SUMMARY
Human epidermal growth factor receptor 2 (ERBB2/HER2) amplification or overexpression occurs in approximately 20% of advanced gastric or gastroesophageal junction adenocarcinomas1–3. Over a decade ago, combination therapy with the anti–HER2 antibody trastuzumab and chemotherapy became the standard first-line treatment for these patients4. Although adding the anti–programmed death 1 (PD-1) antibody pembrolizumab to chemotherapy does not significantly improve efficacy in advanced HER2-negative gastric cancer5, there are preclinical6–19 and clinical20,21 rationales for adding pembrolizumab in HER2-positive disease. Here we describe results of the protocol-specified first interim analysis of the randomized, double-blind, placebo-controlled phase III KEYNOTE-811 study of pembrolizumab plus trastuzumab and chemotherapy for unresectable or metastatic, HER2-positive gastric or gastroesophageal junction adenocarcinoma (ClinicalTrials.gov, NCT03615326)22. We show that adding pembrolizumab to trastuzumab and chemotherapy dramatically reduces tumour size, induces complete responses in some participants, and significantly improves objective response rate.
A pre-existing immune response to tumour antigens is associated with clinical activity of immune checkpoint blockade23. Enabling such a response through use of tumour-targeting antibodies for facilitation of cross-priming or chemotherapeutic agents for inducing immunogenic cell death or reducing immune suppressive populations is a logical next step in the development of combinatorial anticancer therapies. In preclinical models and patient-derived samples, trastuzumab increases HER2 internalization and cross-presentation by dendritic cells6, which in turn stimulates HER2-specific T-cell responses7–9. Trastuzumab has also been shown to upregulate expression of PD-1 and its ligand, PD-L1, induce expression of tumour-infiltrating lymphocytes, and modulate expression of major histocompatibility complex class II10–14. Following treatment with oxaliplatin, dying cancer cells can stimulate dendritic cells, which enhances antigen processing and presentation and facilitates priming of CD8+ tumour-specific T cells15–17. Coadministration of immune checkpoint inhibitors and trastuzumab has been shown to enhance HER2-specific T-cell responses, promote immune cell trafficking, and induce expansion of peripheral memory T cells7,8,14,18,19. The possible mechanistic interaction of these modalities has been demonstrated in a HER2-positive transgenic mouse model in which dual inhibition of PD-1 and HER2 resulted in tumour eradication14.
Clinical efficacy and manageable safety for the combination of pembrolizumab, trastuzumab, and chemotherapy in patients with HER2-positive advanced oesophageal, gastroesophageal, or gastric adenocarcinoma have been demonstrated in two single-arm, phase II studies20,21. To further study the combination, we conducted the randomized, double-blind, phase III KEYNOTE-811 trial of pembrolizumab or placebo in combination with trastuzumab and the investigator’s choice of chemotherapy with 5-fluorouracil and cisplatin or capecitabine and oxaliplatin in participants with previously untreated unresectable or metastatic, HER2-positive gastric or gastroesophageal junction adenocarcinoma (ClinicalTrials.gov, NCT03615326)22. We present results of the protocol-specified first interim analysis of objective response rate for the first 264 participants enrolled (efficacy population) and safety for all enrolled participants who received at least one dose of study treatment as of the June 17, 2020, data cutoff (as-treated population).
Of the 434 participants randomly allocated to treatment between October 5, 2018, and June 17, 2020 (intention-to-treat population), 433 received at least one dose of study therapy—217 of 217 allocated to the pembrolizumab group and 216 of 217 allocated to the placebo group—and were included in the as-treated population. The efficacy population included 133 participants in the pembrolizumab group and 131 in the placebo group. The median (range) study follow-up, defined as the time from randomization to the June 17, 2020, data cutoff was 9.9 months (0.1–19.4) in the intention-to-treat population and 12.0 months (8.5–19.4) in the efficacy population. Calculations of follow-up duration that account for participant death are found in Extended Data Table 1. The disposition for both populations is shown in Extended Data Table 2. Baseline characteristics were as expected and generally balanced between groups in both populations (Extended Data Table 3). In the intention-to-treat population, 81.3% of participants were male, 68.4% had primary tumours in the stomach, 50.2% had an intestinal histologic subtype, and 80.6% had HER2 immunohistochemistry 3+ tumours. The chosen chemotherapy regimen was capecitabine plus oxaliplatin for 86.6% of participants and 5-fluorouracil plus cisplatin for the remaining 13.4%.
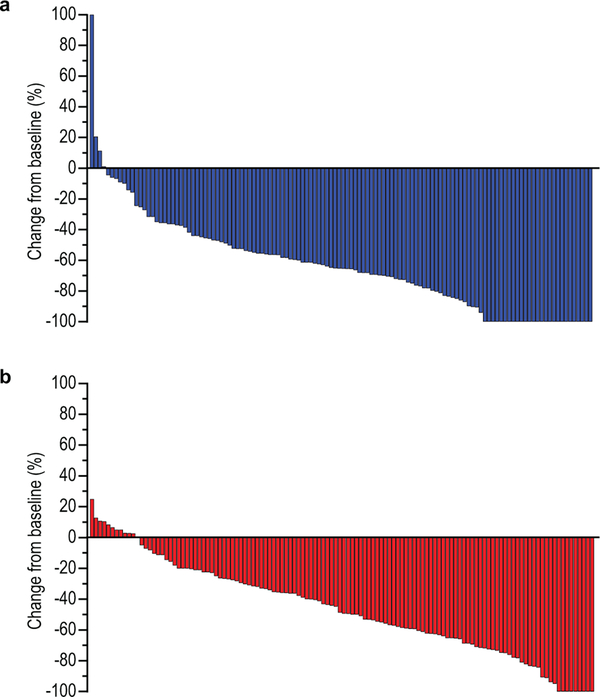
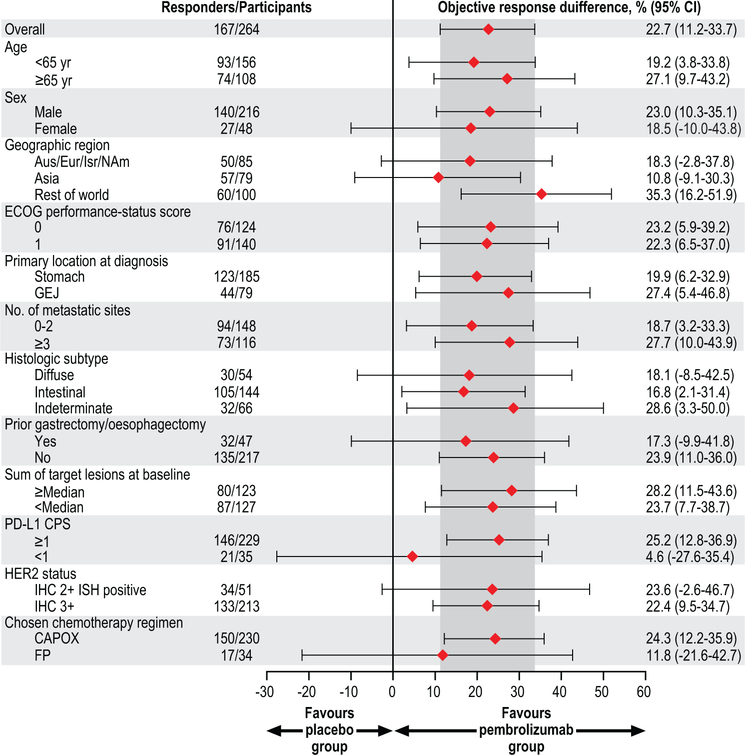
In the efficacy population, we observed an objective response rate assessed per Response Evaluation Criteria in Solid Tumors (RECIST), version 1.1, by blinded, independent central review of 74.4% (95% CI, 66.2–81.6) in the pembrolizumab group and of 51.9% (95% CI, 43.0–60.7) in the placebo group. This resulted in a statistically significant 22.7% improvement in objective response rate in the pembrolizumab group (95% CI, 11.2–33.7; P=0.00006). We found that treatment differences in key participant subgroups were generally consistent with that of the total population (Extended Data Fig. 1). Responses in the pembrolizumab group were also deeper than those in the placebo group (median change from baseline, –65% vs –49%; ≥80% decrease from baseline, 32.3% vs 14.8%) (Fig. 1), and complete responses were more frequent (11.3% vs 3.1%) (Table 1). Among responders, 50.5% in the pembrolizumab group and 44.1% in the placebo group had ongoing response at data cutoff. By Kaplan-Meier estimation, 70.3% of responders in the pembrolizumab group and 61.4% of responders in the placebo group had response duration of at least 6 months and 58.4% and 51.1%, respectively, had response duration of at least 9 months; median response duration was 10.6 months (range, 1.1+ to 16.5+) in the pembrolizumab group and 9.5 months (range, 1.4+ to 15.4+) in the placebo group.
Fig. 1. Best percentage change from baseline in the size of target lesions among participants in the efficacy population.
(a) pembrolizumab group; (b) placebo group. Only those participants in the efficacy population who had RECIST-measurable disease at baseline and at least one evaluable post-baseline measurement are evaluable for change from baseline (N=124 in the pembrolizumab group, N=122 in the chemotherapy group). The treatment regimen included trastuzumab and chemotherapy in both groups. Increases from baseline greater than 100% were truncated at 100%.
Table 1.
Summary of confirmed objective response in the efficacy population
| Variable | Pembrolizumab Group (N=133) |
Placebo Group (N=131) |
|---|---|---|
| Objective response — % (95% CI)* | 74.4 (66.2–81.6) | 51.9 (43.0–60.7) |
| Disease control — % (95% CI)† | 96.2 (91.4–98.8) | 89.3 (82.7–94.0) |
| Best overall response — no. (%) | ||
| Complete response | 15 (11.3) | 4 (3.1) |
| Partial response | 84 (63.2) | 64 (48.9) |
| Stable disease | 29 (21.8) | 49 (37.4) |
| Progressive disease | 5 (3.8) | 7 (5.3) |
| Not evaluable‡ | 0 (0.0) | 2 (1.5) |
| Not assessed‡ | 0 (0.0) | 5 (3.8) |
The treatment regimen included trastuzumab and chemotherapy in both groups.
Objective response was defined as the proportion of participants with a best response of complete or partial response. The estimated treatment difference in objective response between the pembrolizumab and placebo groups was 22.7% (95% CI, 11.2–33.7; P=0.00006) and was calculated using the Miettinen and Nurminen method stratified by geographic region (Australia/Europe/Israel/North America vs Asia vs rest of world), PD-L1 combined positive score (≥1 vs <1), and chemotherapy choice (5-fluorouracil plus cisplatin vs capecitabine plus oxaliplatin).
Disease control was defined as the proportion of participants with a best response of complete response, partial response, or stable disease.
Participants who were not evaluable were those who had at least one postbaseline imaging assessment, none of which could be evaluated for response according to RECIST, version 1.1. Participants who were not assessed included those who had no postbaseline imaging assessment.
In the as-treated population, median (range) treatment duration was 6.2 months (2 days-17.7 months) in the pembrolizumab group and 5.3 months (1 day-17.8 months) in the placebo group. We observed a similar incidence of adverse events in the pembrolizumab and placebo groups; adverse events were of grade 3–5 severity in 57.1% of participants in the pembrolizumab group versus 57.4% in the placebo group and led to discontinuation of any study treatment in 24.4% versus 25.9% (Table 2). The most common adverse events in both groups were diarrhoea (52.5% in the pembrolizumab group vs 44.4% in the placebo group), nausea (48.8% vs 44.4%), and anaemia (41.0% vs 44.0%) (Extended Data Table 4). Adverse events with a possibly immune-mediated cause and/or infusion reactions occurred in 33.6% and 20.8% of participants, respectively; the most common of these events in the pembrolizumab group were infusion-related reactions (18.0% vs 13.0% in the placebo group) and pneumonitis (5.1% vs 1.4%) (Extended Data Table 5). Seven (3.2%) participants in the pembrolizumab group and 10 (4.6%) in the placebo group died from adverse events; these were attributed to study treatment by investigators in two (0.9%) participants in each group and were considered immune-mediated in three (1.4%) and 1 (0.5%) participants, respectively (Extended Data Table 6).
Table 2.
Summary of adverse events in the as-treated population
| Event — no. (%) | Any Cause | Immune-Mediated Events and Infusion Reactions* | ||
|---|---|---|---|---|
| Pembrolizumab Group (N=217) | Placebo Group (N=216) | Pembrolizumab Group (N=217) | Placebo Group (N=216) | |
| Any grade | 211 (97.2) | 212 (98.1) | 73 (33.6) | 45 (20.8) |
| Grade 3–5 | 124 (57.1) | 124 (57.4) | 21 (9.7) | 7 (3.2) |
| Serious | 68 (31.3) | 83 (38.4) | 19 (8.8) | 7 (3.2) |
| Led to death | 7 (3.2) | 10 (4.6) | 3 (1.4) | 1 (0.5) |
| Led to discontinuation of any drug | 53 (24.4) | 56 (25.9) | 12 (5.5) | 5 (2.3) |
| Pembrolizumab or placebo | 12 (5.5) | 15 (6.9) | 7 (3.2) | 2 (0.9) |
| Trastuzumab | 12 (5.5) | 16 (7.4) | 7 (3.2) | 2 (0.9) |
| Any chemotherapy | 50 (23.0) | 44 (20.4) | 12 (5.5) | 5 (2.3) |
| All drugs | 8 (3.7) | 12 (5.6) | 6 (2.8) | 2 (0.9) |
The treatment regimen included trastuzumab and chemotherapy in both groups.
Events with a possible immune-mediated cause and infusion reactions were considered regardless of attribution to study treatment or immune relatedness by the investigator and are based on a list of terms provided by the sponsor.
Discussion
Results of this protocol-specified first interim analysis of KEYNOTE-811 show that adding pembrolizumab to standard therapy with trastuzumab and chemotherapy results in a statistically significant, clinically meaningful improvement in objective response rate compared with trastuzumab and chemotherapy alone as first-line therapy for unresectable or metastatic, HER2-positive gastric or gastroesophageal junction adenocarcinoma. We also found responses in the pembrolizumab group to be deeper and more durable. The safety profile of pembrolizumab plus trastuzumab and chemotherapy was manageable. The observed adverse events were as expected based on previous studies of pembrolizumab and trastuzumab plus chemotherapy4,5,20,21,24, with no new events identified for the combination. Based on these findings, the United States Food and Drug Administration approved pembrolizumab in combination with trastuzumab and fluoropyrimidine- and platinum-containing chemotherapy for first-line treatment of locally advanced or unresectable or metastatic HER2-positive gastric or gastroesophageal junction adenocarcinoma. KEYNOTE-811 is continuing as planned, and results for the primary end points of overall and progression-free survival will be assessed at a later date in accordance with the statistical analysis plan.
Several phase III trials have studied the addition of PD-1 inhibitors to first-line chemotherapy in patients with HER2-negative advanced gastric or gastroesophageal cancer.5,25,26 In these studies, objective response rate in the combination groups ranged from 49% to 60%, representing an improvement of approximately 10% to 15% by adding a PD-1 inhibitor. The 74.4% objective response rate observed when adding pembrolizumab to trastuzumab and chemotherapy in KEYNOTE-811, representing a 22.7% improvement versus trastuzumab and chemotherapy, supports preclinical data suggesting that there may be synergy between dual HER2 and PD-1 inhibition7,8,14,18,19.
To support biomarker analyses, we required all participants in KEYNOTE-811 to provide tissue samples at baseline and included optional collection of an on-treatment biopsy sample; blood samples for biomarker assessment are also captured throughout treatment to support analysis of genetic, plasma, and serum biomarkers and circulating tumour DNA. To-date, the only biomarkers we have explored are MSI-high status and PD-L1 expression. Three participants (0.7%) in the intention-to-treat population were known to have MSI-high tumours, a molecularly defined subset of gastric cancer associated with a great likelihood of response to PD-1 inhibitors27. This low frequency is not surprising because HER2-positive tumours are characterized by chromosomal instability and lower tumour mutational burden28. PD-L1 expression has been shown to correlate with efficacy of pembrolizumab monotherapy in gastric cancer5,24. In KEYNOTE-811, 84.1% of participants had a PD-L1 combined positive score of ≥1. We observed a greater difference in objective response rate in participants with PD-L1 combined positive score ≥1, although the 95% confidence intervals for the combined positive score ≥1 and <1 subgroups overlapped. It will be interesting to see whether the relative benefit of pembrolizumab plus trastuzumab and chemotherapy for overall survival will be impacted by PD-L1 expression.
These promising initial findings of KEYNOTE-811 suggest that the combination of pembrolizumab, trastuzumab, and chemotherapy may be a transformative treatment option for HER2-positive gastric or gastroesophageal junction adenocarcinoma and support exploring the combination in patients with earlier stages of disease. As an example of this approach, an ongoing phase II study is exploring the role of pembrolizumab and trastuzumab as adjuvant therapy for patients with HER2-positive oesophageal or gastric cancer with minimal residual disease (NCT04510285).
METHODS
Study Design and Participants
KEYNOTE-811 is a global randomized, double-blind, placebo-controlled, phase III study designed to assess the efficacy and safety of adding pembrolizumab to standard-of-care therapy with trastuzumab and platinum-based chemotherapy as first-line therapy for HER2-positive advanced gastric or gastroesophageal cancer (ClinicalTrials.gov identifier, NCT03615326) being conducted at 168 medical centres in Australia, Brazil, Chile, China, France, Germany, Guatemala, Ireland, Israel, Italy, Japan, New Zealand, Poland, Russia, South Korea, Spain, Turkey, the United Kingdom, and the United States (Supplementary Information). The study was designed by academic advisors and employees of the study sponsor. An external data and safety monitoring committee oversees the trial by periodically assessing safety and assessing efficacy at prespecified interim analyses. The study protocol and all amendments were approved by the appropriate ethics body at each participating study centre (Supplementary Information). All participants provided written informed consent. This ongoing study is being conducted in accordance with Good Clinical Practice guidelines.
Full eligibility criteria have been published22. Briefly, eligible participants are aged 18 years or older; have previously untreated, unresectable or metastatic, histologically or cytologically confirmed adenocarcinoma of the stomach or gastroesophageal junction that is HER2-positive as assessed by central review; have measurable disease per RECIST, version 1.129, as assessed by the investigator; have ECOG performance-status score of 0 or 130; have life expectancy of more than 6 months; have adequate organ function; and have provided a tumour sample adequate for assessment of programmed death ligand 1 (PD-L1) and microsatellite instability (MSI) status.
Randomization and Treatment
Participants are randomly allocated in a 1:1 ratio to receive either pembrolizumab at a dose of 200 mg or placebo (normal saline or dextrose), both administered intravenously once every 3 weeks, by means of an integrated interactive voice-response and Web-response system. Randomization is stratified by geographic region (Australia/Europe/Israel/North America vs Asia vs rest of world), PD-L1 combined positive score (≥1 vs <1), and investigator’s choice of chemotherapy (5-fluorouracil plus cisplatin vs capecitabine plus oxaliplatin). In the global cohort, all participants receive trastuzumab at a dose of 6 mg per kilogram of body weight intravenously once every 3 weeks following an initial loading dose of 8 mg per kilogram and either 5-flurouracil (800 mg per square meter of body-surface area administered intravenously on days 1–5 of each 3-week cycle) and cisplatin (80 mg per square meter administered intravenously once every 3 weeks) or capecitabine (1000 mg per square meter administered orally twice daily on days 1–14 of each 3-week cycle) and oxaliplatin (130 mg per square meter administered intravenously once every 3 weeks). All treatment is continued for up to 35 cycles (~2 years) or until disease progression, unacceptable toxic effects, investigator decision, or participant withdrawal of consent. If toxicity is clearly attributed to one drug, that drug alone may be discontinued.
Assessments
HER2 status is assessed during screening at a central laboratory using immunohistochemistry (IHC) (Dako HercepTest™) and fluorescence in situ hybridization (FISH) (Dako HER2 FISH pharmDx™ Kit). HER2 positivity was defined as IHC 3+ or IHC 2+ with positive ISH or FISH, with ISH and FISH positivity defined as a ratio of ≥2.0 for the number of HER2 copies to the number of signals for CEP17; a ratio of <2.0 was considered positive if the HER2 copy number was >6. PD-L1 expression in formalin-fixed tumour samples is assessed during screening at a central laboratory using the PD-L1 IHC 22C3 pharmDx assay (Agilent Technologies, Inc.) and characterized according to the combined positive score, calculated as the number of PD-L1–staining cells (tumour cells, lymphocytes, macrophages) divided by the total number of viable tumour cells, multiplied by 100; a minimum of 100 viable tumour cells must be present for a sample to be considered evaluable31. MSI status is determined centrally by polymerase chain reaction using the MSI Analysis System, version 1.2 (Promega) and characterized as MSI-high if 2 or more markers are changed compared to normal controls. Tumour imaging by computed tomography (preferred) or magnetic resonance imaging is scheduled for week 6 and every 6 weeks thereafter. Response is assessed according to RECIST, version 1.1, by blinded independent central review for determination of study endpoints and according to iRECIST32 by the investigator to inform treatment decisions. Adverse events and laboratory abnormalities are collected regularly during study treatment and for up to 30 days thereafter (up to 90 days for serious adverse events), classified according to the Medical Dictionary for Regulatory Affairs, version 23.0, and graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, version 4.03. Following treatment discontinuation, participants are assessed for survival every 12 weeks.
Statistical Analysis
The dual primary endpoints are progression-free survival assessed per RECIST, version 1.1, by blinded independent central review and overall survival. Secondary end points are objective response and duration of response assessed per RECIST, version 1.1, by blinded independent central review and safety. Efficacy is assessed in all randomly allocated participants according to the allocated treatment. Safety is assessed in the as-treated population (i.e., all randomly allocated participants who received ≥1 dose of study treatment according to the treatment received).
The full statistical analysis plan specifies the performance of three interim analyses and a final analysis. An extension of the graphical method of Mauer and Bretz is used to control the family-wise type I error rate at a one-sided α=0.025 across all hypotheses and interim analyses. Planned enrolment in the global cohort is 692 participants and is based on the following assumptions: an enrolment period of 28 months and a ramp-up enrolment period of 6 months; the duration of progression-free survival and overall survival follows an exponential distribution; median progression-free survival is 6.7 months in the placebo group and the true hazard ratio is 0.7; and median overall survival is 13.8 months in the placebo group and the true hazard ratio is 0.75. Per protocol, the first interim analysis was to be performed when the first 260 participants enrolled had at least 8.5 months of follow-up and was to test the objective response hypothesis. With approximately 260 participants, the study has approximately 90% power to detect a difference in objective response of 25 percentage points (73% vs 48%) favouring the pembrolizumab group at one-sided α=0.002. Power and interim analysis calculations were performed using the gsDesign R package version 3.0.1.
Study data were captured in InForm version 4.6 (Oracle). All statistical analyses were performed using SAS version 9.4 (SAS Institute, Inc.). No data from the specified analysis populations were excluded from analysis. The difference in objective response and its 95% CI were calculated using the Miettinen and Nurminen method stratified by the randomization stratification factors of geographic region (Australia/Europe/Israel/North America vs Asia vs rest of world), PD-L1 combined positive score (≥1 vs <1), and investigator’s choice of chemotherapy (5-fluorouracil plus cisplatin vs capecitabine plus oxaliplatin) with strata weighting by sample size. Duration of response was estimated using the Kaplan-Meier method. All reported data are based on the first interim analysis, which had a data cutoff of June 17, 2020.
Extended Data
Extended Data Fig. 1. Treatment difference in objective response in subgroups of the efficacy population.
Response was assessed per RECIST, version 1.1, by blinded, independent central review. The estimated treatment difference between the pembrolizumab and placebo groups in the overall population was calculated using the Miettinen and Nurminen method stratified by geographic region (Australia/Europe/Israel/North America [Aus/Eur/Isr/NAm] vs Asia vs rest of world), PD-L1 combined positive score ([CPS]; ≥1 vs <1), and chemotherapy choice (5-fluorouracil plus cisplatin [FP] vs capecitabine plus oxaliplatin [CAPOX]); differences in subgroups were calculated using the unstratified Miettinen and Nurminen method. The efficacy population included the first 264 participants randomly allocated to treatment. The treatment regimen included trastuzumab and chemotherapy in both groups. Diamonds represent the estimated treatment difference in objective response, the error bars represent the 95% confidence interval (CI) of the estimated treatment difference, and the region shaded in dark grey represents the 95% CI for the treatment difference in the overall population. ECOG, Eastern Cooperative Oncology Group; GEJ, gastroesophageal junction; IHC, immunohistochemistry; ISH, in situ hybridization.
Extended Data Table 1.
Follow-up duration calculated as time from randomization to the date of death or the database cutoff date, whichever was earliest
| Follow-up duration | Pembrolizumab group | Placebo group |
|---|---|---|
|
| ||
| Intention-to-treat population | N=217 | N=217 |
| Median,* months | 8.4 | 7.7 |
| Range, months | 0.1–19.0 | 0.5–17.9 |
| Efficacy population | N=133 | N=131 |
| Median,* months | 11.1 | 10.4 |
| Range, months | 2.2–19.0 | 0.5–17.9 |
Medians were calculated using the observation time method.
Extended Data Table 2.
Disposition of study treatment
| Efficacy population | Intention-to-treat population | |||
|---|---|---|---|---|
| Variable — no. (%) | Pembrolizumab group (N=133) | Placebo group (N=131) | Pembrolizumab group (N=217) | Placebo group (N=217) |
|
| ||||
| Started | 133 | 130 | 217 | 216 |
| Discontinued all study treatment | 79 (59.4) | 93 (71.5) | 90 (41.5) | 112 (51.9) |
| Adverse event | 7 (5.3) | 10(7.7) | 10(4.6) | 14(6.5) |
| Clinical progression | 4 (3.0) | 9 (6.9) | 6 (2.8) | 12 (5.6) |
| Non-study anticancer therapy | 2(1.5) | 3 (2.3) | 2 (0.9) | 3(1.4) |
| Physician decision | 1 (0.8) | 2(1.5) | 1 (0.5) | 2 (0.9) |
| Radiographic progression | 59 (44.4) | 64 (49.2) | 65 (30.0) | 76 (35.2) |
| Withdrawal by participant | 6 (4.5) | 5 (3.8) | 6 (2.8) | 5 (2.3) |
| Continuing any study treatment | 54 (40.6) | 37 (28.5) | 127 (58.5) | 104 (48.1) |
All percentages are calculated out of the number of participants who started study treatment. The treatment regimen included trastuzumab and chemotherapy in both groups.
Extended Data Table 3.
Demographic and disease characteristics at baseline
| Efficacy population | Intention-to-treat population | |||
|---|---|---|---|---|
| characteristic | Pembrolizumab group (N=133) | Placebo group (N=131) | Pembrolizumab group (N=217) | Placebo group (N=217) |
|
| ||||
| Age | ||||
| Median (range) — yr | 62 (19–84) | 61 (32–83) | 62 (19–84) | 63 (32–83) |
| ≥65 yr — no. (%) | 55 (41.4) | 53 (40.5) | 89 (41.0) | 98 (45.2) |
| Male sex — no. (%) | 112 (84.2) | 104 (79.4) | 179 (82.5) | 174 (80.2) |
| Region of enrolment — no. (%) | ||||
| Australia/Europe/lsrael/North America | 41 (30.8) | 44 (33.6) | 67 (30.9) | 67 (30.9) |
| Asia | 40 (30.1) | 39 (29.8) | 76 (35.0) | 75 (34.6) |
| Rest of world | 52 (39.1) | 48 (36.6) | 74 (34.1) | 75 (34.6) |
| ECOG performance-status score — no. (%) | ||||
| 0 | 65 (48.9) | 59 (45.0) | 101 (46.5) | 89 (41.0) |
| 1 | 68 (51.1) | 72 (55.0) | 116(53.5) | 128 (59.0) |
| Primary location at diagnosis — no. (%) | ||||
| Gastroesophageal junction | 37 (27.8) | 42 (32.1) | 62 (28.6) | 75 (34.6) |
| Stomach | 96 (72.2) | 89 (67.9) | 155 (71.4) | 142 (65.4) |
| No. of metastatic sites — no. (%) | ||||
| 0–2 | 71 (53.4) | 77 (58.8) | 117(53.9) | 131 (60.4) |
| ≥3 | 62 (46.6) | 54 (41.2) | 100 (46.1) | 86 (39.6) |
| Histologic subtype — no. (%) | ||||
| Diffuse | 28 (21.1) | 26(19.8) | 48 (22.1) | 39(18.0) |
| Intestinal | 81 (60.9) | 63 (48.1) | 117(53.9) | 101 (46.5) |
| Indeterminate | 24(18.0) | 42 (32.1) | 52 (24.0) | 77 (35.5) |
| Previous gastrectomy or oesophagectomy — no. (%) | ||||
| Yes | 22 (16.5) | 25(19.1) | 34(15.7) | 44 (20.3) |
| No | 111 (83.5) | 106 (80.9) | 183 (84.3) | 173 (79.7) |
| PD-L1 combined positive score — no. (%) | ||||
| ≥1 | 117(88.0) | 112(85.5) | 184 (84.8) | 181 (83.4) |
| <1 | 16(12.0) | 19(14.5) | 33(15.2) | 36(16.6) |
| HER2 status — no. (%) | ||||
| IHC 1 + | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
| IHC 2+ ISH equivocal | 0 (0.0) | 0 (0.0) | 0 (0.0) | 1 (0.5) |
| IHC 2+ ISH positive | 24(18.0) | 27 (20.6) | 36(16.6) | 45 (20.7) |
| IHC 3+ | 109 (82.0) | 104 (79.4) | 180 (82.9) | 170 (78.3) |
| MSI status — no. (%) | ||||
| MSI-high | 1 (0.8) | 1 (0.8) | 2 (0.9) | 1 (0.5) |
| Non-MSI-high | 120 (90.2) | 120 (91.6) | 171 (78.8) | 174 (80.2) |
| Unknown | 12 (9.0) | 10(7.6) | 44 (20.3) | 42(19.4) |
| Sum of target lesions at baseline — no. (%) | ||||
| <Median | 62 (46.6) | 65 (49.6) | 98 (45.2) | 107 (49.3) |
| ≥Median | 62 (46.6) | 61 (46.6) | 107 (49.3) | 101 (46.5) |
| Missing | 9 (6.8) | 5 (3.8) | 12 (5.5) | 9(4.1) |
| Chosen chemotherapy regimen — no. (%) | ||||
| Capecitabine and oxaliplatin | 115(86.5) | 115(87.8) | 189 (87.1) | 187 (86.2) |
| 5-fluorouracil and cisplatin | 18(13.5) | 16(12.2) | 28 (12.9) | 30(13.8) |
The treatment regimen included trastuzumab and chemotherapy in both groups.
Extended Data Table 4.
Adverse events that occurred in 10% or more of participants in either group of the as-treated population
| Pembrolizumab group (N=217) | Placebo group (N=216) | |||
|---|---|---|---|---|
| Event — no.(%) | Any grade | Grade 3–5 | Any grade | Grade 3–5 |
|
| ||||
| Diarrhoea | 114(52.5) | 16(7.4) | 96 (44.4) | 18 (8.3) |
| Nausea | 106 (48.8) | 10(4.6) | 96 (44.4) | 12 (5.6) |
| Anaemia | 89 (41.0) | 19(8.8) | 95 (44.0) | 20 (9.3) |
| Decreased appetite | 67 (30.9) | 5(2.3) | 69 (31.9) | 9 (4.2) |
| Vomiting | 67 (30.9) | 10(4.6) | 59 (27.3) | 4(1.9) |
| Platelet count decreased | 53 (24.4) | 17(7.8) | 61 (28.2) | 15(6.9) |
| Fatigue | 51 (23.5) | 9(4.1) | 43(19.9) | 6(2.8) |
| Neutrophil count decreased | 51 (23.5) | 16(7.4) | 53 (24.5) | 16(7.4) |
| Peripheral sensory neuropathy | 50 (23.0) | 6(2.8) | 40(18.5) | 3(1.4) |
| Aspartate aminotransferase increased | 45 (20.7) | 1 (0.5) | 28 (13.0) | 1 (0.5) |
| Weight decreased | 41 (18.9) | 2 (0.9) | 37(17.1) | 1 (0.5) |
| Palmar-plantar erythrodysaesthesia syndrome | 40(18.4) | 1 (0.5) | 35(16.2) | 2 (0.9) |
| Neutropenia | 34(15.7) | 11 (5.1) | 33(15.3) | 11 (5.1) |
| Constipation | 33(15.2) | 1 (0.5) | 37(17.1) | 0(0.0) |
| Neuropathy peripheral | 33(15.2) | 3(1.4) | 35(16.2) | 5(2.3) |
| Hypokalaemia | 32 (14.7) | 11 (5.1) | 25(11.6) | 14 (6.5) |
| Alanine aminotransferase increased | 31 (14.3) | 1 (0.5) | 22 (10.2) | 1 (0.5) |
| Infusion related reaction | 31 (14.3) | 4 (1.8) | 20 (9.3) | 2 (0.9) |
| Pyrexia | 31 (14.3) | 1 (0.5) | 26(12.0) | 0 |
| Hypoalbuminemia | 30(13.8) | 0(0.0) | 32 (14.8) | 4(1.9) |
| White blood cell count decreased | 27(12.4) | 4 (1.8) | 31 (14.4) | 5(2.3) |
| Malaise | 24 (11.1) | 1 (0.5) | 19(8.8) | 2 (0.9) |
| Stomatitis | 24 (11.1) | 2 (0.9) | 22 (10.2) | 4(1.9) |
| Asthenia | 22 (10.1) | 5(2.3) | 35(16.2) | 8 (3.7) |
| Blood bilirubin increased | 22 (10.1) | 3(1.4) | 12 (5.6) | 1 (0.5) |
| Thrombocytopenia | 20 (9.2) | 7(3.2) | 25(11.6) | 2 (0.9) |
The treatment regimen included trastuzumab and chemotherapy in both groups.
Extended Data Table 5.
Adverse events with a possible immune-mediated cause and infusion reactions in the as-treated population
| Pembrolizumab group (N=217) | Placebo group (N=216) | |||
|---|---|---|---|---|
| Event — no.(%) | Any grade | Grade 3–5 | Any grade | Grade 3–5 |
|
| ||||
| Infusion-related reactions | 39(18.0) | 6 (2.8) | 28 (13.0) | 2 (0.9) |
| Pneumonitis | 11 (5.1) | 3(1.4) | 3(1.4) | 0(0.0) |
| Colitis | 10 (4.6) | 6(2.8) | 4(1.9) | 4(1.9) |
| Hypothyroidism | 10 (4.6) | 0 (0.0) | 6(2.8) | 0 (0.0) |
| Hyperthyroidism | 8 (3.7) | 0 (0.0) | 7 (3.2) | 0 (0.0) |
| Hypophysitis | 3(1.4) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Hepatitis | 2 (0.9) | 2 (0.9) | 2 (0.9) | 0 (0.0) |
| Severe skin reactions | 2 (0.9) | 2 (0.9) | 0 (0.0) | 0 (0.0) |
| Nephritis | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0 (0.0) |
| Thyroiditis | 1 (0.5) | 0 (0.0) | 0 (0.0) | 0(0.0) |
| Type 1 diabetes mellitus | 1 (0.5) | 1 (0.5) | 0 (0.0) | 0 (0.0) |
| Uveitis | 1 (0.5) | 0 (0.0) | 1 (0.5) | 0 (0.0) |
| Myocarditis | 0 (0.0) | 0 (0.0) | 1 (0.5) | 1 (0.5) |
Adverse events with a possible immune-mediated cause and infusion reactions were considered regardless of attribution to study treatment by the investigator. The specific events are based on a list of terms provided by the sponsor. In addition to the specific terms listed, related terms were also included.
Extended Data Table 6.
Adverse events leading to death in the as-treated population
| Pembrolizumab group (N-217) | Placebo group (N=216) | |||||
|---|---|---|---|---|---|---|
| Event — no. (%) | Any cause | Treatment related* | Immune mediated† | Any cause | Treatment related* | Immune mediated† |
|
| ||||||
| Pneumonitis | 2 (0.9) | 1 (0.5) | 2 (0.9) | 0 | 0 | 0 |
| Abdominal infection | 1 (0.5) | 0 | 0 | 0 | 0 | 0 |
| Hepatitis | 1 (0.5) | 1 (0.5) | 1 (0.5) | 0 | 0 | 0 |
| Multiple organ dysfunction syndrome | 1 (0.5) | 0 | 0 | 1 (0.5) | 0 | 0 |
| Myocardial infarction | 1 (0.5) | 0 | 0 | 0 | 0 | 0 |
| Pneumonia | 1 (0.5) | 0 | 0 | 1 (0.5) | 0 | 0 |
| Aspiration | 0 | 0 | 0 | 1 (0.5) | 0 | 0 |
| Cholangitis | 0 | 0 | 0 | 1 (0.5) | 1 (0.5) | 0 |
| Completed suicide | 0 | 0 | 0 | 1 (0.5) | 0 | 0 |
| Craniocerebral injury | 0 | 0 | 0 | 1 (0.5) | 0 | 0 |
| Death | 0 | 0 | 0 | 1 (0.5) | 0 | 0 |
| Gastric cancer | 0 | 0 | 0 | 1 (0.5) | 0 | 0 |
| Myocarditis | 0 | 0 | 0 | 1 (0.5)‡ | 1 (0.5)‡ | 1 (0.5)‡ |
| Respiratory tract infection | 0 | 0 | 0 | 1 (0.5) | 0 | 0 |
As indicated by the investigator.
Adverse events with a possible immune-mediated cause were considered regardless of attribution to study treatment by the investigator. The specific events are based on a list of terms provided by the sponsor. In addition to the specific terms listed, related terms were also included.
Diagnosis of myocarditis was supported by post-mortem histological findings of a CD3-positive T cell population in the lymphocytic infiltrate in the myocardium; there were no features of myocardial infarction.
Supplementary Material
Acknowledgements
This study was funded by Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. Yelena Y. Janjigian was additionally supported by Memorial Sloan Kettering Cancer Center National Institutes of Health/National Cancer Institute Cancer Center Support Grant P30 CA008748. We thank the patients and their families and caregivers for participating in the study; the investigators and site personnel; and the following employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA: Chie-Schin Shih, MD, for study oversight and Melanie A. Leiby, PhD, for medical writing and editorial assistance.
Competing Interest Declaration
Y.Y.J. has received research funding to the institution from Merck Sharp & Dohme, the National Cancer Institute, the United States Department of Defense, Cycle for Survival, Fred’s Team, Rgenix, Bayer, Genentech/Roche, Bristol-Myers Squibb, and Eli Lilly, has served on advisory boards for Rgenix, Merck Serono, Bristol-Myers Squibb, Eli Lilly, Pfizer, Bayer, Imugene, Merck Sharp & Dohme, Daiichi-Sankyo, Zymeworks, SeaGen, Basilea Pharmaceutical and AstraZeneca, and has equity in Rgenix. A.K. has received research funding to the institution from Merck Sharp & Dohme, Ono Pharmaceutical Co., Ltd. and Taiho Pharmaceutical Co., Ltd., and received honoraria for lectures, presentations, speakers bureaus, or educational events from Ono Pharmaceutical Co., Ltd. and Taiho Pharmaceutical Co., Ltd. P.Y. has received research funding to the institution and travel support from Merck Sharp & Dohme. N.L. has received research funding to the institution from Merck Sharp & Dohme. S.L. has received research funding to the institution from Merck Sharp & Dohme, Amgen, Merck Serono, Bayer, Roche, Lilly, AstraZeneca, and Bristol-Myers Squib, consulting fees from Amgen, Merck Serono, Lilly, AstraZeneca, Incyte, Daiichi Sankyo, Bristol-Myers Squibb, and Servier, and payment or honoraria for lectures, presentations, speakers bureaus, or educational events from Roche, Lilly, Bristol-Myers Squibb, Servier, Merck Serono, Pierre-Fabre, GSK, and Amgen. O.K. has received research funding to the institution from Merck Sharp & Dohme. O.B. has received research funding to the institution from Merck Sharp & Dohme, has received honoraria for serving as a speaker from Eli Lilly, travel support from Bristol-Myers Squibb and Roche, participated as an advisor for Bristol-Myers Squibb and Roche, and has a leadership role in the Sociedad Chilena de Oncología Medica. Y.B. has received research funding to the institution from Merck Sharp & Dohme. L.S. has received research funding to the institution from Merck Sharp & Dohme, Beijing Xiantong Biomedical Technology, Qilu Pharmaceutical, ZaiLab Pharmaceutical (Shanghai), Jacobio Pharmaceuticals, and Beihai Kangcheng (Beijing) Medical Technology, has received consulting fees from Merck Sharp & Dohme, Merck, Mingji Biopharmaceutical, Haichuang Pharmaceutical, Harbour BioMed, and Boehringer Ingelheim, has received payment for speakers bureaus from Hutchison Whampoa, Henrui, ZaiLab, and CSTONE Pharmaceutical, and has participated on an advisory board for Rongchang Pharmaceutical, Zailab, and CSTONE Pharmaceutical. Y.T. has received research funding to the institution from Merck Sharp & Dohme. L.S.W. has received research funding to the institution from Merck Sharp & Dohme. J.X. has received research funding to the institution from Merck Sharp & Dohme. K.S. has received research funding to the institution from Merck Sharp & Dohme, Astellas, Lilly, Ono, Sumitomo Dainippon, Daiichi Sankyo, Taiho, Chugai, Medi Science, and Eisai, has received consulting fees from Astellas, Lilly, Bristol-Myers Squibb, Takeda, Pfizer, Ono, Merck Sharp & Dohme, Taiho, Novartis, AbbVie, GlaxoSmithKline, Daiichi Sankyo, Amgen, and Boehringer Ingelheim, and has received honoraria for lectures from Novartis, AbbVie, and Yalkult. S.Q. has received consulting fees from Merck Sharp & Dohme for serving on an advisory board. E.V.C. has received funding to the institution from Merck Sharp & Dohme, Amgen, Bayer, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Ipsen, Lilly, Merck KGaA, Novartis, Roche, and Servier and has received consulting fees from Array, Astellas, AstraZeneca, Bayer, Beigene, Biocartis, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Daiichi Sankyo, Halozyme, GSK, Incyte, Ipsen, Merck Sharp & Dohme, Merck KGaA, Novartis, Pierre-Fabre, Roche, Servier, Sirtex, and Taiho. J.T. has received funding to the institution from Merck Sharp & Dohme, has received consulting fees from Array Biopharma, AstraZeneca, Avvinity, Bayer, Boehringer Ingelheim, Chugai, Daiichi Sankyo, F. Hoffmann-La Roche Ltd., Genentech Inc., HalioDX SAS, Hutchison MediPharma International, Ikena Oncology, IQVIA, Lilly, Menarini, Merck Serono, Merus, Merck Sharp & Dohme, Mirati, Neophore, Novartis, Orion Biotechnology, Peptomyc, Pfizer, Pierre Fabre, Samsung Bioepis, Sanofi, SeaGen, Servier, Taiho, Tessa Therapeutics, and TheraMyc, and has received payment for educational collaboration with Imedex, Medscape Education, MJH Life Sciences, PeerView Institute for Medical Education, and Physicians Education Resource (PER). L.L. receives salary for full-time employment from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA. S.S. receives salary for full-time employment from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and owns stock and/or has stock options in Merck & Co., Inc., Kenilworth, NJ, USA. P.B. receives salary for full-time employment from Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and owns stock and/or has stock options in Merck & Co., Inc., Kenilworth, NJ, USA. H.C.C. has received funding to the institution from Merck Sharp & Dohme. Y.Y.J, A.K., P.Y., N.L., S.L., O.K., O.B., Y.B., L.S., Y.T., L.S.W., J.X., K.S., S.Q., E.V.C., J.T., L.L., S.S., P.B., and H.C.C. received medical writing support for this manuscript (no payment) from Merck Sharp & Dohme.
Footnotes
Reprints and permissions information is available at www.nature.com/reprints.
Supplementary Information is available for this paper.
Data Availability
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued; an exception will be made for this ongoing trial such that data will be made available for request after the protocol-specified primary endpoint analyses have been reported. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to a SAS portal so that the requestor can perform the proposed analyses.
Main Text References
- 1.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Van Cutsem E et al. HER2 screening data from ToGA: targeting HER2 in gastric and gastroesophageal junction cancer. Gastric Cancer 18, 476–484 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cancer Genome Atlas Research Network. et al. Integrated genomic characterization of oesophageal carcinoma. Nature 541, 169–175 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bang YJ et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet 376, 687–697 (2010). [DOI] [PubMed] [Google Scholar]
- 5.Shitara K et al. Efficacy and safety of pembrolizumab or pembrolizumab plus chemotherapy vs chemotherapy alone for patients with first-line, advanced gastric cancer: the KEYNOTE-062 phase 3 randomized clinical trial. JAMA Oncol 6, 1571–1580, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gall VA et al. Trastuzumab increases HER2 uptake and cross-presentation by dendritic cells. Cancer Res 77, 5374–5383, (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Park S et al. The therapeutic effect of anti-HER2/neu antibody depends on both innate and adaptive immunity. Cancer Cell 18, 160–170, (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Taylor C et al. Augmented HER-2 specific immunity during treatment with trastuzumab and chemotherapy. Clin Cancer Res 13, 5133–5143, (2007). [DOI] [PubMed] [Google Scholar]
- 9.zum Buschenfelde C. M., Hermann C, Schmidt B, Peschel C & Bernhard H Antihuman epidermal growth factor receptor 2 (HER2) monoclonal antibody trastuzumab enhances cytolytic activity of class I-restricted HER2-specific T lymphocytes against HER2-overexpressing tumor cells. Cancer Res 62, 2244–2247, (2002). [PubMed] [Google Scholar]
- 10.Chaganty BKR et al. Trastuzumab upregulates PD-L1 as a potential mechanism of trastuzumab resistance through engagement of immune effector cells and stimulation of IFNgamma secretion. Cancer Lett 430, 47–56, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Varadan V et al. Immune signatures following single dose trastuzumab predict pathologic response to preoperative trastuzumab and chemotherapy in HER2-positive early breast cancer. Clin Cancer Res 22, 3249–3259, (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Triulzi T et al. HER2 signaling regulates the tumor immune microenvironment and trastuzumab efficacy. Oncoimmunology 8, e1512942, (2019). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Triulzi T et al. Early immune modulation by single-agent trastuzumab as a marker of trastuzumab benefit. Br J Cancer 119, 1487–1494, (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Stagg J et al. Anti-ErbB-2 mAb therapy requires type I and II interferons and synergizes with anti-PD-1 or anti-CD137 mAb therapy. Proc Natl Acad Sci U S A 108, 7142–7147, (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Apetoh L et al. Toll-like receptor 4-dependent contribution of the immune system to anticancer chemotherapy and radiotherapy. Nat Med 13, 1050–1059, (2007). [DOI] [PubMed] [Google Scholar]
- 16.Ghiringhelli F et al. Activation of the NLRP3 inflammasome in dendritic cells induces IL-1beta-dependent adaptive immunity against tumors. Nat Med 15, 1170–1178, (2009). [DOI] [PubMed] [Google Scholar]
- 17.Tesniere A et al. Immunogenic death of colon cancer cells treated with oxaliplatin. Oncogene 29, 482–491, (2010). [DOI] [PubMed] [Google Scholar]
- 18.Mortenson ED, Park S, Jiang Z, Wang S & Fu YX Effective anti-neu-initiated antitumor responses require the complex role of CD4+ T cells. Clin Cancer Res 19, 1476–1486, (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Muller P et al. Trastuzumab emtansine (T-DM1) renders HER2+ breast cancer highly susceptible to CTLA-4/PD-1 blockade. Sci Transl Med 7, 315ra188, (2015). [DOI] [PubMed] [Google Scholar]
- 20.Janjigian YY et al. First-line pembrolizumab and trastuzumab in HER2-positive oesophageal, gastric, or gastro-oesophageal junction cancer: an open-label, single-arm, phase 2 trial. Lancet Oncol 21, 821–831, (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rha SY et al. Targeting HER2 in combination with anti-PD-1 and chemotherapy confers a significant tumor shrinkage of gastric cancer: A multi-institutional phase Ib/II trial of first-line triplet regimen (pembrolizumab, trastuzumab, chemotherapy) for HER2-positive advanced gastric cancer (AGC). J Clin Oncol 38 (suppl 15), abstract 3081 (2020). [Google Scholar]
- 22.Chung HC et al. First-line pembrolizumab/placebo plus trastuzumab and chemotherapy in HER2-positive advanced gastric cancer: KEYNOTE-811. Future Oncol 17, 491–501 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barrueto L et al. Resistance to checkpoint inhibition in cancer immunotherapy. Transl Oncol 13, 100738 (2020). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shitara K et al. Pembrolizumab versus paclitaxel for previously treated, advanced gastric or gastro-oesophageal junction cancer (KEYNOTE-061): a randomised, open-label, controlled, phase 3 trial. Lancet 392, 123–133 (2018). [DOI] [PubMed] [Google Scholar]
- 25.Boku N et al. Nivolumab plus chemotherapy versus chemotherapy alone in patients with previously untreated advanced or recurrent gastric/gastroesophageal junction (G/GEJ) cancer: ATTRACTION-4 (ONO-4538–37) study. Ann Oncol 31, S1192–S1192 (2020). [Google Scholar]
- 26.Janjigian YY et al. First-line nivolumab plus chemotherapy versus chemotherapy alone for advanced gastric, gastro-oesophageal junction, and oesophageal adenocarcinoma (CheckMate 649): a randomised, open-label, phase 3 trial. Lancet 398, 27–40, (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chao J et al. Assessment of pembrolizumab therapy for the treatment of microsatellite instability-high gastric or gastroesophageal junction cancer among patients in the KEYNOTE-059, KEYNOTE-061, and KEYNOTE-062 clinical trials. JAMA Oncol, doi: 10.1001/jamaoncol.2021.0275 (2021). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Janjigian YY et al. Genetic predictors of response to systemic therapy in esophagogastric cancer. Cancer Discov 8, 49–58 (2018). [DOI] [PMC free article] [PubMed] [Google Scholar]
Methods References
- 29.Eisenhauer EA et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 45, 228–247 (2009). [DOI] [PubMed] [Google Scholar]
- 30.Oken MM et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5, 649–655 (1982). [PubMed] [Google Scholar]
- 31.Kulangara K et al. Clinical utility of the combined positive score for programmed death ligand-1 expression and the approval of pembrolizumab for treatment of gastric cancer. Arch Pathol Lab Med 143, 330–337 (2019). [DOI] [PubMed] [Google Scholar]
- 32.Seymour L et al. iRECIST: guidelines for response criteria for use in trials testing immunotherapeutics. Lancet Oncol 18, e143–e152 (2017). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA (MSD) is committed to providing qualified scientific researchers access to anonymized data and clinical study reports from the company’s clinical trials for the purpose of conducting legitimate scientific research. MSD is also obligated to protect the rights and privacy of trial participants and, as such, has a procedure in place for evaluating and fulfilling requests for sharing company clinical trial data with qualified external scientific researchers. The MSD data sharing website (available at: http://engagezone.msd.com/ds_documentation.php) outlines the process and requirements for submitting a data request. Feasible requests will be reviewed by a committee of MSD subject matter experts to assess the scientific validity of the request and the qualifications of the requestors. In line with data privacy legislation, submitters of approved requests must enter into a standard data-sharing agreement with MSD before data access is granted. Data will be made available for request after product approval in the US and EU or after product development is discontinued; an exception will be made for this ongoing trial such that data will be made available for request after the protocol-specified primary endpoint analyses have been reported. There are circumstances that may prevent MSD from sharing requested data, including country or region-specific regulations. If the request is declined, it will be communicated to the investigator. Access to genetic or exploratory biomarker data requires a detailed statistical analysis plan that is collaboratively developed by the requestor and MSD subject matter experts; after approval of the statistical analysis plan and execution of a data-sharing agreement, MSD will either perform the proposed analyses and share the results with the requestor or will construct biomarker covariates and add them to a file with clinical data that is uploaded to a SAS portal so that the requestor can perform the proposed analyses.