Abstract
Arctigenin (ACT) is a novel anti‐inflammatory lignan extracted from Arctium lappa L, a herb commonly used in traditional Chinese herbal medicine. In this study, we investigated the molecular mechanism whereby ACT inhibits PCV2 infection‐induced proinflammatory cytokine production in vitro and in vivo. We observed that in PCV2 infection+ACT treated PK‐15 cells, proinflammatory cytokine production was significantly reduced, compared to the PCV2‐infected cells. The transfection and luciferase reporter assay confirmed that ACT suppressed NF‐κB signalling pathway activation following PCV2 infection in PK‐15 cells. Furthermore, western blotting demonstrated that ACT suppressed the NF‐κB signal pathway in PCV2 infection‐stimulated PK‐15 cells by inhibiting the translocation of p65 from the cytoplasm to the nucleus and IκBα phosphorylation. BALB/c mice were used as a model to evaluate the anti‐inflammatory effect of ACT in vivo. We found that the BALB/c mice inoculated with PCV2 infection + ACT treated showed a significant reduction of proinflammatory cytokine production in serum, lung and spleen tissue, compared to the PCV2‐infected mice. Western blotting confirmed that ACT suppressed the NF‐κB signal pathway in PCV2‐infected mice by inhibiting the translocation of p65 from the cytoplasm to the nucleus and IκBα phosphorylation in lung tissue. Our studies first demonstrate that ACT inhibits PCV2 infection‐induced proinflammatory cytokine production by suppressing the phosphorylation and nuclear translocation of NF‐κB in vitro and in vivo. These results will help further develop ACT as a Traditional Chinese herbal medicine remedy in the treatment of porcine circovirus‐associated diseases.
Keywords: arctigenin, mice, NF‐κB, PCV2, proinflammatory cytokine
Short abstract
This study investigates the molecular mechanism of ACT inhibits PCV2 infection‐induced proinflammatory cytokine production in vitro and in vivo. The results demonstrate that ACT could inhibit PCV2 infection‐induced proinflammatory cytokine production by suppressing the phosphorylation and nuclear translocation of NF‐κB. This study will help further develop ACT as a Traditional Chinese herbal medicine remedy in the treatment of PCVADs.
1. INTRODUCTION
Porcine circovirus (PCV) belongs to the genus Circovirus of the family Circoviridae, which was characterised as a single‐stranded 1.7‐kb circular DNA virus enclosed in an icosahedral virion with a diameter of approximately 17 nm (Allan & Ellis, 2000). Until now, four genotypes of PCVs have been discovered in the swine population, including porcine circovirus type 1 (PCV1), porcine circovirus type 2 (PCV2), porcine circovirus type 3 (PCV3) and porcine circovirus type 4. It is generally accepted that PCV1 is non‐pathogenic; in contrast, PCV2 and PCV3 are pathogenic to swine (Allan et al., 1998; Palinski et al., 2016; Tischer et al., 1974). PCV4 is identified very recently and future information on this virus is required to understand its potential impact (Zhang et al., 2019). PCV2 is now recognised as the primary pathogen of porcine circovirus‐associated diseases (PCVADs), including several various syndromes in pigs, such as post‐weaning multi‐systemic wasting syndrome, porcine dermatitis, nephropathy syndrome, as well as a reproductive failure (Ellis, 2014; Gillespie et al., 2009). Furthermore, PCV2 can cause immunosuppression in pigs, leading to secondary infection or co‐infection with other swine pathogens, such as porcine reproductive and respiratory syndrome virus, Mycoplasma hyopneumoniae, bacterial septicemia or pneumonia, and swine influenza virus (Dorr et al., 2007; Ramamoorthy & Meng, 2009). Thus, PCV2 is a significant threat that caused high economic losses to the global swine industry in recent decades (Finsterbusch & Mankertz, 2009).
Until now, the mechanism of PCV2 pathogenesis is poorly understood. The innate immune response is the first line of host defence against pathogen invasion (Riera et al., 2016). Toll‐like receptors (TLRs) are key pattern recognition receptors of the innate immune system that recognise pathogen‐associated molecule patterns and induce innate immune responses, including the production of interferons and proinflammatory cytokines (Hertzog et al., 2003; Kawai & Akira, 2010). Transcription analysis showed that PCV2 infection of porcine alveolar macrophages (PAMs) causes up‐regulation of inflammatory‐related genes (Li et al., 2013). Further studies confirmed that PCV2 infection of PAMs induces the expression of IL‐1β and IL‐10 via the TLR9/MyD88/NF‐κB signalling pathway (Han et al., 2017). PCV2 infection of PK‐15 cells induced NF‐κB p65 translocation from the cytoplasm to the nucleus, as well as degradation and phosphorylation of IκBα proteins (Wei et al., 2008). These studies suggest that the transcription factor NF‐κB is commonly activated upon PCV2 infection and plays a crucial role in the induction and regulation of the hosts’ inflammatory response.
Vaccination is considered as the most effective measure to prevent and control viral diseases (Afhgah et al., 2017; Chae, 2012). Currently, many commercial vaccines are available for PCV2. PCV2 vaccines have been shown to be very successful and efficacious to control the prevalence of PCVAD. Due to PCV2 contains many genotypes and PCV2 infection could cause immunosuppression, leading to co‐infection with other swine pathogens (Tanja et al., 2020). Therefore, finding alternative effective measures to prevent and control this disease is urgently required. Arctigenin (ACT) is a phenylpropanoid dibenzylbutyrolactone lignan extracted from the seeds of a Traditional Chinese herbal medicine, Arctium lappa L. ACT has been proven to demonstrate promising biological activities, including immunomodulatory, antiviral, anti‐cancer and anti‐inflammatory activities (Hayashi et al., 2010; Swarup et al., 2008; Gao et al., 2018; Jeong et al., 2011). Our previous studies have demonstrated that ACT can significantly inhibit the proliferation of PCV2 in vitro and in vivo, and suppress the inflammatory response caused by PCV2 infection of experimental piglets (Chen et al., 2016). However, the specific anti‐inflammatory mechanism is not clear.
Herein, we further investigated the molecular mechanism of ACT inhibited PCV2 infection‐induced inflammatory responses. Our results demonstrated that ACT inhibited PCV2 infection‐induced proinflammatory cytokine production mainly through suppressing NF‐κB phosphorylation and nuclear translocation in vitro and in vivo. This study provided a theoretical basis for further development of ACT as a Chinese herbal medicine against PCVADs.
2. MATERIALS AND METHODS
2.1. Virus and cells
The strain PCV2‐WH (GenBank: FJ598044) was kindly provided by the State Key Laboratory of Agricultural Microbiology, Huazhong Agricultural University. The titre of PCV2‐WH was 107TCID50/ml, as determined by immunofluorescence assay. PK‐15 cells free of PCV1 were purchased from China Center for Type Culture Collection (CCTCC, GDC0061), and they were cultured in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 10% fetal bovine serum (FBS), 100 U/ml penicillin and 100 μg/ml streptomycin, and incubated in a humidified incubator at 37°C with 5% CO2.
2.2. Reagents and plasmids
DMEM, FBS, penicillin and streptomycin were purchased from Gibco. Rabbit monoclonal primary antibody, NF‐κB p65 and mouse monoclonal antibody, phospho‐IκBα, were purchased from Cell Signaling Technology (Danvers, MA, USA). Dimethyl sulfoxide (DMSO) was purchased from Sigma. Histone H3 mouse monoclonal antibody, β‐Actin mouse monoclonal antibody, lipopolysaccharide (LPS), reporter plasmid pNF‐κB‐luc and the internal control plasmid pRL‐SV40 were purchased from Beyotime Biotechnology (Jiangsu, China). ACT (purity > 98%) was purchased from Shanghai Ronghe Pharmaceutical Technology Co., Ltd. ACT was dissolved in DMSO at a concentration of 20 mM and stored as a stock solution at –20°C. Before use, the desired concentrations of ACT were diluted with DMEM from the stock solution.
2.3. Anti‐inflammatory activity of ACT in vitro
1.5 × 105 PK‐15 cells were cultured in 24‐well plate at 37°C and maintained in DMEM supplemented with 10% FBS. The PK‐15 cells were randomly divided into four groups as follows: the PCV2 infection group, the ACT‐treated group, the PCV2 infection + ACT‐treated group and the negative control group. After culturing for 24 h, the PK‐15 cells were infected with PCV2 at 1 MOI for 1 h, and the supernatant was replaced with 500 μl of DMEM in the PCV2 infection group. PK‐15 cells were incubated with 500 μl of DMEM containing 62.6 μg/ml ACT in the ACT‐treated group. In the PCV2 infection + ACT‐treated group, PK‐15 cells were infected with PCV2 at 1 MOI for 1 h, and the supernatant was replaced with 500 μl of DMEM containing 62.6 μg/ml ACT. In the negative control group, PK‐15 cells were incubated with 500 μl of DMEM. PK‐15 cells from each group were collected at 0, 6, 12, 18 and 24 h for qRT‐PCR analysis.
2.4. Transfection and luciferase reporter assay
PK‐15 cells were transfected using Attractene Transfection Reagent (QIAGEN) according to the manufacturer's instructions. Approximately 1.5 × 105 cells per well in 24‐well plate were co‐transfected with 100 ng reporter plasmid pNF‐κB‐luc and 10 ng internal control plasmid pRL‐SV40. Twenty‐four hours post‐transfection, the PK‐15 cells were randomly divided into five groups as follows: the PCV2 infection group, the ACT‐treated group, the PCV2 infection + ACT‐treated group, the LPS‐treated group and the negative control group. In the PCV2 infection group, PK‐15 cells were infected with PCV2 at 1 MOI for 1 h, and the supernatant was replaced with 500 μl DMEM. In the ACT‐treated group, PK‐15 cells were incubated with 500 μl of DMEM containing 62.6 μg/ml ACT. In the PCV2 infection + ACT‐treated group, PK‐15 cells were infected with PCV2 at 1 MOI for 1 h, and the supernatant was replaced with 500 μl DMEM containing 62.6 μg/ml ACT. In the LPS‐treated group, PK‐15 cells were incubated with 500 μl of DMEM containing 100 ng/ml LPS. In the negative control group, PK‐15 cells were incubated with 500 μl of DMEM. PK‐15 cells were harvested at 6, 12, 18 and 24 h and lysed. Luciferase activities were determined using the dual‐luciferase assay system according to the manufacturer's instructions.
2.5. Anti‐inflammatory activity of ACT in vivo
Forty‐five 7‐week‐old BALB/c female mice were randomly assigned to three groups: the PCV2 infection group (n = 15), the PCV2 infection + ACT inoculation group (n = 15) and the negative control group (n = 15). In the PCV2 infection group and PCV2 infection + ACT inoculation group, mice were inoculated with PCV2 via an intraperitoneal injection (0.25 ml per mouse), as well as an intranasal administration (0.25 ml per mouse). After 24 h following the injections, mice in the PCV2 infection + ACT inoculation group were inoculated with ACT via an intraperitoneal injection (2.0 mg/kg body weight per mouse) and repeated every day for 5 consecutive days. The mice in the PCV2 infection group were inoculated with DMEM (0.25 ml intraperitoneal injection per mouse) and repeated every day for 5 consecutive days. The mice in the negative group were inoculated with DMEM (0.25 ml intraperitoneal injection per mouse). After 7, 14 and 21 days post‐inoculation, five mice were randomly selected from each group and euthanised. The blood, lung and spleen were collected for further experiments.
2.6. ELISA for the production of proinflammatory cytokines
The levels of IL‐1β, IL‐6, IL‐8 and TNF‐α proinflammatory cytokines in mouse serum were measured using double antibody sandwich ELISA kit (JINGMEI, China), according to the manufacturer's instructions. 50 μl diluted samples (1:40, v/v) were added into each well and then add 100 μl HPR‐conjugate rabbit anti‐mouse IL‐1β polyclonal antibody to each well and incubated for 60 min at 37°C. After shaking out the samples, washing the plate 5 times with washing buffer and dry by pat. Add 50 μl TMB substrate solution to each well respectively, evade the light preservation for 15 min at 37°C. Add stop solution 50 μl to each well and read the optical density using a microplate reader set at 450 nm. The cytokine concentrations were assessed based on the standard curves obtained from the serial 10‐fold dilution of standard provided by the kit.
2.7. Western blot analysis of p65 and p‐IκBα
Nuclear and cytoplasmic proteins from PK‐15 cells and mouse lung tissue were extracted using nuclear and cytoplasmic protein extraction kits (Beyotime), according to the manufacturer's instructions. Protein concentrations were determined using a BCA Protein Assay Kit (Servicebio, China). Equal amounts of protein were separated by SDS‐PAGE and then transferred to a PVDF membrane (Millipore, Bedford, MA, USA). After blocking with 5% non‐fat milk in TBST for 1 h, the membranes were incubated with primary antibodies overnight at 4°C. After washing three times with TBST, the membranes were incubated with secondary antibodies at 37°C for 1 h. Immunoreactive bands were visualised by an enhanced chemiluminescence system (Amersham Biosciences).
2.8. Quantitative RT‐PCR assays
Total RNA was isolated from PK‐15 cells and mouse tissue using the Takara MiniBEST Universal RNA Extraction Kit (Takara), according to the manufacturer's instructions. The RNA samples were then reverse‐transcribed to cDNA using the Primescript RT Reagent Perfect Real‐Time kit (Takara). The PCR primers were designed using Primer Premier 5.0 software, and primers for GAPDH, IL‐1β, IL‐6, IL‐8 and TNF‐α are shown in Table 1. The PCR reaction system consisted of 2 μl cDNA, 10 μl SYBR Premix Ex Taq II, 0.8 μl forward primer, 0.8 μl reverse primer and 6.4 μl ddH2O. qPCR was performed using the following program: 95°C for 15 s, 40 cycles at 95°C for 5 s and 60°C for 30 s followed by the melting curve and plate reading stages, under default settings. The relative expression level of the target genes was calculated using the comparative Ct method. All data were normalised relative to GAPDH. qPCR was performed using the Bio‐Rad CFX Connect Real‐Time System.
TABLE 1.
qRT‐PCR primers used in this study
| Gene | Sequence 5′–3′ | Product length (bp) |
|---|---|---|
| Pig IL‐1β | Forward: TGACGGACCCCAAAAGATGA | 90 |
| Reverse: TGCTGCTGCGAGATTTGAAG | ||
| Pig IL‐6 | Forward: GGGACTGATGCTGGTGACAA | 90 |
| Reverse: ACAGGTCTGTTGGGAGTGGTATC | ||
| Pig IL‐8 | Forward: GCTCCATGGGTGAAGGCTACT | 90 |
| Reverse: CTTCATTGCCGGTGGAAATT | ||
| Pig TNF‐α | Forward: AGAGGCACTCCCCCAAAAGA | 90 |
| Reverse: TGCCACAAGCAGGAATGAGA | ||
| Mouse IL‐1β | Forward: ACTGTGAAATGCCACCTTTTGA | 90 |
| Reverse: TGGAAGCAGCCCTTCATCTT | ||
| Mouse IL‐6 | Forward: TGGGACTGATGCTGGTGACA | 90 |
| Reverse: CTGTTGGGAGTGGTATCCTCTGT | ||
| Mouse IL‐8 | Forward: TCCATGGGTGAAGGCTACTGT | 90 |
| Reverse: CATTGCCGGTGGAAATTCC | ||
| Mouse TNF‐α | Forward: AGAGGCACTCCCCCAAAAGA | 90 |
| Reverse: TGCCACAAGCAGGAATGAGA | ||
| Pig GAPDH | Forward: CTCCACTCACGGCAAATTCA | 90 |
| Reverse: CGCTCCTGGAAGATGGTGAT | ||
| Mouse GAPDH | Forward: GGCAAATTCAACGGCACAGT | 90 |
| Reverse: GGTCTCGCTCCTGGAAGATG |
2.9 Animals Forty‐five seven‐week‐old female BALB/c mice were purchased from the Wuhan Institute of Biological Products Co., LTD. Mouse studies were performed according to the guidelines of this company (No. 00281346). All animal studies were complied with the Hubei Provincial Animal Care and Use Committee and the animal experiment guidelines of the Animal Experimentation Ethics Committee of Huazhong Agricultural University.
2.9. Animals
Forty‐five seven‐week‐old female BALB/c mice were purchased from the Wuhan Institute of Biological Products Co., LTD. Mouse studies were performed according to the guidelines of this company (No. 00281346). All animal studies were complied with the Hubei Provincial Animal Care and Use Committee and the animal experiment guidelines of the Animal Experimentation Ethics Committee of Huazhong Agricultural University.
2.10. Statistical analysis
Statistical analysis of the experimental data was performed using SPSS 20.0 software. Results are presented as the mean ± SD. All experiments were performed in triplicate. The data were analysed by one‐way ANOVA. Individual group means were compared using Duncan's multiple comparison when significant differences were observed. p Values < 0.05 were considered statistically significant.
3. RESULTS
3.1. ACT inhibits the production of PCV2 infection‐stimulated proinflammatory cytokines in vitro
qRT‐PCR experiments were carried out to determine whether ACT inhibited the proinflammatory cytokines activated by PCV2 infection of PK‐15 cells. The results are shown in Figure 1. In the PCV2 infection group, proinflammatory cytokines, including IL‐1β, IL‐6, IL‐8 and TNF‐α mRNA were significantly higher than those of the control group at 6, 12, 18 and 24 h post‐infection (p < 0.01). In contrast, in the PCV2 infection + ACT treated group, the levels of proinflammatory cytokines were significantly lower than those of the PCV2 group at 6, 12, 18 and 24 h post‐infection (p < 0.05 or p < 0.01). These findings demonstrated that ACT inhibited the production of proinflammatory cytokines in PK‐15 cells following PCV2 infection of PK‐15 cells.
FIGURE 1.

ACT inhibits the production of PCV2 infection‐stimulated proinflammatory cytokine in vitro. PK‐15 cells from the DMEM (negative control) incubated, PCV2 infection, ACT treated and PCV2 infection + ACT treated groups were collected after 0, 6, 12, 18 and 24 h post‐incubation. Total RNA was extracted, and real‐time RT‐PCR was used to determine the fold‐change of (a) IL‐1β, (b) IL‐6, (c) IL‐8 and (d) TNF‐ α relative to negative control. Data are presented as mean ± SD. Significant differences are indicated as *p < 0.05, **p < 0.01
3.2. ACT suppresses the PCV2 infection‐stimulated NF‐κB promoter in vitro
NF‐κB luciferase reporter assays were carried out to determine whether ACT suppressed the NF‐κB signal pathway. The results are shown in Figure 2. In the PCV2 infection group, NF‐κB luciferase activities were significantly higher than those of the control group at 6, 12, 18 and 24 h post‐infection (p < 0.01). In contrast, in the PCV2 infection + ACT treated group, NF‐κB luciferase activities were significantly lower than those of the PCV2 infection group at 6, 12, 18 and 24 h post‐infection (p < 0.05 or p < 0.01). These findings demonstrated that ACT significantly suppressed the activation of the NF‐κB signal pathway following PCV2 infection in PK‐15 cells.
FIGURE 2.

Effects of ACT on the PCV2 infection‐stimulated expression of the NF‐κB promoter. PK‐15 cells were co‐transfected with pNF‐kB Luc and pRL‐SV40 for 24 h. Cells from the DMEM (negative control) incubated, PCV2 infection, ACT treated, PCV2 infection + ACT treated and LPS treated were collected after 6, 12, 18 and 24 h post‐incubation. Luciferase activities were determined at (a) 6 h, (b) 12 h, (c) 18 h and (d) 24 h by dual‐luciferase assays. Data are presented as mean ± SD. Significant differences are indicated as *p < 0.05, **p < 0.01
3.3. ACT suppresses NF‐κB phosphorylation and nuclear translocation in vitro
To further characterise whether ACT suppressed the NF‐κB signalling pathway after PCV2 infection of PK‐15 cells, the levels of NF‐κB p65 and p‐IκBα proteins were measured by Western blotting. The results are shown in Figure 3. PCV2 infection of PK‐15 led to the progressive accumulation of p65 in the nucleus in a time‐dependent manner, and the levels of p‐IκBα protein in the cytoplasm were also increased in a time‐dependent manner. In contrast, PCV2 infection + ACT treated PK‐15 cells suppressed the translocation of p65 from the cytoplasm to the nucleus in a time conditional manner, and the levels of p‐IκBα protein in the cytoplasm were also decreased in a time conditional manner. These findings demonstrated that ACT suppressed the NF‐κB signal pathway after PCV2 infection of PK‐15 cells by inhibiting the translocation of p65 from the cytoplasm to the nucleus and the phosphorylation of IκBα.
FIGURE 3.
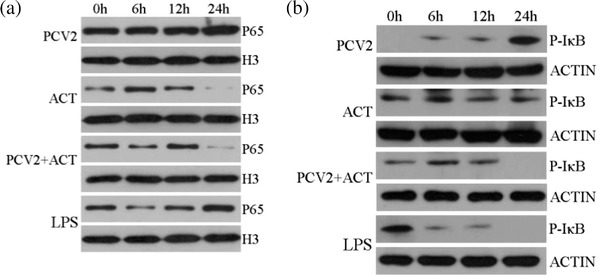
Changes in NF‐κB p65 protein and p‐IκBα protein expression in vitro. PK‐15 cells from the PCV2 infection, ACT treated, PCV2 infection + ACT treated and LPS treated were collected after 6, 12, 18 and 24 h post‐incubation. Nuclear and cytoplasmic proteins from PK‐15 cells were extracted, and the levels of (a) p65 in nuclear and (b) p‐IκBα in cytoplasmic were measured by western blotting
3.4. ACT inhibits the production of PCV2 infection‐stimulated proinflammatory cytokines in mice serum
ELISA was carried out to investigate whether ACT inhibited the production of PCV2 infection‐stimulated proinflammatory cytokines in mice serum. The results are shown in Figure 4. In the PCV2 infection group, the levels of proinflammatory cytokines, including IL‐1β, IL‐6, IL‐8 and TNF‐α in mice serum, were significantly higher than those of the control group at 7, 14 and 21 days post‐infection (p < 0.01). In contrast, in the PCV2 infection + ACT inoculated group, the levels of proinflammatory cytokines, including IL‐1β, IL‐6, IL‐8 and TNF‐α in mice serum, were significantly lower than those of the PCV2 infection group at day 7, 14, and 21 post‐inoculation (p < 0.01). These findings demonstrated that ACT inhibits the production of PCV2 infection‐stimulated proinflammatory cytokines in mice serum.
FIGURE 4.

ACT inhibits PCV2 infection‐activated proinflammatory cytokine production in vivo. BALB/c mice were inoculated with the negative control, PCV2 infection and PCV2 infection + ACT treated. The serum was collected at 0, 7 and 14 days post‐inoculation and ELISA was used to determine the concentrations of (a) IL‐1β, (b) IL‐6, (c) IL‐8 and (d) TNF‐α. Data are presented as mean ± SD. Significant differences are indicated as *p < 0.05, **p < 0.01
3.5. ACT inhibits PCV2 infection stimulated the production of proinflammatory cytokines in mice tissue
qRT‐PCR was performed to evaluate whether ACT inhibited PCV2 infection‐stimulated proinflammatory cytokine production in mouse tissues. The results are shown in Figure 5. In the PCV2 infection group, the levels of proinflammatory cytokines including IL‐1β, IL‐6, IL‐8 and TNF‐α mRNA in mouse lung and spleen tissues were significantly higher than those of the tissues from mice in the control group at 7, 14 and 21 days post‐infection (p < 0.01). In contrast, in PCV2 infection + ACT inoculated mice, the levels of proinflammatory cytokines were significantly lower than those in the mice from the PCV2 infection group at 7, 14 and 21 days post‐infection (p < 0.01). These findings demonstrated that ACT inhibited the PCV2 infection‐stimulated production of proinflammatory cytokines in mouse tissues.
FIGURE 5.

ACT inhibits PCV2 infection‐stimulated proinflammatory cytokine production in vivo. BALB/c mice were inoculated with the negative control, PCV2 infection and PCV2 infection + ACT treated. The spleen and lung tissues were collected at 0, 7 and 14 days post‐inoculation and real‐time RT‐PCR was used to determine the fold‐change of IL‐1β, IL‐6, IL‐8 and TNF‐α in spleen (a–d) and lung (e–h) tissues relative to negative control. Data are presented as mean ± SD. Significant differences are indicated as *p < 0.05, **p < 0.01
3.6. ACT suppresses NF‐κB phosphorylation and nuclear translocation in vivo
To further characterise whether ACT suppressed the NF‐κB signalling pathway after PCV2 infection of mice, the levels of p65 and p‐IκBα proteins in mice lung tissue were measured by western blotting. The results are shown in Figure 6. PCV2 infected mice led to the progressive accumulation of p65 in the nucleus in a time‐dependent manner, and the levels of p‐IκBα protein in the cytoplasm were also increased in a time‐dependent manner. In contrast, in PCV2 infection + ACT inoculated mice suppressed the translocation of p65 from the cytoplasm to the nucleus in a time‐dependent manner, and the levels of p‐IκBα protein in the cytoplasm were also decreased in a time‐dependent manner. These findings demonstrated that ACT suppressed the NF‐κB signal pathway in PCV2 infected mice by inhibiting the translocation of p65 from the cytoplasm to the nucleus and the phosphorylation of IκBα.
FIGURE 6.

Changes of NF‐κB p65 protein and p‐IκBα protein expression levels in vivo. BALB/c mice were inoculated with the negative control, PCV2 infection and PCV2 infection + ACT treated. The lung tissues were collected at 0, 7 and 14 days post‐inoculation, and western blotting was used to measure the protein expression of p65 in nuclear and p‐IκBα in cytoplasmic
4. DISCUSSION
In recent years, numerous studies have shown that ACT exerts a strong anti‐inflammatory effect (Hyam et al., 2013). The molecular mechanism of the anti‐inflammatory effect of ACT has been extensively investigated. Both in vitro and in vivo studies have shown that ACT inactivates NF‐κB by inhibiting p65 nucleus translocation and suppressing IκBα phosphorylation (Cho et al., 2002; Kou et al., 2011). The nuclear factor NF‐κB pathway has been considered a prototypical proinflammatory signalling pathway, based mainly on the role of NF‐κB in the expression of proinflammatory genes (Lawrence, 2009). Our previous studies have demonstrated that ACT significantly inhibited the proliferation of PCV2 in vitro and in vivo and reduced the inflammatory response caused by the PCV2 infection of piglets (Chen et al., 2016). However, the specific anti‐inflammatory mechanism was not clear. This study aimed to investigate further the molecular mechanism of how ACT inhibits the PCV2 infection‐activated inflammatory response.
PCV2 infection regulated the TLR‐MyD88‐NF‐κB signal pathway and caused the phosphorylation and degradation of IκB in the cytoplasm and subsequent NF‐κB protein translocation to the nucleus, as well as induced the expression of genes participating in the inflammatory response (Han et al., 2017; Wei et al., 2008). Our results first found that ACT significantly inhibited the production of proinflammatory cytokines following PCV2 infection of PK‐15 cells. Furthermore, the NF‐κB luciferase reporter assay demonstrated that ACT suppressed the NF‐κB signal pathway activated by PCV2 infection. Western blotting confirmed that ACT suppressed the NF‐κB signal pathway in PCV2 infected PK‐15 cells by inhibiting the translocation of p65 from the cytoplasm to the nucleus and the phosphorylation of IκBα. These results demonstrated that ACT inhibited the production of PCV2 infection‐stimulated proinflammatory cytokines by suppressing NF‐κB phosphorylation and nuclear translocation in vitro.
Mice have been widely used as an animal model to elucidate the in vivo behaviours of virus–host interactions (Ouyang et al., 2018). PCV2 has been shown to replicate in BALB/c mice, and PCV2 infection significantly enhanced proinflammatory cytokine secretion (Wang et al., 2017). It has been reported that infection of mice with PCV2 results in different diseases that vary in severity depending on the viral genotype and mouse strain, indicating difference in the pathogenesis of PCV2, as well as innate and adaptive immune response, between pigs (the natural host) and mice (unnatural animal model) . However, mouse models might be useful for antiviral agent and vaccine research due to the commercial availability of mouse‐specific immunological reagents and the ease of genetic manipulation in mice (Ting et al., 2018). In this study, BALB/c mice were chosen as a model to evaluate whether ACT inhibited the production of PCV2 infection‐stimulated proinflammatory cytokines in vivo. Our results demonstrated that ACT inhibited the production of PCV2 infection‐stimulated proinflammatory cytokines in mice serum and tissue. Western blotting confirmed that ACT suppressed the NF‐κB signal pathway in PCV2 infected mice by inhibiting the translocation of p65 from the cytoplasm to the nucleus and the phosphorylation of IκBα.
Recently studies indicated that multiple routes have been adopted to deliver ACT to animals, which include oral administration, intravenous administration, sublingual administration and hypodermic injection. For oral administration, due to its extensive first‐pass metabolism, oral administration of ACT would be quickly metabolised and the concentrations in plasma were very low and even undetectable in various animal models, suggesting poor oral bioavailability (Gao et al., 2018). Thus, delivering ACT through alternative administration routes might be plausible to bypass the first‐pass metabolism and improve its bioactivities. Researchers have compared the ACT pharmacokinetics and tissue distribution after intravenous, oral, hypodermic and sublingual administration in experimental animals. The results demonstrated substantially improved area under the plasma concentration versus time curve and bioavailability of ACT after hypodermic or sublingual administration compared with oral administration (Li et al., 2017). These results suggested that optimisation of the administration routes for ACT may potentially improve its therapeutic efficacy by increasing the systemic and target organ exposure. It is necessary to study the pharmacokinetic of ACT after different routes of administration in future.
In conclusion, this is the first study to demonstrate that ACT inhibits PCV2 infection‐stimulated production of proinflammatory cytokines by suppressing NF‐κB phosphorylation and nuclear translocation in vitro and in vivo. This study provides information to help developing ACT as a Chinese herbal medicine against PCVADs in clinical settings.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
SUBMISSION DECLARATION AND VERIFICATION
This manuscript has not been published elsewhere; its publication is approved by all authors and tacitly or explicitly by the responsible authorities where the work was carried out, and that, if accepted, it will not be published elsewhere in the same form, in English or in any other language, including electronically without the written consent of the copyright‐holder.
AUTHOR CONTRIBUTIONS
Lijun Wu: Data collection; Collation, Article writing; Inspection. Danna Zhou: Funding acquisition. Runshan Chen: Data curation. Wenhai Yang: Data curation. Bin He: Experimental design; Conceptualization; Formal analysis.
ETHICS STATEMENT
Animal experiments in this study were approved by the Hubei Provincial Animal Care and Use Committee and the animal experiment guidelines of the Animal Experimentation Ethics Committee of Huazhong Agricultural University.
ACKNOWLEDGEMENTS
This project was supported by Hubei Provincial Natural Science Foundation of China (grant no. 2018CFB678); Open Project of Key Laboratory of Animal Embryo Engineering and Molecular Breeding of Hubei Province (grant no. KLAEMB‐2016‐05); Science and Technology Innovation Platform Projects from Science and Technology Department of Hubei Province (grant no. 2018BEC494); Innovation System Construction Project of Wuhan Academy of Agricultural Sciences (grant no. CXJSFW202002‐1) and The Central Government Guides Local Science and Technology Development Project (grant no. 2020ZYYD029).
Wu, L. , Chen, J. , Zhou, D. , Chen, R. , Chen, X. , Shao, Z. , Yang, W. , & He, B. (2022). Anti‐inflammatory activity of arctigenin against PCV2 infection in a mouse model. Veterinary Medicine and Science, 8, 700–709. 10.1002/vms3.693
DATA AVAILABILITY STATEMENT
The date that support the findings of this study are available from the corresponding author upon reasonable request.
REFERENCES
- Afhgah, Z. , Webb, B. , Meng, X J. , & Ramamoorthy, S. (2017). The years of PCV2 vaccines and vaccination: Is eradication a possibility? Veterinary Microbiology, 206, 21–28. [DOI] [PubMed] [Google Scholar]
- Allan, G. M. , & Ellis, J. A. (2000). Porcine circoviruses: A review. Journal of Veterinary Diagnostic Investigation, 12, 3–14. [DOI] [PubMed] [Google Scholar]
- Allan, G. M. , McNeilly, F. , Kennedy, S. , Daft, B. , Clarke, E. G. , Ellis, J. A. , Haines, D. M. , Meehan, B. M. , & Adair, B. M. (1998). Isolation of porcine circovirus‐like viruses from pigs with a wasting disease in the USA and Europe. Journal of Veterinary Diagnostic Investigation, 10, 3–10. [DOI] [PubMed] [Google Scholar]
- Chae, C. (2012). Commercial porcine circovirus type 2 vaccines: Efficacy and clinical application. Veterinary Journal, 194, 151–157. [DOI] [PubMed] [Google Scholar]
- Chen, J. , Li, W. , He, Q. , Yan, W. , Yang, H. , Gong, S. , Gou, Y. , Fu, S. , Chen, X. , Ye, S. , & Qian, Y. 2016. The antiviral activity of arctigenin in traditional Chinese medicine on porcine circovirus type 2. Research in Veterinary Science, 106, 159–164. [DOI] [PubMed] [Google Scholar]
- Cho, M. K. , Park, J. M. , Jang, Y. P. , Kim, Y. C. , & Kim, S. G. (2002). Potent inhibition of lipopolysaccharide‐inducible nitric oxide synthase expression by dibenzylbutyrolactone lignans through inhibition of I‐appaBalpha phosphorylation and of p65 nuclear translocation in macrophages. International Immunopharmacology, 2, 105–116. [DOI] [PubMed] [Google Scholar]
- Dorr, P. M. , Baker, R. B. , Almond, G. W. , Wayne, S. R. , & Gebreyes, W. A. (2007). Epidemiologic assessment of porcine circovirus type 2 coinfection with other pathogens in swine. Journal of the American Veterinary Medical Association, 230, 244–250. [DOI] [PubMed] [Google Scholar]
- Ellis, J. (2014). Porcine circovirus: A historical perspective. Veterinary Pathology, 51, 315–327. [DOI] [PubMed] [Google Scholar]
- Finsterbusch, T. , Mankertz, A. (2009). Porcine circoviruses‐small but powerful. Virus Research, 143, 177–183. [DOI] [PubMed] [Google Scholar]
- Gao, Q. , Yang, M. , & Zou, Z. (2018). Overview of the anti‐inflammatory effects, pharmacokinetic properties and clinical efficacies of arctigenin and arctiin from Arctium lappa L. Acta Pharmacologica Sinica, 39, 787–801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillespie, J. , Opriessnig, T. , Meng, X. J. , Pelzer, K. , & Buechner‐Maxwell, V. (2009). Porcine circovirus type 2 and porcine circovirus‐associated disease. Journal of Veterinary Internal Medicine, 23, 1151–1163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han, J. , Zhang, S. , Zhang, Y. , Chen, M. , & Lv, Y. (2017). Porcine circovirus type 2 increases interleukin‐1beta and interleukin‐10 production via the MyD88‐NF‐kappa B signaling pathway in procine alveolar macrophages in vitro. Journal of Veterinary Science, 181, 83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi, K. , Narutaki, K. , Nagaoka, Y. , Hayashi, T. , & Uesato, S. (2010). Therapeutic effect of arctiin and arctigenin in immunocompetent and immunocompromised mice infected with influenza A virus. Biological & Pharmaceutical Bulletin, 33, 1199–1205. [DOI] [PubMed] [Google Scholar]
- Hertzog, P. J. , O'Neill, L. A. , & Hamilton, J. A. (2003). The interferon in TLR signaling: More than just antiviral. Trends in Immunology, 24, 534–539. [DOI] [PubMed] [Google Scholar]
- Hyam, S. R. , Lee, I. A. , Gu, W. , Kim, K. A. , Jeong, J. J. , Jang, S. E. , Han, M. J. , & Kim, D. H. (2013). Arctigenin ameliorates inflammation in vitro and in vivo by inhibiting the PI3K/AKT pathway and polarizing M1 macrophages to M2‐like macrophages. European Journal of Pharmacology, 708, 21–29. [DOI] [PubMed] [Google Scholar]
- Jeong, J. B. , Hong, S. C. , Jeong, H. J. , & Koo, J. S. (2011). Arctigenin induces cell cycle arrest by blocking the phosphorylation of Rb via the modulation of cell cycle regulatory proteins in human gastric cancer cells. International Immunopharmacology, 11, 1573–1577. [DOI] [PubMed] [Google Scholar]
- Kawai, T. , & Akira, S. (2010). The role of pattern‐recognition receptors in innate immunity: Update on Toll‐like receptors. Nature Immunology, 11, 378–384. [DOI] [PubMed] [Google Scholar]
- Kou, X. J. , Qi, S. M. , Dai, W. X. , Lou, L. , & Yin, Z. M. (2011). Arctigenin inhibits lipopolysaccharide‐induced iNOS expression in RAW264.7 cells through suppressing JAK‐STAT signal pathway. International Immunopharmacology, 11, 1095–1102. [DOI] [PubMed] [Google Scholar]
- Lawrence, T. (2009). The nuclear factor NF‐κB pathway in inflammation. Cold Spring Harbor Perspectives in Biology, 1, 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, J. , Li, X. , Ren, Y. S. , Lv, Y. Y. , Zhang, J. S. , Xu, X. L. , Wang, X. Z. , Yao, J. C. , Zhang, G. M. , & Liu, Z. (2017). Elucidation of arctigenin pharmacokinetics and tissue distribution after intravenous, oral, hypodermic and sublingual administration in rats and beagle dogs: Integration of in vitro and in vivo findings. Frontiers in Pharmacology, 8, 376. 10.3389/fphar. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li, W. , Liu, S. , Wang, Y. , Deng, F. , Yan, W. , Yang, K. , Chen, H. , He, Q. , Charreyre, C. , & Audoneet, J. C. (2013). Transcription analysis of the porcine alveolar macrophage response to porcine circovirus type 2. Bmc Genomics [Electronic Resource], 14, 353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ouyang, T. , Liu, X H. , Ouyang, H. S. , & Ren, L. Z. (2018). Mouse models of porcine circovirus 2 infection. Animal Models and Experimental Medicine, 19, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palinski, R. , Pineyro, P. , Shang, P. , Yuan, F. , Guo, R. , Fang, Y. , Byers, E. , & Hause, B. M. (2016). A novel porcine circovirus distantly related to known circoviruses is associated with porcine dermatitis and nephropathy syndrome and reproductive failure. Journal of Virology, 91, e01879‐16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramamoorthy, S. , & Meng, X. J. (2009). Porcine circoviruses: A minuscule yet mammoth paradox. Animal Health Research Reviews, 10, 1–20. [DOI] [PubMed] [Google Scholar]
- Riera, R. M. , Pérez‐Martínez, D. , & Castillo, F. C. (2016). Innate immunity in vertebrates: An overview. Immunology, 148, 125–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swarup, V. , Ghosh, J. , Mishra, M. K. , & Basu, A. (2008). Novel strategy for treatment of Japanese encephalitis using arctigenin, a plant lignan. Journal of Antimicrobial Chemotherapy, 61, 679–688. [DOI] [PubMed] [Google Scholar]
- Tanja, O. , Anbu, K. , Alessandra, M. , & Chao, T. (2020). Porcine circoviruses: Current status, knowledge gaps and challenges. Virus Research, 286 (198044). [DOI] [PubMed] [Google Scholar]
- Ting, O. , Liu, X. , Hong, S. , & Ren, L. (2018). Mouse models of porcine circovirus 2 infection. Animal Models and Experimental Medicine, 19, 23–28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tischer, I. , Rasch, R. , & Tochtermann, G. (1974). Characterization of papovavirus‐and picornavirus‐like particles in permanent pig kidney cell lines. Zentralblatt fur Bakteriologie, Parasitenkunde, Infektionskrankheiten und Hygiene. Erste Abteilung Originale. Reihe A: Medizinische Mikrobiologie und Parasitologie, 226, 153–167. [PubMed] [Google Scholar]
- Wang, X. , Chen, L. , Yuan, W. , Li, Y. , Li, L. , Li, H. , & Song, Q. (2017). Effect of porcine circovirus type 2 (PCV2) on the function of splenic CD11c+ dendritic cells in mice. Archives of Virology, 162, 1289–1298. [DOI] [PubMed] [Google Scholar]
- Wei, L. , Kwang, J. , Wang, J. , Shi, L. , Yang, B. , Li, Y. , & Liu, J. (2008). Porcine circovirus type 2 induces the activation of nuclear factor kappa B IκBα degradation. Virology, 378, 177–184. [DOI] [PubMed] [Google Scholar]
- Zhang, H. , Hu, W. , Li, J. , Liu, T. , Zhou, J. , Opriessnig, T. , & Xiao, C. (2019). Novel circovirus species identified in farmed pigs designated as Porcine circovirus 4, Hunan province, China. Transboundary and Emerging Diseases, 67, 1057–1061. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The date that support the findings of this study are available from the corresponding author upon reasonable request.


