Abstract
The review briefly summarizes the role of the family of adhesion molecules, JAMs (junctional adhesion molecules), in various cell migration, covering germ cells, epithelial cells, endothelial cells, several leukocytes, and different cancer cells. These functions affect multiple diseases, including reproductive diseases, inflammation-related diseases, cardiovascular diseases, and cancers. JAMs bind to both similar and dissimilar proteins and take both similar and dissimilar effects on different cells. Concluding relevant results provides a reference to further research.
Keywords: junctional adhesion molecules, cell migration, germ cell, epithelial cells, endothelial cells, leukocytes, cancer cells
Introduction
Cell migration plays a pivotal role in tissue organization during development, and several diseases develop due to its dysregulation. The migration properties are partly dictated by cell adhesion and its endocytic regulation, and scaffold cell-dependent migration could be represented among various types of migration (Kawauchi 2012). It is critical to maintain junctional integrity during cell migration and cell extrusion through classic cell–cell junctions member protein such as ZO-1 (Garcia et al., 2018). Transmembrane and associated cytoplasmic proteins in tight junctions show dynamic behavior, including migration within the junction and exchange in and out of the junctions (Chalmers and Whitley 2012). In addition, internalized tight junctions (TJs) proteins are recycled to the plasma membrane or sorted to late endosomes and degradation (Stamatovic et al., 2017).
Junctional adhesion molecule (JAM) is a member of the immunoglobulin superfamily localized at the tight junction of polarized cells and on the cell surface of leukocytes (Ebnet et al., 2004). Several members of the family mediate cell polarity, endothelium permeability, and leukocytes migration through a multitude of homophilic and heterophilic interactions with intrafamily and extrafamily partners (Garrido-Urbani et al., 2014). Several members of the JAM family interact with PDZ domain-containing scaffolding proteins such as ZO-1, claudin, and afadin, regulating cell–cell contact maturation and the generation of junctional complexes such as TJs and adherens junctions (AJs) (Keiper et al., 2005a; Ebnet 2017; Hartmann et al., 2020). In the early years, there existed some inconsistencies in the JAM nomenclature. In this review, we quote the original description of these articles and specific information referring to the review of Mandell and Parkos (Mandell and Parkos 2005).
Germ Cell Motility, Polarization, and Maturation
Germ cells (GCs) migrate spatially distinct locations for proper development with various patterns; nevertheless, the reason and cellular mechanisms that facilitate germ cells motility and guide migration in vivo remain unclear (Kanamori et al., 2019; Grimaldi and Raz 2020). The adhesive interaction between germ and Sertoli cells (SCs) regulates spermatogenesis (Cartier-Michaud et al., 2017).
Among all members and family-related molecules, JAM-C could be the most striking protein modulating germ cell activities. JAM-C localizes to germ/Sertoli cell contacts and participates in acrosome formation and germ cell polarity, specifically round spermatids. Intriguingly, JAM-C is restricted to the apical ectoplasmic specialization (ES) but not at the blood–testis barrier (BTB) in mouse testes. Par6, Cdc42, PKCl, and PATJ are identified as the downstream of engaged JAM-C protein, mediating spermatid polarization in mice and possibly in humans together (Gliki et al., 2004). Furthermore, Par6 formed a stable complex with Pals1 and JAM-C in normal testes, and the tight association of the Par6/Pals1 complex with Src kinase rendered a loss of association of the Par6/Pals1 complex with JAM-C, thereby destabilizing apical ES to facilitate spermatid loss (Wong et al., 2008). Additionally, RA175 formed a ternary complex with JAM-C via interaction with Par-3, which may take effect when the specialized adhesion structures of elongating spermatid form (Fujita et al., 2007). Another demonstrated partner of JAM-C is CAR, and they both are components of apical ES involved in spermatid orientation, facilitating cell movement and orientation in the seminiferous epithelium (Mirza et al., 2006; Yan et al., 2007). Unfortunately, another research instead implied that JAM-C controlled germ cell differentiation without reference to CAR and the interaction between CAR and JAML, meanwhile, appears not to confer transepithelial migration of cells in the BTB (Sultana et al., 2014). Jam-C-deficient males are infertile and fail to produce mature sperm cells with about 50% smaller testes and lacks differentiated elongated spermatids (Gliki et al., 2004). Similarly, Spo11(Cre) mice crossed with floxed JAM-C mice to produce conditional knockouts and showed a strong reduction of JAM-C protein levels in the testis and a spermiogenetic arrest (Pellegrini et al., 2011).
JAM-A is present in the prostate and seminal vesicles and all three regions of the epididymis. JAM-A is located at both Sertoli and a subset of basal GCs, specifically in inter-Sertoli cell junctions and the tails of elongated spermatids within the epididymis of rodents, secreted in epididymosomes in the luminal fluid, and delivered to sperm in vitro (Shao et al., 2008; Wu et al., 2017). When the Sertoli cell tight junction was perturbed in vitro, BTB-associated proteins JAM-A disappear from the cell–cell interface (Xia et al., 2007). At the time of germ cell migration across the BTB during spermatogenesis, the TJ- and AJ-integral membrane proteins (including JAM-1) can be disengaged to facilitate AJ restructuring, accommodating germ cell migration while maintaining the BTB integrity (Yan and Cheng 2005). JAM-A expressed in premeiotic GCs facilitates GC migration through the BTB and then disappears in most GCs resident in the adluminal compartment, and CAR has mechanisms similar to localization and involvement in GC migration (Wang and Cheng 2007; Wang et al., 2007; Tarulli et al., 2008). JAM-A reactivity declined in Sertoli cells from tubules with testicular carcinoma in situ (CIS) and emerged to be strong in seminoma (Tarulli et al., 2013). sGCb1 plays an important role in the restructuring of the adherent junction in the testis, which seems associated with JAM-A (Sarkar et al., 2006). The presence of TGF-β3 and TNFα enhanced the kinetics of endocytosis of JAM-A from the Sertoli cell surface, and TGF-β3 disrupted JAM-A-based TJ fibrils (Xia et al., 2009). More association between P-glycoprotein (P-gp, MDR1) and JAM-A possibly enhanced BTB function (Su et al., 2009). In sperm, Ca2+ homeostasis is sustained by the relative ratios of CASK–PMCA4b and CASK–JAM-A interactions, as JAM-A positively regulates PMCA4b by sequestering CASK under conditions of elevated Ca2+ (Aravindan et al., 2012). Multiple stimuli have been shown to affect JAM-A function. Cyproterone acetate (CPA) could somehow reduce the kinetics of internalization of JAM-A, whose maintenance impliedly requires a very low level of endogenous testosterone (Yan et al., 2008). C-type natriuretic peptide (CNP) produced by Sertoli and germ cells into the BTB microenvironment accelerates endocytosis of JAM-A and opens the BTB transiently to encourage preleptotene spermatocyte migration (Xia et al., 2007). Excess iodine causes loss of spermatogenesis by disrupting the blood–testis barrier and cytoskeleton, with reduced expression of JAM-A in blood–testis barrier proteins (Chakraborty et al., 2020). The commercial polychlorinated biphenyls mixture, Aroclor1254 treatment, could induce increments in JAM-A endocytosis and occludin ubiquitination in primary cultured SCs and rats (Jia et al., 2017). In addition, oxidative damage participates in heat stress-induced downregulation of tight junction proteins in Sertoli cells by inhibiting the CaMKKβ–AMPK axis in boars, which was reversed by N-acetyl-l-cysteine (NAC) (Yang et al., 2020). IL-6 treatment delayed the kinetics of JAM-A, leading to accumulation in Sertoli cells (Zhang et al., 2014). Presently, JAM-A has been regarded as typical BTB-associated integral membrane proteins and several other related proteins mainly including tight junction, ectoplasmic specialization, and adherens junction proteins.
In the mammalian testis, JAM-B occurs in the blood–testis barrier between Sertoli cells and the apical ectoplasmic specializations between Sertoli and germ cells, promoting the transit of developing germ cells across the blood–testis barrier and the timely release of mature spermatids at stage VIII. In MSC-1 cells, the binding of various transcription factors to various cis-acting elements-binding motifs regulates the constitutive expression of JAM-B (Wang and Lui 2009). IL-1alpha promotes JAM-B expression by facilitating the binding of Elk-1 to TG-interacting factor (TGIF) and proximal Sp1 (pSp1) + E2F motifs in a p38-dependent manner, leading to an additive effect on Sp1-and neuron-restrictive silencer factor (NRSF)-mediated JAM-B transactivation (Wang and Lui 2009). Rather, TGF-beta2 inhibits JAM-B transcription via the activation of mothers against decapentaplegic (Smad) proteins, and activated Smads compete with specificity proteins (Sp1 and Sp3) for the TGIF motif, causing JAM-B repression (Wang and Lui 2009). Transforming growth factor-β3 regulates cell junction restructuring via Smad-dependent protein degradation of JAM-B (Zhang and Lui 2015). JAM-A and JAM-B are localized at the BTB, and JAM-C on spermatids interact with JAM-B on the Sertoli cell to foster the morphological polarization of round spermatids to elongated spermatids (Ebnet 2017). Graspin inhibited the PDZ-mediated interactions of GRASP55 with JAMs, resulting in hampered polarized localization of JAM-C in spermatids, the premature release of spermatids, and the affected Golgi morphology of meiotic spermatocytes (Cartier-Michaud et al., 2017). Another member JAM4 protein could function as a cell adhesion molecule rather than a tight-junction protein in the testis during BTB formation; generally, JAM might participate in homophilic cell adhesion between spermatogonia–spermatogonia, spermatogonia–Sertoli cells, and Sertoli cells–Sertoli cells (Nagamatsu et al., 2006).
Germ cells have impressive and special migration characteristics. In adult rat testes, the blood–testis barrier, TJs between Sertoli cells (Byers et al., 1991), in the seminiferous epithelium must “open” (or “disassemble”) to accommodate the migration of preleptotene spermatocytes from the basal to the adluminal compartment that occurs at stage VIII of the epithelial cycle (Xia et al., 2007). To recap, transepithelial migration of male germ cells across the BTB regulates sperm motility and spermatid differentiation, and JAMs have an exact function to regulate it. The roles of JAMs in germ cell motility, polarization, and maturation have been concluded in Table 1.
TABLE 1.
JAMs in germ cell motility, polarization, and maturation.
| Protein | Location | Partner | Final effect |
|---|---|---|---|
| JAM-A | Sertoli cell and a subset of basal GCs | P-glycoprotein | Maintains the BTB integrity |
| Su et al. (2009) | |||
| CASK | |||
| Aravindan et al. (2012) | |||
| JAM-B | BTB between Sertoli and germ cells | JAM-C on spermatids | Promotes germ cells to transit across the BTB |
| Ebnet (2017) | Wang and Lui (2009) | ||
| JAM-C | Germ/Sertoli cell | Par6/Pals | Mediates spermatid polarization and differentiation and produces mature sperm cells |
| Wong et al. (2008) | |||
| Par-3 | |||
| Fujita et al. (2007) | |||
| CAR | |||
| Sultana et al. (2014) |
Epithelial and Epidermis Barrier
Keratinocytes, the predominant cell type of the epidermis, migrate to bring tissue reepithelialization and reinstate the epithelial barrier during wound healing (Garcia et al., 2018; Holt et al., 2021). JAM-A is expressed in epidermis two-folds higher than that in full-thickness skin predominantly located at the cell–cell interface in the epidermis (Wang et al., 2018). JAM-A knockdown promotes keratinocyte proliferation and migration to improve the skin healing process in vivo, regulated by the signaling of FAK-mediated Erk1/2 activation (Wang et al., 2018). Bioactive glass (BG) extracts a posttranscriptional regulation mechanism on the expression of JAM-A to assemble into TJs located along the edge of the cell membrane, involved in mediating the enhanced barrier function of the keratinocyte monolayers (Tang et al., 2021).
JAM-A cis-homodimers encourage the formation of a complex with afadin and PDZ-GEF2 to enhance cell migration by activating the small GTPase Rap1A, whose active levels are decreased by JAM-A depletion or overexpression of cis-dimerization mutants (Mandell et al., 2005; Severson et al., 2008; Severson and Parkos 2009a; Severson and Parkos 2009b; Severson et al., 2009; Luissint et al., 2014). Additionally, Rap1 activity activation requires N-glycosylation of JAM-A, reinforcing barrier function, as glycosylation of N185 is required for JAM-A-mediated reduction of cell migration (Scott et al., 2015). Interestingly, trans-null but not cis-null JAM-A mutant expression decreased Rap2 activity, which implies trans-dimerization of JAM-A as a barrier-inducing molecular switch (Monteiro et al., 2014). A functional complex comprising JAM-A, α3β1 integrin, and tetraspanins CD151 and CD9 regulated collective cell migration of polarized epithelial cells (Thölmann et al., 2022). JAM-A protein is found at the leading edge of repairing corneal epithelial wounds in wild-type mice, while corneal epithelial wound repair was qualitatively normal, but corneal epithelium cells are irregularly shaped in JAM-A null animals (Kang et al., 2007). JAM-A deletion worsened intestinal hyperpermeability and therefore increases intestinal epithelial migration (Meng et al., 2019). Similarly, in oral epithelial cells, ligation of CD24 induces a c-Src kinase-dependent decrease in paracellular permeability mediated by JAM-A and other tight junction proteins, which also affects migrating epithelium of the periodontitis lesion (Ye et al., 2011). The increase in JAM-A expression following Ykt6 knockdown drives prostate epithelial cell motility by stimulating Rap1 and Rac1 small GTPases, regulated by miR-145 (Naydenov et al., 2018).
Alternatively, JAM-C regulates epithelial cell migration at the level of β1 integrin activity but not integrin expression (Mandicourt et al., 2007; Ebnet 2017). JAM-C distributed nonclassically in the apical membranes of Müller cells and retinal pigment epithelial (RPE) (Daniele et al., 2007; Economopoulou et al., 2009), and JAM-C knockdown inhibits human RPE cell migration but not proliferation and decreases the permeability of monolayer hRPE (Hou et al., 2012). JAM3 is expressed in multiciliated cells (MCCs) in the airway epithelium, and JAM3 lacking causes a delay in BB assembly/positioning during MCC differentiation (Mateos-Quiros et al., 2021).
Moreover, JAM-A was often observed for aberrant cytoplasmic expression in diseased gingival tissues and more expression within the leukocytes in disease-associated epithelia (Choi et al., 2014). γδ T cells present epithelial tissue bridge innate and adaptive immunity. More interestingly, the costimulation of JAML with its endogenous ligand CAR or by binding to the stimulatory antibody HL4E10 activates epithelial γδ T cells, leading to cellular proliferation, migration, and adhesion (Witherden et al., 2010; Verdino et al., 2011; Johnson et al., 2021).
Overall, JAMs regulate the migration process of multiple epithelial cells in different but connected ways, whose mechanisms remain to be explored. Conspicuously, JAMs play striking roles in cancer invasion and metastasis as discussed in the following chapter. The roles of JAMs in the epithelial and epidermis barrier have been concluded in Table 2.
TABLE 2.
JAMs in the epithelial and epidermis barrier.
| Protein | Cell type | Partner | Final effect |
|---|---|---|---|
| JAM-A | Epidermis, intestinal epithelial cells, oral epithelial cells, prostate epithelial cells, and MDCK cells | Afadin and PDZ | Inhibits cell migration and induces permeability |
| Severson et al. (2009) | (Tang et al. (2021); | ||
| α3β1 integrin | Meng et al. (2019); | ||
| Thölmann et al. (2022) | Ye et al. (2011); | ||
| Naydenov et al. (2018)) | |||
| Reduces collective cell motility | |||
| (Thölmann et al. (2022)) | |||
| JAM-C | Human RPE cell | β1 integrin | Inhibits migration but not proliferation and decreases the permeability |
| Hou et al. (2012) | (Hou et al. (2012)) | ||
| JAML | Epithelial γδ T cell | CAR | Promotes proliferation, migration, and adhesion |
| Witherden et al. (2010) | (Witherden et al. (2010); | ||
| Verdino et al. (2011); | |||
| Johnson et al. (2021)) |
Endothelial and Endothelial Barrier
JAM-A regulates cell migration through changes in directional persistence under shear flow by cooperating with microtubule-stabilizing pathways in endothelial cells (ECs) (Huang et al., 2006; Severson and Parkos 2009a). Soluble JAM-A blocked cultured endothelial cells migration (Koenen et al., 2009). Signaling through JAM-A is necessary for alpha(v)beta(3)-dependent HUVEC migration, and this effect could be increased by engagement of the ligand-binding site of the integrin by Arg-Gly-Asp-Ser (RGDS) peptide and blocked by phosphoinositide 3-kinase and protein kinase C inhibitors (Naik and Naik 2006). The ternary JAM-A-CD9-αvβ3 integrin complex releases JAM-A upon bFGF stimulation to activate ERK and to regulate endothelial cell migration on vitronectin (Naik et al., 2003; Parise 2003; Cooke et al., 2006; Peddibhotla et al., 2013). Similar to its role in the epithelium, JAM-A dimerization works in close cytoplasmic apposition of complexes containing specific PDZ domain-containing scaffold proteins, which activates small G protein Rap1 to stabilize β1 integrin protein and promotes endothelial cell migration (Severson and Parkos 2009b). Furthermore, N-glycosylation of JAM-A contributes to Rap1 activity, and glycosylation of N185 is required for JAM-A-mediated reduction of cell migration (Scott et al., 2015). ZO-1 and JAM-A assemble into a cooperative unit and then induce the formation of actin/myosin II stress fibers and redistribution of vinculin and PAK2 from adherens junction to focal adhesions in primary EC, regulating endothelial cell migration and angiogenic potential (Tornavaca et al., 2015). Tight junction protein (JAM-A and ZO-1) expression suppressed by rosiglitazone, which is linked to promote endothelial cell migration and induced permeability resulting from rosiglitazone (Ku et al., 2017). miR-145-rich exosomes can inhibit the migration of HUVECs via targeting JAM-A (Yang et al., 2021). The inhibitory effects on cell migration of human retinal capillary endothelial cells (HRCECs) induced by high concentrations of glucose were reversed once the expression of secreted protein acidic and rich in cysteine (SPARC) was inhibited, possibly associated with increased expression of JAM1 (Fu et al., 2019). Incidentally, JAM-A is a prerequisite for inflamed SMCs migration (Azari et al., 2010).
Consistently, soluble matrix-bound forms of JAM-C could be instrumental in guiding migration and adhesion of hematopoietic cells and vascular endothelial cells to the limbal niche, but further studies are warranted to investigate the precise role of JAMs and other IgCAMs in the human limbus (Polisetti et al., 2016). sJAM-C stimulates human microvascular endothelial cell (HMVEC) migration in vitro, dependent on Src, p38, and PI3K (Rabquer et al., 2010). The ubiquitylation of JAM-C by the E3 ligase Casitas B-lineage lymphoma (CBL) and dynamic JAM-C trafficking and degradation are necessary for junctional remodeling during cell migration (Kostelnik et al., 2019). Over-expressed or hypoxia-induced miR-212/132 led to a downregulation of JAM-C in human brain microvascular endothelial cells (BMECs) and resulted in slower migration of BMECs (Burek et al., 2019). JAM-C, expressed by the tumor endothelium, is obligated to transvascular migration of embryonic-endothelial progenitor cells (e-EPCs) (Czabanka et al., 2020).
To conclude, various types of endothelial cells migrate with the regulation of JAMs related to diverse diseases. JAMs mediate a variety of immune cells. Transendothelial migration is another predominant function, and this review will discuss it in the next chapter.
Immune Cells
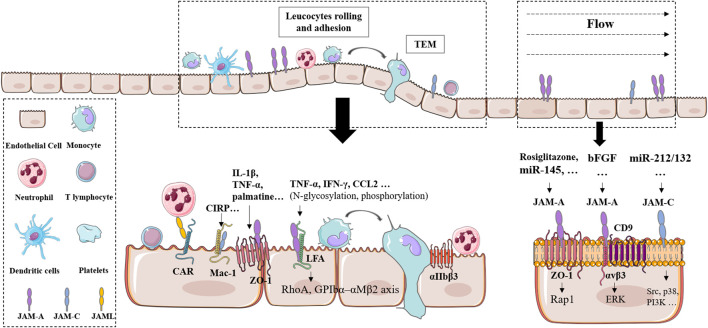
JAMs governing various types of immune cell migration has been a project under the limelight, especially their function in transendothelial migration (TEM) and transepithelial migration (TEpM). The first and second immunoglobulin domains of JAM-A and the I domain of leukocyte function-associated antigen-1 (LFA-1) support the interaction of JAM-A snd LFA-1, which destabilizes the JAM-A homophilic interaction to promote LFA-1-dependent transendothelial migration of T cells and neutrophils (Ostermann et al., 2002; Fraemohs et al., 2004; Wojcikiewicz et al., 2009). N-glycosylation of JAM-A turned out to regulate leukocyte LFA-1 binding (Scott et al., 2015). Furthermore, phosphorylation of JAM-A at Ser-284 activated RhoA to facilitate leukocyte TEM through interactions with the integrin LFA-1, dependent on PI3K-mediated activation of GEF-H1 and p115 RhoGEF (Scott et al., 2016). JAM-A antagonist peptide (JAM-Ap) blocked the interaction of JAM-A with LFA on neutrophils and monocytes/macrophages and attenuated brain ischemia/reperfusion (I/R)-induced neutrophil and monocyte infiltration into the brain parenchyma (Sladojevic et al., 2014). Meanwhile, pro-inflammatory cytokines such as TNF-alpha and IFN-gamma induced JAM redistribution and might further promote TEM of leukocytes (Ozaki et al., 1999). Apart from the typical partnership with LFA-1, JAMs instruct TEM through other mechanisms as follows:
Stem and Progenitor Cells
Wu et al. found that the JAM-A overexpression MSCs (JAM-A(ov) MSCs) migrated into the hair follicle (HF) sheath, and JAM-A promoted MSC proliferation and migration by activating T-cell lymphoma invasion and metastasis 1 (Tiam1) (Wu et al., 2014; Wu et al., 2015). Moreover, a JAM-C-blocking monoclonal antibody induces HSPC mobilization in a JAM-B dependent manner (Arcangeli et al., 2014).
Neutrophil (PMN)
JAM-A mediated neutrophil migration through the endothelium, which is dependent on IL-1β stimulus and only required endothelial-cell JAM-A and not leukocyte JAM-A (Woodfin et al., 2007; Cera et al., 2009). Furthermore, JAM-A mediates TNF-alpha-induced neutrophil transmigration by activating leukocytes and endothelial cells in vivo (Woodfin et al., 2009). JAM-A exclusively secreted from cardiac progenitor cells (CPCs) inhibited the transmigration across inflamed endothelium into the myocardium and affected complement factor 5 (C5aR)-dependent function, reducing oxidative stress and inflammatory response after infarction (Mueller et al., 2013; Liu et al., 2014). JAM-A activity promotes migration of PMNs into the alveolar space, also relevant to increased oxidative stress (Lakshmi et al., 2012). Vasodilator-stimulated phosphoprotein (VASP) is colocalized with ZO-1, occludin, and JAM-1 and may favor PMN transmigration (Comerford et al., 2002). Antihuman JAM mAbs and high-titer polyclonal mouse antiserum generated against recombinant JAM seem to show no functional effect on TEpM, TEpM in the reverse direction, and PMN transmigration across human microvascular endothelial cell monolayers (Liu et al., 2000). Khandoga et al. first identified JAM-A as an endothelial receptor of neutrophil transmigration (Khandoga et al., 2005). PMN infiltration elevated, and the recruitment of leukocytes enhanced in the colonic mucosa of Jam-A −/− mice (Laukoetter et al., 2007). In uterine, mucosal epithelial cells stimulated with LPS and palmatine downregulated expression of JAM1 and could therefore facilitate the TEpM of leukocytes residing in the endometrium, such as neutrophils and macrophages (Hui et al., 2020). Distinguishing from JAM-A, PECAM-1 mediates migration through the endothelial-cell basement membrane when leukocyte PECAM-1 and endothelial-cell PECAM-1 to the same extent regulated via upregulated integrins α6β1 (Dangerfield et al., 2002; Woodfin et al., 2007). The Parkos team discovered that PMN migration into the colonic lumen was reduced in Jam-A −/− mice and Villin-Cre; Jam-A fl/fl mice, along with reduced peritoneal PMN migration in Villin-Cre; and Jam-a fl/fl mice (Flemming et al., 2018; Luissint et al., 2019; Boerner et al., 2021).
JAM-C mAbs and JAM-C/Fc chimeras significantly inhibited neutrophil transmigration, connected with the specific binding of JAM-C to the leukocyte β2-integrin Mac-1 (αMβ2, CD11b/CD18) (Zen et al., 2004). Furthermore, LFA-1/Mac-1-JAM-C bonds can accelerate PMN crawling under high shear stress (Li G. C et al., 2018). Orlova et al. discovered that soluble JAM-C decreased leukocytes TEM and endothelial permeability by modulating VE-cadherin-mediated cell–cell contacts, working together with inhibition of Mac-1 (Chavakis et al., 2004; Orlova et al., 2006). However, Sircar et al. reported that JAM-C had a minimal role in neutrophil transmigration under shear flow conditions in vitro (Sircar et al., 2007). PMN TEM plays a considerable role in various inflammatory diseases. Transgenic mice overexpressing JAM-C under the control of the endothelial-specific promotor Tie2 showed increased leukocyte adhesion and transmigration to inflammatory sites (Aurrand-Lions et al., 2005). Soluble mouse JAM-C reduced neutrophil emigration in the mouse with acute thioglycollate-induced peritonitis and selectively reduced neutrophil infiltration into inflamed joints (Chavakis et al., 2004; Palmer et al., 2007). Cold-inducible RNA-binding protein (CIRP) induces neutrophil reverse transendothelial migration (rTEM) in sepsis by increasing neutrophil elastase (NE) and decreasing JAM-C (Jin et al., 2019). Blockade of JAM-C reduced the aged pro-inflammatory neutrophils in sepsis-induced acute lung injury (ALI), and JAM-C downregulation may contribute to acute pancreatitis (AP)-associated ALI via promoting neutrophil rTEM (Wu et al., 2016; Hirano et al., 2018). NE local proteolytically cleaved EC JAM-C via Mac-1 and therefore drove the lipid chemoattractant leukotriene B4 (LTB4) causing loss of venular JAM-C and promoting neutrophil reverse transendothelial cell migration (rTEM) in vivo (Colom et al., 2015). JAM-C was regarded as a negative regulator of rTEM under conditions of ischemia–reperfusion (I-R) and cisplatin-induced acute kidney injury (AKI) (Woodfin et al., 2011; Cho et al., 2017; Kim et al., 2017).
Zinc metalloproteases cleaved JAML from the neutrophil surface during PMN TEpM, and fusion proteins containing JAML and CAR extracellular domains and antibodies against JAML and CAR inhibited TEpM (Zen et al., 2005; Weber et al., 2014). Zen et al. proposed a revised model of PMN TEpM: sequential Mac-1-mediated binding to JAM-C at desmosomes when PMN cross the TJ, followed by JAML binding to CAR (Zen et al., 2005).
Monocytes and Macrophages
A JAM-A mAb, BV11, was able to block human monocyte migration across bEND-3 cell monolayers in vitro and in the skin inflammatory and meningitis model in vivo (Martìn-Padura et al., 1998; Del Maschio et al., 1999; Williams et al., 1999). Nevertheless, both a monoclonal antibody and polyclonal rabbit IgG to JAM-A decreased slightly in monocyte transmigration (Liu et al., 2000; Schenkel et al., 2004). Activation of lung vascular endothelial ADAM17 and ADAM10 markedly promotes ectodomain shedding of JAM-A to enhance total leukocyte and neutrophil recruitment by LFA-1- and JAM-A-dependent mechanisms (Koenen et al., 2009; Dreymueller et al., 2012). Meanwhile, matrix metalloproteinase (MMP) contributes to the HIV-induced decreased expression of JAM-A and occludin, associated with elevated TEM of HIV-infected monocytes across an in vitro model of the blood–brain barrier (BBB) (Huang et al., 2009). Except for those functions of metalloproteinase in the previous content, Williams et al. found that CD14+CD16+ monocytes selectively transmigrated across the BBB model due to increased JAM-A and ALCAM expression in HIV-infected individuals (Williams et al., 2013; Williams et al., 2015). HIV+ CD14+CD16+ ART-treated monocytes (mature monocytes infected with HIV and treated with ART) preferentially transmigrate across the BBB to CCL2, which was reduced and/or blocked by blocking antibodies against junctional proteins JAM-A significantly (León-Rivera et al., 2021). In addition, buprenorphine limits the chemokine (C–C motif) ligand 2 (CCL2)-mediated monocyte transmigration into the central nervous system (CNS), through decreasing the phosphorylation of the junctional protein JAM-A increase (Carvallo et al., 2015). Also, this process probably links to CCL2-induced JAM-A redistribution via RhoA and Rho kinase (Stamatovic et al., 2012). JAM-1 is necessary for cellular interactions during β2-integrin-dependent leukocyte adhesion and transmigration on the inflammatory endothelium (Chavakis et al., 2003). It was found earlier that monocyte arrest and transmigration attenuated on activated Jam-A −/− ApoE −/− versus Jam-A +/+ ApoE −/− endothelial cells under flow conditions in vitro (Zernecke et al., 2006). Then, Schmitt et al. found an endothelium-specific deficiency in JAM-A reduced mononuclear cell recruitment into the arterial wall, whereas somatic deficiency in JAM-A revealed no significant effects, accompanying endothelial JAM-A increased by oxidized low-density lipoprotein (oxLDL) but repressed by microRNA (miR)-145 (Schmitt et al., 2014a; Schmitt et al., 2014b; Liu et al., 2015). Tantalizingly, recruitment of platelets and monocytes to the inflamed endothelium increased in the blood of platelet-specific (tr)JAM-A-deficiency ApoE −/− mice, benefitted from αIIbβ3 signaling and the GPIbα–αMβ2 axis (Karshovska et al., 2015; Zhao et al., 2017). Ginkgolide B decreased the expression of JAM-A and reduced monocyte transmigration in oxLDL-treated HUVECs, linked to the attenuation of Akt phosphorylation (Liu et al., 2015). Additionally, p-cresol-impaired leukocyte TEM is potentially attributable to reduced membrane expression of JAM-A (Faure et al., 2006). Deep hypothermia and post-hypothermic rewarming regulate leukocyte–endothelial interaction and TEM, associated with JAM-A surface expression (Bogert et al., 2016; Bogert et al., 2020).
Liver irradiation does not lead to recruitment of leukocytes into the parenchyma, possibly related to the radiation-induced increase of JAM-1gene expression in rat livers in vivo and in hepatocytes in vitro (Moriconi et al., 2009). By the way, soluble JAM-C increased motility in hepatic stellate cells (Hintermann et al., 2016). Morphine, methamphetamine (Meth), and morphine- and tat-treatment significantly increased JAM-2 expression, while gp120 alone and in combination with Meth significantly decreased JAM-2 expression (Mahajan et al., 2008a; Mahajan et al., 2008b). All those treatments enhanced the TEM of immunocompetent cells across the BBB. Furthermore, JAM-C Fc chimera inhibited macrophage transmigration across hRPE (Hou et al., 2012). Blocking JAM-C function or JAM-B/-C interaction increased monocyte reverse transmigration in the peritonitis model (Bradfield et al., 2007). Consistently, monoclonal antibodies directed against JAM-C significantly blocked the influx of leukocytes in which cerulein-induced acute pancreatitis was assessed (Vonlaufen et al., 2006). Leukocyte transmigration was suppressed in Jam-C −/− mice and enhanced in mice overexpressing JAM-C in their ECs (Scheiermann et al., 2009). Similar to antibody blockade, overexpression or gene silencing of JAM-C in human endothelium exposed to flow elevated rates of monocyte reverse-transendothelial migration under inflammatory conditions in vitro (Bradfield et al., 2016). Neutralizing antibodies against JAM-C enhanced U937 cell migration through the rheumatoid arthritis (RA) synovial tissue fibroblast monolayer (Rabquer et al., 2008). JAM-C is upregulated by oxLDL and may thereby mediate both leukocyte adhesion and leukocyte TEM (Keiper et al., 2005b). Consequently, blocking JAM-C can assist the emigration of atherogenic monocytes/macrophages in plaques. In addition, exosomal miR-146a-5p that transported into endothelial cells reduced monocyte TEM by binding to the 3’untranslated region (3′UTR) of JAM-C (Hu et al., 2020). Monocytic JAML regulated TEM and TEpM of monocyte-derived THP-1 cells probably via binding to CAR, and this interaction is controlled by phosphorylation of CAR (Guo et al., 2009; Morton et al., 2016). During relapsing–remitting MS (RRMS), JAML has homophilic interaction with the BBB endothelium and heterophilical binding to CAR on the choroidal epithelium forming the blood–CSF barrier, which encourages monocyte and CD8 T-cell migration into the CNS (Alvarez et al., 2015).
Dendritic Cell
JAM-A deficiency selectively increased DCs random motility and transmigration across lymphatic endothelial cells in vitro and enhanced DC migration to lymph nodes in vivo (Cera et al., 2004). H33, a monoclonal antibody against mouse JAM-C, improved the migration of DCs to sites of infection and in draining lymph nodes (Ballet et al., 2014).
Lymphocytes
Under inflammation of the vascular wall, JAM-A mediated lymphocyte recruitment to the endothelium and subsequent TEM, through redistribution of JAM-A receptors toward endothelial junctions (Jaczewska et al., 2014). JAM-1 knockdown of EC inhibited chemokine-dependent TEM of effector memory (EM) CD4+ T cells (Manes and Pober 2011). Under flow conditions, stromal cell-derived factor (SDF)-1alpha triggered transendothelial chemotaxis of activated T cells and arrest on cytokine-costimulated endothelium, which could be inhibited by soluble JAM-A.Fc (sJAM-A.Fc) (Ostermann et al., 2005). Blocking EZH2 or JAM-A reduced T-cell adhesion, migration, and extravasation, and EZH2 was involved in leukocyte adhesion and migration via upregulating JAM-A (Tsou et al., 2018). VE-JAM (JAM-B) was prominently expressed in HEV and distributed at interendothelial boundaries (Palmeri et al., 2000). The Engelhardt team discovered JAM-B, implicated in CD8 T-cell migration to the CNS, as one ligand for α4β1-integrin, but JAM-B deficiency does not affect T-cell transmigration across the BBB in vitro (Martin-Blondel et al., 2015; Tietz et al., 2018). JAM-2 promotes lymphocyte TEM (Johnson-Léger et al., 2002). Recombinant JAM-C binds to Mac-1 on BM-DCs (Zimmerli et al., 2009). Although JAM-C was upregulated in activated human T lymphocytes (Immenschuh et al., 2009), JAM-C-deficiency did not affect DC homing, T-cell activation, and DC migration to lymph nodes (Zimmerli et al., 2009). JAM-C potentially works in the final steps of trafficking and transmigration of antigen-specific autoaggressive T-cells to the islets of Langerhans (Christen et al., 2013). Anti-JAM-C antibodies could reduce migration of normal and malignant JAM-C-expressing B cells to bone marrow, lymph nodes, and spleen, probably associated with blockage of adhesion to their ligand JAM-B (Doñate et al., 2013). Not only that, endothelial-cell-selective adhesion molecule (ESAM)-1, an endothelial TJ protein linked with the JAMs, was predicted to attend cell migrations through the sinus-lining cell layer (Pfeiffer et al., 2008).
Taken together, JAMs have been recognized as important players controlling leukocyte transendothelial migration. According to the notable functions of TEM in various physiological and pathological situations, JAMs play complicated and variable roles (Figure 1).
FIGURE 1.
| Roles of JAMs on endothelial cell migration and leucocytes TEM.
Representative interaction and signaling of JAMs. Abbreviation is attached at the end.
Cancer
A couple of reviews summarized the function of JAMs in cancer in recent years, and this review focused on their roles in cancer invasion and metastasis.
Naik et al. discovered JAM-A expression was downregulated as breast cancer disease progresses and thereupon enhanced cancer cell migration (Naik et al., 2008; Naik and Naik 2008). McSherry et al. observed that knockdown or functional antagonism of JAM-A drove breast cancer cell migration via activation of Rap1 GTPase and β1-integrin (McSherry et al., 2009; McSherry et al., 2011). In the murine 4T1 breast cancer model, administration of Tβ4 and TGF-β1 decreased the sJAM-A levels in murine blood, and a peptide derived from the sequence of the F11R/JAM-A protein, peptide 4D (P4D), blocked the TEM of breast cancer cells in the presence of TNF/IFN and Tβ4 (Bednarek et al., 2020). In human breast cancer cell lines, JAM-A blocked the pro-migratory function of CD146 (Imbert et al., 2012) and connected a unique antihuman CD81 antibody (5A6), which effectively halts tumor cell invasion and migration (Vences-Catalán et al., 2021). MicroRNA-495 stimulated breast cancer cell migration by targeting JAM-A (Cao et al., 2014). In the Rip1Tag2 tumor model, Jam-A −/− DCs had a higher rate of DC migration through the endothelium into the tumor than Jam-A +/+ DCs (Murakami et al., 2010). High JAM-A expression induces EMT of nasopharyngeal carcinoma (NPC) cells in vitro and in vivo via the PI3K/Akt pathway, and lncRNA P73 antisense RNA 1 T (TP73-AS1) could upregulate JAM-A expression (Tian et al., 2015; Dai et al., 2021). Histone deacetylases (HDACs) inhibitors downregulated p63-mediated JAM-A expression, suppressing the proliferation, migration, and invasiveness of human head and neck squamous cell carcinoma (HNSCC) (Kakiuchi et al., 2021). Aberrant expression of JAM-A regulated PVR/CD155 to exacerbate malignancy of uterine cervical adenocarcinoma (Murakami et al., 2021). JAM-A restoration suppressed anaplastic thyroid carcinoma (ATC) cell motility and TEM, related to the level of phosphorylation of p53 and GSK3 α/β proteins (Orlandella et al., 2019). In colorectal cancer (CRC), MIR21 upregulation caused JAM-A downregulation and then activated ERK, AKT, and ROCK pathways in promoting invasiveness and metastasis (Lampis et al., 2021). Furthermore, JAM-A expression declined in renal cancer (Gutwein et al., 2009), gastric cancer (Huang et al., 2014), and multiple myeloma (MM) (Solimando et al., 2018) and impaired these cancer cells migration and invasion. Incidentally, N-glycosylation controlled JAM-A’s effects on the migration of MDA-MB-231 cells (Scott et al., 2015).
Qi et al. reported quantum dots (QDs) or cell-penetrating magnetic nanoparticles-mediated JAM-2 knockdown facilitated inhibition of glioma cell migration, and JAM-2 gene targeted the Notch pathway and regulated cytoskeleton remodeling and migration associated protein gene expression (Qi et al., 2013; Qi et al., 2014). Over-expression of JAM-2 in RKO cells resulted in decreasing growth, migration, adhesion, and invasion by regulating the transcription of MMP-9. Negatively binding of miR-374b and JAM-2 inhibits cervical cancer (CC) cell proliferation, migration, and invasion (Li X et al., 2018). JAM-B secreted by cancer cells could promote progression and invasion in pancreatic cancer (PanCa) by upregulating the c-Src signal and related downstream proteins (Zhang et al., 2020).
JAM-C dephosphorylation at serine 281 increased KLN 205 cell adhesion and migration by activating β3 integrins and deactivating β1 integrins (Mandicourt et al., 2007). In Lewis lung carcinoma cells (LLC1s), treatment with a monoclonal antibody directed against JAM-C reduced the infiltration of macrophages into tumors (Lamagna et al., 2005). Palmitoylation of JAM-C supported the movement to TJs and inhibited A549 lung cancer cells migration (Aramsangtienchai et al., 2017). JAM3 endorsed CRC cell viability, colony formation, and migration (Zhou et al., 2019). Some researchers from Geneva University Hospital recounted JAM-C function in several cancers. Anti-JAM-C antibodies reduced migration of normal and malignant JAM-C-expressing B cells to bone marrow, lymph nodes, and spleen by blocking adhesion of JAM-C-expressing B cells to ligand JAM-B (Doñate et al., 2013) and impaired lymphoma B-cell homing to supportive lymphoid microenvironments by driving the MAPK signaling pathway (Doñate et al., 2016). JAM-C/B combination escalated glioma growth and invasion in vivo, linked to activated c-Src proto-oncogene (Tenan et al., 2010). The dimerization sites E66-K68 of JAM-C affected mouse lung squamous carcinoma KLN 205 cells migration (Garrido-Urbani et al., 2018). Moreover, circKIF4A promoted cell proliferation and migration in ovarian cancer by sponging miR-127 and upregulating JAM3 expression (Sheng et al., 2020). B16 melanoma cell metastasis to the lung was proved to decrease in Jam-C −/− mice and endothelial-specific JAM-C-deficient mice, and treatment with soluble JAM-C created a similar decrease (Langer et al., 2011). JAM-C also promoted HT1080 human fibrosarcoma metastasis (Fuse et al., 2007). However, JAM-3 was proved to suppress migration and promote apoptosis of renal carcinoma cells (Li G. C et al., 2018).
JAML promoted gastric cancer (GC) cell migration and proliferation partially via p38 signaling (Fang et al., 2021). In DC-based cancer immunotherapy, the interaction of JAML and CAR acted a crucial role in the TEM of mouse bone marrow-derived DCs (BMDCs) and human monocyte-derived DCs (MoDCs) (Roh et al., 2018).
In fact, numerous articles depicted the significant function of JAMs in cancer notwithstanding, but there are still many questions. For instance, JAM-A diminished the SLM8 cells line TEM, but JAM-C enhances the A375 cell line TEM conversely (Ghislin et al., 2011). Hence, figuring out how JAMs function in different cancer requires further investigation. The roles of JAMs in cancers have been concluded in Table 3.
TABLE 3.
JAMs in cancers.
| Protein | Cancer | Final effect |
|---|---|---|
| JAM-A | Breast cancer, anaplastic thyroid carcinoma, and colorectal cancer | Inhibits invasion and migration |
| Naik et al. (2008) | ||
| Naik and Naik (2008) | ||
| McSherry et al. (2009) | ||
| McSherry et al. (2011) | ||
| Bednarek et al. (2020) | ||
| Imbert et al. (2012) | ||
| Vences-Catalán et al. (2021) | ||
| Cao et al. (2014) | ||
| Orlandella et al. (2019) | ||
| Lampis et al. (2021) | ||
| Nasopharyngeal carcinoma, head and neck squamous cell carcinoma, uterine cervical adenocarcinoma, renal cancer, gastric cancer, and multiple myeloma | Induces proliferation, migration, and invasiveness | |
| Tian et al. (2015) | ||
| Dai et al. (2021) | ||
| Kakiuchi et al. (2021) | ||
| Murakami et al. (2021) | ||
| Gutwein et al. (2009) | ||
| Huang et al. (2014) | ||
| Solimando et al. (2018) | ||
| JAM-B | Glioma and pancreatic cancer | Promotes progression and invasion |
| Qi et al. (2013) | ||
| Qi et al. (2014) | ||
| Zhang et al. (2020) | ||
| JAM-C | Ovarian cancer, melanoma, and fibrosarcoma metastasis | Promotes cell proliferation and migration |
| Sheng et al. (2020) | ||
| Langer et al. (2011) | ||
| Fuse et al. (2007) | ||
| Renal carcinoma | Suppresses migration | |
| Li N et al. (2018) | ||
| JAML | Gastric cancer | Promotes migration and proliferation |
| Fang et al. (2021) |
Conclusion and Prospective
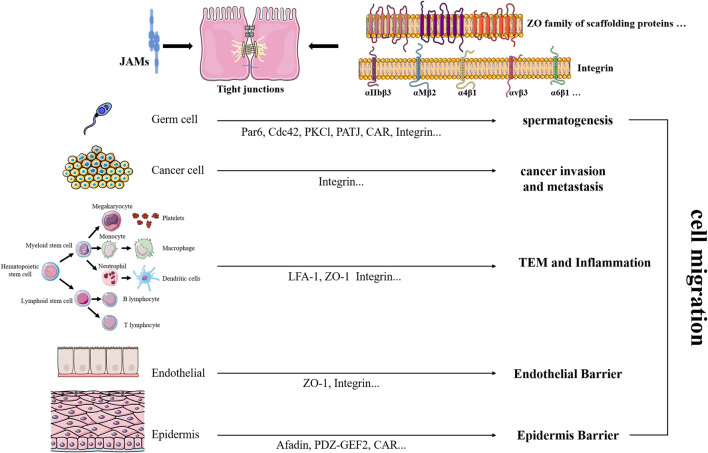
Multiple research studies implicated the principal suppressive effect of JAMs in cell migration with tight junctions. Related inhibitory action was presumably due to the conjunction with the ZO family of scaffolding proteins ZO-1 (Garcia et al., 2018), claudin, afadin (Keiper et al., 2005a; Hartmann et al., 2020; Ebnet 2017), Par6 (Gliki et al., 2004), Par-3 (Fujita et al., 2007), and several integrins (Parise 2003; Naik et al., 2003; Cooke et al., 2006; Peddibhotla et al., 2013; Zen et al., 2004; Karshovska et al., 2015; Zhao et al., 2017; Martin-Blondel et al., 2015; Tietz et al., 2018) (Figure 1). Direct downstream proteins included one integrin ligand such collagen I, collagen IV, and fibronectin (Ebnet 2017).
Another point that deserved discussion should be shear flow, which is important for the migration of endothelial cells as well as leukocytes. Under flow conditions, JAM-A deficiency increased protrusion extension in the direction of flow and enhanced downstream cellular displacement by cooperating with microtubule-stabilizing pathways in ECs (Huang et al., 2006). Whereas, JAM-A deficiency and soluble JAM-A.Fc attenuated monocyte and activated T cells arrest and transmigration (Ostermann et al., 2005; Zernecke et al., 2006). Under shear flow conditions, the antibody against JAM-C scarcely influenced neutrophil transmigration yet elevated rates of monocyte reverse-transendothelial migration in vitro (Sircar et al., 2007; Bradfield et al., 2016).
In addition to the cells described previously, there was a rare report in the central nervous system. For example, Pard3A-dependent JAM-C adhesion promotes germinal zone (GZ) exit OF neuronal cells (Famulski et al., 2010). Nevertheless, JAMs exhibited distinct even opposite functions in different physiological and pathological activities (Ghislin et al., 2011). Since JAMs exhibit unclear roles in one specific cell type, it is more difficult to define their roles under one physiological state or pathological condition. At present, soluble JAMs, the antibody against JAMs, and Jam-deficient mice were developed and were favorable to follow-up studies and potential application. These results remind us that further and detailed examination is necessary for explaining specific influences. Figure 2.
FIGURE 2.
| Regulatory roles of JAMs on cell migration.
Author Contributions
Author JW conceptualized and wrote the manuscript. She solely contributed to the submitted version of the article.
Conflict of Interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s Note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors, and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Abbreviations and Glossary
- AJs
adherens junctions
- AKI
acute kidney injury
- ALI
neutrophil elastase, acute lung injury
- AP
acute pancreatitis
- ATC
anaplastic thyroid carcinoma
- BBB
blood–brain barrier
- BG
bioactive glass
- BMDCs
bone marrow-derived DCs
- BMEC
brain microvascular endothelial cells
- BTB
blood–testis barrier
- C5aR
complement factor 5
- CBL
Casitas B-lineage lymphoma
- CC
cervical cancer
- CCL2
(C–C motif) ligand 2
- CIRP
cold-inducible RNA-binding protein
- CIS
carcinoma in situ
- CNP
C-type natriuretic peptide
- CNS
central nervous system
- CPA
cyproterone acetate
- CPCs
cardiac progenitor cells
- CRC
colorectal cancer
- ECs
endothelial cells
- e-EPCs
embryonic-endothelial progenitor cells
- EM
effector memory
- ES
ectoplasmic specialization
- ESAM
endothelial-cell-selective adhesion molecule
- GC
gastric cancer
- GCs
germ cells
- GZ
germinal zone
- HDACs
histone deacetylases
- HF
hair follicle
- HMVEC
human microvascular endothelial cell
- HNSCC
head and neck squamous cell carcinoma
- HRCECs
human retinal capillary endothelial cells
- I-R
ischemia–reperfusion
- JAMs
junctional adhesion molecules
- JAM-A(ov) MSCs
JAM-A overexpression MSCs
- JAM-Ap
JAM-A antagonist peptide
- LFA-1
leukocyte function-associated antigen-1
- LLC1
Lewis lung carcinoma cells
- LTB4
leukotriene B4
- MCCs
multiciliated cells
- Meth
methamphetamine
- MM
multiple myeloma
- MMP
matrix metalloproteinase
- MoDCs
monocyte-derived DCs
- NAC
N-acetyl-l-cysteine
- NPC
nasopharyngeal carcinoma
- NRSF
neuron-restrictive silencer factor
- oxLDL
oxidized low-density lipoprotein
- PanCa
pancreatic cancer
- P4D
peptide 4D
- P-gp, MDR1
P-glycoprotein
- pSp1
proximal Sp1
- QDs
quantum dots
- RA
rheumatoid arthritis
- RGDS
Arg-Gly-Asp-Ser
- RPE
retinal pigment epithelial
- RRMS
relapsing–remitting MS
- rTEM
reverse transendothelial migration
- SCs
Sertoli cells
- SDF
stromal cell-derived factor
- sJAM-A.Fc
soluble JAM-A.Fc
- Smad
mothers against decapentaplegic
- SPARC
secreted protein acidic and rich in cysteine
- TEM
transendothelial migration
- TEpM
transepithelial migration
- TGIF
TG-interacting factor
- Tiam1
T-cell lymphoma invasion and metastasis 1
- TJs
tight junctions
- TP73-AS1
lncRNA P73 antisense RNA 1 T
- 3′UTR
3’untranslated region
- VASP
vasodilator-stimulated phosphoprotein
References
- Alvarez J. I., Kébir H., Cheslow L., Chabarati M., Larochelle C., Prat A. (2015). JAML Mediates Monocyte and CD8 T Cell Migration across the Brain Endothelium. Ann. Clin. Transl Neurol. 2 (11), 1032–1037. 10.1002/acn3.255 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aramsangtienchai P., Spiegelman N. A., Cao J., Lin H. (2017). S-palmitoylation of Junctional Adhesion Molecule C Regulates its Tight Junction Localization and Cell Migration. J. Biol. Chem. 292 (13), 5325–5334. 10.1074/jbc.M116.730523 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aravindan R. G., Fomin V. P., Naik U. P., Modelski M. J., Naik M. U., Galileo D. S., et al. (2012). CASK Interacts with PMCA4b and JAM-A on the Mouse Sperm Flagellum to Regulate Ca2+ Homeostasis and Motility. J. Cel. Physiol. 227 (8), 3138–3150. 10.1002/jcp.24000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli M.-L., Bardin F., Frontera V., Bidaut G., Obrados E., Adams R. H., et al. (2014). Function of Jam-B/Jam-C Interaction in Homing and Mobilization of Human and Mouse Hematopoietic Stem and Progenitor Cells. Stem Cells 32 (4), 1043–1054. 10.1002/stem.1624 [DOI] [PubMed] [Google Scholar]
- Aurrand-Lions M., Lamagna C., Dangerfield J. P., Wang S., Herrera P., Nourshargh S., et al. (2005). Junctional Adhesion Molecule-C Regulates the Early Influx of Leukocytes into Tissues during Inflammation. J. Immunol. 174 (10), 6406–6415. 10.4049/jimmunol.174.10.6406 [DOI] [PubMed] [Google Scholar]
- Azari B. M., Marmur J. D., Salifu M. O., Cavusoglu E., Ehrlich Y. H., Kornecki E., et al. (2010). Silencing of the F11R Gene Reveals a Role for F11R/JAM-A in the Migration of Inflamed Vascular Smooth Muscle Cells and in Atherosclerosis. Atherosclerosis 212 (1), 197–205. 10.1016/j.atherosclerosis.2010.05.014 [DOI] [PubMed] [Google Scholar]
- Ballet R., Emre Y., Jemelin S., Charmoy M., Tacchini-Cottier F., Imhof B. A. (2014). Blocking Junctional Adhesion Molecule C Enhances Dendritic Cell Migration and Boosts the Immune Responses against Leishmania Major. Plos Pathog. 10 (12), e1004550. 10.1371/journal.ppat.1004550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bednarek R., Selmi A., Wojkowska D., Karolczak K., Popielarski M., Stasiak M., et al. (2020). Functional Inhibition of F11 Receptor (F11R/junctional Adhesion molecule-A/JAM-A) Activity by a F11R-Derived Peptide in Breast Cancer and its Microenvironment. Breast Cancer Res. Treat. 179 (2), 325–335. 10.1007/s10549-019-05471-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boerner K., Luissint A.-C., Parkos C. A. (2021). Functional Assessment of Intestinal Permeability and Neutrophil Transepithelial Migration in Mice Using a Standardized Intestinal Loop Model. JoVE 168. 10.3791/62093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogert N. V., Werner I., Kornberger A., Meybohm P., Moritz A., Keller T., et al. (2016). Influence of Hypothermia and Subsequent Rewarming upon Leukocyte-Endothelial Interactions and Expression of Junctional-Adhesion-Molecules A and B. Sci. Rep. 6, 21996. 10.1038/srep21996 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogert N. V., Werner I., Kornberger A., Vahl C.-F., Beiras-Fernandez A. (2020). Effect of Rewarming on Leukocyte-Endothelial Interaction after Deep Hypothermic Preservation. Ann. Transpl. 25, e919540. 10.12659/aot.919540 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield P. F., Menon A., Miljkovic-Licina M., Lee B. P., Fischer N., Fish R. J., et al. (2016). Divergent JAM-C Expression Accelerates Monocyte-Derived Cell Exit from Atherosclerotic Plaques. PLoS One 11 (7), e0159679. 10.1371/journal.pone.0159679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradfield P. F., Scheiermann C., Nourshargh S., Ody C., Luscinskas F. W., Rainger G. E., et al. (2007). JAM-C Regulates Unidirectional Monocyte Transendothelial Migration in Inflammation. Blood 110 (7), 2545–2555. 10.1182/blood-2007-03-078733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burek M., König A., Lang M., Fiedler J., Oerter S., Roewer N., et al. (2019). Hypoxia-Induced MicroRNA-212/132 Alter Blood-Brain Barrier Integrity through Inhibition of Tight Junction-Associated Proteins in Human and Mouse Brain Microvascular Endothelial Cells. Transl. Stroke Res. 10 (6), 672–683. 10.1007/s12975-018-0683-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers S., Graham R., Dai H. N., Hoxter B. (1991). Development of Sertoli Cell Junctional Specializations and the Distribution of the tight-junction-associated Protein ZO-1 in the Mouse Testis. Am. J. Anat. 191 (1), 35–47. 10.1002/aja.1001910104 [DOI] [PubMed] [Google Scholar]
- Cao M., Nie W., Li J., Zhang Y., Yan X., Guan X., et al. (2014). MicroRNA-495 Induces Breast Cancer Cell Migration by Targeting JAM-A. Protein Cell 5 (11), 862–872. 10.1007/s13238-014-0088-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartier-Michaud A., Bailly A.-L., Betzi S., Shi X., Lissitzky J.-C., Zarubica A., et al. (2017). Genetic, Structural, and Chemical Insights into the Dual Function of GRASP55 in Germ Cell Golgi Remodeling and JAM-C Polarized Localization during Spermatogenesis. Plos Genet. 13 (6), e1006803. 10.1371/journal.pgen.1006803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvallo L., Lopez L., Che F.-Y., Lim J., Eugenin E. A., Williams D. W., et al. (2015). Buprenorphine Decreases the CCL2-Mediated Chemotactic Response of Monocytes. J.I. 194 (7), 3246–3258. 10.4049/jimmunol.1302647 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera M. R., Del Prete A., Vecchi A., Corada M., Martin-Padura I., Motoike T., et al. (2004). Increased DC Trafficking to Lymph Nodes and Contact Hypersensitivity in Junctional Adhesion Molecule-A-Deficient Mice. J. Clin. Invest. 114 (5), 729–738. 10.1172/jci21231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cera M. R., Fabbri M., Molendini C., Corada M., Orsenigo F., Rehberg M., et al. (2009). JAM-A Promotes Neutrophil Chemotaxis by Controlling Integrin Internalization and Recycling. J. Cel Sci 122 (Pt 2), 268–277. 10.1242/jcs.037127 [DOI] [PubMed] [Google Scholar]
- Chakraborty A., Singh V., Singh K., Rajender S. (2020). Excess Iodine Impairs Spermatogenesis by Inducing Oxidative Stress and Perturbing the Blood Testis Barrier. Reprod. Toxicol. 96, 128–140. 10.1016/j.reprotox.2020.06.012 [DOI] [PubMed] [Google Scholar]
- Chalmers A. D., Whitley P. (2012). Continuous Endocytic Recycling of Tight junction Proteins: How and Why? Essays Biochem. 53, 41–54. 10.1042/bse0530041 [DOI] [PubMed] [Google Scholar]
- Chavakis T., Preissner K. T., Santoso S. (2003). Leukocyte Trans-endothelial Migration: JAMs Add New Pieces to the Puzzle. Thromb. Haemost. 89 (1), 13–17. [PubMed] [Google Scholar]
- Chavakis T., Keiper T., Matz-Westphal R., Hersemeyer K., Sachs U. J., Nawroth P. P., et al. (2004). The Junctional Adhesion Molecule-C Promotes Neutrophil Transendothelial Migration In Vitro and In Vivo. J. Biol. Chem. 279 (53), 55602–55608. 10.1074/jbc.M404676200 [DOI] [PubMed] [Google Scholar]
- Cho W., Song J.-Y., Oh S. W., Kim M. G., Ko Y. S., Lee H. Y., et al. (2017). Fate of Neutrophils during the Recovery Phase of Ischemia/Reperfusion Induced Acute Kidney Injury. J. Korean Med. Sci. 32 (10), 1616–1625. 10.3346/jkms.2017.32.10.1616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi Y. S., Kim Y. C., Ji S., Choi Y. (2014). Increased Bacterial Invasion and Differential Expression of Tight-junction Proteins, Growth Factors, and Growth Factor Receptors in Periodontal Lesions. J. Periodontol. 85 (8), e313–e322. 10.1902/jop.2014.130740 [DOI] [PubMed] [Google Scholar]
- Christen S., Coppieters K., Rose K., Holdener M., Bayer M., Pfeilschifter J. M., et al. (2013). Blockade but Not Overexpression of the Junctional Adhesion Molecule C Influences Virus-Induced Type 1 Diabetes in Mice. PLoS One 8 (1), e54675. 10.1371/journal.pone.0054675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colom B., Bodkin J. V., Beyrau M., Woodfin A., Ody C., Rourke C., et al. (2015). Leukotriene B4-Neutrophil Elastase Axis Drives Neutrophil Reverse Transendothelial Cell Migration In Vivo. Immunity 42 (6), 1075–1086. 10.1016/j.immuni.2015.05.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Comerford K. M., Lawrence D. W., Synnestvedt K., Levi B. P., Colgan S. P. (2002). Role of Vasodilator‐stimulated Phosphoprotein in Protein Kinase A‐induced Changes in Endothelial Junctional Permeability. FASEB j. 16 (6), 583–585. 10.1096/fj.01-0739fje [DOI] [PubMed] [Google Scholar]
- Cooke V. G., Naik M. U., Naik U. P. (2006). Fibroblast Growth Factor-2 Failed to Induce Angiogenesis in Junctional Adhesion Molecule-A-Deficient Mice. Atvb 26 (9), 2005–2011. 10.1161/01.Atv.0000234923.79173.99 [DOI] [PubMed] [Google Scholar]
- Czabanka M., Petrilli L. L., Elvers-Hornung S., Bieback K., Albert Imhof B., Vajkoczy P., et al. (2020). Junctional Adhesion Molecule-C Mediates the Recruitment of Embryonic-Endothelial Progenitor Cells to the Perivascular Niche during Tumor Angiogenesis. Ijms 21 (4), 1209. 10.3390/ijms21041209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dai B. Q., Jiang X., Feng L. C. (2021). LncRNA TP73‐AS1 Regulates miR ‐495 Expression to Promote Migration and Invasion of Nasopharyngeal Carcinoma Cells through Junctional Adhesion Molecule A. Kaohsiung J. Med. Sci. 37 (5), 361–370. 10.1002/kjm2.12338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dangerfield J., Larbi K. Y., Huang M.-T., Dewar A., Nourshargh S. (2002). PECAM-1 (CD31) Homophilic Interaction Up-Regulates α6β1 on Transmigrated Neutrophils In Vivo and Plays a Functional Role in the Ability of α6 Integrins to Mediate Leukocyte Migration through the Perivascular Basement Membrane. J. Exp. Med. 196 (9), 1201–1212. 10.1084/jem.20020324 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daniele L. L., Adams R. H., Durante D. E., Pugh E. N., Philp N. J. (2007). Novel Distribution of Junctional Adhesion Molecule-C in the Neural Retina and Retinal Pigment Epithelium. J. Comp. Neurol. 505 (2), 166–176. 10.1002/cne.21489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Maschio A., De Luigi A., Martin-Padura I., Brockhaus M., Bartfai T., Fruscella P., et al. (1999). Leukocyte Recruitment in the Cerebrospinal Fluid of Mice with Experimental Meningitis Is Inhibited by an Antibody to Junctional Adhesion Molecule (JAM). J. Exp. Med. 190 (9), 1351–1356. 10.1084/jem.190.9.1351 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doñate C., Ody C., McKee T., Ruault-Jungblut S., Fischer N., Ropraz P., et al. (2013). Homing of Human B Cells to Lymphoid Organs and B-Cell Lymphoma Engraftment Are Controlled by Cell Adhesion Molecule JAM-C. Cancer Res. 73 (2), 640–651. 10.1158/0008-5472.Can-12-1756 [DOI] [PubMed] [Google Scholar]
- Doñate C., Vijaya Kumar A., Imhof B. A., Matthes T. (2016). Anti-JAM-C Therapy Eliminates Tumor Engraftment in a Xenograft Model of Mantle Cell Lymphoma. J. Leukoc. Biol. 100 (5), 843–853. 10.1189/jlb.1HI1114-549RR [DOI] [PubMed] [Google Scholar]
- Dreymueller D., Martin C., Kogel T., Pruessmeyer J., Hess F. M., Horiuchi K., et al. (2012). Lung Endothelial ADAM17 Regulates the Acute Inflammatory Response to Lipopolysaccharide. EMBO Mol. Med. 4 (5), 412–423. 10.1002/emmm.201200217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ebnet K. (2017). Junctional Adhesion Molecules (JAMs): Cell Adhesion Receptors with Pleiotropic Functions in Cell Physiology and Development. Physiol. Rev. 97 (4), 1529–1554. 10.1152/physrev.00004.2017 [DOI] [PubMed] [Google Scholar]
- Ebnet K., Suzuki A., Ohno S., Vestweber D. (2004). Junctional Adhesion Molecules (JAMs): More Molecules with Dual Functions? J. Cel Sci 117 (Pt 1), 19–29. 10.1242/jcs.00930 [DOI] [PubMed] [Google Scholar]
- Economopoulou M., Hammer J., Wang F., Fariss R., Maminishkis A., Miller S. S. (2009). Expression, Localization, and Function of Junctional Adhesion Molecule-C (JAM-C) in Human Retinal Pigment Epithelium. Invest. Ophthalmol. Vis. Sci. 50 (3), 1454–1463. 10.1167/iovs.08-2129 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Famulski J. K., Trivedi N., Howell D., Yang Y., Tong Y., Gilbertson R., et al. (2010). Siah Regulation of Pard3A Controls Neuronal Cell Adhesion during Germinal Zone Exit. Science 330 (6012), 1834–1838. 10.1126/science.1198480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Y., Yang J., Zu G., Cong C., Liu S., Xue F., et al. (2021). Junctional Adhesion Molecule-like Protein Promotes Tumor Progression and Metastasis via P38 Signaling Pathway in Gastric Cancer. Front. Oncol. 11, 565676. 10.3389/fonc.2021.565676 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure V., Cerini C., Paul P., Berland Y., Dignat-George F., Brunet P. (2006). The Uremic Solute P-Cresol Decreases Leukocyte Transendothelial Migration In Vitro. Int. Immunol. 18 (10), 1453–1459. 10.1093/intimm/dxl077 [DOI] [PubMed] [Google Scholar]
- Flemming S., Luissint A.-C., Nusrat A., Parkos C. A. (2018). Analysis of Leukocyte Transepithelial Migration Using an In Vivo Murine Colonic Loop Model. JCI insight 3 (20). 10.1172/jci.insight.99722 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraemohs L., Koenen R. R., Ostermann G., Heinemann B., Weber C. (2004). The Functional Interaction of the β2 Integrin Lymphocyte Function-Associated Antigen-1 with Junctional Adhesion Molecule-A Is Mediated by the I Domain. J. Immunol. 173 (10), 6259–6264. 10.4049/jimmunol.173.10.6259 [DOI] [PubMed] [Google Scholar]
- Fu Y., Tang M., Xiang X., Liu K., Xu X. (2019). Glucose Affects Cell Viability, Migration, Angiogenesis and Cellular Adhesion of Human Retinal Capillary Endothelial Cells via SPARC. Exp. Ther. Med. 17 (1), 273–283. 10.3892/etm.2018.6970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita E., Tanabe Y., Hirose T., Aurrand-Lions M., Kasahara T., Imhof B. A., et al. (2007). Loss of Partitioning-defective-3/isotype-specific Interacting Protein (par-3/ASIP) in the Elongating Spermatid of RA175 (IGSF4A/SynCAM)-Deficient Mice. Am. J. Pathol. 171 (6), 1800–1810. 10.2353/ajpath.2007.070261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fuse C., Ishida Y., Hikita T., Asai T., Oku N. (2007). Junctional Adhesion Molecule-C Promotes Metastatic Potential of HT1080 Human Fibrosarcoma. J. Biol. Chem. 282 (11), 8276–8283. 10.1074/jbc.M608836200 [DOI] [PubMed] [Google Scholar]
- Garcia M. A., Nelson W. J., Chavez N. (2018). Cell-Cell Junctions Organize Structural and Signaling Networks. Cold Spring Harb Perspect. Biol. 10 (4), a029181. 10.1101/cshperspect.a029181 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrido-Urbani S., Bradfield P. F., Imhof B. A. (2014). Tight junction Dynamics: the Role of Junctional Adhesion Molecules (JAMs). Cell Tissue Res 355 (3), 701–715. 10.1007/s00441-014-1820-1 [DOI] [PubMed] [Google Scholar]
- Garrido-Urbani S., Vonlaufen A., Stalin J., De Grandis M., Ropraz P., Jemelin S., et al. (2018). Junctional Adhesion Molecule C (JAM-C) Dimerization Aids Cancer Cell Migration and Metastasis. Biochim. Biophys. Acta (Bba) - Mol. Cel Res. 1865 (4), 638–649. 10.1016/j.bbamcr.2018.01.008 [DOI] [PubMed] [Google Scholar]
- Ghislin S., Obino D., Middendorp S., Boggetto N., Alcaide-Loridan C., Deshayes F. (2011). Junctional Adhesion Molecules Are Required for Melanoma Cell Lines Transendothelial Migration In Vitro. Pigment Cel Melanoma Res 24 (3), 504–511. 10.1111/j.1755-148X.2011.00856.x [DOI] [PubMed] [Google Scholar]
- Gliki G., Ebnet K., Aurrand-Lions M., Imhof B. A., Adams R. H. (2004). Spermatid Differentiation Requires the Assembly of a Cell Polarity Complex Downstream of Junctional Adhesion Molecule-C. Nature 431 (7006), 320–324. 10.1038/nature02877 [DOI] [PubMed] [Google Scholar]
- Grimaldi C., Raz E. (2020). Germ Cell Migration-Evolutionary Issues and Current Understanding. Semin. Cel Develop. Biol. 100, 152–159. 10.1016/j.semcdb.2019.11.015 [DOI] [PubMed] [Google Scholar]
- Guo Y.-L., Bai R., Chen C. X.-J., Liu D.-Q., Liu Y., Zhang C.-Y., et al. (2009). Role of Junctional Adhesion Molecule-like Protein in Mediating Monocyte Transendothelial Migration. Atvb 29 (1), 75–83. 10.1161/atvbaha.108.177717 [DOI] [PubMed] [Google Scholar]
- Gutwein P., Schramme A., Voss B., Abdel-Bakky M. S., Doberstein K., Ludwig A., et al. (2009). Downregulation of Junctional Adhesion Molecule-A Is Involved in the Progression of clear Cell Renal Cell Carcinoma. Biochem. Biophysical Res. Commun. 380 (2), 387–391. 10.1016/j.bbrc.2009.01.100 [DOI] [PubMed] [Google Scholar]
- Hartmann C., Schwietzer Y. A., Otani T., Furuse M., Ebnet K. (2020). Physiological Functions of Junctional Adhesion Molecules (JAMs) in Tight Junctions. Biochim. Biophys. Acta (Bba) - Biomembranes 1862 (9), 183299. 10.1016/j.bbamem.2020.183299 [DOI] [PubMed] [Google Scholar]
- Hintermann E., Bayer M., Ehser J., Aurrand-Lions M., Pfeilschifter J. M., Imhof B. A., et al. (2016). Murine Junctional Adhesion Molecules JAM-B and JAM-C Mediate Endothelial and Stellate Cell Interactions during Hepatic Fibrosis. Cell Adhes. Migration 10 (4), 419–433. 10.1080/19336918.2016.1178448 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirano Y., Ode Y., Ochani M., Wang P., Aziz M. (2018). Targeting Junctional Adhesion molecule‐C Ameliorates Sepsis‐induced Acute Lung Injury by Decreasing CXCR4+aged Neutrophils. J. Leukoc. Biol. 104 (6), 1159–1171. 10.1002/jlb.3a0218-050r [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt J. R., Zeng W.-Z., Evans E. L., Woo S.-H., Ma S., Abuwarda H., et al. (2021). Spatiotemporal Dynamics of PIEZO1 Localization Controls Keratinocyte Migration during Wound Healing. eLife 10. 10.7554/eLife.65415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hou X., Hu D., Wang Y.-s., Tang Z.-s., Zhang F., Chavakis T., et al. (2012). Targeting of Junctional Adhesion Molecule-C Inhibits Experimental Choroidal Neovascularization. Invest. Ophthalmol. Vis. Sci. 53 (3), 1584–1591. 10.1167/iovs.11-9005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu W., Xu B., Zhang J., Kou C., Liu J., Wang Q., et al. (2020). Exosomal miR-146a-5p from Treponema Pallidum-Stimulated Macrophages Reduces Endothelial Cells Permeability and Monocyte Transendothelial Migration by Targeting JAM-C. Exp. Cel Res. 388 (1), 111823. 10.1016/j.yexcr.2020.111823 [DOI] [PubMed] [Google Scholar]
- Huang H., Cruz F., Bazzoni G. (2006). Junctional Adhesion Molecule-A Regulates Cell Migration and Resistance to Shear Stress. J. Cel. Physiol. 209 (1), 122–130. 10.1002/jcp.20712 [DOI] [PubMed] [Google Scholar]
- Huang J.-y., Xu Y.-y., Sun Z., Wang Z.-n., Zhu Z., Song Y.-x., et al. (2014). Low Junctional Adhesion Molecule A Expression Correlates with Poor Prognosis in Gastric Cancer. J. Surg. Res. 192 (2), 494–502. 10.1016/j.jss.2014.06.025 [DOI] [PubMed] [Google Scholar]
- Huang W., Eum S. Y., András I. E., Hennig B., Toborek M. (2009). PPARα and PPARγ Attenuate HIV‐induced Dysrégulation of Tight junction Proteins by Modulations of Matrix Metalloproteinase and Proteasome Activities. FASEB j. 23 (5), 1596–1606. 10.1096/fj.08-121624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hui W., Feng Y., Baoqi Y., Shuwei D., Ruihua X., Jiongjie H., et al. (2020). Comparative Proteomics Analysis Indicates that Palmatine Contributes to Transepithelial Migration by Regulating Cellular Adhesion. Pharm. Biol. 58 (1), 646–654. 10.1080/13880209.2020.1784961 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Imbert A.-M., Garulli C., Choquet E., Koubi M., Aurrand-Lions M., Chabannon C. (2012). CD146 Expression in Human Breast Cancer Cell Lines Induces Phenotypic and Functional Changes Observed in Epithelial to Mesenchymal Transition. PLoS One 7 (8), e43752. 10.1371/journal.pone.0043752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immenschuh S., Naidu S., Chavakis T., Beschmann H., Ludwig R. J., Santoso S. (2009). Transcriptional Induction of Junctional Adhesion Molecule-C Gene Expression in Activated T Cells. J. Leukoc. Biol. 85 (5), 796–803. 10.1189/jlb.0708422 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaczewska J., Abdulreda M. H., Yau C. Y., Schmitt M. M., Schubert I., Berggren P.-O., et al. (2014). TNF-α and IFN-γ Promote Lymphocyte Adhesion to Endothelial Junctional Regions Facilitating Transendothelial Migration. J. Leukoc. Biol. 95 (2), 265–274. 10.1189/jlb.0412205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia X., Xu Y., Wu W., Fan Y., Wang G., Zhang T., et al. (2017). Aroclor1254 Disrupts the Blood-Testis Barrier by Promoting Endocytosis and Degradation of junction Proteins via P38 MAPK Pathway. Cell Death Dis 8 (5), e2823. 10.1038/cddis.2017.224 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Aziz M., Ode Y., Wang P. (2019). CIRP Induces Neutrophil Reverse Transendothelial Migration in Sepsis. Shock 51 (5), 548–556. 10.1097/shk.0000000000001257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson M. D., Otuki M. F., Cabrini D. A., Rudolph R., Witherden D. A., Havran W. L. (2021). Hspa8 and ICAM‐1 as Damage‐induced Mediators of γδ T Cell Activation. J. Leukoc. Bio 111, 135–145. 10.1002/jlb.3ab0420-282r [DOI] [PubMed] [Google Scholar]
- Johnson-Léger C. A., Aurrand-Lions M., Beltraminelli N., Fasel N., Imhof B. A. (2002). Junctional Adhesion Molecule-2 (JAM-2) Promotes Lymphocyte Transendothelial Migration. Blood 100 (7), 2479–2486. 10.1182/blood-2001-11-0098 [DOI] [PubMed] [Google Scholar]
- Kakiuchi A., Kakuki T., Ohwada K., Kurose M., Kondoh A., Obata K., et al. (2021). HDAC Inhibitors Suppress the Proliferation, Migration and Invasiveness of Human Head and Neck Squamous Cell Carcinoma Cells via P63-mediated T-ight junction M-olecules and P-21-mediated G-rowth A-rrest. Oncol. Rep. 45 (4). 10.3892/or.2021.7997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanamori M., Oikawa K., Tanemura K., Hara K. (2019). Mammalian Germ Cell Migration during Development, Growth, and Homeostasis. Reprod. Med. Biol. 18 (3), 247–255. 10.1002/rmb2.12283 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang L. I., Wang Y., Suckow A. T., Czymmek K. J., Cooke V. G., Naik U. P., et al. (2007). Deletion of JAM-A Causes Morphological Defects in the Corneal Epithelium. Int. J. Biochem. Cel Biol. 39 (3), 576–585. 10.1016/j.biocel.2006.10.016 [DOI] [PubMed] [Google Scholar]
- Karshovska E., Zhao Z., Blanchet X., Schmitt M. M. N., Bidzhekov K., Soehnlein O., et al. (2015). Hyperreactivity of Junctional Adhesion Molecule A-Deficient Platelets Accelerates Atherosclerosis in Hyperlipidemic Mice. Circ. Res. 116 (4), 587–599. 10.1161/circresaha.116.304035 [DOI] [PubMed] [Google Scholar]
- Kawauchi T. (2012). Cell Adhesion and its Endocytic Regulation in Cell Migration during Neural Development and Cancer Metastasis. Ijms 13 (4), 4564–4590. 10.3390/ijms13044564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keiper T., Santoso S., Nawroth P. P., Orlova V., Chavakis T. (2005a). The Role of Junctional Adhesion Molecules in Cell-Cell Interactions. Histol. Histopathol 20 (1), 197–203. 10.14670/hh-20.197 [DOI] [PubMed] [Google Scholar]
- Keiper T., Al‐Fakhri N., Chavakis E., Athanasopoulos A. N., Isermann B., Herzog S., et al. (2005b). The Role of Junctional Adhesion molecule‐C (JAM‐C) in Oxidized LDL‐mediated Leukocyte Recruitment. FASEB j. 19 (14), 2078–2080. 10.1096/fj.05-4196fje [DOI] [PubMed] [Google Scholar]
- Khandoga A., Kessler J. S., Meissner H., Hanschen M., Corada M., Motoike T., et al. (2005). Junctional Adhesion Molecule-A Deficiency Increases Hepatic Ischemia-Reperfusion Injury Despite Reduction of Neutrophil Transendothelial Migration. Blood 106 (2), 725–733. 10.1182/blood-2004-11-4416 [DOI] [PubMed] [Google Scholar]
- Kim S. C., Ko Y. S., Lee H. Y., Kim M.-G., Jo S.-K., Cho W.-Y. (2017). Blocking Junctional Adhesion Molecule C Promotes the Recovery of Cisplatin-Induced Acute Kidney Injury. Korean J. Intern. Med. 32 (6), 1053–1061. 10.3904/kjim.2016.060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koenen R. R., Pruessmeyer J., Soehnlein O., Fraemohs L., Zernecke A., Schwarz N., et al. (2009). Regulated Release and Functional Modulation of Junctional Adhesion Molecule A by Disintegrin Metalloproteinases. Blood 113 (19), 4799–4809. 10.1182/blood-2008-04-152330 [DOI] [PubMed] [Google Scholar]
- Kostelnik K. B., Barker A., Schultz C., Mitchell T. P., Rajeeve V., White I. J., et al. (2019). Dynamic Trafficking and Turnover of JAM-C Is Essential for Endothelial Cell Migration. Plos Biol. 17 (12), e3000554. 10.1371/journal.pbio.3000554 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ku Y. H., Cho B.-J., Kim M. J., Lim S., Park Y. J., Jang H. C., et al. (2017). Rosiglitazone Increases Endothelial Cell Migration and Vascular Permeability through Akt Phosphorylation. BMC Pharmacol. Toxicol. 18 (1), 62. 10.1186/s40360-017-0169-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lakshmi S. P., Reddy A. T., Naik M. U., Naik U. P., Reddy R. C. (2012). Effects of JAM-A Deficiency or Blocking Antibodies on Neutrophil Migration and Lung Injury in a Murine Model of ALI. Am. J. Physiology-Lung Cell Mol. Physiol. 303 (9), L758–L766. 10.1152/ajplung.00107.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamagna C., Hodivala-Dilke K. M., Imhof B. A., Aurrand-Lions M. (2005). Antibody against Junctional Adhesion Molecule-C Inhibits Angiogenesis and Tumor Growth. Cancer Res. 65 (13), 5703–5710. 10.1158/0008-5472.Can-04-4012 [DOI] [PubMed] [Google Scholar]
- Lampis A., Hahne J. C., Gasparini P., Cascione L., Hedayat S., Vlachogiannis G., et al. (2021). MIR21-induced Loss of Junctional Adhesion Molecule A Promotes Activation of Oncogenic Pathways, Progression and Metastasis in Colorectal Cancer. Cell Death Differ 28 (10), 2970–2982. 10.1038/s41418-021-00820-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langer H. F., Orlova V. V., Xie C., Kaul S., Schneider D., Lonsdorf A. S., et al. (2011). A Novel Function of Junctional Adhesion Molecule-C in Mediating Melanoma Cell Metastasis. Cancer Res. 71 (12), 4096–4105. 10.1158/0008-5472.Can-10-2794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laukoetter M. G., Nava P., Lee W. Y., Severson E. A., Capaldo C. T., Babbin B. A., et al. (2007). JAM-A Regulates Permeability and Inflammation in the Intestine In Vivo. J. Exp. Med. 204 (13), 3067–3076. 10.1084/jem.20071416 [DOI] [PMC free article] [PubMed] [Google Scholar]
- León-Rivera R., Veenstra M., Donoso M., Tell E., Eugenin E. A., Morgello S., et al. (2021). Central Nervous System (CNS) Viral Seeding by Mature Monocytes and Potential Therapies to Reduce CNS Viral Reservoirs in the cART Era. mBio 12 (2). 10.1128/mBio.03633-20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G. C., Cao X. Y., Li Y. N., Qiu Y. Y., Li Y. N., Liu X. J., et al. (2018). MicroRNA‐374b Inhibits Cervical Cancer Cell Proliferation and Induces Apoptosis through the P38/ERK Signaling Pathway by Binding to JAM‐2. J. Cel Physiol 233 (9), 7379–7390. 10.1002/jcp.26574 [DOI] [PubMed] [Google Scholar]
- Li N N., Yang H., Wang M., Lü S., Zhang Y., Long M. (2018). Ligand-specific Binding Forces of LFA-1 and Mac-1 in Neutrophil Adhesion and Crawling. MBoC 29 (4), 408–418. 10.1091/mbc.E16-12-0827 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Yin A., Zhang W., Zhao F., Lv J., Lv J., et al. (2018). Jam3 Promotes Migration and Suppresses Apoptosis of Renal Carcinoma Cell Lines. Int. J. Mol. Med. 42 (5), 2923–2929. 10.3892/ijmm.2018.3854 [DOI] [PubMed] [Google Scholar]
- Liu M. L., Nagai T., Tokunaga M., Iwanaga K., Matsuura K., Takahashi T., et al. (2014). Anti‐Inflammatory Peptides from Cardiac Progenitors Ameliorate Dysfunction after Myocardial Infarction. Jaha 3 (6), e001101. 10.1161/jaha.114.001101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X., Sun W., Zhao Y., Chen B., Wu W., Bao L., et al. (20152015). Ginkgolide B Inhibits JAM-A, Cx43, and VE-Cadherin Expression and Reduces Monocyte Transmigration in Oxidized LDL-Stimulated Human Umbilical Vein Endothelial Cells. Oxidative Med. Cell Longevity 2015, 1–10. 10.1155/2015/907926 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Y., Nusrat A., Schnell F. J., Reaves T. A., Walsh S., Pochet M., et al. (2000). Human junction Adhesion Molecule Regulates Tight junction Resealing in Epithelia. J. Cel Sci 113 ( Pt 13) (Pt 13), 2363–2374. 10.1242/jcs.113.13.2363 [DOI] [PubMed] [Google Scholar]
- Luissint A.-C., Nusrat A., Parkos C. A. (2014). JAM-related Proteins in Mucosal Homeostasis and Inflammation. Semin. Immunopathol 36 (2), 211–226. 10.1007/s00281-014-0421-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luissint A.-C., Williams H. C., Kim W., Flemming S., Azcutia V., Hilgarth R. S., et al. (2019). Macrophage-dependent Neutrophil Recruitment Is Impaired under Conditions of Increased Intestinal Permeability in JAM-A-Deficient Mice. Mucosal Immunol. 12 (3), 668–678. 10.1038/s41385-019-0143-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S. D., Aalinkeel R., Sykes D. E., Reynolds J. L., Bindukumar B., Adal A., et al. (2008b). Methamphetamine Alters Blood Brain Barrier Permeability via the Modulation of Tight junction Expression: Implication for HIV-1 Neuropathogenesis in the Context of Drug Abuse. Brain Res. 1203, 133–148. 10.1016/j.brainres.2008.01.093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S. D., Aalinkeel R., Sykes D. E., Reynolds J. L., Bindukumar B., Fernandez S. F., et al. (2008a). Tight junction Regulation by Morphine and HIV-1 Tat Modulates Blood-Brain Barrier Permeability. J. Clin. Immunol. 28 (5), 528–541. 10.1007/s10875-008-9208-1 [DOI] [PubMed] [Google Scholar]
- Mandell K. J., Babbin B. A., Nusrat A., Parkos C. A. (2005). Junctional Adhesion Molecule 1 Regulates Epithelial Cell Morphology through Effects on β1 Integrins and Rap1 Activity. J. Biol. Chem. 280 (12), 11665–11674. 10.1074/jbc.M412650200 [DOI] [PubMed] [Google Scholar]
- Mandell K., Parkos C. (2005). The JAM Family of Proteins. Adv. Drug Deliv. Rev. 57 (6), 857–867. 10.1016/j.addr.2005.01.005 [DOI] [PubMed] [Google Scholar]
- Mandicourt G., Iden S., Ebnet K., Aurrand-Lions M., Imhof B. A. (2007). JAM-C Regulates Tight Junctions and Integrin-Mediated Cell Adhesion and Migration. J. Biol. Chem. 282 (3), 1830–1837. 10.1074/jbc.M605666200 [DOI] [PubMed] [Google Scholar]
- Manes T. D., Pober J. S. (2011). Identification of Endothelial Cell Junctional Proteins and Lymphocyte Receptors Involved in Transendothelial Migration of Human Effector Memory CD4+ T Cells. J.I. 186 (3), 1763–1768. 10.4049/jimmunol.1002835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Blondel G., Pignolet B., Tietz S., Yshii L., Gebauer C., Perinat T., et al. (2015). Migration of Encephalitogenic CD8 T Cells into the central Nervous System Is Dependent on the α4β1-integrin. Eur. J. Immunol. 45 (12), 3302–3312. 10.1002/eji.201545632 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martìn-Padura I., Lostaglio S., Schneemann M., Williams L., Romano M., Fruscella P., et al. (1998). Junctional Adhesion Molecule, a Novel Member of the Immunoglobulin Superfamily that Distributes at Intercellular Junctions and Modulates Monocyte Transmigration. J. Cel. Biol. 142 (1), 117–127. 10.1083/jcb.142.1.117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mateos-Quiros C. M., Garrido-Jimenez S., Álvarez-Hernán G., Diaz-Chamorro S., Barrera-Lopez J. F., Francisco-Morcillo J., et al. (2021). Junctional Adhesion Molecule 3 Expression in the Mouse Airway Epithelium Is Linked to Multiciliated Cells. Front. Cel Dev. Biol. 9, 622515. 10.3389/fcell.2021.622515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry E. A., Brennan K., Hudson L., Hill A. D., Hopkins A. M. (2011). Breast Cancer Cell Migration Is Regulated through Junctional Adhesion Molecule-A-Mediated Activation of Rap1 GTPase. Breast Cancer Res. 13 (2), R31. 10.1186/bcr2853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McSherry E. A., McGee S. F., Jirstrom K., Doyle E. M., Brennan D. J., Landberg G., et al. (2009). JAM-A Expression Positively Correlates with Poor Prognosis in Breast Cancer Patients. Int. J. Cancer 125 (6), 1343–1351. 10.1002/ijc.24498 [DOI] [PubMed] [Google Scholar]
- Meng M., Klingensmith N. J., Liang Z., Lyons J. D., Fay K. T., Chen C.-w., et al. (2019). Regulators of Intestinal Epithelial Migration in Sepsis. Shock 51 (1), 88–96. 10.1097/shk.0000000000001117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza M., Hreinsson J., Strand M.-L., Hovatta O., Söder O., Philipson L., et al. (2006). Coxsackievirus and Adenovirus Receptor (CAR) Is Expressed in Male Germ Cells and Forms a Complex with the Differentiation Factor JAM-C in Mouse Testis. Exp. Cel Res. 312 (6), 817–830. 10.1016/j.yexcr.2005.11.030 [DOI] [PubMed] [Google Scholar]
- Monteiro A. C., Luissint A.-C., Sumagin R., Lai C., Vielmuth F., Wolf M. F., et al. (2014). Trans-dimerization of JAM-A Regulates Rap2 and Is Mediated by a Domain that Is Distinct from the Cis-Dimerization Interface. MBoC 25 (10), 1574–1585. 10.1091/mbc.E14-01-0018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moriconi F., Malik I., Ahmad G., Dudas J., Rave-Fränk M., Vorwerk H., et al. (2009). Effect of Irradiation on Gene Expression of Rat Liver Adhesion Molecules. Strahlenther Onkol 185 (7), 460–468. 10.1007/s00066-009-1964-1 [DOI] [PubMed] [Google Scholar]
- Morton P. E., Hicks A., Ortiz-Zapater E., Raghavan S., Pike R., Noble A., et al. (2016). TNFα Promotes CAR-dependent Migration of Leukocytes across Epithelial Monolayers. Sci. Rep. 6, 26321. 10.1038/srep26321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mueller M., Herzog C., Larmann J., Schmitz M., Hilfiker-Kleiner D., Gessner J. E., et al. (2013). The Receptor for Activated Complement Factor 5 (C5aR) Conveys Myocardial Ischemic Damage by Mediating Neutrophil Transmigration. Immunobiology 218 (9), 1131–1138. 10.1016/j.imbio.2013.03.006 [DOI] [PubMed] [Google Scholar]
- Murakami M., Francavilla C., Torselli I., Corada M., Maddaluno L., Sica A., et al. (2010). Inactivation of Junctional Adhesion Molecule-A Enhances Antitumoral Immune Response by Promoting Dendritic Cell and T Lymphocyte Infiltration. Cancer Res. 70 (5), 1759–1765. 10.1158/0008-5472.Can-09-1703 [DOI] [PubMed] [Google Scholar]
- Murakami T., Takasawa A., Takasawa K., Akimoto T., Aoyama T., Magara K., et al. (2021). Aberrant Expression of Junctional Adhesion molecule‐A Contributes to the Malignancy of Cervical Adenocarcinoma by Interaction with Poliovirus receptor/CD155. Cancer Sci. 112 (2), 906–917. 10.1111/cas.14734 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagamatsu G., Ohmura M., Mizukami T., Hamaguchi I., Hirabayashi S., Yoshida S., et al. (2006). A CTX Family Cell Adhesion Molecule, JAM4, Is Expressed in Stem Cell and Progenitor Cell Populations of Both Male Germ Cell and Hematopoietic Cell Lineages. Mol. Cel Biol 26 (22), 8498–8506. 10.1128/mcb.01502-06 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naik M. U., Naik T. U., Suckow A. T., Duncan M. K., Naik U. P. (2008). Attenuation of Junctional Adhesion Molecule-A Is a Contributing Factor for Breast Cancer Cell Invasion. Cancer Res. 68 (7), 2194–2203. 10.1158/0008-5472.Can-07-3057 [DOI] [PubMed] [Google Scholar]
- Naik M. U., Naik U. P. (2006). Junctional Adhesion Molecule-A-Induced Endothelial Cell Migration on Vitronectin Is Integrin αvβ3 Specific. J. Cel Sci 119 (Pt 3), 490–499. 10.1242/jcs.02771 [DOI] [PubMed] [Google Scholar]
- Naik M. U., Vuppalanchi D., Naik U. P. (2003). Essential Role of Junctional Adhesion Molecule-1 in Basic Fibroblast Growth Factor-Induced Endothelial Cell Migration. Atvb 23 (12), 2165–2171. 10.1161/01.Atv.0000093982.84451.87 [DOI] [PubMed] [Google Scholar]
- Naik U. P., Naik M. U. (2008). Putting the Brakes on Cancer Cell Migration. Cel Adhes. Migration 2 (4), 249–251. 10.4161/cam.2.4.6753 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naydenov N. G., Joshi S., Feygin A., Saini S., Litovchick L., Ivanov A. I. (2018). A Membrane Fusion Protein, Ykt6, Regulates Epithelial Cell Migration via microRNA-Mediated Suppression of Junctional Adhesion Molecule A. Cell Cycle 17 (14), 1812–1831. 10.1080/15384101.2018.1496755 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlandella F. M., Mariniello R. M., Iervolino P. L. C., Auletta L., De Stefano A. E., Ugolini C., et al. (2019). Junctional Adhesion Molecule-A Is Down-Regulated in Anaplastic Thyroid Carcinomas and Reduces Cancer Cell Aggressiveness by Modulating P53 and GSK3 α/β Pathways. Mol. Carcinog 58 (7), 1181–1193. 10.1002/mc.23001 [DOI] [PubMed] [Google Scholar]
- Orlova V. V., Economopoulou M., Lupu F., Santoso S., Chavakis T. (2006). Junctional Adhesion Molecule-C Regulates Vascular Endothelial Permeability by Modulating VE-Cadherin-Mediated Cell-Cell Contacts. J. Exp. Med. 203 (12), 2703–2714. 10.1084/jem.20051730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostermann G., Fraemohs L., Baltus T., Schober A., Lietz M., Zernecke A., et al. (2005). Involvement of JAM-A in Mononuclear Cell Recruitment on Inflamed or Atherosclerotic Endothelium. Atvb 25 (4), 729–735. 10.1161/01.ATV.0000157154.14474.3b [DOI] [PubMed] [Google Scholar]
- Ostermann G., Weber K. S. C., Zernecke A., Schröder A., Weber C. (2002). JAM-1 Is a Ligand of the β2 Integrin LFA-1 Involved in Transendothelial Migration of Leukocytes. Nat. Immunol. 3 (2), 151–158. 10.1038/ni755 [DOI] [PubMed] [Google Scholar]
- Ozaki H., Ishii K., Horiuchi H., Arai H., Kawamoto T., Okawa K., et al. (1999). Cutting Edge: Combined Treatment of TNF-Alpha and IFN-Gamma Causes Redistribution of Junctional Adhesion Molecule in Human Endothelial Cells. J. Immunol. 163 (2), 553–557. [PubMed] [Google Scholar]
- Palmer G., Busso N., Aurrand-Lions M., Talabot-Ayer D., Chobaz-Péclat V., Zimmerli C., et al. (2007). Expression and Function of Junctional Adhesion Molecule-C in Human and Experimental Arthritis. Arthritis Res. Ther. 9 (4), R65. 10.1186/ar2223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmeri D., van Zante A., Huang C.-C., Hemmerich S., Rosen S. D. (2000). Vascular Endothelial junction-associated Molecule, a Novel Member of the Immunoglobulin Superfamily, Is Localized to Intercellular Boundaries of Endothelial Cells. J. Biol. Chem. 275 (25), 19139–19145. 10.1074/jbc.M003189200 [DOI] [PubMed] [Google Scholar]
- Parise L. V. (2003). JAM-1 Regulation of Endothelial Cell Migration: Implications for Angiogenesis. Atvb 23 (12), 2119–2120. 10.1161/01.atv.0000102926.54780.e7 [DOI] [PubMed] [Google Scholar]
- Peddibhotla S. S. D., Brinkmann B. F., Kummer D., Tuncay H., Nakayama M., Adams R. H., et al. (2013). Tetraspanin CD9 Links Junctional Adhesion Molecule-A to αvβ3 Integrin to Mediate Basic Fibroblast Growth Factor-specific Angiogenic Signaling. MBoC 24 (7), 933–944. 10.1091/mbc.E12-06-0481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pellegrini M., Claps G., Orlova V. V., Barrios F., Dolci S., Geremia R., et al. (2011). Targeted JAM-C Deletion in Germ Cells by Spo11-Controlled Cre Recombinase. J. Cel Sci 124 (Pt 1), 91–99. 10.1242/jcs.072959 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer F., Kumar V., Butz S., Vestweber D., Imhof B. A., Stein J. V., et al. (2008). Distinct Molecular Composition of Blood and Lymphatic Vascular Endothelial Cell Junctions Establishes Specific Functional Barriers within the Peripheral Lymph Node. Eur. J. Immunol. 38 (8), 2142–2155. 10.1002/eji.200838140 [DOI] [PubMed] [Google Scholar]
- Polisetti N., Zenkel M., Menzel-Severing J., Kruse F. E., Schlötzer-Schrehardt U. (2016). Cell Adhesion Molecules and Stem Cell-Niche-Interactions in the Limbal Stem Cell Niche. Stem Cells 34 (1), 203–219. 10.1002/stem.2191 [DOI] [PubMed] [Google Scholar]
- Qi L., Liu J., Zhu H., Li Z., Lu K., Li T., et al. (2014). Inhibition of Glioma Proliferation and Migration by Magnetic Nanoparticle Mediated JAM-2 Silencing. J. Mater. Chem. B 2 (41), 7168–7175. 10.1039/c4tb00954a [DOI] [PubMed] [Google Scholar]
- Qi L., Shao W., Shi D. (2013). JAM-2 siRNA Intracellular Delivery and Real-Time Imaging by Proton-Sponge Coated Quantum Dots. J. Mater. Chem. B 1 (5), 654–660. 10.1039/c2tb00027j [DOI] [PubMed] [Google Scholar]
- Rabquer B. J., Amin M. A., Teegala N., Shaheen M. K., Tsou P.-S., Ruth J. H., et al. (2010). Junctional Adhesion Molecule-C Is a Soluble Mediator of Angiogenesis. J.I. 185 (3), 1777–1785. 10.4049/jimmunol.1000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rabquer B. J., Pakozdi A., Michel J. E., Gujar B. S., Haines G. K., Imhof B. A., et al. (2008). Junctional Adhesion Molecule C Mediates Leukocyte Adhesion to Rheumatoid Arthritis Synovium. Arthritis Rheum. 58 (10), 3020–3029. 10.1002/art.23867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roh S.-E., Jeong Y., Kang M.-H., Bae Y.-S. (2018). Junctional Adhesion Molecules Mediate Transendothelial Migration of Dendritic Cell Vaccine in Cancer Immunotherapy. Cancer Lett. 434, 196–205. 10.1016/j.canlet.2018.07.029 [DOI] [PubMed] [Google Scholar]
- Sarkar O., Xia W., Mruk D. D. (2006). Adjudin-mediated junction Restructuring in the Seminiferous Epithelium Leads to Displacement of Soluble Guanylate Cyclase from Adherens Junctions. J. Cel. Physiol. 208 (1), 175–187. 10.1002/jcp.20651 [DOI] [PubMed] [Google Scholar]
- Scheiermann C., Colom B., Meda P., Patel N. S. A., Voisin M.-B., Marrelli A., et al. (2009). Junctional Adhesion Molecule-C Mediates Leukocyte Infiltration in Response to Ischemia Reperfusion Injury. Atvb 29 (10), 1509–1515. 10.1161/atvbaha.109.187559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schenkel A. R., Mamdouh Z., Muller W. A. (2004). Locomotion of Monocytes on Endothelium Is a Critical Step during Extravasation. Nat. Immunol. 5 (4), 393–400. 10.1038/ni1051 [DOI] [PubMed] [Google Scholar]
- Schmitt M. M. N., Fraemohs L., Hackeng T. M., Weber C., Koenen R. R. (2014a). Atherogenic Mononuclear Cell Recruitment Is Facilitated by Oxidized Lipoprotein-Induced Endothelial Junctional Adhesion Molecule-A Redistribution. Atherosclerosis 234 (2), 254–264. 10.1016/j.atherosclerosis.2014.03.014 [DOI] [PubMed] [Google Scholar]
- Schmitt M. M. N., Megens R. T. A., Zernecke A., Bidzhekov K., van den Akker N. M., Rademakers T., et al. (2014b). Endothelial Junctional Adhesion Molecule-A Guides Monocytes into Flow-dependent Predilection Sites of Atherosclerosis. Circulation 129 (1), 66–76. 10.1161/circulationaha.113.004149 [DOI] [PubMed] [Google Scholar]
- Scott D. W., Tolbert C. E., Burridge K. (2016). Tension on JAM-A Activates RhoA via GEF-H1 and P115 RhoGEF. MBoC 27 (9), 1420–1430. 10.1091/mbc.E15-12-0833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scott D. W., Tolbert C. E., Graham D. M., Wittchen E., Bear J. E., Burridge K. (2015). N-glycosylation Controls the Function of Junctional Adhesion Molecule-A. MBoC 26 (18), 3205–3214. 10.1091/mbc.E14-12-1604 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson E. A., Jiang L., Ivanov A. I., Mandell K. J., Nusrat A., Parkos C. A. (2008). Cis-dimerization Mediates Function of Junctional Adhesion Molecule A. MBoC 19 (5), 1862–1872. 10.1091/mbc.e07-09-0869 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson E. A., Lee W. Y., Capaldo C. T., Nusrat A., Parkos C. A. (2009). Junctional Adhesion Molecule A Interacts with Afadin and PDZ-GEF2 to Activate Rap1A, Regulate β1 Integrin Levels, and Enhance Cell Migration. MBoC 20 (7), 1916–1925. 10.1091/mbc.e08-10-1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Severson E. A., Parkos C. A. (2009a). Mechanisms of Outside-In Signaling at the Tight junction by Junctional Adhesion Molecule A. Ann. N.Y Acad. Sci. 1165, 10–18. 10.1111/j.1749-6632.2009.04034.x [DOI] [PubMed] [Google Scholar]
- Severson E. A., Parkos C. A. (2009b). Structural Determinants of Junctional Adhesion Molecule A (JAM-A) Function and Mechanisms of Intracellular Signaling. Curr. Opin. Cel. Biol. 21 (5), 701–707. 10.1016/j.ceb.2009.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao M., Ghosh A., Cooke V. G., Naik U. P., Martin-DeLeon P. A. (2008). JAM-A Is Present in Mammalian Spermatozoa where it Is Essential for normal Motility. Develop. Biol. 313 (1), 246–255. 10.1016/j.ydbio.2007.10.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheng S., Hu Y., Yu F., Tong W., Wang S., Cai Y., et al. (2020). circKIF4A Sponges miR-127 to Promote Ovarian Cancer Progression. Aging 12 (18), 17921–17929. 10.18632/aging.103389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sircar M., Bradfield P. F., Aurrand-Lions M., Fish R. J., Alcaide P., Yang L., et al. (2007). Neutrophil Transmigration under Shear Flow Conditions In Vitro Is Junctional Adhesion Molecule-C Independent. J. Immunol. 178 (9), 5879–5887. 10.4049/jimmunol.178.9.5879 [DOI] [PubMed] [Google Scholar]
- Sladojevic N., Stamatovic S. M., Keep R. F., Grailer J. J., Sarma J. V., Ward P. A., et al. (2014). Inhibition of Junctional Adhesion Molecule-A/LFA Interaction Attenuates Leukocyte Trafficking and Inflammation in Brain Ischemia/reperfusion Injury. Neurobiol. Dis. 67, 57–70. 10.1016/j.nbd.2014.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solimando A. G., Brandl A., Mattenheimer K., Graf C., Ritz M., Ruckdeschel A., et al. (2018). JAM-A as a Prognostic Factor and New Therapeutic Target in Multiple Myeloma. Leukemia 32 (3), 736–743. 10.1038/leu.2017.287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic S. M., Johnson A. M., Sladojevic N., Keep R. F., Andjelkovic A. V. (2017). Endocytosis of Tight junction Proteins and the Regulation of Degradation and Recycling. Ann. N.Y. Acad. Sci. 1397 (1), 54–65. 10.1111/nyas.13346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamatovic S. M., Sladojevic N., Keep R. F., Andjelkovic A. V. (2012). Relocalization of Junctional Adhesion Molecule A during Inflammatory Stimulation of Brain Endothelial Cells. Mol. Cell Biol. 32 (17), 3414–3427. 10.1128/mcb.06678-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Su L., Cheng C. Y., Mruk D. D. (2009). Drug Transporter, P-Glycoprotein (MDR1), Is an Integrated Component of the Mammalian Blood-Testis Barrier. Int. J. Biochem. Cel Biol. 41 (12), 2578–2587. 10.1016/j.biocel.2009.08.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sultana T., Hou M., Stukenborg J.-B., Töhönen V., Inzunza J., Chagin A. S., et al. (2014). Mice Depleted of the Coxsackievirus and Adenovirus Receptor Display normal Spermatogenesis and an Intact Blood-Testis Barrier. Reproduction (Cambridge, England) 147 (6), 875–883. 10.1530/rep-13-0653 [DOI] [PubMed] [Google Scholar]
- Tang F., Li J., Xie W., Mo Y., Ouyang L., Zhao F., et al. (2021). Bioactive Glass Promotes the Barrier Functional Behaviors of Keratinocytes and Improves the Re-epithelialization in Wound Healing in Diabetic Rats. Bioactive Mater. 6 (10), 3496–3506. 10.1016/j.bioactmat.2021.02.041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tarulli G. A., Meachem S. J., Schlatt S., Stanton P. G. (2008). Regulation of Testicular Tight Junctions by Gonadotrophins in the Adult Djungarian Hamster In Vivo. Reproduction (Cambridge, England) 135 (6), 867–877. 10.1530/rep-07-0572 [DOI] [PubMed] [Google Scholar]
- Tarulli G. A., Stanton P. G., Loveland K. L., Meyts E. R.-D., McLachlan R. I., Meachem S. J. (2013). A Survey of Sertoli Cell Differentiation in Men after Gonadotropin Suppression and in Testicular Cancer. Spermatogenesis 3 (1), e24014. 10.4161/spmg.24014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenan M., Aurrand-Lions M., Widmer V., Alimenti A., Burkhardt K., Lazeyras F., et al. (2009). Cooperative Expression of Junctional Adhesion Molecule-C and -B Supports Growth and Invasion of Glioma. Glia 58 (5), NA. 10.1002/glia.20941 [DOI] [PubMed] [Google Scholar]
- Thölmann S., Seebach J., Otani T., Florin L., Schnittler H., Gerke V., et al. (2022). JAM-A Interacts with α3β1 Integrin and Tetraspanins CD151 and CD9 to Regulate Collective Cell Migration of Polarized Epithelial Cells. Cell. Mol. Life Sci. 79 (2), 88. 10.1007/s00018-022-04140-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tian Y., Tian Y., Zhang W., Wei F., Yang J., Luo X., et al. (2015). Junctional Adhesion Molecule-A, an Epithelial-Mesenchymal Transition Inducer, Correlates with Metastasis and Poor Prognosis in Human Nasopharyngeal Cancer. Carcinogenesis 36 (1), 41–48. 10.1093/carcin/bgu230 [DOI] [PubMed] [Google Scholar]
- Tietz S., Périnat T., Greene G., Enzmann G., Deutsch U., Adams R., et al. (2018). Lack of Junctional Adhesion Molecule (JAM)-B Ameliorates Experimental Autoimmune Encephalomyelitis. Brain Behav. Immun. 73, 3–20. 10.1016/j.bbi.2018.06.014 [DOI] [PubMed] [Google Scholar]
- Tornavaca O., Chia M., Dufton N., Almagro L. O., Conway D. E., Randi A. M., et al. (2015). ZO-1 Controls Endothelial Adherens Junctions, Cell-Cell Tension, Angiogenesis, and Barrier Formation. J. Cel. Biol. 208 (6), 821–838. 10.1083/jcb.201404140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsou P. S., Coit P., Kilian N. C., Sawalha A. H. (2018). EZH2 Modulates the DNA Methylome and Controls T Cell Adhesion through Junctional Adhesion Molecule A in Lupus Patients. Arthritis Rheumatol. 70 (1), 98–108. 10.1002/art.40338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vences-Catalán F., Rajapaksa R., Kuo C.-C., Miller C. L., Lee A., Ramani V. C., et al. (2021). Targeting the Tetraspanin CD81 Reduces Cancer Invasion and Metastasis. Proc. Natl. Acad. Sci. USA 118 (24), e2018961118. 10.1073/pnas.2018961118 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdino P., Witherden D. A., Ferguson M. S., Corper A. L., Schiefner A., Havran W. L., et al. (20111993). Molecular Insights into γδ T Cell Costimulation by an Anti-JAML Antibody. Structure 19 (1), 80–89. 10.1016/j.str.2010.10.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vonlaufen A., Aurrand-Lions M., Pastor C., Lamagna C., Hadengue A., Imhof B., et al. (2006). The Role of Junctional Adhesion Molecule C (JAM-C) in Acute Pancreatitis. J. Pathol. 209 (4), 540–548. 10.1002/path.2007 [DOI] [PubMed] [Google Scholar]
- Wang C. Q. F., Cheng C. Y. (2007). A Seamless Trespass: Germ Cell Migration across the Seminiferous Epithelium during Spermatogenesis. J. Cel. Biol. 178 (4), 549–556. 10.1083/jcb.200704061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang C. Q. F., Mruk D. D., Lee W. M., Cheng C. Y. (2007). Coxsackie and Adenovirus Receptor (CAR) Is a Product of Sertoli and Germ Cells in Rat Testes Which Is Localized at the Sertoli-Sertoli and Sertoli-Germ Cell Interface. Exp. Cel Res. 313 (7), 1373–1392. 10.1016/j.yexcr.2007.01.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Y., Lui W.-Y. (2009). Opposite Effects of Interleukin-1α and Transforming Growth Factor-Β2 Induce Stage-specific Regulation of Junctional Adhesion Molecule-B Gene in Sertoli Cells. Endocrinology 150 (5), 2404–2412. 10.1210/en.2008-1239 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zheng J., Han Y., Zhang Y., Su L., Hu D., et al. (2018). JAM-A Knockdown Accelerates the Proliferation and Migration of Human Keratinocytes, and Improves Wound Healing in Rats via FAK/Erk Signaling. Cel Death Dis 9 (9), 848. 10.1038/s41419-018-0941-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber D. A., Sumagin R., McCall I. C., Leoni G., Neumann P. A., Andargachew R., et al. (2014). Neutrophil-derived JAML Inhibits Repair of Intestinal Epithelial Injury during Acute Inflammation. Mucosal Immunol. 7 (5), 1221–1232. 10.1038/mi.2014.12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Anastos K., Morgello S., Berman J. W. (2015). JAM-A and ALCAM Are Therapeutic Targets to Inhibit Diapedesis across the BBB of CD14+CD16+monocytes in HIV-Infected Individuals. J. Leukoc. Biol. 97 (2), 401–412. 10.1189/jlb.5A0714-347R [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams D. W., Calderon T. M., Lopez L., Carvallo-Torres L., Gaskill P. J., Eugenin E. A., et al. (2013). Mechanisms of HIV Entry into the CNS: Increased Sensitivity of HIV Infected CD14+CD16+ Monocytes to CCL2 and Key Roles of CCR2, JAM-A, and ALCAM in Diapedesis. PLoS One 8 (7), e69270. 10.1371/journal.pone.0069270 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams L. A., Martin-Padura I., Dejana E., Hogg N., Simmons D. L. (1999). Identification and Characterisation of Human Junctional Adhesion Molecule (JAM). Mol. Immunol. 36 (17), 1175–1188. 10.1016/s0161-5890(99)00122-4 [DOI] [PubMed] [Google Scholar]
- Witherden D. A., Verdino P., Rieder S. E., Garijo O., Mills R. E., Teyton L., et al. (2010). The Junctional Adhesion Molecule JAML Is a Costimulatory Receptor for Epithelial γδ T Cell Activation. Science 329 (5996), 1205–1210. 10.1126/science.1192698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojcikiewicz E. P., Koenen R. R., Fraemohs L., Minkiewicz J., Azad H., Weber C., et al. (2009). LFA-1 Binding Destabilizes the JAM-A Homophilic Interaction during Leukocyte Transmigration. Biophysical J. 96 (1), 285–293. 10.1529/biophysj.108.135491 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong E. W. P., Mruk D. D., Lee W. M., Cheng C. Y. (2008). Par3/Par6 Polarity Complex Coordinates Apical Ectoplasmic Specialization and Blood-Testis Barrier Restructuring during Spermatogenesis. Proc. Natl. Acad. Sci. 105 (28), 9657–9662. 10.1073/pnas.0801527105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A., Reichel C. A., Khandoga A., Corada M., Voisin M.-B., Scheiermann C., et al. (2007). JAM-A Mediates Neutrophil Transmigration in a Stimulus-specific Manner In Vivo: Evidence for Sequential Roles for JAM-A and PECAM-1 in Neutrophil Transmigration. Blood 110 (6), 1848–1856. 10.1182/blood-2006-09-047431 [DOI] [PubMed] [Google Scholar]
- Woodfin A., Voisin M.-B., Beyrau M., Colom B., Caille D., Diapouli F.-M., et al. (2011). The Junctional Adhesion Molecule JAM-C Regulates Polarized Transendothelial Migration of Neutrophils In Vivo. Nat. Immunol. 12 (8), 761–769. 10.1038/ni.2062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodfin A., Voisin M.-B., Imhof B. A., Dejana E., Engelhardt B., Nourshargh S. (2009). Endothelial Cell Activation Leads to Neutrophil Transmigration as Supported by the Sequential Roles of ICAM-2, JAM-A, and PECAM-1. Blood 113 (24), 6246–6257. 10.1182/blood-2008-11-188375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D., Zeng Y., Fan Y., Wu J., Mulatibieke T., Ni J., et al. (2016). Reverse-migrated Neutrophils Regulated by JAM-C Are Involved in Acute Pancreatitis-Associated Lung Injury. Sci. Rep. 6, 20545. 10.1038/srep20545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu K. Z., Li K., Galileo D. S., Martin-DeLeon P. A. (2017). Junctional Adhesion Molecule A: Expression in the Murine Epididymal Tract and Accessory Organs and Acquisition by Maturing Sperm. Mol. Hum. Reprod. 23 (2), 132–140. 10.1093/molehr/gaw082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Guo X., Yang L., Wang Y., Tang Y., Yang Y., et al. (2014). Mesenchymal Stem Cells with Modification of Junctional Adhesion Molecule a Induce Hair Formation. Stem Cell Transl Med 3 (4), 481–488. 10.5966/sctm.2013-0165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu M., Ji S., Xiao S., Kong Z., Fang H., Zhang Y., et al. (2015). JAM-A Promotes Wound Healing by Enhancing Both Homing and Secretory Activities of Mesenchymal Stem Cells. Clin. Sci. (Lond). 129 (7), 575–588. 10.1042/cs20140735 [DOI] [PubMed] [Google Scholar]
- Xia W., Mruk D. D., Cheng C. Y. (2007). C-type Natriuretic Peptide Regulates Blood-Testis Barrier Dynamics in Adult Rat Testes. Proc. Natl. Acad. Sci. 104 (10), 3841–3846. 10.1073/pnas.0610100104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia W., Wong E. W. P., Mruk D. D., Cheng C. Y. (2009). TGF-β3 and TNFα Perturb Blood-Testis Barrier (BTB) Dynamics by Accelerating the Clathrin-Mediated Endocytosis of Integral Membrane Proteins: A New Concept of BTB Regulation during Spermatogenesis. Develop. Biol. 327 (1), 48–61. 10.1016/j.ydbio.2008.11.028 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Cheng C. Y. (2005). Blood-testis Barrier Dynamics Are Regulated by an Engagement/disengagement Mechanism between Tight and Adherens Junctions via Peripheral Adaptors. Proc. Natl. Acad. Sci. 102 (33), 11722–11727. 10.1073/pnas.0503855102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Mruk D. D., Lee W. M., Cheng C. Y. (2007). Ectoplasmic Specialization: a Friend or a Foe of Spermatogenesis? Bioessays 29 (1), 36–48. 10.1002/bies.20513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan H. H. N., Mruk D. D., Lee W. M., Yan Cheng C. (2008). Blood‐testis Barrier Dynamics Are Regulated by Testosterone and Cytokinesviatheir Differential Effects on the Kinetics of Protein Endocytosis and Recycling in Sertoli Cells. FASEB j. 22 (6), 1945–1959. 10.1096/fj.06-070342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.-R., Li B.-B., Hu Y., Zhang L., Wang X.-Z. (2020). Oxidative Stress Mediates Heat-Induced Changes of Tight junction Proteins in Porcine Sertoli Cells via Inhibiting CaMKKβ-AMPK Pathway. Theriogenology 142, 104–113. 10.1016/j.theriogenology.2019.09.031 [DOI] [PubMed] [Google Scholar]
- Yang W., Yin R., Zhu X., Yang S., Wang J., Zhou Z., et al. (2021). Mesenchymal Stem-Cell-Derived Exosomal miR-145 Inhibits Atherosclerosis by Targeting JAM-A. Mol. Ther. - Nucleic Acids 23, 119–131. 10.1016/j.omtn.2020.10.037 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye P., Yu H., Simonian M., Hunter N. (2011). Ligation of CD24 Expressed by Oral Epithelial Cells Induces Kinase Dependent Decrease in Paracellular Permeability Mediated by Tight junction Proteins. Biochem. Biophysical Res. Commun. 412 (1), 165–169. 10.1016/j.bbrc.2011.07.067 [DOI] [PubMed] [Google Scholar]
- Zen K., Babbin B. A., Liu Y., Whelan J. B., Nusrat A., Parkos C. A. (2004). JAM-C Is a Component of Desmosomes and a Ligand for CD11b/CD18-Mediated Neutrophil Transepithelial Migration. MBoC 15 (8), 3926–3937. 10.1091/mbc.e04-04-0317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zen K., Liu Y., McCall I. C., Wu T., Lee W., Babbin B. A., et al. (2005). Neutrophil Migration across Tight Junctions Is Mediated by Adhesive Interactions between Epithelial coxsackie and Adenovirus Receptor and a Junctional Adhesion Molecule-like Protein on Neutrophils. MBoC 16 (6), 2694–2703. 10.1091/mbc.e05-01-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zernecke A., Liehn E. A., Fraemohs L., von Hundelshausen P., Koenen R. R., Corada M., et al. (2006). Importance of Junctional Adhesion Molecule-A for Neointimal Lesion Formation and Infiltration in Atherosclerosis-Prone Mice. Atvb 26 (2), e10–3. 10.1161/01.ATV.0000197852.24529.4f [DOI] [PubMed] [Google Scholar]
- Zhang H., Yin Y., Wang G., Liu Z., Liu L., Sun F. (2014). Interleukin-6 Disrupts Blood-Testis Barrier through Inhibiting Protein Degradation or Activating Phosphorylated ERK in Sertoli Cells. Sci. Rep. 4, 4260. 10.1038/srep04260 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W., He R., Chen S., Zhang L., Cao G., Yang W., et al. (2020). The JAM-B/c-src/MMP9 Pathway Is Associated with Progression and Regulates the Invasion of Pancreatic Cancer. J. Cancer 11 (11), 3246–3255. 10.7150/jca.40953 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Lui W.-Y. (2015). Transforming Growth Factor-Β3 Regulates Cell junction Restructuring via MAPK-Mediated mRNA Destabilization and Smad-dependent Protein Degradation of Junctional Adhesion Molecule B (JAM-B). Biochim. Biophys. Acta (Bba) - Gene Regul. Mech. 1849 (6), 601–611. 10.1016/j.bbagrm.2015.03.005 [DOI] [PubMed] [Google Scholar]
- Zhao Z., Vajen T., Karshovska E., Dickhout A., Schmitt M. M., Megens R. T. A., et al. (2017). Deletion of Junctional Adhesion Molecule A from Platelets Increases Early-Stage Neointima Formation after Wire Injury in Hyperlipidemic Mice. J. Cel. Mol. Med. 21 (8), 1523–1531. 10.1111/jcmm.13083 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou D., Tang W., Zhang Y., An H.-X. (2019). JAM3 Functions as a Novel Tumor Suppressor and Is Inactivated by DNA Methylation in Colorectal Cancer. Cmar Vol. 11, 2457–2470. 10.2147/cmar.S189937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmerli C., Lee B. P. L., Palmer G., Gabay C., Adams R., Aurrand-Lions M., et al. (2009). Adaptive Immune Response in JAM-C-Deficient Mice: normal Initiation but Reduced IgG Memory. J. Immunol. 182 (8), 4728–4736. 10.4049/jimmunol.0803892 [DOI] [PubMed] [Google Scholar]