Abstract
Background
Extensive use of different nanoparticles caused significant concerns about their biological safety.
Objective
This study aimed to evaluate the effects of cryopreservation on ram semen after adding magnetic nanoparticles (MNPs) to separate X and Y chromosome‐bearing spermatozoa.
Methods
The experimental ram sperms in this research included treated spermatozoa (50 μg/ml MNPs) and non‐treated spermatozoa. DNA damage of spermatozoa was examined using an acridine orange (AO) assay. Sperm viability, membrane functionality, abnormality and malondialdehyde (MDA) level were also measured.
Results
Results indicated that the pre‐treatment of ram semen extender with MNPs did not significantly affect the semen parameters such as viability, membrane functionality, abnormality, as well as lipid peroxidation (LPO) levels and DNA integrity in comparison with the control group (p < 0.05).
Conclusions
These observations suggest that pre‐treatment of ram semen extender with MNPs after semen sexing did not have adverse effects on different semen parameters after cryopreservation.
Keywords: cryopreservation, magnetic nanoparticles, ram, semen, sperm morphology
Short abstract
In the current study, we evaluated the effects of cryopreservation on ram semen after adding magnetic nanoparticles (MNPs), then after thawing some semen parameters were evaluated.
1. INTRODUCTION
Sexed semen plays a crucial role in producing the desired gender of offspring. One great example would be the production of males for meat production (Hossein‐Zadeh et al., 2010). Flow cytometry (Seidel, 2003), percoll and albumin gradient centrifugation (Machado et al., 2009; Wolf et al., 2018) swim up (Azizeddin et al., 2014), Sephadex columns (Vidal et al., 1993) and immunological methods (Bennett & Boyse, 1973; Ellis et al., 2011) have all been used to sex sperm in mammals. But not all of them were very successful. Sperm sexing technology was applied to livestock like ovine species in on effort to raise the chances of birth of selected sex for production purposes such as the use of donkey milk as a substitute for cow milk for allergic children, as a dietary supplement, and as a product used by cosmetic industry. Such a technology can be a basis to organise artificial insemination (AI) program. (Rasad et al., 2019). The majority of X and Y chromosome‐bearing spermatozoa isolation methods in farm animals are based on differences in DNA content by cell sorting. Based on the last reports, this technique harms the sperm quality and its parameters (Johnson et al., 1987; Seidel & Garner, 2002; Suh et al., 2005). Although it was contradicted for ram sperms by de Grraaf et al. (2006). Rath et al. (2013) and Garner (2006) stated that the magnetic nanoparticle is one of the suitable and efficient methods for sperm sexing in domestic animals. Using MNPs for sperm sexing in donkey, Domínguez et al. (2018) indicated that most isolated particles contain X chromosome‐bearing sperm. MNPs have been successfully used to select spermatozoa with better cryopreservation tolerance and fertilisation potential for assisted reproduction in humans (Berteli et al., 2017; Said et al., 2006). Although this approach successfully sorted examined sperm, one problem with sexed sperm is the poor quality of spermatozoa after freezing and their high cost. Therefore, it is important to find methods with low separation cost and higher quality after cryopreservation. No information is available on the quality of ram sperm after X and Y chromosome bearing spermatozoa isolation by magnetic nanoparticles. The aim of this study was to determine the effects of cryopreserving of ram semen sexed with magnetic nanoparticles on selected quality parameters of frozen‐thawed ram semen.
2. MATERIAL AND METHODS
2.1. Chemicals
Unless otherwise indicated, all reagents used in the experiment were obtained from Sigma‐Aldrich Company.
2.2. Experimental design
In our pervious study, semen collected from rams was pre‐treated with magnetic nanoparticles (MNPs) to isolate X and Y chromosome‐bearing spermatozoa (Moradi et al., 2021). In present study, cryopreservation ability was evaluated following the pre‐treated of ram semen with MNPs. The most suitable concentration (50 μg/ml) of MNPs for X and Y chromosome‐bearing spermatozoa along with the group (control) not receiving any MNPs were used to perform cryopreservation process (Moradi et al., 2021). Studied parameters included motility, viability, DNA and membrane integrity and morphological abnormality of sperms.
2.3. Semen collection and initial evaluation
Ejaculates from three adult fertile rams (1–3 years old) were used in this study. Two ejaculates were collected from rams using an artificial vagina twice a week according to AI standard procedures. Semen samples were first tested for volume (in a graduated tube), sperm motility and live sperm per cent after being transported to the lab under standard conditions. To be studied further, neat semen samples with more than 80% total motility were selected. Semen samples were pooled to avoid individual variations (Akhtarshenas et al., 2018).
2.4. Semen cryopreservation
The sperm were cryopreserved after being isolated from X and Y chromosome‐bearing spermatozoa using the MNPs method developed by Domínguez et al. (2018). In our study, briefly speaking, MNPs with diameter of 50 nm were coated with silica. Semen samples in extender (Oviplus, Minitube, Germany) were diluted up to the concentration of 50 million sperm/ml and, then, centrifuged. Next, sperm pellets were suspended mHTF (modified Human Tubal Fluid) to a concentration of 100 million sperm/ml. The group not incubated with MNPs was considered as a control one. After that, the groups were gently mixed for 5 min. Each type of sperms exhibited different interactions between negative charges of MNPs and Z electronic potentials of spermatozoa. Y chromosome bearing spermatozoa, therefore closer to MNPs because of their Zeta electrical potential (–16 m mV). Then, mixtures were placed on an external magnetic field consisting of magnets fixed on polycarbonate. Sperm‐bound MNPs were pulled down to the walls of the falcons (conical tubes), which were considered as Y sperms. And, MNPs‐free spermatozoa were collected into different 50‐ml falcon centrifuge tubes, which were considered as X sperms. The process was repeated 3–4 times until the maximum amounts of nanoparticles were removed. Centrifuged semen pellets were diluted in tris‐base extenders. The tris‐based extender was used as the one containing 105.35 mM citric acid, tris 297.58 mM, 82.59 mM fructose, glycerol 6% v/v and egg yolk 20% v/v dissolved in distilled water (Salamon & Maxwell, 2000). Some ejaculates were pre‐treated with MNPs (50μg/ml), and non‐treated ones were diluted with the tris‐based extender at a final concentration of 400 × 106 sperm/ml. Diluted semen was cooled from 37°C to 4°C for 4–5 h. Then, it was transferred into 0.25 ml french straws. French straws were kept on liquid nitrogen vapours for 10 min and stored in liquid nitrogen (–196°C). Straws containing sperm were thawed in a thawing unit held at 37°C for 30 s to test sperms. The studied parameters included sperm viability, membrane functionality, abnormality, lipid peroxidation and apoptosis test with acridine orange (AO) test.
2.5. Sperm motility
The sperm motility was performed under a phase‐contrast microscope at ×400 total magnification.
2.6. Sperm viability
Sperm viability was investigated by eosin‐nigrosine stain (Evans & Maxwell, 1987). According to this technique, smears of spermatozoa were prepared with staining solution (eosin‐Y 1.67 g, nigrosine 10 g, sodium citrate 2.9 g, dissolved in 100 ml distilled water) (Björndahl et al., 2003). Smears of sperm were examined, and each slide yielded 200 spermatozoa. Sperm were classified based on colours: white (alive) and red (dead) (Memon et al., 2012).
2.7. Spermatozoa membrane integrity
HOS‐test indicates the membrane integrity of the spermatozoa and damage is caused to the sperm membrane during cryopreservation. The HOS solution contained 0.73 g tri‐sodium citrate dihydrate and 1.35 g fructose, dissolved in 100 ml distilled water (osmotic pressure ∼190 mOsmol/kg). The assay was performed by mixing 50 μl of frozen‐thawed semen sample to 500 μl of HOS solution and incubating at 37°C for 60 min. After incubation, 200 spermatozoa per slide were assessed and the percentage of spermatozoa with curled tails (swollen/intact plasma membrane) was calculated (Ahmad et al., 2003).
2.8. Morphological abnormalities
To evaluate sperm abnormalities, we added at least 15 μl of each sample to tubes containing 1 ml of the Hancock solution (62.5 ml of formalin, 150 ml of sodium saline solution, 150 ml of buffer solution and 500 ml of double‐distilled water). One drop of this mixture was placed on a microscope slide and covered with a coverslip. Percentages of the sperm head, tail and total abnormalities were determined by counting 200 sperms per slide under phase contrast microscopy at ×1000 (Zhu et al., 2015).
2.9. Malondialdehyde (MDA) test
MDA levels were measured by thiobarbituric acid (TBA). An MDA testing kit (Teb pajohan Razi) was used. According to the kit instructions, MDA content was measured at 532 nm on the spectrophotometer and expressed as nanomole per milligram.
2.10. Acridine orange (AO) test
The acridine orange (AO) assay measures the ability of sperm nuclear DNA to be denatured by the acid and bound to DNA, with an excitation limit of 502 nm (red) and an emission maximum of 525 nm (green), resulting in a metachromatic change of AO fluorescence from green (native DNA) to red (sperm DNA) (denatured DNA). Fluorochrome AO is a monomer that binds to single‐stranded DNA in double‐stranded DNA (Chisty et al., 2018).
To perform the AO assay, spermatozoa smears were first prepared and fixed overnight in carney's solution (Liu & Baker 1994). The slides were then stained for 5 min in citrate‐phosphate buffer solution with AO stain. Finally, all of them were evaluated using a fluorescent microscope (Leica, type; 11090–137002).
2.11. Statistical analysis
Results are presented as mean ± SD. Statistical differences between the means of the two groups were evaluated by the Student's t‐test. The value of p < 0.05 was considered statistically significant. All data were verified to accomplish the parametric assumptions of homogeneity of variances and normality.
3. RESULTS
3.1. Sperm motility
The total motility of sperms treated with MNPS cryopreservation and thawing was about 51%, and there was no significant difference with the control group (p < 0.05) (Figure 1).
FIGURE 1.

The effect of pre‐treatment of MNPs on sperm total motility
3.2. Sperm viability, abnormality and membrane functionality
The percentages of sperm viability, morphological abnormality and membrane functionality are presented in Figure 2. Mean ± SD percentage of viability, morphological abnormality and membrane functionality of sperm in frozen‐thawed semen pre‐treated with MNPs were not significantly different from those of the frozen‐thawed control semen (p < 0.05).
FIGURE 2.
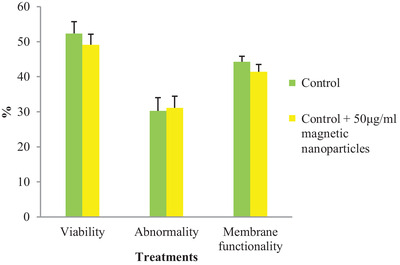
The effects of pre‐treatments of MNPs on sperm morphological parameters. p < 0.05 compared to the controls by independent sample t‐test
3.3. Sperm lipid peroxidation
Figure 3 reports the data on the effects of pre‐treatment with MNPs on MDA production by ram sperm following freeze‐thawing. This analysis did not reveal any significant differences in MDA levels between the control group and the group pre‐treated with MNPs.
FIGURE 3.
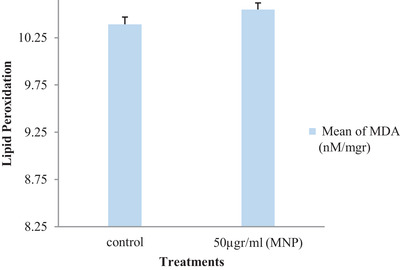
Mean ± SD of lipid peroxidation (MDA) after cryopreservation of ram semen in the control group and groups pre‐treated with MNPs
3.4. Chromatin integrity
DNA integrity of frozen‐thawed ram sperms pre‐treated with MNPs is depicted in Figures 4 and 5. Results show that pre‐treating ram semen extender with MNPs does not impact DNA integrity after freeze‐thawing compared to the control group (p < 0.05).
FIGURE 4.
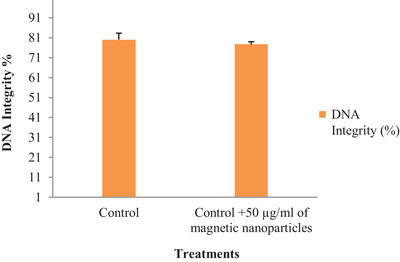
The effects of pre‐treatments of MNPs on sperm DNA integrity
FIGURE 5.

Ram sperm stained with 1% acridine orange (AO). Sperm with normal chromatin structure display green fluorescence and those with an abnormal DNA (single‐strand) shows a yellow to red fluorescence colour
4. DISCUSSION
As a small ruminant, sheep serve as a source of wool, meat and milk. It can be concluded that sperm sexing is very useful to increase the number of desired‐sex offspring during the breeding season. Recently, MNPs as a useful method can separate X from Y‐bearing sperm. After using the MNPs system and subsequent cryopreservation, there was little knowledge on sperm quality. It is argued that flow cytometry can, currently, be used as a commercially available tool to sex sperms, but it is a costly method with extremely low rates of sperms surviving from the cryopreservation processes, which is due to application of UV rays to death of sperms. Different methods for separation X and Y chromosome‐bearing spermatozoa have been studied that are inexpensive and maintain the relative quality of sperm after treatment of samples. The high‐efficiency MNPs have recently been used to isolate X from Y sperm (Domínguez et al., 2018; Moradi et al., 2021). According to previous studies, ram sperms are highly vulnerable to cryopreservation due to high levels of polyunsaturated fatty acids on the cell membrane (Alvarez et al., 1987; Gandini et al., 2000). As a result of such sensitivity, cell membranes are damaged, leading to reduced viability, decreased motility and changed metabolic activity (Ball et al., 2001). Various studies have been performed to improve viability after freeze‐thawing. Different compounds have been added to the semen extenders, ultimately inhibiting oxidative stresses and improving sperm motility in ram (Bucak et al., 2007; Moradi et al., 2013). Our results showed that MNPs could separate X from Y‐bearing sperm without any adverse effect on the viability and integrity of treatment sperm compared with controls.
The content of X chromosome‐bearing sperm was evaluated in this study. The X sperm were treated with 50μg/ml MNPs after separation. Despite the fact that different stages of isolation, such as washing, centrifugation, nanoparticles treatment and nanoparticle separation, were described in detail in the Domínguez et al. (2018) study, we found no significant differences in the amount of viability, abnormality and membrane functionality of sperm and level of MDA, as well as DNA integrity, after being frozen‐thawed. In this study, motility, morphological abnormality and membrane functionality showed relatively acceptable values. Our results indicated that these parameters in treatment and control samples were not changed, and at least the MNPs had no negative effects on sperm in these two samples. The only study investigating X chromosome‐bearing spermatozoa isolation using nanoparticles was the research by Domínguez et al. (2018) donkey semen. In contrast to our findings, Domínguez et al. (2018) reported the lower values of sperm parameters. Several techniques are being used to separate appropriate sperm from inappropriate ones, including a swim‐up, gradient density of percoll and magnetic‐assisted cell sorting methods (Gil et al., 2013; Kaneko et al., 1986; Mortimer, 2000). Recently, research has shown that nanoparticles are capable of isolating spermatozoa with appropriate fertility from defective and damaged sperm. The results showed that after treating pig spermatozoa with MNPs coated with lectin or annexin V for 10–15 min, sperm motility, acrosomal, mitochondrial and plasma membrane integrity increased while ROS production decreased. However, treatment groups showed no difference in the rate of spermatozoa viability with the control group. In addition, it was found that MNPs could be a suitable tool for removing abnormal and defective sperm and improving fertility, especially in male animals under heat stress (Durfey et al., 2019; Odhiambo et al., 2014). Lectin‐coated MNPs can remove the abnormal sperm in bull cattle and improve fertilisation after artificial insemination (Odhiambo et al., 2014). Feugang et al. (2015) used lectin‐coated magnetic iron oxide nanoparticles to isolate dead or dysfunctional spermatozoa from healthy sperms. They observed an increase in sperm motility. Also, MNPs had no toxic effects on the fertility rate of pig sperm and the survival of newborn pigs. On the surface of sperm plasma membranes are lectin and carbohydrate receptor systems, which are used for a variety of functions. Lectins are carbohydrates capable of creating an accumulative system in sperms by binding to carbohydrate plasma membranes. MNPs combined with fluorescent dyes, antibodies or magnetism has been used to isolate apoptotic spermatozoa in semen samples (Feugang et al., 2015). According to recent studies, MNPs improved freezing resistance and fertilisation rate in human sperm samples (Berteli et al., 2017; Said et al., 2006). Treatment of spermatozoa with magnetic iron nanoparticles (Fe3O4‐PVA) at 37°C for 2 h showed that MNPs could influx to cells without any adverse effects on motility and acrosomal reaction (Ben‐David et al., 2006). In other words, since MNPs can effectively extract abnormal and dead sperm, the rate of fertilisation using this technique can be increased.
MDA levels are a good indicator of peroxidation‐induced lipid peroxidation. Also, this measurement can provide a good perspective on the complex process of fatty peroxide formation and decomposition. Additionally, sperm motility is highly related to seminal plasma MDA levels (Zarghami & Khosrowbeygi, 2004), so it is very important to evaluate it after the cryopreservation process. In this study, MDA concentration was measured against the previous study on donkeys. Our results showed no significant differences in MDA levels between the groups pre‐treated with MNPs and the control group after cryopreservation. So, these MNPs did not result in lipid peroxidation on the sperm plasma membrane after different steps. Our findings differ from those reported by Peris‐Frau et al. (2020) who based their findings on a decrease in MDA levels following cryopreservation procedures. According to the findings of this study, there was no substantial difference in post‐cryopreservation DNA integrity between the control group and the group pre‐treated with MNPs, while Domínguez et al. (2018) found a higher value. Different approaches were used in this study and Domínguez et al. (2018) study, which may explain why different responses were observed.
Finally, it should be noted that, based on our results, no differences in semen parameters, MDA levels and DNA integrity were observed between semen samples of control and treated group. Further studies are needed to compare MNPs isolation methods with others and evaluate sperm quality and fertility after the freeze‐thawing process.
5. CONCLUSION
In summary, our study's MNPs had no negative effect on X‐bearing sperm. Even though the flow cytometry and MNPs approaches are useful and effective for X from Y chromosome‐bearing sperm, the MNPs are more accessible and cheaper than flow cell sorter. The MNPs can be produced commercially and used in sperm sexing in sheep industries.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.689
AUTHOR CONTRIBUTIONS
All authors contributed equally.
ETHICS STATEMENT
Animal husbandry and handling were conducted in accordance with the guidelines of Animal Ethics Committee (Permission number: IR.RAZI.REC. 397‐1‐022) of Razi University, Kermanshah, Iran.
CONFLICT OF INTEREST
The author declares that they have no conflict of interest.
ACKNOWLEDGEMENT
This research did not receive any specific grant from funding agencies in the public, commercial, or not‐for‐profit sectors. We would like to express our thanks to Ms. Zohreh Heidari for helping in the semen collection.
Moradi, M. , Hajarian, H. , Karamishabankareh, H. , Soltani, L. , & Soleymani, B. (2022). Pre‐treatment of ram semen extender with magnetic nanoparticles on freeze‐thawed spermatozoa. Veterinary Medicine and Science, 8, 792–798. 10.1002/vms3.689
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding upon reasonable request.
REFERENCES
- Ahmad, Z. , Anzar, M. , Shahab, M. , Ahmad, N. , & Andrabi, S. M. H. (2003). Sephadex and sephadex ionexchange filtration improves the quality and freezability of low‐grade buffalo semen ejaculates. Theriogenology, 59: 1189–1202. 10.1016/S0093-691X(02)01159-7 [DOI] [PubMed] [Google Scholar]
- Akhtarshenas, B. , Karami Shabankareh, H. , Hajarian, H. , Bucak, M. N. , Abdolmohammadi, A. R. , & Dashtizad, M. (2018). The protease inhibitor antipain has a beneficial synergistic effect with trehalose for ram semen cryopreservation. Reproduction in Domestic Animals, 53(6), 1359–1366. 10.1111/rda.13253 [DOI] [PubMed] [Google Scholar]
- Alvarez, J. G. , Touchstone, J. C. , Blasco, L. , & Storey, B. T. (1987). Spontaneous lipid peroxidation and production of hydrogen peroxide and superoxide in human spermatozoa Superoxide dismutase as major enzyme protectant against oxygen toxicity. Journal of Andrology, 8(5), 338–348. 10.1002/j.1939-4640.1987.tb00973.x [DOI] [PubMed] [Google Scholar]
- Azizeddin, A. , Ashkar, F. A. , King, W. A. , & Revay, T. (2014). Enrichment of Y‐chromosome‐bearing bull spermatozoa by swim‐up through a column. Reproduction in Domestic Animals, 49(1), e1–e4. 10.1111/rda.12252 [DOI] [PubMed] [Google Scholar]
- Ball, B. , Medina, V. , Gravance, C. , & Baumber, J. (2001). Effect of antioxidants on preservation of motility, viability and acrosomal integrity of equine spermatozoa during storage at 5C°. Theriogenology, 56(4), 577–589. 10.1016/S0093-691X(01)00590-8 [DOI] [PubMed] [Google Scholar]
- Ben‐David Makhluf, S. , Qasem, R. , Rubinstein, S. , Gedanken, A. , & Breitbart, H. (2006). Loading magnetic nanoparticles into sperm cells does not affect their functionality. Langmuir, 22(23), 9480–9482. 10.1021/la061988z [DOI] [PubMed] [Google Scholar]
- Bennett, D. , & Boyse, E. A. (1973). Sex ratio in progeny of mice inseminated with sperm treated with HY antiserum. Nature, 246(5431), 308–309. 10.1038/246308a0 [DOI] [PubMed] [Google Scholar]
- Berteli, T. S. , Da Broi, M. G. , Martins, W. P. , Ferriani, R. A. , & Navarro, P. A. (2017). Magnetic‐activated cell sorting before density gradient centrifugation improves recovery of high‐quality spermatozoa. Andrology, 5(4), 776–782. 10.1111/andr.12372 [DOI] [PubMed] [Google Scholar]
- Björndahl, L. , Söderlund, I. , & Kvist, U. (2003). Evaluation of the one‐step eosin‐nigrosin staining technique for human sperm vitality assessment. Human Reproduction, 18(4), 813–816. 10.1093/humrep/deg199 [DOI] [PubMed] [Google Scholar]
- Bucak, M. N. , Ateşşahin, A. , Varışlı, Ö. , Yüce, A. , Tekin, N. , & Akçay, A. (2007). The influence of trehalose, taurine, cysteamine and hyaluronan on ram semen: Microscopic and oxidative stress parameters after freeze–thawing process. Theriogenology, 67(5), 1060–1067. 10.1016/j.theriogenology.2006.12.004 [DOI] [PubMed] [Google Scholar]
- Chisty, L. T. , Quaglia, D. , & Webb, M. R. (2018). Fluorescent single‐stranded DNA‐binding protein from Plasmodium falciparum as a biosensor for single‐stranded DNA. PloS One, 13(2), e0193272. 10.1371/journal.pone.0193272 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Graaf, S. P. , Evans G., Maxwell, W. M. , & Justine, O. (2006). In vitro characteristics of fresh and frozen–thawed ram spermatozoa after sex sorting and re‐freezing. Reproduction, Fertility and Development 18(8), 867–874. 10.1071/RD06061 [DOI] [PubMed] [Google Scholar]
- Domínguez, E. , Moreno‐Irusta, A. , Castex, H. R. , Bragulat, A. F. , Ugaz, C. , Clemente, H. , Giojalasa, L. , & Losinno, L. (2018). Sperm sexing mediated by magnetic nanoparticles in donkeys, a preliminary in vitro study. Journal of Equine Veterinary Science, 65, 123–127. 10.1016/j.jevs.2018.04.005 [DOI] [Google Scholar]
- Durfey, C. L. , Swistek, S. E. , Liao, S. F. , Crenshaw, M. A. , Clemente, H. J. , Thirumalai, R. V. K. G. , Steadman, C. S. , Ryan, P. L. , Willard, S. T. , & Feugang, J. M. (2019). Nanotechnology‐based approach for safer enrichment of semen with best spermatozoa. Journal of Animal Science and Biotechnology, 10(1), 1–12. 10.1186/s40104-018-0307-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis, P. J. , Yu, Y. , & Zhang, S. (2011). Transcriptional dynamics of the sex chromosomes and the search for offspring sex‐specific antigens in sperm. Reproduction, 142(5), 609–619. 10.1530/rep-11-0228 [DOI] [PubMed] [Google Scholar]
- Evans, G. & Maxwell, W. M. C. (1987). Salamon's artificial insemination of sheep and goats. Sydney, Australia: Butterworths. [Google Scholar]
- Feugang, J. , Liao, S. , Crenshaw, M. , Clemente, H. , Willard, S. , & Ryan, P. (2015). Lectin‐functionalized magnetic iron oxide nanoparticles for reproductive improvement. Journal of Fertilization: In Vitro, 3(145), 17–19. 10.1186/s12951-016-0168-y [DOI] [Google Scholar]
- Gandini, L. , Lombardo, F. , Paoli, D. , Caponecchia, L. , Familiari, G. , Verlengia, C. , Dondero, F. , & Lenzi, A. (2000). Study of apoptotic DNA fragmentation in human spermatozoa. Human Reproduction, 15(4), 830–839. 10.1093/humrep/15.4.830 [DOI] [PubMed] [Google Scholar]
- Garner, D. L. (2006). Flow cytometric sexing of mammalian sperm. Theriogenology, 65(5), 943–957. 10.1016/j.theriogenology.2005.09.009 [DOI] [PubMed] [Google Scholar]
- Gil, M. , Sar‐Shalom, V. , Sivira, Y. M. , Carreras, R. , & Checa, M. A. (2013). Sperm selection using magnetic activated cell sorting (MACS) in assisted reproduction: A systematic review and meta‐analysis. Journal of Assisted Reproduction and Genetics, 30(4), 479–485. 10.1007/s10815-013-9962-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hossein‐Zadeh, N. G. , Nejati‐Javaremi, A. , Miraei‐Ashtiani, S. R. , & Kohram, H. (2010). Bio‐economic evaluation of the use of sexed semen at different conception rates and herd sizes in Holstein populations. Animal Reproduction Science, 121(1–2), 17–23. 10.1016/j.anireprosci.2010.05.012 [DOI] [PubMed] [Google Scholar]
- Johnson, L. A. , Flook, J. , Hawk, H. , Look, M. , & Pinkel, D. (1987). Flow sorting of X and Y chromosome bearing spermatozoa into two populations. Gamete Research, 16, 1–9. 10.1002/mrd.1120160102 [DOI] [PubMed] [Google Scholar]
- Kaneko, S. , Oshio, S. , Kobanawa, K. , Kobayashi, T. , Mohri, H. , & Iizuka, R. (1986). Purification of human sperm by a discontinuous Percoll density gradient with an innercolumn. Biology of Reproduction, 35(4), 1059–1063. 10.1002/mrd.22831 [DOI] [PubMed] [Google Scholar]
- Liu, D. Y. & Baker, H. W. G. (1994). Disordered acrosome reaction of spermatozoa bound to the zone pellucida: A newly discovered sperm defect causing infertility with reduced sperm‐zona pellucida penetration and reduced fertilization in vitro. Human Reproduction, 9, 1694–1700. 10.1093/oxfordjournals.humrep.a138776 [DOI] [PubMed] [Google Scholar]
- Machado, G. M. , Carvalho, J. O. , Siqueira Filho, E. , Caixeta, E. S. , Franco, M. M. , Rumpf, R. , & Dode, M. A. N. (2009). Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology, 71(8), 1289–1297. 10.1016/j.theriogenology.2009.01.002 [DOI] [PubMed] [Google Scholar]
- Memon, A. A. , Wahid, H. , Rosnina, Y. , Goh, Y. , Ebrahimi, M. , & Nadia, F. (2012). Effect of antioxidants on post thaw microscopic, oxidative stress parameter and fertility of Boer goat spermatozoa in Tris egg yolk glycerol extender. Animal Reproduction Science, 136(1–2), 55–60. 10.1016/j.anireprosci.2012.10.020 [DOI] [PubMed] [Google Scholar]
- Moradi, M. , Hajarian, H. , Karamishabankareh, H. , Soltani, L. , & Soleymani, B. (2021). Recovery of sperms bearing X chromosomes with different concentrations of magnetic nanoparticles in ram. Reproduction in Domestic Animals. 56, 263–269. 10.1111/rda.13807 [DOI] [PubMed] [Google Scholar]
- Moradi, A. , Malekinejad, H. , Farrokhi‐Ardabili, F. , & Bernousi, I. (2013). Royal Jelly improves the sperm parameters of ram semen during liquid storage and serves as an antioxidant source. Small Ruminant Research, 113(2–3), 346–352. 10.1016/j.smallrumres.2013.03.003 [DOI] [Google Scholar]
- Mortimer, D. (2000). Sperm preparation methods. Journal of Andrology, 21(3), 357–366. 10.1002/j.1939-4640.2000.tb03390.x [DOI] [PubMed] [Google Scholar]
- Odhiambo, J. F. , DeJarnette, J. , Geary, T. W. , Kennedy, C. E. , Suarez, S. S. , Sutovsky, M. , & Sutovsky, P. (2014). Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biology of Reproduction, 91(4), 97, 91–10. 10.1095/biolreprod.114.121897 [DOI] [PubMed] [Google Scholar]
- Peris‐Frau, P. , Soler, A. J. , Iniesta‐Cuerda, M. , Martín‐Maestro, A. , Sánchez‐Ajofrín, I. , Medina‐Chávez, D. A. , Fernández‐Santos, M. R. , García‐Álvarez, O. , Maroto‐Morales, A. , Montoro, V. , & Garde, J. J. (2020). Sperm cryodamage in ruminants: Understanding the molecular changes induced by the cryopreservation process to optimize sperm quality. International Journal of Molecular Sciences, 21(8), 2781. 10.3390/ijms21082781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasad, S. , Solihati, N. , Winangun, K. , Avicenna, M. , Yusrina, A. , Melinda, M. , & Rauf, A. (2019). Evaluation of Pasundan Cattle semen quality in three different types of extender. Paper presented at the IOP Conference Series: Earth and Environmental Science. 10.1088/1755-1315/247/1/012011 [DOI]
- Rath, D. , Barcikowski, S. , De Graaf, S. , Garrels, W. , Grossfeld, R. , Klein, S. , Knabe, W. , Knorr, C. , Kues, W. , & Meyer, H. (2013). Sex selection of sperm in farm animals: Status report and developmental prospects. Reproduction, 145(1), R15–R30. 10.1530/rep-12-0151e [DOI] [PubMed] [Google Scholar]
- Said, T. M. , Agarwal, A. , Grunewald, S. , Rasch, M. , Glander, H.‐J. , & Paasch, U. (2006). Evaluation of sperm recovery following annexin V magnetic‐activated cell sorting separation. Reproductive Biomedicine Online, 13(3), 336–339. 10.1016/S1472-6483(10)61437-X [DOI] [PubMed] [Google Scholar]
- Salamon, S. , & Maxwell, W. (2000). Storage of ram semen. Animal Reproduction Science, 62(1–3), 77–111. 10.1016/S0378-4320(00)00155-X [DOI] [PubMed] [Google Scholar]
- Seidel Jr, G. E. (2003). Sexing mammalian sperm – Intertwining of commerce, technology, and biology. Animal Reproduction Science, 79(3–4), 145–156. 10.1016/S0378-4320(03)00162-3 [DOI] [PubMed] [Google Scholar]
- Seidel Jr, G. E. , & Garner, D. L. (2002). Current status of sexing mammalian spermatozoa. Reproduction (Cambridge, England), 124(6), 733–743. 10.1262/jrd.2012-077 [DOI] [PubMed] [Google Scholar]
- Suh, T. , Schenk, J. , & Seidel Jr, G. (2005). High pressure flow cytometric sorting damages sperm. Theriogenology, 64(5), 1035–1048. 10.1016/j.theriogenology.2005.02.002 [DOI] [PubMed] [Google Scholar]
- Vidal, F. , Moragas, M. , Catala, V. , Torello, M. J. , Santalo, J. , Calderon, G. , Gimenez, C. , Barri, P. N. , Egozcue, J. , & Veiga, A. (1993). Sephadex filtration and human serum albumin gradients do not select spermatozoa by sex chromosome: A fluorescent in‐situ hybridization study. Human Reproduction (Oxford, England), 8(10), 1740–1743. 10.1093/oxfordjournals.humrep.a137926 [DOI] [PubMed] [Google Scholar]
- Wolf, C. A. , Brass, K. E. , Rubin, M. I. B. , Pozzobon, S. E. , Mozzaquatro, F. D. , & De La Corte, F. D. (2018). The effect of sperm selection by Percoll or swim‐up on the sex ratio of in vitro produced bovine embryos. Animal Reproduction (AR), 5(3), 110–115. [Google Scholar]
- Zarghami, N. , & Khosrowbeygi, A. (2004). Evaluation of lipid peroxidation as an indirect measure of oxidative stress in seminal plasma. International Journal of Reproductive BioMedicine, 2(1), 34–30. http://journals.ssu.ac.ir/ijrmnew/article‐1‐9‐en.html [Google Scholar]
- Zhu, Z. , Fan, X. , Lv, Y. , Zhang, N. , Fan, C. , Zhang, P. , & Zeng, W. (2015). Vitamin E analogue improves rabbit sperm quality during the process of cryopreservation through its antioxidative action. PloS One, 10(12), e0145383. 10.1371/journal.pone.0145383 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data that support the findings of this study are available from the corresponding upon reasonable request.


