Abstract
Context:
Prolactinoma is the most frequent pituitary tumor among women of childbearing age. Fewer studies have addressed the outcome of prolactinomas after gestation.
Objective:
The aim was to study the spontaneous remission rate and change in tumor size after pregnancy and/or lactation in women with prolactinomas.
Patients and Methods:
Retrospective study conducted at a tertiary care center of north India. Records of 25 women with 31 pregnancies (20 microprolactinomas and 11 macroprolactinomas), who conceived on dopamine agonist (cabergoline) were studied. Cabergoline was stopped at conception in 24 pregnancies and continued in 7. Serum prolactin was noted 3 months after delivery and/or lactation. Magnetic resonance imaging available at last visit after delivery and/or lactation was also noted. Remission was defined as normal serum prolactin after pregnancy and/or lactation without use of cabergoline.
Results:
Among patients in whom cabergoline was stopped during pregnancy (n = 24), 41.6% (n = 10) had prolactin in normal range (achieved remission) after pregnancy and/or lactation. In 25% (n = 6) of women, adenoma size decreased by more than 50%, in 33%(n = 8), there was no change in adenoma size, and in 42% (n = 10), decrease in adenoma size was less than 50% after pregnancy and/or lactation. The median duration of cabergoline treatment before pregnancy among patients who achieved remission was 60 months against 24 months in those who did not achieve remission. The median pre-pregnancy adenoma size was 5.5 mm in women with remission against 8 mm in women who did not achieve remission.
Conclusion:
Pregnancy-induced remission of hyperprolactinemia was seen in 41.6% prolactinomas. Longer duration of dopamine agonist treatment before pregnancy, small pre-pregnancy adenoma size, and lower baseline prolactin were associated with high likelihood of remission, though not statistically significant.
Keywords: Hyperprolactinemia, India, prolactinoma, remission
INTRODUCTION
Prolactinomas are the most common cause of elevated serum prolactin (PRL), accounting for 40% of all pituitary adenomas, and are classified as microprolactinoma (less than 10 mm) and macroprolactinoma (more than 10 mm).[1] Prolactinoma in a young woman usually presents with oligomenorrhea, amenorrhea, galactorrhea, and/or infertility. Most patients conceive within few months of dopamine agonist (DA) treatment.[2] The normal pituitary enlarges during pregnancy; the bulk of enlargement is due to lactotroph hyperplasia in response to placental estrogen.[3] Prolactinomas may also enlarge during pregnancy, the enlargement is less frequently seen in microprolactinoma than in macroprolactinoma. Tumor enlargement may occur in 2% to 3% of patients with microadenomas, 20% to 30% of those with macroadenomas, and 4.7% of those macroadenomas with prior ablative treatment.[4,5] Studies predominantly involving patients with microprolactinomas have documented remission of hyperprolactinemia (HPL) in 10% to 68% of the subjects.[6,7,8] In one Indian study, remission was observed only in 36.4% macroprolactinomas after pregnancy despite a tumor size reduction in 95.8% patients.[9]
The aim of this study was to observe spontaneous remission rate and change in tumor size after pregnancy and/or lactation in women with prolactinomas and not on any DA treatment.
PATIENTS AND METHODS
Study design
This retrospective study was conducted at a tertiary care center of north India. Medical records of patients who were on follow-up of the endocrine clinic from 2016 to 2020 were screened. During this period, 25 women who conceived after treatment with cabergoline (CAB), which is a DA, were included in the study. Of these 25 women (with 31 pregnancies), CAB was stopped at conception in 24 pregnancies. In 7 pregnancies, CAB was continued in view of high risk of enlargement and optic chiasm involvement. The following details were extracted from the records: Age, symptoms, and their duration at the time of diagnosis of prolactinoma. The diagnosis of prolactinoma was based on assay of morning fasting, pooled (3 samples 20 min apart) sample for PRL and magnetic resonance imaging (MRI) evidence of pituitary adenoma. History of treatment for HPL with CAB, its duration, time interval of conception after initiation CAB, mode of conception (spontaneous or assisted), duration of pregnancy, growth of tumor (if any) during pregnancy, mode of delivery, and duration of lactation were recorded. A total of 15 pregnancies were followed by normal breastfeeding and serum PRL was recorded 3 months after stopping breastfeeding. In those who did not breastfeed their babies (n = 6) or ended in abortions (n = 3), serum PRL concentration was recorded 3 months after delivery. Repeat MR imaging (if available as part of standard care, during last hospital visit) after pregnancy and/or lactation were studied [Figure 1]. Serum PRL [normal range 1–27 ng/ml (women); 1–20 ng/ml (men)] was measured using commercial Chemiluminescent Immunoassay (Beckman Coulter Unicel, DXI). Pituitary imaging was done as dynamic contrast enhanced study of sellar, parasellar, and suprasellar region performed on 1.5 tesla MRI (Siemens, Magneton Avanto, MR Scanner, Germany). Precontrast T1 and T2 weighted spin echo coronal and sagittal sections were acquired using a small field of vision (20 × 25 cm), thin (3 mm) slices, and high (256 × 512) resolution. After a bolus injection of intravenous gadolinium, 6 consecutive sets of 3 images were obtained in coronal plane every 10 s to detect small adenomas. Adenomas were categorized into microprolactinomas, if less than 10 mm, or macroprolactinoma, if more than 10 mm in size.[1] The MRI films before and after pregnancy were studied by the same radiologist. Remission of HPL was defined as normalization of serum PRL after delivery and/or lactation without use of DA and regardless of adenoma size.
Figure 1.
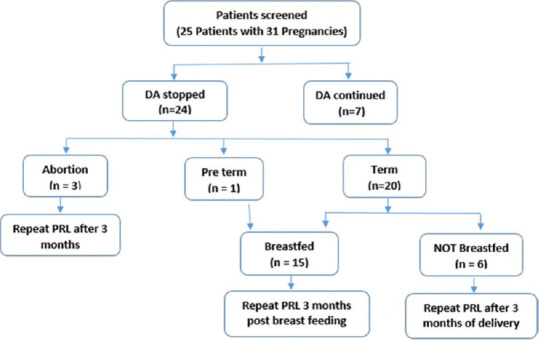
CONSORT diagram showing flow of study design,
Statistical analysis
Statistical Package for Social Sciences statistical software version 20 (IBM SPSS Statistics for Windows, Version 20 Armonk, NY: IBM Corp) was used to analyze the data. The continuous variables are shown in terms of descriptive statistics like mean, SD, median, and interquartile range. The Student's independent t test and Wilcoxon–Mann Whitney U test were used to compare the parameters between two groups. Also the paired t test and Wilcoxon signed rank test have been used to analyze the data before and after pregnancy. All results have been described on 5% level of significance, that is, P value < 0.05 considered as significant.
RESULTS
Twenty-five patients with 31 pregnancies were included in the study with mean age at pregnancy of 29.19 ± 4.92 years. The median duration of CAB treatment before pregnancy was 12 months (IQR 4–72 months). Serum PRL was normal in all pregnancies before conception. The median PRL at initial diagnosis was 107 ng/ml (IQR 70–200) which decreased to 32 ng/ml (IQR 15–90) after delivery. The median maximum adenoma diameter before pregnancy was 9 mm (IQR 5–14 mm) which decreased to median of 5 mm (IQR 4.4–9 mm). Majority of the tumors were microadenomas (n = 20), while 11 were macroadenomas. In 24 pregnancies (4 macroadenomas and 20 micro adenomas), CAB was stopped at conception. In 7 (22.6%) macroprolactinoma pregnancies, CAB was continued throughout pregnancy and lactation. Out of 31 pregnancies, 80.6% (n = 25) delivered at term while as 6.5% (n = 2) had premature delivery (of which one ended in intrauterine death) and 12.5% (n = 4) had early pregnancy loss. There was no significant difference in pregnancy outcomes in patients who continued with CAB as against those who stopped it (p = 0.616).
Among pregnancies not on DA (n = 24), overall, 41.6% (n = 10) patients had PRL in normal range (achieved remission) after delivery and/or lactation, while in 58.3% (n = 14) of patients, PRL continued to raise [Table 1]. In 25% (n = 6) of pregnancies, adenoma size decreased by more than 50%, there was no change in adenoma size in 33% (n = 8), and adenoma size decreased by less than 50% after pregnancy and/or lactation in 42% patients (n = 10) [Table 1].
Table 1.
Clinical imaging and biochemical parameters of patients with and without remission
| Parameter | Remission (n=10) | Not in remission (n=14) | P |
|---|---|---|---|
| Age (years)# | 30.8±4.93 | 29.0±5.4 | 0.404 |
| Duration of treatment before pregnancy (months)+ | 60 (IQR 10-99) | 24 (IQR 4-63) | 0.25 |
| Pre-pregnancy adenoma size (mm)+ | 5.5 (IQR 5-10.5) | 8 (IQR 5-9.3) | 0.977 |
| Post-pregnancy adenoma size (mm)+ | 5 (IQR 3.7-7.05) | 5 (4-6.25) | 0.886 |
| PRL at diagnosis (ng/ml)+ | 96 (38-120) | 108 (70-163) | 0.341 |
| Post-pregnancy PRL (ng/ml)+ | 14 (9.6-20.7) | 72.5 (44.7-99.7) | <0.001 |
| Microadenoma (n) | 8 | 12 | 0.53 |
| Macroadenoma (n) | 2 | 2 | - |
| Decrease in adenoma size (n) | 5 | 11 | 0.153 |
| Preterm delivery (n) | 0 | 1 | 0.531 |
| Abortion (n) | 1 | 2 | 0.640 |
+Median (IQR), #Mean±SD
The median duration of treatment before pregnancy among patients who achieved remission was 60 (IQR 10–99) months as against 24 (IQR 4–63) months in those who did not achieve remission (P = 0.251). The median pre-pregnancy adenoma size was 5.5 (IQR 5–10.5) mm in patients in remission as against 8 (IQR 5–9.3) mm in those not achieving remission (P = 0.977). Initial prolactin at diagnosis was 96 ng/ml (IQR 38–120) in patients who were in remission as against 108 ng/ml (IQR 70–163) in those who were not in remission (P = 0.341). Overall, 8 out of 20 patients with microadenoma and 2 with macroadenoma were in remission as against 12 patients with microadenoma and 2 patients with macroadenoma (P = 0.53) [Table 1]. There was no significant difference in pregnancy outcomes among patients who achieved or did not achieve remission (P = 0.640).
DISCUSSION
In women with prolactinoma, effect of pregnancy on mother (especially growth of prolactinoma) and growing fetus has been studied extensively.[10,11,12] The natural history of prolactinomas after successful childbirth (and lactation) is gaining interest. Many case studies and series described since 1980s have demonstrated favorable effect of pregnancy on prolactinoma and demonstrated both reduction in size of adenoma and normalization of PRL post delivery.[9,13,14] This study revealed that 41.6% of women with prolactinoma were in remission with normalization of PRL after pregnancy and/or lactation and results are consistent with the previous literature demonstrating a remission rate of as low as 9.4% to as high as 68%.[6,7,8,15,16,17,18,19] As early as 1979, Cowden et al.[14] reported a case of amenorrhea/galactorrhea because of HPL in whom bromocriptine-induced pregnancy resulted in normalization of serum PRL after delivery. Subsequently, in 1991, Mornex et al.[13] in a series of 40 HPL women (with enlarged sella in 18) found regression of HPL in 35% women after delivery. Crosignani et al.[20] suggested that pregnancy might be the treatment option for HPL. Comparing a group of 48 HPL women who completed term pregnancy, in 17% of these pregnancies, normalization of PRL was demonstrated as against none in 32 women who did not wish to be pregnant. The same group compared the pregnancy outcome with DA treatment and found normalization of PRL in 29% patients after pregnancy as against 13% of women on DAs.[15] Subsequently, many studies demonstrated remission in HPL after pregnancy.[15,16,17,18,19]
Auriemma et al.[16] reported a remission rate of 66% in microprolactinoma and 70% in macroprolactinoma at 12 and 60 months after delivery. Such high remission may be due to longer prepregnancy treatment (46 months) and including women with non-tumoral HPL. In our study, median duration of CAB treatment before pregnancy who achieved remission was 60 months against 24 months who did not achieved remission. Duration of CAB treatment before pregnancy may have a favorable effect on remission rate as is suggested from higher rates of remission in Auriemma et al.,[16] while as Domingue et al.[17] did not find a significant effect of duration of DA treatment before pregnancy on the final remission rates. Lower serum PRL concentration at diagnosis has been found to be associated with higher chances of remission in few studies.[16,17] However, on linear regression analysis, the effect was not found to be significant.[16] In this study, however, low initial PRL concentrations and small prepregnancy adenoma size were associated with higher chances of remission, though not statistically significant and could be because of small sample size. We observed a remission rate of 41.6% in microadenomas. The remission rate in this study is higher than a meta-analysis by Dekkers et al.,[21] suggesting that pregnancy has an additional effect on remission. In a large study, total number of pregnancies in one woman did not influence remission, though twin pregnancy had a favorable effect on remission.[16] In this study, in 74% of women, there was a decrease in adenoma size after delivery. The decrease was mainly accounted by reduction in size of macroadenomas. Most of the previous studies comparing the imaging findings at presentation and after delivery have found either reduction in size of adenoma in 15% to 95% of cases[6,7,9,17] or no enlargement of tumor after pregnancy.[16] To conclude, we have observed that gestation has a favorable effect on adenoma size as well as PRL concentrations. However, the study is limited by its retrospective nature and sample size.
SUMMARY
Prolactinoma undergoes remission after pregnancy in significant number of patients. Factors like low initial serum PRL concentration, smaller prepregnancy adenoma size, and longer duration of CAB treatment before pregnancy may be associated with higher chances of remission.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERRENCES
- 1.Casanueva FF, Molitch ME, Schlechte JA, Abs R, Bonert V, Bronstein MD, et al. Guidelines of the pituitary society for the diagnosis and management of prolactinomas. Clin Endocrinol (Oxf) 2006;65:265–73. doi: 10.1111/j.1365-2265.2006.02562.x. [DOI] [PubMed] [Google Scholar]
- 2.Maiter D. Prolactinoma and pregnancy: From the wish of conception to lactation. Ann Endocrinol. 2016;77:128–34. doi: 10.1016/j.ando.2016.04.001. [DOI] [PubMed] [Google Scholar]
- 3.Laway BA, Mir SA. Pregnancy and pituitary disorders: Challenges in diagnosis and management. Indian J Endocrinol Metab. 2013;17:996–1004. doi: 10.4103/2230-8210.122608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Molitch ME. Endocrinology in pregnancy: Management of the pregnant patient with a prolactinoma. Eur J Endocrinol. 2015;172:R205–213. doi: 10.1530/EJE-14-0848. [DOI] [PubMed] [Google Scholar]
- 5.Bronstein MD, Salgado LR, de Castro Musolino NR. Medical management of pituitary adenomas: The special case of management of the pregnant woman. Pituitary. 2002;5:99–107. doi: 10.1023/a:1022364514971. [DOI] [PubMed] [Google Scholar]
- 6.Karaca Z, Yarman S, Ozbas I, Kadioglu P, Akturk M, Kilicli F, et al. How does pregnancy affect the patients with pituitary adenomas: A study on 113 pregnancies from Turkey. J Endocrinol Invest. 2018;41:129–41. doi: 10.1007/s40618-017-0709-8. [DOI] [PubMed] [Google Scholar]
- 7.O'Sullivan SM, Farrant MT, Ogilvie CM, Gunn AJ, Milsom SR. An observational study of pregnancy and post-partum outcomes in women with prolactinoma treated with dopamine agonists. Aust N Z J Obstet Gynaecol. 2020;60:405–11. doi: 10.1111/ajo.13070. [DOI] [PubMed] [Google Scholar]
- 8.Rjosk HK, Fahlbusch R, von Werder K. Influence of pregnancies on prolactinomas. Acta Endocrinol (Copenh) 1982;100:337–46. doi: 10.1530/acta.0.1000337. [DOI] [PubMed] [Google Scholar]
- 9.Rastogi A, Bhadada SK, Bhansali A. Pregnancy and tumor outcomes in infertile women with macroprolactinoma on cabergoline therapy. Gynecol Endocrinol. 2017;33:270–73. doi: 10.1080/09513590.2016.1254177. [DOI] [PubMed] [Google Scholar]
- 10.Melmed S, Casanueva FF, Hoffman AR, Kleinberg DL, Montori VM, Schlechte JA, et al. Diagnosis and treatment of hyperprolactinemia: An Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2011;96:273–88. doi: 10.1210/jc.2010-1692. [DOI] [PubMed] [Google Scholar]
- 11.Molitch ME. Prolactinoma in pregnancy. Best Pract Res Clin Endocrinol Metab. 2011;25:885–96. doi: 10.1016/j.beem.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 12.Colao A, Abs R, Bárcena DG, Chanson P, Paulus W, Kleinberg DL. Pregnancy outcomes following cabergoline treatment: Extended results from a 12-year observational study. Clin Endocrinol (Oxf) 2008;68:66–71. doi: 10.1111/j.1365-2265.2007.03000.x. [DOI] [PubMed] [Google Scholar]
- 13.Mornex R, Hugues B. Remission of hyperprolactinemia after pregnancy. N Engl J Med. 1991;324:60. doi: 10.1056/NEJM199101033240116. [DOI] [PubMed] [Google Scholar]
- 14.Cowden EA, Thomson JA. Resolution of hyperprolactinaemia after bromocriptine-induced pregnancy. Lancet. 1979;1:613. doi: 10.1016/s0140-6736(79)91043-2. [DOI] [PubMed] [Google Scholar]
- 15.Crosignani PG, Mattei AM, Severini V, Cavioni V, Maggioni P, Testa G. Long-term effects of time, medical treatment and pregnancy in 176 hyperprolactinemic women. Eur J Obstet Gynecol Reprod Biol. 1992;44:175–80. doi: 10.1016/0028-2243(92)90094-f. [DOI] [PubMed] [Google Scholar]
- 16.Auriemma RS, Perone Y, Di Sarno A, Grasso LF, Guerra E, Gasperi M, et al. Results of a single-center observational 10-year survey study on recurrence of hyperprolactinemia after pregnancy and lactation. J Clin Endocrinol Metab. 2013;98:372–9. doi: 10.1210/jc.2012-3039. [DOI] [PubMed] [Google Scholar]
- 17.Domingue M-E, Devuyst F, Alexopoulou O, Corvilain B, Maiter D. Outcome of prolactinoma after pregnancy and lactation: A study on 73 patients. Clin Endocrinol (Oxf) 2014;80:642–8. doi: 10.1111/cen.12370. [DOI] [PubMed] [Google Scholar]
- 18.Jeffcoate WJ, Pound N, Sturrock ND, Lambourne J. Long-term follow-up of patients with hyperprolactinaemia. Clin Endocrinol (Oxf) 1996;45:299–303. doi: 10.1046/j.1365-2265.1996.00824.x. [DOI] [PubMed] [Google Scholar]
- 19.Bronstein MD. Prolactinomas and pregnancy. Pituitary. 2005;8:31–8. doi: 10.1007/s11102-005-5083-4. [DOI] [PubMed] [Google Scholar]
- 20.Crosignani PG, Mattei AM, Scarduelli C, Cavioni V, Boracchi P. Is pregnancy the best treatment for hyperprolactinaemia? Hum Reprod. 1989;4:910–2. doi: 10.1093/oxfordjournals.humrep.a137010. [DOI] [PubMed] [Google Scholar]
- 21.Dekkers OM, Lagro J, Burman P, Jørgensen JO, Romijn JA, Pereira AM. Recurrence of hyperprolactinemia after withdrawal of dopamine agonists: Systematic review and meta-analysis. J Clin Endocrinol Metab. 2010;95:43–51. doi: 10.1210/jc.2009-1238. [DOI] [PubMed] [Google Scholar]


