Although inflammatory cytokines are implicated in the pathogenesis of cardiac arrhythmias, inflammation is still largely overlooked in the current management of heart rhythm disorders. Now, COVID-19, a systemic inflammatory disease, causes an unexpectedly high prevalence of arrhythmic events, emphasizing the relevance of inflammation in the pathogenesis of cardiac arrhythmias.
Subject terms: Cardiology, Acute inflammation
COVID-19 is associated with an unexpectedly high prevalence of arrhythmic events. In this Comment, Lazzerini et al. discuss how systemic inflammation is linked to cardiac arrhythmias.
Cardiac arrhythmias are a leading cause of morbidity and mortality in Western countries, but the underlying mechanisms are still ill-defined. Over the past decade, systemic inflammation has been shown to promote a wide spectrum of cardiac arrhythmias, particularly atrial fibrillation, long-QT syndrome and Torsades de Pointes and atrioventricular blocks1. Moreover, inflammatory cytokines also seem to be involved in arrhythmogenic cardiomyopathy and other arrhythmogenic syndromes1. Nevertheless, inflammation is still largely overlooked in the management of arrhythmias, and as yet, agents that target the immune-inflammatory system have not become standard treatments as antiarrhythmics1. There is increasing evidence that these medications may be effective in the clinic; however, large randomized placebo controlled trials are yet to be carried out1.
The unexpectedly high prevalence of arrhythmic events after COVID-19 has caused a marked increase in interest in this topic. Suddenly, millions of patients share the same, repetitive and well-defined cause of systemic inflammation along with frequent cardiac arrhythmias2,3.
Arrhythmogenic effects of cytokines
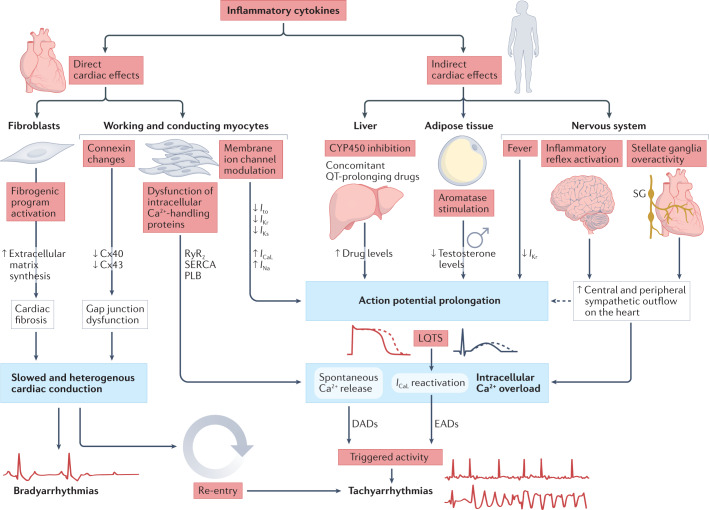
It is well known that inflammatory cytokines, particularly TNF, IL-1 and IL-6, exert important arrhythmogenic effects via several mechanisms, including direct cardiac activities and indirect systemic changes1 (Fig. 1). Direct effects include cardiac remodelling, characterized by structural and electrical changes. Electrical changes occur early (hours to days after exposure to cytokines) and result from complex modulatory activities of inflammatory cytokines on the expression and function of specific ion channels and gap junction-forming connexins, which cause inflammatory cardiac channelopathies, as well as on intracellular proteins responsible for calcium handling in cardiomyocytes, such as ryanodine receptors1. Cytokines can also induce (over weeks or months) structural remodelling by activating myofibroblast-driven synthesis of extracellular matrix responsible for cardiac fibrosis1. Overall, these phenomena lead to a prolonged action potential duration or corrected QT (QTc) interval, enhanced ectopic firing and slowed or heterogeneous propagation of electric impulses throughout the working and conducting myocardium. In turn, this promotes triggered and re-entry-driven tachyarrhythmias and bradyarrhythmias, as well as conduction disturbances1 (Fig. 1).
Fig. 1. Arrhythmogenic effects of inflammatory cytokines.
Inflammatory cytokines, particularly TNF, IL-1 and IL-6, can direct and indirectly promote cardiac arrhythmias. Direct effects consist of complex modulatory activities on the function and expression of cardiomyocyte proteins such as cardiac ion channels, leading to the prolongation of action potentials due to inhibition of outward repolarizing currents (Ito, IKs, IKr) and enhancement of inward depolarizing currents (ICaL, INa); key intracellular Ca2+-handling proteins of the cardiomyocyte, such as ryanodine receptors (RyR), sarco-endoplasmic reticulum calcium ATPase (SERCA) and phospholamban (PLB), favouring spontaneous sarco-endoplasmic reticulum (SR) Ca2+ release; and connexins (particularly connexin 40 (Cx40) and Cx43) responsible for gap-junction dysfunction and abnormal impulse conduction. In addition, inflammatory cytokines can induce cardiac fibrosis by activating myofibroblast-driven extracellular matrix synthesis, further impairing velocity and homogeneity of cardiac conduction. Indirect effects comprise the inhibition of cytochrome p450 (CYP450) in the liver, significantly increasing bioavailability of concomitant QT-prolonging drugs; stimulation of aromatase activity in adipose tissue with enhanced androgen-to-oestrogen conversion, promoting action potential and QTc prolongation (in males) due to reduced testosterone levels; and several effects on the nervous system, including fever and related temperature-mediated changes in cardiac ion channel biophysical properties (such as IKr decrease), and enhanced cardiac sympathetic system activation, via central hypothalamus-mediated (inflammatory reflex) and peripheral (left stellate ganglia activation) pathways, in turn further promoting action potential prolongation and intracellular Ca2+ overload. Overall, these phenomena are responsible for a slowed and heterogenous intra-cardiac conduction, favouring the development of bradyarrhytmias and conduction disturbances, but also re-entry circuit formation, which represents a key electrophysiological mechanism for re-entrant arrhythmias. At the same time, ectopic firing leading to triggered tachyarrhythmias is enhanced, this is due to an increased propensity to early and delayed after depolarizations (EADs and DADs, respectively) because of intracellular Ca2+ overload. These alterations are the result of action potential prolongation, which facilitates inward ICaL reactivation, and Ca2+-handling protein dysfunction-induced spontaneous SR Ca2+ release, respectively. ICaL, l-type calcium current; IKs or IKr, slow or rapid, respectively, component of the delayed-rectifier potassium current; Ito, transient outward potassium current; INa, sodium current; LQTS, long QT-syndrome; SG, stellate ganglia; TdP, Torsades de Pointes.
COVID-19 and cardiac arrhythmias
Large studies have reported an overall prevalence of arrhythmias after SARS-CoV-2 infection that ranges from 10 to 20%, although the incidence is greatly increased in individuals with severe disease. Supraventricular tachyarrhythmias, particularly atrial fibrillation, comprise more than 60% of these arrhythmias. The remaining arrhythmias are ventricular tachyarrhythmias, bradyarrhythmias and conduction defects, which are associated with remarkably high mortality2.
In the initial phases of the pandemic, it was thought that COVID-19-associated arrhythmias mostly resulted from disease-specific mechanisms, such as cardiac injury due to direct viral invasion, or electrophysiological effects of repurposed ‘off-label’ drugs, particularly antimalarials, azithromycin and protease inhibitors. However, it has become evident that direct virus-induced cardiac damage occurs in only a small minority of patients. Moreover, although the above mentioned drugs were progressively abandoned owing to a lack of efficacy, the risk of arrhythmia remained high.
On the basis of these observations, focus then shifted to the potential role of other arrhythmogenic factors, such as tissue hypoxia due to lung damage and the systemic high-grade inflammatory state. Indeed, it was demonstrated that both factors can induce cardiac injury, as reflected by an increase in troponin levels, and the rates and severity of this complication are similar in non-COVID-19 severe pneumonia or sepsis. Here, the contribution of inflammatory cytokines to cardiac injury may be particularly relevant. It has become apparent that several patients with COVID-19, as well as individuals with non-COVID-19 pneumonia, can develop life-threatening arrhythmic events, despite the absence of a severe respiratory impairment, when an intense systemic inflammatory activation was present4. In patients with COVID-19, increased levels of circulating proinflammatory cytokines directly correlated with increased troponin levels3. Moreover, in vitro treatment of cardiomyocytes with IL-6 and IL-1β resulted in disorganization of myofibrils, as well as reduced and erratic beating, similar to what was observed after infection of these cells with SARS-CoV-2 (ref.5). Thus, it is likely that cytokines also increase myocardial electric instability as a result of a direct cell injury, which indicates that the inherent arrhythmogenic effects of inflammatory cytokines are important drivers of COVID-19-associated arrhythmias, similar to what is observed in other inflammatory conditions. Indeed, it was reported that increased levels of IL-6 and IL-10 were predictive of the risk of atrial and ventricular arrhythmias in patients hospitalized with COVID-19 (ref.6). In a larger cohort of almost 1,000 patients hospitalized with COVID-19, an independent association between infection status and QTc prolongation and a direct correlation between IL-6 levels and QTc maximum were shown7. More recently, a retrospective analysis of almost 4,000 patients hospitalized with COVID-19 found that new-onset atrial fibrillation or atrial flutter was associated with inflammatory markers, including IL-6, regardless of patient baseline characteristics8. Notably, the authors also analysed patients who were hospitalized with influenza in the previous 3 years and observed similar rates of atrial fibrillation or atrial flutter, along with comparable levels of IL-6 and troponin8. In a study of arrhythmia prevalence in critically ill patients with COVID-19 or severe pneumonia of other origin, matched according to comorbidities and predisposing risk factors, increased levels of IL-6 were associated with high rates of atrial fibrillation and ventricular tachyarrhythmias9.
Conclusion
COVID-19 has provided us with the unique opportunity to observe how systemic cytokine release increases arrhythmic risk. It is increasingly evident that a very similar increase of arrhythmic events is also found in other inflammatory conditions. Although some clinical studies show beneficial effects of targeting inflammation and inflammatory cytokines to reduce arrhythmic risk1, this field is still largely unexplored. Notably, a large meta-analysis of randomized controlled trials recently demonstrated that therapy with glucocorticoid and/or IL-6 receptor antagonist significantly reduced short-term mortality in patients with severe COVID-19, including cardiovascular death10. Therefore, it is important to consider inflammation as an essential contributor to cardiac arrhythmic events, especially given that its clinical impact is likely to be relevant in conditions beyond COVID-19.
Acknowledgements
This paper was funded by BANDO RICERCA COVID-19 REGIONE TOSCANA, 2021, Progetto PRECARVID.
Competing interests
P.E.L. received a grant from Roche Italia S.p.A. outside the submitted work, in 2018. The other authors declare no conflicts of interest.
Footnotes
These authors contributed equally: Franco Laghi-Pasini, Mohamed Boutjdir, Pier Leopoldo Capecchi.
References
- 1.Lazzerini PE, Capecchi PL, Laghi-Pasini F. Systemic inflammation and arrhythmic risk: lessons from rheumatoid arthritis. Eur. Heart J. 2017;38:1717–1727. doi: 10.1093/eurheartj/ehw208. [DOI] [PubMed] [Google Scholar]
- 2.Coromilas EJ, et al. Worldwide survey of COVID-19-associated arrhythmias. Circ. Arrhythm. Electrophysiol. 2021;14:e009458. doi: 10.1161/CIRCEP.120.009458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wang Y, et al. Cardiac injury and clinical course of patients with coronavirus disease 2019. Front Cardiovasc. Med. 2020;7:147. doi: 10.3389/fcvm.2020.00147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Elsaid O, McCullough PA, Tecson KM, Williams RS, Yoon A. Ventricular fibrillation storm in coronavirus 2019. Am. J. Cardiol. 2020;135:177–180. doi: 10.1016/j.amjcard.2020.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddiq MM, et al. Functional effects of cardiomyocyte injury in COVID-19. J. Virol. 2022;96:e0106321. doi: 10.1128/JVI.01063-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guan H, et al. Arrhythmias in patients with coronavirus disease 2019 (COVID-19) in Wuhan, China: incidences and implications. J. Electrocardiol. 2021;65:96–101. doi: 10.1016/j.jelectrocard.2021.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rubin GA, et al. Cardiac corrected QT interval changes among patients treated for COVID-19 infection during the Early phase of the pandemic. JAMA Netw. Open. 2021;4:e216842. doi: 10.1001/jamanetworkopen.2021.6842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Musikantow DR, et al. Atrial fibrillation in patients hospitalized with COVID-19: incidence, predictors, outcomes, and comparison to influenza. JACC Clin. Electrophysiol. 2021;7:1120–1130. doi: 10.1016/j.jacep.2021.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jirak P, et al. Higher incidence of stroke in severe COVID-19 is not associated with a higher burden of arrhythmias: comparison with other types of severe pneumonia. Front Cardiovasc. Med. 2021;8:763827. doi: 10.3389/fcvm.2021.763827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shankar-Hari M, et al. Association between administration of IL-6 antagonists and mortality among patients hospitalized for COVID-19: a meta-analysis. JAMA. 2021;326:499–518. doi: 10.1001/jama.2021.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]