using the BESTSEL web server.32,33
Results
Structural characterization of peanut and walnut VBPs reveal a conserved α-helical scaffold
Backbone and side-chain assignments for all four VBPs were obtained using standard triple-resonance approaches. Analysis of the resulting chemical shifts using the TALOS prediction algorithm revealed two α-helical regions consistent with the expected VBP motif (Figure 2). The downfield shift (>33 ppm) of the cysteine Cβ peaks, along with the presence of NOESY cross-peaks between the Cβ protons on the opposite CxxxC repeats support the presence of an α-hairpin disulfide bonding pattern (Figures 1, S1). AH1.1 also contains an additional pair of cysteine residues flanking the main CxxxC motif, which were found to form a third disulfide bond that was verified by mass-spectrometry (Figures 1, S1). This information, along with structural restraints derived from the available NMR data was used to determine the 3D structure of all four VBPs. As shown in Figure 2, all four constructs adopt an unambiguous α-hairpin structure with low backbone RMSD values (Figure S1). Few long-range NOE interactions were detected beyond the disulfide bonded cysteines in each of the VBPs; a rather unusual observation for an ordered protein (Fig. S1). Reducing these disulfide bonds resulted in a decrease in peak dispersion in the 1H-15N HSQC spectra of all four peanut/walnut VBPs indicative of a loss of secondary structure (Figure 2). Cα/Cβ chemical shift analysis and CD spectroscopy confirm this conclusion, suggesting that the VBP sequence itself possesses a low degree of conformational stability in the absence of its covalent disulfide linkages (Figures 2, 3). Taken together, this data suggests that AH1.1, JR2.1, JR2.2 and JR2.3 each adopt a common α-hairpin structure maintained almost exclusively by the conserved disulfide bonds. This architecture is common to the VBPs from other plant species such as tomato and buckwheat (Fig. S2),9,10,15 potentially facilitating cross-reactivity across evolutionarily distant species.
Figure 2:
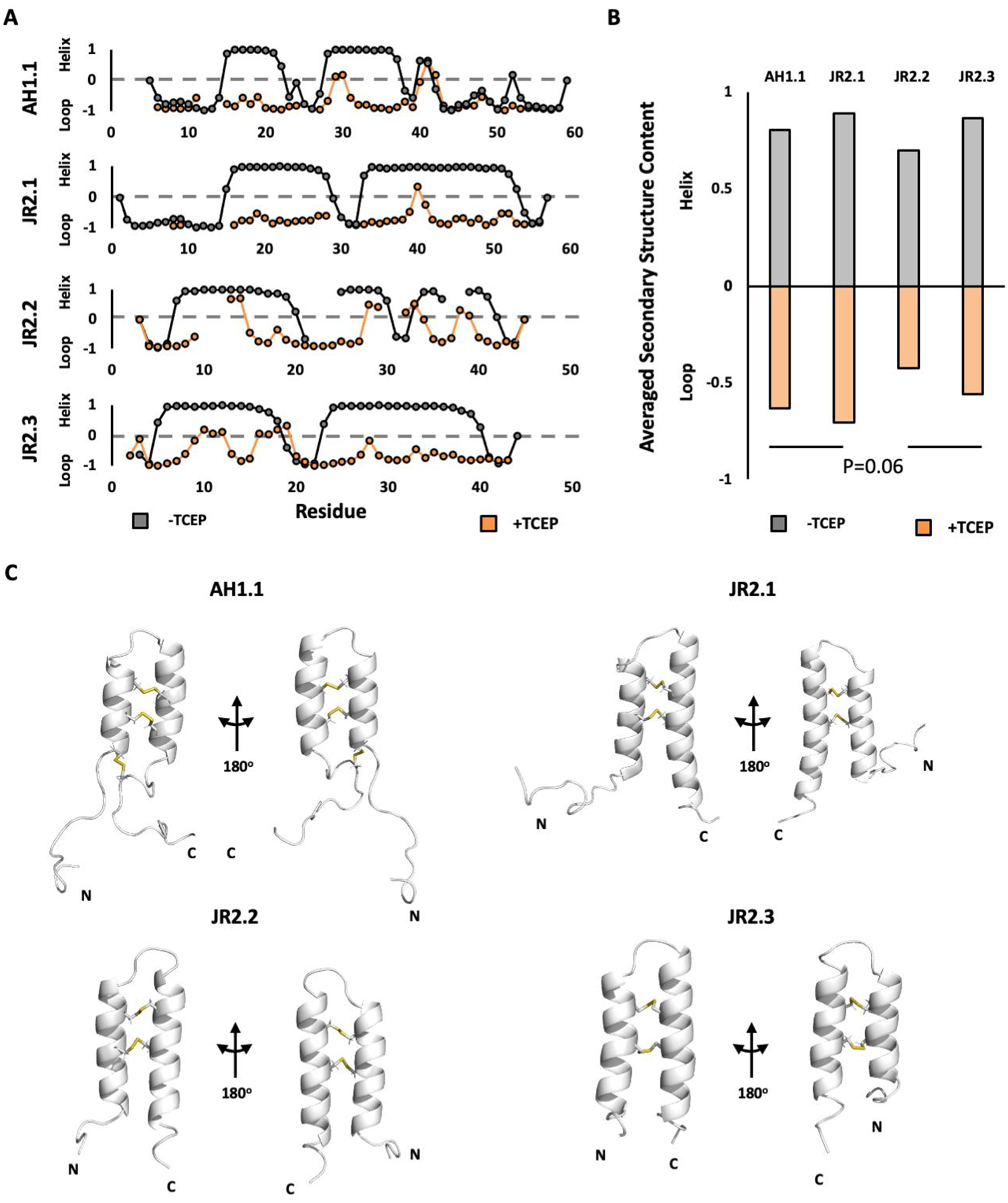
Peanut and walnut VBPs adopt a common α-hairpin structure mediated by conserved disulfide bonds. A) Secondary structure plot for the peanut/walnut VBPs under oxidizing (black) and reducing (orange) conditions, as calculated based on the backbone and side chain NMR chemical shifts using the TALOS algorithm. Average secondary structure content for all α-hairpin residues are shown in B. C) NMR structures of AH1.1, JR2.1, JR2.2, and JR2.3. Cartoon figures depict representative conformations of each structure. Inter-helix disulfides shown as sticks and highlighted in yellow. Complete NMR data tables are available in Figures S1.
Figure 3:

Loss of VBP structure under reducing conditions. A) Representative circular-dichroism spectra of peanut/walnut VBPs in the absence (black) and presence (orange) of the reducing agent tris(2-carbocyethyl)phosphine (TCEP). The local minima at 220/210 nm and 205 nm are indicative of α-helical and random-coil secondary structure respectively. The relative loss of α-helical structure upon addition of TCEP is quantified in B. Data values and error bars represent the mean and standard deviation obtained from at least three trials from two biological replicates.
The disulfide-mediated α-hairpin allows peanut and walnut VBPs to adopt a conserved motif with similar physiochemical properties despite their low sequence identity (Figure 1). Indeed, previous papers using the Structural Database of Allergenic Proteins (SDAP) Property Distance (PD) index - a tool that compares peptide sequences based on the physicochemical properties of their constituent amino acids -30,34,35 identified several potential cross-reactive regions on evolutionarily distant allergen families including peanut/walnut VBPs, their parent 2S albumins, and 7s vicilin domains.17,18 Drawing inspiration from these studies, the surface similarity of the peanut/walnut VBP structures was quantified using the SPADE computational tool developed by Dall’Antonia et al.28,29 The SPADE algorithm takes into account the physical properties of each residue, similar to the PD metric employed for sequence analysis, but includes an additional layer examining the solvent accessible area and spatial positioning of each residue based on the available 3D structures to calculate a surface similarity score (ΔSIM). Comparing the structure of AH1.1 with the three walnut VBPs reveals a high degree of similarity (Figure 4), supporting the potential for VBPs to mediate peanut/walnut cross-reactivity. Repeating this analysis in the reverse direction yields similar results. In both cases the overall surface similarity (Σ[ΔSIM]) values were noticeably higher between JR2.1, JR2.2, and AH1.1 than JR2.3, potentially reflecting subtle differences in the cross-reactive potential of the latter.
Figure 4:

Quantifying structural similarity across peanut and walnut VBPs A) Structure of AH1.1 colored by surface similarity (ΔSIM) values when compared against JR2.1, JR2.2, and JR2.3 as calculated using the SPADE surface comparison algorithm.28,29 Cyan and purple represent regions of low and high surface similarity respectively. Total ΔSIM (Σ[ΔSIM]) for all residues is indicated. B) Structure of WN VBPs colored by surface similarity (ΔSIM) values against AH1.1 as calculated using the SPADE surface comparison algorithm. Total ΔSIM (Σ[ΔSIM]) for all residues is indicated. Additional SPADE comparisons were performed on VBP homologues from tomato (VBP-8) and buckwheat (BWI-2c) (Figure S2).
α-hairpin scaffold supports cross-reactive IgE epitopes despite low sequence identity
Overlapping 15-mers representing the complete sequence of all four VBPs were printed onto peptide microarrays, and assessed for IgE binding using sera from peanut (PN), walnut (WN) and dual-sensitized (PW) patients (Table 1). Three immunoactive peptides were identified in AH1.1 (A4, A12 and A13), which show considerable overlap with previously identified immunodominant epitopes of Ara h 1 (Figure 5),36–38 while seven peptides were identified in the walnut VBPs (JR3, JR5, JR6, JR7, JR8, JR9, JR18) (Table 2). Two trends were clear from the prevalence of IgE binding to the peptide series. First, both PN and WN mono-allergic patients recognized peptides derived from the sequences of both peanut and walnut despite the low sequence identity, suggesting a high degree of cross-reactivity. Second, sera from PN and PW patients displayed higher % IgE binding than the WN patients to all the microarray peptides – including those from the walnut VBPs, further supporting the presence of cross reactivity between the peanut and walnut VBPs.
Figure 5:

Potential IgE-reactive and cross reactive regions on peanut and walnut VBPs. A) Sequence of peanut/walnut VBPs used in the peptide microarray analysis. Residues depicted in the solution-NMR structures are shown in capital letters. Residues from the expression system used to generate the NMR samples but are not present in the peptide microarrays are denoted with brackets. Residues highlighted in blue represent alpha-helices identified in the available NMR structures. Epitopes identified in previous works by Maleki et al.17 and Burks et al.36 are indicated in grey rectangles, whereas cross-reactive peptides identified in this present work are indicated by colored rectangles. B) IgE-reactive peptides mapped onto the structure of peanut/walnut VBPs. Peptides color-coded as in (A).
Table 2:
IgE-reactive PN/WN VBP peptides.
| Peptide # | Amino acid sequence | % Patients IgE binding | Average z value | ||||
|---|---|---|---|---|---|---|---|
| WN | PN | PW | WN | PN | PW | ||
| A4 | VLASVSATHAKSSPY | 25 | 58 | 38 | 2.9 | 3.5 | 2.2 |
| A10 | CQQEPDDLKQKACES | 8 | 42 | 19 | 1 | 5.1 | 6.4 |
| A11 | DDLKQKACESRCTKL | 0 | 0 | 0 | 0.7 | 0.4 | 0.5 |
| A12 | KACESRCTKLEYDPR | 42 | 50 | 50 | 3.1 | 4.2 | 3.2 |
| A13 | RCTKLEYDPRCVYDP | 50 | 92 | 81 | 5.3 | 8.1 | 7.2 |
| JR3 | PRDPREQYRQCQEYC | 92 | 100 | 94 | 28.5 | 24.5 | 27.4 |
| JR4 | EQYRQCQEYCRRQGQ | 0 | 33 | 19 | 0.6 | 2.9 | 2.7 |
| JR5 | CQEYCRRQGQGQRQQ | 17 | 58 | 50 | 2.4 | 4.8 | 4 |
| JR6 | RRQGQGQRQQQQCQI | 50 | 75 | 75 | 6.5 | 17.7 | 16.3 |
| JR7 | GQRQQQQCQIRCEER | 75 | 100 | 75 | 10.9 | 17.2 | 16.3 |
| JR8 | QQCQIRCEERLEEDQ | 67 | 75 | 81 | 7.4 | 9.5 | 11.2 |
| JR9 | RCEERLEEDQRSQEE | 50 | 100 | 69 | 6.2 | 10.3 | 9.5 |
| JR18 | QRRGQEQTLCRRRCE | 50 | 83 | 69 | 4.8 | 8.8 | 6.8 |
Acronyms: WN=walnut; PN=peanut; PW=peanut and walnut
Mapping the individual immunoactive peptides onto the VBP structures suggests this cross-reactivity is mediated by the shared α-hairpin motif (Figure 5). Given the overlapping nature of the microarray peptides, A12 and A13, along with a neighboring peptide which barely missed the IgE binding prevalence threshold (A10) likely encompasses a single conformational epitope. Notable, these peptides incorporates significant regions of the ordered α-hairpin structure and is recognized by all three serum groups (PN, WN, PW), demonstrating the cross-reactive potential of the conserved VBP motif (Figure 5). In contrast, the IgE-reactive peptide A4 encompasses the disordered N-terminal region of AH1.1 (Figure 5), potentially indicating a second epitope that is independent of the α-hairpin motif. With regards to the walnut VBPs, the majority of IgE reactive sequences are localized to JR2.1, with only one peptide (J18) on JR2.2, and none on JR2.3 (Figure 5). Of the IgE-reactive walnut peptides, all were localized to the structured α-hairpin region and were recognized by all three patient groups, confirming the VBP motif as a potent mediator of cross-reactive peanut/walnut IgE binding. Taken together, the data suggest that the conserved α-hairpin structure of JR2.1 and AH1.1 mediates both allergic sensitization and cross-reactive IgE binding across peanut and walnut-allergic patients.
Thermodynamic stability and proteolytic resistance as determinants of VBP allergenicity
Previous studies identified protein stability as an important criteria for allergenicity among some allergen families.39 Similar trends could influence the sensitizing potential of VBP allergens, potentially providing a physical basis for the paucity of IgE binding peptides from JR2.2 and JR2.3.40,41 In the context of food allergens, stability plays a key role in the ability of potential allergens to resist proteolytic digestion, facilitating exposure of the intact antigen to the immune system.42 To assess this hypothesis, all four VBPs were subjected to simulated gastric and duodenal digestion using pepsin (pH 2.0) and trypsin (pH 7.4) respectively. The oxidized form of all four VBP’s were extremely resilient to gastric digestion, with no detectable degradation observed under the conditions employed. Reducing the intramolecular disulfide bonds significantly enhanced proteolysis, with almost complete cleavage observed even following a 20-fold reduction in pepsin concentration (Figure 6). Curiously, both JR2.2 and JR2.3 were found to be significantly more resistant than their counterparts under these conditions. Moving onto the duodenum, some VBPs such as BWI-2c from buckwheat and C2 from pumpkin have been shown to act as potent, irreversible trypsin inhibitors with Ki values in the nm range.7,10,15 However, no such activity was observed for any of the peanut and walnut VBPs tested here (Figure 6). Instead, all four VBPs were readily degraded by trypsin. As with gastric digestion, proteolytic cleavage was noticeably enhanced under reducing conditions, reflecting the loss of the ordered α-hairpin structure though no correlation with IgE binding was observed under either condition.
Figure 6:

Conformational stability of peanut and walnut VBPs contribute to proteolytic stability. Half-life of peanut and walnut VBPs subjected to simulated gastric (A) and duodenal (B) digestion under reducing and oxidizing conditions. C) Half-life of peanut and walnut VBPs subjected to simulated endosomal digestion (reducing conditions only). Faded bars represent conditions under which no appreciable (<10%) digestion was detected. Data values and error bars represent the mean and standard deviation obtained from at least three trials from two biological replicates.
The ability of potential allergens to resist endosomal digestion can also influence the sensitization process, providing an additional avenue through which differences in VBP stability could determine allergenicity.43–45 To assess this hypothesis peanut and walnut VBPs were subjected to simulated endosomal degradation by Cathepsin S (pH 5.4, 2 mM DTT). Both AH1.1 and JR2.1 displayed moderate stability under these conditions, with half-lives in the 30–60 minute range. In contrast JR2.2 and JR2.3 showed negligible (<10%) degradation under the assay conditions, potentially accounting for the lower sensitization potential of the latter. It should be noted that both JR2.2 and JR2.3 retain a greater proportion of their ordered secondary structure under reducing conditions (Figure 3) as assessed using CD, suggesting that the α-hairpin motif may be more resilient in these sequences, contributing to their proteolytic resistance. NMR chemical shift analysis shows a similar trend (Figure 2) though the statistical difference between AH1.1, JR2.1 versus JR2.2, JR2.3 was borderline significant (p=0.06) when assessed using a Student’s T-test. However, it is consistent with both the CD studies and proteolytic stability results, providing a compelling mechanistic link between the global conformational stability of the VBP motif, endosomal degradation kinetics, and allergenicity.
Discussion
This study demonstrates that while the vicilin LSs from peanut and walnut contain a variable number of VBP motifs, each of these adopt a characteristic α-hairpin motif mediated by disulfides between the conserved CxxxC elements. While homologous structures have been described for other VBPs9–11,15, this is the first time such a structural motif has been described for a major food allergen. The unusual nature of these sequences - being maintained almost exclusively by disulfide bonds - allows for significant variability in the amino acids surrounding the conserved CxxxC cysteine residues while retaining a high degree of structural similarity as indicated by the high surface similarity values shown in Figure 3. This gives rise to cross-reactive peanut/walnut IgE epitopes despite the low sequence identity. Indeed, it is worth noting that the sequence identity between JR2.1 and AH1.1 is a mere 17% (Figure 1) - falling well below the threshold commonly considered for cross-reactivity. While the main vicilin/albumin domain of Ara h 1 and Jug r 2 are recognized by both peanut and tree nut-allergic patient IgE,17,18 our work represents the first time that the cross-reactivity potential of the independent VBP domains has been specifically assessed. The structural homology provided by the VBP motif could potentially facilitate cross-reactivity across other plant species. Indeed, comparing the peanut/walnut VBP structures against those of its tomato (VBP-8) and buckwheat (BWI-2c) homologues reveals a high degree of similarity, with Σ[ΔSIM] values comparable to those observed among the peanut/walnut VBPs (Figure S2). A notable exception is JR2.3, whose comparatively low Σ[ΔSIM] values might account for the absence cross-reactive peptides observed in this study. Similarly, the high Σ[ΔSIM] score observed for JR2.2 belies its scarcity of IgE-reactive peptides relative to JR2.1. It should be noted that the cross-reactive α-hairpin structure is mediated primarily by intermediate and long-range disulfide bonds, suggesting that IgE recognition is mediated by conformational rather than linear epitopes: a conjecture supported by the localization of IgE binding to the structured α-hairpin regions of the VBP’s tested (Figure 5). The drastic loss in secondary structure observed under reducing conditions suggests that the peptide microarray may not fully represent these conformational epitopes, potentially accounting for some of the discontinuities in the peptide mapping, or the aforementioned discrepancies concerning JR2.2 and JR2.3. Nonetheless, the structural similarities observed between the various VBP α-hairpins reported in this work and elsewhere, coupled with the high (up to 100%) rate of cross-reactive PN IgE binding observed in JR2.1, suggests that the contribution of these shared α-hairpins to cross-reactivity of these sequences warrants further investigation.
The α-hairpin VBP structure also bears striking similarity with other cysteine-rich seed storage proteins, potentially allowing for cross-reactivity with other allergen families. Indeed, previous studies have employed bioinformatics approaches, including the SDAP PD search tool described previously, to identify several potential peanut/tree-nut cross-reactive sequences on 2S albumins, 7S globulins, 11S globulins, and VBPs based on their biophysical similarity to the immunodominant epitope of Ara h 2, a conjecture which was verified by subsequent microarray studies.17,18 Additionally, antibodies raised against a 13-mer consensus (13cp) sequence derived from these multiple alignments was able to successfully recognize both peanut and walnut VBPs, along with their globulin and albumin counterparts.18 Curiously, a PD search comparing the tomato and buckwheat VBPs shown in Figure S2 against this same 13cp reveals comparable scores to the peanut/walnut VBPs, suggesting that this mode of cross-reactivity is applicable to other food allergen sources beyond the peanut and walnut sequences described in this work. Finally, it should also be noted that IgE-reactive VBPs have also been identified in in almonds (Pru du 8),46 cashew (Ana o 1),47 pistachio (Pis v 3),48,49 sesame (Ses i 3),49 and macadamia nut (Mac i 1).50 While the structure of these IgE-reactive VBPs have yet to be solved, their sequences share similar PD scores when compared against the 13cp as their peanut and walnut homologues. Taken together, these findings establish VBPs as a potential family of pan-allergens whose disulfide-bridged α-helical structure contribute to both allergenicity and cross reactivity across a wide range of species and protein families.
Stability is frequently cited as a biophysical property that can differentiate allergens from non-allergens. A systematic investigation into its role in the sensitization process is complicated by the various definitions of protein stability (eg: resistance to pressure, heat, chemical modification etc.), and the potential contribution of other physiochemical phenomenon such as aggregation, ligand binding, and post-translational modification. Nonetheless, there are several works which demonstrate this trend on both the individual protein and whole-proteome level.39,40,51,52 With regards to food allergens, stability is proposed to enhance resistance to gastrointestinal digestion, facilitating exposure of the intact antigen to the immune system following oral consumption.42 However, recent work suggests that sensitization can also occur via cutaneous or airway exposure, potentially accounting for the low gastrointestinal resistance of profilins and PR-10 allergens.39,53,54 It should be noted the walnut and other plant VBP’s naturally adopt a structured, disulfide-bonded α-hairpin motif when extracted from their natural source material, indicating that this represents the most relevant species in the context of gastrointestinal digestion.9,19 In this form, no correlation was observed between gastrointestinal digestion and IgE reactivity, suggesting that non-oral exposure avenues may play a role in VBP sensitization.
Stability can also be defined by the ability to resist endosomal degradation by antigen-presenting cells. The data herein suggests that resilience under these conditions may be a better predictor of VBP allergic potential. This is consistent with a growing body of work indicating that the kinetics of endosomal digestion, epitope generation, and MHCII presentation can influence the nature of the downstream immune response against a wide range of allergens.43–45 Under this “stability hypothesis”, high but transient MHCII levels facilitating asymptomatic tolerance while a more persistent presentation regime promotes allergic sensitization.43–45 This gives rise to a bimodal relationship between stability and allergenicity. Here, proteins with intermediate stabilities tend to be asymptomatic, as the majority of their epitope fragments are generated within the late endosome when MHCII loading and presentation is most effective, i.e. ‘normal’.55,56 In contrast, proteins with above average stabilities are more likely to generate an allergic response as peak epitope generation occurs after the window for optimal MHCII loading (Figure 7).57–59 However, proteins which stray too far toward either extreme are unlikely to generate an immune response of any kind. This phenomenon has been observed in hypoallergenic variants of the birch pollen allergen Bet v 151 or non-allergic Amb a 1 (ragweed) homologues.60 The observations presented in this work indicate that AH1.1 and JR2.1 lie in the stability regime required for allergic sensitization (Figure 7) while the elevated stability of JR2.2 and JR2.3 inhibits the generation of T-cell epitopes on a reasonable timescale, limiting their allergic potential. These findings provide valuable insight into possible proteomics tools to differentiate allergic VBPs from their non-allergic counterparts and raises the possibility for novel immunotherapeutic strategies centered around destabilized VBP mutants which fall into the intermediate proteolysis regime, increasing the likelihood of a tolerogenic response (Figure 7).
Figure 7:

Proposed model of VBP allergenicity. Following uptake by antigen presenting cells, potential allergens are subjected to endosomal degradation to generate T-cell epitopes. Proteins with an intermediate stability are primarily digested in the late endosome, resulting in efficient MHCII loading and a tolerogenic (IgG/IgG4) immune response as reviewed by Foo et al.59 Proteins with above-average stability experience peak epitope generation in the lysosome, increasing the probability of an allergic (IgE) response due to the scarcity of MHCII. Hyper-stabilized proteins are completely resilient to endosomal digestion and yield only minimal levels of epitope fragments over the endosomal lifecycle, resulting in a diminished immune response. The results reported in this work suggest that extremely immunogenic VBPs such as AH1.1 and JR2.1 belong to the former, while hyper-stabilized homologues such as JR2.2 and JR2.3 fall into the latter category, contributing to their reduced sensitizing potential.
Together, the results suggest that plant VBP’s represent an important family of potential pan-allergens whose conserved α-hairpin fold allows them to mediate cross-reactivity across evolutionarily distant plant species and protein families. The unique bisulfide-mediated architecture of the VBP motif hinders attempts to assess immunogenicity using traditional bioinformatic approaches. Instead, stability in the context of endosomal degradation was identified as a potential predictor of allergenicity within the VBP family, though future work is required to confirm these findings in a clinical setting. Nonetheless these findings provide valuable insight into the mechanism of sensitization and potential proteomic tools to identify and assess allergic VBP’s in future works.
Supplementary Material
Notes, acknowledgements, funding information:
This research was supported in part by the Intramural Research Program of the National Institute of Environmental Health Sciences, (Z01-ES102906, GAM) and the National Institute of Allergy and Infectious Disease (NIAID-R21 AI135397, CHS). Also funded by the US Department of Agriculture: Agricultural Research Service (ARS-6054-43440-044-00D) and the National Institute for Food and Agriculture/National Peanut Board (NIFA-60-6054-7-003 SJM/BKH/JBN). The structures for AH1.1, JR2.1, JR2.2, and JR2.3 submitted to the Protein Data Bank and the Biological Magnetic Resonance Bank under the access codes PBD ID 7LXK BMRB 30875, PDB ID 7LVF BRMB ID 30870, PDB ID 7LVG BMRB 30871, and PDB ID 7LVE BRMB ID 30869 respectively.
Abbreviations Used:
- AEP
asparaginyl endopeptidase
- CD
Circular Dischroism
- ΔSIM
Surface similarity score
- DTT
Dithiothreitol
- HSQC
Heteronuclear Single Quantum Coherence
- LS
Leader sequence
- MHCII
Major Histocompatability Complex, Class 2
- NMR
Nuclear Magnetic Resonance
- NOE
Nuclear Overhauser Effect
- NOESY
Nuclear Overhauser Effect Spectroscopy
- PD
Property Distance
- SDAP
Structural Database of Allergenic Proteins
- TCEP
tris(2-carboxyethyl)phosphine
- VBP
Vicilin-buried peptide.
Footnotes
References
- (1).Burney P; Summers C; Chinn S; Hooper R; Van Ree R; Lidholm J Prevalence and Distribution of Sensitization to Foods in the European Community Respiratory Health Survey: A EuroPrevall Analysis. Allergy Eur. J. Allergy Clin. Immunol 2010, 65 (9), 1182–1188. 10.1111/j.1398-9995.2010.02346.x. [DOI] [PubMed] [Google Scholar]
- (2).Chong KW; Ruiz-Garcia M; Patel N; Boyle RJ; Turner PJ Reaction Phenotypes in IgE-Mediated Food Allergy and Anaphylaxis. Ann. Allergy, Asthma Immunol 2020, 124 (5), 473–478. 10.1016/j.anai.2019.12.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (3).Fischer D; Vander Leek TK; Ellis AK; Kim H Anaphylaxis. Allergy, Asthma Clin. Immunol 2018, 14 (Suppl 2). 10.1186/s13223-018-0283-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (4).Weinberger T; Sicherer S Current Perspectives on Tree Nut Allergy: A Review. J. Asthma Allergy 2018, 11, 41–51. 10.2147/JAA.S141636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (5).Maloney JM; Rudengren M; Ahlstedt S; Bock SA; Sampson HA The Use of Serum-Specific IgE Measurements for the Diagnosis of Peanut, Tree Nut, and Seed Allergy. J. Allergy Clin. Immunol 2008, 122 (1), 145–151. 10.1016/j.jaci.2008.04.014. [DOI] [PubMed] [Google Scholar]
- (6).Garcia BE, Lizaso MT Cross-Reactivity Syndromes in Food Allergy. J Investig Allergol Clin Immunol 2011, 21 (3), 162–170. 10.1016/j.anai.2012.02.006. [DOI] [PubMed] [Google Scholar]
- (7).Yamada K; Shimada T; Kondo M; Nishimura M; Hara-Nishimura I Multiple Functional Proteins Are Produced by Cleaving Asn-Gln Bonds of a Single Precursor by Vacuolar Processing Enzyme. J. Biol. Chem 1999, 274 (4), 2563–2570. 10.1074/jbc.274.4.2563. [DOI] [PubMed] [Google Scholar]
- (8).Wichers HJ; De Beijer T; Savelkoul HFJ; Van Amerongen A The Major Peanut Allergen Ara h 1 and Its Cleaved-off N-Terminal Peptide; Possible Implications for Peanut Allergen Detection. J. Agric. Food Chem 2004, 52 (15), 4903–4907. 10.1021/jf049697o. [DOI] [PubMed] [Google Scholar]
- (9).Zhang J; Payne CD; Pouvreau B; Schaefer H; Fisher MF; Taylor NL; Berkowitz O; Whelan J; Rosengren KJ; Mylne JS An Ancient Peptide Family Buried within Vicilin Precursors. ACS Chem. Biol 2019, 14 (5), 979–993. 10.1021/acschembio.9b00167. [DOI] [PubMed] [Google Scholar]
- (10).Payne CD; Vadlamani G; Fisher MF; Zhang J; Clark RJ; Mylne JS; Rosengren KJ Defining the Familial Fold of the Vicilin-Buried Peptide Family. J. Nat. Prod 2020, 83 (10), 3030–3040. 10.1021/acs.jnatprod.0c00594. [DOI] [PubMed] [Google Scholar]
- (11).Ng YM; Yang Y; Sze KH; Zhang X; Zheng YT; Shaw PC Structural Characterization and Anti-HIV-1 Activities of Arginine/Glutamate-Rich Polypeptide Luffin P1 from the Seeds of Sponge Gourd (Luffa Cylindrica). J. Struct. Biol 2011, 174 (1), 164–172. 10.1016/j.jsb.2010.12.007. [DOI] [PubMed] [Google Scholar]
- (12).Wang R; Gan C; Gao W; He W; Wang X; Peng Y; Zhuo J; Tan J; Peng X; Wu J; Luo G A Novel Recombinant Immunotoxin with the Smallest Ribosome-Inactivating Protein Luffin P1: T-Cell Cytotoxicity and Prolongation of Allograft Survival. J. Cell. Mol. Med 2010, 14 (3), 578–586. 10.1111/j.1582-4934.2009.00840.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (13).Li F; Yang XX; Xia HC; Zeng R; Hu WG; Li Z; Zhang ZC Purification and Characterization of Luffin P1, a Ribosome-Inactivating Peptide from the Seeds of Luffa Cylindrica. Peptides 2003, 24 (6), 799–805. 10.1016/S0196-9781(03)00173-6. [DOI] [PubMed] [Google Scholar]
- (14).Conners R; Konarev AV; Forsyth J; Lovegrove A; Marsh J; Joseph-Horne T; Shewry P; Brady RL An Unusual Helix-Turn-Helix Protease Inhibitory Motif in a Novel Trypsin Inhibitor from Seeds of Veronica (Veronica Hederifolia L.). J. Biol. Chem 2007, 282 (38), 27760–27768. 10.1074/jbc.M703871200. [DOI] [PubMed] [Google Scholar]
- (15).Oparin PB; Mineev KS; Dunaevsky YE; Arseniev AS; Belozersky MA; Grishin EV; Egorov TA; Vassilevski AA Buckwheat Trypsin Inhibitor with Helical Hairpin Structure Belongs to a New Family of Plant Defence Peptides. Biochem. J 2012, 446 (1), 69–77. 10.1042/BJ20120548. [DOI] [PubMed] [Google Scholar]
- (16).Baranov YV; Nolde SB; Samsonova OV; Grishin EV; Vassilevski AA; Egorov TA; Balashova TA; Rogozhin EA; Barinov NA; Arseniev AS; Feofanov AV Disulfide-Stabilized Helical Hairpin Structure and Activity of a Novel Antifungal Peptide EcAMP1 from Seeds of Barnyard Grass (Echinochloa Crus-Galli). J. Biol. Chem 2011, 286 (28), 25145–25153. 10.1074/jbc.m110.200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (17).Maleki SJ; Teuber SS; Cheng H; Chen D; Comstock SS; Ruan S; Schein CH Computationally Predicted IgE Epitopes of Walnut Allergens Contribute to Cross-Reactivity with Peanuts. Allergy Eur. J. Allergy Clin. Immunol 2011, 66 (12), 1522–1529. 10.1111/j.1398-9995.2011.02692.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (18).Nesbit JB; Schein CH; Braun BA; Gipson SAY; Cheng H; Hurlburt BK; Maleki SJ Epitopes with Similar Physicochemical Properties Contribute to Cross Reactivity between Peanut and Tree Nuts. Mol. Immunol 2020, 122 (May), 223–231. 10.1016/j.molimm.2020.03.017. [DOI] [PubMed] [Google Scholar]
- (19).Downs ML; Semic-Jusufagic A; Simpson A; Bartra J; Fernandez-Rivas M; Rigby NM; Taylor SL; Baumert JL; Mills ENC Characterization of Low Molecular Weight Allergens from English Walnut (Juglans Regia). J. Agric. Food Chem 2014, 62 (48), 11767–11775. 10.1021/jf504672m. [DOI] [PubMed] [Google Scholar]
- (20).Aalberse RC; Mueller GA; Derksen NIL; Aalberse JA; Edwards LL; Pomés A; Lidholm J; Rispens T; Briza P Identification of the Amino-Terminal Fragment of Ara h 1 as a Major Target of the IgE-Binding Activity in the Basic Peanut Protein Fraction. Clin. Exp. Allergy 2020, 50 (3), 401–405. 10.1111/cea.13554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (21).Pastorello EA; Farioli L; Pravettoni V; Robino AM; Scibilia J; Fortunato D; Conti A; Borgonovo L; Bengtsson A; Ortolani C Lipid Transfer Protein and Vicilin Are Important Walnut Allergens in Patients Not Allergic to Pollen. J. Allergy Clin. Immunol 2004, 114 (4), 908–914. 10.1016/j.jaci.2004.06.020. [DOI] [PubMed] [Google Scholar]
- (22).Ghosh D; Mueller GA; Schramm G; Edwards LL; Petersen A; London RE; Haas H; Bhattacharya SG Primary Identification, Biochemical Characterization, and Immunologic Properties of the Allergenic Pollen Cyclophilin Cat r 1. J. Biol. Chem 2014, 289 (31), 21374–21385. 10.1074/jbc.M114.559971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (23).Favier A; Brutscher B Recovering Lost Magnetization: Polarization Enhancement in Biomolecular NMR. J. Biomol. NMR 2011, 49 (1), 9–15. 10.1007/s10858-010-9461-5. [DOI] [PubMed] [Google Scholar]
- (24).Yang D; Kay LE TROSY Triple-Resonance Four-Dimensional NMR Spectroscopy of a 46 Ns Tumbling Protein. J. Am. Chem. Soc 1999, 121 (11), 2571–2575. 10.1021/ja984056t. [DOI] [Google Scholar]
- (25).Solyom Z; Schwarten M; Geist L; Konrat R; Willbold D; Brutscher B BEST-TROSY Experiments for Time-Efficient Sequential Resonance Assignment of Large Disordered Proteins. J. Biomol. NMR 2013, 55 (4), 311–321. 10.1007/s10858-013-9715-0. [DOI] [PubMed] [Google Scholar]
- (26).Lee W; Kim JH; Westler WM; Markley JL PONDEROSA, an Automated 3D-NOESY Peak Picking Program, Enables Automated Protein Structure Determination. Bioinformatics 2011, 27 (12), 1727–1728. 10.1093/bioinformatics/btr200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (27).Guntert P; Buchner L Combined Automated NOE Assignment and Structure Calculation with CYANA. J. Biomol. NMR 2015, 62 (4), 453–471. [DOI] [PubMed] [Google Scholar]
- (28).Dall’Antonia F; Keller W SPADE Web Service for Prediction of Allergen IgE Epitopes. Nucleic Acids Res. 2019, 47 (W1), W496–W501. 10.1093/nar/gkz331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (29).Dall’Antonia F; Gieras A; Devanaboyina SC; Valenta R; Keller W Prediction of IgE-Binding Epitopes by Means of Allergen Surface Comparison and Correlation to Cross-Reactivity. J. Allergy Clin. Immunol 2011, 128 (4), 872–879.e8. 10.1016/j.jaci.2011.07.007. [DOI] [PubMed] [Google Scholar]
- (30).Ivanciuc O; Schein CH; Braun W SDAP: Database and Computational Tools for Allergenic Proteins. Nucleic Acids Res. 2003, 31 (1), 359–362. 10.1093/nar/gkg010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (31).Foo ACY; Thompson PM; Perera L; Arora S; DeRose EF; Williams J; Mueller GA Hydrophobic Ligands Influence the Structure, Stability, and Processing of the Major Cockroach Allergen Bla g 1. Sci. Rep 2019, 9 (1), 1–12. 10.1038/s41598-019-54689-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (32).Micsonai A; Wien F; Kernya L; Lee YH; Goto Y; Réfrégiers M; Kardos J Accurate Secondary Structure Prediction and Fold Recognition for Circular Dichroism Spectroscopy. Proc. Natl. Acad. Sci. U. S. A 2015, 112 (24), E3095–E3103. 10.1073/pnas.1500851112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (33).Micsonai A; Wien F; Bulyáki É; Kun J; Moussong É; Lee Y-H; Goto Y; Réfrégiers M; Kardos J BeStSel: A Web Server for Accurate Protein Secondary Structure Prediction and Fold Recognition from the Circular Dichroism Spectra. Nucleic Acids Res. 2018, 46 (W1), W315–W322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (34).Ivanciuc O; Schein CH; Braun W Data Mining of Sequences and 3D Structures of Allergenic Proteins. Bioinformatics 2002, 18 (10), 1358–1364. 10.1093/bioinformatics/18.10.1358. [DOI] [PubMed] [Google Scholar]
- (35).Ivanciuca O; Midoro-Horiuti T; Schein CH; Xie L; Hillman GR; Goldblum RM; Braun W The Property Distance Index PD Predicts Peptides That Cross-React with IgE Antibodies. Mol. Immunol 2009, 46 (5), 873–883. 10.1038/jid.2014.371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (36).Burks WA; Shin D; Cockrell G; Stanley SJ; Helm RM; Bannon GA Mapping and Mutational Analysis of the IgE-Binding Epitopes on Ara h 1, a Legume Vicilin Protein and a Major Allergen in Peanut Hypersensitivity. Eur. J. Biochem 1997, 245 (2), 334–339. 10.1111/j.1432-1033.1997.t01-1-00334.x. [DOI] [PubMed] [Google Scholar]
- (37).Beyer K; Ellman-Grunther L; Järvinen KM; Wood RA; Hourihane J; Sampson HA Measurement of Peptide-Specific IgE as an Additional Tool in Identifying Patients with Clinical Reactivity to Peanuts. J. Allergy Clin. Immunol 2003, 112 (1), 202–207. 10.1067/mai.2003.1621. [DOI] [PubMed] [Google Scholar]
- (38).Cong YJ; Lou F; Xue WT; Li LF; Chen MH Characterisation of the IgE-Binding Immunodominant Epitopes on Ara H1. Food Agric. Immunol 2008, 19 (3), 175–185. 10.1080/09540100802172599. [DOI] [Google Scholar]
- (39).Costa J; Villa C; Verhoeckx K; Cirkovic-Velickovic T; Schrama D; Roncada P; Rodrigues PM; Piras C; Martín-Pedraza L; Monaci L; Molina E; Mazzucchelli G; Mafra I; Lupi R; Lozano-Ojalvo D; Larré C; Klueber J; Gelencser E; Bueno-Diaz C; Diaz-Perales A; Benedé S; Bavaro SL; Kuehn A; Hoffmann-Sommergruber K; Holzhauser T Are Physicochemical Properties Shaping the Allergenic Potency of Animal Allergens? Clin. Rev. Allergy Immunol 2021. 10.1007/s12016-020-08826-1. [DOI] [PubMed] [Google Scholar]
- (40).Cabrera A; Randall TA; Ogburn RN; Mebrahtu B; Johnson JHR; Foo ACY; Fitzgerald MC; Mueller GA Are Allergens More Abundant and/or More Stable than Other Proteins in Pollens and Dust? Allergy Eur. J. Allergy Clin. Immunol 2019, 1267–1269. 10.1111/all.14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (41).Herman RA; Woolhiser MM; Ladics GS; Korjagin VA; Schafer BB; Storer NP; Kan L Stability of a Set of Allergens and Non-Allergens in Simulated Gastric Fluid. Int. J. Food Sci. Nutr 2007, 58 (2), 125–141. 10.1080/09637480601149640. [DOI] [PubMed] [Google Scholar]
- (42).Foster ES; Kimber I; Dearman RJ Relationship between Protein Digestibility and Allergenicity: Comparisons of Pepsin and Cathepsin. Toxicology 2013, 309, 30–38. 10.1016/j.tox.2013.04.011. [DOI] [PubMed] [Google Scholar]
- (43).Constant S; Pfeiffer C; Woodard A; Pasqualini T; Bottomly K; Constant BS; Pfeiffer C; Woodard A; Pasqualini T; Bottomly K Extent of T Cell Receptor Ligation Can Determine the Functional Differentiation of Naive CD4+ T Cells. J. Exp. Med 1995, 182, 1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (44).Langenkamp A; Messi M; Lanzavecchia A; Sallusto F Kinetics of Dendritic Cell Activation: Impact on Priming of TH1,TH2 and Nonpolarized T Cells. Nat. Immunol 2000, 1 (4), 311–316. 10.1038/79758. [DOI] [PubMed] [Google Scholar]
- (45).Hosken NA; Shibuya K; Heath AW; Murphy KM; O’Garra A The Effect of Antigen Dose on CD4+ T Helper Cell Phenotype Development in a T Cell Receptor-Ab-Transgenic Model. J. Exp. Med 1995, 182, 1579–158420– 158422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (46).Kabasser S; Hafner C; Chinthrajah S; Sindher SB; Kumar D; Kost LE; Long AJ; Nadeau KC; Breiteneder H; Bublin M Identification of Pru Du 6 as a Potential Marker Allergen for Almond Allergy. Allergy Eur. J. Allergy Clin. Immunol 2021, 76 (5), 1463–1472. 10.1111/all.14613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (47).Wang F; Robotham JM; Teuber SS; Tawde P; Sathe SK; Roux KH Ana o 1, a Cashew (Anacardium Occidental) Allergen of the Vicilin Seed Storage Protein Family. J. Allergy Clin. Immunol 2002, 110 (1), 160–166. 10.1067/mai.2002.125208. [DOI] [PubMed] [Google Scholar]
- (48).Willison LN; Tawde P; Robotham JM; Penney RM; Teuber SS; Sathe SK; Roux KH Pistachio Vicilin, Pis v 3, Is Immunoglobulin E-Reactive and Cross-Reacts with the Homologous Cashew Allergen, Ana o 1. Clin. Exp. Allergy 2008, 38 (7), 1229–1238. 10.1111/j.1365-2222.2008.02998.x. [DOI] [PubMed] [Google Scholar]
- (49).Barre A; Nguyen C; Granier C; Benoist H; Roug P; Rougé P IgE-Binding Epitopes of Pis v 1, Pis v 2 and Pis v 3, the Pistachio (Pistacia Vera) Seed Allergens. Allergies 2021, 1 (1), 63–91. 10.3390/allergies1010006. [DOI] [Google Scholar]
- (50).Kabasser S; Pratap K; Kamath S; Taki AC; Dang T; Koplin J; Perrett K; Hummel K; Radauer C; Breiteneder H; Lopata AL; Bublin M Identification of Vicilin, Legumin and Antimicrobial Peptide 2a as Macadamia Nut Allergens. Food Chem. 2022, 370 (August 2021), 131028. 10.1016/j.foodchem.2021.131028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (51).Machado Y; Freier R; Scheiblhofer S; Thalhamer T; Mayr M; Briza P; Grutsch S; Ahammer L; Fuchs JEJE; Wallnoefer HGHG; Isakovic A; Kohlbauer V; Hinterholzer A; Steiner M; Danzer M; Horejs-Hoeck J; Ferreira F; Liedl KR; Tollinger M; Lackner P; Johnson CMCM; Brandstetter H; Thalhamer J; Weiss R; Biza P; Grutsch S; Ahammer L; Fuchs JEJE; Wallnoefer HGHG; Isakovic A; Kohlbauer V; Hinterholzer A; Steiner M; Danzer M; Horejs-Hoek J; Ferreira F; Liedl KR; Tollinger M; Lackner P; Johnson CMCM; Brandstetter H; Thalhamer J; Weiss R Fold Stability during Endolysosomal Acidification Is a Key Factor for Allergenicity and Immunogenicity of the Major Birch Pollen Allergen. J. Allergy Clin. Immunol 2016, 137 (5), 1525–1534. 10.1016/j.jaci.2015.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (52).Ogburn RN; Randall TA; Xu Y; Roberts JH; Mebrahtu B; Karnuta JM; Rider SD; Kissling GE; London RE; Pomés A; Arlian L; Fitzgerald MC; Mueller GA Are Dust Mite Allergens More Abundant and/or More Stable than Other Dermatophagoides Pteronyssinus Proteins? J. Allergy Clin. Immunol 2017, 139 (3), 1030–1032.e1. 10.1016/j.jaci.2016.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (53).Tordesillas L; Goswami R; Benedé S; Grishina G; Dunkin D; Järvinen KM; Maleki SJ; Sampson HA; Berin MC Skin Exposure Promotes a Th2-Dependent Sensitization to Peanut Allergens. J. Clin. Invest 2014, 124 (11), 4965–4975. 10.1172/JCI75660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (54).Dolence JJ; Kobayashi T; Iijima K; Krempski J; Drake LY; Dent AL; Kita H Airway Exposure Initiates Peanut Allergy by Involving the IL-1 Pathway and T Follicular Helper Cells in Mice. J. Allergy Clin. Immunol 2018, 142 (4), 1144–1158.e8. 10.1016/j.jaci.2017.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (55).van Niel G; Wubbolts R; Stoorvogel W Endosomal Sorting of MHC Class II Determines Antigen Presentation by Dendritic Cells. Curr. Opin. Cell Biol 2008, 20 (4), 437–444. 10.1016/j.ceb.2008.05.011. [DOI] [PubMed] [Google Scholar]
- (56).Ohkuri T; Nagatomo S; Oda K; So T; Imoto T; Ueda T A Protein’s Conformational Stability Is an Immunologically Dominant Factor: Evidence That Free-Energy Barriers for Protein Unfolding Limit the Immunogenicity of Foreign Proteins. J. Immunol 2010, 185 (7), 4199–4205. 10.4049/jimmunol.0902249. [DOI] [PubMed] [Google Scholar]
- (57).Winter P; Stubenvoll S; Scheiblhofer S; Joubert IA; Strasser L; Briganser C; Soh WT; Hofer F; Kamenik AS; Dietrich V; Michelini S; Laimer J; Lackner P; Horejs-Hoeck J; Tollinger M; Liedl KR; Brandstetter J; Huber CG; Weiss R In Silico Design of Phl p 6 Variants With Altered Fold-Stability Significantly Impacts Antigen Processing, Immunogenicity and Immune Polarization. Front. Immunol 2020, 11, 1–21. 10.3389/fimmu.2020.01824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (58).da Silva ES; Huber S; Alcantara-Neves NM; Asam C; Silveira EF; de Andrade Belitardo EMM; Aglas L; Wallner M; Gadermaier G; Briza P; Karner I; Torres RT; Alvarez JRU; Wuenschmann S; Chapman M; Ferreira F; Pinheiro CS N-Terminal Peptide Deletion Influences Immunological and Structural Features of Blo t 5. Allergy Eur. J. Allergy Clin. Immunol 2020, 75 (6), 1503–1507. 10.1111/all.14176. [DOI] [PubMed] [Google Scholar]
- (59).Foo ACY; Mueller GA Abundance and Stability as Common Properties of Allergens. Front. Allergy 2021, 2 (80), 1–15. 10.3389/falgy.2021.769728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- (60).Wolf M; Aglas L; Twaroch TE; Steiner M; Huber S; Hauser M; Hofer H; Parigiani MA; Ebner C; Bohle B; Briza P; Neubauer A; Stolz F; Wallner M; Ferreira F Endolysosomal Protease Susceptibility of Amb a 1 as a Determinant of Allergenicity. J. Allergy Clin. Immunol 2018, 141 (4), 1488–1491.e5. 10.1016/j.jaci.2017.10.027. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


