Abstract
RATIONALE:
Gabapentin has shown initial promise as an opioid-sparing medication in pain patients as well as a treatment for opioid withdrawal and LC-MS/MS is often used for clinical monitoring. Despite reports of validated tandem masspectormetric methods for the determination of gabapentin and buprenorphine, mechanisms for the collision-induced fragmentation have not been adequetly described.
METHODS:
A rapid analytical method has been developed to determine the gabapentinoid, gabapentin, and partial opioid agonist, buprenorphine in 20 microliters of human serum using liquid chromatography-tandem mass spectrometry (LC-MS/MS) with a chromatographic run time of 2 minutes. A simplified sample cleanup procedure using methanol precipitation of serum proteins/lipids followed by evaporation and reconstitution in mobile phase was demonstrated. Gabapentin and buprenorphine were detected following positive ion electrospray ionization using multiple-reaction-monitoring. The internal standard approach was used for quantitation with labeled gabapentin-D10 and buprenorphine-D4 serving as internal standards. Using organic reaction principals and stable isotope labels, collision-induced fragmentation mechanisms for both gabapentin and buprenorphine are proposed. The method was validated according to the FDA Guidance for Industry – Bioanalytical Method Validation.
RESULTS:
Accuracy was demonstrated by error values ≤15% for buprenorphine and ≤6% for gabapentin. The inter-day precision was ≤4.88% and 15.59% for gabapentin and buprenorphine and the intra-day precision was ≤5.20% and 11.65% for gabapentin and buprenorphine. The lower limit of quantitation corresponded to 10 ng/mL for gabapentin and 1 ng/mL for buprenorphine in serum. Recoveries were 104% ± 2.55 and 85% ± 2.03 for gabapentin and buprenorphine, respectively.
CONCLUSIONS:
Concentrations of gabapentin and buprenorphine were determined for 5 authentic human serum samples to further validate the utility of the method and applicable to therapeutic drug monitoring beyond its use as a drug screening assay. Furthermore, new mechanisms for the collision-induced dissociation of gabapentin and buprenorphine have been proposed.
INTRODUCTION
Opioid abuse is a serious public health problem, with 70.5% of drug overdose deaths caused by prescription pain reliever and/or heroin overdose.1 From the 2019 National Survey on Drug Use and Health conducted by SAMHSA, an estimated 10.1 million people 12 or older were current misusers of pain relievers or heroin users.1 While these data represent a significant decrease in opioid misuse from 2018, opioid related deaths increased in 2019 by 4.6%.1 Buprenorphine continues to be the opioid with highest percentage of users (i.e. 27.8%) who acknowledge minuse of this prescription opioid.2
Although buprenorphine may resolve withdrawal symptoms faster than methadone, the symptoms are still moderate3 and highly variable among patients.4 Buprenorphine detoxification is also complicated by the emergence of withdrawal symptoms post-taper.5,6 Given that withdrawal symptoms are associated with relapse, identifying efficacious treatments for opioid withdrawal are a high priority. Therapeutic ranges for buprenorphine have been defined by Repetto and Repetto as less than 5 ng/mL in plasma, and by Kintz to be 2–20 ng/mL from a clinical study.7,8 Concentations ranging from 0.3–7.7 ng/mL of blood have been reported from 13 death cases involving buprenorphine but involved a concomitant intake of a psychotropic agent; in 9 out of these 13 cases, benzodiazepines were present; and a concomitant intake of narcotics or cocaine was observed in 3 cases.9 Plasma concentration of at least 0.8 ng/mL are needed for avoidance of withdrawal symptoms.10 With a mean therapeutic dose of 4 mg/ day the expected plasma concentration is between 0.7 and 1.6 ng/mL of buprenorphine.10,11 Buprenorphine has a narrow therapeutic range for treatment of pain in nonopioid abusers but, in maintenance therapy, up to 40-fold higher doses may be needed due to development of tolerance.12 For instance, plasma concentrations from a patient undergoing therapy with 32 mg of buprenorphine every other day were 30.0 ng/mL.2 From a pharmacodynamics perspective, efficacy and drug concentration show an inverse relationship to withdrawal symptoms in heroin-dependent patients.10,12 High interindividual variability in plasma levels has been shown, partially because of a polymorphism in the metabolic pathway.13 Buprenorphine blood testing is the desired method for dose optimization.14
Gabapentinoids alleviate certain pain conditions.15 They have been shown to attenuate morphine-induced conditioned place preference in rats16; enhance the analgesic effect of morphine in rats17 and healthy volunteers18; decrease postoperative morphine consumption and movement-related pain after radical mastectomy19; and block, as well as reverse, tolerance to the anti-nociceptive effects of morphine in the rat paw-pressure and tail-flick tests20, suggesting adjunct gabapentinoid use may have opioid-sparing benefits. Gabapentin is absorbed rapidly (tmax 2–3 h) in the GI tract by capacity-limited L-amino acid transport systems.21 Therapeutic concentrations of gabapentin ranged from 2 to 20 μg/mL in treatment-refractory patients with partial seizures.22
Gabapentinoids also have shown initial promise as adjunct treatments for opioid withdrawal.23–25 However, their effectiveness may lead to an increase in use or misuse of gabapentinoids such as gabapentin to self-medicate uncontrolled pain, anxiety, or withdrawal.23,26–33 Patients undergoing substance use disorder treatment have also admitted to misusing gabapentinoids to potentiate the effects of methadone or buprenorphine, as well as to avoid detection during routine urine drug screening.26,27,34 Gabapentin was initially marketed as an agent that had no significant risk for dependence or abuse—hence its lack of classification as a scheduled medication. However, those with a history of substance abuse may misuse any medication, particularly those that affect the dopaminergic reward system.29,34 This was demonstrated by a quality-improvement project in the United States where 22% of the 162 patients undergoing inpatient opioid detoxification reported misusing gabapentin.35 Because of the therapeutic as well as abuse potential of gabapentin, quantitation of both gabapentin and buprenorphine is important in detecting compliance for detoxification with buprenorphine and to indicate appropriate use of gabapentin.
Several methods have been published for the quantitation of buprenorphine (37–39, 42) and gabapentin (44–47). in a variety of human tissues and fluids. These methods are summarized in Table 1. Cao, et al., were the only group to report the simultaneous determination of buprenorphine and gabapentin (49). None of these previous reports using LC-MS/MS described a mechanism for the fragmentation of buprenorphine or gabapentin. In a targeted study, Biri, et al. have proposed a mechanism for the collision-induced dissociation buprenorphine (51), but we have found no reports on a proposed mechanism for the fragmentation of gabapentin.
Table 1.
Previously reported bioanalytical methodology for the determination of gabapentin and buprenorphine
| Analyte | Method | Linear Dynamic Range | Analysis Time | Reference |
|---|---|---|---|---|
| Buprenorphine | LC-HRMS | 20 – 10 000 pg/mL | 6 min | 37 |
| LC-MS/MS | 0.1 – 10 ng/mL | 6 min | 38 | |
| LC-MS/MS | 25 – 10 000 pg/mL | 4 min | 39 | |
| LC-MS/MS | 1 – 100 ng/mL | 34 min | 42 | |
| Gabapentin | LC-fluorescence* | 1000 – 26 000 ng/mL | 10 min | 45 |
| LC-MS/MS | 50 – 10 000 ng/mL | 4 min | 46 | |
| LC-MS/MS | 500 – 50 000 ng/mL | 17 min | 44 | |
| GC-MS | 500 – 10 000 ng/mL | 10 min | 47 | |
| Buprenorphine and Gabapentin | LC-MS/MS | 2 – 80 ng/mL and 100 – 4000 ng/mL | 11 min | 49 |
Pre-column derivatization with o-phthaldialdeyde
trimethylsilylated derivatives
Here we report a validated LC-MS based method to quantitate both gabapentin and buprenorphine using gabapentin-D10, and buprenorphine-D4 as internal standards (Figure 1) in human serum with a linear range of 100 ng/mL to 10,000 ng/mL for gabapentin and 1 ng/mL to 10,000 ng/mL for buprenorphine in 20 μL of serum. This method has simple sample preparation including precipitation of proteins using methanol, evaporation, and filtration followed by LC-MS/MS analysis with a 2 minute run time. Thus, the purpose of this study was development and validation of a rapid method for the simultaneous determination of gabapentin and buprenorphine. Futhermore, mechanisms for collision-induced-dissociation of gabapentin and buprenorphine are also proposed and reported herein for the first time.
Figure 1.
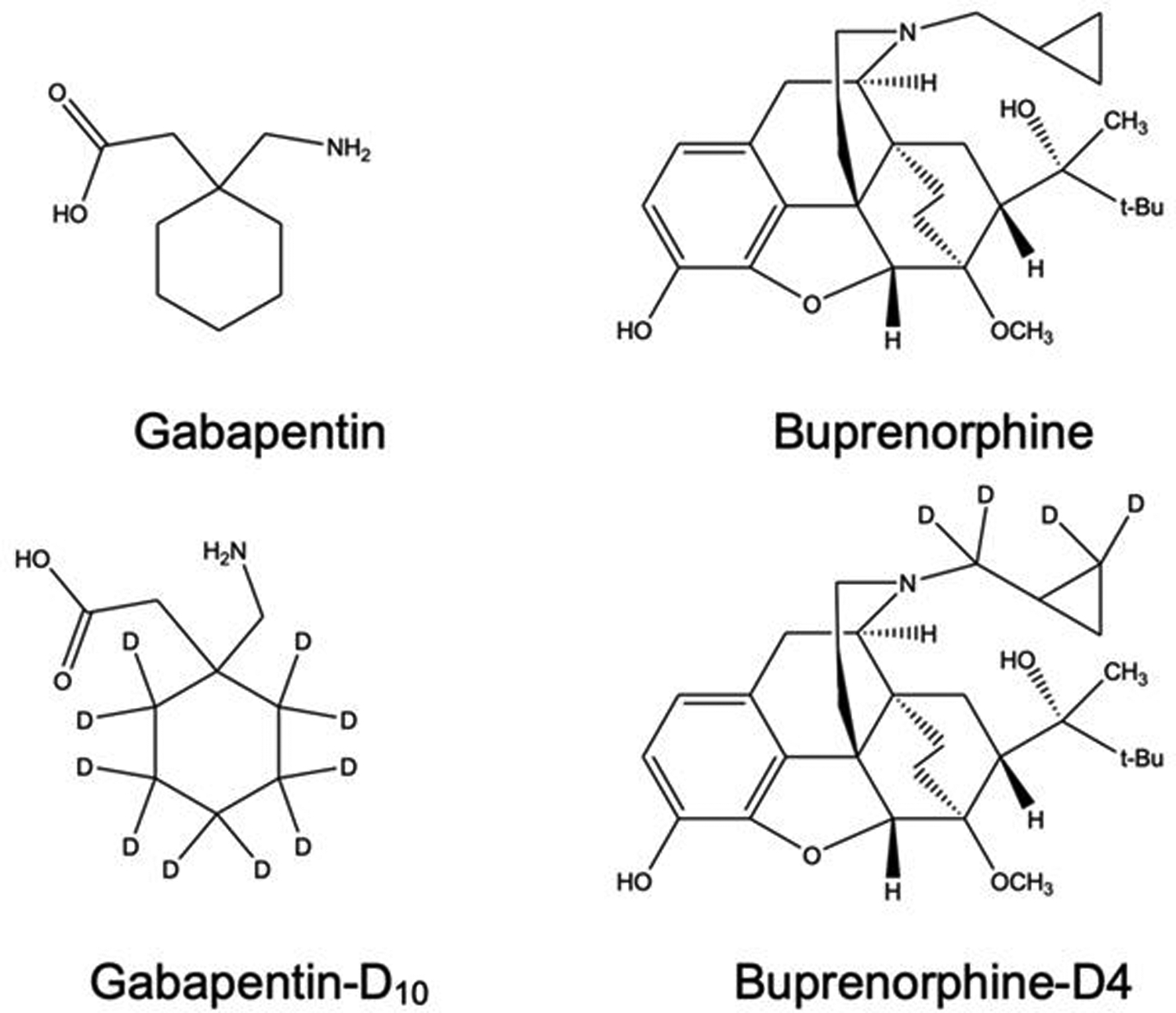
Structure for gabapentin, buprenorphine, and their respective stable isotopes.
2 |. EXPERIMENTAL
2.1. Chemicals.
Gabapentin, gabapentin-D10, buprenorphine, buprenorphine-D4 were purchased from Sigma (St. Louis, MO) as solutions in methanol. LC-MS grade formic acid, ammonium acetate, methanol and acetonitrile were purchased from Fisher Scientific (Asheville, NC), and 0.22 μm PVDF centrifugal filters were purchased from Millipore (Billerica, MA). Human serum was purchased from Innovative Research (Novi, MI).
2.2. Study Design.
The study protocol was approved by and in compliance with the University of Arkansas for Medical Sciences Institutional Research Board (Little Rock, AR), and all participants provided written informed consent before commencing the study. All subjects were adults between the ages of 18–65 who fulfilled DSM-5 criteria for moderate to severe opioid use disorder and submitted a urine sample negative for benzodiazepines and barbiturates prior to starting the study. Subjects were excluded if they reported having had a severe adverse reaction to study medications, had an unstable medical condition or stable medical condition that would interact with study medications or participation, including a current chronic pain or other medical condition that required ongoing opioid agonist treatment (determined by physician assessment), had a major psychiatric disorder (psychosis, schizophrenia, bipolar), current suicidal ideation or suicidal attempt in the past 12 months, had a seizure disorder, had major depression or anxiety disorder requiring psychoactive medication (as determined by physician), physiological dependence on alcohol or drugs other than opioids, tobacco or marijuana (as determined by physician assessment), were pregnant, planned to become pregnant, had inadequate birth control or were lactating, reported ongoing use of OTC or prescription drugs that would have major interaction with study drugs, had liver function tests >3 times normal, BUN and Creatinine outside normal range; ECG abnormalities; or pre-existing severe gastrointestinal narrowing (pathologic or iatrogenic).
Participants received 12 mg of buprenorphine per day for nine days. This was followed by a 10-day detox regimen where the buprenorphine dose was decreased to 1 mg/day.50 Gabapentin treatment began after two day washout period. The gabapentin dose was increased from 100 mg to 1600 mg over a seven day period. Serum samples (2 mL) were obtained for gabapentin/buprenorphine determination following 3 weeks of gabapentine (1600 mg/day).
2.3. Sample Preparation.
Calibration standards were prepared in human serum by placing a known amount of gabapentin and buprenorphine in methanol into a sample vial, evaporating the solution using nitrogen and adding serum. Preparation of serum samples for LC-MS analysis consisted of taking 20 μL of human serum, adding 1 mL of cold methanol containing the internal standards corresponding to 100 ng/mL and vortex-mixing for 20 s. The samples were then centrifuged at 6000 rpm at 4°C for 10 minutes and the supernatant was collected. The pellet was washed with another 1 mL of cold methanol and centrifuged and decanted. The combined supernatants were evaporated at 40°C under nitrogen and the residue reconstituted in 200 μL 95:5 10 mm ammonium formate: methanol, 0.1% formic acid. Samples were then centrifuged at 6000 rpm at 4°C for 10 minutes and the supernatant was collected. Samples were then filtered using 0.22 μm PVDF centrifugal filters and the filtrate was collected after being centrifuged at 12,000 × g for 4 minutes and injected onto the HPLC. To determine the effect of serum volume and extraction solvent on matrix ion effects and recovery 200 μL and 20 μL of serum were used with an extraction solvent of either acetonitrile or methanol. Calibration standards were treated in the same way as samples.
2.4. Chromatography.
The HPLC system was a Waters Acquity series (Waters Corp, Milford, MA) equipped with a sample manager, binary pump, in line degasser, and a column thermostat. The mass spectrometer was a Quattro Premier equipped with an electrospray ionization probe (Waters). Analytical separation was achieved on a Phenomenex Kinetex 5 μm Biphenyl 100Å analytical column (50 × 2.1 mm (i.d.)). The injection volume was 5 μL. Gabapentin and buprenorphine were separated using a linear binary gradient (Mobile phase A: 10 mM ammonium formate, Mobile phase B: methanol containing 0.1% formic acid). The flow rate was 0.4 mL/min, and the gradient was: Initial 5% (B), 1.25 min 95% (B) and held at 95%B for 0.45 minutes, then returned to 5% (B) at 1.85 minutes. The total run time was 2.00 minutes. The dead-time for the column (t0) was estimated using the equation t0 = (5 × 10−4)(L*(dc)2)/F, where L and dc are the column length and internal column diameter in mm. F is the flow rate in mL/min. The retention factor (k) for gabapentin and buprenorphine was calculated using the equation k = (tr/t0) – 1.
2.5. Mass Spectrometry.
Positive ions for gabapentin and gabapentin–D10 were generated using a cone voltage of 25 V, and for 60 V buprenorphine, and 70 V buprenorphine-D4. Product ions were generated using argon collision induced disassociation at a collision energy of 16 eV for gabapentin, and gabapentin-D10, 40 eV for buprenorphine, and 45 eV for buprenorphine-D4, while maintaining a collision cell pressure of 4.8× 10−3 torr. Detection was achieved in the multiple-reaction-monitoring (MRM) mode using the precursor/product ions, m/z 172.0 / 136.9, for gabapentin, 182.0 / 147.2 for gabapentin-D10, 468.4 / 396.3 for buprenorphine, and 472.4 / 400.4 for buprenorphine-D4. Data were acquired by MassLynx software (version 4.1) and calibration curves for the analyte were constructed using calibration samples using gabapentin or buprenorphine to internal standard peak-area ratios via a 1/x weighted least-squares linear regression. Unknown sample peak-area ratios were interpolated using the calibration curve to provide concentrations of gabapentin and buprenorphine.
2.6. Method Validation.
The selectivity was determined by comparing three blank human serum samples, three human serum samples spiked with buprenorphine and gabapentin, and five human serum samples from five different individuals treated with gabapentin and buprenorphine.
To validate linearity, five calibration standard sets of gabapentin and buprenorphine as well as human serum were determined in two independent runs. The peak area ratios for gabapentin and buprenorphine were used to construct calibration curves versus the concentration of gabapentin and buprenorphine respectively with weighted least squares linear regression (1/x). The criteria to confirm linearity and precision was an RSD ≤15% for non-LLOQ samples, and ≤20% for the LLOQ. The criteria to confirm accuracy was a relative error (RE) of 80–120%. The lower limit of quantitation (LLOQ) was defined as the lowest concentration with acceptable precision (RSD), 80–120% accuracy (RE).
To validate inter-day precision and accuracy, 5 sets of calibration standards prepared on two separate days and the precision and accuracy were determined. Intra-day precision and accuracy was determined by comparing 3 sets of calibration standards analyzed on the same day.
The process recoveries of gabapentin and buprenorphine were analyzed three times using a 100 ng/mL concentration. Process recoveries were calculated by comparing the peak areas of extracted serum spiked with gabapentin and buprenorphine before sample preparation with standards containing gabapentin and buprenorphine in mobile phase. Matrix effects were calculated using peak areas of samples spiked post extraction with standards containing gabapentin and buprenorphine and corresponding internal standards in mobile phase ((Post extraction spike/mobile phase std)×100%). Extraction recovery was determined by comparing serum spiked with gabapentin, buprenorphine and corresponding internal standards before and after extraction ((Pre extraction spike/Post extraction spike)×100%).
Post preparative stability was performed under autosampler conditions (4°C) for 4 hours and 24 hours. Freeze thaw stability was evaluated for three cycles by samples being frozen at −20°C for a minimum of 24 hours and thawed at room temperature.
3 |. Results and Discussion
3.1. Method Validation.
An LC-MS/MS based method has been developed to determine gabapentin and buprenorphine in human serum. Figure 2 shows LC-MS/MS chromatograms for control human serum (2A), serum spiked at the LLOQ concentration of 100 ng/mL of gabapentin and buprenorphine (2B), and from a serum sample collected from a patient transitioning from taking buprenorphine and gabapentin to only taking gabapentin (2C). Ion counts in the chromatographic response were obtained from peak heights of the ion transition. These results indicated that this patient was complient with the prescrible dosing regimen for the study. The retention time of gabapentin and gabapentin-D10 were 0.88 and 0.87 minutes and for buprenorphine and buprenorphine-D4 were 1.68 and 1.67 minutes. Given the rapid nature of this separation an estimation of the retention factor (k) is also useful.The lower limit of quantitation gabapentin and buprenorphine are shown in Table 3. The analytical recover was 98% and 110% for gabapentin (100 ng/mL) and buprenorphine (1 ng/mL), respectively. The corresponding imprecision was at these LLOQ values was 4.88% and 15.69%. The intraday analytical recovery and impression was maintained at 80–120% and <20%, respectively. Recovery and impression was 85–115% and <15% for all other calibrators. Figure 2 A and B show the response of gabapentin and buprenorphine for extracted control serum and extracted serum spiked at the LLOQ.
Figure 2.
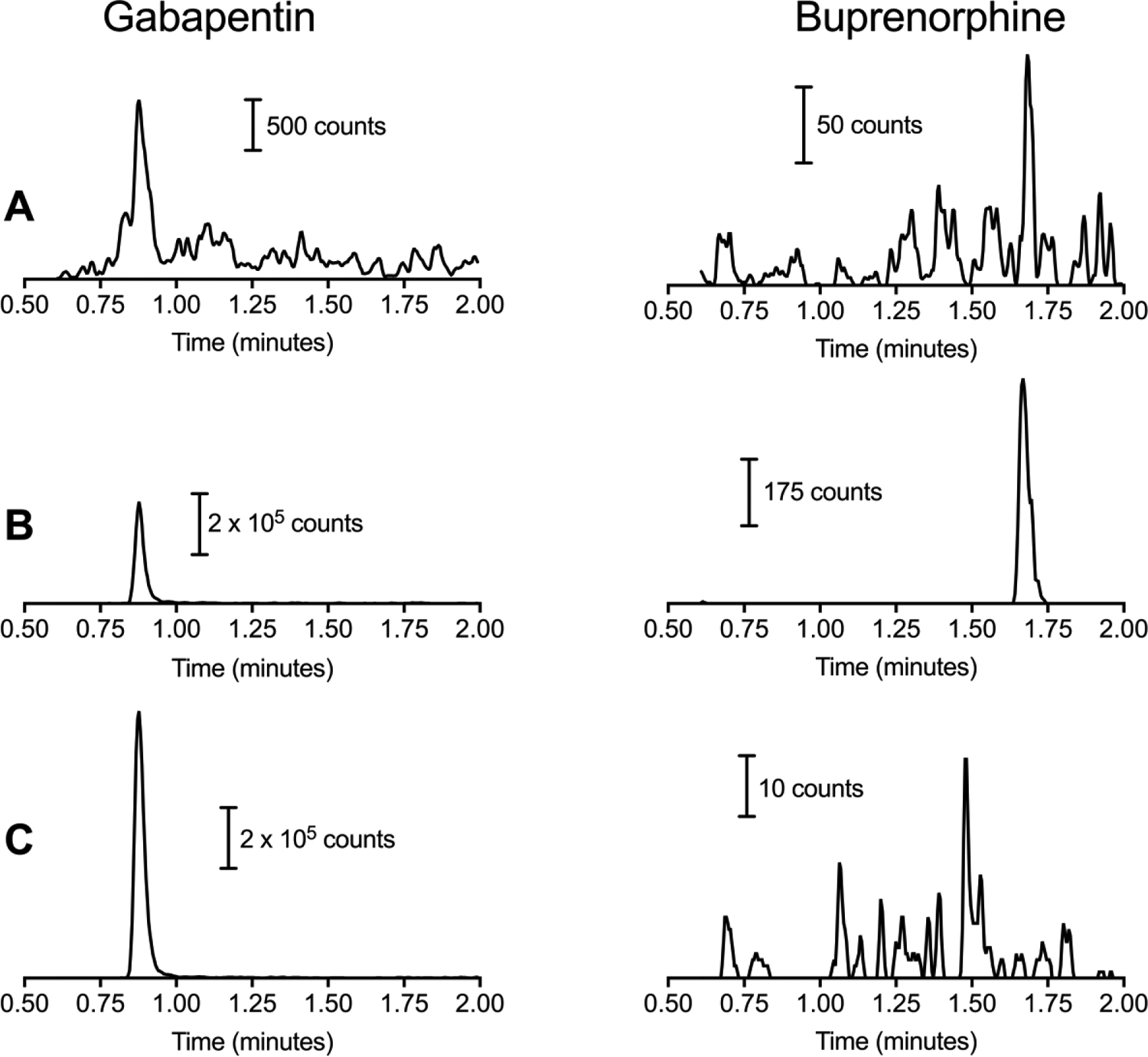
Representative LC-MS/MS chromatograms for gabapentin and buprenorphine. A) represents extracted blank serum, B) serum spiked with gabapentin and buprenorphine at the lower limit of quantitation and C) authentic clinical sample in the subject following progression from buprenorphine to gabapentin.
Table 3.
Inter-day and Intra-day Precisions for Gabapentin and Buprenorphine. Values are calculated as relative standard deviation.
| Gabapentin | ||||||||
|---|---|---|---|---|---|---|---|---|
| Standard Concentration (ng/mL) | Inter-day (n=3) | Intra-day(n=5) | ||||||
| Mean | SD | %RSD | %DFN | Mean | SD | %RSD | %DFN | |
| 100 | 98.0 | 4.78 | 4.88% | −1.95% | 97.5 | 5.17 | 5.30% | −2.47% |
| 300 | 310 | 8.46 | 2.73% | 3.34% | 307 | 9.82 | 3.20% | 2.24% |
| 1000 | 1003 | 32.2 | 3.21% | 0.28% | 1019 | 32.9 | 3.23% | 1.86% |
| 3000 | 2933 | 79.4 | 2.71% | −2.22% | 2940 | 67.8 | 2.31% | −2.01% |
| 10000 | 10056 | 97.8 | 0.97% | 0.56% | 10037 | 88.6 | 0.88% | 0.37% |
| Buprenorphine | ||||||||
| Standard Concentration (ng/mL) | Inter-day (n=3) | Intra-day(n=5) | ||||||
| Mean | SD | %RSD | %DFN | Mean | SD | %RSD | %DFN | |
| 1 | 1.1 | 0.17 | 15.59% | 7.72% | 1.1 | 0.13 | 11.65% | 9.24% |
| 30 | 29 | 2.62 | 8.98% | −2.87% | 29 | 1.92 | 6.67% | −3.99% |
| 100 | 94 | 10.5 | 11.12% | −5.69% | 96 | 8.3 | 8.66% | −4.11% |
| 300 | 292 | 15.8 | 5.40% | −2.59% | 296 | 12.7 | 4.30% | −1.46% |
| 1000 | 979 | 90.7 | 9.26% | −2.08% | 982 | 64.2 | 6.54% | −1.83% |
| 3000 | 2900 | 69.1 | 2.38% | −3.33% | 2887 | 218.3 | 7.56% | −3.75% |
| 10000 | 10025 | 227.0 | 2.26% | 0.25% | 10075 | 276.1 | 2.74% | 0.75% |
Recovery as well as matrix ion effect experiments were performed using both acetonitrile and methanol as extraction solvents and for methanol both 200 μL and 20 μL of serum. When acetonitrile was used for sample treatment, the process recovery for gabapentin overall was 94% but this was due to matrix ion enhancement effect of 164% and an extraction recovery of 58% (loss of 62%) as shown in Table 2. For buprenorphine, the extraction recovery was near 100% at 107% but the matrix ion effects were 140% leading to a process recovery of 150%. To counteract the issues of matrix ion effects, the extraction solvent was changed from acetonitrile to methanol. The matrix ion effects for gabapentin and buprenorphine were 84% and 86% respectively using methanol as an extraction solvent. The extraction recovery for gabapentin was similar in methanol (53%) and acetonitrile (58%). For buprenorphine, there was a loss in recovery of approximately 40% when switching the extraction solvent from acetonitrile to methanol (Table 1). To further improve the matrix ion effects and extraction recovery the amount of serum was decreased tenfold from 200 μL to 20 μL, this led to matrix ion effects of 102% for gabapentin and 101% buprenorphine and improved extraction recovery to 102% and 84% (Table 2).
Table 2.
Matrix ion effects, extraction recovery and process recovery for gabapentin and buprenorphine using acetonitrile and methanol as extraction solvents.
| Gabapentin | Buprenorphine | ||||
|---|---|---|---|---|---|
| Acetonitrile | Methanol | Acetonitrile | Methanol | ||
| 200 μL Serum | 20 μL Serum | 200 μL Serum | 20 μL Serum | ||
| 164% | 84% | 102% | 140% | 86% | 99% |
| 58% | 53% | 102% | 107% | 64% | 84% |
| 94% | 45% | 104% | 150% | 73% | 83% |
Accuracy of the assay was demonstrated by error values ≤7% for buprenorphine at non-LLOQ values and ≤18% at the LLOQ and ≤6% for gabapentin. These are within the FDA’s guidance for Industry for Bioanalytical Method Validation of 15% of the nominal value and 20% of the nominal value at the LLOQ. This confirms using a 1/x weighted least-squares linear regression best describes the concentration-response relationship.
The inter-day and intra-day precisions determined by the RSD values are shown in Table 3 for each individual concentration. The inter-day precision was ≤4.88% for gabapentin and ≤11.12% at non-LLOQ values and 15.59% at the LLOQ for buprenorphine. The intra-day precision was ≤5.30% and ≤11.65% for gabapentin and buprenorphine. The RSD values were all within the FDA guidelines of <15% and <20% for the LLOQ.
Analyte stability was determined for three freeze-thaw cycles at −20°C at three concentrations, 300 ng/mL, 1000 ng/mL and 3000 ng/mL. The average recovery for the first freeze-thaw was 95% for gabapentin, all recoveries were between 85–115%, and 107% for buprenorphine, with 89% (8 out of 9) of the recoveries between 85–115%. For the second freeze thaw the average recovery was 90% for gabapentin with 67% (6 out of 9) of the recoveries between 85–115% for gabapentin, and for buprenorphine 104% with 89% (8 out of 9) recoveries between 85–115%. The average recovery for the third freeze-thaw cycle was 87% with 67% (6 out of 9) of the recoveries between 85–115% and 110% for buprenorphine with all the recoveries between 85–115%. The acceptance criteria stated in the FDA’s guidance for Industry for Bioanalytical Method Validation states that 67% or more of the calibration standards analyzed must have recoveries between 85–115% and that not all replicates of the same concentration can have recoveries outside this range. For all three freeze-thaw cycles this criteria was met confirming freeze thaw stability for gabapentin and buprenorphine.
Analyte stability was further tested for samples stored at 4°C for 4 hours and 24 hours. Three samples (200 ng/mL, 600 ng/mL and 2000 ng/mL) were stored at 4°C for 4 hours. Recoveries from these samples were 85.1%, 98.5%, and 99.1% for gabapentin and 89.5%, 97.7%, and 108.6% for buprenorphine indicating stability at 4°C for 4 hours. Also, three sets of calibration standards were analyzed after being stored at 4°C for 24 hours. The average recovery for gabapentin was 81% ± 2.8%, and for buprenorphine was 95% ± 9.5%. For gabapentin 13% of the calibration standards had recoveries in the range of 85–115% with the remaining calibration standard having recoveries below 85% indicating that the gabapentin samples are not stable for 24 hours at 4°C. For buprenorphine 87% of the calibration standards were within the range of 85–115% indicating that buprenorphine in the sample is stable for 24 hours at 4°C.
3.2. Application of Methodology
Determination of gabapentin and buprenorphine in serum collected from buprenorphine-tapered patients taking gabapentin or placebo shows the robustness and utility of this assay. Gabapentin was detected in four of the five samples analyzed ranging from 2296 ng/mL to 9464 ng/mL (Table 4). Buprenorphine levels were below the limit of detection of 1 ng/mL as defined by the accuracy (80–120%) and precision (RSD<20%) at the lowest calibration standard.
Table 4.
Serum Concentrations of gabapentin and buprenorphine from five human subjects after transitioning to from buprenorphine to gabapentin.*
| Subject ID | Gabapentin Concentration (ng/mL) | Buprenorphine Concentration (ng/mL) |
|---|---|---|
| 1 | 9464 | ND |
| 2 | 2654 | ND |
| 3 | ND | ND |
| 4 | 4038 | ND |
| 5 | 2296 | ND |
100 mg of gabapentin twice a day on day 3 of week 1 followed by increasing doses of gabapentin daily of 200 mg, 400 mg, 600 mg, 800 mg, and 1600 mg though day 7 of week 1. The 1600 mg dose was continued through week 4. buprenorphine dosage was as follows; 4 mg given initially and 4 mg given 30 minutes later on day 1 followed by 12 mg given daily on day 2 through week 2 day 2. The dose was decreased to 8 mg on day 3 and 4 of week 2, 6 mg on days 5–7, 4 mg on week 3 day 1, 2 mg on week 3 days 2–3 and 1 mg on days 4–5 of week 3. Serum samples were collected at the beginning of week 4. ND indicates below the detection limit of 10 ng/mL for both gabapentin and buprenorphine.
3.3. Proposed Fragmentation Mechanism.
Product ion spectra for gabapentin and buprenorphine are shown in Figure 3A and 3B, respectively. A proposed mechanism for the fragmentation of gabapentin (m/z 172/137) is shown in Figure 4A. A charge migration fragmentation mechanism is proposed here with the initial step involving hydrogen migration to the hydroxyl group followed by loss of water from the precursor ion and leaving the charge on the acyl carbon. This is followed by neutral loss of ammonia and formation of the oxocylobutane carbocation product ion. Loss of the nitrogen is required under the nitrogen rule which states that protonated molecules containing exclusively hydrogen, carbon, nitrogen, oxygen, silicon, phosphorus, sulfur, and the halogens must lack a nitrogen or have an even number of nitrogens. Additionally, this mechanism is supported in part by the observed transition for the deuterated form of gabapentin (m/z 182/147; Figure 4B) where the product ion is 35 mass units less than the precursor ion. Similarly a proposed mechanism for the fragmentation of bupronorphine (m/z 468/396) is shown in Figure 5A. Here, a charge retention fragmentation mechanism is proposed with initial neutral loss of 2-methylpropene, followed by hydrogen migration and neutral loss of methane leading to formation of a 3-hydroxy tetrahydrofuran and the product ion. This mechanism is further supported in part by the observed transition for the stable isotope used in these studies as all deuterium atoms are retained in the product ion (Figure 5B). The ruggedness of this method could be improved by adding a second MRM transition as qualitative confirmation of peak identity. Since subjects in the current study were under tight control of their drug regimen, one transition was deemed satisfactory for the current study.
Figure 3.
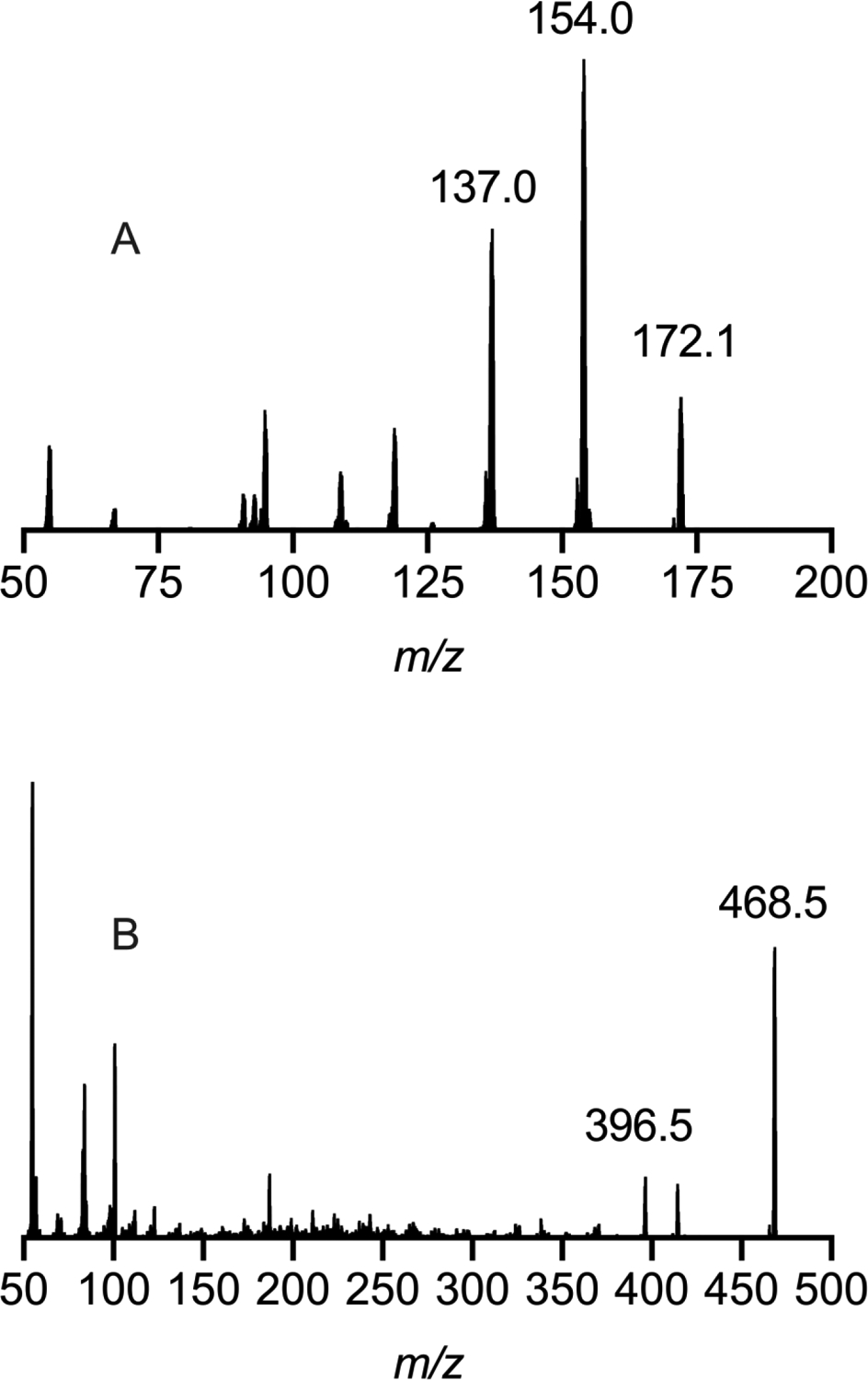
Product ion spectra for gabapentin (A) and buprenorphine (B).
Figure 4.
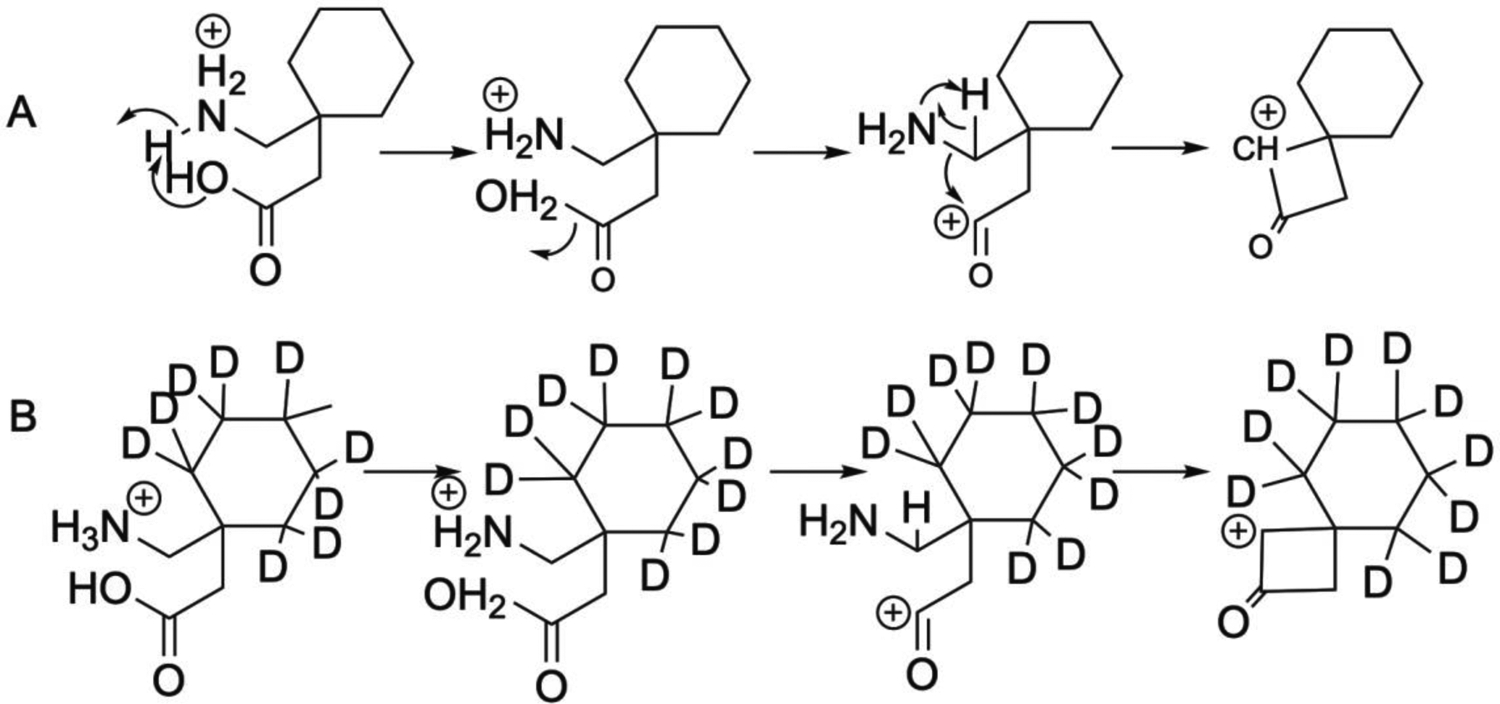
Proposed mechanism for collision-induced fragmentation of gabapentin (A) and its stable isotope (B).
Figure 5.
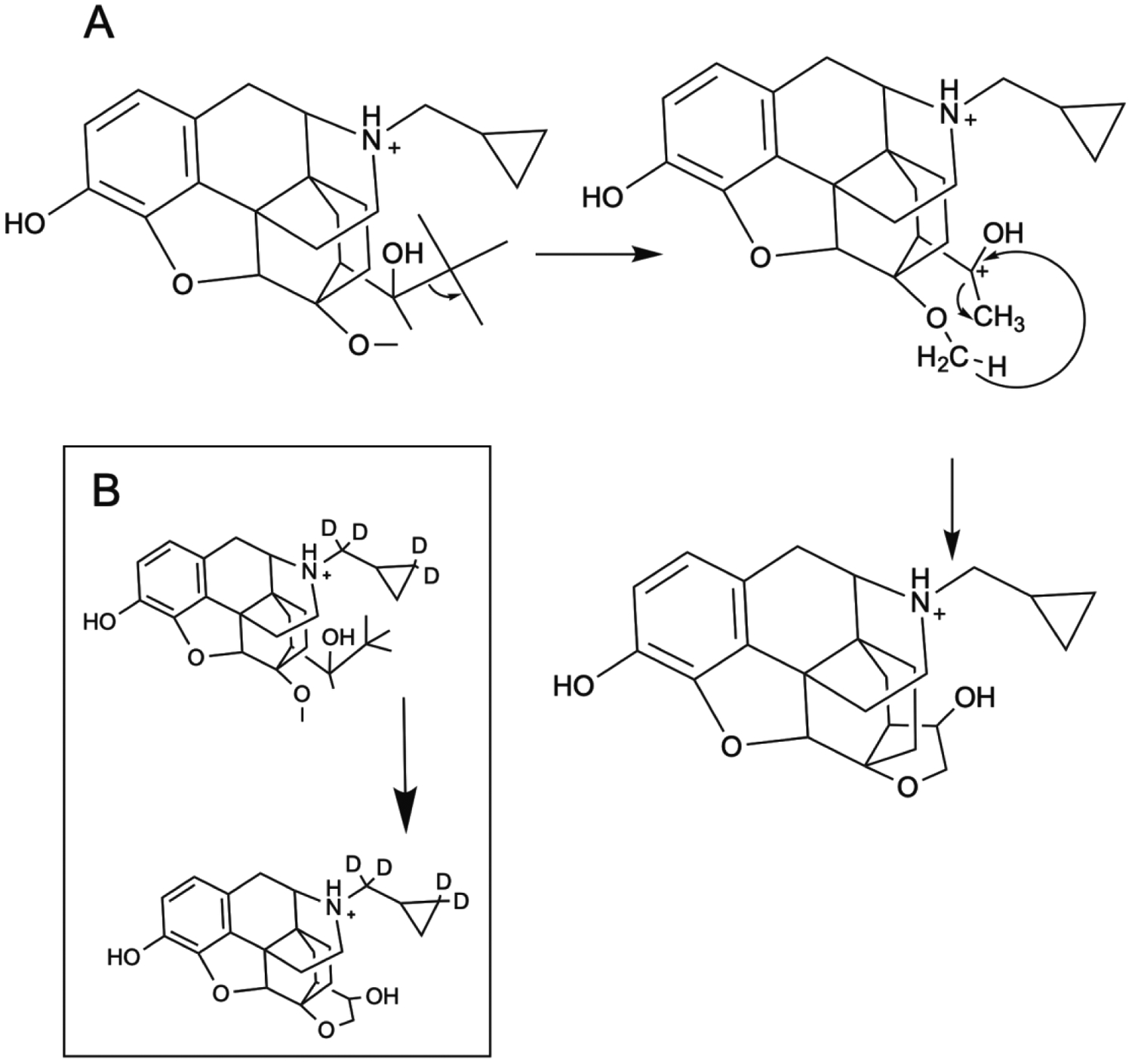
Proposed mechanism for collision-induced fragmentation of buprenorphine (A) and its stable isotope (B).
4 |. CONCLUSIONS
Reported here is an LC-MS/MS based method to determine both gabapentin and buprenorphine in human serum with an analysis time of 2 minutes. This method was validated for precision, accuracy, and recovery in accordance with FDA guidance for industry for bioanalytical validation. Recovery was 104% ± 3.59 for gabapentin and 83% ± 5.54 for buprenorphine when 20 μL of serum and methanol was used as an extraction solvent. Using 200 μL of serum decreased the extraction recovery by 50% for gabapentin and 20% for buprenorphine. Employing 200 μL of serum also led to matrix ion suppression for gabapentin and enhancement for buprenorphine of approximately 15%. The use of acetonitrile as an extraction solvent also produced significant matrix ion enhancement compared to using methanol as the extraction solvent.
With the widespread abuse of prescription opioids and potential therapeutic promise of gabapentin as an adjunct treatment in pain patients as well as during buprenorphine-assisted opioid detoxification, the need for rapid analysis for compliance is necessary. This LC-MS/MS based method described here can be of use for analyzing serum samples from patients undergoing buprenorphine detoxification from prescription opioids. Buprenorphine-specific methods have demonstrated LLOQ well below the 1 ng/mL reported herein.38 And while this higher LLOQ might be a seen as a limitation of the current study, it is fit for the purpose of determining if subjects were misusing buprenorphine.
While there are several reports describing the determination of gabapentin and buprenorphine, we propose a fragmentation mechanism based on movement of electron pair from areas of higher electron density to lower electron density. Biri, et. al. have described fragmentation for buprenorphine and its synthetic precursors.51 Interestingly, Biri, et al. also found an intense response for the m/z 468→396 (loss of C5H12), but proposed an aldehyde in final structure for the product ion where a hydroxy-tetrahydrofuran has been proposed in the current study. Formation of the aldehyde from the C(OH)(tBu)CH3 requires movement of electrons from a low electron density (C-C bond) to an area of higher electron density (C-OH bond), which is counter to organic chemistry principles. Careful selection of stable isotopes for buprenorphine could help with a more definitive fragmentation mechanism than was possible in the current study.
ACKNOWLEDGEMENTS.
This work was supported by the National Institutes of Health DA039088
References
- 1.Center for Behavioral Health Statistics and Quality. (2020). 2019 National Survey on Drug Use and Health: Methodological summary and definitions. Rockville, MD: Substance Abuse and Mental Health Services Administration. Retrieved from https://www.samhsa.gov/data/ [Google Scholar]
- 2.Cicero TJ, Ellis MS, Chilcoat HD. Understanding the use of diverted buprenorphine. Drug and Alcohol Dependence. 2018;193:117–23. [DOI] [PubMed] [Google Scholar]
- 3.Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009(3). [DOI] [PubMed] [Google Scholar]
- 4.Kleber HD. Pharmacologic treatments for opioid dependence: detoxification and maintenance options. Dialogues in clinical neuroscience. 2007;9(4):455–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sanders NC, Mancino MJ, Gentry WB, Guise JB, Bickel WK, Oliveto AH. Randomized, placebo-controlled pilot trial of gabapentin during an outpatient, buprenorphine-assisted detoxification procedure. Experimental and Clinical Psychopharmacology. 2013;21(4):294–302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gowing LR, Ali RL. The place of detoxification in treatment of opioid dependence. Current Opinion in Psychiatry. 2006;19:266–270. [DOI] [PubMed] [Google Scholar]
- 7.Repetto MR, Repetto M. Habitual, toxic, and lethal concentrations of 103 drugs of abuse in humans. Journal of Toxicology-Clinical Toxicology. 1997;35(1):1–9. [DOI] [PubMed] [Google Scholar]
- 8.Kintz P Deaths involving buprenorphine: a compendium of French cases. Forensic Science International. 2001;121(1–2):65–69. [DOI] [PubMed] [Google Scholar]
- 9.Oechsler S, Skopp G. Buprenorphine and major metabolites in blood specimens collected for drug analysis in law enforcement purposes. Forensic Science International. 2010;195(1–3):73–77. [DOI] [PubMed] [Google Scholar]
- 10.Kuhlman JJ, Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93(4):549–559. [DOI] [PubMed] [Google Scholar]
- 11.Chawarski MC, Schottenfeld RS, O’Connor PG, Pakes J. Plasma concentrations of buprenorphine 24 to 72 hours after dosing. Drug and Alcohol Dependence. 1999;55(1–2):157–163. [DOI] [PubMed] [Google Scholar]
- 12.Greenwald MK, Johanson CE, Moody DE, et al. Effects of buprenorphine maintenance dose on mu-opioid receptor availability, plasma concentrations, and antagonist blockade in heroin-dependent volunteers. Neuropsychopharmacology. 2003;28(11):2000–2009. [DOI] [PubMed] [Google Scholar]
- 13.Du J, Xing Q, Xu L, et al. Systematic screening for polymorphisms in the CYP3A4 gene in the Chinese population. Pharmacogenomics. 2006;7(6):831–841. [DOI] [PubMed] [Google Scholar]
- 14.Brunen S, Vincent PD, Baumann P, Hiemke C, Havemann-Reinecke U. Therapeutic drug monitoring for drugs used in the treatment of substance-related disorders: literature review using a therapeutic drug monitoring appropriateness rating scale. Therapeutic Drug Monitoring. 2011;33(5):561–572. [DOI] [PubMed] [Google Scholar]
- 15.Alguacil LF, Salas E, Gonzalez-Martin C. Identification of new drug targets and biomarkers related to obesity and eating disorders: an approach based on reward deficit and addiction. Current Pharmaceutical Design. 2011;17(5):462–470. [DOI] [PubMed] [Google Scholar]
- 16.Andrews N, Loomis S, Blake R, Ferrigan L, Singh L, McKnight AT. Effect of gabapentin-like compounds on development and maintenance of morphine-induced conditioned place preference. Psychopharmacology. 2001;157(4):381–387. [DOI] [PubMed] [Google Scholar]
- 17.Shimoyama M, Shimoyama N, Inturrisi CE, Elliott KJ. Gabapentin enhances the antinociceptive effects of spinal morphine in the rat tail-flick test. Pain. 1997;72(3):375–382. [DOI] [PubMed] [Google Scholar]
- 18.Eckhardt K, Ammon S, Hofmann U, Riebe A, Gugeler N, Mikus G. Gabapentin enhances the analgesic effect of morphine in healthy volunteers. Anesthesiology and Analgesia. 2000;91(1):185–191. [DOI] [PubMed] [Google Scholar]
- 19.Dirks J, Fredensborg BB, Christensen D, Fomsgaard JS, Flyger H, Dahl JB. A randomized study of the effects of single-dose gabapentin versus placebo on postoperative pain and morphine consumption after mastectomy. Anesthesiology. 2002;97(3):560–564. [DOI] [PubMed] [Google Scholar]
- 20.Gilron I, Biederman J, Jhamandas K, Hong M. Gabapentin blocks and reverses antinociceptive morphine tolerance in the rat paw-pressure and tail-flick tests. Anesthesiology. 2003;98(5):1288–1292. [DOI] [PubMed] [Google Scholar]
- 21.Vollmer KO, Vonhodenberg A, Kolle EU. Pharmacokinetics and metabolism of gabapentin in rat, dog, and man. Arzneimittel-Forschung/Drug Research. 1986;36–1(5):830–839. [PubMed] [Google Scholar]
- 22.Lindberger M, Luhr O, Johannessen SI, Larsson S, Tomson T. Serum concentrations and effects of gabapentin and vigabatrin: Observations from a dose titration study. Therapeutic Drug Monitoring. 2003;25(4):457–462. [DOI] [PubMed] [Google Scholar]
- 23.Salehi M, Kheirabadi GR, Maracy MR, Ranjkesh M. Importance of gabapentin dose in treatment of opioid withdrawal. Journal of Clinical Psychopharmacology. 2011;31(5):593–596. [DOI] [PubMed] [Google Scholar]
- 24.Freynhagen R, Backonja M, Schug S, et al. Pregabalin for the treatment of drug and alcohol withdrawal symptoms: A Comprehensive Review. Cns Drugs. 2016;30(12):1191–1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Martinez-Raga J, Sabater A, Perez-Galvez B, Castellano M, Cervera G. Add-on gabapentin in the treatment of opiate withdrawal. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 2004;28(3):599–601. [DOI] [PubMed] [Google Scholar]
- 26.Bastiaens L, Galus J, Mazur C. Abuse of gabapentin is associated with opioid addiction. Psychiatric Quarterly. 2016;87(4):763–767. [DOI] [PubMed] [Google Scholar]
- 27.Baird CRW, Fox P, Colvin LA. Gabapentinoid abuse in order to potentiate the effect of methadone: A Survey among Substance Misusers. European Addiction Research. 2014;20(3):115–118. [DOI] [PubMed] [Google Scholar]
- 28.Schwan S, Sundstrom A, Stjernberg E, Hallberg E, Hallberg P. A signal for an abuse liability for pregabalin-results from the Swedish spontaneous adverse drug reaction reporting system. European Journal of Clinical Pharmacology. 2010;66(9):947–953. [DOI] [PubMed] [Google Scholar]
- 29.Kruszewse SP, Paczynski RP, Kahn DA. Gabapentin-induced delirium and dependence. Journal of Psychiatric Practice. 2009;15(4):314–319. [DOI] [PubMed] [Google Scholar]
- 30.Pittenger C, Desan PH. Gabapentin abuse, and delirium tremens upon gabapentin withdrawal. Journal of Clinical Psychiatry. 2007;68(3):483–484. [DOI] [PubMed] [Google Scholar]
- 31.Schifano F, D’Offizi S, Piccione M, et al. Is there a recreational misuse potential for pregabalin? Analysis of Anecdotal Online Reports in Comparison with Related Gabapentin and Clonazepam Data. Psychotherapy and Psychosomatics. 2011;80(2):118–122. [DOI] [PubMed] [Google Scholar]
- 32.Smith RV, Lofwall MR, Havens JR. Abuse and diversion of gabapentin among nonmedical prescription opioid users in appalachian Kentucky. American Journal of Psychiatry. 2015;172(5):487–488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith BH, Higgins C, Baldacchino A, Kidd B, Bannister J. Substance misuse of gabapentin. British Journal of General Practice. 2012;62(601):406–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mersfelder TL, Nichols WH. Gabapentin abuse, dependence, and withdrawal. 2016;50(3):229–233. [DOI] [PubMed] [Google Scholar]
- 35.Wilens T, Zulauf C, Ryland D, Carrellas N, Catalina-Wellington I. Prescription medication misuse among opioid dependent patients seeking inpatient detoxification. American Journal on Addictions. 2015;24(2):173–177. [DOI] [PubMed] [Google Scholar]
- 36.Belsey SL, Couchman L, Flanagan RJ. Buprenorphine detection in urine using liquid chromatography-high-resolution mass spectrometry: comparison with cloned enzyme donor immunoassay (ThermoFisher) and homogeneous enzyme immunoassay (Immunalysis). Journal of Analytical Toxicology. 2014;38(7):438–443. [DOI] [PubMed] [Google Scholar]
- 37.Liu YZ, Li XH, Xu A, Nasser AF, Heidbreder C. Simultaneous determination of buprenorphine, norbuprenorphine and naloxone in human plasma by liquid chromatography/tandem mass spectrometry. Journal of Pharmaceutical and Biomedical Analysis. 2016;120:142–152. [DOI] [PubMed] [Google Scholar]
- 38.Moody DE, Slawson MH, Strain EC, Laycock JD, Spanbauer AC, Foltz RL. A liquid chromatographic-electrospray ionization-tandem mass spectrometric method for determination of buprenorphine, its metabolite, norbuprenorphine, and a coformulant, naloxone, that is suitable for in vivo and in vitro metabolism studies. Analytical Biochemistry. 2002;306(1):31–39. [DOI] [PubMed] [Google Scholar]
- 39.Wang Y-Y, Shen X-H, Li H, Chen F-J, Fu Y, Ding L. A sensitive, simple and rapid HPLC–MS/MS method for simultaneous quantification of buprenorpine and its N-dealkylated metabolite norbuprenorphine in human plasma. 2013;3(4):221–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Steagall PVM, Pelligand L, Giordano T, et al. Pharmacokinetic and pharmacodynamic modelling of intravenous, intramuscular and subcutaneous buprenorphine in conscious cats. Veterinary Anaesthesia and Analgesia. 2013;40(1):83–95. [DOI] [PubMed] [Google Scholar]
- 41.Liu HC, Lee HT, Hsu YC, et al. Direct injection LC-MS-MS analysis of opiates, methamphetamine, buprenorphine, methadone and their metabolites in oral fluid from substitution therapy patients. Journal of Analytical Toxicology. 2015;39(6):472–480. [DOI] [PubMed] [Google Scholar]
- 42.Eckart K, Rohrich J, Breitmeier D, Ferner M, Laufenberg-Feldmann R, Urban R. Development of a new multi-analyte assay for the simultaneous detection of opioids in serum and other body fluids using liquid chromatography-tandem mass spectrometry. Journal of Chromatography B-Analytical Technologies in the Biomedical and Life Sciences. 2015;1001:1–8. [DOI] [PubMed] [Google Scholar]
- 43.Toh DSL, Limenta LMG, Yee JY, et al. Effect of mushroom diet on pharmacokinetics of gabapentin in healthy Chinese subjects. British Journal of Clinical Pharmacology. 2014;78(1):129–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Deeb S, McKeown DA, Torrance HJ, Wylie FM, Logan BK, Scott KS. Simultaneous analysis of 22 antiepileptic drugs in postmortem blood, serum and plasma using LC-MS-MS with a focus on their role in forensic cases. Journal of Analytical Toxicology. 2014;38(8):485–494. [DOI] [PubMed] [Google Scholar]
- 45.Chollet DF, Goumaz L, Juliano C, Anderegg G. Fast isocratic high-performance liquid chromatographic assay method for the simultaneous determination of gabapentin and vigabatrin in human serum. Journal of Chromatography B. 2000;746(2):311–314. [DOI] [PubMed] [Google Scholar]
- 46.Ifa DR, Falci M, Moraes ME, Bezerra FAF, Moraes MO, de Nucci G. Gabapentin quantification in human plasma by high-performance liquid chromatography coupled to electrospray tandem mass spectrometry. Application to bioequivalence study. Journal of Mass Spectrometry. 2001;36(2):188–194. [DOI] [PubMed] [Google Scholar]
- 47.Ikeda K, Ikawa K, Yokoshige S, Yoshikawa S, Morikawa N. Gas chromatography-electron ionization-mass spectrometry quantitation of valproic acid and gabapentin, using dried plasma spots, for therapeutic drug monitoring in in-home medical care. Biomedical Chromatography. 2014;28(12):1756–1762. [DOI] [PubMed] [Google Scholar]
- 48.Cao LW, Hu Y, Liang SL, Li C, Meng JX. Sensitive and efficient determination of gabapentin in human serum based on capillary electrophoresis with laser-induced fluorescence detection. Journal of Analytical Chemistry. 2013;68(7):639–645. [Google Scholar]
- 49.Cao Z, Kaleta E, Wang P. Simultaneous quantitation of 78 drugs and metabolites in urine with a dilute-and-shoot LC-MS-MS assay. Journal of Analytical Toxicology. 2015;39(5):335–346. [DOI] [PubMed] [Google Scholar]
- 50.Sanders NC, Mancino MJ, Gentry WB, Bickel WK, Guise JB, Oliveto AH. Effects of gabapentin in opioid-dependent individuals during a 10-day buprenorphine detoxification. The 2012 Annual Meeting of the College on Problems of Drug Dependence. Palm Springs, CA. 2012. [Google Scholar]
- 51.Biri B, Kalmár J, Nagy L, Sipos A, Zsuga M, Kéki S. Energy-dependent collision-induced dissociation study of buprenorphine and its synthetic precursors. Rapid Communications Mass Spectrometry. 2011;25(1):41–49. [DOI] [PubMed] [Google Scholar]


