Abstract
Ribonucleic acid (RNA) and proteins play critical roles in gene expression and regulation. The relevant study increases the understanding of various life processes and contributes to the diagnosis and treatment of different diseases. RNA imaging and mapping RNA-protein interactions expand the understanding of RNA biology. However, the existing methods have some limitations. Recently, precise RNA targeting of CRISPR-Cas13 in cells has been reported, which is considered a new promising platform for RNA imaging in living cells and recognition of RNA-protein interactions. In this review, we first described the current findings on Cas13. Furthermore, we introduced current tools of RNA real-time imaging and mapping RNA-protein interactions and highlighted the latest advances in Cas13-mediated tools. Finally, we discussed the advantages and disadvantages of Cas13-based methods, providing a set of new ideas for the optimization of Cas13-mediated methods.
Keywords: CRISPR, Cas13, RNA imaging, RNA-protein interactions, RNA targeting, RNA biology
1 Introduction
CRISPR-Cas (Clustered Regularly Interspersed Short Palindromic Repeat-CRISPR-Associated) systems have received increasing attention in the scientific community due to their accurate targeting and excellent editing ability. The CRISPR-Cas loci encode an adaptive immune system in most archaea and many bacteria, which specifically recognize and cut foreign DNA or RNA sequences for improved protection against viral invaders (Barrangou et al., 2007; Marraffini and Sontheimer, 2010; Koonin and Makarova, 2013; Makarova et al., 2013; Barrangou and Marraffini, 2014).
CRISPR-Cas systems have two principal modules, the adaptive module and the effector module (Koonin et al., 2017). The adaptive module is basically composed of endonuclease Cas1 and structural subunit Cas2 (Amitai and Sorek, 2016; Koonin et al., 2017), whereas the effector module varies widely between CRISPR-Cas types and subtypes (Makarova et al., 2013; Amitai and Sorek, 2016; Mohanraju et al., 2016). Cas effectors consist of Class I and Class II (Murugan et al., 2017). The two classes are further divided into six types (I–VI). In Class I (Type I, III, and IV), the effector is composed of a large multi-subunit complex, whereas in Class II (Type II, V, and VI), the effector is a single-protein endonuclease (Makarova et al., 2015; Mohanraju et al., 2016; O’Connell, 2019). Although Class I is widely distributed in archaea and bacterial genomes (Jackson et al., 2017; Shmakov et al., 2017), many researchers prefer to use Class II due to its high efficiency with just one multidomain protein. Before Cas13 was discovered, DNA targeting was still the research tendency of methods based on CRISPR-Cas systems. In 2015, Shmakov et al. (2015) first reported the existence of Cas13 (formerly named C2c2). Cas13 has fascinating prospects due to its characteristic of specific RNA targeting. To date, Cas13 has been widely used in RNA detection, imaging, and manipulation of RNA biology (O’Connell, 2019).
RNA is an important component of gene expression and regulation and undergoes complex dynamic processes that influence gene expression in different ways (Braselmann et al., 2020). RNA imaging can be used to visualize gene expression and regulation. This strengthens the understanding of cell life activities and provides new methods and ideas for both the diagnosis and treatment of diseases (Darnell et al., 2010; Yamada et al., 2011; Chao et al., 2012; Ke et al., 2013; Lee et al., 2014; Buxbaum et al., 2015). Given that RNA does not fluoresce by itself, a variety of RNA imaging probes have been designed to achieve RNA imaging. However, each approach has limitations. CRISPR-Cas13 is a new tool in RNA imaging in living cells. For example, dCas13a (LwaCas13a) labeled with GFP can achieve imaging stress-induced bulk β-actin mRNA movement (Abudayyeh et al., 2017). With the discovery of more Cas13 proteins, some Cas13-mediated imaging tools have been created and attracted much attention.
The interactions of RNA and its binding proteins (RBPs) have been recognized as one of the real components that regulate cellular functions, such as precise translation of spatiotemporal localization and promotion of correct cell expression (Dreyfuss et al., 2002). RBPs play a vital role in RNA biology and disease treatments (Gebauer et al., 2021). Recently, due to the development of the CRISPR-Cas system, a new hypothesis of identifying RBPs has stood out. Based on the discovery of dCas13 by Zhang’s group, which retained the targeting potential of Cas13 and removed its cleavage ability (Abudayyeh et al., 2017), Cas13 can be used as bait to pull down RBPs. To date, Cas13-mediated methods such as CARPID and CRUIS have been developed, providing new ideas for the detection of RBPs (Yi et al., 2020; Zhang et al., 2020).
In this review, we first described the current findings on Cas13. Then, we discussed various Cas13-mediated imaging tools in the field of RNA imaging and detection of RNA-protein interactions and compared them with conventional tools. Finally, we comprehensively analyzed the advantages and disadvantages of Cas13-based imaging technology, provided relevant suggestions based on existing research, and put forward prospects for future research.
2 CRISPR-Cas13
Cas13 (type VI system) is the only member of the CRISPR-Cas systems that can specifically target RNA, in addition to the Class I Type III systems (Abudayyeh et al., 2016; Tamulaitis et al., 2017). Cas13 has two enzymatically active Higher Eukaryotes and Prokaryotes Nucleotide-binding (HEPN) RNase domains (Anantharaman et al., 2013). One RNase is responsible for crRNA preprocessing, helping to form a mature type VI interference complex, whereas the other one has two HEPN endoRNase domains that mediate the precise cleavage of RNA (Shmakov et al., 2015; Abudayyeh et al., 2016; East-Seletsky et al., 2017; Smargon et al., 2017; Konermann et al., 2018; Yan et al., 2018). Generally, the Cas13 protein families have been divided into four subtypes based on the phylogeny of their effector complexes, namely, Cas13a (previously known as C2c2), Cas13b, Cas13c, and Cas13d (Shmakov et al., 2017; Konermann et al., 2018; Yan et al., 2018). Recently, two new proteins of Cas13 (Cas13X and Cas13Y) have also been reported (Xu et al., 2021).
Cas13a is a large protein that belongs to the type VI-A CRISPR-Cas system (Shmakov et al., 2015). Cas13a contains the nuclease (NUC) lobe and crRNA recognition (REC) lobe. The NUC lobe consists of the HEPN-1 domain, HEPN-2 domain, Helical-2 domain, and Helical-3 domain. The REC lobe consists of an N-terminal domain (NTD), a Helical-1 domain, and a cleft accommodating the crRNA repeat region (Liu et al., 2017a; Liu et al., 2017b; Knott et al., 2017). At present, several Cas13 proteins have been found, such as LshCas13a, LbuCas13a and LwaCas13a (Abudayyeh et al., 2017; Liu et al., 2017a; Liu et al., 2017b). In addition to pre-crRNA processing, these Cas13 proteins also have highly different structures at the architectural and domain organization levels (Knott et al., 2017; Liu et al., 2017a; Liu et al., 2017b). Recent studies showed that LwaCas13a was the most effective protein among fifteen Cas13a orthologs with no significant PFS motif, which provides a potential platform to research RNA targeting (Abudayyeh et al., 2017).
Cas13b is more powerful than Cas13a. Zhang’s group tested 21 orthologs of Cas13a, 15 orthologs of Cas13b and seven orthologs of Cas13c for knockdown activity of Cas13 family members and indicated that the knockdown level of PspCas13b increased continuously relative to LwaCas13a (the average knockdown rate of PspCas13b was 92.3%, while that of LwaCas13a was 40.1%) (Cox et al., 2017). Compared with other Cas13 proteins, Cas13b has many structural differences. First, unlike Cas13a, Cas13c, and Cas13d, the HEPN domains of Cas13b are at the extreme N and C termini of the linear protein (Shmakov et al., 2017; Smargon et al., 2017). Second, the direct repeat of the Cas13b crRNA is at the 3′ end (Abudayyeh et al., 2016; Konermann et al., 2018; Yan et al., 2018). Third, the target RNA is allowed to access the opened central channel of PbuCas13b, whereas Cas13a and Cas13d require a shared solvent-exposed cleft that grasps the target RNA (Liu et al., 2017a; Zhang et al., 2018; Slaymaker et al., 2021).
The flexibility of Cas13d makes it more widely used because the enzyme is smaller than other Cas13 subtypes in terms of body size (Yan et al., 2018). One main reason is the absence of the Helical-1 domain compared to Cas13a (Liu et al., 2017b; Yan et al., 2018). Meanwhile, Cas13d lacks an appreciable sequence that constrains the target flanking sequences. In addition, Cas13d has a special mechanism for crRNA processing. Cas13d shows powerful collateral RNase activity and target cleavage (Yan et al., 2018) and is suitable for in vivo delivery because of its relatively small size (Abudayyeh et al., 2017).
Cas13X and Cas13Y are new family members of Cas13 which were identified from hypersaline samples. Cas13X can be divided into Cas13X.1 and Cas13X.2, while Cas13Y can be divided into Cas13Y.1 to Cas13Y.5. Compared with the conventional Cas13 protein, Cas13X.1 contains only 775 amino acids, which is the smallest Cas13 protein at present. Cas13X.1 was further truncated from 775 to 445 aa, which solves the delivery obstacles of various Cas13-based base editors in vivo. With the combination of ADAR2dd variants and truncated dCas13X.1, new editors used for A-to-I or C-to-U editing of various RNA loci in mammalian cells have been designed and show more advantages compared with REPAIR (Cox et al., 2017) systems and RESCUE systems (Abudayyeh et al., 2019; Xu et al., 2021).
With the further study of Cas13, Zhang’s group mutated conserved catalytic residues in the HEPN domains of Cas13, removing its nuclease activity (Cox et al., 2017). The mutant Cas13 is called CRISPR-catalytically dead CRISPR-Cas13 (dCas13). Due to a lack of cleavage ability, dCas13 becomes a great RNA binding platform for RNA modification, which is guided by gRNA to deliver engineering enzymes to the target region. On this basis, an increasing number of RNA tools based on dCas13 have been developed and widely applied in the fields of cell biology, disease and imaging (Tang et al., 2021).
3 Classic Imaging Methods and CRISPR/Cas13-Mediated Ribonucleic Acid Imaging
In the last dozen years, technological advances in RNA imaging in living cells have revolutionized cell biology. Many tools have been created and widely used for the recognition of numerous types of RNAs, including mRNAs and noncoding RNAs (Urbanek et al., 2014). RNA imaging probes can be classified into two categories: probes imaging exogenous RNAs and probes imaging endogenous RNAs, depending on whether the target RNA needs to be preprocessed. Recently, due to the excellent capability of RNA targeting, several attempts have been made to introduce new Cas13-mediated imaging methods. dLwaCas13a was first demonstrated to label abundant β-actin mRNA molecules after stress (Abudayyeh et al., 2017). Thereafter, a growing number of direct homologs of Cas13 proteins were reported to be applied to RNA imaging (Yang et al., 2019). In this section, we mainly focus on fluorescence-based CRISPR-Cas13 approaches and compare them with other imaging methods.
3.1 Classic Imaging Methods
3.1.1 Probes Imaging Endogenous Ribonucleic Acids
Probes imaging exogenous RNAs in living cells mainly includes Molecular beacons (MBs), Nano-MBs, and Quencher-free probes. MBs are oligonucleotide probes formed by antisense stem-loop with a fluorophore and quenching agent at the end. The stem-loop structures contain recognition sequences binding to target RNA (Tyagi and Kramer, 1996; Xia et al., 2017). With a good fluorophore-quench pair, MB fluorescence can be increased by 20–100 times after hybridization with target RNA (Marras et al., 2002). However, MBs require carrier proteins to transport them into cells and hence cannot be directly used to image RNA in live-cells. In addition, the widespread use of MBs is limited by false-positive signals (FPS) due to their biological stability (Tyagi and Alsmadi, 2004; Chen et al., 2007).
As the quencher and the carrier, nanoparticles can combine with MBs to form Nano-MBs that consist of gold nanoparticles (GNPs) (Dubertret et al., 2001). GNP-Nano-MBs are the most typical Nano-MBs and can track the spatial distribution of target RNAs and quantify their expression (Briley et al., 2015). GNP-Nano-MBs have many advantages: a high signal-to-noise ratio (Dubertret et al., 2001), excellent resistance to enzyme degradation (Seferos et al., 2009), high cellular uptake (Choi et al., 2013), and a longer imaging fluorescence lifetime (Shi et al., 2016). However, GNP-Nano-MBs also contribute to RNA downregulation due to stable binding with targeted mRNA (Seferos et al., 2007; Prigodich et al., 2009).
Quencher-free probes can quench fluorophores by themselves, resulting in low background fluorescence without quenchers. Forced Intercalation Probes (FIT Probes) and Exciton-Controlled Hybridization-sensitive fluorescent Oligonucleotide probes (ECHO Probes) are two classical probes among them. Within the FIT probe, thiazole orange (TO) dyes serve as fluorescent base surrogates and signal hybridization in a single-nucleotide specific manner. The FIT probe can be used to develop brighter probes by incorporating fluorophore intercalators with higher fluorescence (Hövelmann et al., 2013). ECHO probes are sequence-specific and hybridization-sensitive probes that serve as artificial fluorescent nucleobases for RNA detection (Okamoto, 2011). However, ECHO probes are less robust and seldom used because the fluorescence increase strongly relies on the targeted RNA sequence (Okamoto, 2011).
3.1.2 Probes Imaging Exogenous Ribonucleic Acids
Probes imaging exogenous RNAs in living cells mainly include the RNA binding protein-fluorescent protein system (RBP-FP system), bimolecular fluorescence complementation (BiFC) system, RNA aptamer/fluorophore system, and reporter gene system. The RBP-FP system can be applied in mRNA imaging. RBP refers to MS2 coat protein (MCP) binding to MBs (MS2-binding sequence), and FP refers to GFP. In this imaging system, the targeted mRNA is first pretreated by inserting six units of MBs into the 3′-UTR (untranslated region). Subsequently, the MCP-GFP fusion protein is added to the pretreated system, which helps illuminate the specific mRNAs. The RBP-FP system was first used in living yeast to track the transport and localization of ASH1 mRNA particles (Bertrand et al., 1998). However, recent studies have shown that MS2 binding site arrays inhibit 5′ to 3′ degradation of mRNA. which results in the accumulation of 3′ mRNA fragments (Garcia and Parker, 2015, 2016; Heinrich et al., 2017). In addition, the accumulation of MBs fragments has a significant effect on mRNAs with high regulation and short life span (Tutucci et al., 2018). The analysis is often confounded by background fluorescence.
BiFC uses two different RBPs conjugated to two halves of split FPs (RBP1-N-FP and RBP2-C-FP) (Hu et al., 2002). MCP-PCP-based (Wu et al., 2014), PUMHD-based (Yoshimura and Ozawa, 2016), and aptamer-protein-based (Valencia-Burton and Broude, 2007) BiFC systems are among the main types of BiFC systems. This method is suitable to visualize long-lived RNAs, not for real-time visualization, because the fusion of N-FP and C-FP requires time and is irreversible (Wu et al., 2014).
Unlike RBP-FP systems and BiFC systems, RNA aptamer/fluorophore systems enable real-time imaging due to the fast-binding interactions of the aptamer and small molecule fluorophores (Bertrand et al., 1998; Paige et al., 2011; Wu et al., 2014). There are three advantages of the systems: 1) fast imaging speed (Paige et al., 2011), 2) low background noise (Song et al., 2014), and 3) resistance to photobleaching and suitability for long-term tracking of RNAs.
Reporter gene systems are specifically suitable for miRNA imaging (Kim S. et al., 2009; Niu and Chen, 2009; Niu and Chen, 2012). It can be used for imaging pri-miRNA (primary miRNA) cutting, pri-miRNA transcription, ds-miRNA (miRNA-miRNA*), and miRNA function; however, it mostly focuses on the imaging of mature miRNAs (Kim H. J. et al., 2009; Kim S. et al., 2009; Tu et al., 2014; Choi et al., 2016; Wang et al., 2016) (Table 1).
TABLE 1.
Classical RNA imaging methods and Cas13-mediated methods.
| Methods | Application | In vitro/In vivo | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| RBP-FP system | mRNA | In vitro | High resolution | High background signals | Zimyanin et al. (2008), van Gemert et al. (2009), Lionnet et al. (2011), Muramoto et al. (2012), Yoshimura et al. (2012), Hocine et al. (2013), Hayashi et al. (2014), Rino et al. (2014) |
| BiFC system | mRNA | In vitro | Low background signals | Irreversibility; only suitable for visualizing long-lived RNAs; not suitable for visualizing real-time | Shyu and Hu (2008), Yamada et al. (2011) |
| RNA aptamer/fluorophore system | 5S RNAs, 6S RNAs, mRNA | In vitro | Fast imaging; real-time imaging; suitable for long-time tracking of RNAs | High background signals | Paige et al. (2011), Dolgosheina et al. (2014), Shin et al. (2014) |
| Reporter gene system | miRNA | In vitro | Without affecting the properties of RNAs | Only suitable for the visualization of miRNA | Kim H. J. et al. (2009), Ko et al. (2009), Kang et al. (2012), Wang F. et al. (2013) |
| MBs | miRNA, mRNA | In vivo | Wide application | False-positive signals | Kang et al. (2011), Wang Z. et al. (2013), Lee et al. (2015b), Tay et al. (2015); Xia et al. (2017) |
| Nano-MBs | mRNA, miRNA | In vivo | Low background signals; excellent resistance to enzyme degradation; high cellular uptake; longer imaging fluorescence lifetime | RNA downregulation | Riahi et al. (2014), Lee et al. (2015a), Wang et al. (2015) |
| Quencher-free probes | mRNA, 28S rRNA, snoRNA, polyA RNA | In vivo | Robust; high sensitivity and specificity | Easily subject to self-dimerization | Kummer et al. (2011), Okamoto (2011), Oomoto et al. (2015) |
| dCas13a-NF | mRNA | In vivo | High efficiency; robust; low background noise; real-time imaging | Cumbersome design | Abudayyeh et al. (2017) |
| CRISPR-dPspCas13b-mediated imaging | lncRNA, mRNA | In vivo | Yang et al. (2019) | ||
| Imaging using dCas13 and dCas9 | DNA, mRNA | In vivo | Wang et al. (2019), Yang et al. (2019) |
3.2 Methods Based on Cas13
3.2.1 dCas13a-NF
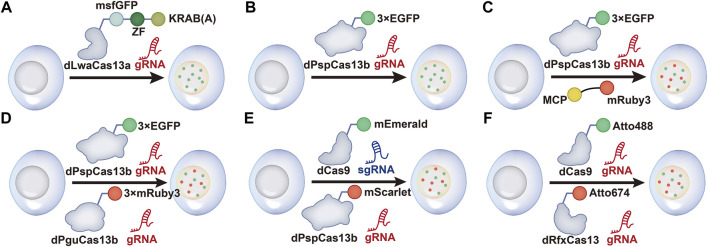
Cas13a was previously used for RNA knockdown and binding. Recently, a method called dCas13a-NF was created by Zhang’s group to image RNA. First, the investigators evaluated 15 orthologs and identified LwaCas13a as the most effective Cas13a that is highly active and lacks PFS in bacteria. dCas13a was generated by inactivating catalytic arginine residues, and a significant enrichment of the corresponding target was presented by pulldown of dCas13a. To reduce background noise resulting from free proteins, a negative-feedback system was then designed based on zinc finger self-targeting and KRAB domain repression. The dCas13a (LwaCas13a) labeled with GFP can effectively re-localize and achieve imaging of β-actin mRNA under stress (Abudayyeh et al., 2017) (Figure 1A).
FIGURE 1.
Schematic of CRISPR/Cas13-mediated RNA imaging methods. (A) dCas13a-GFP-KRAB construction for negative-feedback imaging. The dLwaCas13a incorporates a negative-feedback system based upon zinc finger self-targeting and the KRAB repression domain to image ACTB mRNA. (B) dPspCas13b-3 × EGFP labeling system. dPspCas13b is tagged with several green fluorescent proteins EGFP to image mRNAs. (C) Dual-color RNA labeling using a combination of the dPspCas13b and MS2- MCP systems. A total of 24 × MS2 (24 copies of the MS2 stem loop)-NEAT1-KI HeLa cells are constructed. Then, RNAs are labeled simultaneously by transfecting dPspCas13b-3 × EGFP, gRNAs for NEAT1, and MCP-mRuby3 into the cells. (D) Dual-color RNA labeling by different dCas13b systems. RNAs are labeled with dPspCas13b and dPguCas13b in HeLa cells. (E) Dual-color labeling using dCas9 and dPspCas13b. DNA and RNAs are labeled simultaneously in living cells by combining the dCas9-mEmerald and dPspCas13b-mScarlet systems. (F) Dual-Color labeling using dCas9 and dRfxCas13. Atto488-labeled dCas9 and Atto647-labeled dRfxCas13 are used to image genomic DNA and RNA transcripts.
3.2.2 CRISPR-dPspCas13b-Mediated Imaging
In a separate study, Yang et al. used the co-localization of NEAT1 to screen eight fluorescent protein-labeled dCas13 direct homologs and thus explored their RNA imaging ability. NEAT1 is a moderately expressed long noncoding RNA (lncRNA), and the co-location of multiple NEAT1 molecules on the fluorescence background shows sufficient S/N (signal-to-noise) differentiation, which is suitable for screening RNA imaging. The results showed that dPspCas13b and dPguCas13b were the most effective Cas proteins. However, the mentioned dLwaCas13a protein is not available for NEAT1. Previous studies have discovered that the length and mismatch location of gRNA affected the efficiency of RNA cleavage and labeling (Abudayyeh et al., 2017; Cox et al., 2017).
For RNA imaging, length and targeting position are crucial to the gRNA of dPspCas13b (Yang et al., 2019). It is noteworthy that the length of gRNA is preferably within 20–27 nt, whereas for RNA cleavage, the suitable length is 30 nt. In addition, the investigators assessed RNA-binding specificity. The results indicated that the binding activity of dPspCas13b was more sensitive to mismatches in the middle and direct repeat regions. For example, the mismatches at positions 17 and 18 could lead to the disability of labeling. Thereafter, the optimal gRNA was screened prior to labeling MUC4 mRNA and GCN4 using the dPspCas13b system (Figure 1B). Notably, dRfxCas13d cannot be used due to the generation of abnormal signals (Yang et al., 2019). smFISH further confirmed the efficiency and accuracy of the labeling mRNAs containing repeated sequences in the nucleus and cytoplasm. Compared to the classic MS2-MCP system, the labeling efficiency of CRISPR-dPspCas13b reached approximately 80%, whereas the MS2-MCP system was only 30% (Figure 1C). Importantly, this system can achieve dual-color imaging for single RNAs and image two different RNAs using two orthogonal CRISPR-dCas13 proteins (Figure 1D). However, the steps of pretreatment are tedious and hence require optimization.
3.2.3 Imaging Using dCas13 and dCas9
Previous studies have also shown that fluorescently labeled nuclease-deficient Cas9 (dCas9) protein is excellent at DNA or RNA imaging (Jolly et al., 2002; Chen et al., 2013). Furthermore, it has been reported that the dCas9 protein was fused with an enhanced green fluorescent protein (EGFP) to enrich the fluorescence signal at the target genome site for imaging (Chen et al., 2013). Therefore, the combination of dCas9 and dCas13 provides a new idea for the simultaneous imaging of RNA and DNA in living cells.
Research showed that after stress treatment of cells, dCas9 and dCas13 (dPspCas13b) could realize dual-color imaging of DNA and RNA (Figure 1E). dCas9 successfully labeled MUC4 and SatIII DNAs, and dPspCas13b-mScarlet successfully labeled MUC4 and SatIII RNAs, realizing simultaneous labeling of genomic DNA and transcriptional RNAs (Yang et al., 2019). In addition, Wang et al. (2019) developed a robust, versatile approach named CRISPR live-cell fluorescent (LiveFISH) in situ hybridization using fluorescent oligos, which is suitable for genome tracking in broad cell types. They used another Cas13 protein (RfxCas13d) to bind dCas9, visualizing both the MS2-repeat-tagged mRNA and DNA in real time (Figure 1F).
4 CRISPR/Cas13-Mediated Ribonucleic Acid-Protein Interactions Detection
Revealing the interactions of RNA and RBPs offers essential clues for understanding RNA biology. The interactions are complex, and a particular RNA tends to have fixed protein-binding domains with multiple RBPs. Similarly, a particular protein can also work with different RNAs (Weissinger et al., 2021). When the RNA-RBP interaction is disturbed, it will inevitably lead to many diseases, such as cancer, neurogenic diseases, and leukemia (Tolino et al., 2012; Pereira et al., 2017; Elcheva and Spiegelman, 2021).
Currently, there are a variety of methods for identifying RNA and its binding proteins, which can be broadly divided into two categories, protein-centered and RNA-centered (Ramanathan et al., 2019). Protein-centered methods are used to select a specific protein and observe the RNAs bound to it, such as RNA coimmunoprecipitation (RIP) and cross-linking immunoprecipitation (CLIP) (Gagliardi and Matarazzo, 2016; Lee and Ule, 2018). Furthermore, CLIP has been optimized with the development of mass spectrometry and sequencing technology (Licatalosi et al., 2008). The RNA-centered methods are for selecting a specific RNA and identifying the respective RBPs, such as RNA hybridization capture, RNA tagging based on gene aptamers, and proximity labeling (Tsai et al., 2011; West et al., 2014; Ramanathan et al., 2018). However, these methods have similar disadvantages, including the need for specific antibodies and strict elution conditions. The study of dCas13 provides a new idea for imaging, and some Cas13-mediated RNA-centered methods have been reported. In this section, we highlight Cas13-mediated methods and explore existing methods regarding their advantages and limitations.
4.1 Common Ribonucleic Acid-Centered Methods
RNA-centered methods can roughly be divided into in vitro and in vivo methods (Moore, 2005). In vitro methods usually use in vitro-transcribed (IVT) RNA with specific markers and then add cell extracts and elution proteins and analysis thereafter (Leppek and Stoecklin, 2014; Zheng et al., 2016). However, in vitro methods tend to ignore the influence of the intracellular environment on protein interactions. Moreover, the RNA transcribed in vitro may be different from the actual RNA in cells in terms of structure and morphology (Faoro and Ataide, 2014).
In vivo, endogenous RNA-protein imaging can overcome the described shortcomings. Endogenous methods can be divided into two categories: methods that use protein-RNA cross-linking and do not require protein-RNA cross-linking. The cross-linking methods first use formaldehyde or UV to cross-link the RBPs and RNA into a reversible or irreversible covalent bond, and then the protein is pulled down using a specific probe for elution (Sutherland et al., 2008; Li et al., 2014). Common cross-linking methods include RNA Antisense Purification (RAP), tandem RNA Isolation procedure (TRIP), and peptide nucleic acid (PNA)-assisted identification of RBPs (PAIR) (Zeng et al., 2006; Matia-González et al., 2017; McHugh and Guttman, 2018). Different from the previous cross-linking method, chromatin isolation by RNA purification (ChIRP) and capture hybridization analysis of RNA targets (CHART) use formaldehyde to cross-link RNA to proteins (Chu et al., 2011; Simon et al., 2011) (Table 2).
TABLE 2.
RNA-content methods: common methods and methods based on Cas13.
| Methods | Application | In vitro/In vivo | Advantages | Disadvantages | References |
|---|---|---|---|---|---|
| Biotinylated RNA | mRNA | In vitro | Strong combination between streptavidin beads and biotinylated RNA | Potentially biased toward abundant proteins | Zheng et al. (2016) |
| S1 aptamer | mRNA | In vitro | Simple purification without the need for recombinant protein production | Potentially biased toward abundant proteins; interference with native RBPs formation; unspecific interactions | Leppek and Stoecklin (2014) |
| RAP | lncRNA | In vivo | Strong combination between probe and RNA | High input cell numbers | McHugh et al. (2015) |
| TRIP | mRNA | In vivo | No need of genetic manipulation; UV cross-linking | Careful design and evaluation of ASO; differences in ASO binding sites may reduce efficiency | Matia-González et al. (2017) |
| PAIR | mRNA | In vivo | UV cross-linking | Difficult to product peptide nucleic acid | Zeng et al. (2006) |
| CHART | lncRNA | In vivo | Simple design; split pools of tiling oligonucleotide probes and glutaraldehyde crosslinking ensure the success | High input cell numbers | Chu et al. (2011) |
| RaPID | mRNA | In vivo | Low number of cells needed; interrogate motifs <50 nucleotides | Requires BoxB link to RNA; not all proteins can be detected due to biotinylation; it’s difficult to tell whether the protein is acting directly or indirectly | Ramanathan et al. (2018) |
| CARPID | lncRNA | In vivo | No need of genetic manipulation; Multiple gRNAs are designed to reduce background noise | Need a high abundance of targeted RNA; unstable binding; difficult to detect all the proteins due to the limitation of gRNA | Yi et al. (2020) |
| Cas13-based APEX targeting | hTR | In vivo | No need of genetic manipulation; introduce double-stranded RNA binding domain (dsRBD) to improve the stability of dCas13 complex | Need a high abundance of targeted RNA; unstable binding; difficult to detect all the proteins due to the limitation of gRNA | Han et al. (2020) |
| CRUIS | lncRNA, mRNA | In vivo | No need of genetic manipulation; no restriction on the type of RNA | Need a high abundance of targeted RNA; unstable binding; difficult to detect all the proteins due to the limitation of gRNA | Zhang et al. (2020) |
| CBRPP | lncRNA, mRNA | In vivo | No need of genetic manipulation; no restriction on the type of RNA | Need a high abundance of targeted RNA; unstable binding; difficult to detect all the proteins due to the limitation of gRNA | Li et al. (2021) |
| RPL | snRNA | In vivo | No need of genetic manipulation; no restriction on the type of RNA | Need a high abundance of targeted RNA; unstable binding; difficult to detect all the proteins due to the limitation of gRNA | Lin et al. (2021) |
| CBRIP | snRNA | In vivo | No restriction on the type of RNA; high stability and specificity | Need a high abundance of targeted RNA; difficult to detect all the proteins because of the limitation of gRNA | Chen et al. (2021) |
Recently, a proximity labeling technique has been used to map molecular interactions in living cells (Roux et al., 2012). The technique does not require protein-RNA cross-linking, which uses biotin ligases such as BirA* and BioID2 or ascorbate peroxidases such as APEX and APEX2 to produce biotin around itself, which can mark surrounding proteins for subsequent purification.
The aptamer technique combined with the proximity labeling technique forms a mature technique for mapping RBPs. For example, RNA-protein interaction detection (RaPID) binds biotin ligase to BoxB, which helps deliver the system around the targeted RNA through the λ -n domain, and the surrounding proteins are hence labeled (Ramanathan et al., 2018). Other aptamers, such as MS2, have also been used to investigate RNA-protein interactions combined with ascorbate peroxidase (Han et al., 2020). However, this approach is limited by complex artificial design and biological expression. In addition, with the continuous development of CRISPR systems, dCas13 can directly target RNA of interest at the endogenous level without adaptor fusion (Abudayyeh et al., 2017). Therefore, Cas13-mediated methods are gradually becoming popular in the scientific community.
4.2 Methods Based on Cas13
4.2.1 CARPID
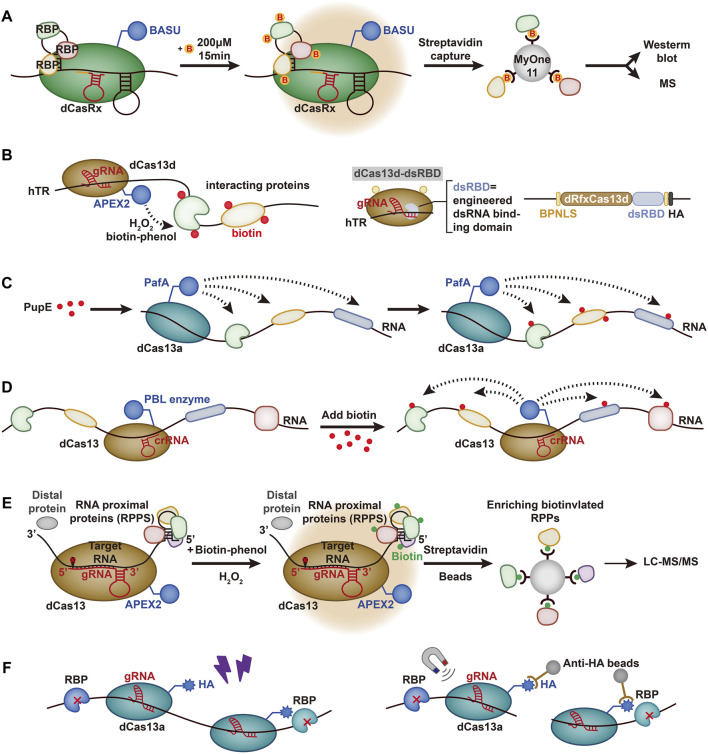
Xist lncRNAs have been identified in mammals and play an important role in gene silencing (Wutz, 2011). In a study conducted by Yi et al. (2020) a system called the CRISPR-assisted RNA-protein interaction detection method (CARPID) was developed to identify RNPs based on CRISPR/Cas13. The CARPID specifically binds the fused BASU biotin ligase to the target lncRNA in living cells and then biotinylates the adjacent proteins and facilitates streptomycin affinity coupling magnetic bead separation (Figure 2A). Furthermore, the isolated proteins were quantitatively analyzed by mass spectrometry. A gRNA array consisting of two gRNA sequences is designed to reduce background noise and enhance the specificity of targeting. To ensure the optimal reaction conditions, experiments such as changing the induction time or changing the location of the biotin ligase are needed.
FIGURE 2.
Schematic representation of RNA-contented methods based on Cas13. (A) CARPID. dCasRx is fused with BASU. Biotin is represented by yellow circles marked with red “B.” CARPID is directed by dCasRx to target the RNA of interest, and the RBPs are biotinylated. (B) RNA–protein interaction mapping via Cas13-based APEX targeting. APEX2 is fused with dCas13d and targeted to the hTR with the help of gRNA. H2O2 is added to cells preloaded with biotin-phenol, which is oxidized by APEX2 to phenoxy and covalently labels proximal endogenous proteins. A sequence-independent double-stranded RNA binding domain (dsRBD) from human protein kinase R (PKR) is fused to the C-terminus of the dRfxCas13d protein to strengthen the stability and targeting ability. A bipartite nuclear localization sequence (BPNLS) is used to optimize nuclear localization. (C) CRUIS. PafA is fused with dCas13a to target the RNA of interest and modifies the surrounding proteins by mediating PupE. (D) CBRPP. PBL is fused with dCas13 and targets specific RNA to covalently tag the surrounding protein. (E) RPL. APEX2 is fused with dCas13 and biotinylates RNA proximal proteins with H2O2 and biotin-phenol mediated by gRNA. The biotinylated proteins are enriched by streptavidin beads and analyzed by liquid chromatography-tandem mass spectrometry (LC-MS/MS). (F) CBRIP. dCas13a is fused with an HA tag and targets a specific RNA. RNA-protein interactions are stabilized by UV crosslinking, and the complexes are enriched by anti-HA beads.
In addition, Yi et al. (2020) demonstrated the high specificity and repeatability of this method. The investigators used different gRNAs to form the experimental group and replicated the experiment in each group. The results showed that at least 447 proteins were shared between the replication groups, and unexpected RBPs, such as TAF15, were found. However, some proteins still fail to pass the detection threshold due to unstable binding between proteins and RNA. Furthermore, this method is still limited by the mediation of biotin ligase, and it is still difficult to operate low abundance RNA. The method requires improvement in future studies.
4.2.2 Cas13-Based APEX Targeting
A new system to study human telomerase RNA (hTR) was developed by Han et al. for the detection of RNA-protein interactions. hTR plays a critical role in regulating cellular senescence as a template for reverse transcription and affects the development of diseases (Chen and Greider, 2004; Theimer and Feigon, 2006). In their study, Han et al. combined RfxCas13d with APEX2 (Konermann et al., 2018) to identify the RBPs of hTR (Theimer and Feigon, 2006; Han et al., 2020) (Figure 2B). They envisioned using dCas13 to send APEX2 near the targeted RBPs for labeling. APEX2 can use hydrogen peroxide (H2O2) as an oxidant to catalyze the one-electron oxidation of biotin-phenol (BP), which helps to pull down the proteins (Hung et al., 2016). However, although RfxCas13d was found to be effective in targeting and cutting hTR, there was no significant enrichment of gRNA co-expressed with dRfxCas13d, which was also demonstrated in other experiments (Li et al., 2021). Therefore, the combination of dRfxCas13d and hTR needs to be further optimized.
To improve the stability and targeting potential of the imaging system, Han et al. introduced a double-stranded RNA binding domain (dsRBD) (Masliah et al., 2013), which was fused to the C-terminus of the dRfxCas13d protein. The results showed that dCas13d-dsRBD-APEX2 can effectively improve the enrichment level (Konermann et al., 2018). However, some limitations still remain. For instance, the results from the CRISPR-based imaging system only partially overlapped with MCP-APEX2, which reflected that background noise was still present (Konermann et al., 2018).
4.2.3 CRISPR-Based RNA-United Interacting System
Based on the development of the PUP-IT system for detecting membrane protein interactions (Liu et al., 2018), Zhang et al. (2020) created the CRISPR-based RNA-United Interacting System (CRUIS) by combining the PUP-IT system with dLwaCas13a (Figure 2C). The dLwaCas13a is used as a tracking tool to target specific RNA, and PUP-IT pulls down the proteins (Abudayyeh et al., 2017). To demonstrate the functioning of the system, Zhang et al. tested RBPs of NORAD (noncoding RNA activated by DNA damage) with CRUIS (Lee et al., 2016; Munschauer et al., 2018). The mass spectrometry results showed that CRUIS performed well in detecting validated RBPs, such as KHSPR and SRSF9, and could also detect unexpected proteins that were not considered RBPs (Munschauer et al., 2018; Zhang et al., 2020).
In addition to lncRNA, Zhang et al. (2020) also used this system to detect p21 mRNA and detected hnRNA-bound proteins such as HNRNPK and HNRNPA1, which also indirectly reflected the maturation mechanism of mRNA. The results indicate that the CRUIS system has a wide range of applications and broad potential. However, the efficiency of single sgRNA protein detection is limited and thus greatly affects the detection efficiency. Moreover, the presence of RNA secondary structures also continues to influence the system (Zhang et al., 2020).
4.2.4 CBRPP
Li et al. (2021) developed a set of systems named CBRPP based on the CRISPR system and PBL fusion selected by three experiments (Li et al., 2021). First, they fused dRfxCas13d and APEX2 to detect the RBPs of ACTB (Lam et al., 2015; Konermann et al., 2018) (Figure 2D). However, non-specific enrichment was found to be displayed regardless of whether the RfxcrRNA, the targeting sequence of ACTB, was transfected, indicating that dRfxCas13d-APEX2-NLS could not bind to the target RNA, which was also proven in the experiment conducted by Yang et al. (2019), Li et al. (2021). They then transfected dPspCas13b-APEX2-NES to detect ACTB mRNA and found the same inefficiency, which could be related to the high expression of dPspCas13b-APEX2-NES (Li et al., 2021). Later, they chose to fuse dPspCas13b with BioID2. The obtained data showed that the dPspCas13b-BioID2-NES system successfully recognized its interacting proteins. In addition, BioID2 can also help to identify transient proteins, and the targeted proteins can accumulate during reimaging, which can reduce the background noise (Li et al., 2021). Li et al. also used this system to detect NORAD in lncRNAs, showing good imaging performance. These findings revealed the strong potential of CBRPP in the imaging system (Ventura, 2016).
4.2.5 RNA Proximity Labeling
Lin et al. (2021) developed a method known as RNA proximity labeling (RPL) based on the fusion of dPspCas13b with the adjacent marker enzyme APEX2, which can lead to biotinylation of proteins within a distance of 25 nm from the targeted RNA by inference (Figure 2E). Cas13b was selected for its high specificity and low miss efficiency, and APEX2 was selected for its high kinetic effects (Lam et al., 2015; Yang et al., 2019). The RPL system was used to analyze the nuclear RNA (ncRNA) U1 and poly(A) tail proximal proteins and can rapidly identify RBPs and uncover novel RBPs such as KPNB1 (Lin et al., 2021). To reduce the background noise of the system, Lin et al. also took some measures, such as selecting the U1 with high abundance (Stark et al., 2001) and designing three kinds of gRNA to be expressed in separate cell lines in combination with different regions. Importantly, the RPL system does not use UV cross-linking or genetic system-based operations, which can effectively avoid the biases of UV cross-linking and genetic interference with RNA function (Baranello et al., 2016; Laprade et al., 2020).
4.2.6 CRISPR-Based RNA Interaction Proteomics
Recently, Chen et al. (2021) developed CRISPR-based RNA interaction proteomics (CBRIP) by combining dLwaCas13a with HA Tag (Figure 2F). Specific RNA is tracked using dCas13, and RBPs and RNA are then cross-linked using UV light. Finally, they are captured by anti-HA beads. The corresponding protein can be detected through mass spectrometry thereafter. Chen et al. proposed that limiting the expression of dCas13 was critical to improving the SNR. Therefore, Tet-on was designed as the promoter of dCas13 based on Dox, and gRNA was continuously induced by the U6 promoter (Han et al., 2020; Chen et al., 2021). A total of 226 proteins were identified as RBPs of U1 snRNA, and RPL7 was verified as a novel U1 binding protein. Meanwhile, the potential of this system for lncRNA was also demonstrated by validating RBM15 as an XIST-binding protein (McHugh et al., 2015). However, the whole CBRIP experiment requires large input cell numbers for maintenance of the capturing efficiency due to UV cross-linking (Chen and Greider, 2004).
5 Conclusion and Challenges
The precise targeting and editable properties of Cas13 make it an excellent tool for RNA imaging and detection of RNA-protein interactions. However, Cas13-mediated RNA imaging and mapping RNA-protein interactions are still in the exploration stage. Compared with other imaging methods, Cas13-mediated tools provide real-time imaging in living cells with lower background noise, better stability, and better imaging efficiency. Although these Cas13-mediated imaging methods have been optimized, there is still room for further improvements in their performance. Screening of Cas13 proteins is still tedious work, and not all proteins can be used for imaging. One reason could be that some Cas13 proteins cannot produce labeling signals. Furthermore, the design process of gRNAs is very difficult, and not all gRNAs can work well. Even small changes in gRNA position will lead to significant differences in imaging effects. In addition, the existing Cas13 imaging methods are only suitable for high abundance RNA whereas they cannot achieve good imaging effect for RNA with low abundance. Moreover, some cells also need to be processed in advance before imaging. For example, SatIII RNA visualization was achieved only after treatment with heat shock or SA (Yang et al., 2019). The mechanism and realization of this technology still require further exploration.
To date, multiple Cas13-mediated RNA-protein detection tools have been reported and initially evaluated. Cas-mediated methods have many advantages. It is noteworthy that Cas13 does not require pre-labeling of target RNA (Zheng et al., 2016), design of antisense probes (McHugh and Guttman, 2018) or insertion of MS2 or BoxB (Ramanathan et al., 2018; Mukherjee et al., 2019), which simplifies the experimental workflow. In addition, the imaging process is performed at the level of endogenous expression and does not involve any genetic manipulation, and the entire imaging process occurs in the living cell, which ensures that the detected interaction is closer to the real intracellular environment. However, the efficiency and specificity of targeting depend on the types of gRNA and Cas proteins, which requires rigorous and complicated screening. A larger Cas13 protein can affect the efficiency, while whether some newfound smaller Cas13 proteins present high efficiency is still questionable. Moreover, the expression level of the Cas13 protein complex in cells also affects the signal-to-noise ratio of the whole experiment (Han et al., 2020; Zhang et al., 2020; Li et al., 2021). One reason might be that the highly expressed Cas13 protein complex leads to a higher rate of labeling for nonspecific proteins. Therefore, inducing the low expression of Cas13 is an important part of the whole imaging system. In addition, the binding of gRNA to target RNA may be competitive with the binding of RBPs, while a single gRNA may reduce the RBP detection rate (Lin et al., 2021). Therefore, a RNA array can be set up as described by Yi et al. (2020) in CARPID. Furthermore, groups of different gRNAs can also be set up to reduce background noise (Lin et al., 2021). Meanwhile, the type of biotin also affects the efficiency of imaging (Li et al., 2021). The labeling of biotin is nonspecific; thus, all proteins within the range may be labeled, which may also cause damage to the binding of RNA and protein.
There are several directions for future optimization of the current approaches for Cas13-mediated RNA imaging and detection of RNA-protein interactions. First, a structural design database should be set up, and the design methods of gRNA should be optimized to simplify the design process of gRNA. With the development of deep learning technology, new gRNA efficiency prediction and design tools are expected to be developed. Second, more Cas13 proteins, especially newfound ones, can be screened out to further improve the targeting ability. Third, current linker proteins can be chemically modified so that they have a stronger signal when they bind to their target. Finally, optimizing the observation imaging equipment can also achieve better visualization results.
However, there are more difficult problems to address. The space structure of RNA changes may affect the targeted efficiency, such as the existence of the secondary structure, which may affect the gRNA combined with the target RNA (Zhang et al., 2020). In addition, the current imaging systems based on Cas13 still target high abundance RNAs, which is still a challenge for low abundance RNA, and the imaging efficiency still needs further increased (Han et al., 2020; Lin et al., 2021). In conclusion, Cas13-mediated methods for RNA imaging and detection of RNA-protein interactions are novel techniques. There is a need for further research to obtain solutions to the described challenges of imaging technology optimization based on the CRISPR system. In the near future, we could expect more reports on the improvement or development of Cas13-mediated methods.
Author Contributions
HC, YW, and NZ were responsible for literature collection and writing. SX, PT and LL helped to perform the literature search and prepared the figures. JD and YD had the idea for the manuscript and critically revised the work.
Funding
This work was funded by the National Natural Science Foundation of China (No. 31701162), the Key Research and Development Program of Anhui Province (No. 202104a07020031) and the College Students’ Innovation and Entrepreneurship Training Program of Anhui Province (No. S202110366054, S202110366098).
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
References
- Abudayyeh O. O., Gootenberg J. S., Essletzbichler P., Han S., Joung J., Belanto J. J., et al. (2017). RNA Targeting with CRISPR-Cas13. Nature 550 (7675), 280–284. 10.1038/nature24049 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudayyeh O. O., Gootenberg J. S., Franklin B., Koob J., Kellner M. J., Ladha A., et al. (2019). A Cytosine Deaminase for Programmable Single-Base RNA Editing. Science 365 (6451), 382–386. 10.1126/science.aax7063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abudayyeh O. O., Gootenberg J. S., Konermann S., Joung J., Slaymaker I. M., Cox D. B. T., et al. (2016). C2c2 is a Single-Component Programmable RNA-Guided RNA-Targeting CRISPR Effector. Science 353 (6299), aaf5573. 10.1126/science.aaf5573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amitai G., Sorek R. (2016). CRISPR-Cas Adaptation: Insights into the Mechanism of Action. Nat. Rev. Microbiol. 14 (2), 67–76. 10.1038/nrmicro.2015.14 [DOI] [PubMed] [Google Scholar]
- Anantharaman V., Makarova K. S., Burroughs A. M., Koonin E. V., Aravind L. (2013). Comprehensive Analysis of the HEPN Superfamily: Identification of Novel Roles in Intra-genomic Conflicts, Defense, Pathogenesis and RNA Processing. Biol. Direct 8, 15. 10.1186/1745-6150-8-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baranello L., Kouzine F., Sanford S., Levens D. (2016). ChIP Bias as a Function of Cross-Linking Time. Chromosome Res. 24 (2), 175–181. 10.1007/s10577-015-9509-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrangou R., Fremaux C., Deveau H., Richards M., Boyaval P., Moineau S., et al. (2007). CRISPR Provides Acquired Resistance against Viruses in Prokaryotes. Science 315 (5819), 1709–1712. 10.1126/science.1138140 [DOI] [PubMed] [Google Scholar]
- Barrangou R., Marraffini L. A. (2014). CRISPR-cas Systems: Prokaryotes Upgrade to Adaptive Immunity. Mol. Cell 54 (2), 234–244. 10.1016/j.molcel.2014.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bertrand E., Chartrand P., Schaefer M., Shenoy S. M., Singer R. H., Long R. M. (1998). Localization of ASH1 mRNA Particles in Living Yeast. Mol. Cell 2 (4), 437–445. 10.1016/s1097-2765(00)80143-4 [DOI] [PubMed] [Google Scholar]
- Braselmann E., Rathbun C., Richards E. M., Palmer A. E. (2020). Illuminating RNA Biology: Tools for Imaging RNA in Live Mammalian Cells. Cell Chem. Biol. 27 (8), 891–903. 10.1016/j.chembiol.2020.06.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briley W. E., Bondy M. H., Randeria P. S., Dupper T. J., Mirkin C. A. (2015). Quantification and Real-Time Tracking of RNA in Live Cells Using Sticky-Flares. Proc. Natl. Acad. Sci. USA 112 (31), 9591–9595. 10.1073/pnas.1510581112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum A. R., Yoon Y. J., Singer R. H., Park H. Y. (2015). Single-molecule Insights into mRNA Dynamics in Neurons. Trends Cell Biol. 25 (8), 468–475. 10.1016/j.tcb.2015.05.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chao J. A., Yoon Y. J., Singer R. H. (2012). Imaging Translation in Single Cells Using Fluorescent Microscopy. Cold Spring Harbor Perspect. Biol. 4 (11), a012310. 10.1101/cshperspect.a012310 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. K., Behlke M. A., Tsourkas A. (2007). Avoiding False-Positive Signals with Nuclease-Vulnerable Molecular Beacons in Single Living Cells. Nucleic Acids Res. 35 (16), e105. 10.1093/nar/gkm593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Gilbert L. A., Cimini B. A., Schnitzbauer J., Zhang W., Li G.-W., et al. (2013). Dynamic Imaging of Genomic Loci in Living Human Cells by an Optimized CRISPR/Cas System. Cell 155 (7), 1479–1491. 10.1016/j.cell.2013.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen B., Shi H., Zhang J., Zhou C., Han M., Jiang W., et al. (2021). CRISPR-Based RNA-Binding Protein Mapping in Live Cells. Biochem. Biophys. Res. Commun. 583, 79–85. 10.1016/j.bbrc.2021.10.059 [DOI] [PubMed] [Google Scholar]
- Chen J.-L., Greider C. W. (2004). Telomerase RNA Structure and Function: Implications for Dyskeratosis Congenita. Trends Biochem. Sci. 29 (4), 183–192. 10.1016/j.tibs.2004.02.003 [DOI] [PubMed] [Google Scholar]
- Choi C. H. J., Hao L., Narayan S. P., Auyeung E., Mirkin C. A. (2013). Mechanism for the Endocytosis of Spherical Nucleic Acid Nanoparticle Conjugates. Proc. Natl. Acad. Sci. 110 (19), 7625–7630. 10.1073/pnas.1305804110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y., Hwang D. w., Kim M. Y., Kim J. Y., Sun W., Lee D. S. (2016). Transgenic Mouse Expressing Optical MicroRNA Reporter for Monitoring MicroRNA-124 Action during Development. Front. Mol. Neurosci. 9, 52. 10.3389/fnmol.2016.00052 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu C., Qu K., Zhong F. L., Artandi S. E., Chang H. Y. (2011). Genomic Maps of Long Noncoding RNA Occupancy Reveal Principles of RNA-Chromatin Interactions. Mol. Cell 44 (4), 667–678. 10.1016/j.molcel.2011.08.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cox D. B. T., Gootenberg J. S., Abudayyeh O. O., Franklin B., Kellner M. J., Joung J., et al. (2017). RNA Editing with CRISPR-Cas13. Science 358 (6366), 1019–1027. 10.1126/science.aaq0180 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darnell D. K., Stanislaw S., Kaur S., Antin P. B. (2010). Whole Mount In Situ Hybridization Detection of mRNAs Using Short LNA Containing DNA Oligonucleotide Probes. RNA 16 (3), 632–637. 10.1261/rna.1775610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolgosheina E. V., Jeng S. C. Y., Panchapakesan S. S. S., Cojocaru R., Chen P. S. K., Wilson P. D., et al. (2014). RNA Mango Aptamer-Fluorophore: A Bright, High-Affinity Complex for RNA Labeling and Tracking. ACS Chem. Biol. 9 (10), 2412–2420. 10.1021/cb500499x [DOI] [PubMed] [Google Scholar]
- Dreyfuss G., Kim V. N., Kataoka N. (2002). Messenger-RNA-Binding Proteins and the Messages They Carry. Nat. Rev. Mol. Cell Biol. 3 (3), 195–205. 10.1038/nrm760 [DOI] [PubMed] [Google Scholar]
- Dubertret B., Calame M., Libchaber A. J. (2001). Single-Mismatch Detection Using Gold-Quenched Fluorescent Oligonucleotides. Nat. Biotechnol. 19 (4), 365–370. 10.1038/86762 [DOI] [PubMed] [Google Scholar]
- East-Seletsky A., O’Connell M. R., Burstein D., Knott G. J., Doudna J. A. (2017). RNA Targeting by Functionally Orthogonal Type VI-A CRISPR-Cas Enzymes. Mol. Cell 66 (3), 373–383. 10.1016/j.molcel.2017.04.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elcheva I. A., Spiegelman V. S. (2021). Targeting RNA-Binding Proteins in Acute and Chronic Leukemia. Leukemia 35 (2), 360–376. 10.1038/s41375-020-01066-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faoro C., Ataide S. F. (2014). Ribonomic Approaches to Study the RNA-Binding Proteome. FEBS Lett. 588 (20), 3649–3664. 10.1016/j.febslet.2014.07.039 [DOI] [PubMed] [Google Scholar]
- Gagliardi M., Matarazzo M. R. (2016). RIP: RNA Immunoprecipitation. Methods Mol. Biol. 1480, 73–86. 10.1007/978-1-4939-6380-5_7 [DOI] [PubMed] [Google Scholar]
- Garcia J. F., Parker R. (2015). MS2 Coat Proteins Bound to Yeast mRNAs Block 5′ to 3′ Degradation and Trap mRNA Decay Products: Implications for the Localization of mRNAs by MS2-MCP System. RNA 21 (8), 1393–1395. 10.1261/rna.051797.115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia J. F., Parker R. (2016). Ubiquitous Accumulation of 3′ mRNA Decay Fragments in Saccharomyces cerevisiae mRNAs with Chromosomally Integrated MS2 Arrays. RNA 22 (5), 657–659. 10.1261/rna.056325.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gebauer F., Schwarzl T., Valcárcel J., Hentze M. W. (2021). RNA-Binding Proteins in Human Genetic Disease. Nat. Rev. Genet. 22 (3), 185–198. 10.1038/s41576-020-00302-y [DOI] [PubMed] [Google Scholar]
- Han S., Zhao B. S., Myers S. A., Carr S. A., He C., Ting A. Y. (2020). RNA-Protein Interaction Mapping via MS2- or Cas13-Based APEX Targeting. Proc. Natl. Acad. Sci. USA 117 (36), 22068–22079. 10.1073/pnas.2006617117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi R., Wainwright S. M., Liddell S. J., Pinchin S. M., Horswell S., Ish-Horowicz D. (2014). A Genetic Screen Based on in vivo RNA Imaging Reveals Centrosome-Independent Mechanisms for Localizing Gurken Transcripts in Drosophila. G3 (Bethesda, Md.) 4 (4), 749–760. 10.1534/g3.114.010462 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinrich S., Sidler C. L., Azzalin C. M., Weis K. (2017). Stem-Loop RNA Labeling Can Affect Nuclear and Cytoplasmic mRNA Processing. RNA 23 (2), 134–141. 10.1261/rna.057786.116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hocine S., Raymond P., Zenklusen D., Chao J. A., Singer R. H. (2013). Single-Molecule Analysis of Gene Expression Using Two-Color RNA Labeling in Live Yeast. Nat. Methods 10 (2), 119–121. 10.1038/nmeth.2305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hövelmann F., Gaspar I., Ephrussi A., Seitz O. (2013). Brightness Enhanced DNA FIT-Probes for Wash-Free RNA Imaging in Tissue. J. Am. Chem. Soc. 135 (50), 19025–19032. 10.1021/ja410674h [DOI] [PubMed] [Google Scholar]
- Hu C.-D., Chinenov Y., Kerppola T. K. (2002). Visualization of Interactions Among bZIP and Rel Family Proteins in Living Cells Using Bimolecular Fluorescence Complementation. Mol. Cell 9 (4), 789–798. 10.1016/s1097-2765(02)00496-3 [DOI] [PubMed] [Google Scholar]
- Hung V., Udeshi N. D., Lam S. S., Loh K. H., Cox K. J., Pedram K., et al. (2016). Spatially Resolved Proteomic Mapping in Living Cells with the Engineered Peroxidase APEX2. Nat. Protoc. 11 (3), 456–475. 10.1038/nprot.2016.018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson S. A., McKenzie R. E., Fagerlund R. D., Kieper S. N., Fineran P. C., Brouns S. J. J. (2017). CRISPR-Cas: Adapting to Change. Science 356 (6333). 10.1126/science.aal5056 [DOI] [PubMed] [Google Scholar]
- Jolly C., Konecny L., Grady D. L., Kutskova Y. A., Cotto J. J., Morimoto R. I., et al. (2002). In Vivo binding of Active Heat Shock Transcription Factor 1 to Human Chromosome 9 Heterochromatin during Stress. J. Cell Biol. 156 (5), 775–781. 10.1083/jcb.200109018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang W. J., Cho Y. L., Chae J. R., Lee J. D., Ali B. A., Al-Khedhairy A. A., et al. (2012). Dual Optical Biosensors for Imaging microRNA-1 during Myogenesis. Biomaterials 33 (27), 6430–6437. 10.1016/j.biomaterials.2012.05.056 [DOI] [PubMed] [Google Scholar]
- Kang W. J., Cho Y. L., Chae J. R., Lee J. D., Choi K.-J., Kim S. (2011). Molecular Beacon-Based Bioimaging of Multiple microRNAs During Myogenesis. Biomaterials 32 (7), 1915–1922. 10.1016/j.biomaterials.2010.11.007 [DOI] [PubMed] [Google Scholar]
- Ke R., Mignardi M., Pacureanu A., Svedlund J., Botling J., Wählby C., et al. (2013). In Situ Sequencing for RNA Analysis in Preserved Tissue and Cells. Nat. Methods 10 (9), 857–860. 10.1038/nmeth.2563 [DOI] [PubMed] [Google Scholar]
- Kim H. J., Chung J.-K., Hwang D. W., Lee D. S., Kim S. (2009). In Vivo imaging of miR-221 Biogenesis in Papillary Thyroid Carcinoma. Mol. Imag. Biol. 11 (2), 71–78. 10.1007/s11307-008-0188-6 [DOI] [PubMed] [Google Scholar]
- Kim S., Hwang D. W., Lee D. S. (2009). A Study of microRNAs In Silico and In Vivo: Bioimaging of microRNA Biogenesis and Regulation. FEBS J. 276 (8), 2165–2174. 10.1111/j.1742-4658.2009.06935.x [DOI] [PubMed] [Google Scholar]
- Knott G. J., East-Seletsky A., Cofsky J. C., Holton J. M., Charles E., O’Connell M. R., et al. (2017). Guide-Bound Structures of an RNA-Targeting A-Cleaving CRISPR-Cas13a Enzyme. Nat. Struct. Mol. Biol. 24 (10), 825–833. 10.1038/nsmb.3466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ko H. Y., Hwang D. W., Lee D. S., Kim S. (2009). A Reporter Gene Imaging System for Monitoring microRNA Biogenesis. Nat. Protoc. 4 (11), 1663–1669. 10.1038/nprot.2009.119 [DOI] [PubMed] [Google Scholar]
- Konermann S., Lotfy P., Brideau N. J., Oki J., Shokhirev M. N., Hsu P. D. (2018). Transcriptome Engineering with RNA-Targeting Type VI-D CRISPR Effectors. Cell 173 (3), 665–676. 10.1016/j.cell.2018.02.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Makarova K. S. (2013). CRISPR-Cas. RNA Biol. 10 (5), 679–686. 10.4161/rna.24022 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koonin E. V., Makarova K. S., Zhang F. (2017). Diversity, Classification and Evolution of CRISPR-Cas Systems. Curr. Opin. Microbiol. 37, 67–78. 10.1016/j.mib.2017.05.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kummer S., Knoll A., Socher E., Bethge L., Herrmann A., Seitz O. (2011). Fluorescence Imaging of Influenza H1N1 mRNA in Living Infected Cells Using Single-Chromophore FIT-PNA. Angew. Chem. Int. Ed. 50 (8), 1931–1934. 10.1002/anie.201005902 [DOI] [PubMed] [Google Scholar]
- Lam S. S., Martell J. D., Kamer K. J., Deerinck T. J., Ellisman M. H., Mootha V. K., et al. (2015). Directed Evolution of APEX2 for Electron Microscopy and Proximity Labeling. Nat. Methods 12 (1), 51–54. 10.1038/nmeth.3179 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laprade H., Querido E., Smith M. J., Guérit D., Crimmins H., Conomos D., et al. (2020). Single-Molecule Imaging of Telomerase RNA Reveals a Recruitment-Retention Model for Telomere Elongation. Mol. Cell 79 (1), 115–126. 10.1016/j.molcel.2020.05.005 [DOI] [PubMed] [Google Scholar]
- Lee F. C. Y., Ule J. (2018). Advances in CLIP Technologies for Studies of Protein-RNA Interactions. Mol. Cell 69 (3), 354–369. 10.1016/j.molcel.2018.01.005 [DOI] [PubMed] [Google Scholar]
- Lee J., Kang H. J., Lee Y. S., Heo H., Gu H.-N., Cho S., et al. (2015a). A Self-Assembling Magnetic Resonance Beacon for the Detection of microRNA-1. Chem. Commun. 51 (33), 7199–7202. 10.1039/c4cc10231b [DOI] [PubMed] [Google Scholar]
- Lee J., Moon S., Lee Y., Ali B., Al-Khedhairy A., Ali D., et al. (2015b). Quantum Dot-Based Molecular Beacon to Monitor Intracellular microRNAs. Sensors 15 (6), 12872–12883. 10.3390/s150612872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee K., Cui Y., Lee L. P., Irudayaraj J. (2014). Quantitative Imaging of Single mRNA Splice Variants in Living Cells. Nat. Nanotech. 9 (6), 474–480. 10.1038/nnano.2014.73 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S., Kopp F., Chang T.-C., Sataluri A., Chen B., Sivakumar S., et al. (2016). Noncoding RNA NORAD Regulates Genomic Stability by Sequestering PUMILIO Proteins. Cell 164 (1-2), 69–80. 10.1016/j.cell.2015.12.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leppek K., Stoecklin G. (2014). An Optimized Streptavidin-Binding RNA Aptamer for Purification of Ribonucleoprotein Complexes Identifies Novel ARE-Binding Proteins. Nucleic Acids Res. 42 (2), e13. 10.1093/nar/gkt956 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Song J., Yi C. (2014). Genome-wide Mapping of Cellular Protein-RNA Interactions Enabled by Chemical Crosslinking. Genom. Proteom. Bioinform. 12 (2), 72–78. 10.1016/j.gpb.2014.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y., Liu S., Cao L., Luo Y., Du H., Li S., et al. (2021). CBRPP: A New RNA-Centric Method to Study RNA-Protein Interactions. RNA Biol. 18 (11), 1608–1621. 10.1080/15476286.2021.1873620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Licatalosi D. D., Mele A., Fak J. J., Ule J., Kayikci M., Chi S. W., et al. (2008). HITS-CLIP Yields Genome-Wide Insights into Brain Alternative RNA Processing. Nature 456 (7221), 464–469. 10.1038/nature07488 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin X., Fonseca M. A. S., Breunig J. J., Corona R. I., Lawrenson K. (2021). In Vivo Discovery of RNA Proximal Proteins via Proximity-Dependent Biotinylation. RNA Biol. 18 (12), 2203–2217. 10.1080/15476286.2021.1917215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionnet T., Czaplinski K., Darzacq X., Shav-Tal Y., Wells A. L., Chao J. A., et al. (2011). A Transgenic Mouse for in vivo Detection of Endogenous Labeled mRNA. Nat. methods 8 (2), 165–170. 10.1038/nmeth.1551 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu L., Li X., Ma J., Li Z., You L., Wang J., et al. (2017a). The Molecular Architecture for RNA-Guided RNA Cleavage by Cas13a. Cell 170 (4), 714–726. 10.1016/j.cell.2017.06.050 [DOI] [PubMed] [Google Scholar]
- Liu L., Li X., Wang J., Wang M., Chen P., Yin M., et al. (2017b). Two Distant Catalytic Sites are Responsible for C2c2 RNase Activities. Cell 168 (1-2), 121–134. 10.1016/j.cell.2016.12.031 [DOI] [PubMed] [Google Scholar]
- Liu Q., Zheng J., Sun W., Huo Y., Zhang L., Hao P., et al. (2018). A Proximity-Tagging System to Identify Membrane Protein-Protein Interactions. Nat. Methods 15 (9), 715–722. 10.1038/s41592-018-0100-5 [DOI] [PubMed] [Google Scholar]
- Makarova K. S., Wolf Y. I., Alkhnbashi O. S., Costa F., Shah S. A., Saunders S. J., et al. (2015). An Updated Evolutionary Classification of CRISPR-Cas Systems. Nat. Rev. Microbiol. 13 (11), 722–736. 10.1038/nrmicro3569 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makarova K. S., Wolf Y. I., Koonin E. V. (2013). The Basic Building Blocks and Evolution of CRISPR-CAS Systems. Biochem. Soc. Trans. 41 (6), 1392–1400. 10.1042/BST20130038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marraffini L. A., Sontheimer E. J. (2010). CRISPR Interference: RNA-Directed Adaptive Immunity in Bacteria and Archaea. Nat. Rev. Genet. 11 (3), 181–190. 10.1038/nrg2749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marras S. A., Kramer F. R., Tyagi S. (2002). Efficiencies of Fluorescence Resonance Energy Transfer and Contact-Mediated Quenching in Oligonucleotide Probes. Nucleic Acids Res. 30 (21), e122. 10.1093/nar/gnf121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah G., Barraud P., Allain F. H.-T. (2013). RNA Recognition by Double-Stranded RNA Binding Domains: A Matter of Shape and Sequence. Cell. Mol. Life Sci. 70 (11), 1875–1895. 10.1007/s00018-012-1119-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matia-González A. M., Iadevaia V., Gerber A. P. (2017). A Versatile Tandem RNA Isolation Procedure to Capture In Vivo Formed mRNA-Protein Complexes. Methods 118–119, 93–100. 10.1016/j.ymeth.2016.10.005 [DOI] [PubMed] [Google Scholar]
- McHugh C. A., Chen C.-K., Chow A., Surka C. F., Tran C., McDonel P., et al. (2015). The Xist lncRNA Interacts Directly with SHARP to Silence Transcription through HDAC3. Nature 521 (7551), 232–236. 10.1038/nature14443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McHugh C. A., Guttman M. (2018). RAP-MS: A Method to Identify Proteins that Interact Directly with a Specific RNA Molecule in Cells. Methods Mol. Biol. 1649, 473–488. 10.1007/978-1-4939-7213-5_31 [DOI] [PubMed] [Google Scholar]
- Mohanraju P., Makarova K. S., Zetsche B., Zhang F., Koonin E. V., van der Oost J. (2016). Diverse Evolutionary Roots and Mechanistic Variations of the CRISPR-Cas Systems. Science 353 (6299), aad5147. 10.1126/science.aad5147 [DOI] [PubMed] [Google Scholar]
- Moore M. J. (2005). From Birth to Death: The Complex Lives of Eukaryotic mRNAs. Science 309 (5740), 1514–1518. 10.1126/science.1111443 [DOI] [PubMed] [Google Scholar]
- Mukherjee J., Hermesh O., Eliscovich C., Nalpas N., Franz-Wachtel M., Maček B., et al. (2019). β-Actin mRNA Interactome Mapping by Proximity Biotinylation. Proc. Natl. Acad. Sci. USA 116 (26), 12863–12872. 10.1073/pnas.1820737116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munschauer M., Nguyen C. T., Sirokman K., Hartigan C. R., Hogstrom L., Engreitz J. M., et al. (2018). The NORAD lncRNA Assembles a Topoisomerase Complex Critical for Genome Stability. Nature 561 (7721), 132–136. 10.1038/s41586-018-0453-z [DOI] [PubMed] [Google Scholar]
- Muramoto T., Cannon D., Gierlinski M., Corrigan A., Barton G. J., Chubb J. R., et al. (2012). Live Imaging of Nascent RNA Dynamics Reveals Distinct Types of Transcriptional Pulse Regulation. PNAS 109 (19), 7350–7355. 10.1073/pnas.1117603109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murugan K., Babu K., Sundaresan R., Rajan R., Sashital D. G. (2017). The Revolution Continues: Newly Discovered Systems Expand the CRISPR-Cas Toolkit. Mol. Cell 68 (1), 15–25. 10.1016/j.molcel.2017.09.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G., Chen X. (2012). Molecular Imaging with Activatable Reporter Systems. Theranostics 2 (4), 413–423. 10.7150/thno.3940 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niu G., Chen X. (2009). Noninvasive Visualization of microRNA by Bioluminescence Imaging. Mol. Imaging Biol. 11 (2), 61–63. 10.1007/s11307-008-0190-z [DOI] [PubMed] [Google Scholar]
- O’Connell M. R. (2019). Molecular Mechanisms of RNA Targeting by Cas13-Containing Type VI CRISPR-Cas Systems. J. Mol. Biol. 431 (1), 66–87. 10.1016/j.jmb.2018.06.029 [DOI] [PubMed] [Google Scholar]
- Okamoto A. (2011). ECHO Probes: A Concept of Fluorescence Control for Practical Nucleic Acid Sensing. Chem. Soc. Rev. 40 (12), 5815–5828. 10.1039/c1cs15025a [DOI] [PubMed] [Google Scholar]
- Oomoto I., Suzuki-Hirano A., Umeshima H., Han Y.-W., Yanagisawa H., Carlton P., et al. (2015). ECHO-Live FISH: In Vivo RNA Labeling Reveals Dynamic Regulation of Nuclear RNA Foci in Living Tissues. Nucleic Acids Res. 43 (19), e126. 10.1093/nar/gkv614 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paige J. S., Wu K. Y., Jaffrey S. R. (2011). RNA Mimics of Green Fluorescent Protein. Science 333 (6042), 642–646. 10.1126/science.1207339 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira B., Billaud M., Almeida R. (2017). RNA-Binding Proteins in Cancer: Old Players and New Actors. Trends Cancer 3 (7), 506–528. 10.1016/j.trecan.2017.05.003 [DOI] [PubMed] [Google Scholar]
- Prigodich A. E., Seferos D. S., Massich M. D., Giljohann D. A., Lane B. C., Mirkin C. A. (2009). Nano-Flares for mRNA Regulation and Detection. ACS Nano 3 (8), 2147–2152. 10.1021/nn9003814 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Majzoub K., Rao D. S., Neela P. H., Zarnegar B. J., Mondal S., et al. (2018). RNA-Protein Interaction Detection in Living Cells. Nat. Methods 15 (3), 207–212. 10.1038/nmeth.4601 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramanathan M., Porter D. F., Khavari P. A. (2019). Methods to Study RNA-Protein Interactions. Nat. Methods 16 (3), 225–234. 10.1038/s41592-019-0330-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riahi R., Wang S., Long M., Li N., Chiou P.-Y., Zhang D. D., et al. (2014). Mapping Photothermally Induced Gene Expression in Living Cells and Tissues by Nanorod-Locked Nucleic Acid Complexes. ACS nano 8 (4), 3597–3605. 10.1021/nn500107g [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rino J., Martin R. M., Carvalho T., Carmo-Fonseca M. (2014). Imaging Dynamic Interactions Between Spliceosomal Proteins and Pre-mRNA in Living Cells. Methods (San Diego, Calif.) 65 (3), 359–366. 10.1016/j.ymeth.2013.08.010 [DOI] [PubMed] [Google Scholar]
- Roux K. J., Kim D. I., Raida M., Burke B. (2012). A Promiscuous Biotin Ligase Fusion Protein Identifies Proximal and Interacting Proteins in Mammalian Cells. J. Cell Biol. 196 (6), 801–810. 10.1083/jcb.201112098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferos D. S., Giljohann D. A., Hill H. D., Prigodich A. E., Mirkin C. A. (2007). Nano-Flares: Probes for Transfection and mRNA Detection in Living Cells. J. Am. Chem. Soc. 129 (50), 15477–15479. 10.1021/ja0776529 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seferos D. S., Prigodich A. E., Giljohann D. A., Patel P. C., Mirkin C. A. (2009). Polyvalent DNA Nanoparticle Conjugates Stabilize Nucleic Acids. Nano Lett. 9 (1), 308–311. 10.1021/nl802958f [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi J., Zhou M., Gong A., Li Q., Wu Q., Cheng G. J., et al. (2016). Fluorescence Lifetime Imaging of Nanoflares for mRNA Detection in Living Cells. Anal. Chem. 88 (4), 1979–1983. 10.1021/acs.analchem.5b03689 [DOI] [PubMed] [Google Scholar]
- Shin I., Ray J., Gupta V., Ilgu M., Beasley J., Bendickson L., et al. (2014). Live-Cell Imaging of Pol II Promoter Activity to Monitor Gene Expression with RNA IMAGEtag Reporters. Nucleic Acids Res. 42 (11), e90. 10.1093/nar/gku297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmakov S., Abudayyeh O. O., Makarova K. S., Wolf Y. I., Gootenberg J. S., Semenova E., et al. (2015). Discovery and Functional Characterization of Diverse Class 2 CRISPR-Cas Systems. Mol. Cell 60 (3), 385–397. 10.1016/j.molcel.2015.10.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shmakov S., Smargon A., Scott D., Cox D., Pyzocha N., Yan W., et al. (2017). Diversity and Evolution of Class 2 CRISPR-Cas Systems. Nat. Rev. Microbiol. 15 (3), 169–182. 10.1038/nrmicro.2016.184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shyu Y. J., Hu C.-D. (2008). Fluorescence Complementation: An Emerging Tool for Biological Research. Trends Biotechnol. 26 (11), 622–630. 10.1016/j.tibtech.2008.07.006 [DOI] [PubMed] [Google Scholar]
- Simon M. D., Wang C. I., Kharchenko P. V., West J. A., Chapman B. A., Alekseyenko A. A., et al. (2011). The Genomic Binding Sites of a Noncoding RNA. Proc. Natl. Acad. Sci. 108 (51), 20497–20502. 10.1073/pnas.1113536108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slaymaker I. M., Mesa P., Kellner M. J., Kannan S., Brignole E., Koob J., et al. (2021). High-Resolution Structure of cas13b and Biochemical Characterization of RNA Targeting and Cleavage. Cell Rep. 34 (10), 108865. 10.1016/j.celrep.2021.108865 [DOI] [PubMed] [Google Scholar]
- Smargon A. A., Cox D. B. T., Pyzocha N. K., Zheng K., Slaymaker I. M., Gootenberg J. S., et al. (2017). Cas13b is a Type VI-B CRISPR-Associated RNA-Guided RNase Differentially Regulated by Accessory Proteins Csx27 and Csx28. Mol. Cell 65 (4), 618–630. 10.1016/j.molcel.2016.12.023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song W., Strack R. L., Svensen N., Jaffrey S. R. (2014). Plug-and-Play Fluorophores Extend the Spectral Properties of Spinach. J. Am. Chem. Soc. 136 (4), 1198–1201. 10.1021/ja410819x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stark H., Dube P., Lührmann R., Kastner B. (2001). Arrangement of RNA and Proteins in the Spliceosomal U1 Small Nuclear Ribonucleoprotein Particle. Nature 409 (6819), 539–542. 10.1038/35054102 [DOI] [PubMed] [Google Scholar]
- Sutherland B. W., Toews J., Kast J. (2008). Utility of Formaldehyde Cross-Linking and Mass Spectrometry in the Study of Protein-Protein Interactions. J. Mass. Spectrom. 43 (6), 699–715. 10.1002/jms.1415 [DOI] [PubMed] [Google Scholar]
- Tamulaitis G., Venclovas Č., Siksnys V. (2017). Type III CRISPR-Cas Immunity: Major Differences Brushed Aside. Trends Microbiol. 25 (1), 49–61. 10.1016/j.tim.2016.09.012 [DOI] [PubMed] [Google Scholar]
- Tang T., Han Y., Wang Y., Huang H., Qian P. (2021). Programmable System of Cas13-Mediated RNA Modification and its Biological and Biomedical Applications. Front. Cell Dev. Biol. 9, 677587. 10.3389/fcell.2021.677587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tay C. Y., Yuan L., Leong D. T. (2015). Nature-Inspired DNA Nanosensor for Real-Time In Situ Detection of mRNA in Living Cells. ACS Nano 9 (5), 5609–5617. 10.1021/acsnano.5b01954 [DOI] [PubMed] [Google Scholar]
- Theimer C. A., Feigon J. (2006). Structure and Function of Telomerase RNA. Curr. Opin. Struct. Biol. 16 (3), 307–318. 10.1016/j.sbi.2006.05.005 [DOI] [PubMed] [Google Scholar]
- Tolino M., Köhrmann M., Kiebler M. A. (2012). RNA-Binding Proteins Involved in RNA Localization and Their Implications in Neuronal Diseases. Eur. J. Neurosci. 35 (12), 1818–1836. 10.1111/j.1460-9568.2012.08160.x [DOI] [PubMed] [Google Scholar]
- Tsai B. P., Wang X., Huang L., Waterman M. L. (2011). Quantitative Profiling of In Vivo-Assembled RNA-Protein Complexes Using a Novel Integrated Proteomic Approach. Mol. Cell Proteom. 10 (4), 007385. 10.1074/mcp.M110.007385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tu Y., Wan L., Zhao D., Bu L., Dong D., Yin Z., et al. (2014). In Vitro and In Vivo Direct Monitoring of miRNA-22 Expression in Isoproterenol-Induced Cardiac Hypertrophy by Bioluminescence Imaging. Eur. J. Nucl. Med. Mol. Imaging 41 (5), 972–984. 10.1007/s00259-013-2596-3 [DOI] [PubMed] [Google Scholar]
- Tutucci E., Vera M., Biswas J., Garcia J., Parker R., Singer R. H. (2018). An Improved MS2 System for Accurate Reporting of the mRNA Life Cycle. Nat. Methods 15 (1), 81–89. 10.1038/nmeth.4502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S., Alsmadi O. (2004). Imaging Native β-Actin mRNA in Motile Fibroblasts. Biophys. J. 87 (6), 4153–4162. 10.1529/biophysj.104.045153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyagi S., Kramer F. R. (1996). Molecular Beacons: Probes that Fluoresce upon Hybridization. Nat. Biotechnol. 14 (3), 303–308. 10.1038/nbt0396-303 [DOI] [PubMed] [Google Scholar]
- Urbanek M. O., Galka-Marciniak P., Olejniczak M., Krzyzosiak W. J. (2014). RNA Imaging in Living Cells - Methods and Applications. RNA Biol. 11 (8), 1083–1095. 10.4161/rna.35506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valencia‐Burton M., Broude N. E. (2007). Visualization of RNA Using Fluorescence Complementation Triggered by Aptamer‐Protein Interactions (RFAP) in Live Bacterial Cells. Curr. Protoc. Cell Biol. 37, 11. 10.1002/0471143030.cb1711s37 [DOI] [PubMed] [Google Scholar]
- van Gemert A. M. C., van der Laan A. M. A., Pilgram G. S. K., Fradkin L. G., Noordermeer J. N., Tanke H. J. (2009). In vivo Monitoring of mRNA Movement in Drosophila Body Wall Muscle Cells Reveals the Presence of Myofiber Domains. PloS one 4 (8), e6663. 10.1371/journal.pone.0006663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura A. (2016). NORAD: Defender of the Genome. Trends Genet. 32 (7), 390–392. 10.1016/j.tig.2016.04.002 [DOI] [PubMed] [Google Scholar]
- Wang F., Song X., Li X., Xin J., Wang S., Yang W., et al. (2013). Noninvasive Visualization of microRNA-16 in the Chemoresistance of Gastric Cancer Using a Dual Reporter Gene Imaging System. PloS one 8 (4), e61792. 10.1371/journal.pone.0061792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Zhang B., Zhou L., Shi Y., Li Z., Xia Y., et al. (2016). Imaging Dendrimer-Grafted Graphene Oxide Mediated Anti-miR-21 Delivery With an Activatable Luciferase Reporter. ACS Appl. Mater. Inter. 8 (14), 9014–9021. 10.1021/acsami.6b02662 [DOI] [PubMed] [Google Scholar]
- Wang H., Nakamura M., Abbott T. R., Zhao D., Luo K., Yu C., et al. (2019). CRISPR-Mediated Live Imaging of Genome Editing and Transcription. Science 365 (6459), 1301–1305. 10.1126/science.aax7852 [DOI] [PubMed] [Google Scholar]
- Wang S., Riahi R., Li N., Zhang D. D., Wong P. K. (2015). Single Cell Nanobiosensors for Dynamic Gene Expression Profiling in Native Tissue Microenvironments. Adv. Mater. 27 (39), 6034–6038. 10.1002/adma.201502814 [DOI] [PubMed] [Google Scholar]
- Wang Z., Zhang K., Shen Y., Smith J., Bloch S., Achilefu S., et al. (2013). Imaging mRNA Expression Levels in Living Cells with PNA·DNA Binary FRET Probes Delivered by Cationic Shell-Crosslinked Nanoparticles. Org. Biomol. Chem. 11 (19), 3159–3167. 10.1039/c3ob26923j [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissinger R., Heinold L., Akram S., Jansen R.-P., Hermesh O. (2021). RNA Proximity Labeling: A New Detection Tool for RNA-Protein Interactions. Molecules 26 (8), 2270. 10.3390/molecules26082270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J. A., Davis C. P., Sunwoo H., Simon M. D., Sadreyev R. I., Wang P. I., et al. (2014). The Long Noncoding RNAs NEAT1 and MALAT1 Bind Active Chromatin Sites. Mol. Cell 55 (5), 791–802. 10.1016/j.molcel.2014.07.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu B., Chen J., Singer R. H. (2014). Background Free Imaging of Single mRNAs in Live Cells Using Split Fluorescent Proteins. Sci. Rep. 4, 3615. 10.1038/srep03615 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wutz A. (2011). Gene Silencing in X-Chromosome Inactivation: Advances in Understanding Facultative Heterochromatin Formation. Nat. Rev. Genet. 12 (8), 542–553. 10.1038/nrg3035 [DOI] [PubMed] [Google Scholar]
- Xia Y., Zhang R., Wang Z., Tian J., Chen X. (2017). Recent Advances in High-Performance Fluorescent and Bioluminescent RNA Imaging Probes. Chem. Soc. Rev. 46 (10), 2824–2843. 10.1039/c6cs00675b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu C., Zhou Y., Xiao Q., He B., Geng G., Wang Z., et al. (2021). Programmable RNA Editing with Compact CRISPR-Cas13 Systems from Uncultivated Microbes. Nat. Methods 18 (5), 499–506. 10.1038/s41592-021-01124-4 [DOI] [PubMed] [Google Scholar]
- Yamada T., Yoshimura H., Inaguma A., Ozawa T. (2011). Visualization of Nonengineered Single mRNAs in Living Cells Using Genetically Encoded Fluorescent Probes. Anal. Chem. 83 (14), 5708–5714. 10.1021/ac2009405 [DOI] [PubMed] [Google Scholar]
- Yan W. X., Chong S., Zhang H., Makarova K. S., Koonin E. V., Cheng D. R., et al. (2018). Cas13d is a Compact RNA-Targeting Type VI CRISPR Effector Positively Modulated by a WYL-Domain-Containing Accessory Protein. Mol. Cell 70 (2), 327–339. 10.1016/j.molcel.2018.02.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang L.-Z., Wang Y., Li S.-Q., Yao R.-W., Luan P.-F., Wu H., et al. (2019). Dynamic Imaging of RNA in Living Cells by CRISPR-Cas13 Systems. Mol. Cell 76 (6), 981–997. 10.1016/j.molcel.2019.10.024 [DOI] [PubMed] [Google Scholar]
- Yi W., Li J., Zhu X., Wang X., Fan L., Sun W., et al. (2020). CRISPR-Assisted Detection of RNA-Protein Interactions in Living Cells. Nat. Methods 17 (7), 685–688. 10.1038/s41592-020-0866-0 [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Inaguma A., Yamada T., Ozawa T. (2012). Fluorescent Probes for Imaging Endogenous β‐actin mRNA in Living Cells Using Fluorescent Protein-Tagged Pumilio. ACS Chem. Biol. 7 (6). 10.1021/cb200474a [DOI] [PubMed] [Google Scholar]
- Yoshimura H., Ozawa T. (2016). Monitoring of RNA Dynamics in Living Cells Using PUM-HD and Fluorescent Protein Reconstitution Technique. Methods Enzymol. 572, 65–85. 10.1016/bs.mie.2016.03.018 [DOI] [PubMed] [Google Scholar]
- Zeng F., Peritz T., Kannanayakal T. J., Kilk K., Eiríksdóttir E., Langel U., et al. (2006). A Protocol for PAIR: PNA-Assisted Identification of RNA Binding Proteins in Living Cells. Nat. Protoc. 1 (2), 920–927. 10.1038/nprot.2006.81 [DOI] [PubMed] [Google Scholar]
- Zhang C., Konermann S., Brideau N. J., Lotfy P., Wu X., Novick S. J., et al. (2018). Structural Basis for the RNA-Guided Ribonuclease Activity of CRISPR-Cas13d. Cell 175 (1), 212–223. 10.1016/j.cell.2018.09.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z., Sun W., Shi T., Lu P., Zhuang M., Liu J.-L. (2020). Capturing RNA-Protein Interaction via CRUIS. Nucleic Acids Res. 48 (9), e52. 10.1093/nar/gkaa143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Cho S., Moon H., Loh T. J., Jang H. N., Shen H. (2016). Detecting RNA-Protein Interaction Using End-Labeled Biotinylated RNA Oligonucleotides and Immunoblotting. Methods Mol. Biol. 1421, 35–44. 10.1007/978-1-4939-3591-8_4 [DOI] [PubMed] [Google Scholar]
- Zimyanin V. L., Belaya K., Pecreaux J., Gilchrist M. J., Clark A., Davis I., et al. (2008). In vivo Imaging of Oskar mRNA Transport Reveals the Mechanism of Posterior Localization. cell 134 (5), 843–853. 10.1016/j.cell.2008.06.053 [DOI] [PMC free article] [PubMed] [Google Scholar]