Abstract
Background:
Norovirus is the world-leading cause of acute gastroenteritis associated with severe symptoms and deaths. However, vaccines against norovirus are currently not available, and medications that specifically target human norovirus infection are still under development. The current study evaluated the virucidal and antiviral activities of epigallocatechin-3-gallate-palmitate (EC16), a compound derived from green tea polyphenols, against murine norovirus (MNV S99, a surrogate for human norovirus).
Method:
Initially, formulation suitability tests were conducted to compare EGCG (epigallocatechin-3-gallate), EC16 and tea polyphenol-palmitate in alcohol solution and hand hygiene formulations. The virucidal activity of EC16 was then tested in hand sanitizer gel and hand sanitizer foam formulations using a TCID50 time-kill suspension assay. In vitro treatment and prevention tests were performed using a 1-hour incubation of EC16 or EGCG with RAW264.7 cells, either post-infection or pre-infection with MNV. Statistical analysis employed two-tailed student t test (alpha=0.05).
Results:
Unlike EC16, both EGCG and tea polyphenol-palmitate showed auto-oxidation (color change) and precipitation in alcohol solution and hand hygiene formulations, and thus less suitable for potential hand hygiene product or new drug development. The time-kill suspension test results demonstrated that EC16 in both sanitizer gel and foam formulations reduced MNV by >99.99% (>log10 4) after 60 sec direct contact. One-hour incubation of EC16 with RAW264.7 cells either before or after MNV infection (i.e., without direct contact with MNV), resulted in >99% (>log10 2) reduction of MNV infectivity.
Conclusion:
EC16 is a candidate for use as a virucidal and antiviral compound to prevent and treat norovirus infection, with potential to be developed as a new drug against norovirus, pending in vivo and clinical tests.
Keywords: EGCG, EGCG-palmitate, green tea, norovirus, gastroenteritis, foodborne illness
Introduction
Nonenveloped viruses, including norovirus, rotavirus, enterovirus, adenovirus, and hepatitis A virus, are major causes of human morbidity and mortality (1). All are resistant to alcohol sanitization, a widely used method for reducing infections. Norovirus is the most common cause of acute gastroenteritis in the United States, associated with 19-21 million illnesses, 56,000-71,000 hospitalizations and 570-800 deaths annually (US CDC, Overview about Norovirus). Norovirus is responsible for the majority of viral gastroenteritis cases among children in a large pediatric hospital setting (2). Gastroenteritis caused by norovirus can be severe and costly to patients and healthcare providers (3). In developing countries, up to 200,000 children under 5 die each year due to norovirus (4).
Norovirus is the leading cause (58%) of foodborne illness in the United States. The majority (62%) of norovirus outbreaks occurred in long-term care facilities and hospitals, followed by restaurants, schools, and cruise ships (5). The cruise ship industry has been consistently impacted by norovirus outbreaks due to the high frequency of human to human contact and contaminated surfaces. A CDC study demonstrated that one cruise ship experienced large norovirus outbreaks during two consecutive 7-day Caribbean cruises. Despite the ship having undergone a one-week cleaning and sanitization procedure after the second cruise trip, acute viral gastroenteritis cases continued to occur in the subsequent four 7-day cruises, highlighting the difficulty in sanitizing a contaminated environment (6).
Despite a 2011 CDC update to the Guidelines for the Prevention and Control of Norovirus Gastroenteritis Outbreaks in Healthcare Settings, norovirus outbreaks are not declining (National Outbreak Reporting System, Outbreaks per year 2009-2018). As of today, there is no vaccine available for norovirus, and there is no specific therapeutic or prophylactic drug against norovirus that has received FDA approval. Efforts to develop antiviral agents against norovirus infection have identified several targets and approaches, including viral entry blockers, structural proteins, nonstructural proteins, protease inhibitors, and polymerase inhibitors (7, 8). There have been encouraging signs from a number of clinical studies. For example, the anti-protozoal drug nitrazoxanide showed significant reduction of norovirus symptom duration in the intervention group (six subjects with median 1.5 days) compared to the placebo group (seven subjects with median 2.5 days) (9). A case report showed nitrazoxanide successfully treated an immunocompromised patient (10). The broad-spectrum antiviral activity of nitrazoxanide has been suggested for treatment of respiratory viral infections such as influenza and COVID-19 (11, 12). However, results from a double-blind, placebo-controlled, randomized trial failed to show nitrazoxanide reduced the duration of hospital stay in severe influenza-like illness (13).
Green tea polyphenols, EGCG in particular, are known to possess antiviral properties (14). However, as strong antioxidants, water-soluble green tea polyphenols are relatively unstable and react readily with oxygen (15). We reported previously that epigallocatechin-3-gallate-palmitate (EC16), a lipid-soluble ester of EGCG, possesses antiviral and virucidal activities against a broad spectrum of human viruses, including herpes simplex virus (HSV), influenza virus, SARS-CoV-2, and surrogates of human norovirus (16-21). In fact, EC16 is a 44-fold more effective inhibitor of influenza A virus than EGCG (22). The unique feature of EC16 is that it is an amphipathic compound with a charged EGCG moiety and an ester linked hydrophobic palmitoyl tail, which forms an anchor on the cell membrane. The EGCG ester increases the compound’s stability and prevents rapid auto-oxidation or metabolism in the digestive system. This property enables EC16 to be a potential candidate agent for therapeutic and prophylactic use against norovirus and other human viruses. Another practical advantage is that EC16 is the major component of tea polyphenol-palmitate (lipid-soluble tea polyphenols referred to as LTP hereafter), an FDA recognized GRAS food additive (23).
A recent study in human intestinal enteroids demonstrated that pre-incubation of green tea extract and human norovirus for 1 h significantly reduced viral replication, consistent with previous reports using human norovirus surrogates (24). The current study evaluated the suitability of EC16, EGCG, and LTP for virucidal formulations based on auto-oxidation, and used a murine norovirus (MNV S99, a human norovirus surrogate) cell infection system to evaluate the virucidal activity of EC16 sanitizer formulations, and as a treatment and prevention model to examine in vitro the antiviral activity of EC16 in comparison to EGCG.
Materials and Methods
EGCG (>90%), EGCG-palmitate (>95%)(EC16), and tea polyphenol-palmitate (LTP) were purchased from Changxing Sanju Biotechnology Co., Ltd, Huzhou, China). Murine norovirus S99 strain was obtained from BioScience Laboratories, Bozeman, MT. The RAW264.7 (ATCC# TIB-71) cell line was purchased from American Type Culture Collection (ATCC, Manassas, VA). Carbopol Ultrez 20 gelling agent was obtained from Voyageur Soap and Candle Co (Surrey, BC Canada), and triethanolamine (TEA) from Carolina Biological Supplies (Burlington, NC). Fetal bovine serum (FBS) was from Atlas Biologicals, Inc. (Fort Collins, CO). Dulbecco’s Minimum Essential Media (DMEM) with high glucose, L-glutamine and sodium pyruvate was from GenDepot (Baker, TX). Cell culture plasticware was purchased from Southern Labware Inc. (Cumming, GA). For virucidal testing of EC16, the hand sanitizer gel was based on ProtecTeaV formulations described previously (20). The hand sanitizer foam was prepared using a commercially available formulation. Denatured ethanol SDA 3C (200 proof) was obtained from Inopak, Ltd. (Ringwood, NJ).
Infection of RAW264.7 cells, viral titer and TCID50 assay
RAW264.7 cells were cultured in DMEM supplemented with 10% fetal calf and 1X penicillin, streptomycin, and amphotericin B (100x from Corning, Corning, NY) at 37°C with 5% CO2. The viral infection assay was performed in 48 well cell culture plates using RAW264.7 cells that had reached 90% confluency. To obtain a viral titer, 50 μl of MNV S99 virus suspension was added to 450 μl DMEM containing 2% FBS (minimum medium, referred to MM hereafter) (i.e., 10−1 viral dilution). A series of 10-fold dilutions of this dilution up to 10−8 was made with MM. From each dilution, 100 μl were loaded in triplicates into a 48-well plate of cells, followed by 1 h incubation for viral absorption. The overlying liquid containing virus was then removed and replaced with MM. Incubation was continued for 48 h prior to beginning observation for cytopathic effect (CPE) through 4-7 days (the time required to complete the viral infection cycle after which no new CPE appears in the wells). The number of wells associated with CPE were entered into Reed & Muench TCID50 calculation calculator software to calculate viral titer and infectivity (25).
Hand sanitizer formulations
EC16, EGCG, or LTP was dissolved in 200 proof denatured ethanol at 10% (w/v) to make EC16, EGCG, and LTP alcohol solution stocks. For the ProtecTeaV hand sanitizer gel formulation, 0.8% (w/v) of EC16, EGCG and LTP were added to the base formulation, comprised of 75% ethanol v/v, 25% purified water containing 0.4% Ultrez 20 and 1 ppm FD&C Blue #1, with 0.04% TEA. For hand sanitizer foam formulation, a commercially available hand sanitizer foam containing 70% alcohol was adjusted to a 0.8% of EC16, EGCG or LTP by direct addition of powder form of the compounds to the foam formulation.
Formulation stability test of the hand sanitizer gel was conducted in 50 ml centrifuge tubes at 37°C in a tissue culture incubator with 90% humidity and 5% CO2 for 30 days (without light) before the tubes were removed for photography. The incubation was then continued for four months. For the ethanol stock solutions and hand sanitizer foam formulations, different formulations in 15 ml centrifuge tubes were placed in room temperature (22°C) for 7 days prior to photography, and continued for four months.
Virucidal time-kill suspension assay
A method similar to one described previously by our group was used (20). For each hand sanitizer formulation, 450 μl of the test formulation was placed in a plastic centrifuge tube, 50 μl of S99 virus was added and mixed for 60 sec. The mix was then immediately neutralized by dilution with MM (i.e., 10−2 viral dilution), and subsequent dilutions were made from this mix up to 10−8; as an untreated viral infectivity control a viral titer was simultaneously performed on the same plate with the tested formulations. The dilutions were loaded onto RAW264.7 cells and incubate for 1 h (250 μl per well, 3 repeats). After 1 hr absorption, the dilutions were removed, and cells were incubated for 48 h prior to initiation of CPE observation, and the virucidal activity rate determined as described above.
Viral Infectivity tests
A method for viral infectivity testing similar to one previously described by our group was used (20). Briefly, MM containing EC16 was prepared by dissolving EC16 in a sugar alcohol (referred to as “carrier” hereafter) and then diluting with a mixture of MM and carrier to 0.1% EC16 in 10% carrier. Being water-soluble, EGCG was added directly to MM to 0.1% immediately prior to use to minimize auto-oxidation. MM without EC16 or EGCG was used for viral titer control.
For the pre-infection (prevention) test, MM containing EC16 or EGCG was added to RAW264.7 cells in a 48 well plate and incubated for 1 h. The MM solution was then aspirated, followed by the addition of a series dilution of MNV S99 to 10−8 in MM (250 μl per well, 3 replicates per dilution). After 1 h absorption, the viral dilutions were replaced with MM, and cells were incubated at 37°C with 5% CO2. The viral infectivity rate was determined as described above in viral titer method
For the post-infection (treatment) test, a series dilution of MNV S99 in MM to 10−8 were incubated with RAW264.7 cells in a 48 well plate (250 μl per well, 3 replicates per dilution). After 1 h virus absorption, the wells were replaced with MM solutions containing EC16 or EGCG and incubated for 1 h. Then the EC16 and EGCG solutions were replaced with MM, the cell culture plates were placed in incubator at 37°C with 5% CO2 before infectivity was determined.
Statistical analyses were performed using two-tailed student t tests to compare group mean log10 reductions (alpha=0.05).
Results
Suitability of hand sanitizer formulations based on stability
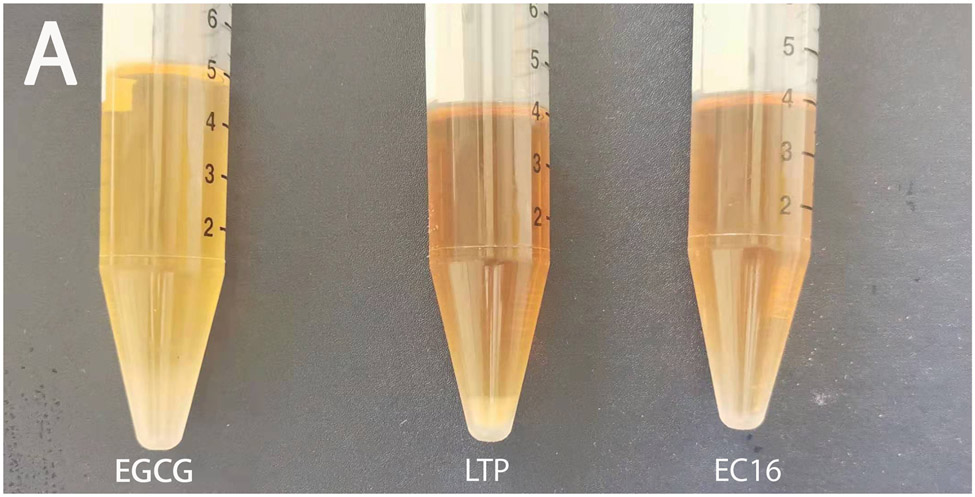
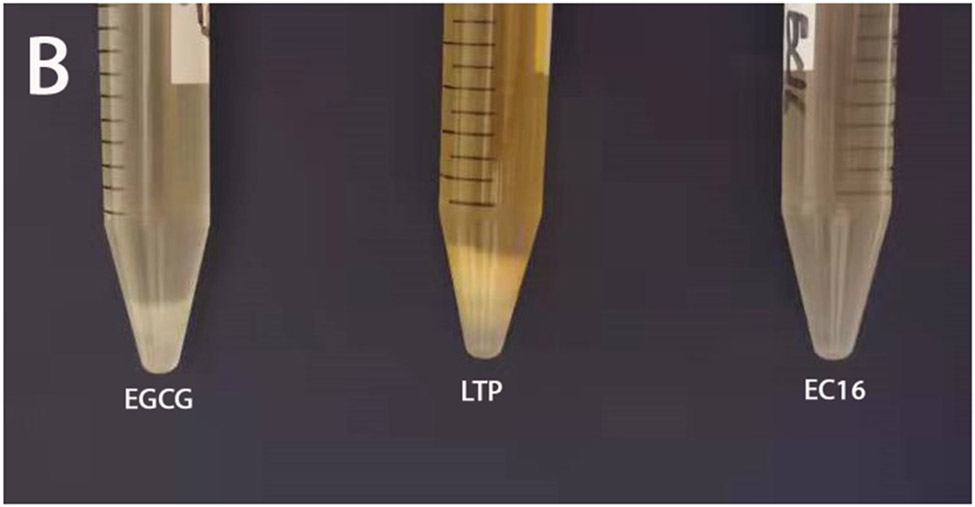
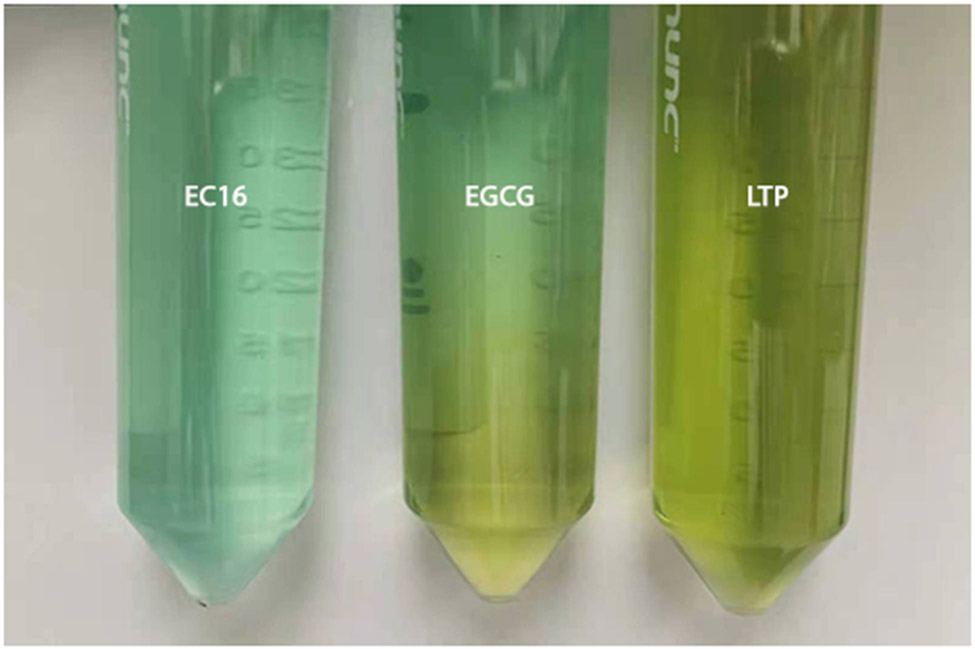
To test whether EC16, EGCG and LTP would be suitable for inclusion in hand sanitizer formulations, 10% w/v stock solutions of EC16, EGCG and LTP were prepared in 100% ethanol. Figure 1A shows both 10% EGCG and LTP formed a precipitate in the 100% ethanol stock solution over a 24 h period, while 10% EC16 remained as a clear solution in 100% ethanol (no precipitation observed after 4 months). In the 70% alcohol hand sanitizer foam formulation, both EGCG and LTP formed significant precipitation, while EC16 remained clear (no precipitation observed after 4 months) (Figure 1B). To observe auto-oxidation/coloration of EC16, EGCG and LTP, the compounds were used to make 75% ethanol ProtecTeaV hand sanitizer gel with 0.8% EC16, EGCG or LTP with added blue dye. These hand sanitizer gel samples were placed at 37°C for 30 days before observation. Figure 2 shows both the EGCG and LTP formulations had a significant color change from blue to brown/green. The EGCG hand gel also had uneven coloration. In contrast, the EC16 hand gel had no significant color change.
Figure 1.
A. Precipitations formed by EGCG and tea polyphenol palmitate, comparing to EC16, after the compounds were dissolved with 100% ethanol at 10% w/v concentration. And kept on the bench for 24 h. B. Precipitations formed by EGCG and LTP, comparing to EC16 after the compounds were mixed with a hand sanitizer foam formulation with 70% alcohol at 0.8% w/v and kept on the bench for 24 h. (EC16 did not form a visible precipitate even after four months; see text).
Figure 2.
Color changes of hand sanitizer gel containing EGCG and LTP in comparison to EC16 after the samples were incubated at 37° C for 30 days. The sample containing EC16 did not show a visible color change, while the sample containing EGCG presented an uneven color gradient with a light brown color in the bottom portion. The sample containing LTP shows an even color of brown/green.
Virucidal activity of EC16 hand sanitizer formulations
Based on the results from stability testing (see above), only ProtecTeaV hand sanitizer containing EC16 and the commercially available hand sanitizer foam containing EC16 were tested for virucidal activity. Direct contact virucidal activity time-kill suspension tests were performed using hand sanitizer gel and foam formulations containing 0.8% of compounds against MNV S99 with a 60 sec direct contact time. Results shown in Figure 3 demonstrated both the EC16 gel and foam hand sanitizers reduced MNV S99 by >4 log10 (>99.99% kill). For the gel and foam, the mean log10 reductions were respectively 4.11 (n=6, SD=1.05) and 4.30 (n=6, SD=0.59), corresponding to geometric mean fold reductions of 99.992 and 99.995%. Both were significantly greater than log10 0 (no reduction)(p<0.0002; one-sample t-test). There was no significant difference in virucidal activity between the two formulations (two-tailed student t test, p=0.107). The result was consistent with our previously published data that evaluated ProtecTeaV hand sanitizer gel formulations against feline calicivirus (20). The virucidal activity of ProtecTeaV hand sanitizer gel containing EC16 against MNV S99 was subsequently validated by an independent GLP laboratory (to be published elsewhere).
Figure 3.
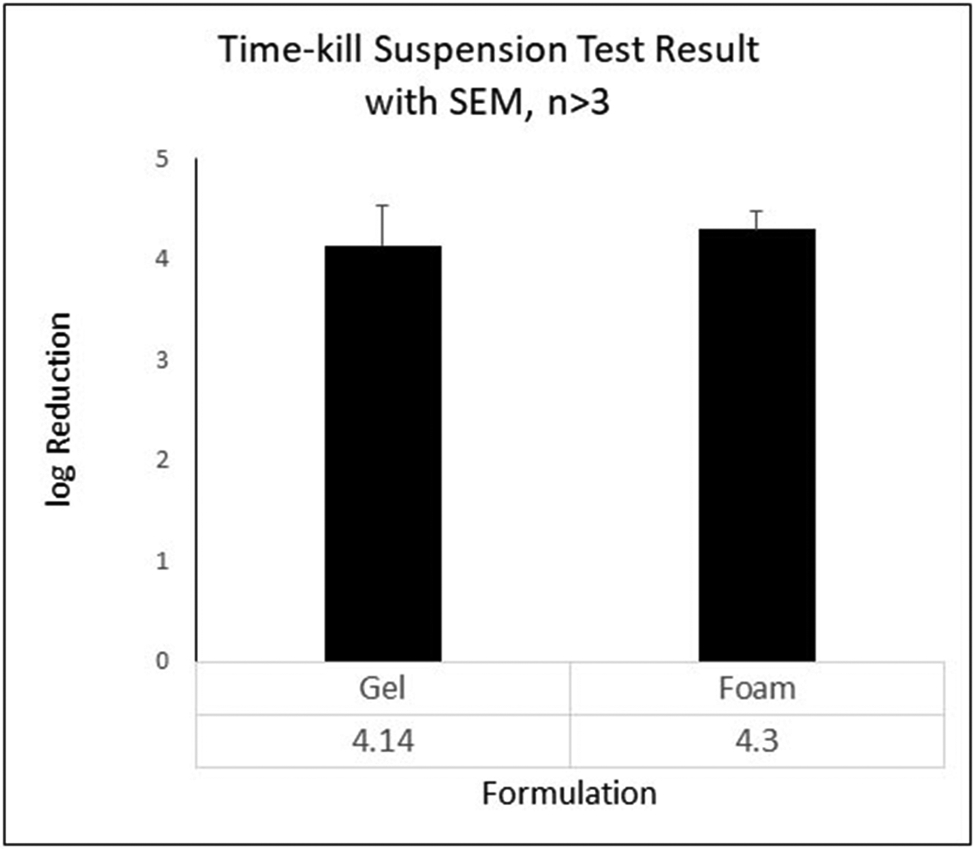
Results of 60 sec time-kill suspension tests of the hand sanitizer gel (left) and foam (right) containing 0.8% EC16. Both formulations (80% strength in test settings) gave a mean log10 reduction >4 of MNV S99 CPE. Data were obtained from 6 (sanitizer) and 12 (foam) independent tests. Bars show SEM. There was no statistical difference between the two hand sanitizer formulations (two-tailed student t test, p=0.107).
Reduction of MNV S99 infectivity without direct contact
Pre-infection treatment without direct contact with MNV S99
To test for a prevention effect on infection, RAW264.7 cells were incubated with MM containing 0.1% w/v EC16 or EGCG for 1 h, and after removing the agent the cells were infected with a series dilution of MNV S99 and CPE was quantified. Figure 4 shows that in three independent experiments pre-treatment with both EC16 and EGCG resulted in a reduction of the log10 CPE. EGCG led to an approximately 9-fold reduction (log10 0.89, SD=1.07) of MNV S99, and EC16 caused about a 100-fold reduction (log10 2.11, SD=0.51). The fold reduction due to EC16 was significantly greater than log10 0 (no reduction)(one-sample t-test, p=0.019; Bonferroni correction to alpha (two tests) 0.0253), whereas the reduction due to EGCG was not (p=0.29). There was no significant difference between the two groups (paired t-test)(p=0.30).
Figure 4.
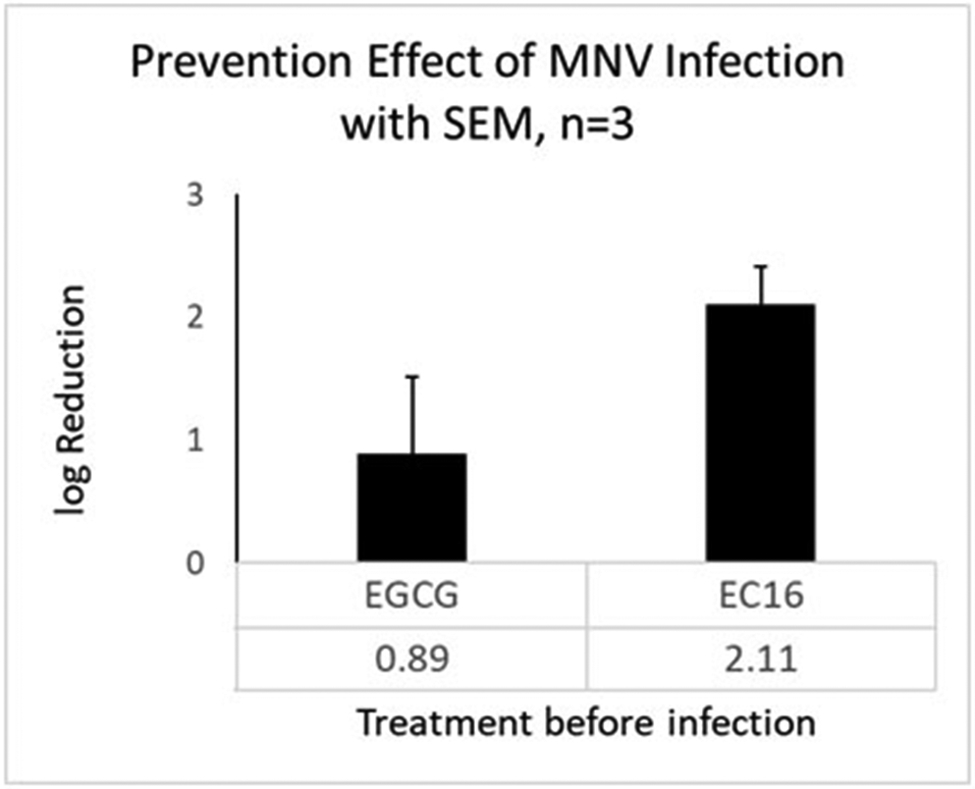
MNV S99 infectivity in RAW264.7 cells pre-treated with 0.1% EGCG (left) and EC16 (right) in MM for 1 h. EGCG resulted in an average of log10 0.89 reduction of viral infectivity (less than 10-fold). EC16 led to an average of log10 2.11 reduction of viral infectivity (more than 100-fold, or >99%). Data were obtained from three independent experiments. Bars show SEM.
Post-infection treatment without direct contact with MNV S99
To test for a treatment effect, MM containing 0.1% w/v EC16 or EGCG was added to RAW264.7 cells that had been infected for 1 h by a series dilution of MNV S99. After 1 h treatment, the MM containing EC16 or EGCG was replaced with MM without EC16 or EGCG, followed by quantitation of CPE. Figure 5 shows that a log10 reduction resulted from EC16 and EGCG in three independent experiments. EGCG led to an approximately 10-fold reduction (log10 1.11, SD=0.51) of MNV S99, and EC16 caused about 100-fold reduction (log10 2.03, SD=0.39). The reduction due to EC16 was significant (one-sample t-test, p=0.012), whilst the reduction due to EGCG was not (p=0.063). The two treatment groups were significantly different in the log10 reduction (paired t-test, p=0.047).
Figure 5.
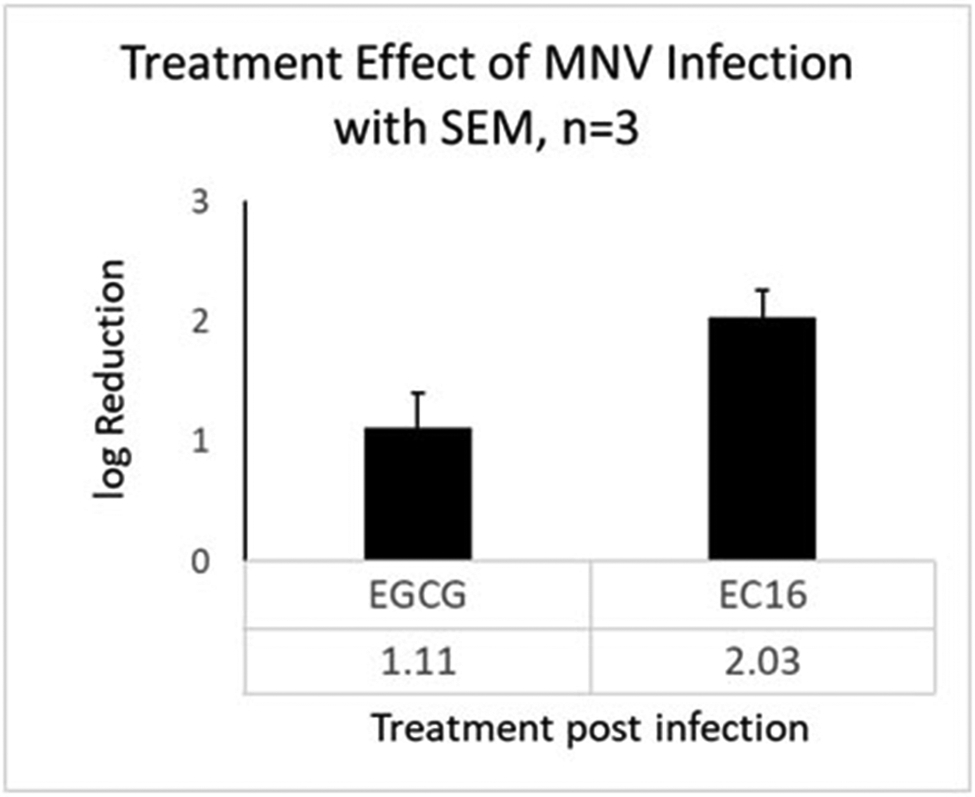
Results of post-infection treatment of 0.1% EGCG (left) and EC16 (right) in MM. RAW264.7 cells were infected by MNV S99 for 1 h prior to media change to 1 h incubation of MM containing EGCG of EC16. EGCG resulted in an average of log10 1.11 reduction of viral infectivity (>10-fold). EC16 led to an average of log10 2.03 reduction of viral infectivity (more than 100-fold, or >99%). Data were obtained from three independent experiments. Bars show SEM.
Discussion
Unlike various other human viruses, human norovirus is difficult to culture in vitro and the propagation yield is relatively low. Researchers have therefore relied on porcine enteric calicivirus, Tulane virus, feline calicivirus (FCV), and murine norovirus (MNV) as surrogates for human norovirus to conduct in vitro and in vivo studies or tests for virucidal products (26). An animal model has been created to closely resemble human norovirus symptoms using the interferon receptor deficient mouse strain STAT1−/−. In vivo studies using this model showed that infection with MNV induced symptoms similar to those of human norovirus disease (27, 28). This established model could be applied to proof-of-concept studies toward new drug development against human norovirus disease and prevention once suitable candidates have been identified by in vitro testing.
The current study evaluated the suitability of EC16, EGCG and LTP for hand hygiene-disinfectant alcohol formulations using direct contact time-kill tests, and also the effects of EC16 and EGCG in vitro for treatment and prevention using the cell culture MNV infection system. Figure 1A demonstrates that while EC16 formed a clear solution in 100% ethanol, both EGCG and LTP formed a precipitate in 100% ethanol. This result suggests that EGCG and certain components of LTP either have relatively low solubility in the organic solvent, or rapidly form precipitating products, and therefore may not be suitable for alcohol-based formulations. Even when the water content was increased to 30% in a hand sanitizer foam formulation, both EGCG and LTP still formed a precipitate (Figure 1B). Further, in a water-containing hand sanitizer gel formulation, both EGCG and LTP demonstrated chemical instability as evidenced by a color change within 30 days at 37°C (Figure 2). The color change and auto-oxidation of EGCG has been well-documented previously (29). In fact, EGCG dissolved in phosphate buffer saline at 37°C completely converts to unstable EGCG auto-oxidation products (EAOPs) within 4 hours (29). In contrast, EC16 appeared to be stable in the hand sanitizer gel formulation without significant color change (Figure 2). Since LTP is a mixture of palmitate esters of different green tea polyphenols, its stability could be reduced by polyphenol esters other than EC16. Therefore, EC16 was deemed best suited for alcohol-based formulations used in hand hygiene and surface disinfectant products. Indeed, both hand sanitizer gel and foam containing EC16 resulted in a >99.99% (>log10 4) reduction of MNV S99 infectivity in 60 sec direct contact time-kill suspension tests (Figure 3). These results were consistent with our previously reported test results that showed >99.99% (>log10 4) time-kill reduction of feline calicivirus, another surrogate for human norovirus (20). Further, they have been confirmed by an independent GLP laboratory (data not shown, manuscript in preparation).
The advantage of EC16 in comparison to EGCG was also evident in both pre and post-infection assays designed to evaluate prevention and intervention effects using the MNV S99 cell infection system, without direct contact of the agent with the virus. A significant >log10 2 reduction in viral infectivity was achieved with a single dose of 0.1% EC16 treatment of RAW264.7 cells either before or after MNV S99 infection (Figures 4 and 5). That is, a one-hour pre-treatment of cells with 0.1% EC16 was able to block >99% of MNV S99 infection without direct contact with the virus, and a one-hour incubation of infected RAW264.7 cells with 0.1% EC16 reduced MNV S99 replication by >99%. In contrast, the water-soluble EGCG only reduced infection by approximately 10-fold under the same conditions, and the effect was not significant. The mechanisms underlying these differences could be due to the chemical characteristics of the two agents. The long chain fatty acyl group of EC16 not only stabilizes the EGCG from auto-oxidation, but also enables EC16 to attach to the cell membrane for a relatively long-term effect. If EC16 attaches to the epithelial cells in the digestive system, the antiviral effect would be superior to EGCG, which does not possess a hydrophobic moiety for membrane anchoring. In addition, EC16 more readily permeates the cell membrane, and therefore could enter the cytoplasm and perform in situ antiviral functions. We previously showed that esters of EGCG with either saturated or monounsaturated fatty acids are able to enter the cytoplasm of human epidermal keratinocytes and release free EGCG by intracellular esterase-mediated hydrolysis (30). There is a difference in the time of release depending on the fatty acid; the saturated fatty acyl EGCG ester (EGCG-stearate) released most free EGCG in 6 h, while the monounsaturated EGCG ester (EGCG-oleate) only took 1 h (30). This difference could be due to the flexibility of oleate compared to a more rigid structure of stearate to penetrate the double lipid layer of the cell membrane. EC16 is an ester of palmitate, a fully saturated fatty acid with 16 carbons. EC16 is likely to attach to the cell membrane via the palmitate moiety as anchor and slowly enter the cytoplasm prior to release of free EGCG, thereby exerting a prolonged antiviral effect (31). Compared to the hydrophilic EGCG, EC16 could have a significant duration of attachment on the cell membrane, and only gradually enter the cytoplasm. In addition to stability, EC16 has a significantly higher bioavailability than EGCG (32) and less toxicity than EGCG (33). Thus, although both EC16 and EGCG are able to bind to cell receptors to inhibit viral attachment (34), EC16 is a better candidate for therapeutic and prophylactic use against norovirus infection.
Here we report, for the first time, that the antiviral activity of EC16 could be used in prevention and treatment of norovirus infection, pending future proof-of-concept in vitro and in vivo studies prior to human trials. In addition, the virucidal property of EC16 is suitable for use in hand hygiene and disinfectant products. It was also found that EGCG has lower antiviral activity comparing to EC16, and it is not suitable for virucidal formulations due to rapid auto-oxidation.
Acknowledgement
This work was supported in part by NIH grant R42AI124738 to Camellix, LLC and Augusta University Research Institute. The authors thank Lester Sampath for his expertise and advice in the formulations and tests. Research reported in this publication was supported by the National Institute Of Allergy And Infectious Diseases of the National Institutes of Health under Award Number R42AI124738. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References Cited
- 1.Rodríguez-Lázaro D, Cook N, Ruggeri FM, Sellwood J, et al. Virus hazards from food, water and other contaminated environments. FEMS Microbiol Rev. 2012; 36: 786–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cunliffe NA, Booth JA, Elliot C, et al. Healthcare-associated viral gastroenteritis among children in a large pediatric hospital, United Kingdom. Emerg Infect Dis. 2010; 16: 55–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piednoir E, Bessaci K, Bureau-Chalot F, et al. Economic impact of healthcare-associated rotavirus infection in a paediatric hospital. J Hosp Infect., 2003; 55: 190–5. [DOI] [PubMed] [Google Scholar]
- 4.Patel MM, Widdowson MA, Glass RI, et al. Systematic literature review of role of noroviruses in sporadic gastroenteritis. Review Emerg Infect Dis. 2008;14: 1224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Vega E, Barclay L, Gregoricus N, et al. Genotypic and Epidemiologic Trends of Norovirus Outbreaks in the United States, 2009 to 2013. J Clin Microbiol. 2014; 52: 147–155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Isakbaeva ET, Widdowson MA, Beard RS, et al. Norovirus Transmission on Cruise Ship. Emerg Infect Dis. 2005; 11: 154–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Netzler NE, Tuipulotu DE, and White PA. Norovirus antivirals: Where are we now? Med Res Rev. 2019; 39: 860–886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ali ES, Rajapaksha H, Carr JM, et al. Norovirus drug candidates that inhibit viral capsid attachment to human histo-blood group antigens. Antiviral Res. 2016; 133: 14–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rossignol JF and El-Gohary YM. Nitazoxanide in the treatment of viral gastroenteritis: a randomized double-blind placebo-controlled clinical trial. Clinical Trial Aliment Pharmacol Ther. 2006; 24:1423–30. [DOI] [PubMed] [Google Scholar]
- 10.Siddiq DM, Koo HL, Adachi JA, et al. Norovirus gastroenteritis successfully treated with nitazoxanide. Case Reports J Infect. 2011; 63: 394–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lokhande AS and Devarajan PV. A review on possible mechanistic insights of Nitazoxanide for repurposing in COVID-19. Eur J Pharmacol. 2021; 891: 173748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahmoud DB, Shitu Z, and Mostafa A. Drug repurposing of nitazoxanide: can it be an effective therapy for COVID-19? J Genet Eng Biotechnol. 2020; 18: 35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gamiño-Arroyo AE, Guerrero ML, McCarthy S, et al. Efficacy and Safety of Nitazoxanide in Addition to Standard of Care for the Treatment of Severe Acute Respiratory Illness. Clin Infect Dis. 2019; 69: 1903–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu J, Xu Z, Zheng W. A Review of the Antiviral Role of Green Tea Catechins. Molecules. 2017; 22: 1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu S. Compounds derived from epigallocatechin-3-gallate (EGCG) as a novel approach to the prevention of viral infections. Inflammation & Allergy - Drug Target. 2015; 14: 13–18. [DOI] [PubMed] [Google Scholar]
- 16.Zhao M, Jiang J, Zheng R, Fu B, Pearl H, Dickinson D, and Hsu S. A proprietary topical preparation containing EGCG-stearate and glycerin with inhibitory effects on herpes simplex virus: case report. Inflammation & Allergy – Drug Targets. 2012; 11: 364–368. [DOI] [PubMed] [Google Scholar]
- 17.Zhao M, Zheng R, Jiang J, et al. Topical Lipophilic EGCG on Herpes Labialis: A Phase II Clinical Trial of AverTeaX. OOOO, 2015; 120: 717–724. [DOI] [PubMed] [Google Scholar]
- 18.de Oliveira A, Adams SD, Lee LH, et al. Inhibition of herpes simplex virus type 1 with the modified green tea polyphenol palmitoyl-epigallocatechin gallate. Food Chem Toxicol. 2012; 52: 207–215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dickinson D, Xayaraj S, Dickinson S, et al. Effect of Novel Formulations using Lipophilic Epigallocatechin-3-Gallate against Influenza Virus Infection. Microbiology & Infectious Diseases, 2018; 2: 1–8. [Google Scholar]
- 20.Widjaja N, Dickinson D, Shao X, et al. Persistent Virucidal Activity in Novel Alcohol-Based Sanitizer Formulation (ProtecTeaV) for Potential Use Against Norovirus. Microbiology & Infectious Diseases, 2018; 2: 1–8. [Google Scholar]
- 21.Hurst BL, Dickinson D, Hsu S. Epigallocatechin-3-Gallate (EGCG) Inhibits Sars-Cov-2 Infection in Primate Epithelial Cells. Microbiol Infect Dis. 2021; 5: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Mori S, Miyake S, Kobe T, et al. Enhanced anti-influenza A virus activity of (−)-epigallocatechin-3-O-gallate fatty acid monoester derivatives: effect of alkyl chain length. Bioorg Med Chem Lett. 2008; 18: 4249–4252. [DOI] [PubMed] [Google Scholar]
- 23.USFDA GRAS Notice 772: GRAS Notice for Oil-Soluble Green Tea Extract (Green Tea Catechin Palmitate). 2019. PDF: https://www.fda.gov/media/126906/download. FDA “no question (approval)” letter: https://www.fda.gov/media/120299/download [Google Scholar]
- 24.Randazzo W, Costantini V, Morantz EK, et al. Human Intestinal Enteroids to Evaluate Human Norovirus GII.4 Inactivation by Aged-Green Tea. Front Microbiol. 2020; 11: 1917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lindenbach BD. Measuring HCV infectivity produced in cell culture and in vivo. Methods Mol Biol. 2009; 510: 329–336. [DOI] [PubMed] [Google Scholar]
- 26.Rocha-Pereira J, Neyts J, and Jochmans D. Norovirus: Targets and tools in antiviral drug discovery. Biochem Pharmacol. 2014; 91: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kahan SM, Liu G, Reinhard MK, et al. Comparative murine norovirus studies reveal a lack of correlation between intestinal virus titers and enteric pathology. Virology. 2011; 421: 202–210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Strong DW, Thackray LB, Smith TJ, et al. Protruding domain of capsid protein is necessary and sufficient to determine murine norovirus replication and pathogenesis in vivo. J Virol. 2012; 86: 2950–2958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wei Y, Chen P, Ling T, et al. Certain (−)-epigallocatechin-3-gallate (EGCG) auto-oxidation products (EAOPs) retain the cytotoxic activities of EGCG. Food Chem. 2016; 204: 218–226. [DOI] [PubMed] [Google Scholar]
- 30.Chen P, Dickinson D, and Hsu S. Lipid-soluble green tea polyphenols: stabilized for effective formulations. Green Tea and Health Research. In Helen McKinley and Mark Jamieson (Eds.). Handbook of Green Tea and Health Research. (2009) 45–61. Nova Publishers. Hauppauge, NY. USA. [Google Scholar]
- 31.Cai ZY, Li XM, Liang JP, et al. Bioavailability of Tea Catechins and Its Improvement. Molecules. 2018; 23: 2346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dai W, Ruan C, Zhang Y, et al. Bioavailability enhancement of EGCG by structural modification and nanodelivery: A review. Journal of Functional Foods. 2020; 65: 103732. [Google Scholar]
- 33.Zhou F, Xu S, Shen T, et al. Antioxidant effects of lipophilic tea polyphenols on diethylnitrosamine/phenobarbital–induced hepatocarcinogenesis in rats. In Vivo, 2014; 28: 495–503. [PubMed] [Google Scholar]
- 34.Falco I, Randazzo W, Rodriguez-Diaz J, et al. Antiviral activity of aged green tea extract in model food systems and under gastric conditions. Int. J. Food Microbiol 2019; 2: 101–106. [DOI] [PubMed] [Google Scholar]