Abstract
Background
The emergence of methicillin‐resistant Staphylococcus aureus (MRSA) and methicillin‐resistant Staphylococcus pseudintermedius (MRSP) have a significant health impact on people with direct or supportive occupations in veterinary medicine including veterinarians, animal handlers, laboratory personnel and pet owners.
Objectives
This cross‐sectional survey was conducted to determine the prevalence of and risk factors for S. aureus, S. pseudintermedius, MRSA and MRSP in dogs in Bangladesh.
Methods
A total of 358 swab samples were collected from different body sites of 150 dogs attending a university teaching veterinary hospital between January and June 2018. Standard bacteriological procedures were followed to isolate Staphylococcus, and identification was confirmed to the species level by PCR to detect the nuc gene. MRSA and MRSP were confirmed by the presence of the mecA gene.
Results
The prevalence of coagulase‐positive S. aureus and S. pseudintermedius in dogs were 16% and 45.3%, respectively. S. aureus and S. pseudintermedius isolates displayed the highest resistance against nalidixic acid (95.2% and 91%, respectively) and erythromycin (89.3% and 84.7%, respectively). Notably, all the staphylococcal isolates showed resistance to ≥3 antimicrobial classes. The prevalence of MRSA and MRSP in dogs was 8.7% and 6%, respectively. Multivariable logistic regression analysis identified the following variables as risk factors for MRSA colonisation in dogs: dogs with dermatitis (odds ratio [OR], 12.24, 95% CI: 3.12–57.33; p < 0.001) and history of antibiotic use (OR 8.73, 95% CI: 2.23–43.10; p < 0.001). Presence of otitis (OR 14.22; 95% CI: 1.64–103.58; p = 0.008) and oral lesions (OR 9.48, 95% CI: 1.14–64.82; p = 0.002) were identified as the significant risk factors for the carriage of MRSP.
Conclusions
The circulation of multidrug‐resistant S. aureus and S. pseudintermedius is a serious concern to dogs and humans. To our knowledge, this is the first report of S. pseudintermedius and MRSP affecting dogs in Bangladesh.
Keywords: carriage, dogs, MRSA, MRSP, risk factors
The emergence of methicillin‐resistant Staphylococcus aureus (MRSA) and Staphylococcus pseudintermedius (MRSP) have a significant health impact on occupational people and pet owners. Although the occurrence of MRSA and MRSP in humans and animals has been reported in many other countries, the information on the magnitude of S. aureus and S. pseudintermedius infection in dogs in Bangladesh and their antimicrobial resistance pattern is limited, if not absent. This manuscript, for the first time, describes the circulation of mecA gene bearing S. pseudintermedius in dogs in Bangladesh.

1. INTRODUCTION
Staphylococci represent significant opportunistic bacterial pathogens in humans and animals. The most common species associated with companion animal infections are coagulase‐positive Staphylococcus aureus and Staphylococcus pseudintermedius (Weese & van Duijkeren, 2010). S. aureus causes many clinical conditions in humans and animals, ranging from mild skin infections to life‐threatening bacteremia (Kong et al., 2016; O'Gara, 2017). S. pseudintermedius, a skin commensal, is frequently isolated from dogs with cutaneous and wound infections (Weese & van Duijkeren, 2010). Humans are not permanently colonised with S. pseudintermedius, but can become transient carriers if they come in close contact with infected dogs. Therefore, zoonotic transmission of S. pseudintermedius from dogs to humans is a public health concern (Paul et al., 2011). Moreover, humans colonised with S. pseudintermedius may act as a vehicle for transmission between animals which is also an important concern.
Since the inception of antibacterial drugs into the practice of modern medicine, resistant staphylococci have evolved in response to antibiotic selective pressure. Many staphylococcal species exhibit some degree of antimicrobial resistance (Schwarz et al., 2017). Moreover, a number of reports on companion animals colonised or infected with multiple drug‐resistant organisms, such as methicillin‐resistant S. aureus (MRSA) and methicillin‐resistant S. pseudintermedius (MRSP) have been published (Algammal et al., 2020; Murphy et al., 2009). Over the past decade, there has been significant concern about antimicrobial resistance that accrete considerable public health attention, namely the emergence and spread of MRSA and MRSP in humans and animals (Guardabassi et al., 2013). In human medicine, methicillin resistance in S. aureus strains have contributed to the scope of multidrug resistance since the early 1960s (Barber, 1961). Infection with MRSA in small animals, particularly in dogs, has been recorded in many countries, with wound infections, surgical site infections, pyoderma, pyogenic endocarditis, suppurative pneumonia, osteomyelitis, septic arthritis, otitis and urinary tract infections (Algammal et al., 2020; Weese & van Duijkeren, 2010). Over the past few years, S. pseudintermedius has gained importance due to the increasing rate of resistance to methicillin and non‐β‐lactam antibiotics. There are several reports published on S. aureus and S. pseudintermedius isolates showing resistance to many antimicrobials authorised for use in veterinary medicine (Perreten et al., 2010; Weese & van Duijkeren, 2010). Both MRSA and MRSP infections have been shown to occur in humans and animals that have high zoonotic and zooanthroponotic potential. Similarly, pets are increasingly considered potential reservoirs of MRSA and MRSP in cases of refractory or recurrent human infections (Loeffler & Lloyd, 2010).
Dogs are regarded as one of the best ancient companion animals and remain in close contact with humans. Like other parts of the world, the tendency towards rearing dogs is increasing nowadays in Bangladesh. Thus human–animal behavioural relationships are becoming more intimate which may create a potential chance to transmit zoonotic pathogens like S. aureus and S. pseudintermedius from dogs to humans. However, information on the magnitude of S. aureus and S. pseudintermedius infection in dogs in Bangladesh and their antimicrobial resistance pattern is limited, if not absent. As these organisms are usually resistant to a wide range of antimicrobial agents (Algammal et al., 2020), clinical management of animals infected with MRSA and MRSP represents a great challenge to the veterinary profession. The objectives of this study were to determine the prevalence of S. aureus and S. pseudintermedius in dogs in Bangladesh, their antimicrobial resistance pattern, and identify the risk factors associated with MRSA and MRSP colonisation in dogs.
2. MATERIALS AND METHODS
2.1. Collection and preparation of samples
To determine coagulase‐positive S. aureus and S. pseudintermedius in dogs, we conducted a cross‐sectional survey on the dogs admitted to a Teaching Veterinary Hospital (TVH) from January to July 2018. The average number of dogs admitted daily to the TVH is approximately 10. All the dogs were registered to the hospital for the purpose of treatment or vaccination or for general health check‐up. The health status of the dogs was determined based on clinical examinations by the veterinarian on duty. Immediately upon admission swab samples were taken from the perineum and mouth from each healthy dog. One additional swab from each of the infection sites was collected if there were any skin wounds, dermatitis, abscess or ear infections. A questionnaire was used to collect animal demographic and clinical data from the dog owners by interviewing directly. The first author (EAR) conducted the interviews and collected specimens with a prior consent from the dog owners. A sterile cotton swab was rotated several times against the oral mucosa, the surface of the perineal area and/or the infection site to collect a sample from a particular site. Swabs from a body site were placed individually in 5 ml Mueller Hinton broth (MHB) (Oxoid Ltd., Basingstoke, UK) supplemented with 6.5% NaCl and stored at 4˚C until processing. All procedures were carried out under an approval of the Ethics Committee of CVASU [Approval no. CVASU/Dir (R&E) EC/2019/39 (2/8)].
2.2. Isolation and identification of S. aureus and S. pseudintermedius
The swabs placed in MHB were incubated overnight at 37°C for primary selective enrichment. From this broth culture, 10 μl was streaked onto 5% bovine blood agar and incubated at 37°C for 24 h. Three to five colonies on blood agar plates displaying the characteristic appearance of staphylococci (medium‐sized, smooth, pigmented or non‐pigmented, raised and haemolytic) were further subcultured onto Mannitol salt agar (Oxoid Ltd., Basingstoke, UK) and incubated at 37°C for 24 h (Weese & van Duijkeren, 2010). The presumptive colonies of staphylococci on Mannitol salt agar were further tested by Gram's staining, catalase and tube coagulase tests. Before conducting the coagulase test all suspected staphylococci isolates were further sub‐cultured on blood agar plates at 37°C for 24 h. All coagulase‐positive isolates were investigated for the confirmation of S. aureus and S. pseudintermedius by PCR targeting the nuc gene as described previously (Sasaki et al., 2010). Bacterial genomic DNA was extracted from the freshly grown cultures on blood agar plate using boiling lysis method (Millar et al., 2000). All PCR‐confirmed S. aureus and S. pseudintermedius were grown in 5 ml brain heart infusion broth (BHIB) (Oxoid Ltd., Basingstoke, UK) and stored at –80°C for further analysis.
2.3. Antimicrobial susceptibility testing of S. aureus and S. pseudintermedius
All S. aureus and S. pseudintermedius isolates were screened for antimicrobial susceptibility against 14 antimicrobials representing 8 different classes using agar disk diffusion method. The following antimicrobials (Oxoid Ltd., Basingstoke, UK) were used: amoxicillin + clavulanic acid (30 μg), ampicillin (10 μg), cefaclor (30 μg), cefoxitin (10 μg), ciprofloxacin (10 μg), erythromycin (15 μg), gentamicin (30 μg), nalidixic acid (10 μg), oxacillin (5 μg), penicillin (10 IU), streptomycin (100 μg), sulfamethoxazole‐trimethoprim (1.25 + 23.75 μg), tetracycline (30 μg) and vancomycin (30 μg). The zone of inhibition around each disk was measured and interpreted as susceptible (S), intermediate (I) or resistant (R) according to Clinical and Laboratory Standards Institute (CLSI) guidelines for veterinary pathogens (CLSI, 2008). In the case of nalidixic acid, the interpretation was made based on earlier study described by Vaez et al. (2011). Methicillin resistance was determined by measuring zone diameter around oxacillin and cefoxitin disks (Schissler et al., 2009). S. aureus and S. pseudintermedius isolates showing resistance against ≥3 antimicrobial classes were defined as multidrug resistant (MDR) (Magiorakos et al., 2012).
2.4. Detection of the mecA gene
All oxacillin‐ and cefoxitin‐resistant S. aureus and S. pseudintermedius isolates were further tested for the presence of the mecA gene by PCR as described earlier (Larsen et al., 2008). Nuclease‐free water and an in‐house MRSA strain were used as negative and positive control, respectively.
2.5. Statistical analysis
A dog was considered positive for S. aureus or S. pseudintermedius when samples from at least one of the different body sites tested positive for the organism. The prevalence was calculated considering the number of positive dogs as the numerator divided by the number of dogs sampled as the denominator. Data were analysed using ‘R’ Program (version 3.5.1) (R CoreTeam, 2016). All possible risk factors were analysed for four target outcomes: the presence of S. aureus, S. pseudintermedius, MRSA and MRSP. First, univariable analysis was performed to identify possible risk factors for the four outcomes mentioned. Any factor having a p value of ≤0.20 was entered into multivariable logistic regression model. Forward stepwise selection approach was used to build the model. Variables with p value of 0.05 were considered significant and kept in the final model. The logistic regression analysis was performed using the glmer function from the lme4 package (Bates et al., 2014) in R version 3.5.1 (R CoreTeam, 2016). The 95% confidence interval of the prevalence values was calculated by the modified Wald method using the Graph Pad Quick Calcs online tool (www.graphpad.com/quickcalcs/).
3. RESULTS
3.1. Distribution of S. aureus, S. pseudintermedius, MRSA and MRSP
A total of 358 samples were collected from 150 dogs. Among them, 146 were from 73 healthy dogs, and 212 from 77 clinically sick dogs. Of the total samples obtained, 300 were from oral and perineal regions and the rest were from clinical cases of dermatitis (n = 28), skin wound (n = 22) and otitis (n = 8). An overview of the samples collected from different body sites, isolation frequency of S. aureus and S. pseudintermedius from the samples and the distribution of MRSA and MRSP is shown in Table 1. Out of the 150 dogs, 24 (16%; 95% CI: 10.9%–22.8%) and 68 (45.3%; 95% CI: 37.6%–53.3%) were positive for S. aureus and S. pseudintermedius, respectively. Overall, coagulase‐positive staphylococci were isolated from 142 (39.7%; 95% CI: 34.7%–44.8%) out of the 358 samples (Table 1). A total of 28 and 111 isolates were confirmed as S. aureus and S. pseudintermedius, respectively. However, the remaining three isolates were not confirmed by PCR either S. aureus or S. pseudintermedius. Of the S. aureus and S. pseudintermedius isolates, 13 (46.4%; 95% CI: 29.5%–64.2%) and 9 (8.1%; 95% CI: 4.1%–14.9%) were positive for the mecA gene, and thus classified as MRSA and MRSP, respectively. The overall prevalence of MRSA in dogs was 8.7% (95% CI: 5.0%–14.4%) and MRSP was 6% (95% CI: 3%–11.2%).
TABLE 1.
Distribution of S. aureus, S. pseudintermedius, methicillin‐resistant S. aureus and methicillin‐resistant S. pseudintermedius in different body sites of clinically healthy and sick dogs
| Body sites | No. sample a | No. coagulase‐positive Staphylococcus (%, 95% CI) | No. S. aureus (%, 95% CI) | No. S. pseudintermedius (%, 95% CI) | No. MRSA(%, 95% CI) | No. MRSP(%, 95% CI) |
|---|---|---|---|---|---|---|
| Perineal | 150 | 61 b (40.7, 33.1–48.7) |
9 (6.0) (3.0–11.2) |
51 (34.0) (26.9–41.9) |
3 (33.3) (11.7–64.9) |
3 (5.9) (1.4–16.5) |
| Oral | 150 |
47 b (31.3) (24.4–39.2) |
3 (2.0) (0.4–6.0) |
43 (28.7) (22.0–36.4) |
2 (66.7) (20.2–94.4) | 1 (2.3) (0.0–13.2) |
| Dermatitis | 28 |
16 b (57.1) (39.1–73.5) |
8 (28.6) (15.1–47.2) |
7 (25.0) (12.4–43.6) |
5 (62.5) (30.4–86.5) | 2 (28.6) (7.6–64.8) |
| Skin Wound | 22 |
13 (59.1) (38.7–76.8) |
7 (31.8) (16.2–52.9) |
6 (27.3) (12.9–48.4) |
2 (28.6) (7.6–64.8) | 1 (16.7) (1.1–58.2) |
| Otitis | 8 |
5 (62.5) (30.4–86.5) |
1 (12.5) (0.1–49.2) |
4 (50.0) (21.5–78.5) |
1 (100) (16.8–100.0) | 2 (50.0) (15.0–85.0) |
| Total | 358 | 142 (39.7) (34.7–44.8) |
28 (7.8) (5.4–11.1) |
111 (31.0) (26.4–36.0) |
13 (46.4) (29.5–64.2) | 9 (8.1) (4.1–14.9) |
Abbreviations: CI, confidence interval; MRSA, methicillin‐resistant Staphylococcus aureus; MRSP, methicillin‐resistant Staphylococcus pseudintermedius.
Considered as denominator to calculate prevalence for the specific organism or its specific antibiotic‐resistant type (MRSA and MRSP).
Three staphylococci isolates were not confirmed by PCR as S. aureus or S. pseudintermedius.
3.2. Antimicrobial susceptibility profiles
All S. aureus and S. pseudintermedius isolates were found to be MDR (Figures 1b and 1d, 2 and 3). The highest resistance in S. aureus and S. pseudintermedius was observed against nalidixic acid (95.2% and 91.0%, respectively) followed by erythromycin (89.3% and 84.7%, respectively) (Figure 1a and 1c). Resistance against oxacillin was detected in 72.5% in S. aureus and 79.3% in S. pseudintermedius. Similarly, in both cases, majority of the isolates displayed resistance against tetracycline and vancomycin (Figure 1a and 1c). The antibiogram profiles of methicillin‐resistant and methicillin‐sensitive isolates are displayed in Figures 2 and 3, respectively.
FIGURE 1.
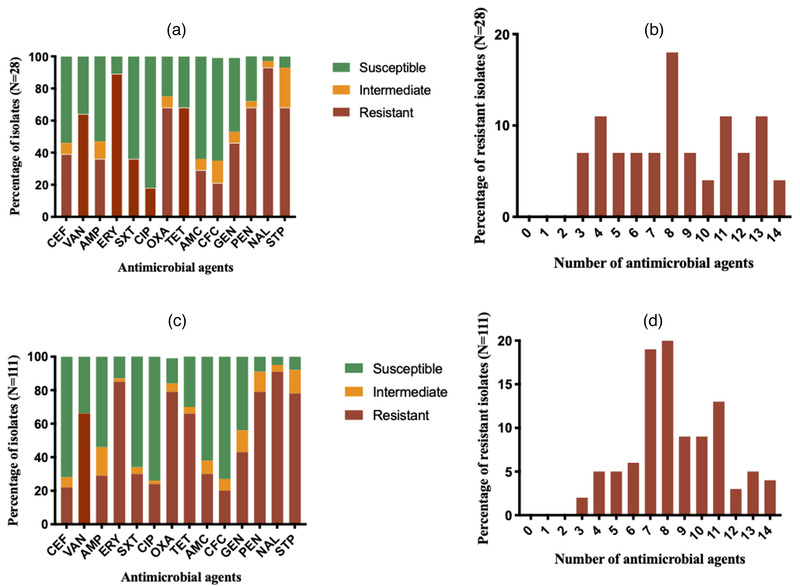
Antimicrobial resistance profile of S. aureus (a, b) and S. pseudintermedius (c, d). CEF: cefoxitin; VAN: vancomycin; AMP: ampicillin; ERY: erythromycin; SXT: sulfamethoxazole‐trimethoprim; CIP: ciprofloxacin; OXA: oxacillin; TET: tetracycline; AMC: amoxicillin + clavulanic acid; CFC: cefaclor; GEN: gentamicin; NAL: nalidixic acid; PEN: penicillin; STP: streptomycin.
FIGURE 2.
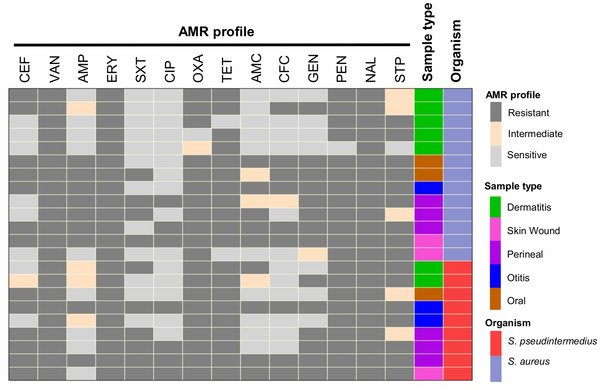
Heat map showing distribution of antimicrobial resistance profile of methicillin‐resistant S. aureus and methicillin‐resistant S. pseudintermedius isolates (mecA gene positive). Each row represents one isolate. CEF: cefoxitin; VAN: vancomycin; AMP: ampicillin; ERY: erythromycin; SXT: sulfamethoxazole‐trimethoprim; CIP: ciprofloxacin; OXA: oxacillin; TET: tetracycline; AMC: amoxicillin + clavulanic acid; CFC: cefaclor; GEN: gentamicin; NAL: nalidixic acid; PEN: penicillin; STP: streptomycin.
FIGURE 3.
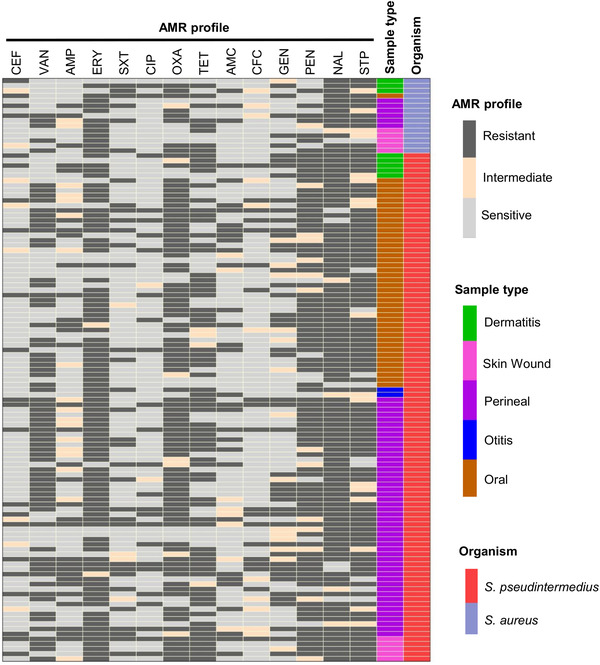
Heat map showing distribution of antimicrobial resistance profile of methicillin‐sensitive S. aureus and methicillin‐sensitive S. pseudintermedius isolates (mecA gene negative). Each row represents one isolate. CEF: cefoxitin; VAN: vancomycin; AMP: ampicillin; ERY: erythromycin; SXT: sulfamethoxazole‐trimethoprim; CIP: ciprofloxacin; OXA: oxacillin; TET: tetracycline; AMC: amoxicillin + clavulanic acid; CFC: cefaclor; GEN: gentamicin; NAL: nalidixic acid; PEN: penicillin; STP: streptomycin.
3.3. Risk factors associated with the carriage of S. aureus and MRSA in dogs
The univariable analysis identified five potential risk factors (p ≤ 0.20) associated with the carriage of S. aureus in dogs (Supplementary Table S1). In the subsequent multivariable analysis, three variables were identified as significant risk factors associated with the carriage of S. aureus. The significantly associated variables were ‘presence of dermatitis’ (OR 10.07, 95% CI 3.42–32.77, p < 0.001), ‘history of antibiotic use in the past one month’ (OR 4.50, 95% CI 1.54–14.07, p < 0.001) and ‘presence of skin wound’ (OR 3.46, 95% CI 1.06–11.15, p = 0.006) (Table 2).
TABLE 2.
Multivariable logistic regression model for assessing the risk factors independently associated with the S. aureus and methicillin‐resistant S. aureus (MRSA) from different body sites of both clinically healthy and sick dogs
| Outcome variable | Explanatory variable | Description | OR (95% CI) | p Value |
|---|---|---|---|---|
| S. aureus | Dermatitis | Yes | 10.07 (3.42–32.77) | <0.001 |
| No | 1 | Reference | ||
| Antibiotic use | Yes | 4.50 (1.54–14.07) | <0.001 | |
| No | 1 | Reference | ||
| Skin wound | Yes | 3.46 (1.06–11.15) | 0.006 | |
| No | 1 | Reference | ||
| MRSA | Dermatitis | Yes | 12.24 (3.12–57.33) | <0.001 |
| No | 1 | Reference | ||
| Antibiotic use | Yes | 8.73 (2.23–43.10) | <0.001 | |
| No | 1 | Reference |
Abbreviation: OR, odds ratio.
Seven out of the fourteen variables were identified in the univariable analysis as the potential risk factors for the carriage of MRSA in dogs (Supplementary Table S2). Two variables, ‘presence of dermatitis’ (OR 12.24, 95% CI 3.12–57.33, p < 0.001) and ‘history of antibiotic use’ (OR 8.73, 95% CI 2.23–43.10, p < 0.001) were retained significant in the final model (Table 2).
3.4. Risk factors associated with the carriage of S. pseudintermedius and MRSP in dogs
Of the fourteen variables tested in the univariable analysis (Supplementary Tables S3 and S4), six and five were eligible (p < 0.20) for multivariable analysis for the carriage of S. pseudintermedius and MRSP, respectively. In multivariable analysis for S. pseudintermedius carriage, two were retained in the final model: ‘presence of dermatitis’ (OR 3.16, 95% CI 1.33–7.91, p = 0.011) and ‘presence of skin wound’ (OR 3.02, 95% CI 1.16–8.54, p = 0.027) (Table 3). Similarly, in the multivariable analysis for MRSP, two factors were found to be significantly associated: ‘presence of otitis’ (OR 14.22, 95% CI 1.64–103.58, p = 0.008) and ‘presence of oral lesions’ (OR 9.48, 95% CI 1.14–64.82, p = 0.002) (Table 3).
TABLE 3.
Multivariable logistic regression model for assessing the risk factors independently associated with the S. pseudintermedius and methicillin‐resistant S. pseudintermedius (MRSP) from different body sites of both clinically healthy and sick dogs
| Outcome variable | Explanatory variable | Description | OR (95% CI) | p Value |
|---|---|---|---|---|
| S. pseudintermedius | Dermatitis | Yes | 3.16 (1.33–7.91) | 0.011 |
| No | 1 | Reference | ||
| Skin wound | Yes | 3.02 (1.16–8.54) | 0.027 | |
| No | 1 | Reference | ||
| MRSP | Otitis | Yes | 14.22 (1.64–103.58) | 0.008 |
| No | 1 | Reference | ||
| Oral lesions | Yes | 9.48 (1.14–64.82) | 0.002 | |
| No | 1 | Reference |
Abbreviation: OR, odds ratio.
4. DISCUSSION
This cross‐sectional study reveals a very high prevalence of MDR S. aureus and S. pseudintermedius along with MRSA and MRSP in dogs. Although the prevalence of MRSA has been reported in few studies (Afroz et al., 2008; Habibullah et al., 2017), the prevalence of MRSP has seemingly never been reported in dogs in Bangladesh. It is important to know the prevalence of multidrug‐resistant bacteria in pets because it contributes to clinical management of diseased dogs. Moreover, the zoonotic potential of MRSP is well known (Guardabassi et al., 2013; Paul et al., 2011) and it helps increase pet owner awareness.
The results of this study show that coagulase‐positive staphylococci were isolated from 39.7% of samples collected from different body sites of 150 dogs. The proportion of coagulase‐positive isolates in this study is comparatively lower than an earlier report of the United States (70%) (Griffeth et al., 2008). However, Sasaki et al. (2007) reported 52.6% coagulase‐positive S. aureus in dogs in Japan, which is nearly similar to our findings. As staphylococci are predominant commensal pathogens of dogs, the prevalence estimates may be influenced by many factors such as species, breed, age, sex, managements, clinical condition, geographical location etc.
The overall frequency of S. aureus (16%) carriage in dogs was in close agreement with previously reported studies in the United States where they sampled dogs at veterinary hospitals (Griffeth et al., 2008; Iverson et al., 2015). Also, Hanselman et al. (2009) reported 14% prevalence in household dogs in Canada. The overall prevalence of S. pseudintermedius (45.3%) in dogs is somewhat similar to the previously reported prevalence in Brazil (38.4%) (Penna et al., 2010), the United States (53%) (Iverson et al., 2015) and Tunisia (55%) (Gharsa et al., 2013). On the contrary, very high prevalence of S. pseudintermedius in dogs was reported in Japan (89.50%) (Kawakami et al., 2010), Poland (87.6 %) (Garbacz et al., 2013), the United Kingdom (87.5%) (Fazakerley et al., 2009), Canada (87.4%) (Rubin et al., 2011) and Korea (61.15%) (Yoon et al., 2010). As a skin commensal, S. aureus and S. pseudintermedius are frequently isolated from the nares, mouth, pharynx, forehead, groin and anus of healthy dogs and cats (Beck et al., 2012; Garbacz et al., 2013). The isolation rate of S. pseudintermedius from perineum and oral cavity/mucosa/mouth were 51% and 43%, respectively, in our study. Moreover, these two body sites are recognised as the most common colonisation sites for S. pseudintermedius in dogs reported in different studies (Garbacz et al., 2013; Paul et al., 2011; van Duijkeren et al., 2011). The carriage frequency of S. pseudintermedius in different body sites found in this study is consistent with the previous findings of Hanselman et al. (2009) and Beck et al. (2012) who reported that the nose and rectum of the healthy dogs generally carried 46% and 47.6% S. pseudintermedius, respectively. The variation of carriage percentages among different studies might be due to the variations in sample size, sample collection technique, culture methods, breeds of dogs, health status, environments, management as well as geographical location. The prevalence of S. aureus and S. pseudintermedius in dogs with dermatitis were 28.6% and 25% in this study, which is comparatively lower than the previous report (45%) (Beck et al., 2012).
Both S. aureus and S. pseudintermedius exhibited great diversity of resistance against the 14 antimicrobials tested in this study. This type of diverse antimicrobial resistance profile of these two organisms was previously reported by a number of studies (Algammal et al., 2020; Couto et al., 2011; Davis et al., 2014; Garbacz et al., 2013; Kern & Perreten, 2013; Perkins et al., 2020; Perreten et al., 2010).
Most of the S. aureus isolates in this study showed resistance to erythromycin (89.3%) and nalidixic acid (95.2%). High resistance frequency of S. aureus against erythromycin was reported in several previous studies (Davis et al., 2014; Garbacz et al., 2013; Morris et al., 2006; Schmitz et al., 2000). It may be due to repeated exposure to the same antimicrobial(s) and/or lower doses of the drug made the organism more tolerant against these antimicrobials. Moreover, the use of similar groups of antimicrobials that have similar modes of action against a particular organism may encourage development of more resistance. In this study, the S. aureus displayed high proportion of susceptibility to ciprofloxacin (82%) which is similar to the reports of Raviglione et al. (1990). The high rate of susceptibility to ciprofloxacin may be due to less frequent use of these antimicrobials in dogs. Surprisingly, S. aureus and S. pseudintermedius exhibited higher resistance to tetracycline compared with previous studies (Morris et al., 2006; Gharsa et al., 2013; Garbacz et al., 2013). This higher resistance profile of this antibiotic is probably due to either cross‐transmission of resistant organisms or acquisition of resistance genes. Nalidixic acid, erythromycin and tetracycline are less frequently used in pet animal practices in Bangladesh but the resistances of these antimicrobials are alarming for future treatment guidelines. However, vancomycin is reserved as last resort drugs for MRSA and MRSP infected patients in both pets and human clinics (Guardabassi & Prescott, 2015; Wunderink et al., 2003).
In this study, the prevalence of MRSA was found to be 8.7%. Similar prevalence (8%) was also reported at pet hospitals in the United States (Iverson et al., 2015). Depending on the study area and sample size, high proportion of MRSA (>50%) was reported in the United States, some Asian countries and Malta (Stefani et al., 2012). Whereas, intermediate frequency of MRSA (25–50%) was reported in African countries, China and some part of Europe (Mejìa et al., 2010; Stefani et al., 2012; Vincze et al., 2014). Moreover, the global trend of MRSA prevalence was comparatively higher than our present study. This variation in reported prevalence studies from different geographical locations might be due to sample size of conducted study, breed characteristics, frequency of disease condition, repeated exposure of antimicrobials, different human–animal behavioural relationships, accessibility and availability of veterinary care as well as diverse antimicrobial stewardship practices in different countries.
The prevalence of MRSP (6%) in dogs in this study is close to the prevalence reported by several previous studies (Ishihara et al., 2010; Beck et al., 2012; Gold et al., 2014). Kawakami et al. (2010) and Perreten et al. (2010) reported the prevalence of MRSP was 66.5% in Japan and 72.8% in Europe in different clinically diseased dogs, which is much higher than our observation in Bangladesh. There have been higher prevalence reports in dogs presenting with pyoderma to dermatology referral clinics. Beck et al. (2012) reported that the prevalence of MRSP was 45.2% when dogs suffer from pyoderma and others disease condition. The percentage is higher in diseased dogs because concurrent infection can occur when a dog is infected with a resistant strain. Moreover, the dog may spread the infection if it comes in close contact with other dogs. So, long‐term treatment of chronic disease condition using topical or systemic antimicrobials may encourage the development of MRSP very rapidly. On the contrary, several studies revealed that the prevalence of MRSP in healthy dogs was 4.5% (Canada) (Hanselman et al., 2009), 4.0% (USA) (Abraham et al., 2007), 3.9% (Denmark) (Paul et al., 2011), 2.1% (Canada) (Hanselman et al., 2008) and 1% (USA) (Iverson et al., 2015) at veterinary hospital which is comparatively lower than our study. However, administration of broad‐spectrum antimicrobials, particularly, concurrent use of β‐lactams and fluoroquinolones in pet animals might play significant roles in the emergence of MRSA and MRSP in dogs (Guardabassi et al., 2013). Any mecA gene‐encoding bacteria have the ability to produce β‐lactamase enzymes which are the main trigger to inhibit the function of β‐lactam antimicrobials (Bush & Bradford, 2020). Overuse of fluoroquinolones may encourage the mutation of nucleotide resulting the emergence of resistant strains (Bakken, 2004).
We identified dermatitis and antibiotic use as the risk factors associated with higher prevalence of MRSA in dogs. Long‐time use of antimicrobials in chronic infections might be a reason behind this association (Ventrella et al., 2017). On the other hand, selection pressure exerted by the use of antimicrobial therapy is a well‐documented risk factor for MRSA (Weber et al., 2003; Eckholm et al., 2013; Gnanamani et al., 2017). Presence of otitis and oral lesions in dogs were found as risk factors for the carriage of MRSP. However, some studies reported that dogs with chronic skin and ear diseases that visited veterinary clinics more frequently and received topical or systemic antimicrobial medication or glucocorticoids were at higher risk of MRSP infection (van Duijkeren et al., 2011; Weese et al., 2012; Lehner et al., 2014). The imprudent use of broad‐spectrum antimicrobials in the treatment of MRSA and MRSP infection must be unsanctioned, and a standard treatment guideline should be based on the laboratory reports of the antimicrobial susceptibility testing. However, during an emergency and urgent situation, veterinarians do not always get the chance to perform culture and sensitivity testing during choosing antimicrobials. Therefore, keeping a cumulative hospital antibiogram would be highly beneficial to guide empiric antimicrobial therapeutic decisions (Fowler et al., 2016).
Potential transmission of MRSA and MRSP from pet animals to owners (Hanselman et al., 2009), suggests their high presence in companion animals. Moreover, the veterinary care providers may act as a vehicle for transmission between patients via contaminated hands or clothes. There is also an increased risk of environmental contamination posing risk of exposure to animal patients while in the hospital. Furthermore, the veterinary healthcare providers may behave as transitory carriers, bringing MDR organism's home with them and exposing their own pets.
The study has some limitations. We sampled the dogs that were registered to the hospital during the study period without considering the statistical sample size calculation. Additionally, we could not perform detailed genotyping of the isolates due to resource constraints. It would be very valuable to know the sequence types of S. aureus and S. pseudintermedius circulating in dogs. Furthermore, this study was conducted in a particular region of the country although the prevalence of staphylococci may vary according to geographical locations. These limitations should be addressed in future research.
5. CONCLUSION
The carriage rate of S. aureus and S. pseudintermedius in pet dogs in Bangladesh appear to be 16% and 45.3%, respectively. The overwhelming majority of the strains belonging to both the species are not only MDR but a significant section of them also carrying the mecA gene, a marker for the methicillin resistance. The prevalence of MRSA in dogs with dermatitis and with the history of antibiotic use might be higher while the presence of otitis and oral lesions seem to be positively associated a higher prevalence for MRSP.
AUTHOR CONTRIBUTIONS
Eaftekhar Ahmed Rana: conceptualisation; data curation; formal analysis; investigation; methodology; project administration; writing – original draft. Md Zohorul Islam: conceptualization; data curation; formal analysis; supervision; visualisation; writing – review & editing. Tridip Das: data curation; visualisation; writing – review & editing. Avijit Dutta: writing – review & editing. Abdul Ahad: project administration; writing – review & editing. Paritosh Kumar Biswas: formal analysis; writing – review & editing. Himel Barua: conceptualisation; data curation; formal analysis; methodology; project administration; supervision; writing – review & editing.
ETHICAL STATEMENT
The authors confirm that the ethical policies of the journal, as noted on the journal's author guidelines page, have been adhered to and the appropriate ethical review committee approval has been received. All procedures were carried out under an approval of the Ethics Committee of CVASU (Approval no. CVASU/Dir (R&E) EC/2019/39 (2/8)).
PLACE OF STUDY
The study was conducted at Sahedul Alam Quaderi Teaching Veterinary Hospital (SAQ‐TVH) at Chattogram Veterinary and Animal Sciences University (CVASU), Bangladesh. The microbiological investigation was conducted at microbiology laboratory of the Department of Microbiology and Veterinary Public Health, CVASU, Bangladesh.
CONFLICT OF INTEREST
The authors have no conflict of interest to declare.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.701.
Supporting information
Supporting Information
Supporting Information
Supporting Information
Supporting Information
ACKNOWLEDGEMENTS
The authors would like to thank the Sahedul Alam Quaderi Teaching Veterinary Hospital (SAQTVH) of the Chattogram Veterinary and Animal Sciences University (CVASU) for supporting the collection of the clinical spicimens from dogs. This study was financially supported by Advanced Study and Research, CVASU, Bangladesh and National Science and Technology Fellowship under the Ministry of Science and Technology, People's Republic of Bangladesh.
Rana, E. A. , Islam, M. Z. , Das, T. , Dutta, A. , Ahad, A. , Biswas, P. K. , & Barua, H. (2022). Prevalence of coagulase‐positive methicillin‐resistant Staphylococcus aureus and Staphylococcus pseudintermedius in dogs in Bangladesh. Veterinary Medicine and Science, 8, 498–508. 10.1002/vms3.701
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available within the article and its supplementary materials
REFERENCES
- Abraham, J. L. , Morris, D. O. , Griffeth, G. C. , Shofer, F. S. , & Rankin, S. C. (2007). Surveillance of healthy cats and cats with inflammatory skin disease for colonization of the skin by methicillin‐resistant coagulase‐positive staphylococci and Staphylococcus schleiferissp. schleiferi . Veterinary Dermatology, 18(4), 252–259. 10.1111/j.1365-3164.2007.00604.x [DOI] [PubMed] [Google Scholar]
- Afroz, S. , Kobayashi, N. , Nagashima, S. , Alam, M. M. , Hossain, A. B. , Rahman, M. A. , Rafiqul Islam, M. , Binte Lutfor, A. , Muazzam, N. , Khan, M. A. H. , Shamsuzzaman, A. K. M. , Chan Mahmud, M. , Musa, A. K. M. , Akram Hossain, M. , & Paul, S. K. (2008). Genetic characterization of Staphylococcus aureus isolates carrying Panton‐Valentine leukocidin genes in Bangladesh. Japanese Journal of Infectious Diseases, 61(5), 393–396. [PubMed] [Google Scholar]
- Algammal, A. M. , Hetta, H. F. , Elkelish, A. , Alkhalifah, D. H. H. , Hozzein, W. N. , Batiha, G. E. S. , El Nahhas, N. , & Mabrok, M. A. (2020). Methicillin‐Resistant Staphylococcus aureus (MRSA): One health perspective approach to the bacterium epidemiology, virulence factors, antibiotic‐resistance, and zoonotic impact. Infection and Drug Resistance, 13, 3255. doi: 10.2147/IDR.S272733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakken, J. S. (2004). The fluoroquinolones: How long will their utility last? Scandinavian Journal of Infectious Diseases, 36(2), 85–92. 10.1080/00365540410019039 [DOI] [PubMed] [Google Scholar]
- Barber, M. (1961). Methicillin‐resistant staphylococci. Journal of Clinical Pathology, 14(4), 385. 10.1136/jcp.14.4.385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates, D. , Mächler, M. , Bolker, B. , & Walker, S. (2014). Fitting linear mixed‐effects models using lme4. arXiv preprint arXiv:1406.5823.
- Beck, K. M. , Waisglass, S. E. , Dick, H. L. , & Weese, J. S. (2012). Prevalence of meticillin‐resistant Staphylococcus pseudintermedius (MRSP) from skin and carriage sites of dogs after treatment of their meticillin‐resistant or meticillin‐sensitive staphylococcal pyoderma. Veterinary Dermatology, 23(4), 369‐75, e66‐7. 10.1111/j.1365-3164.2012.01035.x [DOI] [PubMed] [Google Scholar]
- Bush, K. & Bradford, P. A. (2020). Epidemiology of β‐lactamase‐producing pathogens. Clinical Microbiology Reviews, 33(2), 00047‐19. 10.1128/CMR.00047-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clinical and Laboratory Standards Institute (CLSI) . (2008). Performance standards for antimicrobial disk and dilution susceptibility tests for bacteria isolated from animals: Approved standard, 3rd ed. CLSI document M31‐A3. Clinical and Laboratory Standards Institute, Wayne, PA, USA. [Google Scholar]
- Couto, N. , Pomba, C. , Moodley, A. , & Guardabassi, L. (2011). Prevalence of meticillin‐resistant staphylococci among dogs and cats at a veterinary teaching hospital in Portugal. Veterinary Record, 169(3), 72‐72. 10.1136/vr.c6948 [DOI] [PubMed] [Google Scholar]
- Davis, J. A. , Jackson, C. R. , Fedorka‐Cray, P. J. , Barrett, J. B. , Brousse, J. H. , Gustafson, J. , & Kucher, M. (2014). Carriage of methicillin‐resistant staphylococci by healthy companion animals in the US. Letters in Applied Microbiology, 59(1), 1–8. 10.1111/lam.12254 [DOI] [PubMed] [Google Scholar]
- Eckholm, N. G. , Outerbridge, C. A. , White, S. D. , & Sykes, J. E. (2013). Prevalence of and risk factors for isolation of meticillin‐resistant Staphylococcus spp. from dogs with pyoderma in northern California, USA. Veterinary Dermatology, 24(1), 154‐e34. 10.1111/j.1365-3164.2012.01051.x [DOI] [PubMed] [Google Scholar]
- Fazakerley, J. , Nuttall, T. , Sales, D. , Schmidt, V. , Carter, S. D. , Hart, C. A. , & McEwan, N. A. (2009). Staphylococcal colonization of mucosal and lesional skin sites in atopic and healthy dogs. Veterinary Dermatology, 20(3), 179–184. 10.1111/j.1365-3164.2009.00745.x [DOI] [PubMed] [Google Scholar]
- Fowler, H. , Davis, M. A. , Perkins, A. , Trufan, S. , Joy, C. , Buswell, M. , McElwain, T. F. , Moore, D. , Worhle, R. , & Rabinowitz, P. M. (2016). Survey of veterinary antimicrobial prescribing practices, Washington State 2015. Veterinary Record, 179(25), 651. 10.1136/vr.103916 [DOI] [PubMed] [Google Scholar]
- Garbacz, K. , Żarnowska, S. , Piechowicz, L. , & Haras, K. (2013). Staphylococci isolated from carriage sites and infected sites of dogs as a reservoir of multidrug resistance and methicillin resistance. Current Microbiology, 66(2), 169–173. 10.1007/s00284-012-0254-9 [DOI] [PubMed] [Google Scholar]
- Gharsa, H. , Slama, K. B. , Gómez‐Sanz, E. , Lozano, C. , Klibi, N. , Jouini, A. , Messadi, L. , Boudabous, A. , & Torres, C. (2013). Antimicrobial resistance, virulence genes, and genetic lineages of Staphylococcus pseudintermedius in healthy dogs in Tunisia. Microbial Ecology, 66(2), 363–368. 10.1007/s00248-013-0243-y [DOI] [PubMed] [Google Scholar]
- Gold, R. M. , Cohen, N. D. , & Lawhon, S. D. (2014). Amikacin resistance in Staphylococcus pseudintermedius isolated from dogs. Journal of Clinical Microbiology, 52(10), 3641–3646. 10.1128/JCM.01253-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gnanamani, A. , Hariharan, P. & Paul‐Satyaseela, M. (2017). Staphylococcus aureus: Overview of bacteriology, clinical diseases, epidemiology, antibiotic resistance and therapeutic approach. Frontiers in Staphylococcus aureus, 4, 28. 10.5772/67338 [DOI] [Google Scholar]
- Griffeth, G. C. , Morris, D. O. , Abraham, J. L. , Shofer, F. S. , & Rankin, S. C. (2008). Screening for skin carriage of methicillin‐resistant coagulase‐positive staphylococci and Staphylococcus schleiferi in dogs with healthy and inflamed skin. Veterinary Dermatology, 19(3), 142–149. 10.1111/j.1365-3164.2008.00663.x [DOI] [PubMed] [Google Scholar]
- Guardabassi, L. , & Prescott, J. F. (2015). Antimicrobial stewardship in small animal veterinary practice: From theory to practice. Veterinary Clinics: Small Animal Practice, 45(2), 361–376. 10.1016/j.cvsm.2014.11.005 [DOI] [PubMed] [Google Scholar]
- Guardabassi, L. , Larsen, J. , Weese, J. S. , Butaye, P. , Battisti, A. , Kluytmans, J. , Lloyd, D. H. , & Skov, R. L. (2013). Public health impact and antimicrobial selection of meticillin‐resistant staphylococci in animals. Journal of Global Antimicrobial Resistance, 1(2), 55–62. 10.1016/j.jgar.2013.03.011 [DOI] [PubMed] [Google Scholar]
- Habibullah, A. , Rahman, A. M. M. T. , Haydar, M. R. , Nazir, K. H. M. N. H. , & Rahman, M. T. (2017). Prevalence and molecular detection of methicillin‐resistant Staphylococcus aureus from dogs and cats in Dhaka City. Bangladesh Journal of Veterinary Medicine, 15(1), 51–57. 10.3329/bjvm.v15i1.34055 [DOI] [Google Scholar]
- Hanselman, B. A. , Kruth, S. A. , Rousseau, J. , & Weese, J. S. (2009). Coagulase positive staphylococcal colonization of humans and their household pets. The Canadian Veterinary Journal, 50(9), 954. [PMC free article] [PubMed] [Google Scholar]
- Hanselman, B. A. , Kruth, S. , & Weese, J. S. (2008). Methicillin‐resistant staphylococcal colonization in dogs entering a veterinary teaching hospital. Veterinary Microbiology, 126(1‐3), 277–281. 10.1016/j.vetmic.2007.06.015 [DOI] [PubMed] [Google Scholar]
- Ishihara, K. , Shimokubo, N. , Sakagami, A. , Ueno, H. , Muramatsu, Y. , Kadosawa, T. , Yanagisawa, C. , Hanaki, H. , Nakajima, C. , Suzuki, Y. , & Tamura, Y. (2010). Occurrence and molecular characteristics of methicillin‐resistant Staphylococcus aureus and methicillin‐resistant Staphylococcus pseudintermedius in an academic veterinary hospital. Applied and Environmental Microbiology, 76(15), 5165–5174. 10.1128/AEM.02780-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iverson, S. A. , Brazil, A. M. , Ferguson, J. M. , Nelson, K. , Lautenbach, E. , Rankin, S. C. , Morris, D. O. , & Davis, M. F. (2015). Anatomical patterns of colonization of pets with staphylococcal species in homes of people with methicillin‐resistant Staphylococcus aureus (MRSA) skin or soft tissue infection (SSTI). Veterinary Microbiology, 176(1‐2), 202–208. 10.1016/j.vetmic.2015.01.003 [DOI] [PubMed] [Google Scholar]
- Kawakami, T. , Shibata, S. , Murayama, N. , Nagata, M. , Nishifuji, K. , Iwasaki, T. , & Fukata, T. (2010). Antimicrobial susceptibility and methicillin resistance in Staphylococcus pseudintermedius and Staphylococcus schleiferi subsp. coagulans isolated from dogs with pyoderma in Japan. Journal of Veterinary Medical Science, 72(12), 1615–1619. 10.1292/jvms.10-0172 [DOI] [PubMed] [Google Scholar]
- Kern, A. , & Perreten, V. (2013). Clinical and molecular features of methicillin‐resistant, coagulase‐negative staphylococci of pets and horses. Journal of Antimicrobial Chemotherapy, 68(6), 1256–1266. 10.1093/jac/dkt020 [DOI] [PubMed] [Google Scholar]
- Kong, E. F. , Johnson, J. K. & Jabra‐Rizk, M. A. (2016). Community‐associated methicillin‐resistant Staphylococcus aureus: An enemy amidst us. PLoS Pathogens, 12(10),1005837. 10.1371/journal.ppat.1005837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsen, A. R. , Stegger, M. , & Sørum, M. (2008). spa typing directly from a mecA, spa and pvl multiplex PCR assay—A cost‐effective improvement for methicillin‐resistant Staphylococcus aureus surveillance. Clinical Microbiology and Infection, 14(6), 611–614. 10.1111/j.1469-0691.2008.01995.x [DOI] [PubMed] [Google Scholar]
- Lehner, G. , Linek, M. , Bond, R. , Lloyd, D. H. , Prenger‐Berninghoff, E. , Thom, N. , Straube, I. , Verheyen, K. , & Loeffler, A. (2014). Case–control risk factor study of methicillin‐resistant Staphylococcus pseudintermedius (MRSP) infection in dogs and cats in Germany. Veterinary Microbiology, 168(1), 154–160. 10.1016/j.vetmic.2013.10.023 [DOI] [PubMed] [Google Scholar]
- Loeffler, A. , & Lloyd, D. H. (2010). Companion animals: A reservoir for methicillin‐resistant Staphylococcus aureus in the community? Epidemiology & Infection, 138(5), 595–605. 10.1017/S0950268809991476 [DOI] [PubMed] [Google Scholar]
- Magiorakos, A. P. , Srinivasan, A. , Carey, R. B. , Carmeli, Y. , Falagas, M. E. , Giske, C. G. , Harbarth, S. , Hindler, J. F. , Kahlmeter, G. , Olsson‐Liljequist, B. , & Paterson, D. L. (2012). Multidrug‐resistant, extensively drug‐resistant and pandrug‐resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clinical Microbiology and Infection, 18(3), 268–281. 10.1111/j.1469-0691.2011.03570.x [DOI] [PubMed] [Google Scholar]
- Mejia, C. , Zurita, J. , & Guzman‐Blanco, M. (2010). Epidemiology and surveillance of methicillin‐resistant Staphylococcus aureus in Latin America. Brazilian Journal of Infectious Diseases, 14, 79–86. 10.1590/S1413-86702010000800003 [DOI] [PubMed] [Google Scholar]
- Millar, B. C. , Jiru, X. U. , Moore, J. E. , & Earle, J. A. (2000). A simple and sensitive method to extract bacterial, yeast and fungal DNA from blood culture material. Journal of Microbiological Methods, 42(2), 139–147. 10.1016/S0167-7012(00)00174-3 [DOI] [PubMed] [Google Scholar]
- Morris, D. O. , Rook, K. A. , Shofer, F. S. , & Rankin, S. C. (2006). Screening of Staphylococcus aureus, Staphylococcus intermedius, and Staphylococcus schleiferi isolates obtained from small companion animals for antimicrobial resistance: A retrospective review of 749 isolates (2003–04). Veterinary Dermatology, 17(5), 332–337. 10.1111/j.1365-3164.2006.00536.x [DOI] [PubMed] [Google Scholar]
- Murphy, C. , Reid‐Smith, R. J. , Prescott, J. F. , Bonnett, B. N. , Poppe, C. , Boerlin, P. , Weese, J. S. , Janecko, N. , & McEwen, S. A. (2009). Occurrence of antimicrobial resistant bacteria in healthy dogs and cats presented to private veterinary hospitals in southern Ontario: A preliminary study. The Canadian Veterinary Journal, 50(10), 1047. [PMC free article] [PubMed] [Google Scholar]
- O'Gara, J. P. (2017). Into the storm: Chasing the opportunistic pathogen Staphylococcus aureus from skin colonisation to life threatening infections. Environmental Microbiology, 19(10), 3823–3833. 10.1111/1462-2920.13833 [DOI] [PubMed] [Google Scholar]
- Paul, N. C. , Moodley, A. , Ghibaudo, G. , & Guardabassi, L. (2011). Carriage of methicillin‐resistant Staphylococcus pseudintermedius in small animal veterinarians: Indirect evidence of zoonotic transmission. Zoonoses and Public Health, 58(8), 533–539. 10.1111/j.1863-2378.2011.01398.x [DOI] [PubMed] [Google Scholar]
- Penna, B. , Varges, R. , Medeiros, L. , Martins, G. M. , Martins, R. R. , & Lilenbaum, W. (2010). Species distribution and antimicrobial susceptibility of staphylococci isolated from canine otitis externa. Veterinary Dermatology, 21(3), 292–296. 10.1111/j.1365-3164.2009.00842.x [DOI] [PubMed] [Google Scholar]
- Perkins, A. V. , Sellon, D. C. , Gay, J. M. , Lofgren, E. T. , Moore, D. A. , Jones, L. P. & Davis, M. A. , (2020). Prevalence of methicillin‐resistant Staphylococcus pseudintermedius on hand‐contact and animal‐contact surfaces in companion animal community hospitals. The Canadian Veterinary Journal, 61(6), 613. [PMC free article] [PubMed] [Google Scholar]
- Perreten, V. , Kadlec, K. , Schwarz, S. , GrönlundAndersson, U. , Finn, M. , Greko, C. , Moodley, A. , Kania, S. A. , Frank, L. A. , Bemis, D. A. , Iurescia, M. , Battisti, A. , Duim, B. , Wagenaar, J. A. , Van Duijkeren, E. , Weese, J. S. , Fitzgerald, J. R. , Rossano, A. , Guardabassi, L. , & Franco, A. (2010). Clonal spread of methicillin‐resistant Staphylococcus pseudintermedius in Europe and North America: An international multicentre study. Journal of Antimicrobial Chemotherapy, 65(6), 1145–1154. 10.1093/jac/dkq078 [DOI] [PubMed] [Google Scholar]
- R Core Team . (2016). R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at: https://www.r‐project.org/. [Google Scholar]
- Raviglione, M. C. , Boyle, J. F. , Mariuz, P. , Pablos‐Mendez, A. , Cortes, H. , & Merlo, A. (1990). Ciprofloxacin‐resistant methicillin‐resistant Staphylococcus aureus in an acute‐care hospital. Antimicrobial Agents and Chemotherapy, 34(11), 2050–2054. 10.1128/AAC.34.11.2050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubin, J. E. , & Chirino‐Trejo, M. (2011). Prevalence, sites of colonization, and antimicrobial resistance among Staphylococcus pseudintermedius isolated from healthy dogs in Saskatoon, Canada. Journal of Veterinary Diagnostic Investigation, 23(2), 351–354. 10.1177/104063871102300227 [DOI] [PubMed] [Google Scholar]
- Sasaki, T. , Kikuchi, K. , Tanaka, Y. , Takahashi, N. , Kamata, S. , & Hiramatsu, K. (2007). Methicillin‐resistant Staphylococcus pseudintermedius in a veterinary teaching hospital. Journal of Clinical Microbiology, 45(4), 1118–1125. 10.1128/JCM.02193-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki, T. , Tsubakishita, S. , Tanaka, Y. , Sakusabe, A. , Ohtsuka, M. , Hirotaki, S. , Kawakami, T. , Fukata, T. , & Hiramatsu, K. (2010). Multiplex‐PCR method for species identification of coagulase‐positive staphylococci. Journal of Clinical Microbiology, 48(3), 765–769. 10.1128/JCM.01232-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schissler, J. R. , Hillier, A. , Daniels, J. B. , Cole, L. K. , & Gebreyes, W. A. (2009). Evaluation of Clinical Laboratory Standards Institute interpretive criteria for methicillin‐resistant Staphylococcus pseudintermedius isolated from dogs. Journal of Veterinary Diagnostic Investigation, 21(5), 684–688. 10.1177/104063870902100514 [DOI] [PubMed] [Google Scholar]
- Schmitz, F. J. , Sadurski, R. , Kray, A. , Boos, M. , Geisel, R. , Köhrer, K. , Verhoef, J. , & Fluit, A. C. (2000). Prevalence of macrolide‐resistance genes in Staphylococcus aureus and Enterococcus faecium isolates from 24 European university hospitals. Journal of Antimicrobial Chemotherapy, 45(6), 891–894. 10.1093/jac/45.6.891 [DOI] [PubMed] [Google Scholar]
- Schwarz, S. , Loeffler, A. & Kadlec, K. (2017). Bacterial resistance to antimicrobial agents and its impact on veterinary and human medicine. Advances in Veterinary Dermatology, 8, 95–110. 10.1002/9781119278368.ch5.1 [DOI] [PubMed] [Google Scholar]
- Stefani, S. , Chung, D. R. , Lindsay, J. A. , Friedrich, A. W. , Kearns, A. M. , Westh, H. , & MacKenzie, F. M. (2012). Meticillin‐resistant Staphylococcus aureus (MRSA): Global epidemiology and harmonisation of typing methods. International Journal of Antimicrobial Agents, 39(4), 273–282. 10.1016/j.ijantimicag.2011.09.030 [DOI] [PubMed] [Google Scholar]
- Vaez, H. , Tabaraei, A. , Moradi, A. , & Ghaemi, E. A. (2011). Evaluation of methicillin resistance Staphylococcus aureus isolated from patients in Golestan province‐north of Iran. African Journal of Microbiology Research, 5(4 ), 432–436. 10.5897/AJMR10.877 [DOI] [Google Scholar]
- van Duijkeren, E. , Catry, B. , Greko, C. , Moreno, M. A. , Pomba, M. C. , Pyörälä, S. , Ruzauskas, M. , Sanders, P. , Threlfall, E. J. , Torren‐Edo, J. , & Törneke, K. (2011). Review on methicillin‐resistant Staphylococcus pseudintermedius . Journal of Antimicrobial Chemotherapy, 66(12), 2705–2714. 10.1093/jac/dkr367 [DOI] [PubMed] [Google Scholar]
- Vincze, S. , Stamm, I. , Kopp, P. A. , Hermes, J. , Adlhoch, C. , Semmler, T. , Wieler, L H. , Lübke‐Becker, A. , & Walther, B. (2014). Alarming proportions of methicillin‐resistant Staphylococcus aureus (MRSA) in wound samples from companion animals, Germany 2010–2012. PloSONE, 9(1), e85656. 10.1371/journal.pone.0085656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventrella, G. , Moodley, A. , Grandolfo, E. , Parisi, A. , Corrente, M. , Buonavoglia, D. & Guardabassi, L. (2017). Frequency, antimicrobial susceptibility and clonal distribution of methicillin‐resistant Staphylococcus pseudintermedius in canine clinical samples submitted to a veterinary diagnostic laboratory in Italy: A 3‐year retrospective investigation. Veterinary Microbiology, 211, 103–106. 10.1016/j.vetmic.2017.09.015 [DOI] [PubMed] [Google Scholar]
- Weber, S. G. , Gold, H. S. , Hooper, D. C. , Karchmer, A. W. , & Carmeli, Y. (2003). Fluoroquinolones and the risk for methicillin‐resistant Staphylococcus aureus in hospitalized patients. Emerging Infectious Diseases, 9(11), 1415. 10.3201/eid0911.030284 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weese, J. S. , & van Duijkeren, E. (2010). Methicillin‐resistant Staphylococcus aureus and Staphylococcus pseudintermedius in veterinary medicine. Veterinary Microbiology, 140(3‐4), 418–429. 10.1016/j.vetmic.2009.01.039 [DOI] [PubMed] [Google Scholar]
- Weese, J. S. , Faires, M. C. , Frank, L. A. , Reynolds, L. M. , & Battisti, A. (2012). Factors associated with methicillin‐resistant versus methicillin‐susceptible Staphylococcus pseudintermedius infection in dogs. Journal of the American Veterinary Medical Association, 240(12), 1450–1455. 10.2460/javma.240.12.1450 [DOI] [PubMed] [Google Scholar]
- Wunderink, R. G. , Rello, J. , Cammarata, S. K. , Croos‐Dabrera, R. V. , & Kollef, M. H. , 2003. Linezolid vs vancomycin: Analysis of two double‐blind studies of patients with methicillin‐resistant Staphylococcus aureus nosocomial pneumonia. Chest, 124(5), 1789–1797. 10.1016/S0012-3692(15)33412-7 [DOI] [PubMed] [Google Scholar]
- Yoon, J. W. , Lee, K. J. , Lee, S. Y. , Chae, M. J. , Park, J. K. , Yoo, J. H. , & Park, H. M. (2010). Antibiotic resistance profiles of Staphylococcus pseudintermedius isolates from canine patients in Korea. Journal of Microbiology and Biotechnology, 20(12), 1764–1768. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information
Supporting Information
Supporting Information
Supporting Information
Data Availability Statement
The data supporting the findings of this study are available within the article and its supplementary materials


