Abstract
Objectives
The purpose of the present study was to evaluate the postoperative analgesic efficacy of fentanyl patches versus subcutaneous tramadol after canine ovariectomy, with and without unilateral mastectomy.
Materials and methods
A total of 40 female dogs were included in the present study, all of which were domesticated, healthy and 4–12 years of age. The animals were divided into four groups (n = 10 per group) based on the surgery and the analgesic protocol used: the TO group only underwent ovariectomy, and received postoperative tramadol; the TM group underwent both ovariectomy and mastectomy, and received postoperative tramadol; the FO group only underwent ovariectomy, and received fentanyl patches; and the FM group underwent both ovariectomy and mastectomy, and received fentanyl patches. Postoperative pain was evaluated every 4 h for 24 h using a numeric analogue scale (NAS) and a modified Glasgow Composite Measure Pain Scale Short Form (CMPS‐SF).
Results
The results of the present study showed that patients in all four groups tolerated postoperative surgical stress well. Analysis of variance for repeated measures did not show significant differences in the NAS scores and in Glasgow CMPS‐SF between groups in terms of pain scores or rescue analgesia.
Clinical significance
These results indicated that the analgesic effect of the fentanyl patch was similar to that of subcutaneous (SC) tramadol in female dogs after ovariectomy, with and without unilateral mastectomy, suggesting that the fentanyl patch may represent a valid supplementary tool for the control of postoperative pain in animals after surgery.
Keywords: fentanyl patch, ovariectomy analgesia, tramadol, unilateral mastectomy
The analgesic effect of the fentanyl patch was similar to that of subcutaneous (SC) tramadol in female dogs after ovariectomy, with and without unilateral mastectomy. Considering the cost, availability, restrictions, and side effects associated with the fentanyl patch, this study is encouraging and could spark interest, leading to a possible association between routine postoperative analgesia and fentanyl patch.

1. INTRODUCTION
Ovariectomy is one of the most performed elective surgical procedures in small animals (Rutteman et al., 2001). This surgery involves both somatic and visceral pathways and is sometimes performed concurrently with mastectomy, resulting in extensive tissue damage and severe postoperative pain (Mathews, 2000). Fentanyl is a potent mu opioid receptor agonist, with a wide margin of safety and minimal effects on the cardiovascular and respiratory systems in canines (Reed et al., 2011). The short duration of action has limited its use in the surgical setting to perioperative injections, rescue analgesia, or constant rate infusions (CRI) (Pekcan & Koc, 2010). Therefore, to improve and extend the analgesic effect of fentanyl, additional delivery technologies have been developed for use in humans, including the ‘fentanyl patch’, which has been utilised in an extra‐label manner in veterinary medicine (Egger et al., 1998; Gilbert et al., 2003; Kyles et al., 1998, 1996). Fentanyl patches have a prolonged duration of action and also reduce the side effects of oral and parenteral administration (Kukanich & Clark, 2012). Previous studies regarding the use of fentanyl patches in dogs have reported higher pain scores in the immediate postoperative period (0–6 h) and lower scores starting 12–18 h after the application of the patch (Robinson et al., 1999), indicating the necessity of applying the patch at least 18 h before surgery in order to reach the ideal hematic concentration required for an appropriate analgesic effect during the immediate postoperative period. Fentanyl patches provide the sustained delivery of fentanyl, which can be useful for both the control of postoperative pain and as antinociception during painful surgeries, such as orthopaedic procedures (Lafuente et al., 2005). To the authors’ knowledge, the use of fentanyl patch during canine mastectomy has not been described. It is expected that by using this patch, a good analgesia could be maintained in postoperative period. In the present study, we selected tramadol as a positive control for the fentanyl patch because it is a widely used and well‐known drug used for the management of acute pain in small animals, providing the same analgesic effect as morphine at equipotent doses, and because of its immunomodulatory properties (Mastrocinque & Fantoni, 2003). Tramadol is a synthetic opioid with low mu opioid agonist affinity (6000 times less than morphine), and its efficacy is due to two different mechanisms, it interacts with mu opioid receptors and affects serotonin and norepinephrine reuptake (G. L. Costa et al., 2015, 2019; G. Costa et al., 2018, 2021). In fact, Teixeira (Monteiro et al., 2009) showed that the intramuscular (IM) administration of tramadol (3 mg/kg) at 8‐h intervals appeared to be effective for the management of postoperative pain during the first 24 h in most of the dogs undergoing unilateral mastectomy with or without ovariohysterectomy (OHE). The aim of the present study was to compare the early postoperative analgesic efficacy of fentanyl patches versus tramadol after ovariectomy in canines, with and without unilateral mastectomy.
2. MATERIALS AND METHODS
2.1. Study design
This study model is a randomised non‐blinded clinical trial.
2.2. Inclusion and exclusion criteria
For the present study, 40 female dogs meeting the following requirements were recruited: aged 4–12 years, weighing 11–30 kg, no known previous systemic pathologies, and classified as a low anaesthetic risk [American Society of Anaesthesiology (ASA) class 2]. Of the 40 female dogs, 20 were selected for elective ovariectomy, and 20 were selected for ovariectomy performed concurrently with unilateral mastectomy due to mammary tumours, diagnosed on clinical examination and which were staged according to the tumour node metastasis (TNM) classification system (Teixeira et al., 2013). Informed consent was obtained from the pet owners prior to participation in the study. The day before surgery, patients underwent general preoperative examinations, as well as thoracic radiographs, abdominal ultrasound scans, and routine blood tests (complete blood count and metabolic panel). Dogs were included if they were healthy, as determined by history, physical examination, complete blood count, and serum chemistry. Dogs that presented with ulcerated nodules, and those with evidence of lung metastasis, were excluded from the study. Of the 40 female dogs, 20 (10 selected for ovariectomy and 10 for ovariectomy and unilateral mastectomy) received fentanyl patches, which were placed on the necks of dogs previously shaved and disinfected, 24 h before surgery to optimise the effects and to reach the highest plasma concentration (Freise et al., 2012) in the postoperative period. The patches were fixed with stitches and adhesive for 72 h (Bellei et al., 2011; Hofmeister & Egger, 2004) according to the protocol presented by Gilbert et al. (2003) as follows: < 10 kg = 25 mcg/h; 10–20 kg = 50 mcg/h; 20–30 kg = 75 mcg/h; and 30–40 kg = 100 mcg/h (Hellyer et al., 2006).
2.3. Study groups
The female dogs were randomly (StatView statistical software, JMP) assigned into four groups (n = 10 per group): the TO group only underwent ovariectomy and received postoperative tramadol (Altadol, Formevet S.r.l., Milano, Italy at a dosage of 5 mg/kg); the TM group underwent both ovariectomy and mastectomy and received postoperative tramadol (5 mg/kg); the FO group only underwent ovariectomy and received fentanyl patches (Durogesic, Jannsen, Milano, Italy); and the FM group underwent both ovariectomy and mastectomy and received fentanyl patches. Food was withheld for 12 h prior to the surgery. The time period between patch application and surgery was chosen based on a study by Kukanich and Clark (2012), who observed that plasma fentanyl concentrations increased slowly during the first 24 h and remained in a steady‐state period for the next 48 h.
2.4. Anaesthesia and surgical procedures
Patients in all four groups were pre‐medicated 20 min before surgery with IM dexmedetomidine (Dexdomitor, Vetoquinol Italia SRL, Bertinoro, Italy) at a dosage of 3 μg/kg and IM methadone hydrochloride (Semfortan, Eurovet Animal Health BV, Bladel, The Netherlands) at a dosage of 0.25 mg/kg mixed in the same syringe. When the sedative effect was achieved, a 24G intravenous (IV) catheter was inserted into the cephalic vein to start standard maintenance fluid therapy using ringer lactate 5 ml/kg/h (Ringer Lattato, BBraun Milano Spa, Milano, Italy), and to allow for rapid drug administration, if necessary. This was followed by the administration of 100% oxygen via a face mask using a Mapleson F circuit. Anaesthesia was induced in all dogs by the administration of IV propofol (Vetofol, Esteve, Barcelona, Spain) at a dosage of 4 mg/kg (SD 1 mg/kg). When anaesthesia was achieved, the patients were intubated and connected to the anaesthesia machine using a Mapleson F respiratory circuit. Anaesthesia was maintained throughout the surgery by the administration of sevoflurane (EtSev 2,5%; SevoFlo, Ecuphar Italia S.r.l., Milano, Italy) through the respiratory circuit. Systemic analgesia was administered as a 1 μg/kg bolus of sufentanil citrate (50 μg/ml) followed by a maintenance dose constant‐rate IV infusion of 1.5 μg/kg/h until the surgery was completed (G. L. Costa et al., 2019). The following vital parameters were monitored throughout the surgery: heart rate, electrocardiographic trace, pulse oximetry, end‐tidal carbon dioxide concentration, end‐tidal percentage of sevoflurane, non‐invasive blood pressure, and body temperature (GE‐Datex Ohmeda Carestation 620 Anaesthesia Cart, GE‐Datex Ohmeda B 450 Monitor). The skin at the surgical site was shaved and aseptically prepared, and the animals were placed in the dorsal recumbency position. Throughout the procedure, heat dispersion was minimised by placing the patient on an air‐heated mat (Bair Hugger model 505; 3 M Italia). All surgeries were performed by the same surgeon and the same surgical team, in full compliance with the leges artis. In the immediate postoperative period, all female dogs received subcutaneous (SC) meloxicam (Metacam, Boehringer Ingelheim Italia S.p.A.) at a dosage of 0.20 mg/kg. Additionally, SC tramadol (Altadol) was administered at a dosage of 5 mg/kg in the TO and TM groups.
2.5. Postoperative pain evaluation
During the postoperative period, immediately after the reappearance of the righting reflex and the assumption of sternal decubitus position, patients in the TO and TM groups received SC tramadol injections every 8 h. To assess pain in veterinary patients, we used the numerical analogue scale (NAS) and the Glasgow Composite Measure Pain Scale Short Form (CMPS‐SF) (Hellyer et al., 2006; Reid et al., 2007). The NAS consisted of a 10‐cm line representing ‘no sedation or no pain’ at the left end and ‘the most sedation or worst pain possible’ at the right end, ranging from 0 to 10 points. An observer was responsible for placing a mark on the number that corresponded to the degree of sedation and pain for the animal. The NAS score was defined as the distance between the left end of the scale and the mark (Flecknell & Waterman‐Pearson, 2000; Sarrau et al., 2007). The CMPS‐SF was developed using a psychometric methodology, and takes into account postural attitudes and facial expressions, vocalisations, mood, the response to manipulation and palpation of the painful area, physical activity and gait, and the subject's behaviour towards the surrounding environment (Flecknell & Waterman‐Pearson, 2000; Morton et al., 2005; Tranquili et al., 2007). These scales were considered the most appropriate assessments for the evaluations performed in the present study, the aim of which was to compare the analgesic efficacy of the fentanyl patch versus SC tramadol after ovariectomy, with and without unilateral mastectomy. In all groups, the level of postoperative pain and discomfort was assessed using the NAS and Glasgow CMPS‐SF scores every 4 h for 24 h (Reid et al., 2007) immediately after the end of the surgery. The patients with NAS and Glasgow CMPS‐SF scores >4 received rescue analgesia with IM methadone at a dosage of 0.2 mg/kg (Reid et al., 2007).
2.6. Data analysis
All data collected were entered into a database created with an Excel spreadsheet, and data analysis was performed using Stata MP16 software. Continuous variables were described as median and interquartile range (IQR) and as media on figures, and categorical variables as proportions. The skewness and kurtosis test was used to evaluate the normality of continuous variables, and a normalisation model was used for those not normally distributed. One‐way analysis of variance (ANOVA) was used to compare continuous variables between groups, with Bonferroni correction to compare two groups at a time. The ANOVA for repeated measures test was used to compare continuous variables between groups and detection time. The Fisher's exact test was used to compare proportions between groups. To assess the relation between the difference between t24 and t0 of NAS and CMPS‐SF scores and age, weight and groups multivariate linear regression models were built; correlation coefficients were reported with the indication of 95% confidence interval (95% CI). For all tests, a two‐sided p‐value < 0.05 was considered statistically significant.
3. RESULTS
All surgical procedures lasted on average 25 min (SD 5 min) for the simple ovariectomy and 50 min (SD 7 min) for the ovariectomy performed concurrently with mastectomy. The population for the present study consisted of 40 female dogs: 10 (25.0%) in the FM group, 10 (25.0%) in the FO group, 10 (25.0%) in the TM group, and 10 (25.0%) in the TO group. The characteristics of the population by group are listed in Table 1.
TABLE 1.
Sample characteristics by group
| FM | FO | TM | TO | Total | p‐Value | |
|---|---|---|---|---|---|---|
| Weight | 23.5 (14–25) | 19.5 (14–22) | 20 (15–29) | 21.5 (12–23) | 21.5 (14–25) | 1.000 |
| Age | 9 (8–11) | 5.5 (4–6) | 9.5 (9–11) | 5 (5–6) | 7 (5–9) | 0.000 |
Note: groups: FM, fentanyl patch, ovariectomy and mastectomy; FO, fentanyl patch, ovariectomy; TM, tramadol, ovariectomy and mastectomy; TO, tramadol, ovariectomy.
Analysis of variance (ANOVA) for repeated measures did not show significant differences in the NAS scores (p = 0.279) between groups or between detection time and group (p = 0.867). However, significant differences were observed between detection times (p < 0.0001, Figure 1). Comparisons between individual groups are shown in Table 2.
FIGURE 1.
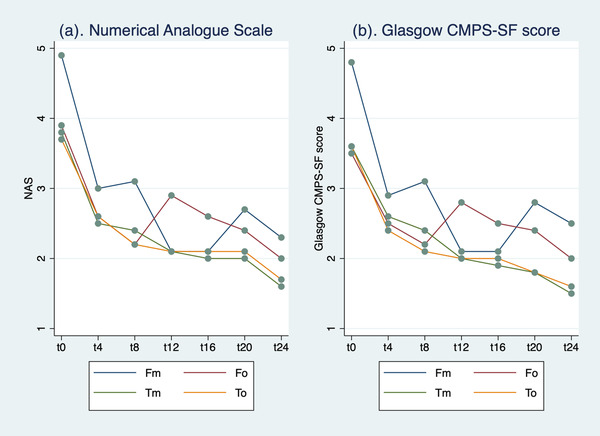
(a) Average values of numerical analogue scale (NAS) scores, by group and detection time and (b) Glasgow Composite Measure Pain Scale Short Form (CMPS‐SF) score mean values, by group and detection time
TABLE 2.
Analysis of variance (ANOVA) for repeated measures of the comparison of numeric analogue scale (NAS) scores comparing one group at a time
| Comparison between groups | Comparison between time | Comparison between group and time | |
|---|---|---|---|
| FM vs. FO | 0.517 | 0.000 | 0.368 |
| FM vs. TM | 0.145 | <0.0001 | 0.829 |
| FM vs. TO | 0.106 | <0.0001 | 0.745 |
| FO vs. TM | 0.350 | <0.0001 | 0.708 |
| FO vs. TO | 0.294 | <0.0001 | 0.889 |
| TM vs. TO | 0.961 | 0.0001 | 0.992 |
Note: groups: FM, fentanyl patch, ovariectomy and mastectomy; FO, fentanyl patch, ovariectomy; TM, tramadol, ovariectomy and mastectomy; TO, tramadol, ovariectomy.
ANOVA for repeated measures did not show any significant differences in the Glasgow CMPS‐SF scores between groups (p = 0.103) or between detection time and group (p = 0.660). Significant differences, however, were observed in the comparison between detection times (p < 0.0001), as shown in Figure 1. Comparisons between individual groups are shown in Table 3.
TABLE 3.
Analysis of variance (ANOVA) for repeated measures of Glasgow Composite Measure Pain Scale Short Form (CMPS‐SF) comparison of values comparing one group at a time
| Comparison between groups | Comparison between time | Comparison between group and time | |
|---|---|---|---|
| FM vs. FO | 0.302 | 0.000 | 0.270 |
| FM vs. TM | 0.062 | <0.0001 | 0.645 |
| FM vs. TO | 0.031 | <0.0001 | 0.655 |
| FO vs. TM | 0.348 | <0.0001 | 0.448 |
| FO vs. TO | 0.235 | <0.0001 | 0.788 |
| TM vs. TO | 0.294 | 0.0001 | 0.889 |
Note: groups: FM, fentanyl patch, ovariectomy and mastectomy; FO, fentanyl patch, ovariectomy; TM, tramadol, ovariectomy and mastectomy; TO, tramadol, ovariectomy.
The proportion of dogs undergoing rescue analgesia is described in Table 4; no statistically significant differences were found between groups (p > 0.05).
TABLE 4.
Proportion of dogs undergoing rescue analgesia, per group and detection time
| FM | FO | TM | TO | Total | p‐Value | |
|---|---|---|---|---|---|---|
| T0 | 4 (40.0) | 3 (30.0) | 2 (20.0) | 2 (20.0) | 11 (27.5) | 0.865 |
| T4 | 2 (20.0) | 1 (10.0) | 1 (10.0) | 1 (10.0) | 5 (12.5) | 1.000 |
| T8 | 2 (20.0) | 0 (0.0) | 1 (10.0) | 0 (0.0) | 3 (7.5) | 0.595 |
| T12 | 0 (0.0) | 2 (20.0) | 0 (0.0) | 0 (0.0) | 2 (5.0) | 0.231 |
| T16 | 0 (0.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 1 (2.5) | 1.000 |
| T20 | 1 (10.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 2 (5.0) | 1.000 |
| T24 | 1 (10.0) | 1 (10.0) | 0 (0.0) | 0 (0.0) | 2 (5.0) | 1.000 |
No statistically significant relations were found between the difference between t24 and t0 of NAS and CMPS‐SF scores and determinants in analysis (p > 0.05; Table 5).
TABLE 5.
Analysis of determinants of numeric analogue scale (NAS) and Glasgow Composite Measure Pain Scale Short Form (CMPS‐SF) scores in multivariate linear regression models
| Determinants | Coefficients | 95%CI | p‐Value |
|---|---|---|---|
| NAS | |||
| Age | 0.13 | −0.43 to 0.68 | 0.647 |
| Weight | 0.04 | −0.07 to 0.16 | 0.461 |
Group
|
1.24 0.30 1.10 |
−1.62 to 4.09 −1.68 to 2.29 −1.72 to 3.92 |
0.384 0.758 0.434 |
| CMPS‐SF scores | |||
| Age | 0.14 | −0.39 to 0.66 | 0.592 |
| Weight | 0.03 | −0.08 to 0.14 | 0.582 |
Group
|
1.37 0.10 0.84 |
−1.33 to 4.08 −1.78 to 1.99 −1.84 to 3.52 |
0.310 0.914 0.527 |
4. DISCUSSION
The results of the present study suggest that fentanyl patches may provide postoperative analgesic effects equivalent to those of tramadol in dogs that have undergone ovariectomy, with and without unilateral mastectomy, for 24 h post‐surgery. This means that the fentanyl patch is a useful alternative for postoperative pain management in dogs. In fact, the use of transdermal patches for analgesia may be considered a valid form of pain therapy in small animals; in particular, it contributes to the postoperative well‐being of patients undergoing painful surgeries, and offers the advantages of being non‐invasive and relatively inexpensive while guaranteeing a sufficient analgesic effect for a sufficient period. Until transdermal patches become available for veterinary use, those for human use represent a valid therapy for postsurgical pain in dogs. Compared with SC drugs, the patch may be useful in biting dogs or that refused food post‐surgery. Postoperative analgesia, especially in painful procedures such as mastectomy, is very important because pain can negatively affect patients’ recoveries (Hansen, 2005; Mwangi et al., 2018). These negative effects include protein imbalance, reduced food intake, release of stress hormones, self‐mutilation, weight loss, delayed surgical site healing, immunosuppression, and increased blood pressure (Gaynor, 1999). In the present study, no side effects or complications occurred in any of the patients. The team of veterinarians involved in the present study ensured that each patient received the utmost attention and postoperative pain management. The fentanyl patch was found to be as effective as 5 mg/kg SC tramadol in dogs after ovariectomy and unilateral mastectomy. However, although the fentanyl patch can be included in the anaesthesiology protocol, it cannot be used for pre‐emptive analgesia during surgery, given the individual variables which influence its absorption and the transdermal administration of the drug (Rialland et al., 2012). Some studies have shown that the absorption of fentanyl is related to the patient's body temperature or accidental ingestion of the patch (Pettifer & Hosgood, 2014; Schmiedt & Bjorling, 2007). In our study, fentanyl patches were well tolerated and did not cause any adverse effects, which was likely because the dogs were in a standardised postoperative hospital setting and were under close veterinary surveillance. No negative or other side effects were reported. The use of long‐acting analgesic, such as the fentanyl patch, ensures satisfactory analgesic coverage that lasts for at least 24 h postoperatively (Cicirelli, Lacalandra, et al., 2021; Grubb & Lobprise, 2020), which guarantees adequate pain management.
Effective postoperative pain control can be achieved with multi‐modal analgesia using a combination of agents with different mechanisms of action (Cicirelli, Debidda, Maggio, Caira, Lacalandra, et al., 2021; Cicirelli, Debidda, Maggio, Caira, Mrenoshki, et al., 2021). Multi‐modal anaesthesia, which was utilised in the fentanyl groups, includes drugs administered both systemically and regionally, and is considered the most effective approach to provide pain relief (Cicirelli, Lacalandra, et al., 2021; Grubb & Lobprise, 2020). This type of anaesthesia includes single or combination drugs administered at varying dosages and routes, and for varying times; it is a less standardised approach, but is better suited to individual patients’ needs (Campagnol et al., 2012).
5. CONCLUSIONS
These findings could be a starting point for further investigations in dogs that cannot take oral medications during the postoperative period. For future studies it will be important to increase the number of dogs enrolled to improve this topic. Moreover, considering the cost, availability, restrictions, and side effects associated with the fentanyl patch, this study is encouraging and could spark interest, leading to a possible association between routine postoperative analgesia and fentanyl patch.
CONFLICTS OF INTEREST
The authors declare no conflict of interest.
ETHICS STATEMENT
The present study was performed in accordance with the ethical guidelines of the Animal Welfare Committee, and institutional review board approval of the study was obtained with approval number 16/19 (21/10/2019). Animal procedures were performed following Directive 2010/63/Eu of the European Parliament (Italian DL 26/2014).
AUTHORS' CONTRIBUTIONS
Data curation; Daniela Mrenoshki. Investigation: Vincenzo Cicirelli. Methodology: Vincenzo Cicirelli. Resources: Giulio G. Aiudi. Supervision: Giovanni M. Lacalandra.
PEER REVIEW
The peer review history for this article is available at https://publons.com/publon/10.1002/vms3.691
ACKNOWLEDGEMENT
Authors are grateful to Francesco Bianchi for data analysis and statistical support.
Cicirelli, V. , Aiudi, G. G. , Mrenoshki, D. , & Lacalandra, G. M. (2022). Fentanyl patch versus tramadol for the control of postoperative pain in canine ovariectomy and mastectomy. Veterinary Medicine and Science, 8, 469–475. 10.1002/vms3.691
This research received no external funding
DATA AVAILABILITY STATEMENT
The data presented in this study are available on request from the corresponding author.
REFERENCES
- Bellei, E. , Roncada, P. , Pisoni, L. , Joechler, M. , & Zaghini, A. (2011). The use of fentanyl‐patch in dogs undergoing spinal surgery: Plasma concentration and analgesic efficacy. Journal of Veterinary Pharmacology, 34, 437–441. [DOI] [PubMed] [Google Scholar]
- Campagnol, D. , Teixeira‐Neto, F. J. , Monteiro, E. R. , Restitutti, F. , & Minto, B. W. (2012). Effect of intraperitoneal or incisional bupivacaine on pain and the analgesic requirement after ovariohysterectomy in dogs. Veterinary Anaesthesia and Analgesia, 39(4), 426–430. [DOI] [PubMed] [Google Scholar]
- Cicirelli, V. , Debidda, P. , Maggio, N. , Caira, M. , Lacalandra, G. M. , & Aiudi, G. G. (2021). Ultrasound‐guided funicular block: Ropivacaine injection into the tissue around the spermatic cord to improve analgesia during orchiectomy in dogs. Animals, 11(5), 1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli, V. , Debidda, P. , Maggio, N. , Caira, M. , Mrenoshki, D. , Aiudi, G. G. , & Lacalandra, G. M. (2021). Use of spinal anaesthesia with anaesthetic block of intercostal nerves compared to a continuous infusion of sufentanyl to improve analgesia in cats undergoing unilateral mastectomy. Animals, 11(3), 887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicirelli, V. , Lacalandra, G. M. , & Aiudi, G. G. (2021). The effect of splash block on the need for analgesia in dogs subjected to video‐assisted ovariectomy. Veterinary Medicine and Science, 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Costa, G. , Musicò, M. , Spadola, F. , Cortigiani, S. , Leonardi, F. , Cucinotta, G. , & Interlandi, C. (2018). Effects of tramadol slow injection vs fast bolus in the therapeutic balance of the foot in bovine. Large Animal Review, 24, 19–21 [Google Scholar]
- Costa, G. , Spadola, F. , Lentini, M. , Lubian, E. , & Leonardi, F. (2021). Comparison of analgesia and ataxia degree obtained between three dosages of tramadol in cattle. Large Animal Review, 27(2), 65–68 [Google Scholar]
- Costa, G. L. , Cristarella, S. , Quartuccio, M. , & Interlandi, C. (2015). Anti‐nociceptive and sedative effects of romifidine, tramadol and their combination administered intravenously slowly in ponies. Veterinary Anaesthesia and Analgesia, 42, 220–225. [DOI] [PubMed] [Google Scholar]
- Costa, G. L. , Nastasi, B. , Spadola, F. , Leonardi, F. , & Interlandi, C. (2019). Effect of levobupivacaine, administered intraperitoneally, on physiological variables and on intrasurgery and postsurgery pain in dogs undergoing ovariohysterectomy. Journal of Veterinary Behavior, 30, 33–36 1 [Google Scholar]
- Gilbert, D. B. , Motzel, S. L. , & Das, S. R. (2003). Postoperative pain management using fentanyl patches in dogs. Contemporary Topics in Laboratory Animal Science, 42(4), 21–26. [PubMed] [Google Scholar]
- Egger, C. M. , Duke, T. , Archer, J. , & Cribb, P. H. (1998). Comparison of plasma fentanyl concentrations by using three transdermal fentanyl patch sizes in dogs. Veterinary Surgery, 27(2), 159–166 PubMed. [DOI] [PubMed] [Google Scholar]
- Flecknell, P. , & Waterman‐Pearson, A. (2000). Pain management in animals. WB Saunders. [Google Scholar]
- Freise, K. J. , Savides, M. C. , Riggs, K. L. , Owens, J. G. , Newbound, G. C. , & Clark, T. P. (2012). Pharmacokinetics and dose selection of a novel, longacting transdermal fentanyl solution in healthy laboratory Beagles. Journal of Veterinary Pharmacology and Therapeutics, 35(2), 21–26. [DOI] [PubMed] [Google Scholar]
- Gaynor, J. S. (1999). Is postoperative pain management important in dogs and cats? Veterinary Medicine, 3, 254–257. [Google Scholar]
- Gilbert, D. B. , Motzel, S. L. , & Das, S. R. (2003). Postoperative pain management using fentanyl patches in dogs. Contemporary Topics in Laboratory Animal Science, 42(4), 21–26 PubMed. [PubMed] [Google Scholar]
- Grubb, T. , & Lobprise, H. (2020). Local and regional anaesthesia in dogs and cats: Overview of concepts and drugs (Part 1). Veterinary Medicine and Science, 6, 2. 209–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansen, B. D. (2005). Analgesia and sedation in the critically ill. Veterinary Emergency and Critical Care, 15, 285–294. [Google Scholar]
- Hellyer, P. W. , Uhrig, S. R. , & Robinson, N. G. (2006). Canine acute pain scale and feline acute pain scale. Colorado State University Veterinary Medical Center. [Google Scholar]
- Hofmeister, E. H. , & Egger, C. M. (2004). Transdermal fentanyl patches in small animals. American Animal Hospital Association, 40(6), 468–78. [DOI] [PubMed] [Google Scholar]
- Kukanich, B. , & Clark, T. P. (2012). The history and pharmacology of fentanyl: Relevance to a novel, long‐acting transdermal fentanyl solution newly approved for use in dogs. Journal of Veterinary Pharmacology and Therapeutics, 35, 3–19. [DOI] [PubMed] [Google Scholar]
- Kyles, A. E. , Hardie, E. M. , & Hansen, B. D. (1998). Comparison of transdermal fentanyl and intramuscular oxymorphone on post‐operative behavior after ovariohysterectomy in dogs. Research in Veterinary Science, 65, 245–251. [DOI] [PubMed] [Google Scholar]
- Kyles, A. E. , Papich, M. , & Hardie, E. M. (1996). Disposition of transdermally administered fentanyl in dogs. American Journal of Veterinary Research, 57, 715–719. [PubMed] [Google Scholar]
- Lafuente, M. P. , Franch, J. , Durall, I. , Díaz‐Bertrana, M. C. , & Márquez, R. M. (2005). Comparison between meloxicam and transdermally administered fentanyl for treatment of postoperative pain in dogs undergoing osteotomy of the tibia and fibula and placement of a uniplanar external distraction device. Journal of the American Veterinary Medical Association, 227(11), 1768–1774 PubMed. [DOI] [PubMed] [Google Scholar]
- Mastrocinque, S. , & Fantoni, D. T. (2003). A comparison of preoperative tramadol and morphine for the control of early postoperative pain in canine ovariohysterectomy. Veterinary Anaesthesia and Analgesia, 30(4), 220–228. [DOI] [PubMed] [Google Scholar]
- Mathews, K. A. (2000). Pain assessment and general approach to management. Veterinary Clinics of North America. Small Animal Practice, 30, 729–755. [DOI] [PubMed] [Google Scholar]
- Monteiro, E. R. , Junior, A. R. , Assis, H. M. , Campagnol, D. , & Quitzan, J. G. (2009). Comparative study on the sedative effects of morphine, methadone, butorphanol or tramadol, in combination with acepromazine, in dogs. Veterinary Anaesthesia and Analgesia, 36(1), 25–33 [DOI] [PubMed] [Google Scholar]
- Morton, C. , Reid, J. , Scott, E. , Holton, L. , & Nolan, A. (2005). Application of a scaling model to establish and validate an interval level pain scale for assessment of acute pain in dogs. American Journal of Veterinary Research, 66, 2154–2166. [DOI] [PubMed] [Google Scholar]
- Mwangi, W. E. , Mogoa, E. M. , Mwangi, J. N. , Mbuthia, P. G. , & Mbugua, S. W. (2018). A systematic review of analgesia practices in dogs undergoing ovariohysterectomy. Veterinary World, 11(12), 1725–1735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pekcan, Z. , & Koc, B. (2010). The post‐operative analgesic effects of epidurally administered morphine and transdermal fentanyl patch after ovariohysterectomy in dogs. Veterinary Anaesthesia and Analgesia, 37(6), 557–565. [DOI] [PubMed] [Google Scholar]
- Pettifer, G. R. , & Hosgood, G. (2014). The effect of inhalant anesthetic and body temperature on peri‐anesthetic serum concentrations of transdermally administered fentanyl in dogs. Veterinary Anaesthesia and Analgesia, 31(2), 109–120. PubMed. [DOI] [PubMed] [Google Scholar]
- Reed, F. , Burrow, R. , Poels, K. L. , Godderis, L. , Veulemans, H. A. , & Mosing, M. (2011). Evaluation of transdermal fentanyl patch attachment in dogs and analysis of residual fentanyl content following removal. Veterinary Anaesthesia and Analgesia, 38(4), 407–412. [DOI] [PubMed] [Google Scholar]
- Reid, J. , Nolan, A. M. , Hughes, J. M. L. , Lascelles, D. , Pawson, P. , & Scott, E. M. (2007). Development of the short‐form Glasgow Composite Measure Pain Scale (CMPS‐SF) and derivation of an analgesic intervention score. Animal Welfare, 16(S), 97–104 [Google Scholar]
- Rialland, P. , Authier, S. , Guillot, M. , Del Castillo, J. R. , Veilleux‐Lemieux, D. , Frank, D. , Gauvin, D. , & Troncy, E. (2012). Validation of orthopedic postoperative pain assessment methods for dogs: A prospective, blinded, randomized, placebo‐controlled study. Plos One. 7(11), e49480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robinson, T. M. , Kruse‐Elliott, K. T. , Markel, M. D. , Pluhar, G. E. , Massa, K. , & Bjorling, D. E. (1999). A comparison of transdermal fentanyl versus epidural morphine for analgesia in dogs undergoing major orthopedic surgery. Journal of the American Animal Hospital Association, 35, 95–100. [DOI] [PubMed] [Google Scholar]
- Rutteman, G. R. , Withrow, S. J. , & MacEwen, E. G. (2001). Tumors of the mammary gland. In Withrow S. J. & MacEwan E. G. (Eds.), Small animal clinical oncology (3rd ed., pp. 455–477). WB Saunders. [Google Scholar]
- Sarrau, S. , Jourdan, J. , Dupuis‐Soyris, F. , & Verwaerde, P. (2007). Effects of postoperative ketamine infusion on pain control and feeding behaviour in bitches undergoing mastectomy. Journal of Small Animal Practice, 48, 670–676. [DOI] [PubMed] [Google Scholar]
- Schmiedt, C. W. , & Bjorling, D. E. (2007). Accidental prehension and suspected transmucosal or oral absorption of fentanyl from a transdermal patch in a dog. Veterinary Anaesthesia and Analgesia, 34(1), 70–73 PubMed. [DOI] [PubMed] [Google Scholar]
- Teixeira, R. C. , Monteiro, E. R. , Campagnol, D. , Coelho, K. , Bressan, T. F. , & Monteiro, B. S. (2013). Effects of tramadol alone, in combination with meloxicam or dipyrone, on postoperative pain and the analgesic requirement in dogs undergoing unilateral mastectomy with or without ovariohysterectomy. Veterinary Anaesthesia and Analgesia, 40, 641–649. [DOI] [PubMed] [Google Scholar]
- Tranquili, W. J. , Thurmon, J. C. , & Grimm, K. A. (2007). Lumb & Jones veterinary anesthesia and analgesia, Blackwell Publishing. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.


