Abstract
Objectives
To describe a novel technique for partial anatomic penile amputation using a thoracoabdominal stapler in dogs and to report any associated short‐term peri‐operative complications and clinical outcomes associated with the procedure.
Materials and methods
Medical records from a tertiary referral hospital were reviewed for dogs undergoing penile amputation and scrotal urethrostomy between October 2007 and December 2019. Data collected included patient signalment, clinical signs on presentation, indication for penile amputation, surgical technique, duration of surgery, post‐operative complications, and short‐term outcome.
Results
Nine dogs were included in the study. Indications for surgery were for treatment of chronic paraphimosis and priapism (n = 3), recurrent urethral prolapse (n = 2), balanoposthitis (n = 1), masses arising from the penis, prepuce, and/or urethra (n = 2), and penile trauma (n = 1). All dogs underwent a scrotal urethrostomy followed by a partial penile amputation with a thoracoabdominal stapler. All dogs suffered mild post‐operative haemorrhage from the urethrostomy stoma. On recovery from general anaesthesia, 2/9 dogs were painful and another 2/9 dogs were dysphoric. Two dogs experienced incisional complications with mild swelling around the urethrostomy stoma. One dog experienced an infection of the penile amputation site 21 days after surgery. The short‐term outcomes for this procedure were excellent in 8/9 dogs. These outcomes were based on owner assessment of comfort and monitoring throughout the recovery period, manual palpation of the surgical site at the time re‐evaluation, and surgeon visualization of successful voluntary urination 14–35 days after surgery.
Clinical significance
Use of a thoracoabdominal stapler is effective in achieving partial anatomic penile amputation in dogs.
Keywords: Penile amputation, Phallectomy, Thoracoabdominal stapler, Soft tissue surgery
The objective of this study was to describe a complete penile amputation technique using a thoracoabdominal stapler in dogs. The use of a thoracoabdominal stapler is an effective and efficient technique to achieve partial penile amputation in dogs in small animal practice.
1. INTRODUCTION
Penile amputation with concurrent pre‐scrotal, scrotal, or perineal urethrostomy is the treatment of choice for diseases of the prepuce, glans penis, os penis, and penile urethra that are refractory to medical management, or have resulted in irreversible pathologic changes to the structure and function of those locoregional tissues. Examples of such diseases include persistent balanoposthitis, recurrent urethral prolapse, chronic paraphimosis, priapism, penile trauma, and neoplasia of the prepuce, penis, and penile urethra (Burrow et al., 2011). Location of the site for penile amputation is determined by the location and extent of the pathology. Partial penile amputation has been described by Hobson et al. as a treatment for small, non‐neoplastic distal penile and preputial lesions (Hobson, 1993). Alternatively, complete penile amputation is recommended for larger distal penile and preputial lesions, lesions located at a level over or proximal to the os penis, and neoplastic lesions requiring radical excision (Hobson, 1993). A common surgical approach to partial penile amputation consists of transecting the penile body proximal to the os penis with a scalpel blade, ligating major penile arterial vessels, apposing the tunica albuginea, and performing a pre‐scrotal, scrotal, or perineal urethrostomy (Burrow et al., 2011). Complications associated with partial penile amputations are primarily associated with the scrotal urethrostomy, which includes urethral stricture, dehiscence of the stoma site, infection of the stoma site, chronic lower urinary tract infections, transient haematuria, and blood clot formation at the stoma site (Bilbrey et al., 1991; Boothe, 2003).
Understanding of canine urethral and vascular anatomy is crucial to the planning of any surgical intervention of the penile tissues. The penile segment of the canine urethra begins at the ischial arch and extends through all three divisions of the penis: the root, the body, and the glans. Within all three divisions, the urethra is circumferentially surrounded by the corpus spongiosum. As it traverses through the glans, it lies ventral to the os penis within the urethral groove of the bone, and finally terminates at the external urethral orifice. The blood supply to the penis is primarily provided by the internal pudendal artery as it terminates as the artery of the penis. The artery of the penis divides into the artery of the bulb, deep artery of the penis, and dorsal artery of the penis, the last of which further subdivides into the preputial branch, superficial branch, and deep branch. The penile blood supply is intricate and complex because many of these arterial channels anastomose with each other, making meticulous haemostasis crucial to any surgical intervention of the penile tissues (Evans & de Lahunta, 2013).
Thoracoabdominal (TA) staplers are hand‐held surgical devices used in conjunction with separately packaged cartridges that engage two or three rows of staggered sterile titanium staples depending on the colour and application of the desired cartridge. The use of TA staplers has been previously reported in the performance of lung lobectomies (Walshaw, 1994), liver lobectomies (Lewis et al., 1990), partial gastrectomies (Clark, 1994), end‐to‐end intestinal anastomoses (Ullman, 1994), typhlectomies (Clark & Pavletic, 1992), nephrectomies (Baniel & Schein, 1996), pancreatectomies (Bellah, 1994), and surgical removal of atrial masses associated with the right auricular appendage (Monnet & Orton, 1994). To the authors’ knowledge, no literature exists describing or evaluating the novel procedure described in this study. This retrospective case‐series describes nine partial penile amputation cases using a TA stapler. The TA stapler is preferentially used in human medicine for its ability to provide superior tissue apposition attained using a simple and reliable technique resulting in shortened operating times (Halevy & Sadé, 1983; Zhang et al., 2013). In veterinary medicine, TA staplers have been described to decrease intra‐operative haemorrhage, decrease tissue handling and necrosis, and shorten the length of the time required to achieve tissue apposition compared to hand sewing with sutures (Risselada et al., 2010; Rosser et al., 2012). For these reasons, the authors hypothesize that the use of a TA stapler provides an easy, effective, efficient, less traumatic, and precise alternative that allows for superior intra‐operative haemostasis and decreased operative time compared to traditional techniques. The proposed technique allows for ease in ligating and transecting the penile tissues, and less intra‐operative cavernous bleeding. The study describes the procedure and the indications for surgery, post‐operative complications, and short‐term outcomes of patients that underwent penile amputation with a TA stapler.
2. MATERIALS AND METHODS
Medical records between October 4th 2007 and December 22nd 2019 from a tertiary referral centre were reviewed for dogs that underwent a partial penile amputation using a TA stapler, followed by a scrotal urethrostomy. Data collected included signalment, clinical signs on presentation, indications for penile amputation, surgical technique, duration of the surgical procedure, peri‐operative complications, post‐operative complications, and short‐term outcomes. For the purpose of this study, short‐term outcomes were assessed through recheck examinations over a 14‐ to 35‐day period after surgery. Parameters used to assess outcome were patient comfort level both at rest and during micturition, as well as successful and consistent urine voiding in the absence of stranguria after surgery. Dogs were excluded from this study if their medical records were incomplete or if a penile amputation was performed using scalpel excision and a hand‐sewn closure.
All nine dogs were pre‐medicated, induced, and maintained under general anaesthesia with an individualized, patient‐specific anaesthetic protocol developed by a board‐certified anaesthesiologist on the basis of each dog's American Society of Anaesthesiologists (ASA) status.
In all nine cases, a standard scrotal urethrostomy was performed prior to amputation of the penis. Each patient was placed in dorsal recumbency, and the proposed surgery site underwent sterile preparation prior to draping. An appropriately sized red rubber urinary catheter was inserted into the urethral orifice and passed retrograde to the level of the urinary bladder. A full‐thickness elliptical skin incision was made with a #10 scalpel blade around the scrotum with the long axis in the cranial‐to‐caudal direction. Haemostasis was achieved using monopolar electrocautery and/or digital pressure. All nine dogs had previously been castrated, and removal of the scrotum was performed as described. The subcutaneous tissues were dissected until the scrotal remnant was removed. The retractor penis muscle was exposed, elevated from its attachments on the ventral urethra, and retracted laterally. The urethra was visibly distended with the aid of the red rubber urinary catheter. A stab incision was made with a #11 scalpel blade along the ventral midline of the urethra. Tenotomy scissors were used to extend the incision to a length that was five to eight times the diameter of the urethra (Smeak, 2000). The red rubber urinary catheter was removed from the urethra. Note that 4‐0 monofilament, absorbable suture material (Monocryl) was used to place a simple interrupted suture in the cranial and caudal aspect of the stoma on midline, followed by another two interrupted sutures placed approximately 3 mm adjacent to, and on either side of, the initial midline suture. A separate simple continuous pattern was used to appose the urethral mucosa to the skin on either side of stoma, taking care to pass the needle first through the urethral mucosa then through the skin.
Following the scrotal urethrostomy, a second elliptical skin incision was made with a #10 scalpel blade around the base of the penis and prepuce, taking care to leave at least 1 cm of skin between the caudal aspect of the penile amputation site and the cranial aspect of the scrotal urethrostomy. Allis tissue forceps were used to clamp the cranial aspect of the prepucial folds, which provided traction as the penis and prepuce were pulled caudally and dissected from the underlying connective tissue (Hobson 1993). Once an appropriate amputation site was determined caudal to the os penis, the dorsal penile vessels were individually ligated with slowly absorbable, monofilament suture material (PDS). Using a TA 55 stapler in dogs larger than 20 kg and a TA 30 stapler in dogs smaller than 20 kg, the stapler was loaded with either 4.8 mm staples (green cartridge) or 3.5 mm staples (blue cartridge). The TA stapler was placed across the penile shaft at a level caudal to the os penis, the device was fired, and a scalpel blade was used to transect the penile tissue distal to the staple line (Figures 1 and 2). The penile stump was assessed for haemorrhage and was allowed to retract caudally between the connective tissues and towards the urethrostomy site (Figure 3). The deep subcutaneous tissues were apposed with rapidly absorbable monofilament suture material (Monocryl) in a simple continuous pattern. The intradermal layer was closed with rapidly absorbable monofilament suture material (Monocryl) in a continuous horizontal mattress pattern. Depending on surgeon preference, external skin sutures were also placed in an interrupted cruciate pattern, using non‐absorbable monofilament suture material (Nylon).
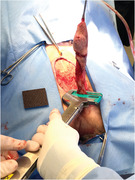
FIGURE 1.
Placement and subsequent firing of the thoracoabdominal (TA) stapler caudal to the os penis
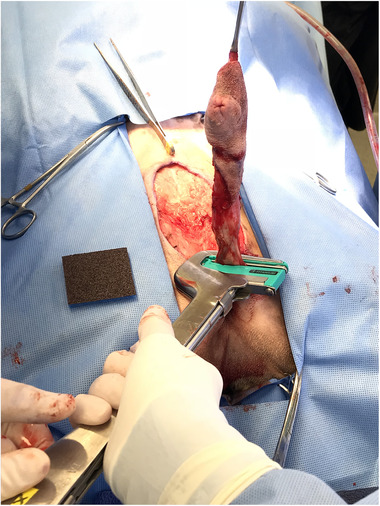
FIGURE 2.
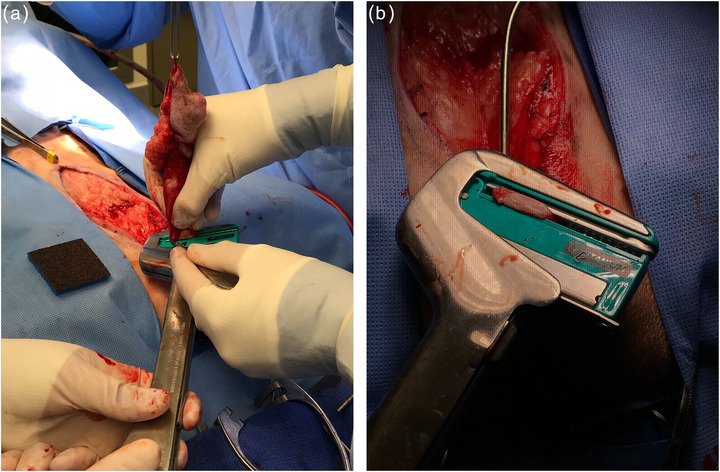
Transection of the penile shaft distal to the staple line (a). The staple line and associated tissues remain in the stapler before the locking pin is opened and the tissues are released (b)
FIGURE 3.
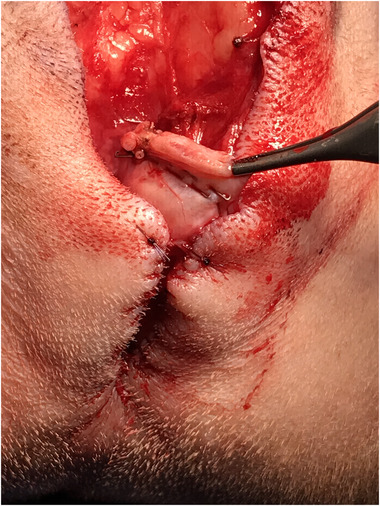
Assessment of the penile stump for haemorrhage after transection
3. RESULTS
Nine dogs underwent a partial penile amputation using a TA stapler following a scrotal urethrostomy. The details of each case are summarized in Table 1.
TABLE 1.
Patient information, stapler size, staple size, and complications for nine dogs that underwent penile amputation using a thoracoabdominal stapler
| Case | Age (years) | Sex | Breed | Presenting signs | Diagnosis | Hosp. (days) | Cartridge length used | Staple length used | Duration of surgical procedure | Surgical complications | Post‐operative complications |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 1 | MC | Boxer | Chronic paraphimosis | Idiopathic priapism | 4 | 55 | 4.8 mm (green) | 85 min | None | Swollen stoma site |
| 2 | 8 | MC | Mixed breed dog | Chronic paraphimosis, necrotic glans penis | Idiopathic priapism | 3 | 30 | 3.5 mm (blue) | 90 min | None | None |
| 3 | 7 | MC | Miniature Schnauzer | Chronic paraphimosis, self‐traumatization | Idiopathic priapism | 2 | 30 | 3.5 mm (blue) | 60 min | None | None |
| 4 | 5 | MC | English Bulldog | Recurrent urethral prolapse | Recurrent urethral prolapse | 2 | 55 | 3.5 mm (blue) | 90 min | None | None |
| 5 | 2 | MC | English Bulldog | Recurrent urethral prolapse | Recurrent urethral prolapse | 3 | 55 | 3.5 mm (blue) | ? | None | Amputation site dehiscence |
| 6 | 11 | MC | Mixed breed dog | Dysuria, stranguria | Fibrosarcoma | 5 | 30 | 3.5 mm (blue) | 75 min | None | None |
| 7 | 12 | MC | Boston Terrier | Prepucial mass | Balanoposthitis | 3 | 30 | 3.5 mm (blue) | 90 min | None | None |
| 8 | 9 | MC | Mixed breed dog | Penile mass | Mast cell tumour | 4 | 30 | 3.5 mm (blue) | 85 min | None | None |
| 9 | 2 | MC | Standard Poodle | Penile trauma, acute paraphimosis | Penile trauma | 2 | 90, 55 | 4.8 mm (green), 3.5 mm (blue) | ? | Inadequate compression of staples | Swollen stoma site |
Abbreviation: MC, male castrated.
The dogs in this study were between the ages of 14 months to 12 years, with a median age of 6 years and 4 months. Three dogs underwent penile amputation and scrotal urethrostomy as treatment for idiopathic priapism, two dogs for recurrent urethral prolapse, two dogs for penile or urethral masses, one dog for chronic inflammation of the prepuce and glans penis, and one dog for paraphimosis secondary to trauma. The penile amputation was performed caudal to the os penis in all cases. Smaller patients accommodated a smaller stapler size (TA 30 stapler) and required staples with a shorter leg length (3.5 mm staples). Larger patients accommodated the medium sized stapler (TA 55 stapler) and required staples with a longer leg length (4.8 mm staples). In one dog, a TA 90 stapler was used with 4.8 mm staples, and adequate compression of the tissues was not achieved after the device was fired. With the original staple line left intact, a TA 55 stapler was subsequently used with 3.5 mm staples which were placed proximal to the original line, and this accomplished adequate haemostasis prior to transection of the penile shaft.
The length of time required to perform both the scrotal urethrostomy and penile amputation using a TA stapler ranged from 60 min to 90 min, with a median time of 105 min. No peri‐operative complications occurred with the exception of the single case requiring repeat stapling. No peri‐anaesthetic complications were observed. Mild post‐operative haemorrhage was observed at the scrotal urethrostomy site in all cases, which resolved with non‐steroidal anti‐inflammatory (NSAID) and antibiotic therapy, monitoring, and regular nursing care that included simple monitoring and maintenance of a clean peri‐surgical area using warm compresses when necessary. Two dogs experienced post‐operative incisional complications at the stoma site. The first was due to self‐trauma from the edge of his Elizabethan collar, which resulted in mild haemorrhage and swelling of the stoma site. The second dog experienced a stoma site complication due to licking the incision after removing his Elizabethan collar, resulting in swelling of the stoma site and mild discomfort during micturition. In both cases, the stoma site swelling resolved within 14 days with NSAID and antibiotic therapy while ensuring compliance in maintaining an Elizabethan collar. One dog experienced post‐operative incisional complications at the amputation site from licking the incision after removing his Elizabethan collar. Sedated examination 7 days after hospital discharge revealed incisional skin dehiscence with a subcutaneous haematoma present at the level of the penile amputation. On examination, it was evident that the penile stump appeared healthy, and only the skin and subcutaneous layers of the amputation site required management. The patient was subsequently hospitalized for daily open wound management for 5 days. After four wound dressing changes over a 5‐day hospitalization period, the wound was closed primarily on the fifth day. The incision site cultured positive for haemolytic Escherichia coli that was susceptible to cephalexin. Twenty‐one days after hospital discharge for wound management (35 days after surgery), the wound was well healed after completing the 3‐week course of antimicrobial therapy.
The seven dogs with non‐neoplastic indications for penile amputation were clinically doing well when rechecked 14–35 days after surgery, with no discernible complications. The dog with a penile mast cell tumour was also doing well when assessed 2 weeks after surgery. Given that each of these patients was assessed to be comfortable at rest (with no pain or irritation of the surgery sites), able to urinate voluntarily, and were comfortable during said urinations without any evidence of discomfort or straining at 14–35 days after surgery, the outcome for each of these dogs was considered excellent. Outcome for the dog with a urethral fibrosarcoma is undetermined due to lack of follow‐up after discharge.
4. DISCUSSION
The focus of this study pertains to the technical approach, procedural length, and patient outcomes specifically regarding partial penile amputation with a TA stapler. This study shows that penile amputations can be performed successfully, safely, and effectively using a TA stapler.
Prior to firing a TA stapler, the staple cartridges are slid into the U‐shaped opening of the TA stapler, which is also the end of the device through which tissues are inserted. Once activated, the locking pin closes the open end of the ‘U’ and secures the tissues in place. When the device trigger is depressed, the staples are released from the cartridge, engaged through the tissues, and pushed against the anvil to form a B‐shaped conformation. This staple shape seals the vessels and tissues along the staple line without disrupting microcirculation by preserving the capillaries that pass through the centre of each staple. This, in turn, allows for a reliable, secure, non‐crushing tissue closure that promotes tissue healing along the cut surface while ensuring excellent haemostasis (Tobias, 2007).
Of the nine cases, only one peri‐operative complication was encountered in which the initial attempt to place a staple line across the base of the penile shaft resulted in inadequate compression of the tissues and resultant haemorrhage. Initially, a TA‐90 stapler was used with 4.8 mm staples. Although the 4.8 mm staple cartridge failed to sufficiently engage the penile tissue, it provided enough compression to allow a 3.5 mm cartridge to be placed 2–3 mm proximal to the first staple line using a TA‐55 stapler. This additional line of staples provided adequate compression of the tissues with no further haemorrhage. We believe that this complication occurred as a result of user error, with the suspicion that the trigger was not depressed fully when firing the first line of staples. In another patient of similar body size, the 4.8 mm staples were successfully used with a TA‐55 stapler, which suggests that the staple size is likely not a contributing factor of this peri‐operative complication. Given that this complication only occurred in a single patient, we can conclude that user error is more likely the cause. No additional peri‐operative complications occurred in this case, nor in any of the other cases in this series.
Post‐operative complications were rare. Two of the nine cases had moderate swelling of the stoma site a few days after the surgery, which is a previously reported post‐operative complication (Bilbrey et al., 1991). Absorbable suture was used for the initial three simple interrupted sutures at the cranial aspect of the stoma, followed by two simple continuous runs of suture along the lateral margins of the stoma. Given that the post‐operative swelling did not occur preferentially at either the location of the interrupted sutures or the continuous sutures, it is unlikely that the suture pattern is related to this particular complication. In a study performed by Burrow et al. (2011) post‐operative haemorrhage from the stoma site was appreciated regardless of urethrostomy closure technique. Swelling and bruising of the urethrostomy site is reported to occur in approximately 29% of cases (Bilbrey et al., 1991). Mild post‐operative haemorrhage was observed in all cases in this study, and this is considered to be an expected observation after surgery given that the haemorrhage resolved with minimal conservative management and regular nursing care. Given that this was observed in all cases with no deleterious effects, mild post‐operative haemorrhage can be considered a sequela rather than a complication for this specific procedure. Self‐limiting post‐operative haemorrhage is reported to occur in greater than 70% of cases (Bilbrey et al., 1991). It is important to note that these complications associated with the stoma are unrelated to the penile amputation procedure. Therefore, it is unlikely that the stomal complications are attributed to the novel penile amputation technique using a TA stapler described in this study.
Similarly, the single post‐operative complication associated with the penile amputation site discussed in this study was determined to be a haematoma coupled with an infection, which is also unrelated to the partial penile amputation technique described herein. The current literature does not report post‐operative infection rates in dogs after partial penile amputation. However, based on this study, it can be concluded that infection is not a common occurrence after partial penile amputation. Given that the subcutaneous haematoma observed in this case was noted after a known history of self‐trauma, it is difficult to discern if the haematoma developed as a consequence of trauma or inadequate haemostasis. Based on this study, it can be concluded that haematoma formation is not a common occurrence after partial penile amputation. The current veterinary literature does not report intra‐operative complications specifically associated with penile amputations performed using traditional transection and hand‐sewn closure. This limits our ability to compare complication rates between the traditional technique and the novel technique described in this study. Aside from user error when operating a TA stapler, no other intra‐operative complications were observed when performing penile amputations in this study, which suggests that it is at least as safe and effective as traditional transection and hand‐sewn closure.
There is no validated outcome measure that currently exists for penile amputation in dogs. Although no formal outcome classification system was used in the study, it was agreed upon that the following criteria would be used to evaluate short‐term outcome: (a) successful voluntary urination, (b) comfort during rest, and (c) comfort during urination. An ‘excellent’ outcome was defined as meeting all three criterion, which all of the dogs in the study did.
Thoracoabdominal staplers may offer certain advantages over scalpel transection and hand‐sewn closure for penile amputation. Peri‐operative bleeding is minimal since haemostasis is achieved prior to tissue transection when using the TA stapler. This difference of establishing haemostasis prior to transection compared to afterwards (as seen with the traditional hand‐sewn technique) offers the ability to prevent significant haemorrhage from the highly vascular cavernous tissues when they are incised. With hand‐sewn closure, haemostasis is not fully achieved until the entire cut surface is sutured in order to tamponade the haemorrhage; this leaves opportunity for blood loss in the time between starting and finishing the tissue apposition. Additionally, with enough experience, we believe that stapling for penile amputations may also be faster, resulting in decreased surgical time and time under general anaesthesia. In summary, the TA stapler effectively seals canine penile tissue and provides a means for performing a penile amputation followed by urethrostomy. The technique offers a safe and simple alternative for penile amputation compared to traditional transection followed by hand‐sewn closure. Additional research is warranted to investigate the efficacy and safety of both techniques in a larger number of clinical patients.
The estimated cost for a TA 60 stapler handpiece is $80, with the blue (3.5 mm) and green (4.8 mm) cartridges costing $40 and $50, respectively (Covidien Inc., Mansfield, MA, USA). Although most surgical practices are likely to keep TA staplers in stock, these financial figures may provide rationale for general practitioners to consider purchasing a TA stapler for their practice.
Limitations of this study include the small sample size, lack of short‐term outcome in one case, and lack of long‐term outcomes in all cases. Outcome data are crucial in understanding the consequences of this procedure and ensuring that this novel technique is not resulting in any long‐term morbidity to the patients that underwent penile amputation with a TA stapler. Furthermore, this study does not offer comparison to data collected from patients that underwent penile amputation using the traditional hand‐sewn technique. Variables measuring anaesthetic time, peri‐operative haemorrhage, and outcome assessment from the hand‐sewn technique would allow for investigators to explore which of the two techniques may be superior. Although this information was not investigated in this case series, it offers good rationale for performing a follow‐up study comparing both surgical techniques.
AUTHOR CONTRIBUTIONS
Patel ‐ Design, data collection, data analysis and processing, literature review, authorship, and critical review.
Jones ‐ Design, data collection, data analysis and processing, supervision of project, authorship, and critical review.
McLoughlin ‐ Conception, design, data collection, data analysis and processing, supervision of project, authorship, and critical review.
Howard ‐ Design, data collection, data analysis and processing, supervision of project, authorship, and critical review.
ETHICS STATEMENT
Submission of the manuscript by the corresponding author and accompanying authors is an original work and not published previously and is not being considered for publication by another source.
TRANSPARENT PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.723
CONFLICT OF INTEREST
The authors declare no conflict of interest.
Patel, N. , Jones, S. C. , McLoughlin, M. A. , & Howard, J. (2022). Partial penile amputation using a thoracoabdominal stapler in nine dogs. Veterinary Medicine and Science, 8, 437–444. 10.1002/vms3.723
DATA AVAILABILITY STATEMENT
Data was generated from The Ohio State Veterinary Medical Center medical records department. The data supporting the findings of this study are available from the corresponding author by request.
REFERENCES
- Baniel, J. , & Schein, M. (1996). Partial nephrectomy performed with linear stapling device—An experimental study. Scandinavian Journal of Urology and Nephrology, 30, 253–255. [DOI] [PubMed] [Google Scholar]
- Bellah, J. R. (1994). Surgical stapling of the spleen, pancreas, liver, and urogenital tract. Veterinary Clinics of North America: Small Animal Practice, 24, 375–394. [DOI] [PubMed] [Google Scholar]
- Boothe, H. W. (2003). Penis, prepuce and scrotum. In Slatter D., & Saunders W. B. (Eds.), Textbook of small animal surgery (3rd ed., pp. 1531–1542). W B Saunders Co Ltd. [Google Scholar]
- Bilbrey, S. A. , Birchard, S. J. , & Smeak, D. D. (1991). Scrotal urethrostomy: A retrospective review of 38 dogs (1973—1988). Journal of the American Animal Hospital Association, 27, 560—564. [Google Scholar]
- Burrow, R. D. , Gregory, S. P. , Giejda, A. A. , & White R. N. (2011). Penile amputation and scrotal urethrostomy in 18 dogs. Veterinary Record, 169(25), q657. [DOI] [PubMed] [Google Scholar]
- Clark, G. N. (1994). Gastric surgery with surgical stapling instruments. Veterinary Clinics of North America: Small Animal Practice, 24, 279–304. [DOI] [PubMed] [Google Scholar]
- Clark, G. N. , & Pavletic, M. M. (1992). Typhlectomy in dogs using a stapling instrument. Journal of the American Animal Hospital Association, 28, 511–517. [Google Scholar]
- Evans, H. E. , & de Lahunta, A. (2013). The urogenital system. In Saunders W. B. (Ed.), Miller's anatomy of the dog (4th ed., pp. 376–382). Saunders. [Google Scholar]
- Halevy, A. , & Sadé, J. (1983). The use of thoracoabdominal staplers in ENT surgery. European Archives of Oto‐Rhino‐Laryngology, 237, 185–190. [DOI] [PubMed] [Google Scholar]
- Hobson, H. P. (1993). Surgical pathophysiology of the penis. In Bojrab M. J., Saunders W. B., Lea & Febiger (Eds.), Disease mechanisms in small animal surgery (2nd ed., pp. 552–559). Teton NewMedia; [Google Scholar]
- Lewis, D. D. , Bellenger, C. R. , Lewis, D. T. , & Latter, M. R. (1990). Hepatic lobectomy in the dog—A comparison of stapling and ligation techniques. Veterinary Surgery, 19, 221–225. [DOI] [PubMed] [Google Scholar]
- Monnet, E. , & Orton, E. C. (1994). Surgical stapling devices in cardiovascular surgery. Veterinary Clinics of North America: Small Animal Practice, 24, 367–374. [DOI] [PubMed] [Google Scholar]
- Risselada, M. , Ellison, G. W. , Bacon, N. J. , Polyak, M. M. R. , van Gilder, J. , Kirkby, K. , & Kim, S. E. (2010). Comparison of 5 surgical techniques for partial liver lobectomy in the dog for intraoperative blood loss and surgical time. Veterinary Surgery, 39, 856–862. [DOI] [PubMed] [Google Scholar]
- Rosser, J. M. , Brounts, S. , Livesey, M. , & Wiedmeyer, K. (2012). Comparison of single layer staple closure versus double layer hand‐sewn closure for equine pelvic flexure enterotomy. The Canadian Veterinary Journal, 53(6), 665–669. [PMC free article] [PubMed] [Google Scholar]
- Smeak, D. D. (2000). Urethrotomy and urethrostomy in the dog. Clinical Techniques in Small Animal Practice, 15, 25–34. [DOI] [PubMed] [Google Scholar]
- Tobias, K. M. (2007). Surgical stapling devices in veterinary medicine: a review. Veterinary Surgery, 36(4), 341–349. [DOI] [PubMed] [Google Scholar]
- Ullman, S. L. (1994). Surgical stapling of the small intestine. Veterinary Clinics of North America: Small Animal Practice, 24, 305–322. [DOI] [PubMed] [Google Scholar]
- Walshaw, R. (1994). Stapling techniques in pulmonary surgery. Veterinary Clinics of North America: Small Animal Practice, 24, 334–366. [DOI] [PubMed] [Google Scholar]
- Zhang, X. , Liu, Z. , Li, Q. , Liu, X. , Li, H. , Liu, W. , Li, Q. , Guo, Z. , & Zeng, Z. (2013). Using a linear stapler for pharyngeal closure in total laryngectomy. European Archives of Oto‐Rhino‐Laryngology, 270, 1467–1471. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data was generated from The Ohio State Veterinary Medical Center medical records department. The data supporting the findings of this study are available from the corresponding author by request.


