Abstract
Background
Reinfection after primary SARS-CoV-2 infection is uncommon in adults, but little is known about the risks, characteristics, severity, or outcomes of reinfection in children. We aimed to assess the risk of SARS-CoV-2 reinfection in children and compare this with the risk in adults, by analysis of national testing data for England.
Methods
In our prospective, national surveillance study to assess reinfection of SARS-CoV-2 in children in England, we used national SARS-CoV-2 testing data to estimate the risk of reinfection at least 90 days after primary infection from Jan 27, 2020, to July, 31, 2021, which encompassed the alpha (B.1.1.7) and delta (B.1.617.2) variant waves in England. Data from children up to age 16 years who met the criteria for reinfection were included. Disease severity was assessed by linking reinfection cases to national hospital admission data, intensive care admission, and death registration datasets.
Findings
Reinfection rates closely followed community infection rates, with a small peak during the alpha wave and a larger peak during the delta wave. In children aged 16 years and younger, 688 418 primary infections and 2343 reinfections were identified. The overall reinfection rate was 66·88 per 100 000 population, which was higher in adults (72·53 per 100 000) than children (21·53 per 100 000). The reinfection rate after primary infection was 0·68% overall, 0·73% in adults compared with 0·18% in children age younger than 5 years, 0·24% in those aged 5–11 years, and 0·49% in those aged 12–16 years. Of the 109 children admitted to hospital with reinfection, 78 (72%) had comorbidities. Hospital admission rates were similar for the first (64 [2·7%] of 2343) and second episode (57 [2·4%] of 2343) and intensive care admissions were rare (seven children for the first episode and four for reinfections). There were 44 deaths within 28 days after primary infection (0·01%) and none after reinfection.
Interpretation
The risk of SARS-CoV-2 reinfection is strongly related to exposure due to community infection rates, especially during the delta variant wave. Children had a lower risk of reinfection than did adults, but reinfections were not associated with more severe disease or fatal outcomes.
Funding
UK Health Security Agency.
Introduction
When exposed to SARS-CoV-2, children are more likely than are adults to develop a mild, transient illness and less likely to develop severe disease, require admission to hospital or intensive care, or die of COVID-19.1 Following infection, children develop robust humoral and cellular responses to SARS-CoV-2, targeted primarily against the spike protein of the virus, irrespective of whether they develop symptomatic illness or remain asymptomatic after infection.2 Additionally, immunity is sustained for at least 12 months, potentially longer than in adults, with evidence of cross-protection against new SARS-CoV-2 variants.2 Reinfection following primary SARS-CoV-2 infection is uncommon but has been described mainly in adults, even in the presence of SARS-CoV-2 antibodies.3, 4, 5 Additionally, breakthrough infections have been reported in vaccinated adults, with increasing risk with time since vaccination.5 An increased risk of reinfection has also been reported following the emergence of the delta (B.1.617.2) variant, which is more transmissible than previous SARS-CoV-2 variants and able to at least partially evade both natural and vaccine-induced immunity.6 However, little is known about the rate, risk factors, characteristics, severity, or outcomes of SARS-CoV-2 reinfections in children with the exception of a few case reports in immunocompromised children.7, 8
In England, the first cases of SARS-CoV-2 emerged in late January, 2020, and infections increased rapidly, leading to a national lockdown including school closures in March, 2020.9 Cases peaked in mid-April and then declined gradually, leading to easing of lockdown measures, including partial reopening of some school class years during June and July, 2020, and full reopening of all school years albeit with strict infection controls in September, 2020.10 Early in the pandemic, testing for SARS-CoV-2 was limited to symptomatic cases in health-care settings, but community testing became available from June, 2020, initially for individuals with typical symptoms of COVID-19 (fever, new-onset cough, or loss of taste or smell) but soon became available for anyone wishing to be tested, with a 7-day rolling average of in excess of 100 000 tests per day by June 28, 2020.11
Research in context.
Evidence before this study
We searched PubMed with the terms “COVID-19” or “SARS-CoV-2” with “reinfection” to identify publications in English relating to SARS-CoV-2 reinfections from Jan 1, 2020, to Nov 15, 2021. We did not find national studies with data on reinfection in children with the exception of case reports, which were mainly reports about immunocompromised children.
There were few publications relating to SARS-CoV-2 reinfections, and these related to adults, with no conclusions for reinfections in children. Published studies reported very low rates of reinfection during the first few months after primary infection in adults. COVID-19 vaccines provide effective immune protection against SARS-CoV-2 infection, but recent studies have reported increasing risk of breakthrough infection with time since primary vaccination due to waning immunity. Several SARS-CoV-2 variants, including the beta, gamma, delta, and omicron variants have been shown to partially evade immunity after natural infection and vaccination, potentially increasing the risk of reinfections and breakthrough infections.
Added value of this study
We used national SARS-CoV-2 testing data during the first 19 months of the pandemic to estimate the risk of reinfection in children compared with adults during a period that encompassed both the alpha and the delta variant waves in England. We found that the risk of reinfection tracked with the risk of SARS-CoV-2 exposure and, therefore, closely reflected community infection rates, with most reinfections occurring during the delta variant wave. While acknowledging the limitation of using national testing data, we found that children had a lower risk of reinfection than did adults, and that the risk of reinfection in children increased with age. Reinfections were not associated with severe disease in terms of hospital admissions or intensive care admissions and there were no fatalities within 28 days of reinfection in children.
Implications of all the available evidence
SARS-CoV-2 reinfections are rare in children, especially younger children, and in England, occurred mainly during the delta wave. Reinfections were not associated with more severe disease or fatal outcomes in children. COVID-19 vaccination will provide further protection against primary infections and reinfections in children.
We aimed to assess the risk of SARS-CoV-2 reinfection in children compared with adults, by analysing national testing data for England during the first 19 months of the pandemic, which included the alpha variant wave during the winter of 2020–21 and the delta variant wave during summer 2021.
Methods
Study design and participants
We did a prospective, national surveillance study to assess reinfection with SARS-CoV-2 in children up to age 16 years in England. This protocol was subject to an internal review by the UK Health Security Agency (UKHSA) Research Support and Governance Office and was classified as surveillance that was being undertaken as part of UKHSA's responsibility to respond to the COVID-19 current pandemic. UKHSA has legal permission, provided by Regulation 3 of The Health Service (Control of Patient Information) Regulations 2002, to collect confidential patient information) under Sections 3 (i) (a to c), 3 (i) (d) (i) and (ii), and 3 (3) as part of its outbreak response activities.
Procedures
The UKHSA and its predecessor, Public Health England, collects and compiles all SARS-CoV-2 tests performed nationally through community and health-care settings using the electronic Second Generation Surveillance System, which includes information on patient's sex, age, and the region in which the test was performed. We obtained results for all positive COVID-19 diagnostic tests done in England from Jan 27, 2020, to July 31, 2021. Primary or first infections were defined as the first ever positive SARS-CoV-2 RT-PCR or rapid antigen or lateral flow device (LFD) test result for an individual. Reinfection was defined as a subsequent positive SARS-CoV-2 RT-PCR or LFD test result at least 90 days from the previous positive test, as per most other European countries, and applying no additional criteria.12 Individuals testing positive for SARS-CoV-2 by LFD testing were advised to take a confirmatory RT-PCR test. Consequently, those who subsequently tested PCR negative within 3 days were removed from the reinfection's dataset. If an individual tested positive for SARS-CoV-2 on multiple occasions with a minimum interval of 90 days between each episode, then only the first and second episodes were included in the analysis. Individuals who tested positive within 90 days were not included (even if they received a negative result between two positive tests).
We used the Secondary Uses Service (SUS), which is a national electronic database of hospital admissions that provides timely updates of ICD-10 codes for completed hospital stays for all NHS hospitals in England, to identify children who were admitted to hospital or to an intensive care unit (ICU) within 21 days of testing positive for SARS-CoV-2 infection. We also used ICD-10 codes recorded for primary and secondary diagnoses in SUS to identify children with comorbidities. Finally, we linked cases to electronic death registrations provided by the Office for National Statistics (ONS) to identify children who died within 28 days of testing positive for SARS-CoV-2, or children who died within 60 days of testing positive for SARS-CoV-2 or with COVID-19 recorded on their death certificate.
Data analysis
The data are presented in absolute numbers of SARS-CoV-2 cases identified during the study period, and we also report the percentage of laboratory-confirmed reinfection cases per primary infection in different age groups and rate of reinfection in the population. Variables of interest such as reporting symptoms at the time of testing, sex, and hospital admissions are represented as proportions within the study population. We calculated rates of primary infection and reinfection per 100 000 population using the ONS 2020 mid-year estimates for population denominators.13 We compared numbers and rates of primary infection and reinfections by age in years in children and by age group (<5 years, 5–11 years or primary school-aged, and 12–16 years or secondary school-aged) and compared rates with adults aged 17 years and older, who were eligible for COVID-19 vaccination from December, 2020, in a structured rollout that started with those aged 80 years and older along with health and social care workers, and then extended down the age groups and those with specific underlying comorbidities.14 COVID-19 vaccine uptake by age and time was obtained from the online NHS England COVID-19 Vaccination archive.15 Data were stored in a secure server used by authorised UKHSA employees. A χ2 test was used to assess the difference in proportion between primary infection cases and reinfection cases who did not report any symptoms at the time of testing. We used Stata to obtain a p value from the χ2 test, and p<0·05 was considered statistically significant. We used SQL Server Management Studio (version 18.9.2) to access and link data from all of the different data sources. Individuals with missing NHS numbers were excluded from the study because linkage to the SUS would not have been possible. We cleaned and organised our final dataset on Oct 14, 2021, using Microsoft Access and analysed using Stata (version 15.0).
Results
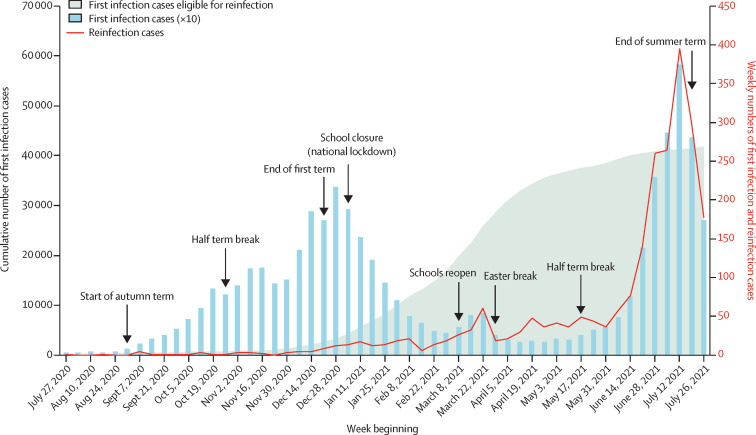
During the surveillance period from Jan 27, 2020, to July 31, 2021, primary infection cases were low during the summer of 2020, then increased from the end of August just before the start of the Autumn school term and peaked at the end of December, 2020, following the emergence and rapid spread of the alpha variant across England from the middle of November, 2020 (figure 1 ). A national lockdown implemented in January, 2021, including school closures, was associated with a rapid decline in cases, with a small peak in March, 2021, when children returned to school while adults remained in national lockdown, followed by a larger peak starting from the end of May, 2021, with the easing of national lockdown alongside the emergence and rapid spread of the delta variant nationally.
Figure 1.
Weekly cumulative count of SARS-CoV-2 primary infections eligible for reinfection (allowing for 90 days interval), weekly count or primary infections (multiplied by 10), and weekly count or possible reinfection cases, in children up to age 16 years in England
Reinfection rates were very low in children during the alpha wave, at a time when the number of primary infection cases eligible for reinfections was also low. There was a small increase in reinfection cases during March, 2021 (given the relatively small number of infections in the community at that time) compared with the low number of reinfections in the December wave, when community rates were high. This difference was due to the cumulative count of primary cases eligible for reinfection increasing rapidly by almost 6-fold in March, 2021, compared with the number of cumulative cases during the winter peak in December, 2020 (figure 1). Reinfection cases then stabilised for 2 months before increasing rapidly from the end of May, 2021, and following the same trajectory as community infection rates during the delta wave, reaching a peak at the same time as primary infection rates in the middle of July, 2020. Weekly primary infection and reinfection cases subsequently declined after schools closed for the summer holidays.
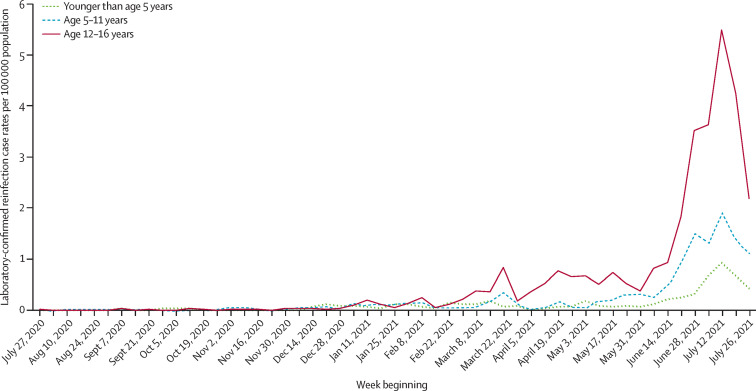
Children aged 16 years or younger had not exceeded a weekly reinfection rate of 1 per 100 000 population individuals by the week beginning June 14, 2021 (figure 2 ). During the alpha wave, reinfections occurred mainly in older adults (age 80 years and older). As the adult population increasingly became vaccinated from December, 2020 (appendix p 3), the age distribution of reinfection cases changed; at the emergence of the delta variant, the reinfection risk was highest in younger adults (age 17–39 years), who remained largely unvaccinated or partially vaccinated with a single vaccine dose. During the peak delta wave, reinfection rates in 12–16 year-olds peaked in the week beginning July 12, 2021, and mirrored reinfection rates in those aged 50–59 years, 93% of whom had been vaccinated with a first dose, and 82% of whom had been vaccinated with a second dose, by June 1, 2021 (appendix pp 1–2).
Figure 2.
Weekly rates of SARS-CoV-2 possible reinfection cases per 100 000 population in different age groups in children
In children, at the peak of reinfections in the week beginning July 12, 2021, there was an age-related gradient, with the lowest reinfection rate of 0·9 per 100 000 population in children younger than age 5 years compared with 1·9 per 100 000 population in those aged 5–11 years and 5·5 per 100 000 population in those aged 12–16-years. These rates were 23, 11, and four times lower than in adults aged 20–29 years, who were unvaccinated and had the highest reinfection rate at the time (figure 2; appendix p 2).
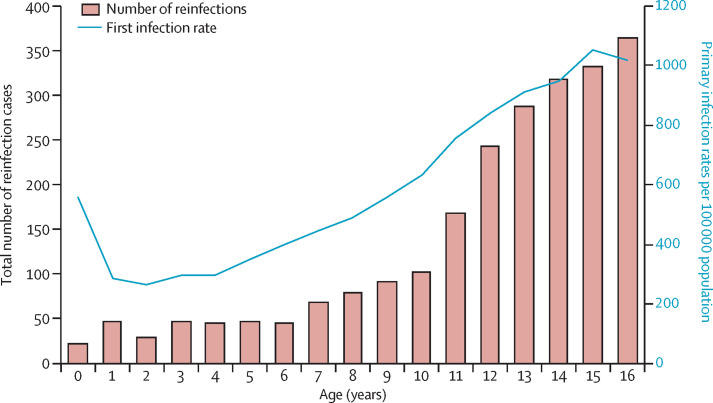
During the 19-month surveillance period (January, 2020, to July, 2021), primary childhood infection rates were higher in infants (those younger than age 1 year) than in those aged 1 and 2 years, before increasing with age up to 16 years (figure 3 ). However, reinfections were lowest in infants (23 cases) and remained low among those aged 1–6 years with an average of 43 cases per year group, before increasing with age, reaching 364 cases among those aged 16 years (figure 3).
Figure 3.
Total count of possible SARS-CoV-2 reinfection cases and SARS-CoV-2 primary infection rates by year of age from Jan 27, 2020, to July 31, 2021
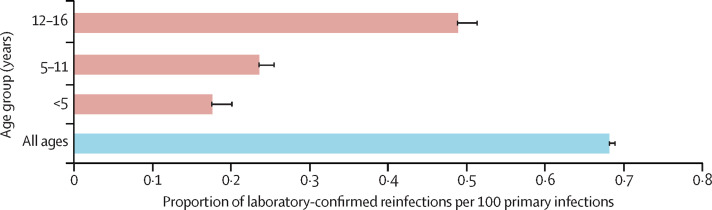
In England, primary infections in children started increasing in February, 2020, with the first reinfection in a child identified in late July, 2020. By the end of July, 2021, there were 688 418 primary infections and 2343 reinfections in children under 16 years in England. There were no differences in sex or ethnicity between primary and secondary infections (table ). The overall reinfection rate for this period was 66·88 per 100 000 population, being higher in adults (72·53 per 100 000 population) than in children (21·53 per 100 000 population). Reinfection rates after primary infection over the same period were 0·68 per 100 primary infections overall, 0·73 per 100 primary infections in adults, and 0·34 per 100 primary infections in children. Reinfection rates by age group are summarised in figure 4 .
Table.
Demographic characteristics of primary SARS-CoV-2 infection and reinfection cases in children up to age 16 years in England, UK
| First infection (n=688 418) | Reinfections (n=2343) | |
|---|---|---|
| Sex | ||
| Female | 340 028/682 009 (49·9%) | 1174/2334 (50·3%) |
| Male | 341 981/682 009 (50·1%) | 1160/2334 (49·7%) |
| Available | 682 009/688 418 (99·1%) | 2334/2343 (99·6%) |
| Missing | 6409/688 418 (0·9%) | 9/2343 (0·4%) |
| Age, years | ||
| 0 | 33 688/680 174 (5·0%) | 23 (0·98%) |
| 1 | 17 746/680 174 (2·6%) | 47 (2·0%) |
| 2 | 17 224/680 174 (2·5%) | 30 (1·3%) |
| 3 | 19 766/680 174 (2·9%) | 48 (2·0%) |
| 4 | 20 656/680 174 (3·0%) | 45 (1·9%) |
| 5 | 24 192/680 174 (3·6%) | 48 (2·0%) |
| 6 | 27 768/680 174 (4·1%) | 45 (1·9%) |
| 7 | 31 609/680 174 (4·6%) | 69 (2·9%) |
| 8 | 35 916/680 174 (5·3%) | 79 (3·4%) |
| 9 | 39 962/680 174 (5·9%) | 92 (3·9%) |
| 10 | 44 531/680 174 (6·5%) | 103 (4·4%) |
| 11 | 52 314/680 174 (7·7%) | 168 (7·2%) |
| 12 | 58 963/680 174 (8·7%) | 243 (10·4%) |
| 13 | 61 798/680 174 (9·1%) | 288 (12·3%) |
| 14 | 63 053/680 174 (9·3%) | 318 (13·6%) |
| 15 | 67 154/680 174 (9·9%) | 333 (14·2%) |
| 16 | 63 834/680 174 (9·4%) | 364 (15·5%) |
| Available | 680 174/688 418 (98·8%) | 2343 (100·0%) |
| Missing | 8244/688 418 (1·2%) | 0 |
| Ethnicity | ||
| Asian | 89 096/619 388 (14·4%) | 289/1973 (14·6%) |
| Black | 24 662/619 388 (4·0%) | 74/1973 (3·8%) |
| Mixed | 35 021/619 388 (5·7%) | 93/1973 (4·7%) |
| Other | 11 987/619 388 (1·9%) | 40/1973 (2·0%) |
| White | 458 622/619 388 (74·0%) | 1477/1973 (74·9%) |
| Available | 619 388/688 418 (90·0%) | 1973/2343 (84·2%) |
| Missing | 69 030/688 418 (10·0%) | 370/2343 (15·8%) |
| Region | ||
| East Midlands | 56 547 (8·2%) | 197 (8·4%) |
| East of England | 68 034 (9·9%) | 197 (8·4%) |
| London | 112 792 (16·4%) | 363 (15·5%) |
| North East | 41 070 (6·0%) | 176 (7·5%) |
| North West | 119 899 (17·4%) | 472 (20·1%) |
| South East | 92 228 (13·4%) | 244 (10·4%) |
| South West | 41 825 (6·1%) | 145 (6·2%) |
| West Midlands | 78 114 (11·3%) | 249 (10·6%) |
| Yorkshire and The Humber | 77 909 (11·3%) | 300 (12·8%) |
Data are n (%), or n/N (%).
Figure 4.
Laboratory-confirmed reinfection rates with 95% CIs by age group from Jan 27, 2020, to July 31, 2021
Of the 2343 children with reinfection, 109 (4·7%) were admitted to hospital at either episode (of first or second infection) and 78 (72%) of those hospitalised were identified as having an underlying comorbidity. Hospital admission rates in children with reinfection were similar for the first (64 [2·7%] of 2343) and second infection (57 [2·4%] of 2343) and included 12 children who were admitted to hospital in both infection periods, who all had additional health issues.
While reviewing the ICD-10 codes provided with hospital admission, 77% (49 of 64) and 72% (41 of 57) of children admitted to hospital had other codes unrelated to COVID-19 diagnosis recorded at first and second infection. Additionally, five children were admitted to hospital specifically for injuries during a first COVID-19 infection and a further five during a reinfection. The median age of children admitted to hospital was slightly lower for primary infection cases (11 years, IQR 7–14) than for reinfection cases (13 years, 10–15). The proportion of children reporting asymptomatic infection at the time of testing was higher in reinfection cases than in primary cases (1038 [49·1%] 2113 vs 235 914 [37·0%] of 638 389; p<0·0001).
Admission to an intensive care unit was rare after primary infection (n=7) or reinfection (n=4). Notably, all four children admitted to an ICU following reinfection had also required intensive care during their primary infection. All four children had multiple and severe multisystem co-morbidities and, despite detailed case note review, ascertaining the contribution of SARS-CoV-2 infection to the illness that eventually led to the intensive care admission was not possible.
There were 44 deaths within 28 days of testing positive for SARS-CoV-2 (44 [0·01%] of 688 418 primary infections), with a further nine deaths occurring within 60 days of testing positive or with COVID-19 specified as a cause of death on the death certificate (53 [0·01%] 688 418 primary infections). All deaths occurred following primary infection and there were no deaths within 28 days or 60 days of positive testing for SARS-CoV-2 after reinfection.
Discussion
SARS-CoV-2 reinfections in children were uncommon and closely followed community infection rates in England, with most reinfections occurring during the delta wave in the summer of 2021. Children had a lower risk of reinfection than did adults overall, especially when compared with unvaccinated younger adults. Among children, reinfections were low up to age 6 years and then increased steadily up to age 16 years, broadly consistent with their risk of primary infection. Severe infection in children, as evidenced by hospital admissions or intensive care admissions, was rare following either primary infection or reinfection, with a high prevalence of comorbidities among children admitted to hospital with reinfection and no deaths reported up to 28 days after reinfection episode.
Several important factors need to be considered when assessing the risk of reinfection in this cohort. First, reinfection cases were based on sequential positive tests with an interval of at least 90 days and did not require a negative PCR result between infection episodes or a genetically distinct SARS-CoV-2 strain at each episode, as specified in other definitions.3 As of Oct 31, 2021, there were only 441 confirmed reinfections with genetically distinct strains in England.16 We used a pragmatic definition applied to surveillance data that allowed sufficient time for clinical recovery and for the initial PCR test to become negative before a second infection was diagnosed to rule out persistent infections, while acknowledging that reinfection might rarely occur within 90 days of primary infection. The population reinfection rates in England increased sharply following the emergence of the omicron variant from November, 2021, onwards. From this point reinfections accounted for increased proportion of all first infections and reinfections in all ages.17
Secondly, only limited testing for SARS-CoV-2 was available during the early stages of the pandemic, with testing restricted primarily to symptomatic individuals attending health-care settings. Because children are significantly less likely than are adults to develop symptomatic infection, severe disease, or be admitted to hospital for COVID-19, earlier infections would probably not have been confirmed in a laboratory.18 Our serosurveillance in educational settings estimated that 11·2% of primary school students (age 5–11 years) had been infected with SARS-CoV-2 by June, 2020,19 and 12·8% of secondary school students (age 11–16 years) by September, 2020.20 Thirdly, community testing became more widely available from June, 2020, including for children, but was primarily targeted to symptomatic individuals with typical COVID-19 symptoms (fever, new-onset cough, and loss of taste or smell). Because children are more likely than are adults to develop a mild, transient illness when exposed to SARS-CoV-2 (and therefore, not seek medical attention for testing or treatment) and to develop non-respiratory symptoms,21 they would potentially be less likely than adults to undergo testing even after community testing was available. Fourthly, national testing data would not include asymptomatic primary infections or reinfections unless individuals were tested because they were concerned over exposure to a case, and children are more likely to be asymptomatic—and therefore, less likely to be tested— than are adults.22 Fifthly, the introduction of twice-weekly rapid home testing with LFDs for secondary school pupils since March, 2021, will probably have enhanced case ascertainment, including asymptomatic infections, leading to reduced virus introduction and in-school transmission in 11–16 year-olds. Finally, the risk of reinfection is also likely to vary with time since primary infection and with different SARS-CoV-2 variants. However, comparison of relative risks between variants is not possible because they emerged at different times, such that both the number of people with primary infections who became susceptible to reinfection, and the interval between primary infection and reinfection, were far greater during the delta wave in July, 2021, than in the alpha wave in December, 2020.
Furthermore, comparison with adults is hampered by differential exposure risks over time, with prolonged periods of national lockdown, which at times included school closures. Schools remained open for in-person teaching for some age groups during national lockdown in June–July, 2020, November, 2020, and March–May, 2021, but closed during January–February, 2021, when England was experiencing the second pandemic wave due to the alpha variant.23 Among school-aged children, testing rates were heavily influenced by school closure due to lockdowns or school term breaks.24 The national rollout of COVID-19 vaccines from December, 2020, with gradual extension of eligibility down the age groups to those aged 12 and older, also affected comparison of risk and rates over time.
While keeping these considerations in mind, we have for the first time estimated the risk of reinfection in children under 16 years and compared them to the risk in adults over a 19-month period. Reinfections occur frequently after infection with seasonal coronaviruses, which cause the common cold (229E, OC43, NL63, and HKU1) because of short-lasting, poorly cross-protective immunity between infections.25 Reinfections have also been reported with SARS-CoV-2, although the risk was previously estimated to be very low during the first few months after primary infection in adults and even lower in children, although data on reinfections are scarce in the paediatric population.3, 26, 27 Our study confirms a lower risk of reinfection in children compared with in adults, especially unvaccinated young adults and, within the childhood age groups, reinfection rates broadly tracked with primary infection rates by age in years except in infants (younger than age 1 year) who had a higher primary infection rate than reinfection rate. This result is likely to be a testing artefact because of screening in newborns for SARS-CoV-2 and as part of infection screens in young infants presenting to hospital with fever or an infectious illness.28
While the vast majority of individuals develop robust protection after SARS-CoV-2 exposure, there is wide heterogeneity in the immune response after primary infection, in terms of neutralising antibodies and T and B cell repertoire, which might render a small proportion of individuals susceptible to reinfection, especially those who were asymptomatic or had mild illness with their primary infection.3 Reinfections might also occur because of waning immunity with time since primary infection; while most children and adults retain high antibody concentrations with virus neutralising activity for at least 12 months after primary infection, they do wane to undetectable concentrations in a small proportion of individuals.2 Waning of immunity has also been reported among vaccinated adults, resulting in increasing risk of breakthrough infections with time since vaccination,29 although this risk appears to be lower in mRNA-vaccinated adults with previous SARS-CoV-2 infection than in mRNA-vaccinated adults without previous infection.30
Importantly, the risk of reinfection appears to be associated with emergence of new variants—such as the beta, gamma, delta and, more recently, omicron variants—which can at least partially evade immunity from previous infection as well as vaccination.5 In our cohort, reinfection rates were much higher during the delta wave than the alpha wave, which might be because the alpha variant is antigenically similar to previously circulating SARS-CoV-2 strains while the delta variant, like the beta variant, are antigenically distant.31 This observation is consistent with the lower neutralising activity of antibodies after natural infection,31 or vaccination,32, 33 as well as lower vaccine effectiveness after mRNA vaccination34 against the delta variant than the alpha and previous variants. Reassuringly, previous infection or COVID-19 vaccination (and more so, a combination of the two) have been shown to be highly protective against severe disease and death due to COVID-19, irrespective of the responsible variant, with studies reporting higher rates of asymptomatic infection and mild illness following reinfection than primary infection in the same individuals.3, 4, 34 This result was also observed in our cohort of children who were more likely to be asymptomatic with their reinfection than with their primary infection.
However, the picture of SARS-CoV-2 infection and reinfection is everchanging with the emergence of new variants. The omicron variant was first detected in England in late November, 2021, and outcompeted delta to become the dominant variant nationally a few weeks later.35 omicron possesses multiple new mutations compared with previous SARS-CoV-2 variants, including some in the spike protein, which enhance its ability to further evade both natural and vaccine-induced immunity.36 Omicron's potential for rapid transmission with a shorter incubation period has led to record numbers of daily reported cases and elevated numbers of reinfection cases in England.37 Early data from South Africa, where the variant was first identified, reassuringly indicate a decrease in disease severity with the omicron compared with the delta variant,38 which is likely to be due to a combination of factors, including virus characteristics, previous SARS-CoV-2 infection with other variants, and COVID-19 vaccination. Our ongoing national surveillance will continue to monitor the risks, severity, and outcomes of reinfection with this new variant.
In our cohort of children with reinfection, the risk of hospital admission was similar to overall admission rates for confirmed childhood COVID-19,39 while ICU admissions were rare and less than in primary infection cases, and mainly in those with severe underlying comorbidities. In the UK, COVID-19 mRNA vaccination is recommended for all those aged 12–15 years and at-risk 5–11-year-olds with underlying co-morbidities, which will help reduce their risk of severe disease after primary infection or reinfection.40
In addition to the limitations already discussed, we did not collect detailed clinical data on all the reinfection cases to assess disease severity compared with primary infection. Among those who had been admitted to hospital, we were unable to distinguish whether the children were admitted because of COVID-19 illness or if the virus was identified incidentally during routine screening of children attending hospital for other illnesses. The emergence of new variants also made it difficult to assess waning of natural immunity over time. We cannot comment on the risk of reinfection with the omicron variant, which emerged after the study period. As children continue to be vaccinated against COVID-19, assessing long-term protection after natural infection will become more difficult. Finally, we did not assess the risk of complications, such as paediatric inflammatory multisystem syndrome temporally associated with SARS-CoV-2 (PIMS-TS, also known as multisystem inflammatory syndrome in children; MIS-C) and long COVID, after primary SARS-CoV-2 infection or reinfection. Further studies are needed to evaluate the risk of such complications after reinfection compared with primary infection in children.
In children younger than 16 years old, the risk of SARS-CoV-2 reinfection is strongly related to the risk of exposure and, therefore, community infection rates. In England, reinfection rates were higher during the delta wave than the alpha wave, most likely the result of a combination of waning immunity over time since primary infection and partial immune evasion by the delta variant compared with the alpha variant. In children, the risk of reinfection increased with age. Most children had mild infection with very low hospital admission rates after primary infection or reinfection. COVID-19 vaccination will help reduce the risk of severe disease and improve outcomes after primary infection and reinfection, especially in children with underlying comorbidities who might have been over-represented among reinfection cases. The emergence of new variants emphasises the importance of ongoing surveillance of reinfections.
This online publication has been corrected. The corrected version first appeared at thelancet.com/child-adolescent on April 18, 2022
Data sharing
The national datasets used in this research contain personal identifiable information which was used to link test results and are not available to the public. Aggregated positive test numbers are published daily on the UK coronavirus dashboard (https://coronavirus.data.gov.uk/) and the UK Health Security Agency publishes data on numbers of possible reinfections in the National flu and COVID-19 surveillance reports (https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports-2021-to-2022-season). Any additional data requests should be made through the corresponding author.
Declaration of interests
We declare no competing interests.
Contributors
AAM, HC, and SNL were involved in the methodology, formal analysis, investigation, data curation, writing the original draft, reviewing and editing the manuscript, designing of tables and graphs, and verified the underlying data. JS, GS, RS, SO'B, AB, and JL were involved in data curation, and reviewing and editing the manuscript. SNL, HC, KB, and MER were involved in conceptualisation, methodology, supervision, and reviewing and editing the manuscript. All authors had full access to all data in the study, and accept responsibility for the decision to submit for publication.
Supplementary Material
References
- 1.Gaythorpe KAM, Bhatia S, Mangal T, et al. Children's role in the COVID-19 pandemic: a systematic review of early surveillance data on susceptibility, severity, and transmissibility. Sci Rep. 2021;11 doi: 10.1038/s41598-021-92500-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dowell AC, Butler MS, Jinks E, et al. Children develop robust and sustained spike-specific immune responses to SARS-CoV-2 infection. Nat Immunol. 2022;23:40–49. doi: 10.1038/s41590-021-01089-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Boyton RJ, Altmann DM. Risk of SARS-CoV-2 reinfection after natural infection. Lancet. 2021;397:1161–1163. doi: 10.1016/S0140-6736(21)00662-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fakhroo A, AlKhatib HA, Al Thani AA, Yassine HM. Reinfections in COVID-19 patients: impact of virus genetic variability and host immunity. Vaccines (Basel) 2021;9 doi: 10.3390/vaccines9101168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Milne G, Hames T, Scotton C, et al. Does infection with or vaccination against SARS-CoV-2 lead to lasting immunity? Lancet Respir Med. 2021;9:1450–1466. doi: 10.1016/S2213-2600(21)00407-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edara VV, Pinsky BA, Suthar MS, et al. Infection and vaccine-induced neutralizing-antibody responses to the SARS-CoV-2 B.1.617 variants. N Engl J Med. 2021;385:664–666. doi: 10.1056/NEJMc2107799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marquez L, Koy T, Spinler JK, et al. Reinfection with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) B.11.7 variant in an immunocompromised adolescent. Infect Control Hosp Epidemiol. 2021:1–2. doi: 10.1017/ice.2021.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yadav SP, Wadhwa T, Thakkar D, Kapoor R, Rastogi N, Sarma S. COVID-19 reinfection in two children with cancer. Pediatr Hematol Oncol. 2021;38:403–405. doi: 10.1080/08880018.2020.1855276. [DOI] [PubMed] [Google Scholar]
- 9.Ladhani SN, Amin-Chowdhury Z, Davies HG, et al. COVID-19 in children: analysis of the first pandemic peak in England. Arch Dis Child. 2020;105:1180–1185. doi: 10.1136/archdischild-2020-320042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mensah AA, Sinnathamby M, Zaidi A, et al. SARS-CoV-2 infections in children following the full re-opening of schools and the impact of national lockdown: prospective, national observational cohort surveillance, July–December 2020, England. J Infect. 2021;82:67–74. doi: 10.1016/j.jinf.2021.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.UK Coronavirus Dashboard 2021. https://coronavirus.data.gov.uk/details/testing
- 12.European Centre for Disease Prevention and Control . European Centre for Disease Prevention and Control; Stockholm: 2021. Reinfection with SARS-CoV-2: implementation of a surveillance case definition within the EU/EEA. [Google Scholar]
- 13.Office for National Statistics Population estimates for the UK, England and Wales, Scotland and Northern Ireland: mid-2020. 2021. https://www.ons.gov.uk/peoplepopulationandcommunity/populationandmigration/populationestimates/bulletins/annualmidyearpopulationestimates/mid2020
- 14.Department of Health & Social Care Joint Committee on Vaccination and Immunisation: advice on priority groups for COVID-19 vaccination, 30 December 2020. 2021. https://www.gov.uk/government/publications/priority-groups-for-coronavirus-covid-19-vaccination-advice-from-the-jcvi-30-december-2020/joint-committee-on-vaccination-and-immunisation-advice-on-priority-groups-for-covid-19-vaccination-30-december-2020 mid2020
- 15.NHS COVID-19 vaccinations archive. 2021. https://www.england.nhs.uk/statistics/statistical-work-areas/covid-19-vaccinations/covid-19-vaccinations-archive/
- 16.UK Health Security Agency Weekly national influenza and COVID-19 surveillance report. Week 44 report (up to week 43 data) 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1031083/Weekly_Flu_and_COVID-19_report_w44.pdf
- 17.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 33. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1043807/technical-briefing-33.pdf
- 18.Yasuhara J, Kuno T, Takagi H, Sumitomo N. Clinical characteristics of COVID-19 in children: a systematic review. Pediatr Pulmonol. 2020;55:2565–2575. doi: 10.1002/ppul.24991. [DOI] [PubMed] [Google Scholar]
- 19.Ladhani SN, Baawuah F, Beckmann J, et al. SARS-CoV-2 infection and transmission in primary schools in England in June–December, 2020 (sKIDs): an active, prospective surveillance study. Lancet Child Adolesc Health. 2021;5:417–427. doi: 10.1016/S2352-4642(21)00061-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ladhani SN, Ireland G, Baawuah F, et al. SARS-CoV-2 infection, antibody positivity and seroconversion rates in staff and students following full reopening of secondary schools in England: a prospective cohort study, September–December 2020. EClinicalMedicine. 2021;37 doi: 10.1016/j.eclinm.2021.100948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Christophers B, Gallo Marin B, Oliva R, Powell WT, Savage TJ, Michelow IC. Trends in clinical presentation of children with COVID-19: a systematic review of individual participant data. Pediatr Res. 2022;91:494–501. doi: 10.1038/s41390-020-01161-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sah P, Fitzpatrick MC, Zimmer CF, et al. Asymptomatic SARS-CoV-2 infection: a systematic review and meta-analysis. Proc Natl Acad Sci USA. 2021;118 doi: 10.1073/pnas.2109229118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ladhani SN, Ireland G, Baawuah F, et al. Emergence of SARS-CoV-2 Alpha (B.1.1.7) variant, infection rates, antibody seroconversion and seroprevalence rates in secondary school students and staff: active prospective surveillance, December 2020 to March 2021, England. J Infect. 2021;83:573–580. doi: 10.1016/j.jinf.2021.08.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.UK Health Security Agency Weekly influenza and COVID-19 surveillance graphs. 2021. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1034350/Weekly_COVID-19_and_Influenza_Surveillance_Graphs_w46.pdf
- 25.Edridge AWD, Kaczorowska J, Hoste ACR, et al. Seasonal coronavirus protective immunity is short-lasting. Nat Med. 2020;26:1691–1693. doi: 10.1038/s41591-020-1083-1. [DOI] [PubMed] [Google Scholar]
- 26.Pilz S, Chakeri A, Ioannidis JP, et al. SARS-CoV-2 re-infection risk in Austria. Eur J Clin Invest. 2021;51 doi: 10.1111/eci.13520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Sanchez-Montalva A, Fernandez-Naval C, Anton A, et al. Risk of SARS-CoV-2 infection in previously infected and non-infected cohorts of health workers at high risk of exposure. J Clin Med. 2021;10 doi: 10.3390/jcm10091968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mileto D, Fenizia C, Cutrera M, et al. SARS-CoV-2 mRNA vaccine BNT162b2 triggers a consistent cross-variant humoral and cellular response. Emerg Microbes Infect. 2021;10:2235–2243. doi: 10.1080/22221751.2021.2004866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mizrahi B, Lotan R, Kalkstein N, et al. Correlation of SARS-CoV-2-breakthrough infections to time-from-vaccine. Nat Commun. 2021;12 doi: 10.1038/s41467-021-26672-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abu-Raddad LJ, Chemaitelly H, Ayoub HH, et al. Association of prior SARS-CoV-2 infection with risk of breakthrough infection following mRNA vaccination in Qatar. JAMA. 2021;326:1930–1939. doi: 10.1001/jama.2021.19623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dupont L, Snell LB, Graham C, et al. Neutralizing antibody activity in convalescent sera from infection in humans with SARS-CoV-2 and variants of concern. Nat Microbiol. 2021;6:1433–1442. doi: 10.1038/s41564-021-00974-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Choi A, Koch M, Wu K, et al. Serum neutralizing activity of mRNA-1273 against SARS-CoV-2 variants. J Virol. 2021;95 doi: 10.1128/JVI.01313-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Noori M, Nejadghaderi SA, Arshi S, et al. Potency of BNT162b2 and mRNA-1273 vaccine-induced neutralizing antibodies against severe acute respiratory syndrome-CoV-2 variants of concern: a systematic review of in vitro studies. Rev Med Virol. 2021 doi: 10.1002/rmv.2277. published online July 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lopez Bernal J, Andrews N, Gower C, et al. Effectiveness of Covid-19 vaccines against the B.1.617.2 (Delta) variant. N Engl J Med. 2021;385:585–594. doi: 10.1056/NEJMoa2108891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.UK Health Security Agency SARS-CoV-2 variants of concern and variants under investigation in England. Technical briefing 34. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1050236/technical-briefing-34-14-january-2022.pdf
- 36.Kannan S, Shaik Syed Ali P, Sheeza A. Omicron (B.1.1.529)—variant of concern—molecular profile and epidemiology: a mini review. Eur Rev Med Pharmacol Sci. 2021;25:8019–8022. doi: 10.26355/eurrev_202112_27653. [DOI] [PubMed] [Google Scholar]
- 37.UK Health Security Agency Weekly influenza and COVID-19 surveillance graphs. 2022. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/1046282/weekly-covid-19-and-influenza-surveillance-graphs-week-2-2022.pdf
- 38.Abdullah F, Myers J, Basu D, et al. Decreased severity of disease during the first global omicron variant Covid-19 outbreak in a large hospital in Tshwane, South Africa. Int J Infect Dis. 2021;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.American Academy of Pediatrics Children and COVID-19: State-Level Data Report. 2021. https://www.aap.org/en/pages/2019-novel-coronavirus-covid-19-infections/children-and-covid-19-state-level-data-report/
- 40.NHS Who can get a coronavirus (COVID-19) vaccine. 2021. https://www.nhs.uk/conditions/coronavirus-covid-19/coronavirus-vaccination/who-can-get-the-vaccine/
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The national datasets used in this research contain personal identifiable information which was used to link test results and are not available to the public. Aggregated positive test numbers are published daily on the UK coronavirus dashboard (https://coronavirus.data.gov.uk/) and the UK Health Security Agency publishes data on numbers of possible reinfections in the National flu and COVID-19 surveillance reports (https://www.gov.uk/government/statistics/national-flu-and-covid-19-surveillance-reports-2021-to-2022-season). Any additional data requests should be made through the corresponding author.