Abstract
BACKGROUND
Children with sickle cell disease (SCD) are at increased risk for bloodstream infections (BSIs), mainly because of functional asplenia. Immunizations and antibiotic prophylaxis have reduced the prevalence of invasive bacterial infections, but contemporary analysis of BSI in children with SCD is limited.
METHODS
We conducted a retrospective cohort study of children aged <18 years with SCD who had blood cultures collected at our institution from 2010 to 2019 to identify BSI. Probable contaminant organisms were identified and not included as BSI. We calculated the annual incidence of BSI at our institution with 95% confidence intervals (CIs) and used multivariate logistic regression to evaluate associations.
RESULTS
There were 2694 eligible patients with 19 902 blood cultures. Excluding repeated cultures and contaminant cultures, there were 156 BSI episodes in 144 patients. The median age at BSI was 7.5 years. The average incidence rate of BSI was 0.89 per 100 person-years (95% CI 0.45–1.32). The most common pathogens were Streptococcus pneumoniae (16.0%), Streptococcus viridans group (9.0%), Escherichia coli (9.0%), Staphylococcus aureus (7.7%), Bordetella holmesii (7.7%), Haemophilus influenzae (7.1%), and Salmonella species (6.4%). Odds of BSI were higher with sickle cell anemia genotypes (odds ratio [OR] 1.88; 95% CI 1.20–2.94) and chronic transfusions (OR 2.66; 95% CI 1.51–4.69) and lower with hydroxyurea (OR 0.57; 95% CI 0.39–0.84).
CONCLUSIONS
BSI remains a risk for children with SCD. Overall incidence, risk factors, and spectrum of pathogens are important considerations to guide prevention and empirical treatment of suspected infection in SCD.
What’s Known on This Subject:
Children with sickle cell disease are at risk for bloodstream infection. Antibiotic prophylaxis and advancements in pneumococcal immunization have reduced but have not eliminated risk. Central venous catheters pose an additional infection risk.
What This Study Adds:
We reviewed all blood cultures obtained over a 10-year period after the licensure of the 13-valent pneumococcal conjugate vaccine. In this study, we provide a contemporary update on potential causes of bloodstream infections in sickle cell disease.
Children with sickle cell disease (SCD) are at increased risk for bloodstream infections (BSIs), particularly with encapsulated bacteria, such as Streptococcus pneumoniae and Haemophilus influenzae. This infection risk is related to impaired or absent splenic function, other immune defects, and anatomic predisposition as occurs with osteomyelitis.1–4 The placement of a central venous catheter (CVC) also increases the risk of BSI.5–8 The Cooperative Study of Sickle Cell Disease, which was conducted in an era before routine penicillin prophylaxis or pneumococcal immunizations, provided a prospective longitudinal assessment of bacteremia risk, revealing that incidence rates were highest in patients with hemoglobin SS aged <3 years and decreased with age during the first 2 decades of life, predominantly because of S pneumoniae and H influenzae.9,10 Later retrospective studies from 1993 to 2010 demonstrated S pneumoniae, Salmonella, Escherichia coli, and Staphylococcus aureus as causes of bacteremia in SCD.11,12
Interventions to prevent BSI, namely prophylactic penicillin in children aged <5 years and the introduction of the 23-valent pneumococcal polysaccharide vaccine (PPSV23) in the mid-1980s, the H influenzae type b vaccine in 1990, the 7-valent pneumococcal conjugate vaccine (PCV7) in 2000, and the 13-valent pneumococcal conjugate vaccine (PCV13) in 2010, have reduced the incidence of invasive bacterial infections in children with SCD.13–19 Despite these advances, BSI remains a concern in children with SCD. Increased prevalence of microorganisms not included in current immunizations,20–23 low rates of penicillin adherence, and antibiotic resistance may be contributors to invasive infection.13,24,25 Large-scale studies of BSI in children with SCD in the current era are needed to understand the contemporary infection rate, guide empirical antibiotic therapy during febrile episodes, and guide policy decisions for targeted immunization schedules.
Our aims for this study are (1) to determine the annual incidence and associated features of BSI in children with SCD at our health care system over a 10-year period from 2010 to 2019 and (2) to delineate the specific microorganisms responsible for BSI in this era.
Methods
We conducted a retrospective cohort study to review all blood cultures from children with SCD obtained at Children’s Healthcare of Atlanta (CHOA) in Atlanta, Georgia, from January 1, 2010, to December 31, 2019. CHOA is the primary pediatric health care system in the metropolitan region, with 3 academic hospitals that provide outpatient, emergency, and inpatient care to the vast majority (>95%) of children with SCD in the Atlanta metropolitan area. Two tertiary care hospitals use CHOA laboratories, whereas 1 nontertiary hospital uses the laboratory at Grady Health System. This study was approved by the Institutional Review Board of CHOA and the Research Oversight Committee of Grady.
The Sickle Cell Disease Clinical Database of CHOA is a comprehensive prospective database housed in Research Electronic Data Capture (REDCap), capturing use data from all patients with a laboratory-confirmed diagnosis of SCD with ≥1 health care encounter at CHOA from 2010 onward. Data capture includes the laboratory-verified SCD genotype, SCD treatment history (hydroxyurea, chronic transfusions, hematopoietic stem cell transplant), and dates of all health care use at CHOA. The SCD database is linked to CHOA’s enterprise electronic health record database (Epic Systems, Verona, WI), allowing comprehensive data searches for this manually validated SCD population. All blood cultures reported for patients with SCD at CHOA laboratories from January 2010 to December 2019 and at the Grady laboratory from June 2013 to December 2019 were identified through the electronic health record. Blood cultures from Grady from January 2010 to May 2013 were obtained directly from Grady microbiology laboratory records.
All patients in the SCD database aged 0 to 18 years, including those without blood cultures, contributed to incidence rate determination. Patients with at least 1 blood culture during the 10-year period were included for specific review of culture results as well as age, sex, SCD genotype, and treatment history. SCD genotypes were categorized into 2 groups: sickle cell anemia (SCA) genotypes (hemoglobin SS, Sβ0 thalassemia, SD, and SO-Arab) and non-SCA genotypes (hemoglobin SC, Sβ+ thalassemia, and Staphylococcus epidermidis). Patients were censored 21 days before hematopoietic stem cell transplant, and all subsequent blood cultures were excluded. Any patients with a concomitant diagnosis of cancer were identified through our institution’s tumor registry and were censored at the date of their cancer diagnosis. Deaths that occurred within the same health care encounter during which the blood culture was collected were identified, and medical record review was performed to determine if death was attributable to infection or another cause.
Blood cultures at CHOA laboratories were processed in automated, continuous monitor systems (Bactec FX; Becton-Dickinson, Franklin Lakes, NJ). Isolates were identified by phenotypic tests or matrix-assisted laser desorption ionization mass spectrometry time of flight (Bruker Microflex MS; Bruker Daltonics, Billerica, MA). Blood cultures at the Grady Health System laboratory were performed with the BacT/ALERT 3D system (bioMérieux; Durham, NC). Isolates were identified by phenotypic tests or matrix-assisted laser desorption ionization mass spectrometry time of flight (Vitek-MS system; bioMérieux). Antimicrobial susceptibility testing and interpretation were in compliance with Clinical and Laboratory Standards Institute standards M07 and M100, respectively, for the appropriate years.26,27 Minimum inhibitory concentration was performed by using the MicroScan Walkaway (Danaher Corp, Pasadena, CA) or Vitek 2 (bioMérieux) system.
Blood cultures that were collected within 14 days of a previous culture and either had the same result or a negative result after a positive one were considered to represent repeat testing from the same episode and were excluded. Contaminated blood cultures with positive results were defined as cultures in which (1) the identified organism is one commonly associated with common skin flora contamination (eg, coagulase-negative Staphylococcus, Bacillus, Micrococcus, common skin flora, or polymicrobial)8,28 and (2) the culture source was not from a CVC. Any culture organism from blood obtained from a CVC was considered a true BSI, regardless of the organism’s identity, including polymicrobial results.
For identified cases of BSI, an individual medical record review was performed to determine if the patient had an active prescription for antibiotic prophylaxis at the time of the BSI or had a history of surgical splenectomy and, in cases of S pneumoniae, to review immunization records.
The serotypes of S pneumoniae and H influenzae isolates were determined by the Centers for Disease Control and Prevention Emerging Infections Program, which provides surveillance for the 20-county Atlanta area.29 Serotypes were determined by quellung reaction before 2016 and by whole genome sequencing in 2016 onward.
Statistical Analysis
For each calendar year from 2010 to 2019, the incidence rate of BSI was calculated by dividing the number of BSI episodes by the amount of person-time for all patients in the SCD database in that year. We defined person-years as the follow-up time for each child in that year until they were diagnosed with BSI or until the end of a particular calendar year, whichever came first. Subsequent BSIs in the same year were treated as independent events. Overall and age-stratified (<5 years and 5–18 years) annual incidence rates with 95% confidence intervals (CIs) were calculated. Characteristics of patients with BSI were compared with those of patients with negative blood culture results by using 2-tailed Student’s t test, Pearson χ2 test, and Fisher’s exact test, as appropriate for the data. A 2-sided P value of .05 was assumed to reflect statistical significance. Covariates that had an adjusted odds ratio (OR) revealing a change >10% in the crude estimate or those of clinical importance were used in the final multivariate logistic regression models with forward stepwise selection. Traditional model diagnostics (eg, goodness-of-fit tests and residuals plots) were used to evaluate model fit. Statistical analysis was performed by using SAS software version 9.3.1 (SAS Institute, Inc, Cary, NC).
Results
Study Population
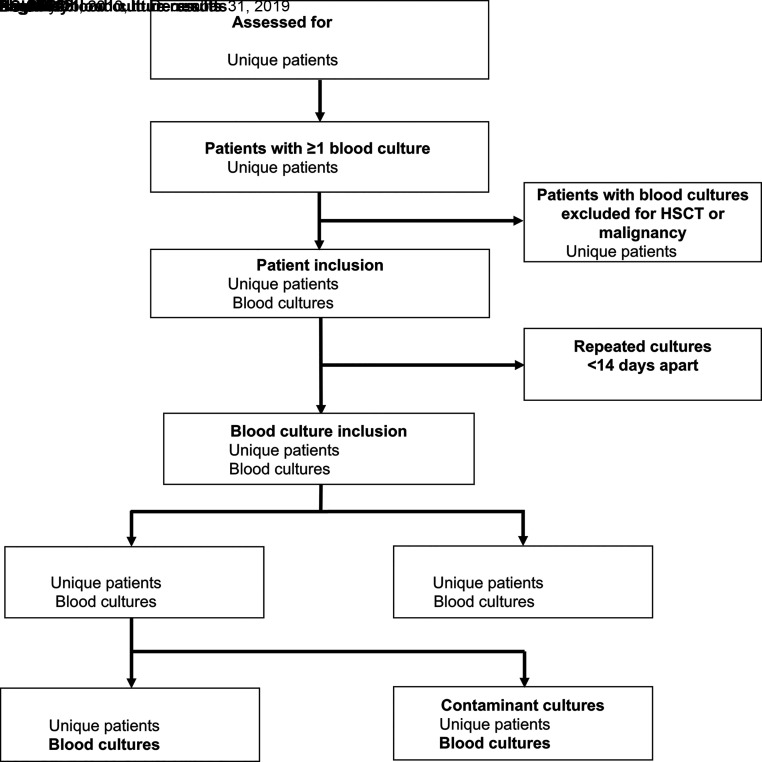
In the 10-year period, which capture 3624 patients with SCD, 2694 (74.3%) patients with a total of 19 902 blood cultures met inclusion criteria, as shown in Fig 1. When repeat blood cultures were excluded, there remained 15 208 unique cultures. After exclusion of positive culture results from presumed contaminant growth (n = 168 cultures), there were 156 episodes of BSI (1.0% of blood cultures) among 144 patients. For BSI, the blood culture sources were peripheral blood in 123 and CVC in 33 (21.1%).
FIGURE 1.
Flow diagram for identification of BSIs among a cohort of children with SCD, 2010–2019. HSCT, hematopoietic stem cell transplant.
Baseline characteristics of the study population are summarized in Table 1. The mean age was slightly older for those with positive versus negative blood culture results (8.4 vs 7.5 years; P = .049). BSIs were more prevalent in patients with SCA (versus a non-SCA genotype) and patients on chronic transfusion therapy. Among BSI episodes, 25 (16.0%) occurred in patients with splenectomy, and 78 (50.0%) occurred in patients on antibiotic prophylaxis (71 on penicillin, 4 on amoxicillin, 3 on other).
TABLE 1.
Demographic and Clinical Features of Children With SCD Who Had Health Care Encounters That Included Blood Culture
| Characteristic | BSI Episode | Blood Culture Without BSI | P |
|---|---|---|---|
| Age, mean (SE), y | 8.4 (0.5) | 7.5 (0.04) | .049 |
| Age ≤60 mo, n (%) | 60 (38.5) | 6147 (40.8) | .55 |
| Sex (female), n (%) | 79 (50.6) | 7108 (47.2) | .4 |
| SCA genotype, n (%) | 131 (84) | 11 299 (75.1) | .01 |
| SS or Sβ0 thalassemia | 131 (84) | 11 252 (74.8) | — |
| SD | 0 (0) | 14 (0.09) | — |
| SO-Arab | 0 (0) | 32 (0.21) | — |
| SC Harlem | 0 (0) | 1 (0.007) | — |
| Non-SCA genotypes, n (%) | |||
| SC | 21 (13.5) | 2950 (19.6) | — |
| Sβ+ thalassemia | 4 (0.64) | 761 (5.1) | — |
| SE | 0 (0) | 42 (0.28) | — |
| Hydroxyurea therapy, n (%) | 38 (24.4) | 4663 (30.9) | .08 |
| Chronic transfusion therapy, n (%) | 14 (8.9) | 425 (2.8) | <.0001 |
| Mortality, n (%) | 3 (1.9) | 11 (0.07) | <.0001 |
Patients with a BSI episode (n = 156) are compared with those without a BSI (n = 15 052). —, not applicable.
Incidence Rates of BSI
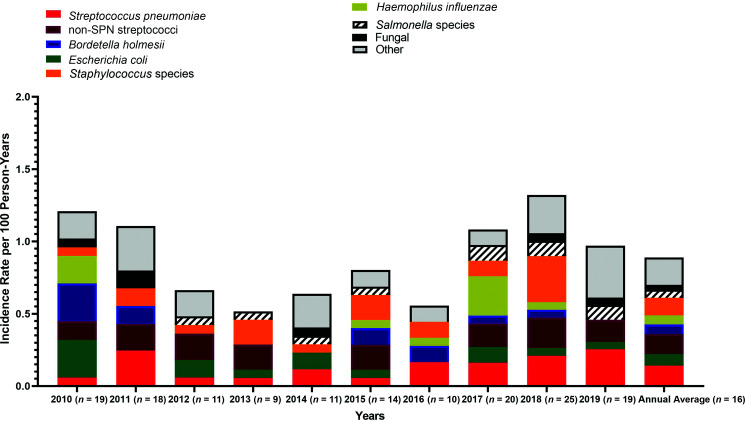
The average annual incidence of BSI over the 10-year period was 0.89 per 100 person-years (95% CI 0.45–1.32). The incidence rate for children aged <5 years was 1.42 per 100 person-years (95% CI 0.31–2.52), compared with an incidence rate of 0.78 per 100 person-years (95% CI 0.29–1.26) for children aged ≥5 years. The incidence rates for the most common organisms in each year are shown in Fig 2. There was no major change in the other less common organisms over the 10-year period.
FIGURE 2.
Annual incidence rates of BSI episodes among children with SCD, 2010–2019. SPN, S pneumoniae.
Risk Factors for BSI
In the multivariate analysis shown in Table 2, the final model included age, sex, SCD genotype, hydroxyurea, and chronic transfusion therapy. The odds of BSI were significantly higher for those with SCA compared with those with non-SCA genotypes (OR 1.88 [95% CI 1.20–2.94]) and for those receiving chronic transfusion therapy (OR 2.66 [95% CI 1.51–4.69]). Hydroxyurea therapy was associated with significantly lower odds of BSI (OR 0.57 [95% CI 0.39–0.84]).
TABLE 2.
Adjusted Multivariate Analysis for BSI and Associated Features Among Children With SCD
| OR | 95% CI | |
|---|---|---|
| Age, y | 1.03 | 1.01–1.06 |
| Sex, female | 1.17 | 0.85–1.61 |
| SCA genotype | 2.13 | 1.33–3.40 |
| Hydroxyurea therapy | 0.56 | 0.38–0.83 |
| Chronic transfusion therapy | 2.68 | 1.52–4.74 |
Microbiologic Findings
A list of all microorganisms associated with BSI is shown in Table 3. The most prevalent pathogen was S pneumoniae (n = 25, 16.0% of BSIs), followed by Staphylococcus (n = 12 for S aureus, n = 5 for S epidermidis, n = 3 for other coagulase-negative Staphylococcus) and Streptococcus (n = 23 viridans group, n = 1 Streptococcus pyogenes). Prevalent Gram-negative organisms included E coli (n = 14, 9.0%), Bordetella holmesii (n = 12, 7.7%), H influenzae (n = 11, 7.1%), and Salmonella species (n = 10, 6.4%). H influenzae included serotypes f (n = 6) and a (n = 1) and nontypeable H influenzae (n = 3) and was not tested in 1 case.
TABLE 3.
Microorganisms Isolated From Cases of BSI Among Children With SCD (n = 156)
| Microorganism Group | n = 156, Frequency (%) | Culture from CVC (n = 33), n (%) |
|---|---|---|
| Gram-negative bacteria | ||
| Enterobacteriaceae | ||
| E coli | 14 (9.0) | 3 (9.1) |
| Salmonella speciesa | 10 (6.4) | 0 (0) |
| Klebsiella pneumoniae | 2 (1.3) | 0 (0) |
| Pantoea agglomerans | 2 (1.3) | 0 (0) |
| Citrobacter koseri | 1 (0.6) | 0 (0) |
| Morganella morganii | 1 (0.6) | 0 (0) |
| Shigella sonnei | 1 (0.6) | 0 (0) |
| Miscellaneous Gram-negative (non-Enterobacteriaceae) | ||
| B holmesii | 12 (7.7) | 1 (3.0) |
| H influenzae | 11 (7.1) | 1 (3.0) |
| Moraxella speciesa | 4 (2.6) | 0 (0) |
| Acinetobacter speciesa | 3 (1.9) | 0 (0) |
| Stenotrophomonas maltophilia | 1 (0.6) | 0 (0) |
| Pseudomonas aeruginosa | 1 (0.6) | 0 (0) |
| Pseudomonas stutzeri | 1 (0.6) | 0 (0) |
| Gram-positive bacteria | ||
| SPN | 25 (16.0) | 1 (3.0) |
| Viridans Streptococcus group (non-SPN) | ||
| Viridans Streptococcus groupa | 14 (9.0) | 1 (3.0) |
| Streptococcus mitis group | 9 (5.8) | 0 (0) |
| S pyogenes | 1 (0.6) | 0 (0) |
| Staphylococcus aureus | ||
| Methicillin-susceptible SA | 8 (5.1) | 1 (3.0) |
| Methicillin-resistant SA | 4 (2.6) | 1 (3.0) |
| Staphylococcus Epidermidis | 5 (3.2) | 5 (15.1) |
| Coagulase-negative staphylococci (non-SE) | ||
| Staphylococcus capitis | 1 (0.6) | 1 (3.0) |
| Staphylococcus hominis | 2 (1.3) | 2 (6.1) |
| Propionibacterium speciesa | ||
| Cutibacterium (Propionibacterium) acnes | 6 (3.9) | 6 (18.2) |
| Propionibacterium speciesa | 1 (0.6) | 1 (3.0) |
| Bacillus species, not Bacillus anthracis | 1 (0.6) | 1 (3.0) |
| Abiotrophia and Granulicatella species | ||
| Abiotrophia defectiva | 1 (0.6) | 0 (0) |
| Abiotrophia and Granulicatella species | 2 (1.3) | 1 (3.0) |
| Gemella species | ||
| Gemella haemolysans | 1 (0.6) | 0 (0) |
| Gemella morbillorum | 1 (0.6) | 0 (0) |
| Rothia speciesa | 1 (0.6) | 0 (0) |
| Miscellaneous | ||
| Polymicrobial | 3 (1.9) | 2 (6.1) |
| Yeasta | ||
| Candida albicans | 4 (2.6) | 4 (100) |
| Candida tropicalis | 1 (0.6) | 1 (100) |
| Candida parapsilosis | 1 (0.6) | 1 (100) |
| Total | 156 (100) | 33 (100) |
SA, S aureus; SE, S epidermidis; SPN, S pneumoniae.
No further identification.
Serotypes of S pneumoniae isolates were determined for 24 of 25 cases (Table 4), whereas 1 isolate was from a child who resided outside the Centers for Disease Control and Prevention surveillance area for S pneumoniae. Pneumococcal BSI occurred in 7 (25%) children <24 months of age, in 7 (28%) children 24 to 59 months of , and in 11 (44%) aged ≥60 months. There were 12 (48%) isolates of serotypes included in PPSV23 (10A, 12F, 15B/C, 22F). No serotypes included in PCV13 were isolated. Among children who had a PPSV23 serotype, 6 (50%) had received at least 1 PPSV23 immunization (5 with 1 dose, 1 with 2 doses), whereas among children with a non-PPSV23 serotype, 9 (75%) had at least 1 PPSV23 immunization (P = .20).
TABLE 4.
Serotypes of S pneumoniae BSI Among Children With SCD, at CHOA, 2010–2019
| Serotype | Frequency (%) |
|---|---|
| PPSV23 serotypes (n = 12) | |
| 10A | 1 (4.2) |
| 12F | 1 (4.2) |
| 15B/C | 7 (28.0) |
| 22F | 3 (12.5) |
| Non-PPSV23 serotypes (n = 12) | |
| 6C | 1 (4.2) |
| 7C | 1 (4.2) |
| 15A | 2 (8.3) |
| 23B | 3 (12.5) |
| 35B | 3 (12.5) |
| 38 | 2 (8.3) |
The serotype of 1 S pneumoniae isolate was not determined.
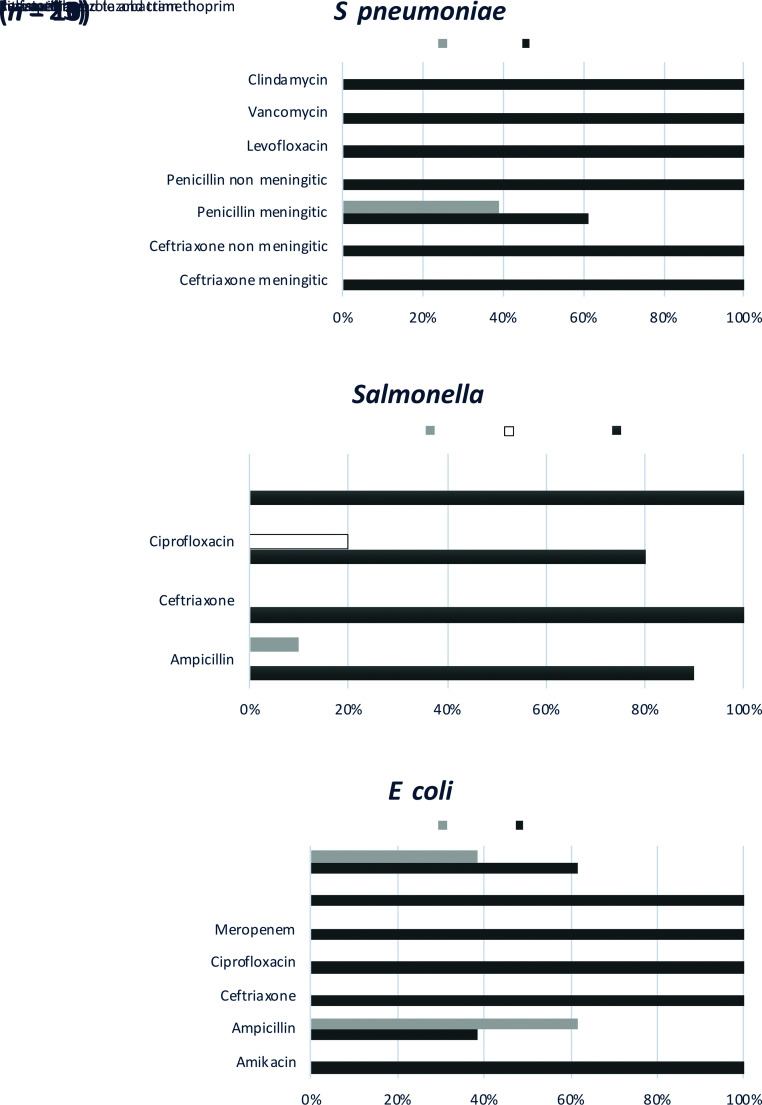
Antimicrobial susceptibility was available for select pathogens (Fig 3). For S pneumoniae, all isolates tested (n = 23) revealed susceptibility to ceftriaxone at both nonmeningitis (<1 μg/mL) and meningitis (≤0.5 μg/mL) breakpoints and to penicillin at nonmeningitis breakpoints; however, only 61% were susceptible to penicillin at the meningitis breakpoint (≥0.12 μg/mL).30 Susceptibility to ceftriaxone was observed in 100% of Salmonella and E coli isolates. Susceptibility to ampicillin was observed in 9 of 10 (90%) of Salmonella and 5 of 13 (38.5%) of E coli isolates.
FIGURE 3.
Antimicrobial susceptibility testing among isolates of S pneumoniae, Salmonella, and E coli.
Mortality
There were 14 deaths within the study inclusion cohort: 3 deaths during illnesses associated with a BSI and 11 deaths during illness in which blood culture results at CHOA were negative. Two deaths were attributable to S pneumoniae sepsis, and 1 death in a patient with CVC-associated BSI (methicillin-resistant S aureus) was attributed to a coexistent non-SCD medical condition. Within the non-BSI group, 2 deaths occurred in patients transferred to CHOA after positive blood culture results reported by outside hospitals (1 H influenzae, 1 Gram-negative rod), 2 deaths were associated with ceftriaxone-induced hemolytic anemia, and 7 deaths were not associated with an known infection or antibiotic adverse event. Mortality was significantly higher within the BSI group (1.9% of BSI events versus 0.09% of non-BSI events; P < .0001). In multivariate analysis of all mortality, the odds of death were significantly higher in those with BSI (OR 22.4 [CI 6.1–82.6]), whereas age, sex, genotype, and hydroxyurea were not associated with mortality.
Discussion
The present comprehensive review of blood culture findings among nearly 2700 children provides a contemporary update to the incidence of and specific pathogens associated with invasive BSI in children with SCD. Significant advances have occurred in infection prevention since the Cooperative Study of Sickle Cell Disease (1978–1994),31 including antibiotic prophylaxis in children aged <5 years and the development of pneumococcal conjugate vaccines. The BSI incidence rates of 0.89 per 100 person-years overall and 1.42 per 100 person-years in children <5 years are lower than the rates in the Cooperative Study of Sickle Cell Disease, which ranged from 7.87 per 100 person-years for children with hemoglobin SS <3 years age to 0.63 per 100 person-years for children with hemoglobin SS aged 10 to 19 years.9 However, our findings demonstrate the ongoing prevalence of BSI in children and adolescents of all ages with SCD. These rates are informative to guiding the prevention, identification, and empirical treatment of infection in SCD.
In this contemporary cohort, organisms classically known to affect individuals with SCD and asplenia, such as S pneumoniae, H influenzae, Salmonella, and E coli, continue to be among the most common pathogens identified. Our study period is unique because it began only 1 month before the licensure of PCV13 in February 2010, which replaced PCV7 in the routine childhood immunization schedule.19 We found no S pneumoniae serotypes included in PCV7 or PCV13, suggesting a change in the epidemiology of infection in children with SCD concurrent with population-wide immunization practices. However, nearly half of the 25 pneumococcal BSIs were serotypes that were included in PPSV23. Almost all cases of vaccine-included serotypes occurred in children with either no doses or only 1 primary dose of PPSV23 without a booster dose. Regardless of PPSV23 immunization status, children with SCD remain at risk for pneumococcal BSI because non-PPSV23 serotypes may also cause invasive infection. The importance of antibiotic prophylaxis thus must be considered in children at highest risk of pneumococcal infection. Notably, when considering all BSIs in our series, 50% occurred in individuals who were prescribed antibiotic prophylaxis. This observation is not a reflection of the effectiveness of prophylaxis because our study was not designed to evaluate this measure. Additionally, this observation in our BSI group likely reflects our clinical practice of prescribing antibiotic prophylaxis in higher-risk patients (younger age or those undergoing splenectomy).
In addition to pneumococcus, several Gram-negative bacteria and organisms associated with CVC contamination were found. B holmesii, a fastidious Gram-negative organism, was the third most common BSI organism in this study.32 Although not identified until 1995, B holmesii bacteremia has been described predominantly among individuals with functional or anatomic asplenia, including those with SCD, typically without a severe disease course, and therefore should be included as a consideration in the empirical treatment of infection in SCD.
Risk factors for BSI identified in our cohort included SCA (hemoglobin SS or Sβ0 thalassemia) and chronic transfusion therapy, whereas hydroxyurea revealed a significant risk reduction in the multivariate analysis. A similar observation with hydroxyurea was reported in the Realizing Effectiveness Across Continents with Hydroxyurea (REACH) trial in sub-Saharan Africa, which revealed lower incidence rates of infection and septicemia in children receiving hydroxyurea. A potential explanation may be that hydroxyurea therapy is associated with increased access to medical care and therefore also to preventive care in SCA. Among patients who are chronically transfused, the increased risk of BSI may be related in part to the frequent use of CVCs among patients receiving this therapy. The benefits of vascular access with CVCs must be balanced against the increased infectious risks in a population with asplenia that is already at increased risk of invasive bacterial infection. BSI in the presence of a CVC may lead to increased hospitalization, longer duration of exposure to antibiotics, or surgical procedures to remove the CVC in some cases.
Within this cohort, there were 4 deaths at our institution related to infection: 2 deaths from S pneumoniae sepsis and 2 deaths from septic shock in patients with Gram-negative bacteremia in cultures obtained at outside hospitals. One other death that occurred in a patient with a CVC-associated BSI was attributed to complications of a comorbid non-SCD medical condition. Given that our review does not include cultures from surrounding outside hospitals, our study likely underrepresents the frequency of death due to infection. This highlights an important limitation of the study because the analysis was based on results of blood cultures drawn at CHOA facilities and does not capture blood culture results from other hospitals, thus underestimating the true incidence of BSI as compared with a population-based surveillance study.
Given the risk of BSI in children with SCD, national consensus guidelines recommend that all individuals with SCD and fever should have a blood culture obtained and treatment with empirical antibiotics.33 Our study revealed that ∼2% of blood culture results from children with SCD were positive, half of which represented true BSI; the other half, culture contamination. Considerations for empirical antibiotic choice should include adequate treatment of S pneumoniae as well as other Gram-positive and Gram-negative bacteria. Although antibiotic susceptibility results in our cohort revealed high susceptibility to ceftriaxone, empirical antibiotic therapy should reflect local susceptibility patterns; thus, these findings may not be extrapolated to other geographic regions. The risk of adverse events, including drug-induced immune hemolytic anemia, also bear consideration. For individuals with a CVC, a broader range of organisms must be considered. Past studies of CVCs in individuals with SCD have shown a rate of infection from 1.5 to 5.5 infections per 1000 catheter days, underscoring the infectious risks associated with these devices and the need for vigilance with BSI prevention and empirical treatment in these patients.5,34–36
Conclusions
This 10-year cohort study is one of the largest reviews of blood culture results and BSI in children with SCD. Although infection and its associated mortality have decreased substantially over the past decades, children with SCD remain at risk for BSI, particularly those with SCA genotypes or a CVC. The findings of this study continue to inform clinical practices for immunization, prevention, and treatment of BSI in SCD.
Acknowledgments
We acknowledge the Georgia Emerging Infectious Program and the Centers for Disease Control and Prevention for information on bacterial serotyping; Thomas Adamkiewicz, MD, MSCR, FRCPC, FAAP and Folashade Omole, MD, FAAFP, Morehouse School of Medicine, for ongoing collaborations involving invasive pneumococcal infections in patients with Sickle Cell Disease; Children’s Healthcare of Atlanta analytics and the Population Discovery team.
Glossary
- BSI
bloodstream infection
- CHOA
Children’s Healthcare of Atlanta
- CI
confidence interval
- CVC
central venous catheter
- OR
odds ratio
- PCV7
7-valent pneumococcal conjugate vaccine
- PCV13
13-valent pneumococcal conjugate vaccine
- PPSV23
23-valent pneumococcal polysaccharide vaccine
- SCA
sickle cell anemia
- SCD
sickle cell disease
Footnotes
Dr Yee conceptualized and designed the study, drafted the initial manuscript, and reviewed and revised the manuscript; Ms Lai and Mr Mallis designed the data collection instruments, collected data, and reviewed and revised the manuscript; Dr Bakshi conceptualized the study, contributed to data analysis, and critically reviewed the manuscript for important intellectual content; Drs Grossman and Jaggi coordinated and supervised data collection and critically reviewed the manuscript for important intellectual content; Drs Wang and Jerris designed and maintained the microbiology data collection instruments, provided data, and critically reviewed the manuscript for important intellectual content; Dr Lane conceptualized and designed the study and the clinical database instruments and critically reviewed the manuscript for important intellectual content; Dr Yildirim conceptualized and designed the study, conducted the data analyses, and reviewed and revised the manuscript; and all authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Deidentified individual participant data (including data dictionaries) will be made available, in addition to study protocols, the statistical analysis plan, and the informed consent form. The data will be made available after publication to researchers who provide a methodologically sound proposal for use in achieving the goals of the approved proposal. Proposals should be submitted to memcphe@emory.edu.
Dr Yildirim’s current affiliation is Section of Infectious Diseases and Global Health, Department of Pediatrics, Yale School of Medicine and Yale Institute of Global Health, Yale University, New Haven, CT.
FUNDING: Supported by a grant from the Abraham J. & Phyllis Katz Foundation. Dr Yee received funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award 1K23HL146901-01A1. Dr Bakshi received funding from the National Heart, Lung, and Blood Institute of the National Institutes of Health under award 1K23HL140142-02. Dr Yildirim received funding from Center for Childhood Infections and Vaccines at Emory University and Children’s Healthcare of Atlanta. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health. Funded by the National Institutes of Health (NIH).
References
- 1. Pearson HA, Spencer RP, Cornelius EA. Functional asplenia in sickle-cell anemia. N Engl J Med. 1969;281(17):923–926 [DOI] [PubMed] [Google Scholar]
- 2. Battersby AJ, Knox-Macaulay HH, Carrol ED. Susceptibility to invasive bacterial infections in children with sickle cell disease. Pediatr Blood Cancer. 2010;55(3):401–406 [DOI] [PubMed] [Google Scholar]
- 3. Larcher VF, Wyke RJ, Davis LR, Stroud CE, Williams R. Defective yeast opsonisation and functional deficiency of complement in sickle cell disease. Arch Dis Child. 1982;57(5):343–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Booth C, Inusa B, Obaro SK. Infection in sickle cell disease: a review. Int J Infect Dis. 2010;14(1):e2–e12 [DOI] [PubMed] [Google Scholar]
- 5. Yeral M, Boga C, Oguzkurt L, Asma S, Kasar M, Kozanoglu I. Short-term central venous catheter complications in patients with sickle cell disease who undergo apheresis. J Thromb Thrombolysis. 2014;37(2):97–101 [DOI] [PubMed] [Google Scholar]
- 6. Rincón-López EM, Navarro Gómez ML, Hernández-Sampelayo Matos T, et al. ; RETRO-DREP Study Group . Low-risk factors for severe bacterial infection and acute chest syndrome in children with sickle cell disease. Pediatr Blood Cancer. 2019;66(6):e27667. [DOI] [PubMed] [Google Scholar]
- 7. Woods D, Hayashi RJ, Binkley MM, Sparks GW, Hulbert ML. Increased complications of chronic erythrocytapheresis compared with manual exchange transfusions in children and adolescents with sickle cell disease. Pediatr Blood Cancer. 2017;64(11):e26635. [DOI] [PubMed] [Google Scholar]
- 8. Chang TP, Kriengsoontorkij W, Chan LS, Wang VJ. Predictors for bacteremia in febrile sickle cell disease children in the post-7-valent pneumococcal conjugate vaccine era. J Pediatr Hematol Oncol. 2013;35(5):377–382 [DOI] [PubMed] [Google Scholar]
- 9. Zarkowsky HS, Gallagher D, Gill FM, et al. Bacteremia in sickle hemoglobinopathies. J Pediatr. 1986; 109(4):579–585 [DOI] [PubMed] [Google Scholar]
- 10. Gill FM, Sleeper LA, Weiner SJ, et al. ; Cooperative Study of Sickle Cell Disease . Clinical events in the first decade in a cohort of infants with sickle cell disease. Blood. 1995;86(2):776–783 [PubMed] [Google Scholar]
- 11. Baskin MN, Goh XL, Heeney MM, Harper MB. Bacteremia risk and outpatient management of febrile patients with sickle cell disease. Pediatrics. 2013;131(6):1035–1041 [DOI] [PubMed] [Google Scholar]
- 12. Williams TN, Uyoga S, Macharia A, et al. Bacteraemia in Kenyan children with sickle-cell anaemia: a retrospective cohort and case-control study. Lancet. 2009;374(9698):1364–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Adamkiewicz TV, Sarnaik S, Buchanan GR, et al. Invasive pneumococcal infections in children with sickle cell disease in the era of penicillin prophylaxis, antibiotic resistance, and 23-valent pneumococcal polysaccharide vaccination. J Pediatr. 2003;143(4): 438–444 [DOI] [PubMed] [Google Scholar]
- 14. Adamkiewicz TV, Silk BJ, Howgate J, et al. Effectiveness of the 7-valent pneumococcal conjugate vaccine in children with sickle cell disease in the first decade of life. Pediatrics. 2008;121(3):562–569 [DOI] [PubMed] [Google Scholar]
- 15. Narang S, Fernandez ID, Chin N, Lerner N, Weinberg GA. Bacteremia in children with sickle hemoglobinopathies. J Pediatr Hematol Oncol. 2012;34(1): 13–16 [DOI] [PubMed] [Google Scholar]
- 16. Gaston MH, Verter JI, Woods G, et al. Prophylaxis with oral penicillin in children with sickle cell anemia. A randomized trial. N Engl J Med. 1986;314(25):1593–1599 [DOI] [PubMed] [Google Scholar]
- 17. Livorsi DJ, Macneil JR, Cohn AC, et al. Invasive Haemophilus influenzae in the United States, 1999-2008: epidemiology and outcomes. J Infect. 2012;65(6): 496–504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Centers for Disease Control and Prevention . Pneumococcal disease. In: Hamborsky J, Kroger A, Wolfe C, eds.. Epidemiology and Prevention of Vaccine-Preventable Diseases. 13th ed. Washington, DC: Public Health Foundation; 2015:255–274 [Google Scholar]
- 19. Centers for Disease Control and Prevention (CDC) . Invasive pneumococcal disease in young children before licensure of 13-valent pneumococcal conjugate vaccine - United States, 2007. MMWR Morb Mortal Wkly Rep. 2010;59(9):253–257 [PubMed] [Google Scholar]
- 20. McCavit TL, Quinn CT, Techasaensiri C, Rogers ZR. Increase in invasive Streptococcus pneumoniae infections in children with sickle cell disease since pneumococcal conjugate vaccine licensure. J Pediatr. 2011;158(3): 505–507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Oligbu G, Fallaha M, Pay L, Ladhani S. Risk of invasive pneumococcal disease in children with sickle cell disease in the era of conjugate vaccines: a systematic review of the literature. Br J Haematol. 2019;185(4):743–751 [DOI] [PubMed] [Google Scholar]
- 22. Yildirim I, Shea KM, Little BA, Silverio AL, Pelton SI; Members of the Massachusetts Department of Public Health . Vaccination, underlying comorbidities, and risk of invasive pneumococcal disease. Pediatrics. 2015;135(3):495–503 [DOI] [PubMed] [Google Scholar]
- 23. Yee ME, Bakshi N, Graciaa SH, et al. Incidence of invasive Haemophilus influenzae infections in children with sickle cell disease. Pediatr Blood Cancer. 2019;66(6):e27642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Patel NG, Lindsey T, Strunk RC, DeBaun MR. Prevalence of daily medication adherence among children with sickle cell disease: a 1-year retrospective cohort analysis. Pediatr Blood Cancer. 2010;55(3):554–556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Warren MD, Arbogast PG, Dudley JA, et al. Adherence to prophylactic antibiotic guidelines among Medicaid infants with sickle cell disease. Arch Pediatr Adolesc Med. 2010;164(3): 298–299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Clinical and Laboratory Standards Institute . Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically. 11th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2018 [Google Scholar]
- 27. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing. 30th ed. Wayne, PA: Clinical and Laboratory Standards Institute; 2020 [Google Scholar]
- 28. Hall KK, Lyman JA. Updated review of blood culture contamination. Clin Microbiol Rev. 2006;19(4):788–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Centers for Disease Control and Prevention, Division of Preparedness and Emerging Infections (DPEI) . Emerging infections program. 2018. Available at: https://www.cdc.gov/ncezid/dpei/eip/index.html. Accessed August 31, 2021
- 30. Clinical and Laboratory Standards Institute . Performance Standards for Antimicrobial Susceptibility Testing; Eighteenth Informational Supplement. Wayne, PA: Clinical and Laboratory Standards Institute; 2008 [Google Scholar]
- 31. National Heart, Lung, and Blood Institute . Cooperative Study of Sickle Cell Disease (CSSCD). 2008. Available at: https://biolincc.nhlbi.nih.gov/studies/csscd/. Accessed June 15, 2021
- 32. Shepard CW, Daneshvar MI, Kaiser RM, et al. Bordetella holmesii bacteremia: a newly recognized clinical entity among asplenic patients. Clin Infect Dis. 2004;38(6):799–804 [DOI] [PubMed] [Google Scholar]
- 33. National Heart, Lung, and Blood Institute . Evidence-Based Management of Sickle Cell Disease: Expert Panel Report, 2014. Bethesda, MD: US Department of Health and Human Services, National Institutes of Health; 2014 [Google Scholar]
- 34. Wagner SC, Eschelman DJ, Gonsalves CF, Bonn J, Sullivan KL. Infectious complications of implantable venous access devices in patients with sickle cell disease. J Vasc Interv Radiol. 2004;15(4):375–378 [DOI] [PubMed] [Google Scholar]
- 35. Alkindi S, Matwani S, Al-Maawali A, Al-Maskari B, Pathare A. Complications of PORT-A-CATH® in patients with sickle cell disease. J Infect Public Health. 2012;5(1):57–62 [DOI] [PubMed] [Google Scholar]
- 36. Jeng MR, Feusner J, Skibola C, Vichinsky E. Central venous catheter complications in sickle cell disease. Am J Hematol. 2002;69(2):103–108 [DOI] [PubMed] [Google Scholar]