Abstract
Objective:
To evaluate the potential link between serum LH concentrations on the day of oocyte triggering and pregnancy outcome during controlled ovarian hyperstimulation.
Materials and Methods:
In this retrospective cross-sectional study, data of women ≤42 years undergoing fresh embryo transfer cycles and who had downregulated with GnRH antagonist protocol in a 12-month period was reviewed. Patients with incomplete hospital records were excluded. Women were divided into four groups based on the percentiles of the serum LH level on the day of oocyte triggering: <1.49 (<25th percentile), 1.49–2.59 (25–50th percentile), 2.60–4.60 (50–75th percentile), and >4.60 IU/L (>75th percentile). Clinical pregnancy was considered the primary outcome, while chemical pregnancy and implantation rate were the most important secondary outcomes which were compared between the four groups.
Results:
Four hundred and nighty-three women of 1003 infertile women, who were initially assessed for eligibility, met the inclusion criteria. Finally, 426 women were analyzed. Levels of progesterone were significantly correlated with the level of LH on the day of trigger in the >4.60 IU/L group (r = 0.20, P = 0.034). Furthermore, the levels of estradiol were significantly correlated with the level of LH on the day of trigger in the <1.49 IU/L (r = 0.21, P = 0.026). The number of retrieved oocytes, 2PNs (two pronucleis), number, and quality of total embryos were similar between groups (P > 0.05). With regard to oocyte maturity rate, fertilization proportion, fertilization rate, chemical pregnancy rate, and clinical pregnancy rate, there was no difference between varied LH levels in the four groups (P > 0.05). The only observed difference was the implantation rate that was significantly higher in the 2.60–4.60 IU/L group than the <1.49 IU/L group (P < 0.05).
Conclusions:
Our result could not show the potential link between LH concentrations during GnRH antagonist cycles and pregnancy outcomes. However, very low LH levels during ovarian stimulation period may negatively affect the implantation rate.
Keywords: ART, GnRH antagonist, luteinizing hormone, ovarian stimulation, pregnancy outcome
INTRODUCTION
Although assisted reproductive technology (ART) and some important factors involved in its improvement were developed quickly, there are still several controversies in the field of controlled ovarian hyperstimulation (COH). During COH, the prevention of the premature luteinizing hormone (LH) rise is critical for reasonable ART outcomes.[1] Conventionally, gonadotropin-releasing hormone (GnRH) agonists were used to avoid the premature surge of endogenous LH before the maturation of follicles.[2] Recently, it has been revealed that GnRH antagonists can induce a rapid and adjustable suppression of LH release.[1] Moreover, previous studies showed the important effects of LH levels during ovarian stimulation protocols on the follicular growth as well as clinical outcome.[3,4] Indeed, the rise and fall of LH levels during the follicular phase have a negative impact on oocyte quality and its consequent fertilization capacity.[5] Even though the LH concentration differs significantly in response to ovarian stimulation protocols from patients to patients,[6] it has been reported that LH levels were declined to <0.5 IU/L after suppression during the late phases of stimulation.[7] However, most patients attained sufficient ovarian response with the aforementioned LH level;[7] the other studies indicated that optimal follicular development occurs at the LH concentrations between 1.2 and 5.0 IU/L.[5,8]
The aim of the current study was to assess the potential link between the LH levels on the day of oocyte triggering and ovarian response in addition to ART outcome after ovarian hyperstimulation with GnRH antagonist.
MATERIALS AND METHODS
Subjects
This retrospective cross-sectional study was conducted at Yazd Reproductive Sciences Institute. The study was confirmed by the Ethics Committee of Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences (IR.SSU.RSI.REC.1397.015). Informed consent was not obtained due to the retrospective nature of the study. The data of 426 women who sought infertility treatment at the infertility clinic of Yazd Reproductive Sciences Institute, Yazd, Iran was consecutively reviewed from February 2018 to February 2019 using the census method. They were candidates for in vitro fertilization (IVF) or intracytoplasmic sperm injection (ICSI). The women were eligible to include the study if they were ≤42 years; those undergoing fresh embryo transfer cycles and who had downregulated with GnRH antagonist protocol. Patients with complete hospital records were included. All information included women's age along with type; cause and duration of infertility and also the ART method was gathered. Furthermore, the hormone levels LH, anti-Müllerian hormone (AMH), estradiol (E2), and progesterone were collected. All laboratory information in terms of number of retrieved oocytes, metaphase II (MII) oocytes, two pronuclear (2PNs), and the number and quality of obtained and transferred embryos were noted. The patients were followed up until clinical pregnancy. Based on the percentiles of LH levels on the day of trigger, women were assigned into four groups: group 1: LH <25th percentile (<1.49 IU/L), group 2: LH = 25–50th percentile (1.49-2.59 IU/L), group 3: LH = 50–75th percentile (2.60–4.60), and group 4: LH >75th percentile (>4.60 IU/L).[9,10,11] The number of women in each group was 106, 109, 105, and 106, respectively.
Treatment protocol
The starting dose of gonadotropin had been adjusted according to patient's age, basal AMH, body mass index, and also ovarian response, which assessed using serial transvaginal ultrasonography during COH. From 150 to 300 IU/day recombinant human follicle stimulating hormone (rFSH, Gonal-F, Merck Serono S.A., Switzerland) was administered subcutaneously for 5 days. Once the follicular maturation occurred and a leading follicle (≥14 mm) was detected in ultrasonography, 0.25 mg per day of subcutaneous GnRH antagonist (Cetrotide, Merck Serono, Germany) was injected. Oocyte triggering was done using 1500 IU human chorionic gonadotropin (hCG) (Pregnyl, Organon, Netherland) and 0.2 mg of subcutaneous GnRH-a (Decapeptyl, 0.1 mg) when at least two follicles with a mean diameter of 17 mm were observed in ultrasonography. Oocyte retrieval was performed 36 h later by follicle aspiration through transvaginal ultrasound-guided single-lumen needle aspiration. A maximum of two embryos of good or excellent quality were transferred 48–72 h after oocyte retrieval, using an embryo transfer Labotect catheter (Labor-Technik-Göttingen GmbH, Gottingen, Germany). Embryo grading was as follows from A (the best) to D (the worst) quality. Grade A: embryos with at least seven blastomeres (seven to nine blastomeres) and maximum of 20% cytoplasmic fragmentation; Grade B: embryos with seven to nine cells and over 20% fragmentation; Grade C: embryos with four to six cells and a maximum of 20% fragmentation; and Grade D: embryos with four to six cells and over 20% fragmentation.[12] All women received Cyclogest Ovaginal pessaries (Cox Pharmaceuticals, Barnstaple, UK) 400 mg twice daily for luteal phase support until the start of menstruation or for 8 weeks following embryo transfer in women who get clinical pregnancy.
Outcome parameters
Clinical pregnancy was measured as the primary outcome and defined by detecting fetal heart activity in transvaginal ultrasonography 5 weeks after positive beta hCG. Secondary outcomes include chemical pregnancy that was considered as serum beta hCG >50 IU/L, 14 days after embryo transfer; implantation rate which was calculated as the number of intrauterine gestational sacs observed by transvaginal ultrasonography divided by the total number of transferred embryos; fertilization rate by means of the number of 2PNs divided by the total number of MII oocytes; and fertilization proportion as the number of 2PNs divided by the number of oocytes retrieved.
Hormone assays
A venous blood sample was collected from all participants and serum LH; progesterone and E2 levels were measured on the day of trigger. The hormonal assays were done by a commercial ELISA kit (JTC Diagnosemittel UG, Voehl, Germany) for LH with an intraassay coefficient of variation of <5% and interassay coefficient of variation of <7.1%. E2 and progesterone levels were assessed using ELISA kit (AccuBind® ELISA, CA, USA) based on the kit instruction. Intraassay coefficient of variation for E2 was <10%; interassay coefficient of variation was <4.9%. Also, intraassay coefficient of variation for progesterone was <15.4%; interassay coefficient of variation was <9%.
Statistical analysis
Statistical analysis was performed using the statistical package for the social science version 20 for windows (SPSS Inc., Chicago. IL, USA). Between-group differences without normal distribution were calculated by Kruskal–Wallis test. Differences between noncontinuous variables were analyzed using the Chi-square test. The Spearman's rank correlation was used to evaluate the relationships between AMH, estradiol and progesterone concentrations and LH levels. The significance level was set equal to 0.05. The test power was determined 90% using power analysis and sample size software 15 (NCSS, LLC, USA).
RESULTS
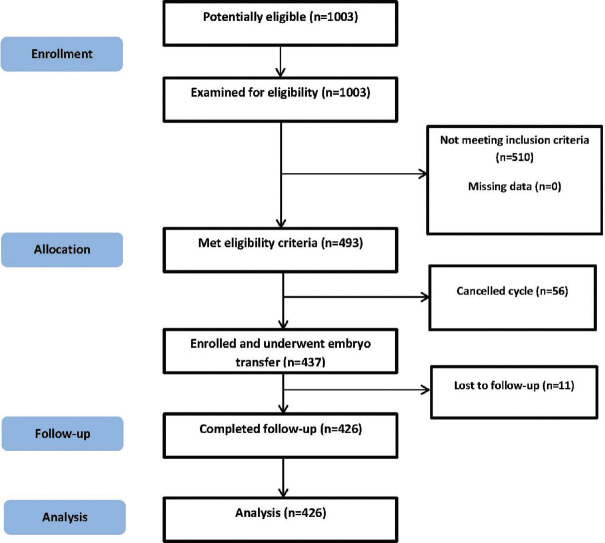
A total of 1003 infertile women were supposed to participate in the study and were assessed for eligibility criteria. Of those, 493 women met inclusion criteria. Among women undergoing IVF/ICSI cycles, 56 women were excluded due to the risk of ovarian hyperstimulation, lack of good-quality oocytes for injection, or suitable embryos for transfer. Eleven women were lost to follow-up and finally 426 women were analyzed [Figure 1]. Patient characteristics in four study groups are listed in Table 1. There were no differences among the groups regarding age (P = 0.915), duration of infertility (P = 0.257), infertility type (P = 0.471), and method of ART (P = 0.145). The mean levels of AMH, estradiol, and progesterone were significantly higher in the >4.60 IU/L group compared with the other groups (P = 0.001). In addition, Spearman's rank correlation test showed a highly significant correlation between estradiol and progesterone with LH in all women (r = 0.20, P = 0.000; r = 0.18, P = 0.000, respectively). However, among groups, the levels of progesterone were significantly correlated with the level of LH on the day of trigger in the >4.60 IU/L group (r = 0.20, P = 0.034). Furthermore, the levels of estradiol were significantly correlated with the level of LH on the day of trigger in the <1.49 IU/L (r = 0.21, P = 0.026). The number of retrieved oocytes, 2PNs (two pronucleis), and number and quality of total embryos were similar between groups (P > 0.05) [Table 2]. With regard to oocyte maturity rate, fertilization proportion, fertilization rate, chemical pregnancy rate, and clinical pregnancy rate, there was no difference between varied LH levels in the four groups (P > 0.05) [Table 3].
Figure 1.
Flowchart of participants’ enrollment, allocation, follow-up, and analysis
Table 1.
Patients’ characteristics based on the LH concentration
| Variable | Concentration of LH (IU/l) | P | |||
|---|---|---|---|---|---|
|
| |||||
| <1.49 (n=106) | 1.49-2.59 (n=109) | 2.60-4.60 (n=105) | >4.60 (n=106) | ||
| Age (yr)* | 31.22±4.17 | 31.25±5.25 | 31.61±5.03 | 30.95±4.53 | 0.915 |
| Duration of infertility (yr)* | 6.03±3.79 | 7.24±4.75 | 6.90±4.08 | 7.00±4.46 | 0.257 |
| Infertility type† | |||||
| Primary | 80 (75.5) | 87 (79.8) | 74 (70.5) | 79 (74.5) | 0.471 |
| Secondary | 26 (24.5) | 22 (20.2) | 31 (29.5) | 27 (25.5) | |
| Infertility diagnosis† | |||||
| Male factor | 57 (53.8) | 44 (40.4) | 31 (29.5) | 38 (35.8) | 0.013 |
| PCOS | 9 (8.5) | 13 (11.9) | 23 (21.9) | 25 (23.6) | |
| Tubal factor | 1 (0.9) | 1 (0.9) | 4 (3.8) | 1 (0.9) | |
| Unexplained | 13 (12.3) | 21 (19.3) | 17 (16.2) | 14 (13.2) | |
| Mixed | 19 (17.9) | 20 (18.3) | 23 (21.9) | 20 (18.9) | |
| Endometriosis | 4 (3.8) | - | 2 (1.9) | 3 (2.8) | |
| Decreased ovarian reserve | 3 (2.8) | 10 (9.2) | 5 (4.8) | 5 (4.7) | |
| ART method† | |||||
| IVF | 14 (13.2) | 7 (6.4) | 11 (10.5) | 19 (17.9) | 0.145 |
| ICSI | 90 (84.9) | 102 (93.6) | 92 (87.6) | 86 (81.1) | |
| Mixed | 2 (1.9) | - | 2 (1.9) | 1 (0.9) | |
*Data are presented as mean±SD, Kruskal-Wallis test. †Data are presented as number (%), Chi-square test. PCOS: Polycystic ovary syndrome; ART: Assisted reproductive technology; IVF: In vitro fertilization; ICSI: Intracytoplasmic sperm injection
Table 2.
Cycle characteristics and laboratory data based on LH concentration
| Variable | Concentration of LH (IU/l) | P | |||
|---|---|---|---|---|---|
|
| |||||
| <1.49 (n=106) | 1.49-2.59 (n=109) | 2.60-4.60 (n=105) | >4.60 (n=106) | ||
| No. of oocytes retrieved* | 7.84±4.02 | 6.88±3.41 | 7.35±3.50 | 7.46±3.65 | 0.247 |
| No. of MII oocytes* | 6.44±3.30 | 5.44±2.93 | 5.97±3.26 | 5.92±3.27 | 0.172 |
| No. of 2PNs* | 4.34±2.71 | 3.60±2.26 | 3.85±2.41 | 3.97±2.32 | 0.197 |
| No. of total embryos* | 3.78±2.51 | 3.20±2.20 | 3.43±2.24 | 3.16±2.08 | 0.183 |
| No. of transferred embryos* | 1.83±0.36 | 1.75±0.45 | 1.77±0.42 | 1.79±0.43 | 0.444 |
| Quality of embryo transferred† | |||||
| A | 29 (27.4) | 23 (21.1) | 37 (35.2) | 25 (23.6) | 0.299 |
| B | 62 (58.5) | 62 (56.9) | 51 (48.6) | 62 (58.5) | |
| C | 14 (13.2) | 23 (21.1) | 14 (13.3) | 18 (17) | |
| D | 1 (0.9) | 1 (0.9) | 3 (2.9) | 1 (0.9) | |
| AMH (ng/mL)* | 4.24±2.89 | 3.67±2.71 | 4.47±2.57 | 4.94±3.29 | 0.001 |
| Estradiol on the day of trigger (pg/mL)‡ | 930 (IQR=862) | 1026 (IQR=689) | 1156 (IQR=754) | 1297 (IQR=1098) | 0.001 |
| Progesterone on the day of trigger (ng/mL)* | 0.49±0.48 | 0.45±0.55 | 0.62±0.92 | 0.69±0.50 | 0.000 |
*Data are presented as mean±SD, Kruskal-Wallis test. †Data are presented as number (%), Chi-square test. ‡Data are presented as median (IQR). MII: Metaphase II; 2PN: two pronuclear; quality of embryos A-D as described in materials and methods; AMH: anti-Mullerian hormone; IQR: interquartile range
Table 3.
The outcome of ovarian stimulation based on LH concentration
| Variable | Concentration of LH (IU/l) | P | |||
|---|---|---|---|---|---|
|
| |||||
| <1.49 (n=106) | 1.49-2.59 (n=109) | 2.60-4.60 (n=105) | >4.60 (n=106) | ||
| Oocyte maturity rate* | 0.84±0.16 | 0.80±0.22 | 0.81±0.20 | 0.81±0.22 | 0.986 |
| Fertilization proportion* | 0.59±0.26 | 0.55±0.25 | 0.55±0.23 | 0.58±0.25 | 0.563 |
| Fertilization rate* | 0.69±0.25 | 0.69±0.27 | 0.68±0.24 | 0.72±0.24 | 0.772 |
| Implantation rate† | 18/195 (9) | 22/191 (11) | 31/186 (16) | 22/190 (11) | 0.030‡ |
| Chemical pregnancy rate† | 19 (17.9) | 28 (25.7) | 25 (24) | 20 (18.9) | 0.432 |
| Clinical pregnancy rate† | 17 (16) | 27 (24.8) | 23 (21.9) | 20 (18.9) | 0.420 |
*Data are presented as mean±SD, Analysis of Kruskal-Wallis. †Data are presented as number (%), Chi-square test. ‡Difference between group 1 (<1.49) and 3 (2.60-4.60)
The only observed difference was implantation rate that was significantly higher in the 2.60–4.60 IU/L group than the <1.49 IU/L group (P < 0.05) [Table 3]. There was no significant difference between the other groups in terms of the implantation rate [Table 3].
DISCUSSION
In our study of 426 GnRH-antagonist cycles, we assessed pregnancy outcome in different LH levels. The result showed that clinical outcomes were not affected by LH concentrations.
In the majority of published studies, it was investigated whether the threshold LH level during the ovarian stimulation affects the clinical outcome. However, the results have not been consistent. Therefore, it is important to focus on the trend of LH alteration in the GnRH antagonist protocol. Until now, in spite of a large number of studies, the optimum concentrations of exogenous or endogenous LH in GnRH antagonist protocol remain controversial. Some reports could not find any relationship between endogenous LH and the chance of ongoing pregnancy among women undergoing GnRH antagonist multiple dose protocol.[13] Principally, it has been supposed that consequent LH suppresses after GnRH antagonist administration might lead to adverse clinical outcomes.[14] In addition, it is revealed that both excessive and inadequate LH suppression by a GnRH antagonist regimen can decrease the chance of reaching pregnancy,[15,16] affirming the theory of “favorable LH window.”[17] In contrast with our findings, Depalo and colleagues found that women with positive pregnancy tests had significantly lower LH levels at the beginning of the stimulation period and the day of trigger.[18] Another study measured serum LH level 24 h before and after the first GnRH antagonist injection in 70 poor responders and egg donor women or who diagnosed with PCOS. The result showed no significant difference in LH concentrations between 2 days among the three groups. The authors concluded that LH changes above or below 50% had no effect on the laboratory and clinical outcome.[19] On the other hand, Huirne and colleagues compared the fluctuation of the serum LH level in five groups with different dosages of GnRH–antagonist administration with regard to clinical outcome. They concluded that no pregnancies were detected in either very high or low LH levels, which confirms the optimum window hypothesis.[15] Nonetheless, we found a nonsignificant reduced clinical pregnancy rate in women with low LH (<1.49 IU/L). Indeed, our results could not show the optimal clinical pregnancy rate in the 1.49–2.59 IU/L and 2.60–2.60 IU/L groups compared to the women with low (<1.49 IU/L) or high (>4.60 IU/L) LH concentration. Nevertheless, other studies stated undesirable outcomes in women with low LH concentrations or who experience a sudden fall in LH levels from the reference point.[20] It is also stated that the administration of the GnRH antagonist in women who have sustained low LH levels during the stimulation period cause further LH suppression which leads to poor clinical outcome.[1] In our study, estradiol and progesterone levels were significantly different between the four LH groups. However, estradiol and progesterone levels were not significantly higher among women who achieved pregnancy than those who did not. In line with our results, Depalo and colleagues could not show that estradiol levels were significantly higher in women who become pregnant. Moreover, they found a progressive linear increase in progesterone concentration during stimulation in women with positive pregnancy outcomes.[18] Additionally, we found no differences between numbers of retrieved oocytes, MII oocytes, 2PNs, and transferred embryos among the various LH concentrations. Huang et al.[21] reported that high LH levels during GnRH antagonist protocol may affect the number of retrieved oocytes. However, no effects on clinical outcomes were found.
In another study, Hosein Rashidi and colleagues[19] found a significant relationship between the LH level and M1 oocytes. Despite the fact that the required amount of LH for follicle and oocyte maturation is not recognized,[22] it has been shown that excessive LH concentration damage follicular development.[23] In addition, the other studies found that the higher number of follicles >18 mm was achieved when LH concentrations were between 25th and 75th percentile.[10] Furthermore, another report showed higher percentages of follicles >15 mm, more retrieved oocytes, and good quality embryos in the LH <0.5 IU/L group.[24]
The main limitation of our study was its retrospective nature, as a result of which not all data were at our disposal.
In conclusion, our result could not show the relationship between LH concentrations during GnRH antagonist cycles and pregnancy outcome. However, it was shown that the LH levels during the ovarian stimulation period should not remain very low which may negatively affect the implantation rate.
Financial support and sponsorship
This study is financially supported by Yazd Reproductive Sciences Institute, Shahid Sadoughi University of Medical Sciences, Yazd, Iran.
Conflicts of interest
There are no conflicts of interest.
REFERRENCES
- 1.Liu M, Liu S, Li L, Wang P, Li H, Li Y. LH levels may be used as an indicator for the time of antagonist administration in gnrh antagonist protocols-A proof-of-concept study. Front Endocrinol (Lausanne) 2019;10:67. doi: 10.3389/fendo.2019.00067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siristatidis CS, Gibreel A, Basios G, Maheshwari A, Bhattacharya S. Gonadotrophin-releasing hormone agonist protocols for pituitary suppression in assisted reproduction. Cochrane Database of Systematic Reviews. 2015;11 doi: 10.1002/14651858.CD006919.pub4. CD006919. DOI: 10.1002/14651858.CD006919.pub4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gizzo S, Andrisani A, Noventa M, Manfe S, Oliva A, Gangemi M, et al. Recombinant LH supplementation during IVF cycles with a GnRH-antagonist in estimated poor responders: A cross-matched pilot investigation of the optimal daily dose and timing. Mol Med Rep. 2015;12:4219–29. doi: 10.3892/mmr.2015.3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Wong PC, Qiao J, Ho C, Ramaraju GA, Wiweko B, Takehara Y, et al. Current opinion on use of luteinizing hormone supplementation in assisted reproduction therapy: An Asian perspective. Reprod Biomed Online. 2011;23:81–90. doi: 10.1016/j.rbmo.2011.03.023. [DOI] [PubMed] [Google Scholar]
- 5.Raju GA, Chavan R, Deenadayal M, Gunasheela D, Gutgutia R, Haripriya G, et al. Luteinizing hormone and follicle stimulating hormone synergy: A review of role in controlled ovarian hyper-stimulation. J Hum Reprod Sci. 2013;6:227–34. doi: 10.4103/0974-1208.126285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kol S. Individualized treatment from theory to practice: The private case of adding LH during GnRH antagonist-based stimulation protocol. Clin Med Insights Reprod Health. 2014;8:59–64. doi: 10.4137/CMRH.S17788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Alviggi C, Clarizia R, Mollo A, Ranieri A, De Placido G. Who needs LH in ovarian stimulation? Reprod Biomed Online. 2011;22(Suppl 1):S33–41. doi: 10.1016/S1472-6483(11)60007-2. [DOI] [PubMed] [Google Scholar]
- 8.O’Dea L, O’Brien F, Currie K, Hemsey G. Follicular development induced by recombinant luteinizing hormone (LH) and follicle-stimulating hormone (FSH) in anovulatory women with LH and FSH deficiency: Evidence of a threshold effect. Curr Med Res Opin. 2008;24:2785–93. doi: 10.1185/03007990802374815. [DOI] [PubMed] [Google Scholar]
- 9.Humaidan P, Bungum L, Bungum M, Andersen CY. Ovarian response and pregnancy outcome related to mid-follicular LH levels in women undergoing assisted reproduction with GnRH agonist down-regulation and recombinant FSH stimulation. Hum Reprod. 2002;17:2016–21. doi: 10.1093/humrep/17.8.2016. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran A, Jamdade K, Kumar P, Adiga SK, Bhat RG, Ferrao SR. Is there a need for luteinizing hormone (LH) estimation in patients undergoing ovarian stimulation with gonadotropin-releasing hormone (GnRH) antagonists and recombinant follicle-stimulating hormone (rFSH)? J Clin Diagn Res. 2014;8:90–2. doi: 10.7860/JCDR/2014/5728.3778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Benmachiche A, Benbouhedja S, Zoghmar A, Humaidan P. Low LH level on the day of GnRH agonist trigger is associated with reduced ongoing pregnancy and live birth rates and increased early miscarriage rates following IVF/ICSI treatment and fresh embryo transfer. Front Endocrinol (Lausanne) 2019;10:639. doi: 10.3389/fendo.2019.00639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Depa-Martynow M, Jedrzejczak P, Pawelczyk L. Pronuclear scoring as a predictor of embryo quality in in vitro fertilization program. Folia Histochem Cytobiol. 2007;45(Suppl 1):S85–9. [PubMed] [Google Scholar]
- 13.Kolibianakis EM, Collins J, Tarlatzis B, Papanikolaou E, Devroey P. Are endogenous LH levels during ovarian stimulation for IVF using GnRH analogues associated with the probability of ongoing pregnancy? A systematic review. Hum Reprod Update. 2006;12:3–12. doi: 10.1093/humupd/dmi030. [DOI] [PubMed] [Google Scholar]
- 14.A double-blind, randomized, dose-finding study to assess the efficacy of the gonadotrophin-releasing hormone antagonist ganirelix (Org 37462) to prevent premature luteinizing hormone surges in women undergoing ovarian stimulation with recombinant follicle stimulating hormone (Puregon). The ganirelix dose-finding study group. Hum Reprod. 1998;13:3023–31. [PubMed] [Google Scholar]
- 15.Huirne JA, van Loenen AC, Schats R, McDonnell J, Hompes PG, Schoemaker J, et al. Dose-finding study of daily GnRH antagonist for the prevention of premature LH surges in IVF/ICSI patients: Optimal changes in LH and progesterone for clinical pregnancy. Hum Reprod. 2005;20:359–67. doi: 10.1093/humrep/deh601. [DOI] [PubMed] [Google Scholar]
- 16.Kolibianakis EM, Albano C, Kahn J, Camus M, Tournaye H, Van Steirteghem AC, et al. Exposure to high levels of luteinizing hormone and estradiol in the early follicular phase of gonadotropin-releasing hormone antagonist cycles is associated with a reduced chance of pregnancy. Fertil Steril. 2003;79:873–80. doi: 10.1016/s0015-0282(02)04920-8. [DOI] [PubMed] [Google Scholar]
- 17.Balasch J, Fabregues F. Is luteinizing hormone needed for optimal ovulation induction? Curr Opin Obstet Gynecol. 2002;14:265–74. doi: 10.1097/00001703-200206000-00004. [DOI] [PubMed] [Google Scholar]
- 18.Depalo R, Trerotoli P, Chincoli A, Vacca MP, Lamanna G, Cicinelli E. Endogenous luteinizing hormone concentration and IVF outcome during ovarian stimulation in fixed versus flexible GnRH antagonist protocols: An RCT. Int J Reprod Biomed (Yazd) 2018;16:175–82. [PMC free article] [PubMed] [Google Scholar]
- 19.Hosein Rashidi B, Kabodmehri R, Shariat M, Shahrokh Tehraninejad E, Abdollahi A, Bagheri M, et al. Luteinizing hormone changes in gonadotropin-releasing hormone antagonist protocol in in vitro fertilization cycles: A cross-sectional study. Int J Reprod Biomed. 2019;17:209–16. doi: 10.18502/ijrm.v17i3.4520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yanaihara A, Yorimitsu T, Motoyama H, Ohara M, Kawamura T. The decrease of serum luteinizing hormone level by a gonadotropin-releasing hormone antagonist following the mild IVF stimulation protocol for IVF and its clinical outcome. J Assist Reprod Genet. 2008;25:115–8. doi: 10.1007/s10815-008-9205-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang Q, Nong Y, Zhang X, Huang L, Tang T, Huang J, et al. Effects of increasing serum luteinizing hormone levels during early phase of the gonadotropin-releasing hormone antagonist protocol on clinical outcomes of the in vitro fertilization cycle. Gynecol Endocrinol. 2021:1–5. doi: 10.1080/09513590.2021.1955341. doi: 10.1080/09513590.2021.1955341. [DOI] [PubMed] [Google Scholar]
- 22.Howles CM. Role of LH and FSH in ovarian function. Mol Cell Endocrinol. 2000;161:25–30. doi: 10.1016/s0303-7207(99)00219-1. [DOI] [PubMed] [Google Scholar]
- 23.Hillier SG. Current concepts of the roles of follicle stimulating hormone and luteinizing hormone in folliculogenesis. Hum Reprod. 1994;9:188–91. doi: 10.1093/oxfordjournals.humrep.a138480. [DOI] [PubMed] [Google Scholar]
- 24.Merviel P, Antoine JM, Mathieu E, Millot F, Mandelbaum J, Uzan S. Luteinizing hormone concentrations after gonadotropin-releasing hormone antagonist administration do not influence pregnancy rates in in vitro fertilization-embryo transfer. Fertil Steril. 2004;82:119–25. doi: 10.1016/j.fertnstert.2003.11.040. [DOI] [PubMed] [Google Scholar]