Abstract
COVID-19 (Coronavirus Disease 2019), illness with associated comorbidities and corticosteroid therapy makes the host immunocompromised and prone to opportunistic microbial infections. As the world continues to struggle with the pandemic of COVID-19, an increase in cases of opportunistic fungal infections have been reported from all over the world during the second wave of COVID-19 like aspergillosis, mucormycosis, and candidiasis. Scedosporium apiospermum is an emerging pathogen that is usually associated with mycetoma, pulmonary infection, and central nervous infections. It has been rarely associated with fungal rhinosinusitis (FRS). In this study, a rare case of FRS caused by S.apiospermum in an immunocompromised post-Covid-19 diabetic woman is reported.
Keywords: Fungal rhinosinusitis, Scedosporium apiospermum, MALDI-TOF/MS, Posaconazole
Introduction
Scedosporium species are ubiquitous, filamentous fungi commonly isolated from soil, polluted water bodies, and sewage. The genus Scedosporium consists of two medically important species Scedosporium apiospermum and Scedosporium prolificans.1 Recent molecular studies have shown that Scedosporium is a species complex comprising of five distinct groups, which are S. auranticum, Pseudoallescheria minutispora, S. dehoogii, S. apiospermum, and Pseudoallescheria boydii, the last group consisting of four subgroups which are P. boydii, P. angusta, P. ellipsoidea, and P. fusoidea. Also, S. prolificans is now renamed as Lomentospora prolificans.2
Scedosporiasis represents a broad spectrum of clinical diseases caused by agents of the genus Scedosporium. Infections caused by these organisms can be localized, extend to the surrounding tissues or disseminate via a hematogenous route to distant organs. The range of diseases caused by these fungi is broad, ranging from transient colonization of the respiratory tract to the involvement of abnormal airways, broncho-pulmonary reaction, and invasive localized disease. These infections include skin and soft tissue infections with extension to tendons, ligaments, bone, septic arthritis, osteomyelitis, pneumonia, endocarditis, brain abscess, sinusitis, otomycosis, keratitis, chorioretinitis, and endophthalmitis.1 Rhinosinusitis is a rare presentation of Scedosporium infections. According to Indian researchers, only six cases of fungal rhinosinusitis (FRS) caused by Pseudoallescheria boydii (teleomorph of Scedosporium apiospermum) have been reported from India to date. In a post-COVID-19 case, various fungal infections such as mucormycosis, candidiasis, aspergillosis, have been reported till now. Scedosporium apiospermum infection has not been reported much. This will be the second case report of Scedosporium apiospermum infection in a post-COVID-19 patient and the first case has been reported by Singla et al.3 Treatment of Scedosporium is challenging because of their resistance to various antifungals. In a study done in China, it was susceptible to the azole group of drugs. Voriconazole was the most active antifungal against Scedosporium species followed by posaconazole. Amphotericin B has been found to have limited activity. Itraconazole was found to have wide intraspecific diversity.2
Case report
A 64-year-old female patient, a known case of diabetes mellitus and hypertension was admitted to a local regional hospital with a provisional diagnosis of post-COVID viral pneumonia. During her stay in the hospital, she developed right-sided facial swelling, facial pain, and right-side headache. Computed tomography was done which showed inflammatory mucosal thickening in bilateral maxillary sinuses and sphenoid sinuses. Soft tissue swelling with fat stranding in the right maxillary, frontonasal and infraorbital region along with blockage of right osteomeatal complex was seen. However, no erosion was seen along the walls of paranasal sinuses (PNS).
Histopathological examination revealed fungal elements suggestive of Aspergillus spp. The patient was managed as a case of acute invasive fungal sinusitis. She underwent bilateral functional endoscopic sinus surgery (FESS), was put on injection amphotericin B, and was subsequently discharged. However, the patient had persisting symptoms along with swelling of the right side of the face for which she reported to our hospital. On ENT (ear, nose, and throat) examination post-FESS changes were seen. After examination in the oral and maxillofacial surgery (OMFS) OPD, multiple draining sinuses were observed in the alveolar mucosa of the maxilla Fig (1a, b) along with boggy swelling of the alveolar and palatal mucosa. Patient consent was obtained for images and inclusion in the study.
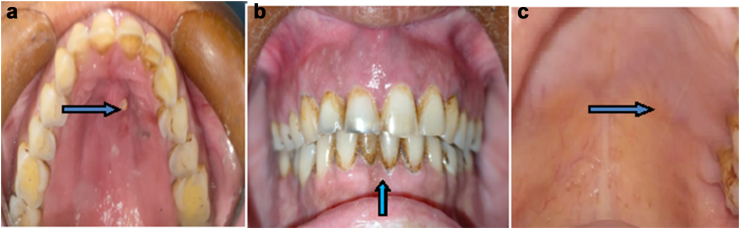
Fig. 1.
a: Boggy swelling in anterior maxilla involving the palatal mucosa with active draining abscess. b: Grade II tooth mobility present with respect to 11, 12, 13, 21, 22, and 23. c: Post-op follow up after three months.
A pus sample from the draining sinuses and a necrotic bone sample from the right maxillary alveolus was sent to our lab for KOH mount, fungal stain, and culture. No fungal elements were seen on KOH and fungal stain (Grocott's methenamine silver stain) on the pus sample. But KOH mount and fungal stain (GMS) from necrotic bone showed hyaline septate hyphae with acute angle branching (Fig. 3a) (Fig. 3b). The culture was done on sabouraud dextrose agar (SDA) at room temperature. Fungal growth was initially observed after 72 h as whitish-grey coloured colonies with cottony texture. Matrix-assisted laser desorption ionization-time of flight mass spectrometry (MALDI-TOF MS) was performed to identify the isolate. It identified the fungus as Scedosporium apiospermum with a score of 99.9% within 15 min (Fig. 3f). After 6–7 days of culture, colonies became greyish-brown in the center and grey–white at the periphery with a fluffy texture. The reverse side was orange in color (Fig. 3e). LPCB mount done from slide culture showed clavate to ovoid conidia which we rounded above with truncate bases. Conidia were elongated, simple, or branched conidiophores, and few were seen laterally over hyphae. It also shows septate hyphae with acute angle branching (Fig. 3c and d). The colony morphology of Aspergillus can vary with different species. Usually, colonies are velvety, have a floccose texture, and are pigmented on front and reverse. Colonies grow at very rapid growth and mature in three to four days. On microscopy, it has long conidiophores with uniseriate to biseriate heads bearing conidia which varies with different species. Colony morphology and microscopic features of Pseudoallescheria boydii and Scedoaporium apiospermum are quite similar so to differentiate between them we need molecular techniques.
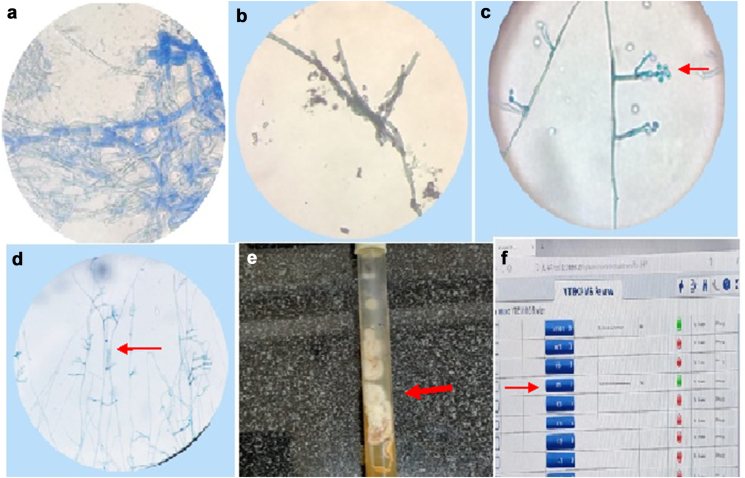
Fig. 3.
a: KOH mount with LPCB stain showing irregular branching with septate hyaline hyphae. b: Grocott's methenamine silver (GMS) stain showing acute branching in hyaline septate hyphae (x400). c: LPCB mount of Scedosporium apiospermum from slide culture showing clavate to ovoid conidia which are rounded above with truncate bases. Conidia are born on elongated, simple, or branched conidiophores and few are seen laterally over hyphae. Conidia having truncated base (x400). d: LPCB mount of slide culture showing acute angle branching of the hyphae (black arrow) (x400). e: Sabourauds dextrose agar (SDA) with white- to brown-colored growth seen with cottony texture after seven days of incubation. f: Fungal isolate from culture was identified as Scedosporium apiospermum to the accuracy of 99.9% by MALDI-TOF/MS.
Before the lab reports were out, the patient was thought to be a case of mucormycosis and was empirically started on injection amphotericin B (150 mg/kg). However, based on the lab reports, the injection of amphotericin B was stopped and the patient was managed with surgical debridement and antifungal treatment, which included syrup posaconazole (400 mg BD). Repeat computed tomography was carried out to find out the extent of invasion. It depicted bony destruction of the maxillary alveolus, maxilla, and hard palate along with changes suggesting the involvement of maxillary, ethmoidal, and sphenoidal sinus (Fig. 2a, b and c). The patient underwent repeat FESS for endoscopic clearance of the ethmoidal and sphenoidal sinuses followed by sequestrectomy, surgical debridement, and partial bilateral maxillectomy by the OMFS team. Patient was put on injectable antibiotics (injection Magnex (1.5 g/day), injection Flagyl (500 mg/TDS), injection Targocid (400 mg/TDS) and syrup posaconazole (400 mg BD) for three months.
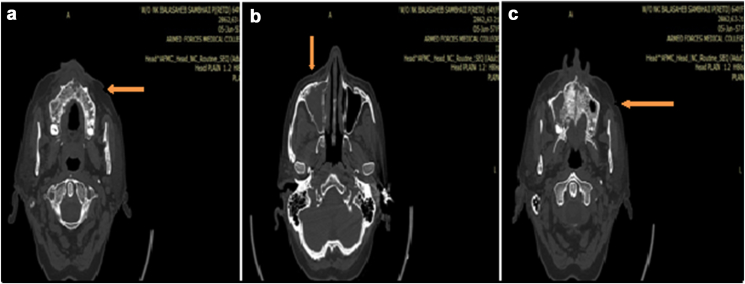
Fig. 2.
a: NCCT head and face reveals the necrotizing anterior maxilla with loss of cortical plates. b: NCCT image reveals the lesion involving leading to inflammation in the whole of the right maxillary sinus. c: NCCT image reveals extension of necrotizing lesion involving the whole of the maxilla.
The patient responded well to injectable antibiotics and antifungals. The patient was discharged after one week. The patient came for review after two weeks subsequently and was found to be stable and responding well to treatment. On further follow-up, post-op healing was satisfactory with no evidence of recurrence (Fig. 1c).
Discussion
Scedosporium is a species of fungus classified in the phylum Ascomycota. Mostly it is associated with eumycetoma.4 Sinus and CNS infections by Scedosporium apiospermum are not so common. Most of the reports of S. apiospermum sinusitis have involved the maxillary sinuses, with a few instances of the ethmoid, frontal, and sphenoid sinus involvement. In our case report, patient has presented with maxillary sinusitis which remains the most common involved sinus as per the literature.5 The source of the infection could be explained by understanding the epidemiology of Scedosporium species. Agricultural areas, as well as playgrounds and soils in urban surroundings, are found to be densely colonized with Scedosporium species.6, 3
Scedosporium species has got similar clinical as well as histopathological presentation as that of Aspergillus, so usually, it gets misdiagnosed as Aspergillus. Both appear as septate hyaline hyphae with acute angle branching on histopathological examination.7 A reporting pathologist needs to correlate the histopathology findings with microbiological diagnosis as an erroneous assumption of Aspergillus infection could lead to ineffective therapy with antifungal, and hence poor outcome.8 Colony morphology on culture and sporulation by culture can help in differentiating Aspergillus species and Scedosporium species infection; however, it can also sometimes lead to confusion.9 Hence, accurate identification of clinical isolates at the species level becomes of utmost importance as these species differ in their antifungal susceptibility pattern.10 Matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF/MS) can be used for fast and accurate diagnosis of the species from the clinical isolate.11 Species identification can be done by macroscopic and microscopic examination of the colonies but it should be always confirmed by molecular methods such as fungal PCR, genus/species-specific multiplex PCR. In our case, we used MALDI-TOF/MS and phenotypic methods to establish the diagnosis.12
Fungal sinusitis due to Scedosporium apiospermum has rarely been reported. In COVID -19 pandemic, mostly the isolates which have been reported are Aspergillus spp, Candida spp, and Mucor spp.11 In our study, patient has shown improvement after treatment with surgical debridement and antifungal treatment with posaconazole, and this is further strengthened by a study done on the treatment of Scedosporium apiospermum in a case of brain abscess.13 However, due to the scarcity of data and the potential publication bias, no solid recommendations can be provided for the therapy of Scedosporium infection. In vitro and in vivo data shows that S. apiospermum is resistant to amphotericin B and flucytosine and demonstrates variable susceptibility to itraconazole, voriconazole, posaconazole and micafungin.14
This case enlightens us to consider rare fungal infections as a differential diagnosis in a post-COVID-19 patient with an immunocompromised state. This case also highlights the importance of culture for fungal isolation over histopathological examination and the role of MALDI-TOF/MS in identification up to species level. Prompt diagnosis and early treatment are imperative in such patients for a better prognosis. This study brings out the role of Posaconazole in the treatment of fungal rhinosinusitis (FRS).
Disclosure of competing interest
The authors have none to declare.
Acknowledgements
Sub/LT Tarun Khan who helped with processing of the samples.
References
- 1.Cortez K.J., Roilides E., Quiroz-Telles F., et al. Infections caused by Scedosporium spp. Clin Microbiol Rev. 2008 Jan;21(1):157–197. doi: 10.1128/CMR.00039-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wang H., Wan Z., Li R., Lu Q., Yu J. Molecular identification and susceptibility of clinically relevant Scedosporium spp. in China. BioMed Res Int. 2015;2015:109656. doi: 10.1155/2015/109656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singla S., Singh V., Bansal A., Sharma J., Wadhwa Arora T., Sarma D.S. Post COVID-19 acute invasive fungal rhinosinusitis caused by Scedosporium apiospermum: a covert pathogen. Int J Otorhinolaryngol Head Neck Surg. 2021 Jun 23;7:1187. [Google Scholar]
- 4.Sahi H., Avery R.K., Minai O.A., et al. Scedosporium apiospermum (Pseudoallescheria boydii) infection in lung transplant recipients. J Heart Lung Transplant Off Publ Int Soc Heart Transpl. 2007 Apr;26(4):350–356. doi: 10.1016/j.healun.2007.01.011. [DOI] [PubMed] [Google Scholar]
- 5.Vibhute P.G., Surabhi V.R., Gomez A., et al. In: Clinical Mycology. 2nd ed. Anaissie E.J., McGinnis M.R., Pfaller M.A., editors. Churchill Livingstone; Edinburgh: 2009. Chapter 6 - radiology of fungal infections.https://www.sciencedirect.com/science/article/pii/B9781416056805000062 [Internet]. [cited 2021 Sep 14]. p. 109–59. Available from: [Google Scholar]
- 6.Ramirez-Garcia A., Pellon A., Rementeria A., et al. Scedosporium and Lomentospora: an updated overview of underrated opportunists. Med Mycol. 2018 Apr 1;56(suppl l_1):S102–S125. doi: 10.1093/mmy/myx113. [DOI] [PubMed] [Google Scholar]
- 7.Jiang Y., Gohara A.F., Mrak R.E., Muldrew K.L. In: Taliani G., editor. vol. 2020. 2020 Jun 24. Misidentification of Scedosporium boydii infection as Aspergillosis in a patient with chronic renal failure; p. 9727513. (Case Rep Infect Dis). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Naik P.P., Bhatt K., Richards E.C., et al. A rare case of fungal rhinosinusitis caused by Scedosporium apiopermum. Head Neck Pathol. 2021 Sep;15(3):1059–1063. doi: 10.1007/s12105-020-01248-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guarro J., Kantarcioglu A.S., Horré R., et al. Scedosporium apiospermum: changing clinical spectrum of a therapy-refractory opportunist. Med Mycol. 2006 Jun 1;44(4):295–327. doi: 10.1080/13693780600752507. [DOI] [PubMed] [Google Scholar]
- 10.Lackner M., de Hoog G.S., Verweij P.E., et al. Species-specific antifungal susceptibility patterns of Scedosporium and Pseudallescheria species. Antimicrob Agents Chemother. 2012 May;56(5):2635–2642. doi: 10.1128/AAC.05910-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sitterlé E., Giraud S., Leto J., et al. Matrix-assisted laser desorption ionization-time of flight mass spectrometry for fast and accurate identification of Pseudallescheria/Scedosporium species. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014 Sep;20(9):929–935. doi: 10.1111/1469-0691.12574. [DOI] [PubMed] [Google Scholar]
- 12.Chen S.C.-A., Halliday C.L., Hoenigl M., Cornely O.A., Meyer W. Scedosporium and Lomentospora infections: contemporary microbiological tools for the diagnosis of invasive disease. J Fungi Basel Switz. 2021 Jan 4;7(1):23. doi: 10.3390/jof7010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mellinghoff I.K., Winston D.J., Mukwaya G., Schiller G.J. Treatment of Scedosporium apiospermum brain abscesses with posaconazole. Clin Infect Dis. 2002 Jun 15;34(12):1648–1650. doi: 10.1086/340522. [DOI] [PubMed] [Google Scholar]
- 14.Tortorano A.M., Richardson M., Roilides E., et al. ESCMID and ECMM joint guidelines on diagnosis and management of hyalohyphomycosis: Fusarium spp., Scedosporium spp. and others. Clin Microbiol Infect Off Publ Eur Soc Clin Microbiol Infect Dis. 2014 Apr;20(suppl 3):27–46. doi: 10.1111/1469-0691.12465. [DOI] [PubMed] [Google Scholar]